Abstract
Both the incidence and prevalence of stroke in Asia are steadily increasing, and the burden of stroke is particularly high in Asian countries. Although strokes in Asians and Caucasians share many common features, there are some differences that are probably due to differences in lifestyle and genetic background. While there have been advances in the stroke classification system, the assignment of Asian stroke patients to etiological categories has received little attention. The current classification system may not be well suited to Asian patients with ischemic stroke because the proportions and relative importance of stroke subtypes may differ with race and ethnicity. This review addresses concerns about the use of the current stroke classification system in Asian patients with ischemic stroke, and proposes a classification system that is more specific to the Asian population, in conjunction with discussing advances in diagnostic techniques.
Keywords: stroke, ischemic stroke, subtype, Asian, classification
INTRODUCTION
Ischemic stroke is a heterogeneous disease with various cardiac, arterial, hemodynamic, rheological, and other systemic abnormalities. Categorizing patients into classes congruent with their pathophysiology is the key to understanding stroke, providing appropriate treatment, and preventing recurrence.
Various classification systems are applied to the subtypes of ischemic stroke, each of which had its own strengths and weaknesses. The OCSP (Oxfordshire Community Stroke Project classification, Bamford classification) relies primarily on clinical syndromes based on the extent and vascular localization of the stroke: total or partial anterior circulation, lacunar, and posterior circulation syndrome.1 The following three etiological classifications have been developed due to acute and secondary preventive therapies being developed based on the understanding of the mechanisms underlying the stroke type: the NINDS stroke data bank subtype,2 the Lausanne Stroke Registry,3 and the Trial of Org 10172 in Acute Stroke Treatment (TOAST).4 These classifications are based on both clinical and laboratory data, including neuroimaging and vascular and cardiac workups. The three most common etiologies of stroke are atherosclerotic, cardioembolic, and lacunar. With advances in stroke imaging and diagnostic techniques, and the availability of new epidemiological data, evidence-based algorithms have recently been developed to assign etiological categories in the presence of multiple mechanisms. These new categories include the Stop Stroke Study-TOAST5 and the A-S-C-O (phenotypic).6
While these classification systems have been applied in large studies worldwide, it might be necessary to reassess how Asian stroke patients are assigned to the etiological categories. This review addresses concerns about the use of stroke classifications in Asian stroke patients, and proposes a classification system that is more specific to the Asian population, in conjunction with discussing advances in diagnostic techniques.
THE CURRENT CLASSIFICATION SYSTEM MAY NOT BE WELL SUITED TO ASIAN PATIENTS WITH ISCHEMIC STROKE
Both the incidence and prevalence of stroke in Asia are increasing steadily, probably due to lifestyle changes and the aging of the population. Moreover, the burden of stroke is particularly high in Asia,7 given that almost two-thirds of the deaths worldwide due to stroke occur in Asian countries. The clinical features and epidemiological data related to stroke in Asians are different from those in Caucasians. However, the classification of Asian patients with ischemic stroke has received little attention. The various reasons why a classification system more specific to the Asian population needs to be developed are discussed below.
First, the proportions and relative importance of stroke subtypes are well known to differ with race and ethnicity (Fig. 1).8,9,10,11 While cardioembolism is the most common (25-30%) cause of ischemic stroke in Western countries, atherosclerotic stroke accounts for up to 25-65% of strokes in Asian countries.10 The prevalence of small-vessel disease is also higher in Asians than in Caucasians; this subtype accounts for up to one-half of ischemic strokes in Asians patients, but only one-fifth of those in Caucasian patients.10 As a result, the vast majority of Asian patients with ischemic stroke are classified as having disease of large or small cerebral arteries.
Fig. 1. Stroke subtypes by racial and ethnic groups. *Data from southern Californians (Modified from Bang et al.8), †Data from South Koreans. AF: atrial fibrillation.
