Keywords: nerve regeneration, tetrandrine, ischemic cerebrovascular disease, vascular dementia, N-methyl-D-aspartic acid receptor 2B, N-methyl-D-aspartate receptor 2B phosphorylation at tyrosine 1472, interleukin-1β, neuronal necrosis, neural regeneration
Abstract
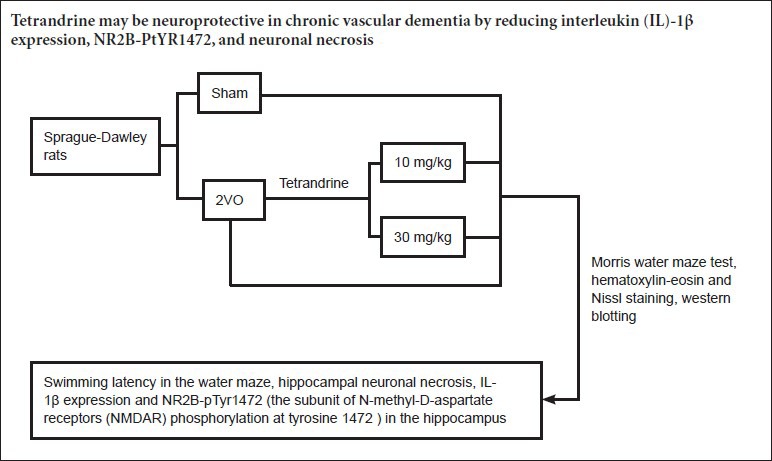
Tetrandrine is one of the major active ingredients in Menispermaceae Stephania tetrandra S. Moore, and has specific therapeutic effects in ischemic cerebrovascular disease. Its use in vascular dementia has not been studied fully. Here, we investigated whether tetrandrine would improve behavioral and cellular impairments in a two-vessel occlusion rat model of chronic vascular dementia. Eight weeks after model establishment, rats were injected intraperitoneally with 10 or 30 mg/kg tetrandrine every other day for 4 weeks. Behavioral assessment in the Morris water maze showed that model rats had longer escape latencies in training trials, and spent less time swimming in the target quadrant in probe trials, than sham-operated rats. However, rats that had received tetrandrine showed shorter escape latencies and longer target quadrant swimming time than untreated model rats. Hematoxylin-eosin and Nissl staining revealed less neuronal necrosis and pathological damage, and more living cells, in the hippocampus of rats treated with tetrandrine than in untreated model rats. Western blot assay showed that interleukin-1β expression, and phosphorylation of the N-methyl-D-aspartate 2B receptor at tyrosine 1472, were lower in model rats that received tetrandrine than in those that did not. The present findings suggest that tetrandrine may be neuroprotective in chronic vascular dementia by reducing interleukin-1β expression, N-methyl-D-aspartate receptor 2B phosphorylation at tyrosine 1472, and neuronal necrosis.
Introduction
Patients with vascular dementia often present with impairments in cognition, linguistic ability and memory (Doruk et al., 2010; Jiwa et al., 2010; Loganathan et al., 2010). Tyrosine phosphorylation of the N-methyl-D-aspartate receptor (NMDAR) has been implicated in nerve cell loss, developmental problems, cancers and ischemic brain injury (Manzerra et al., 2001; Haughey and Mattson, 2002; Viviani et al., 2006). Inflammatory cytokines modulate several neurological functions (Moghaddam et al., 2015; Pang et al., 2015; Yang et al., 2015). Recent studies have shown that the cytokine interleukin (IL)-1β regulates various classic neurotransmitter systems (Balosso et al., 2008; Paul and Kang, 2013; Cassol et al., 2014; Kåhlin et al., 2014) and enhances NMDAR functions (Viviani et al., 2006). Recombinant IL-1β potentiates neuronal death induced by NMDAR activation in primary hippocampal neurons, and increases phosphorylation of NMDAR subtype 2B (NR2B) at tyrosine 1472 (pTyr1472) (Viviani et al., 2003, 2006). Therefore, hypophosphorylation of NR2B may provide a mechanistic explanation for vascular dementia, and be a potential therapeutic target.
