Abstract
Vibrio cholerae of serogroup O1 and O139, the etiological agent of the diarrheal disease cholera, expresses the extracellular Zn-dependent metalloprotease hemagglutinin (HA)/protease also reported as vibriolysin. This enzyme is also produced by non-O1/O139 (non-cholera) strains that cause mild, sporadic illness (i.e. gastroenteritis, wound or ear infections). Orthologs of HA/protease are present in other members of the Vibrionaceae family pathogenic to humans and fish. HA/protease belongs to the M4 neutral peptidase family and displays significant amino acid sequence homology to Pseudomonas aeruginosa elastase (LasB) and Bacillus thermoproteolyticus thermolysin. It exhibits a broad range of potentially pathogenic activities in cell culture and animal models. These activities range from the covalent modification of other toxins, the degradation of the protective mucus barrier and disruption of intestinal tight junctions. Here we review (i) the structure and regulation of HA/protease expression, (ii) its interaction with other toxins and the intestinal mucosa and (iii) discuss the possible role(s) of HA/protease in the pathogenesis of cholera.
1. Introduction
Cholera is an acute, water-borne diarrheal disease caused by the facultative, Gram-negative bacterium Vibrio cholerae of serogroup O1 of the classical and El Tor biotype and serogroup O139, which originated from the El Tor biotype and exhibits a distinct lipopolysaccharide [1]. The O1 V. cholerae serogroup contains a common A antigen and can be subdivided in Ogawa and Inaba serotypes on the basis of serotype-specific antigens B and C, respectively [2]. Mankind has experienced seven recorded cholera pandemics. The seventh and current pandemic is characterized by the predominance of O1 strains of the El Tor biotype with sporadic emergence of serogroup O139. Approximately 5 million cases of cholera and 130,000 deaths occur annually (http://www.cdc.gov/cholera/general). Endemic cholera continues to be a major public health problem in vast regions of South Asia and Africa. Introduction of virulent V. cholerae O1 in non-endemic areas with low sanitation can result in rapidly spreading outbreaks as occurred in 2010 in Haiti [3]. The typical symptoms of this illness include a profuse rice-watery diarrhea and vomiting. If untreated, this condition can lead to severe dehydration, electrolyte imbalance, and death.
V. cholerae O1 and O139 strains expresses two major virulence factors: (i) cholera toxin (CT) and (ii) the toxin co-regulated pilus (TCP). The TCP is a type IV pilus that mediates adherence and microcolony formation and is required for intestinal colonization in neonate mice and humans [4-6]. CT is an ADP-ribosyltransferase responsible for the profuse rice-watery diarrhea typical of this disease [2, 7, 8]. It is composed of one A subunit, which catalyzes NAD-dependent ADP-ribosylation of host adenylate cyclase and five B subunits that carry the ganglioside GM1 receptor binding site [9]. CT is well recognized as the major secretogenic factor causing the clinical symptoms of cholera. However, V. cholerae produces additional toxic factors, such the zonula occludens toxin (Zot) [10, 11], the accessory cholera enterotoxin (Ace) [12], the repeat toxin (RTX) [13], hemolysin (HlyA) [14] and the metalloprotease hemagglutinin(HA)/protease [15]. The contributions of these secondary factors to the pathogenesis of cholera has been difficult to dissect due to strain diversity and the lack of a single animal model fully mimicking the disease as it occurs in humans. It is likely, however, that expression of the above auxiliary toxins could modulate the course of an infection, which is known to vary in clinical symptoms from asymptomatic or mild to severe and life-threatening.
Extracellular metalloproteases are widely distributed among bacteria and pathogenic vibrios (reviewed in [16-18]). In this article we concisely summarize our knowledge of the structure, regulation and pathogenic activities of HA/protease, a Zn-dependent metalloprotease with mucinase activity [15, 19, 20]. We show that HA/protease exhibits a broad range of potentially pathogenic activities in cell culture and animal models. These activities include the covalent modification of other toxins, the degradation of the protective mucus barrier and disruption of intestinal tight junctions. A critical assessment of published in vitro and in vivo studies suggests that HA/protease can enhance the pathogenesis of cholera by (i) increasing the activity of other toxic factors (ii) providing access of vibrios or their toxic factors to the microvilli underlying the protective mucus barrier and (iii) facilitating the dissemination of infecting vibrios along the gastrointestinal tract.
2. Structure and substrate specificity of HA/protease
An extracellular protein with hemagglutinating and proteolytic activities was initially purified from V. cholerae strain CA401 and denoted cholera lectin [21]. The protease was subsequently demonstrated to be a metalloprotease [19] acting on several physiologically relevant substrates such as fibronectin and mucin and was also shown to cleave lactoferrin and nick the A subunit of the Escherichia coli heat labile toxin (LT) [20]. Cloning and sequencing of the hapA gene encoding HA/protease showed that the protein is highly homologous to Pseudomonas aeruginosa elastase (LasB) [15, 22], a metalloprotease known to degrade components of the extracellular matrix during acute and chronic P. aeruginosa infection, breach epithelial cell tight junctions and cleave pulmonary surfactants [23]. HA/protease also shows significant amino acid sequence homology to Vibrio vulnificus elastase (VvpE), which contributes to local tissue damage during vibriosis caused by this human pathogen [24]. The domain structure of HA/protease is shown in Fig. 1. The amino acid sequence of HA/protease begins with a signal peptide followed by a propeptide. The propeptide includes a fungalysin/thermolysin propeptide (FTP) domain and a PepSY domain. These domains are suggested to have chaperone and/or a protease inhibitor functions that prevent the activation of the enzyme prior to its secretion into the extracellular milieu. N-terminal sequencing of HA/protease indicated that the propeptide is cleaved to generate a mature protease starting at A196. The amino acid sequence of the mature protein places HA/protease within the M4 thermolysin family of Zn-dependent secreted eubacterial endopeptidases containing a HEXXH motif, which has been shown in crystallographic studies to form part of the metal-binding site. Finally, the amino acid sequence of HA/protease ends with a prepeptidase C-terminal domain. This domain is found at the C-terminus of secreted bacterial peptidases and is commonly not present in the active enzyme. The difference between the predicted molecular weight of the mature HA/protease (47 kDa) and the actual molecular weight of purified HA/protease (32 kDa) suggests that the prepeptidase C-terminal domain is indeed removed during HA/protease maturation [15]. The crystal structure of HA/protease has been deduced by homology modeling with P. aeruginosa LasB [25].
