Abstract
Common marmoset (Callithrix jacchus) monkeys are a resource for biomedical research and their use is predicted to increase due to the suitability of this species for transgenic approaches. Identification of abnormal neurodevelopment due to genetic modification relies upon the comparison with validated patterns of normal behavior defined by unbiased methods. As scientists unfamiliar with nonhuman primate development are interested to apply genomic editing techniques in marmosets, it would be beneficial to the field that the investigators use validated methods of postnatal evaluation that are age and species appropriate. This review aims to analyze current available data on marmoset physical and behavioral postnatal development, describe the methods used and discuss next steps to better understand and evaluate marmoset normal and abnormal postnatal neurodevelopment
Keywords: behavior, developmental stages, common marmoset, neurodevelopment, assessment scales
Introduction
Common marmoset (Callithrix jacchus) monkeys are a valuable species for modeling human diseases ('t Hart, Abbott, Nakamura, & Fuchs, 2012; Okano, Hikishima, Iriki, & Sasaki, 2012; Tardif, Abee, & Mansfield, 2011a) particularly neurodegenerative ones, including Parkinson's disease (Ando et al., 2008; Gnanalingham, Smith, Hunter, Jenner, & Marsden, 1993), Huntington's disease (Kendall et al., 1998), Alzheimer's disease (Baker, Ridley, Duchen, Crow, & Bruton, 1993; Maclean, Baker, Ridley, & Mori, 2000), and multiple sclerosis ('t Hart et al., 2000; Genain & Hauser, 1997).
Marmoset models of neurodegenerative disorders have been typically induced by neurotoxin dosing (Eslamboli et al., 2007), direct intracerebral delivery of viral vectors encoding for gene mutations of interest (Kirik, Zinchenko, Shestakov, & Babykin, 2003) or by exposure to specific antibodies (Brok et al., 2000; Genain et al., 1995, 1996). Advances in pluripotent stem cells (Thomson et al., 1996; Tomioka et al., 2010) combined with transgenic (Chan & Yang, 2009; Sasaki et al., 2009) and more recently, additional genomic editing techniques (Kishi, Sato, Sasaki, & Okano, 2014) (Doudna & Charpentier, 2014) present the opportunity of creating models carrying genetic mutations linked to neurodegenerative diseases.
Marmoset monkeys present several advantages for genetic modification approaches compared to rhesus monkeys (see Table 1). For example, marmosets frequently give birth to twins in contrast to rhesus that usually have single births, therefore marmosets can double the number of genetically modified subjects in a shorter period of time. In addition, marmosets in captivity have a life span of approximately 16 years while rhesus is 35 years, which facilitates studies where age may impact disease onset.
Table 1. Summary and Comparison of Natural Characteristics Between Rhesus Monkeys and Common Marmosets.
| Rhesus Macaque | Common Marmoset | |
|---|---|---|
| Births | Single | Twins or triplets common |
| Gestation | 5.5 months | 4.5 months |
| Adulthood | 5 years | 2 years |
| Considered aged | ≥20 years | ≥8 years |
| Lifespan* | ∼35 years | ∼16 years |
| Zoonotic potential | Macacine herpes virus 1 | None |
| Mean adult body weight** | Male, 8.8 kg female 7.4 kg | Male and female, 0.38 kg |
| Handling and care | Can be aggressive | Relatively easy |
Marmoset's lifespan information is based in current peerreviewed literature (Abbott, Barnett, Colman, Yamamoto, & Schultz-Darken, 2003; Ross, Davis, Dobek, & Tardif, 2012; Tardif, Mansfield, Ratnam, Ross, & Ziegler, 2011b) and a retrospective review of electronic health heath records at the Wisconsin National Primate Research Center (WNPRC). These records include 1,397 individual common marmosets housed at WNPRC in the past 25 years. The oldest recorded age is 16.6 years. Other marmoset colonies report life span at 12.4 years (Nishijima et al., 2012), 13.7 years (Lacreuse, Mong, & Hara, 2015; Ross et al., 2012).
Weights reported here are average weights of the two species at the WNPRC (see text regarding variation in body weight). Note that marmosets are not sexually dimorphic.
In that regard, for many age-related disorders, it is not clear when and what types of prodromal symptoms first appear. Genomic edited monkeys present an opportunity to systematically study disease onset. Discrete neurological changes that differentiate levels of immaturity and/or deficits may not be evident by behavior observation alone and may require additional assessments to detect, different from tests usually applied to fully grown adult animals. Thus, there is a real need for tools to identify and quantify normal marmoset neurodevelopment.
This review aims to provide a historical perspective on the understanding of marmoset neurobehavior, analyze currently available data on marmoset physical and behavioral postnatal development, describe the methods used and discuss next steps to better understand and evaluate marmoset normal and abnormal postnatal neurodevelopment. Normal variation exists between individuals and colonies, and aspects of later growth can be affected by variations in earlier development (Ross, Power, Artavia, & Tardif, 2013; Tardif et al., 1998). Therefore, we also attempt to point out where there is a need to collect additional data on factors of variation to augment the existing developmental studies in order to better define the time points of normal marmoset neurobehavioral development. This will aid in designing studies so that true variation can be minimized in order to detect real differences when comparing genomic edited monkeys to ‘normal’ controls.
First Descriptions of Marmoset Postnatal Assessment
The first studies characterizing marmoset development involved early captive colonies established from animals exported from South America. In 1976, principal countries exporting new-world nonhuman primates banned or severely restricted the trade, resulting in greater reliance of breeding colonies in captivity. Epple (1974, 1975a,b) was a pioneer in establishing successful breeding colonies of Callithricidae and was among the first to note certain behaviors and vocalizations of individual species of the Callithrix genus.
Stevenson and Poole (1976) published a comprehensive description of the behavior repertoire of common marmosets building on some of Epple's descriptions. Movements and action sequences were characterized into behavior patterns. The collected data were first analyzed by creating a descriptive ethogram of the species that included sitting and resting postures, locomotion patterns, facial expressions and head movements, scent-marking, interaction with objects, social acts involving contact, grooming, patterns involving piloerection, startle response, tail positions, and vocalizations. Next the authors performed a detailed analysis of each behavior in different contexts (i.e., general activity patterns of adult pairs; prey capture; play between adults with young; sexual behavior; and intra- or inter-group aggressive behaviors.) They qualified their results obtained in captivity, as a restricted environment and compared them to descriptions for various species in the wild. This was the first comprehensive report of marmoset behaviors and laid the foundation to assess marmoset development.
Marmosets as Part of a Family
Ingram (1977) described variables affecting complex infant-family relationships, and in doing so identified some infant developmental milestones (Fig. 1). The family unit variables that Ingram considered were age and sex of the infant, birth order, family size, parity of mother, and generations in captivity. Measurable behavioral variables included time spent suckling versus carried, infant or adult initiation of behaviors, time infant spent near versus far from adults, number of infant approaches versus leaving, and the percentage of parental rejections to infant initiation behaviors.
Figure 1.

Common marmoset parent is shown with a 1-month old infant. Marmoset interactions within the family unit have an important role during neurodevelopment. Picture credit: Jordana Lenon, WNPRC.
Ingram's research contributed greatly to the understanding of when infant marmoset behaviors emerged, occurred, or terminated, indicating a timeline for maturity. In her description, suckling decreased until 8 weeks of age at which point it was no longer present. Infant carrying by parents and older siblings decreased to a minimum around 6 weeks of age, and rejections of infant approaches began to increase at that time, along with distance from parents. Additionally, infants had a strong role (60%) in the maintenance of proximity to caregivers throughout the four-month observational period.
Ximenes and Sousa (1996) documented how family composition can influence specific infant interactions that in turn affect developmental outcomes. They observed six families including 28 total infants that were followed from the age of 8 weeks to 3 years. Observations occurred twice weekly during this time. The investigators tallied nursing episodes and noted the nursing initiator, number of actual nursing attempts, and the nursing terminator. In addition, the amount of time each infant spent on the mother, father, or siblings, if present, was recorded. The presence of siblings was found to influence the relationship between parents and infants. For example, nursing episode termination and unsuccessful nursing attempts were more frequent in families with no older siblings, than in a family group with one or more sibling helpers present. Infants in families without older siblings present nursed for shorter durations per nursing bout and weaning occurred later, compared to infants with older siblings in the family who nursed more, received more milk per nursing, and were weaned sooner.
Tardif and colleagues (Tardif, Power, Oftedal, Power, & Layne, 2001; Tardif, Layne, Cancino, & Smucny, 2002) described the potential impact of number of infants on these variables. They demonstrated that outcomes on nursing behavior as well as time spent off carriers are affected by whether there are 1, 2, or 3 infants per litter. Additionally, when there are triplet infants, survival depended more upon perfect ability for crawling, righting, and vertical orientation rather than initial birth weight.