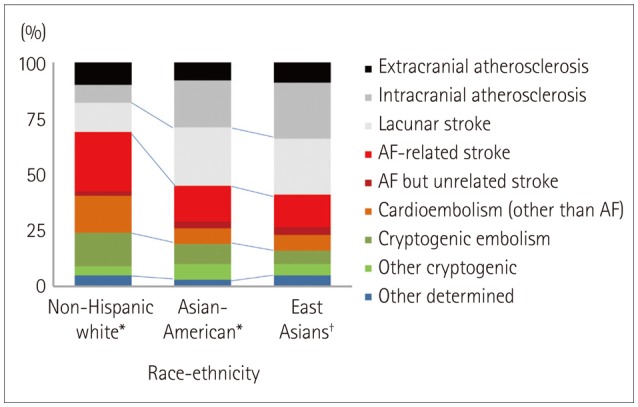
Second, the relative distribution of intracranial, extracranial, and coronary atherosclerosis may differ between Asians and Caucasians. It was reported that the stroke burden is disproportionately high in East Asia, Africa, and South America, while the ischemic heart burden is higher in the Middle East, North America, Australia, and much of Europe.12 In addition, the prevalence of intracranial atherosclerosis is high in Asians,13 causing 30-50% of strokes in Asia,14 whereas it is the cause of only 8-10% of strokes in North America.15 Due to this type of stroke receiving little attention in stroke classification systems, and the relatively low frequency of intracranial atherosclerosis in Western countries, patients with intracranial atherosclerosis are often classified as having cryptogenic embolism. However, recent high-resolution MRI and pathological studies have revealed the presence of intracranial arterial plaques in these patients.16,17,18 In contrast, patients with a milder degree of intracranial stenosis or large and deep infarcts are likely to be classified as having other cryptogenic causes.
Third, specific stroke etiologies should be considered in certain stroke populations due to the presence of genetic differences between populations. For example, sickle cell disease can cause stenosis in cerebral vessels, and can result in stroke in blacks but is very rare in East Asians. In contrast, the prevalence of moyamoya disease (MMD) is higher in Asians than in Caucasians. Recent genome-wide linkage and exome analyses identified the RNF213 mutation as the most important for susceptibility to MMD among East Asian people.19,20,21 The number of East Asian patients with MMD has been estimated to be more than 53,800.22,23
DETAILED SUBTYPING IS NEEDED TO GUIDE ACUTE TREATMENT AND PREVENT STROKE IN ASIANS
The current classification systems have focused on identifying the atherosclerotic and cardioembolic subtypes. This is because guidelines for stroke prevention emphasize the use of antiplatelet agents and statins for atherosclerotic strokes and anticoagulants for patients with atrial fibrillation (AF) based on the results of large clinical trials. However, from a therapeutic point of view, AF might be more complicated in Asian patients with ischemic stroke.
Atherosclerotic subtype
It might be beneficial to divide the atherosclerotic subtype into isolated intracranial and extracranial (with or without intracranial) atherosclerosis subtypes. Differences in clinical and neuroimaging features and risk factors have been reported between extracranial (e.g., cervical carotid) and intracranial atherosclerosis.24,25,26 Previous studies have found atherosclerosis to be frequently localized to either the intracranial or the extracranial arterial system, rather than occurring in both systems.24,27 More importantly, intracranial stenosis can be