Fig. 1. HA/protease domain architecture.
The predicted signal peptide sequence (residues 1-24) is indicated in italics. The propeptide sequence is underlined and includes the FTP and PepSY domains typical of the M4 peptidase family. The Zn binding residues H343, H347 and E367 are shown in bold font. Conserved active site residues E344 and H426 are indicated with an asterisk. The prepeptidase C-terminal domain is typical of secreted bacterial proteases but is commonly not present in the active peptidase. The predicted cleavage sites of the signal peptide and propeptide are indicated by arrows above the sequence.
3. Regulation of HA/protease expression
3.1. Transcription regulation
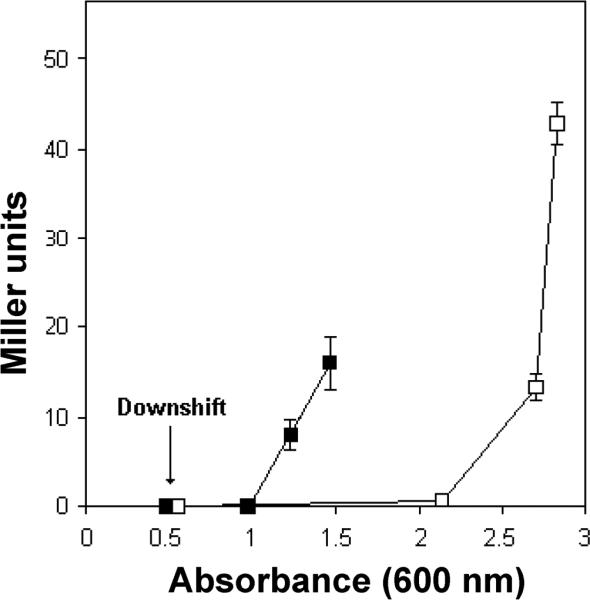
In V. cholerae, the transcription of hapA is activated under culture conditions that integrate nutrient limitation, entry into stationary phase and high cell population density (Fig. 2). Transcription of hapA requires the quorum sensing regulator HapR [26, 27] and the RNA polymerase alternative sigma factor RpoS (σS) [27, 28]. The cAMP receptor protein (CRP) acts upstream of HapR and RpoS to integrate the nutrient limitation and cell density stimuli [27, 29, 30]. The regulatory circuit responsible for HA/protease expression is shown in Fig. 3. Briefly, conditions of nutrient limitation result in elevation of the intracelllular cAMP pool and activation of RpoS and CRP [27]. Activation of CRP furtther enhances the transcription of rpoS [27]. In parallel, CRP activates HapR expression to integrate nutritional and population cell density signals [29-31]. HapR activates the transcription of hapA directly and indirectly by increasing the expression of RpoS [32]. The end result of these regulatory connections is that maximal expression of HA/protease occurs in cultures entering stationary phase at high cell density. In rich laboratory media, vibrios enter quorum sensing mode and express hapA at cell densities higher than 2 × 108 cells/mL [33]. It is well established that V. cholerae can reach high titers in the human gut and cholera patients can shed up to 109 virulent vibrios per mL in the rice-watery stool. This points to the conclusion that vibrios can reach the cell density required for the expression of HA/protease in the environment of the small intestine.
Fig. 2. The combined effect of nutrient limitation and high population cell density on the transcription of hapA.
The El Tor biotype strain AJB2 containing a chromosomally-integrated hapA-lacZ promoter fusion [27] was used to monitor the transcription of hapA. Three cultures of this reporter strain were grown in tryptic soy broth (TSB) to an absorbance at 600 nm 0.5 (arrow). At this stage, one half of each culture was withdrawn, centrifuged and the cell pellet resuspended in 0.25X TSB (nutritional downshift). Transcription of hapA was monitored by measuring β-galactosidase activity (Miller Units). Symbols: ■, cells in 0.25X TSB; □, cells remaining in 1X TSB.
Fig. 3. Regulation of HA/protease expression.
Nutrient limitation results in expression and activation of the RNA polymerase alternative σ subunit S (σS or RpoS) and the cAMP receptor protein (CRP). Nutrient limitation and high population cell density concertedly activate expression of the quorum sensing regulator HapR. HapR and σS activate the transcription of hapA encoding HA/protease which is secreted via the type II secretion system.
It is noteworthy that the expression of HA/protease and CT are invesely regulated. Activation of CRP and expression of HapR at high cell density have been shown to negatively regulate the transcription of ctxAB and tcpA encoding CT and TCP, respectively [34, 35].
3.2. Secretion of HA/protease
Secretion of HA/protease into the extracellular medium is a two stage process. First, the preproprotein or primary translation product is translocated through the cytoplasmic membrane with removal of its signal peptide by a Sec-dependent mechanism. Second, the proprotein folds in the periplasmic space and is secreted across the outer membrane via the type II secretion system, a multiprotein complex encoded by 12 eps (extracellular protein secretion) genes [36, 37]. Removal of the HA/protease propeptide and C-terminal processing is presumed to occur in the extracellular medium through an autocatalytic mechanism [15]. The Eps complex colocalizes with the flagellum at the old pole of the cell after cell division to deliver HA/protease and other cargo proteins to the extracellular mileu [38]. The fully assembled cholera holotoxin is also secreted to the medium through the Eps pathway [37]. These findings suggests a level of coordination in the secretion of the polar flagellum, CT and HA/protease. Consistent with this view, atoxigenic V. cholerae O1 (ΔctxAB) mutants commonly secrete more HA/protease into the culture medium [39, 40].