Tetrandrine (Tet) is a major active ingredient in Menispermaceae Stephania tetrandra S. Moore (Wang et al., 2004). Numerous studies support the notion that Tet is a potential therapeutic candidate against cancer (Shi et al., 2015; Wu et al., 2015), inflammation (Chen, 2002) and brain ischemia/reperfusion injury (Liu et al., 2001). Additionally, Tet can protect the liver, heart, small bowel and brain from ischemia/reperfusion injury (Chen et al., 2011). Tet is a calcium channel blocker, and can inhibit destructive factors in ischemia/reperfusion injury, such as lipid peroxidation, generation of reactive oxygen species, production of cytokines and inflammatory mediators, neutrophil recruitment and platelet aggregation (Chen et al., 2011). Therefore, Tet has potential as a protective agent in ischemic cerebrovascular disease. In the present study, we established an animal model of vascular dementia to explore the association of IL-1β and NR2B phosphorylation, and investigated the molecular mechanism underlying the therapeutic effects of Tet against dementia in terms of NR2B phosphorylation.
Materials and Methods
Animals
A total of 40 specific-pathogen-free male Sprague-Dawley rats, aged 4 weeks and weighing 200 ± 10 g, were obtained from the Experimental Animal Center of Chongqing Medical University, China (certificate No. SCXK (Yu) 2012-0001). Rats were housed under a 12 hour light/dark cycle (lights on from 7:30 a.m.) at 24 ± 1°C and 55 ± 5% relative humidity. Food, vitamin supplements and distilled water were available throughout the light/dark cycle. The protocol was approved by the Animal Care Committee of the First Affiliated Hospital of Chongqing Medical University, China. The rats were equally and randomly divided into four groups: sham, two-vessel occlusion (2VO), 10 mg/kg Tet, and 30 mg/kg Tet.
Establishment of vascular dementia model by 2VO
Rats were anesthetized with 10% chloral hydrate (0.8 mL/200 g). The 2VO surgery was carried out as described previously (Baune et al., 2008). Briefly, a ventral median incision was made to expose the common carotid arteries, which were then separated from adjacent vessels and nerves, and occluded with No. 1 thread. The procedure for the sham-operated rats was identical except that the arteries were not occluded. After 7 days of recovery, animals were housed separately.
Drug administration
In the Tet groups, Tet (Mansite Pharmaceutical Co., Ltd., Chengdu, China; powder, purity > 98%, CAS No. 518-34-3; solvent, double-distilled water) was administered intraperitoneally (10 mg/kg or 30 mg/kg) once every other day for 4 weeks, from the eighth week after surgery (Liu et al., 2001).
Morris water maze
After 4 weeks of Tet administration, the Morris water maze task was conducted for 5 consecutive days. The time taken to climb onto the hidden platform was defined as the escape latency. Rats performed four training trials per day, with entry from each of the four quadrants. When the platform was found within 120 seconds, the rat was allowed to remain there for 20 seconds; if the rat failed to find the hidden platform within 120 seconds, it was placed on it and allowed to remain there for 20 seconds. The mean swimming time in the four quadrants was used to assess the learning and memory ability of the rats. Each day, following the final training trial, the platform was removed. The rats were released at the farthest position from where the platform had been, and allowed to swim for 60 seconds. The time spent in the target quadrant was recorded to evaluate cognitive performance (Xiong et al., 2006). Swimming was monitored by a video camera linked to a computer-based image analyzer (SLY-WMS Morris Water Maze System; Zhenghua Biological Equipment Co., Ltd., Huaibei, Anhui Province, China).
Histopathological observation
After the final behavioral test, three randomly selected rats in each group were perfused transcardially with PBS followed by cold 4% paraformaldehyde (Kinoshita et al., 1991; Mehraein et al., 2011; Chen et al., 2014). The brains were removed, embedded in paraffin, sectioned and processed for hematoxylin-eosin and Nissl staining. Serial coronal sections (5 μm thick) were taken at the level of the hippocampus and cerebral cortex. Nissl bodies appeared purple-blue and allowed identification of the basic neuronal structure (Shang et al., 2006). Photographs of the hippocampal CA1 region were taken using a camera (SX30 IS; Canon, Tokyo, Japan) attached to a microscope (BX51; Olympus, Beijing, China). Healthy neurons in the center of the CA1 region, showing clear boundaries without cellular shrinkage or cytoplasmic disintegration (Liu et al., 2007), were counted using Image Pro Plus 6.0 (Media Cybernetics Co., Silver Spring, MD, USA) in five randomly selected fields at 200× magnification, and the mean taken.