caused by diverse conditions, including MMD, dissection, vasculitis, and reversible cerebral vasoconstriction syndrome (Fig. 2). In contrast, atherosclerosis is the main cause of cervical carotid stenosis, with only rare exceptions of carotid dissection, fibromuscular dysplasia, Takayasu arteritis, and radiation arteritis being differentiated clinically. In addition, intracranial atherosclerotic stroke can be caused by branch occlusive disease as well as artery-to-artery embolism or hemodynamic impairment.28,29 The risk factors, vessel wall pathology, and treatment strategies may differ between these two subtypes of intracranial atherosclerotic stroke.28,30,31,32,33 Patients with branch occlusive disease often show a mild degree of stenosis and are misclassified as having lacunar stroke or other cryptogenic stroke (Fig. 3). However, high-resolution MRI studies have demonstrated intracranial plaques occluding perforating arteries, which suggests that statin plays a role in these patients. Our ongoing serial follow-up high-resolution MRI study in patients with intracranial atherosclerotic stroke is currently addressing this issue (clinicaltrial.gov identifier NCT02458755). Asian investigators are concerned about the definition of stroke subtypes in these patients, and have suggested that lesion size limitations should not be strictly applied to cases of small-vessel occlusions, and that the degree of stenosis in atherosclerotic vessels should not be limiting in determining the presence of intracranial atherosclerotic stroke.10
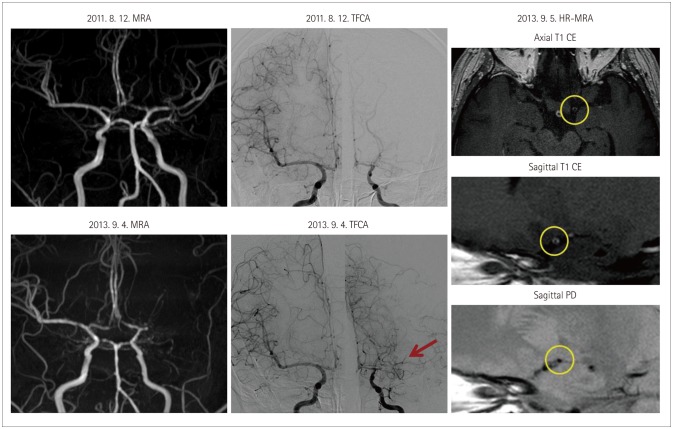
Fig. 2. Recurrent infarcts with progression of intracranial stenosis. A 32-year-old female experienced recurrent left middle cerebral artery (MCA) infarcts (three times) within 2 years. Serial time-of-flight magnetic resonance angiography (MRA) showed the progression of stenosis in the left MCA. Transfemoral cerebral angiography (TFCA) showed no stenosis in the distal internal carotid artery or basal collaterals suggestive of moyamoya disease (MMD) (arrow). However, high-resolution (HR) MRI revealed a smaller outer diameter, concentric enhancement, and the absence of focal plaques in the stenotic segment (circle). A genetic investigation revealed that the RNF213 mutation was associated with MMD (p.Arg4810Lys). CE: contrast enhanced.
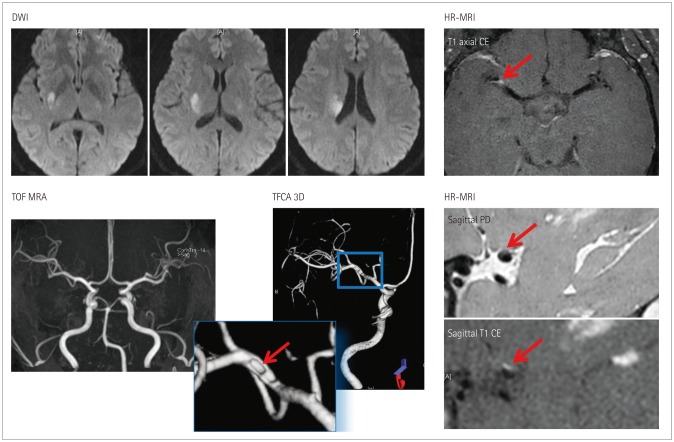
Fig. 3. Subcortical infarction but no significant stenosis. A 48-year-old female experienced left hemiparesis. Diffusion-weighted imaging (DWI) shows a deep infarct in the right basal ganglia and corona radiata. MRA shows no significant stenosis in the relevant vessels. TFCA and high-resolution (HR) MRA show a small plaque in the superior half of the middle cerebral artery (arrow). CE: contrast enhanced, MRA: magnetic resonance angiography, PD: proton density, TFCA: transfemoral cerebral angiography, TOF: time of flight, 3D: three dimensional.