4. Pathogenic activities of HA/protease identified in vitro
4.1. Nicking and activation of cholera toxin
The A subunit of CT containing the ADP-ribosyltransferase activity consists of domain A1 and A2. Enzyme activity is located in domain A1 while the C-terminus of domain A2 inserts into the doughnut-like pentameric B subunit ring. Upon delivery of CT into the intestinal lumen and prior to endocytosis by epithelial cells, the A subunit is activated by cleavage at a serine protease site into A1 and A2 polypeptide that remain bridged by a disulphide bond [41]. It is recognized that this activation could be carried out by host proteases since treatment of CT with trypsin, a common protease of the small intestine, generates a polypeptide similar in size to the A1 peptide [42]. However, it was shown that HA/protease is capable of nicking and activating CT [42]. Biochemical studies identified the nicking site at R192 for the homologous LT A subunit [43]. An independent study using purified HA/protease from non-O1 V. cholerae identified the nicking site at the junction of T193/I194 for both LT and CT A subunits [44]. Based on our knowledge of the regulation of CT and HA/protease expression (section 3.1), it is likely that a significant fraction of CT is nicked and activated by host proteases during infection prior to the onset of HA/protease secretion. Thus, we suggest that expression of HA/protease can potentially contribute to the activation of CT and fluid secretion by processing those CT molecules remaining unnicked late in infection.
4.2. HA/protease and the El Tor cytolysin/hemolysin
Current V. cholerae pandemic strains secrete a an extracellular pore-forming toxin, designated cholera hemolysin (HlyA) or V. cholerae cytolysin/hemolysin. The toxin is synthesized as a 82 kDa preprotoxin, its signal peptide removed during secretion into the culture medium to yield a 79 kDa protoxin, which is subsequently processed into a 65 kDa monomer by removal of the propeptide [45]. The crystal structure of the mature HlyA monomer and the amphipathic β-barrel heptamer has been solved [46, 47]. The monomer interacts with a cell surface receptor, assembles into its β-barrel heptameric form and inserts into the membrane lipid bilayer of host cells [48].
The role of HlyA in the pathogenesis of pandemic cholera remains ill-defined. The finding that some non-O1/O139 serogroup V. cholerae strains that do not produce CT can still cause watery diarrhea suggested that HlyA could play a secondary role in promoting intestinal fluid secretion [49]. In vitro studies have shown that HlyA induces cell vacuolation of cultured Vero cells [50-53]. Furthermore, ex vivo electrophysiological studies in Ussing chambers have shown that HlyA triggers Cl− secretion from intact intestinal mucosa, a critical step preceding the onset of intestinal fluid secretion [54]. This result is consistent with previous in vivo studies showing that purified HlyA induced fluid secretion when injected into rabbit ileal loops, administered by intraintestinal injection to infant rabbits, or orally inoculated to suckling mice [55]. Recently, HlyA was shown to be responsible for lethality, developmental delay and intestinal vacuolation in a novel nematode (Caenorhabditis elegans) model of infection [56]. Microarray studies have shown that the hlyA gene encoding hemolysin is highly expressed in V. cholerae grown in rabbit ileal loops [57]. Thus, HlyA is a well-recognized member of the V. cholerae arsenal of toxic factors that can modulate the severity of cholera or cause gastroenteritis due to infection with non-cholera vibrios.
It has been shown that HA/protease, trypsin, chymotrypsin, subtilisin, papain, and thermolysin can process pro-HlyA to the 65 kDa active and mature form of the protein [58]. Thus, similar to the processing of CT, HA/protease could contribute concertedly with host intestinal proteases to the activation of HlyA. This view, however, has been contested by the finding that expression of HlyA is negatively regulated by the quorum sensing regulator HapR at the level of transcription and at the posttranslational level by HA/protease [59]. It was shown that HA/protease degrades HlyA to diminish V. cholerae hemolytic activity [59]. Therefore, the above study suggests that both CT and HlyA are expressed during infection at low cell density and their transcription coordinately repressed at high cell density by quorum sensing. In contrast to CT, however, HA/protease could degrade rather than activate remaining HlyA protein late in infection.
4.3. Cleavage of intestinal tight junction-associated proteins
Epithelial cells (enterocytes) lining the intestinal mucosa are attached to one another by an intercellular apical junction complex located beneath the apical surface consisting of a tight junction, an adherence junction and a desmosome (reviewed in [60]). Tight junctions function to seal adjacent epithelial cells and limit the passage of molecules and ions through the space between cells known as the paracellular pathway. Alterations in tight junction function can be demonstrated by measuring transepithelial electrical resistance in Ussing chambers as shown for V. cholerae Zot [10, 61]. A concentrated culture supernatant of a V. cholerae strain deleted for genes encoding CT, Ace, Zot and HlyA was shown to disrupt paracellular barrier function in cultured Mardin Darby Canine Kidney (MDCK-I) epithelial cells [62]. The cytotoxic factor was purified and identified by microsequencing as HA/protease [62]. The changes in transepithelial electrical resistance induced by HA/protease were accompanied by significant morphological changes, rearrangement of the tight junction-associated protein ZO-1 and F-actin filaments [62]. ZO-1 is a component of the mature tight junction located at the cytoplasmic side of the plasma membrane that interacts with the cytoskeleton of epithelial cells. The primary target of HA/protease was subsequently demonstrated to be occludin [63]. Occludin is a transmembrane proteins that spans the plasma membrane four times, allow adjacent cells to interact through its extracellular loops [60] and interacts with ZO-1. Cleavage of occludin by HA/protease resulted in rearrangement of ZO-1, the F-actin cytoskeleton and disruption of paracellular barrier function [63]. Production of endogenous nitric oxide protected MDCK-I cells from the cytotoxic effect of HA/protease [64]. Subsequent studies with polarized T84 cells confirmed that HA/protease acts to decrease transepithelial electrical resistance of cultured cells [39].