Play Refining Skillfulness
Chalmers and Locke-Haydon (1984) analyzed the relationship between amount of play and skillfulness in 10 infant marmosets from 6–22 weeks of age. Skillful behaviors were defined as behaviors that helped the infant gain or retain a desirable outcome (i.e., resource, location, interaction, or goal). Chalmers and Locke-Hayden measured these behaviors during tests administered to the infant (skill tests) and during behavioral observation of the family group (play sessions).
The skill tests conceived and used by Chalmers and Locke-Hayden were the aperture, wheel, and food tests. Each infant was assessed on these at weeks 6, 10, 14, 18, and 22 in between the play session observation weeks. The aperture test required the infant to willingly enter into a test cage that was attached to the living cage and then, accurately reach for a food reward (i.e., without touching the sides of the aperture) provided by an examiner. The aperture test evaluated sensorimotor skills involved in accurate reaching, learning ability, and motivation. The wheel test involved a similar test cage yet, the food reward was located on a shelf on the opposing side of the cage from where the infant enters. To obtain the food the infant needed to navigate a wheel turning away from the food at variable speeds and subsequently use a rope to ascend to the food reward. The wheel test evaluated acquisition of agility, attraction to food reward, and fear of motor sounds in the wheel mechanism. The food test evaluated infants at an age when they were able to eat solid food. This test created an artificially generated conflict situation between the infant and mother where food was presented to the pair in a standardized fashion. The experimenter alternated presentation of food to either the infant or mother and noted whether that individual ultimately retained it. In addition any infant communicative gestures, vocalizations, or avoidance of the conflict were recorded. Using the outcomes, they assessed an infant's ability to take food from others or to resist having food taken from them. Results of the three tests showed that with increasing age infants became more adept at negotiation of a moving obstacle, reached more accurately, exhibited less frustration, ended food tests in possession of food more often while making more attempts for food. The skill tests have the potential to be incorporated into a more comprehensive battery of tests for marmoset neurodevelopment.
The play sessions consisted of 2 hr observations twice weekly of each infant's behavior while in the undisturbed family group at 3-week intervals in between the weeks of skill testing. Age periods were defined as 7–9, 11–13, 15–17, and 19–21 weeks. The investigators measured “perseverance” by the number of occasions when the infant was able to continue an ongoing behavior, despite interruptions by other family members. They also noted the percentage of attempts that were successful in climbing onto parents and older siblings, as well as infant success at climbing off, without being stopped or forcibly removed. Other noted behaviors were the number of threats directed towards the infant and the number of infant tantrums based on “scream calls.” Significant increases in frequency and duration of play, as well as acrobatic skills were found when comparing marmosets 7–9 weeks old to 15–17 weeks old. Similarly, the frequency of eating solid food increased for the same age period and again at 19–21 weeks of age.
Although Chalmers and Locke-Hayden do not explicitly discuss variability between individuals tested, the coefficient of variation provided for each of the figures of their report illustrates variability of the data obtained during the behavioral observations and administered tests, between individuals and at the different time points, as it would be expected with any diagnostic behavioral test.
Early Deprivation Studies on Development
The above-mentioned studies highlight the importance of the family unit composition and opportunities for play in marmoset successful maturation. Dettling, Feldon, and Pryce (2002) further demonstrated the impact of familial interactions in marmoset development by studying the effects of early deprivation in marmosets, using the postnatal environmental manipulation paradigm originally developed in the rat (Ogawa et al., 1994). Two sets of twins in seven families were evaluated. In each twin set, one infant was given deprivation experience as an early life stressor, while the other infant was not. Deprivation was defined as removal of the infants from the family for a certain time period, with short manual handling before being placed in a plastic cage within an isolation chamber where no visual or auditory stimulation was available. Deprivation was administered daily from postnatal day 1 to day 28 for a total of 9 hr weekly, as two 30-min, one 60-min, two 90-min, and two 120-min sessions. Body weights as well as urinary cortisol and catecholamine titers, were measured on postnatal day 2 and day 28. The investigators also measured suckling position, parental carrying, anogenital licking, aggression, proximity to parent or infant twin, infant distress vocalizations, tail hair piloerection, and social or solitary play during home cage observations of 60 min each, 3 times per week for the first 8 weeks of life. Data analysis considered for comparison animal experience (deprivation vs. intact) and age, (first 4 weeks vs. second 4 weeks). The investigators found significant increases in cortisol, epinephrine, and norepinephrine in the repeated acute deprivation infants compared to controls when combining age groups. In addition, they found that these infants spent more time suckling in the first 4 weeks, and were smaller, demonstrated increased distress vocalizations, and played less socially than control infants in both age periods. This study emphasized particular infant behavioral measures that may detect developmental deficits and demonstrated that early life stressors can impact infant development in common marmosets.
In a follow up study, Dettling and colleagues (Dettling, Schnell, Maier, Feldon, & Pryce, 2007) investigated the long-term effects of early deprivation measured under basal and social stress conditions. Deprivation was given to one of each dizygotic twin pair with the other twin as control in order to minimize differences in genetic background and family units. The authors found that early deprivation main effects were decreased movement and time spent exploring during adolescence, although many differences were evident only when comparing subjects within the same families. Significant interaction effects in early deprivation and parentage suggested that juveniles exposed to early deprivation in life had increased basal levels of contact time with parents, basal and social stress blood pressure, decreased locomotor activity, and during a social stress challenge, decreased contact calling with increased exploration of novelty. This study provided evidence for long-term effects of early environment on bio-behavioral traits and states in marmosets and the importance of including parental factors in developmental studies.
Stress associated with early deprivation can affect developmental outcomes (Tardif et al., 1998). Johnson and colleagues (Johnson, Kamilaris, Calogero, Gold, & Chrousos, 1996) specifically compared early parenting styles with effect on growth and development. Frequency of positive parental behaviors correlated well with stature of the monkeys at 10 and 20 weeks of age. Juveniles reared with negative parenting behaviors present were smaller in body weight, knee-heel length, and head-tail length. These juveniles also demonstrated atypical social behavior and had altered hypothalamic-pituitary-adrenal (HPA) function when challenged with exogenous cortisol. The investigators concluded that the quality of early care affects later growth during the juvenile period and that poor care during early life led to a growth delay that was apparently reversible with ensuing catch-up growth in young adulthood.
Early deprivation was also used by Koshiba et al. to model autism spectral disorders-like behaviors and identify measures of change in social and emotional development (Koshiba et al., 2013). The investigators analyzed peer sociality using vocalizations and behavioral response and compared subjects with normal and atypical rearing. The marmosets' rearing conditions were either parent with sibling (P2; n = 13), alone by parent (P1; n = 4) or alone by human (H1; n = 6). The tests evaluated the subjects' response to an unfamiliar peer in four different social contexts: acoustic or visual contact only, visual and acoustic contact with no mesh, and visual and acoustic contact in open mesh. An additional test for the P2 group included a familiar peer in each of these contexts. Specific assessment parameters measured were: 1) active measures using head-central velocity, head azimuth velocity, and closest quadrisection preference; 2) positive social measures of synchronized approach-to-other frequency, positive-emotional vocalization frequency, and morphology of the positive calls; 3) negative social measures such as spontaneous approach-to-other frequency, negative-emotional vocalization frequency, and upper-body alert behavior; and 4) immobile behavior measures including freezing, farthest and central quadrisection preferences, and angle of face to peers. The responses were grouped into three developmental ages: 30–60, 80–100, and 101–130 days. Principal components analysis (PCA) was conducted based on a correlation matrix of behavioral parameters to generate PCA scores.
Analysis of PCA scores using factor-loading vectors at each developmental stage revealed qualitative and subtle differences among the rearing groups and social context delays were found to be measurable using this assessment scale. The investigators found that attention and spontaneous approach developed during 80–100 days of life in the normal-reared P2 with either the unfamiliar and familiar sibling context of the test. P1 reared marmosets showed attention and spontaneous approach later, while human (H1) reared did not exhibit this pattern at any of the measured ages. Additionally, marmosets from groups P1 and H1 exhibited vocalizations that differed from the P2 group. Specifically the twitter and short contact calls (see Vocal development section for details on calls) emitted to affiliated animals were of higher frequency for marmosets 80–100 and 101–130 days old in the P2 group, whereas in the P1 and H1 groups these were infrequent suggesting immaturity or lack of context for use of these vocalization. In contrast, the aggressive e-calls were present in all groups and ages when meeting unfamiliar peers. The similar deficits in P1 and H1 suggest that the skills may be better acquired through interactions with siblings apart from parental example. This may be because of stage specific behavior phenotype and learning ability. Further discussion on using these methods for developmental assessment appears in a subsequent section (see Specific early assessment scales—Socio-emotional development assessment).