Lacunar subtype
The pathogenic mechanisms of lacunar stroke are similar to those of hypertensive intracranial hemorrhage, and patients with lacunar stroke often experience hemorrhagic stroke.34 The two subclinical subtypes of lacunar stroke are cerebral deep microbleeds (red type) and leukoaraiosis (white type).35 Cerebral microbleeds are more common in Asians than in Caucasians and are associated with intracranial hemorrhage as well as ischemic stroke (Fig. 4),36 while leukoaraiosis may be caused by silent, acute lacunar infarcts.37,38 Thus, the optimal treatment strategies may differ between the two conditions, and differential risk factors have been reported.39,40,41 The Rotterdam Scan study showed that use of antiplatelet agents is related to the presence of cerebral microbleeds.42 One prospective study found that the risk of subsequent intracranial bleeding increased with the number of cerebral microbleeds, and that the high risk and mortality of intracranial bleeding outweighed the modest benefit of antithrombotic agents in patients with at least five cerebral microbleeds.43
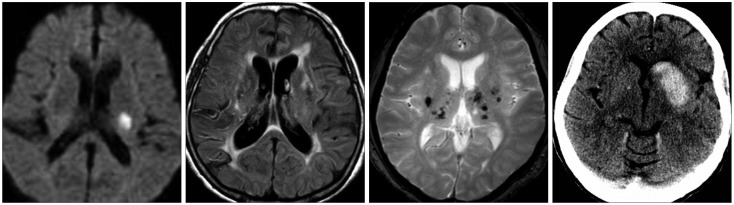
Fig. 4. A 59-year-old male presented with right hemiparesis. Diffusion-weighted imaging shows a small acute lacunar infarct in the left corona radiata. The magnetic resonance angiography findings were normal. Gradient-echo imaging shows multiple cerebral microbleeds in deep regions bilaterally. Two years later he was readmitted to the Department of Neurosurgery due to intracranial bleeding while taking dual antiplatelet agents.
Cardioembolic subtype
While the use of anticoagulants is the standard treatment applied to prevent stroke in patients with AF, AF may not always be the cause of stroke in AF patients. In fact, one-sixth of strokes in AF patients were reported to be unrelated to AF and showed clinical and echocardiographic characteristics distinct from those with AF-related stroke.44 Most patients with AF-unrelated stroke experienced recurrent strokes despite receiving adequate anticoagulation treatment with warfarin (Fig. 5). Because the prevalence of micro- and macroangiopathy is higher in Asians than in Caucasians, more Asian patients with AF are classified as having undetermined etiology with two or more causes, and differentiation of AF-related vs. -unrelated stroke may be more important in Asians than in Caucasians.
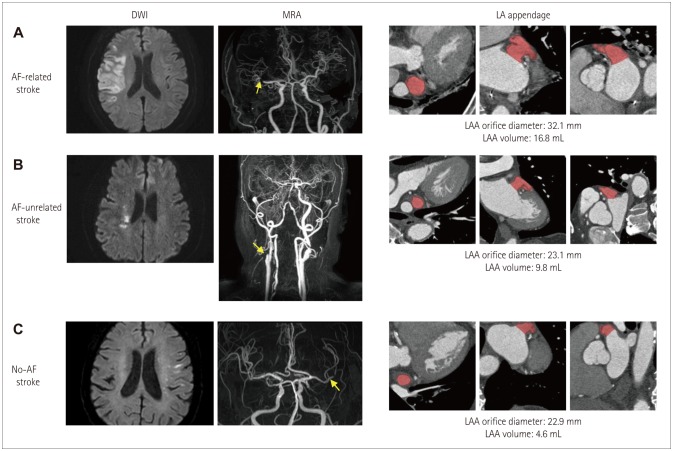
Fig. 5. Typical cases of atrial fibrillation (AF)-related stroke (A), AF-unrelated stroke (B), and no-AF stroke (C). Left panel, DWI. Middle panel, MRA. Right panel, multidetector cardiac computed tomography. A: AF-related stroke with a larger left atrial appendage (LAA) orifice diameter (32.1 mm) and larger LAA volume (16.8 mL). This patient had an infarction and occlusion in the right MCA territory, but no stenosis or occlusion in other intra- and extracranial vessels was noted. B: AF-unrelated stroke with a smaller LAA diameter (23.1 mm) and smaller LAA volume (9.8 mL). This patient had a rightsided border zone infarction with severe right cervical carotid occlusion. C: No-AF stroke with a smaller LAA orifice diameter (22.9 mm) and smaller LAA volume (4.6 mL). This patient had left cortical small infarcts with left MCA stenosis. DWI: diffusion-weighted imaging, MCA: middle cerebral artery, MRA: magnetic resonance angiography.