4.4. Role of HA/protease in biofilm development
Biofilms are sessile communities characterized by cells that are attached to a substratum or to each other and are embedded in a self-produced matrix consisting of exopolysaccharide, proteins and extracellular DNA. Clinical biofilms exhibit enhanced resistance to clearance by innate host defense mechanisms (i.e. bile, antimicrobial peptides) and antibiotics [65, 66]. Similar to other bacterial pathogens, V. cholerae is capable of forming stress-resistant biofilms during infection [67]. The V. cholerae biofilm protein RbmA is expressed on the surface of cells that make the matrix exopolysaccharide and functions to enhance cell-to-cell adhesion [68]. A recent study showed that HA/protease participates in limited proteolysis of RbmA to a form capable of promoting interaction between biofilm cells and bystander planktonic cells thereby recruiting cells to the growing biofilm [69].
5. HA/protease and cholera pathogenesis
5.1. Role of HA/protease in intestinal fluid secretion
To date, it has not been possible to connect clinical symptoms in cholera patients to any factor other than CT. However, it is clear that clinical symptoms vary widely between clinical isolate that produce CT. These differences can be due to host susceptibility and the expression of additional toxic factors that can significantly alter the course of an infection. The activities of HA/protease described above suggests that this metalloprotease can increase the severity of a cholera infection by multiple mechanisms. We have shown that HA/protease enhances fluid accumulation in the rabbit ileal loop model [40]. We suggested that this activity could be due to the combined effect of activating CT and degradation of the mucus blanket to provide access of the toxin to the underlying microvilli as suggested by Crowther et al. [70]. This result was in agreement with experiments demonstrating that pretreatment of ileal loops with purified HA/protease enhanced V. cholerae enterotoxicity [71]. In this experiment, however, pretreatment of ileal loops with HA/protease enhanced fluid accumulation caused by live vibrios and not by purified CT. This finding suggests that the predominant role of HA/protease is to facilitate the delivery of CT in the vicinity of its ganglioside GM1 receptor rather than to assist the diffusion of toxin through the protective mucus barrier. A more recent study showed that purified HA/protease induced an hemorrhagic fluid response accompanied with significant tissue damage and inflammation [72]. In experiments conducted in the suckling mouse model, HA/protease induced significant fluid accumulation and caused morphological changes in the small intestine [73]. It remains to be demonstrated if sufficient HA/protease is expressed during infection to elicit similar responses during clinical cholera.
5.2. Role of HA/protease in pathogen penetration of the protective mucus barrier
To cause disease, vibrios must swim toward the intestinal mucosa and penetrate the protective mucus barrier. The mucin-specific adhesin GbpA has been shown to mediate initial adherence of vibrios to the protective mucus blanket [74, 75]. Flagellar motility has been suggested to facilitate the initial penetration of the mucus gel. This view is suppported by the observation that pretreatment of mice with the mucolytic agent N-acetyl-L-cysteine partially restored colonization capacity to non-motile mutants [76]. However, motility is not sufficient for bacteria to effectively penetrate the mucus gel. This conception is supported by studies showing that the polar flagellum is a less effective locomotion organelle in a high viscocity medium [77] and tends to break in the viscous mucus gel [78]. Penetration of the mucus barrier could be as well facilitated by the activity of mucolytic enzymes. We have shown that expression of HA/protease enhanced the penetration of a mucin gel in vitro using a column assay [79]. As suggested above, bacterial penetration of the mucus gel is required for the delivery of secreted CT in close proximity to its GM1 receptor in the the microvilli.
5.3. Role of HA/protease in bacterial detachment and dissemination
HA/protease was hypothesized to function as a “detachase” during infection based on the observation that hapA mutants remained attached for longer periods to Henle-407 cells [80], exhibited enhanced adherence to differentiated mucin-secreting HT29-18N2 cells [81] and elevated association with intestinal tissue compared to wild type [82]. The “detachase activity” of HA/protease could partly result from its mucinase activity [20]. In addition, HA/protease was shown to degrade the GbpA adhesin required for attachment of V. cholerae to intestinal mucin [83]. The concerted activity of HA/protease and motility could allow vibrios to detach, disseminate along the gastrointestinal tract and establish secondary infection foci to increase the severity of cholera [84]. The role of HA/protease could be more complex as suggested by experiments conducted in an adult mice model. This study showed that accessory toxins that included HA/protease contributed to the persistence of V. cholerae for a longer period in the small intestine [85]. However, the contribution of HA/protease to this phenotype was not dissected from other factors such as hemolysin and the multifunctional autoprocessing RTX toxin.