Visual Development
Head cocking is a typical marmoset behavior associated with visual exploration and recognition of objects from various angles (Rogers, Stafford, & Ward, 1993). It is defined as the fixation of an object in the binocular visual field while rotating the head in its caudal-rostral axis (Menzel, 1980). Head cocking onset occurs during a critical period for visual development and its monitoring and quantification of how it changes overtime can be used as a surrogate measure of visual ability (Kaplan & Rogers, 2006; Menzel & Menzel, 1980). It should be noted that retinal development of the marmoset fovea at birth is similar to humans, although less developed than macaques, but then undergoes rapid postnatal development compared to other primates so that the fovea may be mature within 2–3 months after birth. (Hendrickson, Troilo, Possin, & Springer, 2006; Hendrickson, Troilo, Djajadi, Possin, & Springer, 2009; Springer, Troilo, Possin, & Hendrickson, 2011). This is paralleled by a rapid growth of primary visual cortex (V1) from birth to approximately 3 months, followed by synapse remodeling, reaching adult characteristics by 9 months (Fritschy & Garey, 1986a; Missler, Eins, Merker, Rothe, & Wolff, 1993a; Missler, Wolff, Merker, & Wolff, 1993b; Oga, Aoi, Sasaki, Fujita, & Ichinohe, 2013).
The occurrence and magnitude of head-cocking behavior during the first 2 months of life was described by Kaplan and Rogers (2006). The investigators studied 15 infant marmosets, four times daily from birth until day 60 postnatal. They collected data on the presence of head cocking, degree of maximal rotation and the distance to the object being fixated. The investigators found that during the first couple of weeks of life little to none of head cocking is observed. On average the behavior onset is on postnatal day 13 greatly increasing at 24 days, and then maintaining it at 24-day levels until day 60 postnatal. The amount of rotation in head cocking also changed over time. Infants less than 20 days of age rotated their heads a maximum angle of 45 degrees, while during 25–34 days postnatal marmosets showed more incidences of a maximum angle of 90 degrees. From this, the investigators hypothesized that the use of head cocking behavior, specifically in changes to degree of angle of head tilt, allows for more retinal input to the developing visual cortex.
Izumi, Tsuchida, and Yamaguchi (2012) assessed early marmoset visual development in 58 infants that were family-reared and 37 infants that were hand-reared from a total of 46 litters. In general all infants had a similar onset of eye-closure reflex (present at birth), head cocking (at 12–14 days), or avoidance of impending collision (around 3 weeks by closing eyes). Interestingly while family reared marmosets had onset of visual tracking behavior at day 11, the hand-reared group was significantly later (>3days later). The authors hypothesized that the presence of optical motion is necessary for this behavior to develop, as marmoset infants ride on caregivers and are in constant motion, compared to hand-reared infants who cling to a stationary surrogate.
Vocal Development
Marmosets, like many primates, utilize vocalizations to communicate information about social and emotional status (Stevenson & Rylands, 1988). Many vocalizations are thought to be in response to unexpected movements as well as threatening situations (Jones, 1997). Marmoset vocalizations fall into two general categories: alarm calls (tsik, er-er) and contact calls (phee, twitter, trill, chirp). The most common alarm calls are known as tsiks, and consist of brief descending sounds given alone or in series, and staccatos, which are a series of short ascending calls. These calls are brief and elicit a fleeting behavior from group members and play an important role in mate attraction, maintenance of group cohesion, territorial defense, and location of lost group members (Jones, 1997; Lazaro-Perea, 2001). Additionally, er-er calls are used as aggressive communication between individuals. Phee calls, whirr-trill, and chirps are contact calls used to keep track of group members and are often made in friendly contexts. The phee call is the most frequently used contact call, resembling a high-pitched whistle and often given in a series of one to five repetitions, with each bout lasting approximately two seconds. The trill call is low pitched and its cyclic frequency fluctuations result in a vibrato sound (Jones, 1997). Chatter is a low vibrating sound often given to indicate aggression and dominance over food resources. Similarly, the twitter call may be a territorial call in addition to indicating alertness (Brum, Voss, Kollmer, & Todt, 2003). Lastly, marmoset infants produce a high-pitched, sustained, unique distress call during the attachment phase of development used to indicate distress and encourage parental attentiveness (Jones, 1997).
Pistorio, Vintch, and Wang (2006) digitally recorded and analyzed vocalizations of nine infant marmosets aged 3–25 weeks during a brief parental separation period. Infant marmosets exhibited a high rate of calling (with the highest rate in weeks 3–4), the use of many call types in the absence of context, and the use of many calls specific to infants. The calls decreased in rate, became more contextual, and changed to adult specific calls as the infants matured (around 15–25 weeks). Pistorio et al. postulated that the gradual transformation in vocalization over this period is likely due to experience-based plasticity and learning.
Developmental Timelines
Reports of timelines of marmoset development aimed at identifying milestones and rating maturational changes began appearing in the early 1990's. Missler et al. (1992) and Yamamoto (1993) independently created descriptive categories of marmoset development. Further effort to define marmoset developmental stages was continued by de Castro Leão, Duarte Doria Neto, and de Sousa (2009), who utilized clustering analysis methods to further divide the Yamamoto timeline into distinct groups and subgroups. In this section we describe each of the three timelines separately, followed by a comparative discussion of their characteristics. See Figure 2 for a graphic summary of how the stages overlap.
Figure 2.
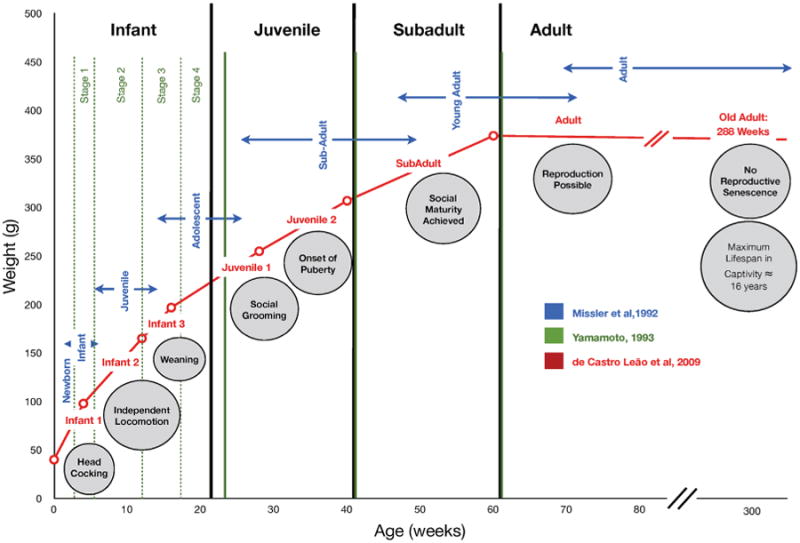
Graphic representation of common marmoset development based on descriptions by Missler et al. (1992), Yamamoto (1993), and de Castro Leão et al. (2009). Note there are small differences in onset and termination of defined stages depending upon the number of stages that were defined for each time frame, but the general overlap of stages is in agreement. Furthermore, all timelines agree on the progression of discrete milestones of development. These milestones are inserted in the figure along the age timeline below the defined stages (see Tables 2–4 for details). The relatively wide range in the age to reach a certain milestone suggests that further data is needed to narrow these down more precisely in order to detect subtle differences.
Missler Et Al. (1992)
Missler and colleagues proposed in 1992 that marmoset physical and social development be divided into 7 distinct post-natal stages from birth through adulthood. Data for this study was acquired using a questionnaire consisting of 106 questions regarding many aspects of marmoset development. The questionnaire consisted of four major physiological and behavioral developmental groupings, including feeding behavior, motor, visual, social, and sexual development. Responses to the questionnaire were obtained from 18 experts who had maintained colonies of C. jacchus at some point during their careers. The questionnaire gathered information about infant suckling, nursing, first consumption of solid foods, carrier-related motor behaviors and grasping reflexes, development of visual system (including head cocking) and social interactions (solitary and group play behaviors), including grooming, twin-fights, copulations, and genital displaying. Based on the collected data, the investigators proposed a 7-stage timeline to differentiate marmoset development (see Table 2). The first period, newborn (first postnatal week), includes the development of basic motor functions. During the second period, infant (first month), the transition to solid food takes place, head cocking develops, and more complex movements are performed. During the third period, juvenile (1–3 months), most developmental and behavioral characteristics are present; social grooming, scent marking, and genital display are all common occurrences. During the fourth period, adolescent (4–6 months), skills established in the third period are elaborated upon and adult facial coloration, pattern, and ear tufts are achieved. During the fifth period, sub-adult (6–12 months), twin-fights may ensue, followed by more complex behavioral patterns such as copulations and genital displaying. Males and females achieve sexual maturity during the sixth period, young adult (16–18 months), but females are typically reproductively suppressed due to the presence of a dominant, breeding female in the group. The seventh period of development, adult (>16–18 months), includes the onset of adult social and reproductive behaviors as well as breeding for those that become dominant in their social group.