Consideration of the above characteristics leads to the conclusion that the optimal treatment strategies may differ among patients with the same stroke subtype. Owing to the paucity of evidence, current guidelines do not provide detailed treatment strategies according to the subclassification of stroke subtype. Future studies should investigate different treatment strategies for the various subclassifications, such the optimal dose of statins for intracranial vs. extracranial atherosclerosis, the use of antithrombotics for white vs. red phenotypes of lacunar stroke, and the addition of antiplatelet agents to anticoagulation for AF-related vs. -unrelated stroke (Table 1). In the meantime, continuous efforts are needed to individualize the treatments provided to Asian patients with ischemic stroke.
Table 1. Stroke subtyping and related issues in Asians.
| Traditional subtype | Detailed subtypes | Relevant issues | |
|---|---|---|---|
| Atherosclerotic | Extracranial (with or without intracranial) | ||
| Intracranial * | BOD | Degree of significant stenosis | |
| Role of statins | |||
| Non-BOD | Prevalence of mimicking conditions (dissection, MMD, vasculitis, or RCVS)* | ||
| Lacunar | Isolated lacunar | Lesion size | |
| Red (multiple CMBs)* | Role of antithrombotics in patients with multiple CMBs | ||
| White (leukoaraiosis)* | |||
| Cardioembolic | AF | Related to AF | |
| Unrelated to AF (2 or more causes)* | Role of additional antiplatelet agents in AF plus other mechanisms | ||
| Other than AF | |||
*Prevalence higher in Asians than in Caucasians.
AF: atrial fibrillation, BOD: branch occlusive disease, CMBs: cerebral microbleeds, MMD: moyamoya disease, RCVS: reversible cerebral vasoconstriction syndrome.
ADVANCES IN DIAGNOSTIC TECHNIQUES MAY BE PARTICULARLY HELPFUL IN ASIAN PATIENTS
The application of advanced diagnostic technologies may reduce the proportion of patients diagnosed with cryptogenic stroke.45 These techniques could also play a role in diagnosing patients with known vascular and cardiac abnormalities.
High-resolution MRI can visualize wall pathology (i.e., plaque, dissection, or vasculitis), and it has been shown to be effective in differentiating MMD and intracranial atherosclerosis.46,47 A recent high-resolution MRI study found that plaques were present in only 26 of 95 Korean patients with isolated stenotic lesions in the middle cerebral artery with no or minimal atherosclerotic risk factors, while the remaining 69 patients had nonatherosclerotic high-resolution MRI features, such as MMD, dissection, or vasculitis, suggesting a role for nonatherosclerotic pathologies in this population.48 Therefore, patients should not be classified as having atherosclerotic subtype simply because they have stenotic lesions on relevant proximal vessels. This is especially true in patients with a relatively healthy risk-factor profile and in Asian populations whose intracranial arteries are prone to dissection and carriers of the RNF213 mutation are more common. Although intracranial artery dissection is less common than cervical artery dissection in adults of European ethnicity, intracranial artery dissection is reportedly more common in Asian populations.49 Unlike pediatric MMD, it is often difficult to angiographically differentiate adult MMD from intracranial atherosclerosis. In adult patients with intracranial stenosis and the RNF213 mutation, typical angiographic findings of MMD (i.e., basal collaterals) are not necessarily prominent, and some patients develop typical angiographic findings of MMD on follow-up angiography.50 Therefore, high-resolution MRI or a follow-up vascular study might be valuable for demonstrating interval changes of basal collaterals and luminal stenosis in these patients.