5.4. Role of HA/protease in vaccine reactogenicity
Current cholera vaccines under evaluation comprise two broad categories: inactivated whole cells and live-genetically attenuated vaccines. Unexpectedly, the first generation of live genetically-attenuated vaccine candidates of the El Tor biotype deleted for genes encoding CT exhibited significant reactogenicity consisting of mild diarrhea, nausea, vomiting, abdominal cramps and fever [86]. Moreover, a multiple deletion strains lacking CT, Zot, Ace and HlyA still exhibited reactogenicity in healthy volunteers [87, 88]. Contrary to clinical cholera, reactogenicity appeared to result from an inflammatory process [89, 90]. The aforementioned activities of HA/protease in cell culture and animal models suggested that it could play a role in reactogenicity. Indeed, clinical trials involving a live genetically-attenuated vaccine candidate deleted for hapA confirmed that production of HA/protease contributes to reactogenicity [91, 92]. In addition, a non-motile (nonflagellated) live genetically-attenuated vaccine candidate was also shown to be less reactogenic [93]. The above studies, however, could not distinguish between HA/protease and motility having a direct or indirect contribution to reactogenicity. Cell culture assays have shown that V. cholerae flagellins are proinflammatory [94, 95]. Furthermore, a recent study using an infant rabbit model of reactogenicity confirmed the induction of proinflammatory cytokines and reactogenicity symptoms by V. cholerae flagellins [96]. Taken together, we suggest that HA/protease contributes to reactogenicity by degrading the protective mucus blanket and exposing the underlying epithelial cells to proinflammatory flagellins. This view does not rule out the possibility of elevated expression of HA/protease during infection resulting in direct damage to the intestinal mucosa. Moreover, we can not exclude the possibility of HA/protease activating or providing access of an unidentified toxin to the microvilli or even targeting an anti-inflammatory molecule produced by the gut microbiota.
6. Conclusions
Cholera is the major vibriosis affecting humans and a major global health threat. Although the clinical symptoms of cholera are largely due to the production of CT, V. cholerae produces an arsenal of accessory toxins such as HA/protease that could dramatically influence the course of an infection. Furthermore, accessory toxins could eventually gain a more predominant role in pandemic cholera through pathogen evolution. HA/protease is a prototype Zn-dependent metalloprotease produced by the major human pathogen V. cholerae of serogroup O1 and O139, non-O1/O139 strains and other pathogenic vibrios. HA/protease exhibits a broad range of pathogenic activities that can potentially increase the severity of cholera. In Fig. 4 we summarize the potential targets of HA/protease in the small intestine during infection and how these interactions could alter the outcome of an infection. These targets include both bacterial factors (i.e. CT, HlyA, RbmA, GbpA) and host proteins (i.e. occludin, mucin). It is noteworthy that a significant fraction of V. cholerae predicted genes encode proteins of unknown function. Thus, we could not exclude the possibility of HA/protease contributing to pathogenicity by acting in concert with an unidentified toxic factor. The lack of an animal model fully representing cholera in humans and the elevated cost of volunteer studies has hampered extrapolating the in vitro and preclinical activities of HA/protease to clinical cholera. Since HA/protease appears to act on multiple targets during infection, its role in disease is complex and could be strain-dependent. To date, the only study on the role of HA/protease in humans are the clinical trials involving hapA mutants [91, 92]. These studies demonstrated that HA/protease is expressed during human infection and has a discernible pathological effect on the host. Though unrelated to cholera, a recent study suggested that purified HA/protease exhibits antitumor activity [97]. This finding could potentially open a new chapter on HA/protease research.
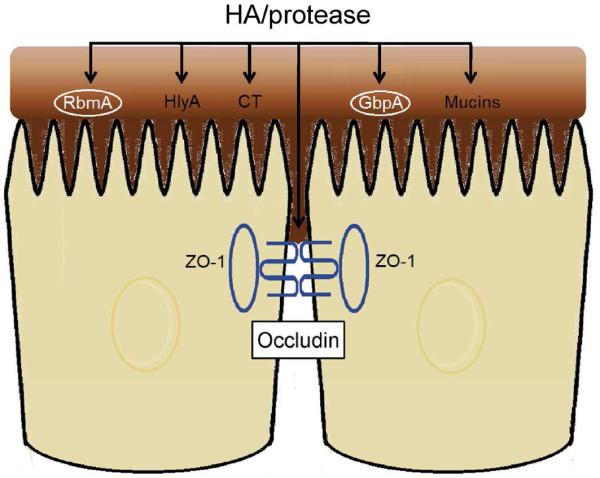
Fig. 4. HA/protease targets in the small intestine during cholera infection.
HA/protease can act at multiple targets during infection. These include the soluble toxins HlyA and CT, the cell-associated proteins RbmA and GbpA (circled in white font), and host proteins occludin and mucin(s). Expression of HA/protease in the gut can modulate the pathogenicity of cholera by multiple mechanisms. Activation of CT and degradation of the mucus barrier facilitates delivery of CT in close proximity to the microvilli to enhance transcellular fluid secretion. Cleavage of occludin disrupts tight junctions and could promote paracellular fluid secretion. Limited proteolysis of RbmA enhances the formation of biofilms resistant to host-specific stresses. Degradation of the GbpA adhesin facilitates bacterial dissemination by dissociating infecting vibrios from mucus. Finally, degradation of the mucus blanket exposes epithelial cells to proinflammatory flagellins.