Western blot assay
IL-1β, NR2B and NR2B-pTyr1472 protein expression in the hippocampus was evaluated by western blot assay. The total protein per hemi-hippocampus in three rats from each group was extracted with radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Nanjing, China) and centrifuged at 12,000 × g for 10 minutes. The supernatant was collected, mixed with loading buffer, and boiled for 5 minutes. Polyacrylamide gel electrophoresis was conducted in an 8% separating gel and 5% stacking gel at 60 V for 150 minutes. Proteins were transferred to a 0.45 μm polyvinylidene difluoride membrane. The membrane was blocked with 5% skimmed milk for 2 hours, incubated at 4°C overnight with rabbit anti-rat IL-1β polyclonal antibody (1:200; Bios Company, Nanjing, China), rabbit anti-rat NR2B polyclonal antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and rabbit anti-rat NR2B-pTyr1472 monoclonal antibody (1:500; Santa Cruz Biotechnology). After three washes with a mixture of Tris-buffered saline and 0.1% Tween-20, the membrane was incubated with horseradish peroxidase-labeled goat anti-rabbit secondary antibody (ZB-2301, 1:5,000; Zhongshan Golden Bridge Biotechnology Company, Beijing, China) for 1.5 hours at 37°C, and washed three times with Tris-buffered saline and Tween-20. Samples were developed with BeyoECL Plus (P1008, Beyotime Institute of Biotechnology). Bands were then scanned and optical density values were analyzed in a gel imaging system (ChemiDOC XRS; Bio-Rad, Hercules, CA, USA). The experiment was performed in triplicate.
Statistical analysis
Data are expressed as the mean ± SD. Group differences were compared by one-way analysis of variance followed by Bonferroni's multiple comparison test, using IBM SPSS Statistics (version 20.0). P < 0.05 was considered statistically significant.
Results
Tet improved cognition in rat models of vascular dementia
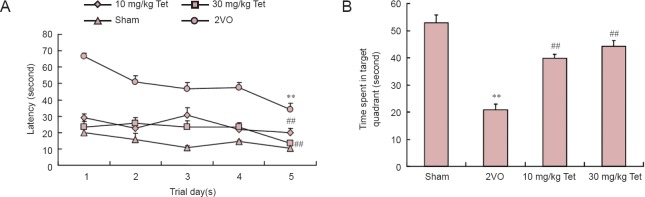
In all groups, the mean latency for finding the hidden platform decreased over 5 days (Figure 1A). Latency was longest in the 2VO group, and significantly shorter in both Tet groups (P < 0.01, vs. 2VO). The time spent in the target quadrant by rats in the 2VO group was 40.84% of that in the sham group (P < 0.01; Figure 1B). However, administration of Tet at 10 mg/kg or 30 mg/kg resulted in improvements in target quadrant swimming times of 36.95% and 43.3%, respectively (P < 0.01, vs. 2VO).
Figure 1.
Effects of Tet on Morris water maze performance in rat models of vascular dementia.
(A) Mean latency to find the hidden platform. (B) Swimming time in target quadrant after removal of platform (cut-off time, 60 seconds). Data are the mean ± SD (n = 10 rats per group). **P < 0.01, vs. sham group; ##P < 0.01, vs. 2VO group (one-way analysis of variance and Bonferroni post hoc test). Tet: Tetrandrine; 2VO: two-vessel occlusion.