Efforts have been made to find and validate possible biomarkers that can reliably predict the risk of stroke in patients with AF, including risk schemes51 and serological52,53 and genetic54 biomarkers. The use of cardiac imaging biomarkers is another approach for differentiating between cardiogenic and noncardiogenic stroke. For example, AF patients with the "chicken wing" type of left atrial appendage (LAA) morphology on multidetector cardiac computed tomography are reportedly less likely to have an embolic event after controlling for comorbidities and CHADS2 score.55 Both other56 and our studies57 have shown that the LAA volume and LAA orifice diameter are both greater in patients with AF-related stroke than in those with AF-unrelated stroke and those without AF (Fig. 5). Therefore, physicians should consider the possibility of an AF-unrelated mechanism if multidetector cardiac computed tomography shows such findings and no thrombus.
Targeted selection and judicious use of the appropriate tests in the workup of stroke are crucial. An extensive pathogenic workup may paradoxically increase the prevalence of cause-undetermined cases (i.e., cases with ≥2 determined causes). Thus, advanced vascular techniques should be applied to patients with milder stenosis for demonstrating vulnerable plaques, and to those with a relatively healthy risk-factor profile in order to preclude nonatherosclerotic stenosis in which specific treatment may be needed. In addition, antithrombotic usage could be guided by the findings of cardiac imaging (the use of antiplatelet agents in addition to oral anticoagulants to prevent AF-unrelated stroke) or gradientecho imaging (while avoiding the use of aggressive antithrombotics in patients with lacunar stroke and multiple cerebral microbleeds).
CONCLUSIONS
This review of the literature has addressed the need for a more-detailed stroke classification system and the systematic application of advanced diagnostic tests in the evaluation of stroke etiology in Asian patients. The continuing advances in technology mean that more diagnostic tests will become available, but this does not mean that it will be possible to apply these advanced techniques in routine clinical practice. Continuous efforts are needed to refine the approach applied for the workup of Asian patients with ischemic stroke.
Footnotes
Conflicts of Interest: The author has no financial conflicts of interest.
References
- 1.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 2.Gross CR, Shinar D, Mohr JP, Hier DB, Caplan LR, Price TR, et al. Interobserver agreement in the diagnosis of stroke type. Arch Neurol. 1986;43:893–898. doi: 10.1001/archneur.1986.00520090031012. [DOI] [PubMed] [Google Scholar]
- 3.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 4.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 6.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis. 2009;27:502–508. doi: 10.1159/000210433. [DOI] [PubMed] [Google Scholar]
- 7.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang OY, Saver JL, Liebeskind DS, Pineda S, Yun SW, Ovbiagele B. Impact of metabolic syndrome on distribution of cervicocephalic atherosclerosis: data from a diverse race-ethnic group. J Neurol Sci. 2009;284:40–45. doi: 10.1016/j.jns.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 10.Kim BJ, Kim JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke. 2014;16:8–17. doi: 10.5853/jos.2014.16.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehndiratta MM, Khan M, Mehndiratta P, Wasay M. Stroke in Asia: geographical variations and temporal trends. J Neurol Neurosurg Psychiatry. 2014;85:1308–1312. doi: 10.1136/jnnp-2013-306992. [DOI] [PubMed] [Google Scholar]
- 12.Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124:314–323. doi: 10.1161/CIRCULATIONAHA.111.018820. [DOI] [PubMed] [Google Scholar]
- 13.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 14.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995;45:659–663. doi: 10.1212/wnl.45.4.659. [DOI] [PubMed] [Google Scholar]