Supplementary Material
Acknowledgments
Research on HA/protease conducted in the author's laboratory has been funded by Public Health Service Grants from the National Institutes of Allergy and Infectious Disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albert MJ. Vibrio cholerae O139 Bengal. J Clin Microbiol. 1994;32(10):2345–9. doi: 10.1128/jcm.32.10.2345-2349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaper JB, Morris JG, Jr., Levine MM. Cholera. Clin Microbiol Rev. 1995;8(1):48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali A, et al. Recent clonal origin of cholera in Haiti. Emerg Infect Dis. 2011;17(4):699–701. doi: 10.3201/eid1704.101973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrington DA, et al. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168(4):1487–92. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacket CO, et al. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect Immun. 1998;66(2):692–5. doi: 10.1128/iai.66.2.692-695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64(7):2853–6. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein RA, Dorner F. Cholera enterotoxin (choleragen). Pharmacol Ther. 1985;27(1):37–47. doi: 10.1016/0163-7258(85)90063-4. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein RA, LoSpalluto JJ. Crystalline cholera toxin and toxoid. Science. 1972;175(4021):529–30. doi: 10.1126/science.175.4021.529. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths SL, Finkelstein RA, Critchley DR. Characterization of the receptor for cholera toxin and Escherichia coli heat-labile toxin in rabbit intestinal brush borders. Biochem J. 1986;238(2):313–22. doi: 10.1042/bj2380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano A, et al. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci U S A. 1991;88(12):5242–6. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano A, et al. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest. 1995;96(2):710–20. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trucksis M, et al. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci U S A. 1993;90(11):5267–71. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, et al. Identification of a vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci U S A. 1999;96(3):1071–6. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagamune K, Yamamoto K, Honda T. Cloning and sequencing of a novel hemolysis gene of Vibrio cholerae. FEMS Microbiol Lett. 1995;128(3):265–9. doi: 10.1111/j.1574-6968.1995.tb07534.x. [DOI] [PubMed] [Google Scholar]
- 15.Hase CC, Finkelstein RA. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J Bacteriol. 1991;173(11):3311–7. doi: 10.1128/jb.173.11.3311-3317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hase CC, Finkelstein RA. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993;57(4):823–37. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinoda S, Miyoshi S. Proteases produced by vibrios. Biocontrol Sci. 2011;16(1):1–11. doi: 10.4265/bio.16.1. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi S. Extracellular proteolytic enzymes produced by human pathogenic vibrio species. Front Microbiol. 2013;4:339. doi: 10.3389/fmicb.2013.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth BA, Boesman-Finkelstein M, Finkelstein RA. Vibrio cholerae soluble hemagglutinin/protease is a metalloenzyme. Infect Immun. 1983;42(2):639–44. doi: 10.1128/iai.42.2.639-644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkelstein RA, Boesman-Finkelstein M, Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci U S A. 1983;80(4):1092–5. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein RA, Hanne LF. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982;36(3):1199–208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hase CC, Finkelstein RA. Comparison of the Vibrio cholerae hemagglutinin/protease and the Pseudomonas aeruginosa elastase. Infect Immun. 1990;58(12):4011–5. doi: 10.1128/iai.58.12.4011-4015.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuang Z, et al. Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A. PLoS One. 2011;6(11):e27091. doi: 10.1371/journal.pone.0027091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77(5):1723–33. doi: 10.1128/IAI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutfullah G, et al. Homology modeling of hemagglutinin/protease [HA/P (vibriolysin)] from Vibrio cholerae: sequence comparision, residue interactions and molecular mechanism. Protein J. 2008;27(2):105–14. doi: 10.1007/s10930-007-9113-0. [DOI] [PubMed] [Google Scholar]
- 26.Jobling MG, Holmes RK. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol Microbiol. 1997;26(5):1023–34. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- 27.Silva AJ, Benitez JA. Transcriptional regulation of Vibrio cholerae hemagglutinin/protease by the cyclic AMP receptor protein and RpoS. J Bacteriol. 2004;186(19):6374–82. doi: 10.1128/JB.186.19.6374-6382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yildiz FH, Schoolnik GK. Role of rpoS in stress survival and virulence of Vibrio cholerae. J Bacteriol. 1998;180(4):773–84. doi: 10.1128/jb.180.4.773-784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benitez JA, Silva AJ, Finkelstein RA. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect Immun. 2001;69(10):6549–53. doi: 10.1128/IAI.69.10.6549-6553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang W, et al. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology. 2007;153(Pt 9):2964–75. doi: 10.1099/mic.0.2007/006668-0. [DOI] [PubMed] [Google Scholar]
- 31.Liang W, et al. Cyclic AMP post-transcriptionally regulates the biosynthesis of a major bacterial autoinducer to modulate the cell density required to activate quorum sensing. FEBS Lett. 2008;582(27):3744–50. doi: 10.1016/j.febslet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, et al. Interplay among cyclic diguanylate, HapR, and the general stress response regulator (RpoS) in the regulation of Vibrio cholerae hemagglutinin/protease. J Bacteriol. 2011;193(23):6529–38. doi: 10.1128/JB.05166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, et al. A highly specific cell-based high-throughput screening assay for ligands of cyclic adenosine monophosphate receptor protein in gram-negative bacteria. Assay Drug Dev Technol. 2013;11(6):382–7. doi: 10.1089/adt.2013.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skorupski K, Taylor RK. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci U S A. 1997;94(1):265–70. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, et al. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 2002;99(5):3129–34. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikora AE. Proteins secreted via the type II secretion system: smart strategies of Vibrio cholerae to maintain fitness in different ecological niches. PLoS Pathog. 2013;9(2):e1003126. doi: 10.1371/journal.ppat.1003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandkvist M, Bagdasarian M, Howard SP. Characterization of the multimeric Eps complex required for cholera toxin secretion. Int J Med Microbiol. 2000;290(4-5):345–50. doi: 10.1016/S1438-4221(00)80038-7. [DOI] [PubMed] [Google Scholar]