Table 2. Description of the Missler et al. (1992) Marmoset Development Timeline.
| Missler Timeline | Age | Associated Behaviors |
|---|---|---|
| Newborn | <1 week | Suckled each hour, grasping reflex, climbing on carrier's back, interchange between mom and dad, open eyes/respond to light |
| Infant | 1–4 weeks | Commence eating solid food (3–4 W), interchange between all group members, jumping short distances, fixation on objects, head cocking commences (4–10 days), siblings recognize each other, inquiry of environment individually, auto-grooming (2–3 W), scent marking start (3–4W) |
| Juvenile | 4–12 weeks [1–3 months] | Eat solid food regularly (4–6 W), able to capture living prey (8–9 W), fine visual object discrimination (6–12 W) highly interactive play behavior, social grooming (8–10 W), start genital display (3 M) |
| Adolescent | 12–24 weeks [4–6 months] | Regular Scent marking, first mountings |
| Sub-adult | 24–48 weeks [6–12 months] | Twin fighting commences, complex behaviors, copulation, genital display, female fertility and physical maturity |
| Young adult | 48–64 weeks [12–16 months] | Fully-developed mount, fully-developed genital display, social maturity and competence |
| Adult | Over 64 weeks [>16 months] | Mating possible |
Missler et al. significantly advanced the field's ability to quantify marmoset maturation by proposing the first timeline that identified a wide range of subtle behaviors that had not been previously described in marmoset development. The description of the discrete changes allowed for comparison between normal and maladaptive marmoset physical and behavioral development. Missler et al. strengthened the integrity of the timeline by relating developmental stages with previous reports of physiological changes such as synapse formation and elimination in the visual cortex as noted in the visual development section (Aitkin, Merzenich, Irvine, Clarey, & Nelson, 1986; Fritschy & Garey, 1986a,b, 1988). Developmental periods one and two are defined by accelerated synapse formation, period four is defined by rapid synaptic pruning and periods five through seven are characterized by a slow rate of synapse pruning. Further physical development, including the sequence of dental maturation complemented the proposed developmental stages. Eruption begins during the infant stage and ceases with the appearance of molar teeth during the juvenile stage. Furthermore, the investigators related the stages of development with adult body proportions and adult facial expressions.
Yamamoto (1993)
In 1993, Yamamoto described four developmental stages by directly observing and quantifying behaviors of nine marmosets over time. The six female and three male monkeys originated from five families, and consisted of four sets of twins and a single infant from the captive breeding colony at the Nucleo de Primatologia of the Universidade Federal do Rio Grande do Norte. The infants were observed through a one-way mirror 3 hr weekly from birth through week 6, and then 3hr every other week until week 22. Yamamoto collected data on time on a carrier, frequencies and durations of rejections, frequencies of infants' attempts to climb on caregivers, approaches and leaves of infants from caregivers and first time appearances of scent-marking and agonistic behaviors. She also quantified time spent in proximity to caregivers, in physical contact with caregivers or twin, grooming, in social behavior or solitary behavior.
Yamamoto first matched the collected data to the traditionally accepted four stages of development: infant, juvenile, sub-adult, and adult (see Table 3). These stages have also been accepted for other Callithrichid species, including Cebuella (Soini, 1982), Saguinus (Cleveland & Snowdon, 1984), and Leontopithecus (Hoage, 1982). Then, using observational data, Yamamoto further subdivided C. jacchus into four additional sub-stages of development: weeks 2–4, 5–10, 12–16, and 18–22. Weeks 2–4 are marked by considerable dependence on carriers, and very brief periods of time “off” of a carrier. During weeks 5–10, infants begin to leave the carrier's back spontaneously, carriers begin rejecting infant approaches, infants ingest solid foods, and weaning occurs between weeks 8–10. The third period, weeks 12–16, are characterized by physical independence, still spending large amounts of time near or in direct physical contact other group members. The fourth period is defined by stable relationships between caregivers and infants, as well as the onset of solitary play and grooming behavior.
Table 3. Description of the Yamamoto (1993) Marmoset Developmental Timeline.
| Yamamoto Timeline | Age | Associated Behaviors |
|---|---|---|
| Infant 1 | 2–4 weeks [up to 1 month] | Dependence on caregiver, 96% of time on caregivers back, caregiver tolerant, time “off“ rare, brief periods “off’ infant began to explore their physical environment through solitary play, spent more time on dad than mom |
| Infant 2 | 5–10 weeks [1–2 months] | Carried 29.5% of time, began leaving spontaneously (due to both infant attempts to leave and caregiver rejection), learned to cope with rejection, infants approached caregivers more than caregivers approached infants, weaning occurred at the end of the period, infants able to feed themselves from weeks 5–6, 8.5% solitary play, grooming and social play occasionally seen. Agonistic behaviors appear, dad has higher tolerance for carrying infant |
| Infant 3 | 12–16 weeks [3–4 months] | Physically quite independent, carrying and nursing cease, eat solid foods unaided, spent 38.2% time near caregiver or in physical contact (14.8%) with them, approached caregivers more (26.2%) than previously—suggests increase in proximity and physical contact due to infants' initiative |
| Infant 4 | 18–22 weeks [4+ to 5+ months] | Relationship with caregivers remained stable, grooming most common activity (10.2%) |
| Juvenile | 22–40 weeks [5+ to 10 months] | Trend toward interacting with other group members besides parents |
| Sub-adult | 40–60 weeks [10–15 months] | Mastered most of adult behavioral repertoire |
| Adult | Over 60 weeks [>15 months] | Reach sexual maturity |
An interesting aspect of the Yamamoto timeline is that it characterized the actions of both the infant and caregivers regarding the infant's behavioral development and intensively analyzed behavior from two weeks through adolescence of marmoset development. It focused on both the amount of interaction and the type of interaction taking place between infants and caregivers, quantifying which caregiver the infants spends the most time on during the first stage of life, and also quantifying when the infant is able to “cope with rejection” from its carrier.
de Castro Leão Et Al. (2009)
de Castro Leão et al. (2009) took a different approach from Missler et al. (1992) and Yamamoto (1993) on how to define developmental stages. The investigators based their timeline on 9,200 entries of marmoset weight and age data collected between 1985 and 2003 at an outdoor-caged captive breeding colony at the Nucleo de Primatologia of the Universidade Federal do Rio Grande do Norte. Although the total number of animals was not reported, the entries per each 3 year period were 1985–1988: n = 991; 1989–1992: n = 4,097; 1993–1996: n = 1,919; 1997–2000: n = 1,931; 2000: n = 200; 2001: n = 28; 2003: n = 34. Taking as a working frame of reference the four classical developmental stages, the investigators utilized mathematical clustering to further identify stages and sub-stages of the data without a distinction being made between an independent and dependent variable. Specifically, the clustering methods were K-means algorithm (Duda, Hart, & Stork, 2002) and artificial neural network-self-organizing maps (SOM) (Kohonen, 1982). Based on their analysis de Castro Leão et al. (2009) proposed a four-stage traditional timeline, further divided into eight sub-stages.
The proposed classification for the ontogenetic development in common marmosets was based on mean weights (see Table 4). The body weight clusters manifest themselves in three sub-stages within the infant stage, and two sub-stages within each of the juvenile, and three adult sub-stages. The first stage, infant I (40.07 g) notes some off-episodes and scent marking behaviors. Infant II (97.88 g) is associated with vocalizations, piloerection, wrestling play with “open mouth face,” independent locomotion, solid ingestion, and self-feeding. Infant III (163.38 g) involves social play and wrestling. The juvenile timeframe is divided into two juvenile periods. The Juvenile I (197.68g) stage includes the onset of puberty in females and social grooming behaviors and the Juvenile II (255.54g) stage includes the onset of puberty in males. The Sub-Adult (307.97g) stage may have ovulation and copulatory behaviors occurring. Lastly, the adult stage is subdivided into two categories, Young Adult (374.63 g) stage involving reproduction activities and the Older Adult (352.77g) stage involving hearing loss and general senescence.
Table 4. Description of the de Castro Leão et al. (2009) Marmoset Development Timeline.
| de Castro Leão Timeline | Age | Weight (g) | Associated Behaviors |
|---|---|---|---|
| Infant 1 | 0–4 weeks [0–1 month] | 40 g | Carrying, nursing, feeding, play, agonism, scent marking |
| Infant 2 | 4–12 weeks [1–3 months] | 98 g | Piloerection begins, vocalization, open-mouth face, independent locomotion, solid ingestion, self feeding |
| Infant 3 | 12–16 weeks [3–4 months] | 165 g | Weaning completed, social play |
| Juvenile 1 | 16–28 weeks [4–7 months] | 197.68 g | Increase in female estradiol, social grooming |
| Juvenile 2 | 28–40 weeks [7–10 months] | 255.43 g | Male increase in testicular size and testosterone levels |
| Sub-adult | 40–60 weeks [10–15 months] | 307.97 g | Ovulation, copulation |
| Adult | 60–288 weeks [15–72 months] | 374 g | Reproduction |
| Older adult | over 288 weeks [>72 months] | Declining weight | Hearing loss, cartilage aging, general aging, but no reproductive senescence |
de Castro Leão et al. was the first to utilize weight as a discrete variable to further analyze the accepted four stage developmental scheme of Callithrix jacchus. The resulting eight-stage timeline coincides with known time periods for the onset of behaviors including carrying, nursing, feeding, play and agonistic behaviors. These results allowed for the development of a prediction weight curve, which can be useful when selecting subjects for long term experiments and for aging free-ranging animals. It is also advantageous because the standardized weight groupings can provide an index of health or age for comparison in experimental procedures and field studies.