- 16.Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol. 2013;34:299–304. doi: 10.3174/ajnr.A3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu WH, Li ML, Gao S, Ni J, Yao M, Zhou LX, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol. 2012;71:195–198. doi: 10.1002/ana.22626. [DOI] [PubMed] [Google Scholar]
- 18.Majidi S, Sein J, Watanabe M, Hassan AE, Van de Moortele PF, Suri MF, et al. Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. AJNR Am J Neuroradiol. 2013;34:2259–2264. doi: 10.3174/ajnr.A3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet. 2011;56:34–40. doi: 10.1038/jhg.2010.132. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011;6:e22542. doi: 10.1371/journal.pone.0022542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimura M, Sonobe S, Nishijima Y, Niizuma K, Sakata H, Kure S, et al. Genetics and biomarkers of Moyamoya disease: significance of RNF213 as a susceptibility gene. J Stroke. 2014;16:65–72. doi: 10.5853/jos.2014.16.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Hashikata H, Inoue K, Matsuura N, Mineharu Y, Kobayashi H, et al. A rare Asian founder polymorphism of Raptor may explain the high prevalence of Moyamoya disease among East Asians and its low prevalence among Caucasians. Environ Health Prev Med. 2010;15:94–104. doi: 10.1007/s12199-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Hitomi T, Kobayashi H, Harada KH, Koizumi A. Distribution of moyamoya disease susceptibility polymorphism p.R4810K in RNF213 in East and Southeast Asian populations. Neurol Med Chir (Tokyo) 2012;52:299–303. doi: 10.2176/nmc.52.299. [DOI] [PubMed] [Google Scholar]
- 24.Bang OY, Lee PH, Yoon SR, Lee MA, Joo IS, Huh K. Inflammatory markers, rather than conventional risk factors, are different between carotid and MCA atherosclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1128–1134. doi: 10.1136/jnnp.2004.054403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang OY, Kim JW, Lee JH, Lee MA, Lee PH, Joo IS, et al. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology. 2005;65:296–298. doi: 10.1212/01.wnl.0000168862.09764.9f. [DOI] [PubMed] [Google Scholar]
- 26.López-Cancio E, Galán A, Dorado L, Jiménez M, Hernández M, Millán M, et al. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) study. Stroke. 2012;43:2712–2719. doi: 10.1161/STROKEAHA.112.661702. [DOI] [PubMed] [Google Scholar]
- 27.Akins PT, Pilgram TK, Cross DT, 3rd, Moran CJ. Natural history of stenosis from intracranial atherosclerosis by serial angiography. Stroke. 1998;29:433–438. doi: 10.1161/01.str.29.2.433. [DOI] [PubMed] [Google Scholar]
- 28.Ryoo S, Park JH, Kim SJ, Kim GM, Chung CS, Lee KH, et al. Branch occlusive disease: clinical and magnetic resonance angiography findings. Neurology. 2012;78:888–896. doi: 10.1212/WNL.0b013e31824c4699. [DOI] [PubMed] [Google Scholar]
- 29.Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. 2014;16:27–35. doi: 10.5853/jos.2014.16.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–1147. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 31.Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke. 2011;42:2957–2959. doi: 10.1161/STROKEAHA.111.618132. [DOI] [PubMed] [Google Scholar]
- 32.Nah HW, Kang DW, Kwon SU, Kim JS. Diversity of single small subcortical infarctions according to infarct location and parent artery disease: analysis of indicators for small vessel disease and atherosclerosis. Stroke. 2010;41:2822–2827. doi: 10.1161/STROKEAHA.110.599464. [DOI] [PubMed] [Google Scholar]
- 33.Turan TN, Derdeyn CP, Fiorella D, Chimowitz MI. Treatment of atherosclerotic intracranial arterial stenosis. Stroke. 2009;40:2257–2261. doi: 10.1161/STROKEAHA.108.537589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005;128(Pt 11):2507–2517. doi: 10.1093/brain/awh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 36.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995–1001. doi: 10.1161/STROKEAHA.111.000038. [DOI] [PubMed] [Google Scholar]
- 37.Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008;39:1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- 38.Conklin J, Silver FL, Mikulis DJ, Mandell DM. Are acute infarcts the cause of leukoaraiosis? Brain mapping for 16 consecutive weeks. Ann Neurol. 2014;76:899–904. doi: 10.1002/ana.24285. [DOI] [PubMed] [Google Scholar]
- 39.Park JH, Ryoo S, Kim SJ, Kim GM, Chung CS, Lee KH, et al. Differential risk factors for lacunar stroke depending on the MRI (white and red) subtypes of microangiopathy. PLoS One. 2012;7:e44865. doi: 10.1371/journal.pone.0044865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, et al. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43:2916–2922. doi: 10.1161/STROKEAHA.112.658369. [DOI] [PubMed] [Google Scholar]
- 41.Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84:825–832. doi: 10.1212/WNL.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66:714–720. doi: 10.1001/archneurol.2009.42. [DOI] [PubMed] [Google Scholar]
- 43.Soo YO, Yang SR, Lam WW, Wong A, Fan YH, Leung HH, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255:1679–1686. doi: 10.1007/s00415-008-0967-7. [DOI] [PubMed] [Google Scholar]
- 44.Kim SJ, Ryoo S, Kwon S, Park YK, Kim JP, Lee GY, et al. Is atrial fibrillation always a culprit of stroke in patients with atrial fibrillation plus stroke? Cerebrovasc Dis. 2013;36:373–382. doi: 10.1159/000355571. [DOI] [PubMed] [Google Scholar]
- 45.Bang OY, Ovbiagele B, Kim JS. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke. 2014;45:1186–1194. doi: 10.1161/STROKEAHA.113.003720. [DOI] [PubMed] [Google Scholar]
- 46.Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, Kim KH, et al. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke. 2014;45:2457–2460. doi: 10.1161/STROKEAHA.114.004761. [DOI] [PubMed] [Google Scholar]
- 47.Kim YJ, Lee DH, Kwon JY, Kang DW, Suh DC, Kim JS, et al. High resolution MRI difference between moyamoya disease and intracranial atherosclerosis. Eur J Neurol. 2013;20:1311–1318. doi: 10.1111/ene.12202. [DOI] [PubMed] [Google Scholar]
- 48.Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke. 2015;46:697–703. doi: 10.1161/STROKEAHA.114.008181. [DOI] [PubMed] [Google Scholar]
- 49.Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015;14:640–654. doi: 10.1016/S1474-4422(15)00009-5. [DOI] [PubMed] [Google Scholar]
- 50.Bang OY, Ryoo S, Kim SJ, Yoon CH, Cha J, Yeon JY, et al. Adult Moyamoya disease: a burden of intracranial stenosis in East Asians? PLoS One. 2015;10:e0130663. doi: 10.1371/journal.pone.0130663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wysokinski WE, Ammash N, Sobande F, Kalsi H, Hodge D, McBane RD. Predicting left atrial thrombi in atrial fibrillation. Am Heart J. 2010;159:665–671. doi: 10.1016/j.ahj.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 52.Okuyama H, Hirono O, Liu L, Takeishi Y, Kayama T, Kubota I. Higher levels of serum fibrin-monomer reflect hypercoagulable state and thrombus formation in the left atrial appendage in patients with acute ischemic stroke. Circ J. 2006;70:971–976. doi: 10.1253/circj.70.971. [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez-Yáñez M, Arias-Rivas S, Santamaría-Cadavid M, Sobrino T, Castillo J, Blanco M. High pro-BNP levels predict the occurrence of atrial fibrillation after cryptogenic stroke. Neurology. 2013;81:444–447. doi: 10.1212/WNL.0b013e31829d8773. [DOI] [PubMed] [Google Scholar]
- 54.Jickling GC, Stamova B, Ander BP, Zhan X, Liu D, Sison SM, et al. Prediction of cardioembolic, arterial, and lacunar causes of cryptogenic stroke by gene expression and infarct location. Stroke. 2012;43:2036–2041. doi: 10.1161/STROKEAHA.111.648725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 56.Lee JM, Shim J, Uhm JS, Kim YJ, Lee HJ, Pak HN, et al. Impact of increased orifice size and decreased flow velocity of left atrial appendage on stroke in nonvalvular atrial fibrillation. Am J Cardiol. 2014;113:963–969. doi: 10.1016/j.amjcard.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 57.Jeong WK, Choi JH, Son JP, Lee S, Lee MJ, Choe YH, et al. Volume and morphology of left atrial appendage as determinants of stroke subtype in patients with atrial fibrillation. Heart Rhythm. 2015 doi: 10.1016/j.hrthm.2015.12.026. [DOI] [PubMed] [Google Scholar]