- 38.Scott ME, Dossani ZY, Sandkvist M. Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway. Proc Natl Acad Sci U S A. 2001;98(24):13978–83. doi: 10.1073/pnas.241411198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mel SF, et al. Association of protease activity in Vibrio cholerae vaccine strains with decreases in transcellular epithelial resistance of polarized T84 intestinal epithelial cells. Infect Immun. 2000;68(11):6487–92. doi: 10.1128/iai.68.11.6487-6492.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva AJ, et al. Contribution of hemagglutinin/protease and motility to the pathogenesis of El Tor biotype cholera. Infect Immun. 2006;74(4):2072–9. doi: 10.1128/IAI.74.4.2072-2079.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lencer WI, et al. Proteolytic activation of cholera toxin and Escherichia coli labile toxin by entry into host epithelial cells. Signal transduction by a protease-resistant toxin variant. J Biol Chem. 1997;272(24):15562–8. doi: 10.1074/jbc.272.24.15562. [DOI] [PubMed] [Google Scholar]
- 42.Booth BA, Boesman-Finkelstein M, Finkelstein RA. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect Immun. 1984;45(3):558–60. doi: 10.1128/iai.45.3.558-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichinose Y, et al. The protease from Vibrio cholerae nicks arginine at position 192 from the N-terminus of the heat-labile enterotoxin a subunit from enterotoxigenic Escherichia coli. Eur J Epidemiol. 1992;8(5):743–7. doi: 10.1007/BF00145394. [DOI] [PubMed] [Google Scholar]
- 44.Naka A, et al. Nicking sites in a subunit of cholera toxin and Escherichia coli heat-labile enterotoxin for Vibrio cholerae hemagglutinin/protease. Toxicon. 1998;36(7):1001–5. doi: 10.1016/s0041-0101(97)00135-9. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto K, et al. Two-step processing for activation of the cytolysin/hemolysin of Vibrio cholerae O1 biotype El Tor: nucleotide sequence of the structural gene (hlyA) and characterization of the processed products. Infect Immun. 1990;58(12):4106–16. doi: 10.1128/iai.58.12.4106-4116.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutta S, et al. Three-dimensional structure of different functional forms of the Vibrio cholerae hemolysin oligomer: a cryo-electron microscopic study. J Bacteriol. 2010;192(1):169–78. doi: 10.1128/JB.00930-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De S, Olson R. Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc Natl Acad Sci U S A. 2011;108(18):7385–90. doi: 10.1073/pnas.1017442108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chattopadhyay K, Bhattacharyya D, Banerjee KK. Vibrio cholerae hemolysin. Implication of amphiphilicity and lipid-induced conformational change for its pore-forming activity. Eur J Biochem. 2002;269(17):4351–8. doi: 10.1046/j.1432-1033.2002.03137.x. [DOI] [PubMed] [Google Scholar]
- 49.Morris JG., Jr. Non-O group 1 Vibrio cholerae: a look at the epidemiology of an occasional pathogen. Epidemiol Rev. 1990;12:179–91. doi: 10.1093/oxfordjournals.epirev.a036052. [DOI] [PubMed] [Google Scholar]
- 50.Figueroa-Arredondo P, et al. [Cytotoxic effect of Vibrio cholerae non-O1 on Vero cells]. Rev Latinoam Microbiol. 1994;36(4):277–81. [PubMed] [Google Scholar]
- 51.Figueroa-Arredondo P, et al. Cell vacuolation caused by Vibrio cholerae hemolysin. Infect Immun. 2001;69(3):1613–24. doi: 10.1128/IAI.69.3.1613-1624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidal JE, et al. Culture supernatants from V. cholerae O1 El Tor strains isolated from different geographic areas induce cell vacuolation and cytotoxicity. Salud Publica Mex. 2009;51(1):39–47. doi: 10.1590/s0036-36342009000100009. [DOI] [PubMed] [Google Scholar]
- 53.Coelho A, et al. Cytotoxic cell vacuolating activity from Vibrio cholerae hemolysin. Infect Immun. 2000;68(3):1700–5. doi: 10.1128/iai.68.3.1700-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Debellis L, et al. The Vibrio cholerae cytolysin promotes chloride secretion from intact human intestinal mucosa. PLoS One. 2009;4(3):e5074. doi: 10.1371/journal.pone.0005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichinose Y, et al. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987;55(5):1090–3. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cinar HN, et al. Vibrio cholerae hemolysin is required for lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans. PLoS One. 2010;5(7):e11558. doi: 10.1371/journal.pone.0011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Q, Dziejman M, Mekalanos JJ. Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. Proc Natl Acad Sci U S A. 2003;100(3):1286–91. doi: 10.1073/pnas.0337479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagamune K, et al. In vitro proteolytic processing and activation of the recombinant precursor of El Tor cytolysin/hemolysin (pro-HlyA) of Vibrio cholerae by soluble hemagglutinin/protease of V. cholerae, trypsin, and other proteases. Infect Immun. 1996;64(11):4655–8. doi: 10.1128/iai.64.11.4655-4658.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsou AM, Zhu J. Quorum sensing negatively regulates hemolysin transcriptionally and posttranslationally in Vibrio cholerae. Infect Immun. 2010;78(1):461–7. doi: 10.1128/IAI.00590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009;1788(4):832–41. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 61.Baudry B, et al. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect Immun. 1992;60(2):428–34. doi: 10.1128/iai.60.2.428-434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Z, et al. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb Pathog. 1996;21(2):111–23. doi: 10.1006/mpat.1996.0047. [DOI] [PubMed] [Google Scholar]
- 63.Wu Z, Nybom P, Magnusson KE. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol. 2000;2(1):11–7. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 64.Wu Z, et al. Endogenous nitric oxide in MDCK-I cells modulates the Vibrio cholerae haemagglutinin/protease (HA/P)-mediated cytotoxicity. Microb Pathog. 1998;24(5):321–6. doi: 10.1006/mpat.1998.0201. [DOI] [PubMed] [Google Scholar]