Considerations Regarding the Three Developmental Timelines
A comparison between the timelines (Fig. 1) highlight a general overlap of stages and an agreement on the progression of discrete milestones of development, as well as some issues that should be considered for their application.
At the time of publication the Missler timeline (1992) was novel and became an essential resource to define the main developmental stages. A lingering concern is that as the data used for the analysis was acquired with a questionnaire, it may not have yielded the most accurate and unbiased results. Additionally, the timeline excludes many behaviors associated with interactions between family members, which play a critical role in marmoset development.
Yamamoto (1993) described an in-depth analysis of the first 22 weeks of life that was not addressed by Missler et al., however the observations were performed in only nine marmosets. The timeline does not define the stages of development using any discrete variable, but rather relies on traditionally accepted stages of Callithrix development in conjunction with observations.
A comparison between Yamamoto and Missler timelines revealed that the four stages broadly coincide through the adolescent period [e.g., week 2–4, newborn and infant; 5–10, juvenile, 12–16, juvenile and adolescent and 18–22 adolescent]. Physical and behavioral developments outlined in Missler et al. correlate well with the interactions between infants and caregivers in the Yamamoto timeline. For example, during weeks 5–10 the infant establishes physical independence, which catalyzes the onset of grooming, eating solid food, and head cocking behavior. Additionally, carrying and nursing ceases in weeks 12–16 and the infants spend most time off caregivers, allowing for adequate time to spend in solitary and social play behavior with both the infant's twin as well as older siblings. The independence from the carrier also allows for the onset of other behaviors learned from group observation such as scent marking. Altogether these two timelines complement each other well, describing aspects of marmoset development from birth through adulthood with consideration for both infant and care-giver behavior.
Compared to the Missler et al. and Yamamoto timelines in which the staging was based on the observation of accomplishing major milestones, the advantage of de Castro Leão et al. (2009) is the added objective criteria that body weight lends to the delineation of stages. The database of weights used by the investigators included many weight entries over a long period of time and from one breeding colony in Rio Grande de Norte. The use of weight entries from 1985 to 2003 (from unknown total number of animals) all obtained from the same population may have resulted in skewed data, not representative of the marmoset population as a whole. It should be noted that the four main clusters used by Leão et al. had been first established by Yamamoto from the same colony, limiting the population used to represent the current postnatal development timelines for the common marmoset. Captive marmosets can be heavier than wild marmosets in all age classes except infants (Araújo et al., 2000). In addition, Ross et al. and Power et al. described that the weight of captive populations has increased over time and also that development of obesity in captivity is influenced by early behaviors, such as licking efficiency and early interest in solid food. (Power, Ross, Schulkin, & Tardif, 2012; Power, Ross, Schulkin, Ziegler, & Tardif, 2013; Ross et al., 2013). Therefore, body weight provides a good metric for clustering methods and algorithm analysis only when used along with other developmental markers and comparisons of stages, and when weight is evaluated within each defined population.
Both the Missler and de Castro Leão timelines outlined marmoset stages from infant through adulthood into seven stages, to which de Castro Leão et al. added an 8th older adult category, yet there are differences between the lengths of the individual stages. In the Missler timeline the first stage is the first week of life, and in de Castro Leão et al. the first stage is the first month of life. Subsequent stages of the de Castro Leão timeline are at later time points than concurrent stages noted in the Missler timeline. de Castro Leão et al. and Missler et al. used different variables to create each timeline, and understandably the divisions from birth to adulthood differ.
Through our analysis emerges that, regardless how the marmoset developmental stage is called, appropriate identification of behavioral milestones plus information on body weight can help match a subject with a corresponding developmental period. In that regard, specific assessment scales (discussed in the next section) can help recognize milestone achievement, beyond simple subject observation.
Specific Early Assessment Scales
Assessment scales are common tools for evaluation of human newborns, such as the Brazelton Newborn Behavioral Assessment Scale (NBAS) (Als, Tronick, Lester, & Brazelton, 1977; Brazelton & Nugent, 1995), and infants and toddlers, such as the Bayley Mental Development (MDI) and Psychomotor Development Indices (PDI) (Bayley, 1963). These scales are both used to describe normal human neurodevelopment as well as to assess neurodevelopmental outcomes related to premature birth (Greene, Patra, Nelson, & Silvestri, 2012) and other environmental factors. Schneider and Suomi (1992) were the first to apply this concept for the development if an assessment scale for neonatal rhesus monkeys based on the Brazelton Newborn Behavioral Assessment Scale. The rhesus neurodevelopment scale has proven useful for investigating prenatal factors such as prenatal maternal stress (Schneider, Roughton, Koehler, & Lubach, 1999) or alcohol consumption effects on the offspring (Schneider, Roughton, & Lubach, 1997). Schneider and Coe (1993) also adapted this scale to assess development in squirrel monkeys suggesting that its applicability is not limited to old world species of nonhuman primates.
Following this premise, early marmoset development assessment scales were created to systematically evaluate subjects with discrete measures in order to identify differences between individuals and /or experimental groups. The scales are based on previously reported human and nonhuman primates assessment scales and adjusted to marmoset species typical behaviors. Each scale provides different but complementary information. The Early postnatal survival assessment (Tardif et al., 2002) covers the first week of life, while the Primate postnatal neurodevelopment assessment scale for marmosets (PPNAS-M) assesses motor, cognitive, sensorial, and emotional measures in the first month of life (Braun, Schultz-Darken, Schneider, Moore, & Emborg, 2015). The motor development scale evaluates motor skills from birth to maturation of adult-like abilities (Wang, Fang, & Gong, 2014), whereas the Socio-emotional development assessment (Koshiba et al., 2013) evaluates changes in peer sociality in marmosets 1–4 months of age. Below we describe each assessment scale individually followed by a discussion on the assessments as complementary tools.
Early Survival Assessment Scale (Up to 7 Days Postnatal)
Tardif et al. (2002) created an assessment method for the first week after birth aimed to quantify marmoset development and predict infant survival. The evaluations were adapted from a seven-task behavioral assessment of physical development by King, Fobes, and Fobes (1974) for neonatal behavior in Squirrel monkeys and Cotton-Top tamarins. Eighty-six marmoset monkeys from 42 litters (number of families not indicated) at the Southwest National Primate Research Center were tested during the first 24–36 hr post-birth. The evaluations include species-specific survival behaviors for infant common marmosets consisting of five motor skills, (crawling within 2 min, clasping-righting abilities from two different orientations and ability to remain in contact with fur-covered cylinder during quick movement, grasping reflex to hold onto experimenter finger for 60 s when raised off substrate, and vertical orienting to 180 degrees on inclined fur-covered surface) and two motor and sensory skills (rooting by turning head and placing mouth on stimulus tip and auditory orienting to turn head in direction of jingled keys). The investigators correlated these tasks with infant weights, morphometric measurements, and infant survival to postnatal day 7.
Weight measurements correlated best with crawling scores, while longer knee-heel lengths correlated with perfect grasping scores. The investigators found that the particular combination of scores from crawling, the two righting tasks, and vertical orientation was the most accurate predictor of infant survival to 7 days. Weight and size in itself did not predict infant survival in triplets, agreeing with Missler et al. (1992) that low birth weight is not the only relevant factor in predicting early postnatal death in marmosets. As such, this scale is a valid method for assessment of neurodevelopment milestones at birth, which helps predict infant marmoset survival.
Primate Postnatal Neurodevelopment Assessment Scale for Marmosets (First 30 Days Postnatal)
Beyond the initial post-birth days, the entire first month of life is identified as a critical period for neurodevelopment in nonhuman primates (King et al., 1974; Missler et al., 1992; Tardif et al., 2002). Aiming to characterize that developmental stage, our group (Braun et al., 2015) created a Primate Postnatal Neurobehavioral Assessment Scale for the marmoset monkey (PPNAS-M). The scale was adapted from the PNNAS for rhesus macaques (Schneider & Suomi, 1992) which was based on human infant assessment methods (Als et al., 1977; Bayley, 1963; Brazelton & Nugent, 1995). Twenty-four healthy infant marmosets from 12 different families housed in the marmoset colony at the Wisconsin National Primate Research Center were tested with this scale at approximately 15 and 30 days after birth. The PPNAS-M consists of 41 non-invasive behavioral evaluations, which are grouped into five main testing categories: visual orienting, auditory and spatial orienting, reflexes, righting and body strength, and temperament tests. The infant assessments involve a 10-min testing session.
Tests for the visual orienting category utilize a bright green ball with a smiley face to assess the infant's ability to maintain eye contact with the object, in order to evaluate visual follow as well as reach and grasp. The auditory and spatial orienting testing category evaluates the ability of the infant to orient towards a novel, repetitive auditory stimulus and includes startle response and orientation to the stimulus. The reflex testing category aims to assess primitive motor responses and include palmar and plantar grasping as well as Galant's reflex. The body righting and strength category evaluates muscle tone, response to restraint, and head orientation in the prone and supine positions. Lastly, the temperament testing category rates the infant's predominant state, coordination, speed and quality of responses, and conformity and self-calming behavior. Each of the 41 tests is scored on a 3 point scale with 0.5 increments (0, 0.5, 1, 1.5, 2). Data analysis comparing results between time points is performed for individual tests, as well as for totals of each of the five testing categories. In addition, groupings based on principal factor analysis are generated to assess how well the marmoset factors fit with those of rhesus.
The total PPNAS-M scores were significantly increased over time, achieving a mean of 51.4% of the total possible score at day 15 and 74.2% at day 30. Separate analysis of each of the five testing categories also showed significant increases over time. Principal component analysis defined four item groups (Orientation, State Control, Motor Maturity, and Sensory Sensitivity) with five variables each. Orientation and State Control factors were highly similar at both ages and correlated highly with previous item groupings used with rhesus macaques. Interestingly certain individual assessment tests, including reach and grasp, response to inversion, palmar and plantar grasping, head orientation in prone or supine positions, self-calming behavior and fearfulness did not show significant change from 15 to 30 days. As the scores for these tests were already high in the first testing session, it can be inferred that maturation for that specific challenge was already achieved prior to 15 days postnatal in a “normally” developed marmoset. Overall, the PPNAS-M provides a valuable battery of tests to measure dimensions of state modulation, arousal, orientation, attention, and neuromotor maturity during the critical first month of marmoset development. Because the PPNAS-M was based on human and nonhuman primate scales, it facilitates translational and interspecies comparisons.
Motor Development Scale (First 8 Weeks)
Wang et al. (2014) developed a motor assessment scale based on human infant motor assessments (Alberta Infant Motor Scale) widely used by physicians, occupational and physical therapists (Piper, Pinnell, Darrah, Maguire, & Byrne, 1992) which were adapted to the natural characteristics of marmosets. The investigators evaluated 11 newborn marmosets from age 1–8 weeks. The weekly measurements of gross motor ability included: (1) testing grasping ability of a small stick; (2) observations in an open field to assess righting reflex, postural control, and locomotion; (3) placement of smooth inclined 45° plane for negative geotaxis skill; (4) use of vertical rod for climbing ability; (5) observation in a small mesh cage to test hanging and jumping behaviors; and (6) placement of 10 cm barriers to test barrier-crossing.
By using these assessment methods, the investigators found that marmosets have a critical period for motor development in postnatal weeks 2–5 and most of their motor skills are acquired by 8 weeks of age. At 1 week of age, all marmosets were able to grasp, hang from the cage, and show the righting reflex. For postural control, marmosets were able to raise their heads during the first 2 weeks, stood with forelimb support from 3 weeks, and were able to sit similar to adults by 5 weeks of age. Locomotion measures showed marmosets able to only crawl during the first 3 weeks, walking in the 4th week, and running by 6 weeks of age. Most marmosets could hold onto the inclined plane in 2–3 weeks and showed negative geotaxis in the 4th week, successfully orienting upward. Marmosets could hold onto a smooth vertical rod from 3 weeks and climb it successfully by the 8th week. They could stand on a barrier at 4 weeks and were not able to cross the barrier successfully until 8 weeks of age. Marmoset exhibited the head to tail sequence of motor development similar to neuronal mechanisms described for human motor development. The motor development scale provides tools for assessment that complements the motor evaluation of the PPNAS-M, and expands the age range for motor evaluation.
Socio-Emotional Development Assessment (1–4 Months)
Progress in affective neuroscience and social neurobiology depends on quantitative measurements of socio-emotional development in addition to sensory-motor development. These types of evaluation tools are needed to monitor animal models of mental and mood disorders, such as autism or schizophrenia. A possible candidate to fill this need is the Socio-emotional development assessment developed by Koshiba et al. (2013) (see Early deprivation section for details). The investigators applied this testing system to study peer sociality in marmosets with normal and atypical rearing, in order to assess behavioral similarities with autism spectral disorders in children.
Their behavioral recordings, that included activity, positive and negative social measures, and immobile behavior and were evaluated by principal components analysis, provide the basis for detecting subtle differences in social interactions in marmosets. The investigators found that attention and spontaneous approach to unfamiliar peers normally develop during 80–100 days of life and that the characteristics of the twitter and short contact calls emitted to affiliated animals depend on the rearing conditions.
Comparison Between Neurodevelopmental Assessment Methods
Although standardized delineation and staging of marmoset behavioral and physiological development is important, the ability to quantify and assess maturation over time is an even more powerful tool, especially as marmoset monkeys become evermore popular vehicles for modeling biomedical diseases.
Tardif et al. (2002) scale was the first to formally use behavioral tasks to quantify marmoset development. The scale is useful for prediction of marmoset neonate survival in the first week of life. As such, it does not allow for comprehensive maturation analysis overtime, limiting its application.
Wang et al. (2014) scale used assessments similar to the Alberta Infant Motor Scale (Piper et al., 1992) to specifically characterize development of gross motor functions. The obtained results showing development of adult-like motor ability by 8 weeks of age substantiate the published Missler et al., Yamamoto, and de Castro Leão et al. timelines for marmosets indicating independent locomotion at approximately 2 months of age. In addition, their assessments of grasping, hanging, righting reflex, and postural control complemented well with the PPNAS-M assessment scale for marmosets.
As we described before, the PPNAS-M is based on an adaptation of the Schneider rhesus monkey scale, consists of 34 additional tests in addition to those used in Tardif et al. (2002), and thus describes marmoset maturation beyond the early postnatal period (Braun et al., 2015). The PPNAS-M both complements and expands upon the three marmoset developmental timelines of Missler et al. (1992), Yamamoto (1993), and de Castro Leão et al. (2009) for the infant stage. Thus far it has only been utilized to assess maturation during the first month of life; however, with some adjustment, the assessment method could be expanded to more age-appropriate behaviors that occur in the remaining months of infancy and into the juvenile stage. Marmosets compared to rhesus monkeys and humans have relatively passive maternal care, which affects the persistence of infant behaviors that facilitate parental- infant interactions (i.e., grasping and rooting) (Braun et al., 2015; King et al., 1974; Rothe, 1973). Therefore, when using genetically modified marmosets as models for human diseases, it is critical to compare their achievement of developmental milestones to normal marmoset cohorts before interpreting the results to the human disease condition.
Interestingly, during the validation of the scale, Braun et al. (2015) noted compelling maturation in the types of vocalizations between 2 and 4 weeks of age, which may be unique to marmosets. Specifically, the authors recorded the types of call of 24 marmosets over a period of one minute. They noted a shift from a majority of infants using short phee calls at the 15-day time point to more complex combinations of calls at the 30-day time point; including, long phee calls, an increase in distress calls, and the appearance of aggressive er'er calls. These findings complement Koshiba et al. (2013) description of normal development of more affiliative t-calls (twitter, trill, etc.) in later developmental stages but lack of these in marmosets from an atypical rearing.
Based on this analysis the assessment scales discussed above are appropriate tools for marmoset evaluation with specific limitations due to their specific sensitivity aimed to a relatively restricted age group or neurodevelopmental sphere. Together, these assessment methods are powerful devices to systematically evaluate marmoset neurobehavioral development sensory-motor as well as socio-emotional spheres.
Conclusions and Next Steps
Overall our review shows that collectively there are valuable tools and data that can be used to evaluate marmoset monkey postnatal development. We advocate for the use of a combination of outcome measures from the three timelines as criteria to match observations in marmosets with neurodevelopmental stages. The evaluation should include considerations of the factors that may contribute to developmental variation. The design of new studies will need to control for factors that influence development of normal infants from different populations when comparing age-matched normal controls to genomically edited monkey models for human diseases. Finally, we also argue that the evaluation of marmosets with appropriate neonatal and postnatal scales equivalent to the ones used in human and other primate species is necessary to identify early changes that characterize normal neurodevelopment and help identify maladaptive responses.
Evaluations of adult motor, cognitive, sensorial and emotional function (Eslamboli, Baker, Ridley, & Annett, 2003; Kendall et al., 1998; Ridley, Hardy, Maclean, & Baker, 2001; Takemoto, Izumi, Miwa, & Nakamura, 2011; Verhave, Vanwersch, van Helden, Smit, & Philip-pens, 2009) have been used and will be additionally important in assessment of marmoset neurodegenerative and psychological disease models. Development of adult age-appropriate assessment scales will become a necessary tool to examine the onset of motor and non-motor pathology as well as the response to treatment when using marmoset models of neurodegenerative disease.
Advances in magnetic resonance imaging (MRI) have facilitated in vivo brain characterization of adult infant and even fetal marmoset monkeys. MRI brain atlases are emerging with correlations with postmortem histological sections (Hikishima et al., 2013; Newman et al., 2009) or population standardized templates (Hikishima et al., 2011). New research combining refined neurobehavioral assessment with novel brain imaging technology (Bock, Hashim, Kocharyan, & Silva, 2011; Bock, Kocharyan, Liu, & Silva, 2009) and circulating molecular markers of gene expression (Hou et al., 2015) are warranted to deepen and expand our understanding of marmoset neurobehavioral development or degeneration and will be key to the study of genetically modified monkey models of neurological disorders.
Acknowledgments
This research was supported by NIH grant R24 OD019803, P51OD011106 and P51OD011106-53S2 (Wisconsin National Primate Research Center, University of Wisconsin-Madison), pilot funding from UL1TR000427 (to the Wisconsin Institutes for Clinical and Translational Research), bridge funding from the UW-Madison Vice Chancellor for Research and Graduate Education as well as the Welton Sophomore Scholarship, and Honors Senior Thesis Award (KB). This research was conducted at a facility constructed with support from Research Facilities Improvement Program grants RR15459-01 and RR020141-01.
Contract grant sponsor: NIH
Contract grant numbers: R24 OD019803, P51OD011106, P51OD011106-53S2
Contract grant sponsor: UL1TR000427
Contract grant sponsor: UW-Madison Vice Chancellor
Contract grant sponsor: Research Facilities Improvement Program
Contract grant numbers: RR15459-01, RR020141-01
Footnotes
Conflict of interest: None.
References
- 't Hart BA, van Meurs M, Brok HPM, Massacesi L, Bauer J, Boon L, … Laman JD. A new primate model for multiple sclerosis in the common marmoset. Immunology Today. 2000;21(6):290–297. doi: 10.1016/S0167-5699(00)01627-3. [DOI] [PubMed] [Google Scholar]
- 't Hart BA, Abbott DH, Nakamura K, Fuchs E. The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discovery Today. 2012;17(21-22):1160–1165. doi: 10.1016/j.drudis.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comparative Medicine. 2003;53(4):339–350. [PubMed] [Google Scholar]
- Aitkin LM, Merzenich MM, Irvine DR, Clarey JC, Nelson JE. Frequency representation in auditory cortex of the common marmoset (Callithrix jacchus jacchus) Journal of Comparative Neurology. 1986;252(2):175–185. doi: 10.1002/cne.902520204. [DOI] [PubMed] [Google Scholar]
- Als H, Tronick E, Lester BM, Brazelton TB. The Brazelton Neonatal Behavioral Assessment Scale (BNBAS) Journal of Abnormal Child Psychology. 1977;5(3):215–231. doi: 10.1007/BF00913693. [DOI] [PubMed] [Google Scholar]
- Ando K, Maeda J, Inaji M, Okauchi T, Obayashi S, Higuchi M, et al. Tanioka Y. Neurobehavioral protection by single dose l-deprenyl against MPTP-induced parkinsonism in common marmosets. Psychopharmacology (Berl) 2008;195(4):509–516. doi: 10.1007/s00213-007-0929-2. [DOI] [PubMed] [Google Scholar]
- Araújo A, Arruda MF, Alencar AI, Albuquerque F, Nascimento MC, Yamamoto ME. Body weight of wild and captive common marmosets (Callithrix jacchus) International Journal of Primatology. 2000;21(2):317–324. doi: 10.1023/A:1005433722475. [DOI] [Google Scholar]
- Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Experimental induction of beta-amyloid plaques and cerebral angiopathy in primates. Annals of the New York Academy of Sciences. 1993;695:228–231. doi: 10.1111/j.1749-6632.1993.tb23057.x. [DOI] [PubMed] [Google Scholar]
- Bayley N. An introduction to development assessment in the 1st year—Illingworth, Rs. Journal of Child Psychology. 1963;4(2):134–134. [Google Scholar]
- Bock NA, Kocharyan A, Liu JV, Silva AC. Visualizing the entire cortical myelination pattern in marmosets with magnetic resonance imaging. Journal of Neuroscience Methods. 2009;185(1):15–22. doi: 10.1016/j.jneumeth.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock NA, Hashim E, Kocharyan A, Silva AC. Visualizing myeloarchitecture with magnetic resonance imaging in primates. Annals of the New York Academy Sciences. 2011;1225(Suppl 1):E171–E181. doi: 10.1111/j.1749-6632.2011.06000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Schultz-Darken N, Schneider M, Moore CF, Emborg ME. Development of a novel postnatal neurobehavioral scale for evaluation of common marmoset monkeys. American Journal of Primatology. 2015;77:401–417. doi: 10.1002/ajp.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TB, Nugent JK. Neonatal behavioral assessment scale. New York, NY: Cambridge University Press; 1995. [Google Scholar]
- Brok HP, Uccelli A, Kerlero De Rosbo N, Bontrop RE, Roccatagliata L, de Groot NG, et al. Hart BA. Myelin/oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis in common marmosets: the encephalitogenic T cell epitope pMOG. Journal Immunology. 2000;165(2):24–36. doi: 10.4049/jimmunol.165.2.1093. [DOI] [PubMed] [Google Scholar]
- Brum H, Voss K, Kollmer I, Todt D. Acoustic communication in noise: regulation of call characheristics in New World monkey. Journal of Experimental Biology. 2003;207:443–448. doi: 10.1242/jeb.00768. [DOI] [PubMed] [Google Scholar]
- Chalmers NR, Locke-Haydon J. Correlations among measures of playfulness and skillfulness in captive common marmosets (Callithrix jacchus jacchus) Developmental Psychobiology. 1984;17(2):191–208. doi: 10.1002/dev.420170209. [DOI] [PubMed] [Google Scholar]
- Chan AW, Yang SH. Generation of transgenic monkeys with human inherited genetic disease. Methods. 2009;49(1):78–84. doi: 10.1016/j.ymeth.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland J, Snowdon CT. Social development during the first twenty weeks in the cotton-top tamarin (Saguinus o. oedipus) Animal Behaviour. 32(2):432–444. doi: 10.1016/S0003-3472(84)80279-1. [DOI] [Google Scholar]
- de Castro Leão A, Duarte Doria Neto A, de Sousa MB. New developmental stages for common marmosets (Callithrix jacchus) using mass and age variables obtained by K-means algorithm and self-organizing maps (SOM) Computers in Biology and Medicine. 2009;39(10):853–859. doi: 10.1016/j.compbiomed.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (callithrix jacchus, primates) and analysis of its effects on early development. Biological Psychiatry. 52(11):1037–1046. doi: 10.1016/S0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Schnell CR, Maier C, Feldon J, Pryce CR. Behavioral and physiological effects of an infant-neglect manipulation in a bi-parental, twinning primate: impact is dependent on familial factors. Psychoneuroendocrinology. 2007;32(4):331–349. doi: 10.1016/j.psyneuen.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213) doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Duda RO, Hart PE, Stork DG. Pattern classification. 2. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- Epple G. Pheromones in primate reproduction and social behavior. Advanced Behavior Biology. 1974;11:131–155. doi: 10.1007/978-1-4684-3069-1_6. [DOI] [PubMed] [Google Scholar]
- Epple G. The behavior of marmoset monkeys (Callithricidae) Primate Behavior. 1975a;4:195–239. [Google Scholar]
- Epple G. Parental behavior in Saguinus fuscicollis ssp. (Callithricidae) Folia Primatol (Basel) 1975b;24(2-3):221–238. doi: 10.1159/000155691. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Baker HF, Ridley RM, Annett LE. Sensorimotor deficits in a unilateral intrastriatal 6-OHDA partial lesion model of Parkinson's disease in marmoset monkeys. Experimental Neurology. 2003;183(2):418–429. doi: 10.1016/s0014-4886(03)00139-0. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, et al. Kirik D. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain. 2007;130(Pt 3):799–815. doi: 10.1093/brain/awl382. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Garey LJ. Quantitative changes in morphological parameters in the developing visual cortex of the marmoset monkey. Developmental Brain Research. 29(2):173–188. doi: 10.1016/0165-3806(86)90093-3. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Garey LJ. Postnatal development of quantitative morphological parameters in the lateral geniculate nucleus of the marmoset monkey. Brain Research. 1986b;395(2):157–168. doi: 10.1016/s0006-8993(86)80195-0. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Garey LJ. Postnatal development of dendrites of relay neurons in the lateral geniculate nucleus of the marmoset (Callithrix jacchus): a quantitative Golgi study. Journal of Comparative Neurology. 1988;268(2):234–247. doi: 10.1002/cne.902680208. [DOI] [PubMed] [Google Scholar]
- Genain CP, Hauser SL. Creation of a model for multiple sclerosis in Callithrix jacchus marmosets. Journal of Molecular Medicine (Berlin) 1997;75(3):187–197. doi: 10.1007/s001090050103. [DOI] [PubMed] [Google Scholar]
- Genain CP, Nguyen MH, Letvin NL, Pearl R, Davis RL, Adelman M, et al. Hauser SL. Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. Journal of Clinical Investigation. 1995;96(6):2966–2974. doi: 10.1172/JCI118368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genain CP, Abel K, Belmar N, Villinger F, Rosenberg DP, Linington C, et al. Hauser SL. Late complications of immune deviation therapy in a nonhuman primate. Science. 1996;274(5295):2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Smith LA, Hunter AJ, Jenner P, Marsden CD. Alterations in striatal and extrastriatal D-1 and D-2 dopamine receptors in the MPTP-treated common marmoset: an autoradiographic study. Synapse. 1993;14(2):184–194. doi: 10.1002/syn.890140212. [DOI] [PubMed] [Google Scholar]
- Greene MM, Patra K, Nelson MN, Silvestri JM. Evaluating preterm infants with the Bayley-III: patterns and correlates of development. Research in Developmental Disabilities. 2012;33(6):1948–1956. doi: 10.1016/j.ridd.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Troilo D, Possin D, Springer A. Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. Journal of Comparative Neurology. 2006;497(2):270–286. doi: 10.1002/cne.20996. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Troilo D, Djajadi H, Possin D, Springer A. Expression of synaptic and phototransduction markers during photoreceptor development in the marmoset monkey Callithrix jacchus. Journal of Comparative Neurology. 2009;512(2):218–231. doi: 10.1002/cne.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikishima K, Quallo MM, Komaki Y, Yamada M, Kawai K, Momoshima S, et al. Okano H. Population-averaged standard template brain atlas for the common marmoset (Callithrix jacchus) Neuroimage. 2011;54(4):2741–2749. doi: 10.1016/j.neuroimage.2010.10.061. [DOI] [PubMed] [Google Scholar]
- Hikishima K, Sawada K, Murayama AY, Komaki Y, Kawai K, Sato N, et al. Okano H. Atlas of the developing brain of the marmoset monkey constructed using magnetic resonance histology. Neuroscience. 2013;230:102–113. doi: 10.1016/j.neuroscience.2012.09.053. [DOI] [PubMed] [Google Scholar]
- Hoage R. Social and physical maturation in captive lion tamarins. Leontopithecus rosalia rosalia (Primates: Callitrichidae) Smithsonian Contributions to Zoology. 1982;354:1–56. [Google Scholar]
- Hou Z, Jiang P, Swanson SA, Elwell AL, Nguyen BK, Bolin JM, et al. Thomson JA. A cost-effective RNA sequencing protocol for large-scale gene expression studies. Science Reports. 2015;5 doi: 10.1038/srep09570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JC. Interactions between parents and infants, and the development of independence in the common marmoset (Callithrix jacchus) Animal Behaviour. 25(0):811–827. doi: 10.1016/0003-3472(77)90035-5. [DOI] [Google Scholar]
- Izumi A, Tsuchida J, Yamaguchi C. Effects of rearing conditions on early visual development in common marmosets. Developmental Psychobiology. 2012;54(7):700–705. doi: 10.1002/dev.20619. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Calogero AE, Gold PW, Chrousos GP. Effects of early parenting on growth and development in a small primate. Pediatric Research. 1996;39(6):999–1005. doi: 10.1203/00006450-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Jones CB. Quantitative analysis of marmoset vocal communication. In: Pryce CR, Scott L, Schnell C, editors. Marmosets and tamarins in biological and biomedical research: proceedings of workshop organised by european marmoset research group. Salisbury, UK: DSSD Imagery; 1997. pp. 41–151. [Google Scholar]
- Kaplan G, Rogers LJ. Head-cocking as a form of exploration in the common marmoset and its development. Developmental Psychobiol. 2006;48(7):551–560. doi: 10.1002/dev.20155. [DOI] [PubMed] [Google Scholar]
- Kendall AL, Rayment FD, Torres EM, Baker HF, Ridley RM, Dunnett SB. Functional integration of striatal allografts in a primate model of Huntington's disease. Nature Medicine. 1998;4(6):727–729. doi: 10.1038/nm0698-727. [DOI] [PubMed] [Google Scholar]
- King JE, Fobes JT, Fobes JL. Development of early behaviors in neonatal squirrel monkeys and cotton-top tamarins. Developmental Psychobiology. 1974;7(2):97–109. doi: 10.1002/dev.420070202. [DOI] [PubMed] [Google Scholar]
- Kirik IA, Zinchenko VV, Shestakov SV, Babykin MM. Trans- and cis-acting autorepressors of the prqR gene in Synechocystis cyanobacteria sp. PCC6803. Molecular Biology (Mosk) 2003;37(6):1035–1044. [PubMed] [Google Scholar]
- Kishi N, Sato K, Sasaki E, Okano H. Common marmoset as a new model animal for neuroscience research and genome editing technology. Developmenatal, Growth & Differentiation. 2014;56(1):53–62. doi: 10.1111/dgd.12109. [DOI] [PubMed] [Google Scholar]
- Kohonen T. Self-organized formation of topologically correct feature maps. Biological Cybernetics. 1982;43(1):59–69. [Google Scholar]
- Koshiba M, Senoo A, Mimura K, Shirakawa Y, Karino G, Obara S, et al. Nakamura S. A cross-species socio-emotional behaviour development revealed by a multivariate analysis. Science Reports. 2013;3 doi: 10.1038/srep02630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Mong JA, Hara Y. Neurocognitive effects of estrogens across the adult lifespan in nonhuman primates: State of knowledge and new perspectives. Hormones Behavior. 2015 doi: 10.1016/j.yhbeh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Lazaro-Perea C. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defense and assessment of neighbors. Animal Behaviour. 2001;62:11–21. [Google Scholar]
- Maclean CJ, Baker HF, Ridley RM, Mori H. Naturally occurring and experimentally induced beta-amyloid deposits in the brains of marmosets (Callithrix jacchus) Journal of Neural Transmission. 2000;107(7):799–814. doi: 10.1007/s007020070060. [DOI] [PubMed] [Google Scholar]
- Menzel CR, Menzel EW., Jr Head-cocking and visual exploration in marmosets (Saguinus fuscicollis) Behaviour. 1980;75(3/4):219–234. doi: 10.2307/4534082. [DOI] [Google Scholar]
- Menzel CR. Head-cocking and visual perception in primates. Animal Behavior. 1980;28 Pt1:151–159. doi: 10.1016/s0003-3472(80)80020-0. [DOI] [PubMed] [Google Scholar]
- Missler M, Wolff JR, Rothe H, Heger W, Merker HJ, Treiber A, et al. Crook G. Developmental biology of the common marmoset: proposal for a “postnatal staging”. Journal of Medical Primatology. 1992;21(6):285–298. [PubMed] [Google Scholar]
- Missler M, Eins S, Merker HJ, Rothe H, Wolff JR. Pre- and postnatal development of the primary visual cortex of the common marmoset. I. A changing space for synaptogenesis. Journal of Comparative Neurology. 1993;333(1):41–52. doi: 10.1002/cne.903330104. [DOI] [PubMed] [Google Scholar]
- Missler M, Wolff A, Merker HJ, Wolff JR. Pre- and postnatal development of the primary visual cortex of the common marmoset. II. Formation, remodelling, and elimination of synapses as overlapping processes. Journal of Comparative Neurology. 1993;333(1):53–67. doi: 10.1002/cne.903330105. [DOI] [PubMed] [Google Scholar]
- Newman JD, Kenkel WM, Aronoff EC, Bock NA, Zametkin MR, Silva AC. A combined histological and MRI brain atlas of the common marmoset monkey, Callithrix jacchus. Brain Research Reviews. 2009;62(1):1–18. doi: 10.1016/j.brainresrev.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, Kitajima S. Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology. 2012;13(4):439–443. doi: 10.1007/s10522-012-9388-1. [DOI] [PubMed] [Google Scholar]
- Oga T, Aoi H, Sasaki T, Fujita I, Ichinohe N. Postnatal development of layer III pyramidal cells in the primary visual, inferior temporal, and prefrontal cortices of the marmoset. Front Neural Circuits. 2013;7 doi: 10.3389/ncir.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K. Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacology, Biochemistry, and Behavior. 1994;49(4):961–967. doi: 10.1016/0091-3057(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Okano H, Hikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin in Fetal & Neonatal Medicine. 2012;17(6):336–340. doi: 10.1016/j.siny.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Piper MC, Pinnell LE, