Abstract
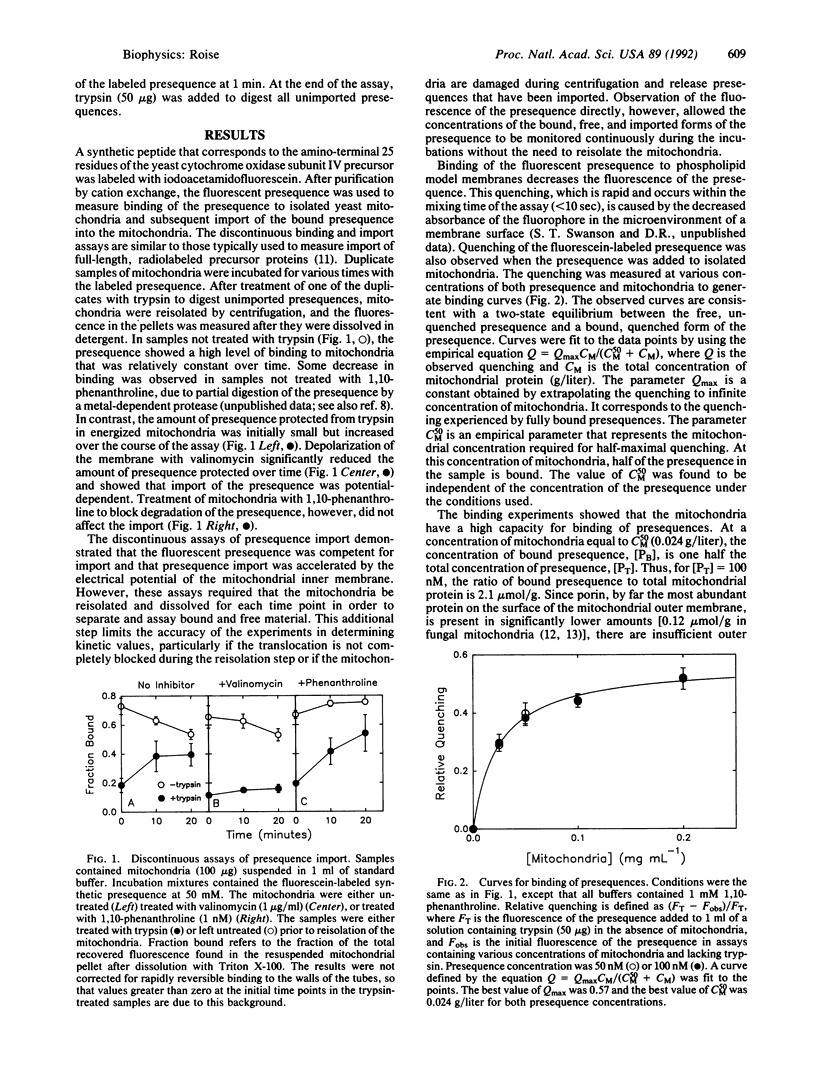
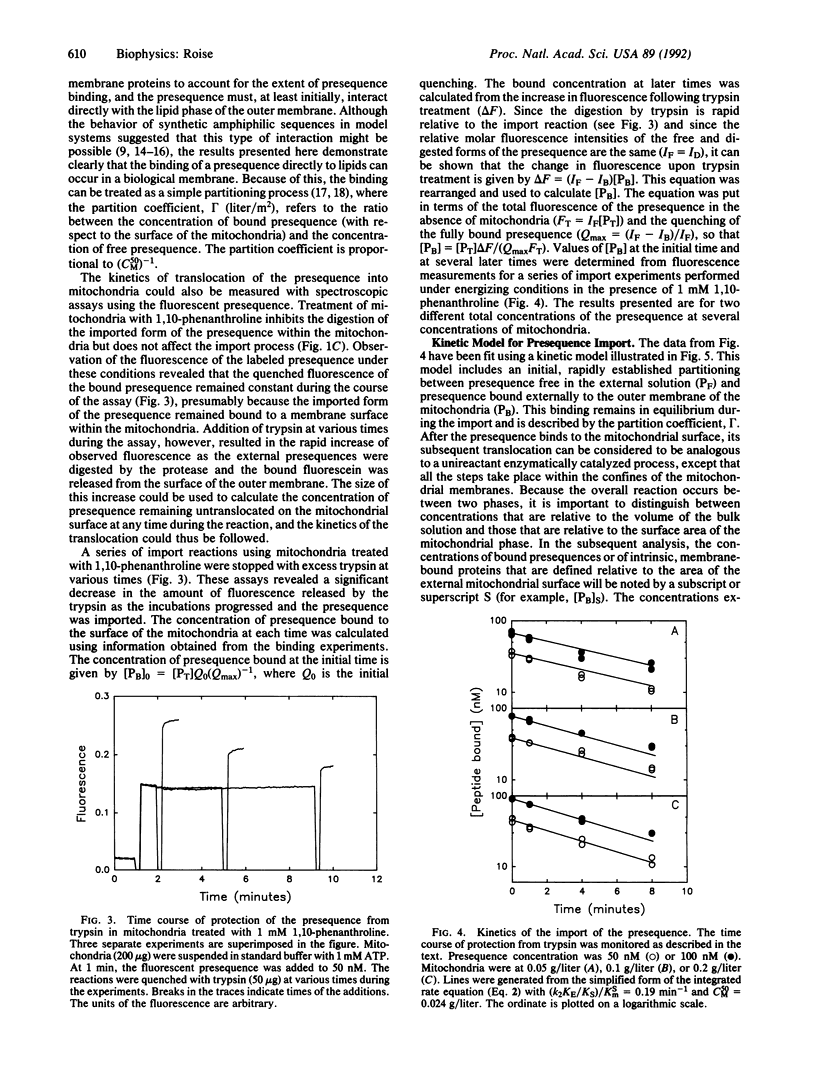
The mechanism of interaction of a presequence with isolated yeast mitochondria was examined. A synthetic peptide corresponding to a matrix-targeting signal was covalently labeled with a fluorescent probe. Binding of the presequence to the surface of the mitochondria and translocation of the presequence into the interior of the mitochondria could then be monitored directly in solution by measuring changes in the steady-state fluorescence of the attached fluorophore. The binding step was rapid and reversible. Quantitation of the binding under equilibrium conditions suggested that the initial association of the presequence with the surface of the mitochondria occurred by partitioning of the presequence directly into the lipid bilayer of the outer membrane. Subsequent translocation of the bound presequence into the mitochondria was monitored by measuring the rate of disappearance of presequences sensitive to digestion by added trypsin. The efficiency of translocation was high, and the rate of the translocation was dependent on the electrical potential across the inner membrane. At physiological concentrations of presequence, the rate displayed first-order kinetics with respect to the concentration of bound presequence and had a rate constant of 0.19 min-1 at 20 degrees C. Several kinetic models for the translocation of the presequence are presented that are consistent with the experimental results.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albery W. J., Knowles J. R. Evolution of enzyme function and the development of catalytic efficiency. Biochemistry. 1976 Dec 14;15(25):5631–5640. doi: 10.1021/bi00670a032. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Epand R. M., Hui S. W., Argan C., Gillespie L. L., Shore G. C. Structural analysis and amphiphilic properties of a chemically synthesized mitochondrial signal peptide. J Biol Chem. 1986 Aug 5;261(22):10017–10020. [PubMed] [Google Scholar]
- Freitag H., Neupert W., Benz R. Purification and characterisation of a pore protein of the outer mitochondrial membrane from Neurospora crassa. Eur J Biochem. 1982 Apr;123(3):629–636. doi: 10.1111/j.1432-1033.1982.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Furuya S., Mihara K., Aimoto S., Omura T. Cytosolic and mitochondrial surface factor-independent import of a synthetic peptide into mitochondria. EMBO J. 1991 Jul;10(7):1759–1766. doi: 10.1002/j.1460-2075.1991.tb07700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Glaser S. M., Cumsky M. G. Localization of a synthetic presequence that blocks protein import into mitochondria. J Biol Chem. 1990 May 25;265(15):8817–8822. [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Pfaller R., Söllner T., Griffiths G., Horstmann H., Pfanner N., Neupert W. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature. 1990 Dec 13;348(6302):610–616. doi: 10.1038/348610a0. [DOI] [PubMed] [Google Scholar]
- Ono H., Tuboi S. The cytosolic factor required for import of precursors of mitochondrial proteins into mitochondria. J Biol Chem. 1988 Mar 5;263(7):3188–3193. [PubMed] [Google Scholar]
- Pain D., Murakami H., Blobel G. Identification of a receptor for protein import into mitochondria. Nature. 1990 Oct 4;347(6292):444–449. doi: 10.1038/347444a0. [DOI] [PubMed] [Google Scholar]
- Pak Y. K., Weiner H. Import of chemically synthesized signal peptides into rat liver mitochondria. J Biol Chem. 1990 Aug 25;265(24):14298–14307. [PubMed] [Google Scholar]
- Reid G. A., Schatz G. Import of proteins into mitochondria. Yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J Biol Chem. 1982 Nov 10;257(21):13056–13061. [PubMed] [Google Scholar]
- Riezman H., Hay R., Gasser S., Daum G., Schneider G., Witte C., Schatz G. The outer membrane of yeast mitochondria: isolation of outside-out sealed vesicles. EMBO J. 1983;2(7):1105–1111. doi: 10.1002/j.1460-2075.1983.tb01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Schatz G. Mitochondrial presequences. J Biol Chem. 1988 Apr 5;263(10):4509–4511. [PubMed] [Google Scholar]
- Schwarz G., Stankowski S., Rizzo V. Thermodynamic analysis of incorporation and aggregation in a membrane: application to the pore-forming peptide alamethicin. Biochim Biophys Acta. 1986 Sep 25;861(1):141–151. doi: 10.1016/0005-2736(86)90573-0. [DOI] [PubMed] [Google Scholar]
- Skerjanc I. S., Shore G. C., Silvius J. R. The interaction of a synthetic mitochondrial signal peptide with lipid membranes is independent of transbilayer potential. EMBO J. 1987 Oct;6(10):3117–3123. doi: 10.1002/j.1460-2075.1987.tb02621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Griffiths G., Pfaller R., Pfanner N., Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989 Dec 22;59(6):1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Tamm L. K. Incorporation of a synthetic mitochondrial signal peptide into charged and uncharged phospholipid monolayers. Biochemistry. 1986 Nov 18;25(23):7470–7476. doi: 10.1021/bi00371a032. [DOI] [PubMed] [Google Scholar]
- Tamm L. K. Membrane insertion and lateral mobility of synthetic amphiphilic signal peptides in lipid model membranes. Biochim Biophys Acta. 1991 Jul 22;1071(2):123–148. doi: 10.1016/0304-4157(91)90021-n. [DOI] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