- 65.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 67.Faruque SM, et al. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci U S A. 2006;103(16):6350–5. doi: 10.1073/pnas.0601277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berk V, et al. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 2012;337(6091):236–9. doi: 10.1126/science.1222981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith DR, et al. In situ proteolysis of the Vibrio cholerae matrix protein RbmA promotes biofilm recruitment. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1512424112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crowther RS, et al. Vibrio cholerae metalloproteinase degrades intestinal mucin and facilitates enterotoxin-induced secretion from rat intestine. Biochim Biophys Acta. 1987;924(3):393–402. doi: 10.1016/0304-4165(87)90153-x. [DOI] [PubMed] [Google Scholar]
- 71.Ichinose Y, et al. The effect on enterotoxicity of protease purified from Vibrio cholerae O1. FEMS Microbiol Lett. 1994;115(2-3):265–71. doi: 10.1111/j.1574-6968.1994.tb06649.x. [DOI] [PubMed] [Google Scholar]
- 72.Ghosh A, et al. Enterotoxigenicity of mature 45-kilodalton and processed 35-kilodalton forms of hemagglutinin protease purified from a cholera toxin gene-negative Vibrio cholerae non-O1, non-O139 strain. Infect Immun. 2006;74(5):2937–46. doi: 10.1128/IAI.74.5.2937-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bardakchian EA, et al. Ultrastructural changes in the small intestine of suckling mice, caused by vibrio cholerae hemagglutinin/protease. Bull Exp Biol Med. 2008;145(4):490–4. doi: 10.1007/s10517-008-0126-2. [DOI] [PubMed] [Google Scholar]
- 74.Wong E, et al. The Vibrio cholerae colonization factor GbpA possesses a modular structure that governs binding to different host surfaces. PLoS Pathog. 2012;8(1):e1002373. doi: 10.1371/journal.ppat.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhowmick R, et al. Intestinal adherence of Vibrio cholerae involves a coordinated interaction between colonization factor GbpA and mucin. Infect Immun. 2008;76(11):4968–77. doi: 10.1128/IAI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Millet YA, et al. Insights into Vibrio cholerae intestinal colonization from monitoring fluorescently labeled bacteria. PLoS Pathog. 2014;10(10):e1004405. doi: 10.1371/journal.ppat.1004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atsumi T, et al. Effect of viscosity on swimming by the lateral and polar flagella of Vibrio alginolyticus. J Bacteriol. 1996;178(16):5024–6. doi: 10.1128/jb.178.16.5024-5026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Z, et al. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc Natl Acad Sci U S A. 2008;105(28):9769–74. doi: 10.1073/pnas.0802241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silva AJ, Pham K, Benitez JA. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology. 2003;149(Pt 7):1883–91. doi: 10.1099/mic.0.26086-0. [DOI] [PubMed] [Google Scholar]
- 80.Finkelstein RA, et al. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992;60(2):472–8. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benitez JA, et al. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect Immun. 1997;65(8):3474–7. doi: 10.1128/iai.65.8.3474-3477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robert A, et al. Tagging a Vibrio cholerae El Tor candidate vaccine strain by disruption of its hemagglutinin/protease gene using a novel reporter enzyme: Clostridium thermocellum endoglucanase A. Vaccine. 1996;14(16):1517–22. doi: 10.1016/s0264-410x(96)00105-3. [DOI] [PubMed] [Google Scholar]
- 83.Jude BA, et al. Levels of the secreted Vibrio cholerae attachment factor GbpA are modulated by quorum-sensing-induced proteolysis. J Bacteriol. 2009;191(22):6911–7. doi: 10.1128/JB.00747-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nielsen AT, et al. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog. 2006;2(10):e109. doi: 10.1371/journal.ppat.0020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olivier V, Salzman NH, Satchell KJ. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect Immun. 2007;75(10):5043–51. doi: 10.1128/IAI.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levine MM, et al. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988;56(1):161–7. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michalski J, et al. CVD110, an attenuated Vibrio cholerae O1 El Tor live oral vaccine strain. Infect Immun. 1993;61(10):4462–8. doi: 10.1128/iai.61.10.4462-4468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tacket CO, et al. Safety and immunogenicity of live oral cholera vaccine candidate CVD 110, a delta ctxA delta zot delta ace derivative of El Tor Ogawa Vibrio cholerae. J Infect Dis. 1993;168(6):1536–40. doi: 10.1093/infdis/168.6.1536. [DOI] [PubMed] [Google Scholar]
- 89.Silva TM, et al. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and Q139 Vibrio cholerae. Infect Immun. 1996;64(6):2362–4. doi: 10.1128/iai.64.6.2362-2364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou X, et al. Induction of interleukin-8 in T84 cells by Vibrio cholerae. Infect Immun. 2004;72(1):389–97. doi: 10.1128/IAI.72.1.389-397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benitez JA, et al. Preliminary assessment of the safety and immunogenicity of a new CTXPhi-negative, hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect Immun. 1999;67(2):539–45. doi: 10.1128/iai.67.2.539-545.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia L, et al. The vaccine candidate Vibrio cholerae 638 is protective against cholera in healthy volunteers. Infect Immun. 2005;73(5):3018–24. doi: 10.1128/IAI.73.5.3018-3024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kenner JR, et al. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J Infect Dis. 1995;172(4):1126–9. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 94.Harrison LM, et al. Vibrio cholerae flagellins induce Toll-like receptor 5-mediated interleukin-8 production through mitogen-activated protein kinase and NF-kappaB activation. Infect Immun. 2008;76(12):5524–34. doi: 10.1128/IAI.00843-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xicohtencatl-Cortes J, et al. Identification of proinflammatory flagellin proteins in supernatants of Vibrio cholerae O1 by proteomics analysis. Mol Cell Proteomics. 2006;5(12):2374–83. doi: 10.1074/mcp.M600228-MCP200. [DOI] [PubMed] [Google Scholar]
- 96.Rui H, et al. Reactogenicity of live-attenuated Vibrio cholerae vaccines is dependent on flagellins. Proc Natl Acad Sci U S A. 2010;107(9):4359–64. doi: 10.1073/pnas.0915164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ray T, Chakrabarti MK, Pal A. Hemagglutinin protease secreted by V. cholerae induced apoptosis in breast cancer cells by ROS mediated intrinsic pathway and regresses tumor growth in mice model. Apoptosis. 2016;21(2):143–54. doi: 10.1007/s10495-015-1194-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.