Tet improved hippocampal pathology in rat models of vascular dementia
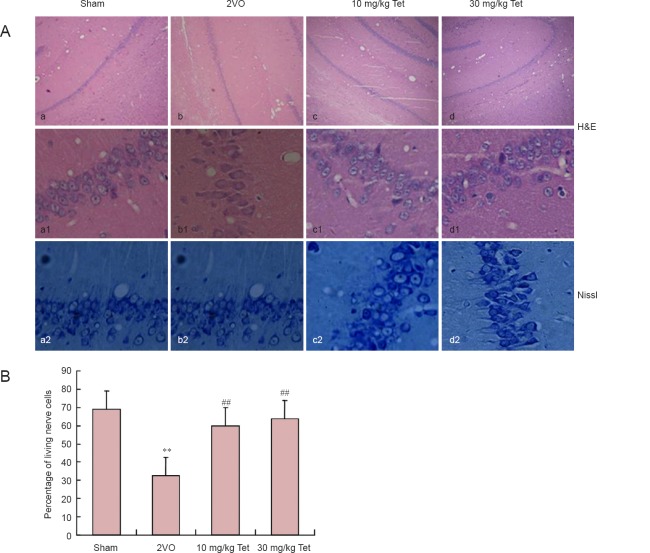
Hematoxylin-eosin and Nissl staining showed distinct layers in the structure of the hippocampus in the sham-operated rats. Cells had well-defined boundaries with no shrinkage or cytoplasmic disintegration (Figure 2A-a1). In comparison, the hippocampi of rats in the 2VO group showed neurons undergoing colliquative necrosis (Figure 2A-b1), characterized by cytoplasmic dissolution, nuclear shrinkage or disappearance, cell membrane rupture, cell lysis and tissue disorganization. The percentage of living cells in the hippocampal CA1 region of rats in the 2VO group was significantly lower than that in the sham group (P < 0.01). However, model rats that received Tet had a higher percentage of living cells than the 2VO group (P < 0.01), with no significant difference between the 10 and 30 mg/kg doses. Furthermore, there were fewer Nissl granules in animals that underwent 2VO surgery compared with the sham group (Figure 2A-a2–d2), and administration of 10 or 30 mg/kg Tet resulted in a noticeable recovery in the number of Nissl granules (Figure 2A-c2, 2d2).
Figure 2.
Effect of Tet on hippocampal CA1 pathology in rat models of vascular dementia.
(A) H&E (upper panel, 40× magnification; middle panel, 200× magnification) and Nissl staining (lower panel, 200× magnification) of hippocampal CA1 showing atrophic or dying cells (arrows). Scale bars: Upper panel, 5 μm; middle and lower panels, 50 μm. (B) Percentage of living cells in CA1 (mean ± SD, n = 3 rats per group). **P < 0.01, vs. sham group; ##P < 0.01, vs. 2VO group (one-way analysis of variance and Bonferroni post hoc test). Tet: Tetrandrine; 2VO: two-vessel occlusion; H&E: hematoxylin-eosin.
Tet increased IL-1β expression and NR2B phosphorylation in hippocampus of rat models of vascular dementia
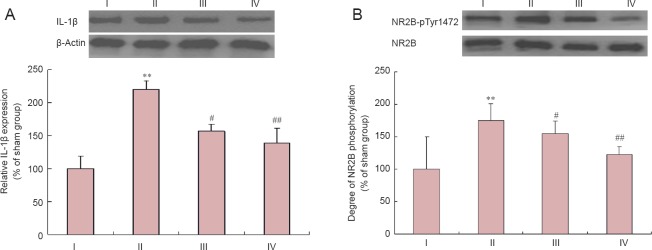
Western blot assay showed that IL-1β expression was significantly higher in the 2VO group than in the sham group (P < 0.01) (Figure 3). After administration of Tet at 10 mg/kg (P < 0.05) or 30 mg/kg (P < 0.01), IL-1β expression was lower than that in the 2VO group (Figure 3A). Interestingly, the NR2B-pTyr1472/NR2B ratio followed a similar trend to changes in IL-1β expression. NR2B phosphorylation was significantly greater in the 2VO group than in the sham group (P < 0.01). Administration of Tet at a dose of 10 mg/kg (P < 0.05) or 30 mg/kg (P < 0.01) resulted in lower NR2B phosphorylation levels than in the 2VO group (Figure 3B).
Figure 3.
Relative IL-1β expression (A) and degree of NR2B phosphorylation (B) in the hippocampus (western blot assay).
Data are expressed as the mean ± SD (n = 3 rats per group). The experiment was performed in triplicate. **P < 0.01, vs. sham group; #P < 0.05, ##P < 0.01, vs. 2VO group (one-way analysis of variance and Bonferroni post hoc test). I: Sham; II: 2VO; III: 10 mg/kg Tet; IV: 30 mg/kg Tet. IL-1β: Interleukin-1β; NR2B: N-methyl-D-aspartate receptor 2B; NR2B-pTyr1472: NR2B phosphorylated at tyrosine 1472.
Discussion
We performed bilateral surgical occlusion of the common carotid arteries to establish a rat model of chronic vascular dementia (Wang et al., 2004; Ivy et al., 2010; Valadares et al., 2010). The model resulted in impairments in spatial learning and memory as assessed in the Morris water maze. Longer escape latencies and shorter swim times in the target quadrant were observed in the model rats than in sham-operated rats. This condition was markedly improved after administration of 10 or 30 mg/kg Tet, which resulted in a 49.4% and 55.6% improvement in escape latency, respectively, and a 40.1% and 64.0% increase in target quadrant swimming time. Hematoxylin-eosin and Nissl staining revealed colliquative necrosis in hippocampi of the 2VO group, but less necrosis in the Tet groups than in the 2VO group. The percentages of living cells in the hippocampal CA1 region in the 2VO, 10 mg/kg Tet and 30 mg/kg Tet groups were 46.8%, 86.4% and 91.3%, respectively. These results demonstrated the success of the model, and indicated the potential therapeutic effects of Tet for chronic vascular dementia (Li et al., 2007; He et al., 2011).
High IL-1β levels are correlated with poor cognitive performance (Munoz et al., 2007; Baune et al., 2008), as reflected in our water maze experiment. Our results were consistent with previous reports of 2VO dementia models showing higher IL-1 expression than sham-operated animals (Zhang et al., 2013; Ben Menachem-Zidon et al., 2014; Rivera-Escalera et al., 2014), suggesting that IL-1 is an important molecular mediator in the development of experimental dementia in the rat. It is interesting to note that the ratio of NR2B-pTyr1472 to NR2B showed a similar trend of change to IL-1β levels among the experimental groups. Recent studies have shown that IL-1β regulates various classical neurotransmitter systems (Balosso et al., 2008) and can markedly enhance NMDAR function (Viviani et al., 2003; Salter and Kalia, 2004; Viviani et al., 2006). Overstimulation of the NMDAR represents a key event in Alzheimer's disease, the most common cause of dementia worldwide (Kaul et al., 2001; Haughey and Mattson, 2002). In the present study, hypophosphorylation of NR2B-pTyr1472 may contribute to the induction of typical necrosis, characterized by cytoplasmic dissolution, nuclear shrinkage or disappearance, cell membrane rupture, cell lysis, and Nissl body disappearance. Viviani et al. (2006) showed that recombinant IL-1β potentiates neuronal death induced by NMDAR activation in primary hippocampal neurons with increased phosphorylation of NR2B at tyrosine 1472. Given this, we propose that IL-1β may contribute to neuronal necrosis by potentiating NR2B tyrosine phosphorylation. This provides a possible mechanistic explanation for vascular dementia-related inflammation, which is in contrast to previous studies suggesting that inflammatory disorders can increase the risk of Alzheimer's disease by altering the amyloid-β burden in the brain (Kennedy et al., 2014; Loeffler, 2014; Ostrovskaya et al., 2014).
Tet is an anti-inflammatory Chinese herb that has already been used for the treatment of cancer (Chen et al., 2014; Kang et al., 2014; Wu et al., 2014; Mei et al., 2015; Xiao et al., 2015) and cerebral ischemia/reperfusion injury (Liu et al., 2001; Chen et al., 2011; Ruan et al., 2013). Our results provide evidence of the therapeutic effects of Tet against vascular dementia in a rat model. Further study of the relationship between IL-1β and the NR2B-pTyr1472/NR2B ratio at the cellular level is warranted (Jing et al., 2010; Wang et al., 2011; Di Filippo et al., 2013; Hwang et al., 2013; Rai et al., 2013). We have investigated only one of several inflammatory pathways involving IL-1β; the mechanisms underlying the therapeutic effects of Tet in vascular dementia need further elucidation.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81070886.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Slone-Murphy J, Pack M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, Vezzani A. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1β. Brain. 2008;131:3256–3265. doi: 10.1093/brain/awn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, Berger K. Association between IL-8 cytokine and cognitive performance in an elderly general population-The MEMO-Study. Neurobiol Aging. 2008;29:937–944. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Menahem YB, Hur TB, Yirmiya R. Intra-hippocampal transplantation of neural precursor cells with transgenic over-expression of IL-1 receptor antagonist rescues memory and neurogenesis impairments in an Alzheimer's disease model. Neuropsychopharmacology. 2014;39:401–414. doi: 10.1038/npp.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28:1579–1591. doi: 10.1097/QAD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tsai YH, Tseng SH. The potential of tetrandrine as a protective agent for ischemic stroke. Molecules. 2011;16:8020–8032. doi: 10.3390/molecules16098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li P, Yang S, Tong N, Zhang J, Zhao X. Tetrandrine enhances the anticancer effects of arsenic trioxide in vitro. Int J Clin Pharmacol Ther. 2014;52:416–424. doi: 10.5414/CP201939. [DOI] [PubMed] [Google Scholar]
- Chen YJ. Potential role of tetrandrine in cancer therapy. Acta Pharmacol Sin. 2002;23:1102–1106. [PubMed] [Google Scholar]
- Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampà C, Costa C, Tantucci M, Zianni E, Boraso M, Siliquini S, de Iure A, Ghiglieri V, Colcelli E, Baker D, Sarchielli P, Fusco FR, Di Luca M, Calabresi P. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis. 2013;52:229–236. doi: 10.1016/j.nbd.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Doruk H, Naharci MI, Bozoglu E, Isik AT, Kilic S. The relationship between body mass index and incidental mild cognitive impairment, Alzheimer's disease and vascular dementia in elderly. J Nutr Health Aging. 2010;14:834–838. doi: 10.1007/s12603-010-0113-y. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31:S55–61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- He FQ, Qiu BY, Zhang XH, Li TK, Xie Q, Cui DJ, Huang XL, Gan HT. Tetrandrine attenuates spatial memory impairment and hippocampal neuroinflammation via inhibiting NF-κB activation in a rat model of Alzheimer's disease induced by amyloid-β(1-42) Brain Res. 2011;1384:89–96. doi: 10.1016/j.brainres.2011.01.103. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Chen JC, Chan YC. Effects of C-phycocyanin and Spirulina on salicylate-induced tinnitus, expression of NMDA receptor and inflammatory genes. PLoS One. 2013;8:e58215. doi: 10.1371/journal.pone.0058215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TZ. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing T, Wu L, Borgmann K, Surendran S, Ghorpade A, Liu J, Xiong H. Soluble factors from IL-1β-stimulated astrocytes activate NR1a/NR2B receptors: implications for HIV-1-induced neurodegeneration. Biochem Biophys Res Commun. 2010;402:241–246. doi: 10.1016/j.bbrc.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem. 2010;115:814–828. doi: 10.1111/j.1471-4159.2010.06958.x. [DOI] [PubMed] [Google Scholar]
- Kåhlin J, Mkrtchian S, Ebberyd A, Hammarstedt-Nordenvall L, Nordlander B, Yoshitake T, Kehr J, Prabhakar N, Poellinger L, Fagerlund MJ, Eriksson LI. The human carotid body releases acetylcholine, ATP and cytokines during hypoxia. Exp Physiol. 2014;99:1089–1098. doi: 10.1113/expphysiol.2014.078873. [DOI] [PubMed] [Google Scholar]
- Kang OH, An HJ, Kim SB, Mun SH, Seo YS, Joung DK, Choi JG, Shin DW, Kwon DY. Tetrandrine suppresses pro-inflammatory mediators in PMA plus A23187-induced HMC-1 cells. Int J Mol Med. 2014;33:1335–1340. doi: 10.3892/ijmm.2014.1683. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kennedy K, Tucci MA, Benghuzzi HA. Comparison of potential preventive therapeutic agents green tea, thymoquinone, and dilinoleoylphosphatidylcholine on human neuroblastoma cells. Biomed Sci Instrum. 2014;50:132–139. [PubMed] [Google Scholar]
- Kinoshita A, Yamada K, Hayakawa T. Wound healing following stab injury on rat cerebral cortex. Neurol Res. 1991;13:184–188. doi: 10.1080/01616412.1991.11739988. [DOI] [PubMed] [Google Scholar]
- Li Z, Ni K, Du G. Simultaneous analysis of six effective components in the anti-Alzheimer's disease effective component group of Xiao-Xu-Ming Decoction. Se Pu. 2007;25:80–83. [PubMed] [Google Scholar]
- Liu C, Wu J, Gu J, Xiong Z, Wang F, Wang J, Wang W, Chen J. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2007;86:423–430. doi: 10.1016/j.pbb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zhou SW, Xue CS. Effect of tetrandrine on neutrophilic recruitment response to brain ischemia/reperfusion. Acta Pharmacol Sin. 2001;22:971–975. [PubMed] [Google Scholar]
- Loeffler DA. Should development of Alzheimer's disease-specific intravenous immunoglobulin be considered? J Neuroinflammation. 2014;11:198. doi: 10.1186/s12974-014-0198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan S, Phutane V, Jhirwal OP, Varghese M. Progression of vascular depression to possible vascular dementia. J Neuropsych Clin N. 2010;22 doi: 10.1176/appi.neuropsych.22.4.451-t.e34. 451-t.e434-451.e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzerra P, Behrens MM, Canzoniero LMT, Wang XQ, Heidinger V, Ichinose T, Yu SP, Choi DW. Zinc induces a Src family kinase-mediated up-regulation of NMDA receptor activity and excitotoxicity. Proc Natl Acad Sci U S A. 2001;98:11055–11061. doi: 10.1073/pnas.191353598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehraein F, Talebi R, Jameie B, Joghataie MT, Madjd Z. Neuroprotective effect of exogenous melatonin on dopaminergic neurons of the substantia nigra in ovariectomized rats. Iran Biomed J. 2011;15:44–50. [PMC free article] [PubMed] [Google Scholar]
- Mei L, Chen Y, Wang Z, Wang J, Wan J, Yu C, Liu X, Li W. Synergistic anti-tumour effects of tetrandrine and chloroquine combination therapy in human cancer: a potential antagonistic role for p21. Br J Pharmacol. 2015;172:2232–2245. doi: 10.1111/bph.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam A, Child C, Bruckner T, Gerner HJ, Daniel V, Biglari B. Posttraumatic inflammation as a key to neuroregeneration after traumatic spinal cord injury. Int J Mol Sci. 2015;16:7900–7916. doi: 10.3390/ijms16047900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz L, Ranaivo HR, Roy SM, Hu W, Craft JM, McNamara LK, Chico LW, Van Eldik LJ, Watterson DM. A novel p38α MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer's disease mouse model. J Neuroinflammation. 2007;4:21. doi: 10.1186/1742-2094-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrovskaya RU, Vakhitova YV, Kuzmina US, Salimgareeva MK, Zainullina LF, Gudasheva TA, Vakhitov VA, Seredenin SB. Neuroprotective effect of novel cognitive enhancer noopept on AD-related cellular model involves the attenuation of apoptosis and tau hyperphosphorylation. J Biomed Sci. 2014;21:74. doi: 10.1186/s12929-014-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Tien LT, Zhu H, Shen J, Wright CF, Jones TK, Mamoon SA, Bhatt AJ, Cai Z, Fan LW. Interleukin-1 receptor antagonist reduces neonatal lipopolysaccharide-induced long-lasting neurobehavioral deficits and dopaminergic neuronal injury in adult rats. Int J Mol Sci. 2015;16:8635–8654. doi: 10.3390/ijms16048635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Kang S. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res. 2013;62:681–688. doi: 10.1007/s00011-013-0620-5. [DOI] [PubMed] [Google Scholar]
- Rai S, Kamat PK, Nath C, Shukla R. A study on neuroinflammation and NMDA receptor function in STZ (ICV) induced memory impaired rats. J Neuroimmunol. 2013;254:1–9. doi: 10.1016/j.jneuroim.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Rivera-Escalera F, Matousek SB, Ghosh S, Olschowka JA, O’Banion MK. Interleukin-1β mediated amyloid plaque clearance is independent of CCR2 signaling in the APP/PS1 mouse model of Alzheimer's disease. Neurobiol Dis. 2014;69:124–133. doi: 10.1016/j.nbd.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan L, Huang HS, Jin WX, Chen HM, Li XJ, Gong QJ. Tetrandrine attenuated cerebral ischemia/reperfusion injury and induced differential proteomic changes in a MCAO mice model using 2-D DIGE. Neurochem Res. 2013;38:1871–1879. doi: 10.1007/s11064-013-1093-1. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Shang YZ, Miao H, Cheng JJ, Qi JM. Effects of amelioration of total flavonoids from stems and leaves of Scutellaria baicalensis Georgi on cognitive deficits, neuronal damage and free radicals disorder induced by cerebral ischemia in rats. Biol Pharm Bull. 2006;29:805–810. doi: 10.1248/bpb.29.805. [DOI] [PubMed] [Google Scholar]
- Shi C, Ahmad Khan S, Wang K, Schneider M. Improved delivery of the natural anticancer drug tetrandrine. Int J Pharm. 2015;479:41–51. doi: 10.1016/j.ijpharm.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Valadares CT, Fukuda MTH, Françolin-Silva AL, Hernandes AS, Almeida SS. Effects of postnatal protein malnutrition on learning and memory procedures. Nutr Neurosci. 2010;13:274–282. doi: 10.1179/147683010X12611460764769. [DOI] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Lemos JR, Iadecola C. Herbal alkaloid tetrandrine: fron an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol Sci. 2004;25:120–123. doi: 10.1016/j.tips.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Wang W, Mei XP, Wei YY, Zhang MM, Zhang T, Wang W, Xu LX, Wu SX, Li YQ. Neuronal NR2B-containing NMDA receptor mediates spinal astrocytic c-Jun N-terminal kinase activation in a rat model of neuropathic pain. Brain Behav Immun. 2011;25:1355–1366. doi: 10.1016/j.bbi.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin JL, Huang J, Yang JQ, Sun WJ, Wan LH, He BC. The role of IGFBP-5 in mediating the anti-proliferation effect of tetrandrine in human colon cancer cells. Int J Oncol. 2015;46:1205–1213. doi: 10.3892/ijo.2014.2800. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wang G, Xu S, Li Y, Tian Y, Niu H, Yuan F, Zhou F, Hao Z, Zheng Y, Li Q, Wang J. Effects of tetrandrine on glioma cell malignant phenotype via inhibition of ADAM17. Tumor Biol. 2014;35:2205–2210. doi: 10.1007/s13277-013-1293-y. [DOI] [PubMed] [Google Scholar]
- Xiao W, Jiang Y, Men Q, Yuan L, Huang Z, Liu T, Li W, Liu X. Tetrandrine induces G1/S cell cycle arrest through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis in vivo. Int J Oncol. 2015;46:360–368. doi: 10.3892/ijo.2014.2735. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Liu C, Wang F, Li C, Wang W, Wang J, Chen J. Protective effects of breviscapine on ischemic vascular dementia in rats. Biol Pharm Bull. 2006;29:1880–1885. doi: 10.1248/bpb.29.1880. [DOI] [PubMed] [Google Scholar]
- Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015;12:26. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YY, Fan YC, Wang M, Wang D, Li XH. Atorvastatin attenuates the production of IL-1β, IL-6, and TNF-β in the hippocampus of an amyloid β1-42-induced rat model of Alzheimer's disease. Clin Interv Aging. 2013;8:103–110. doi: 10.2147/CIA.S40405. [DOI] [PMC free article] [PubMed] [Google Scholar]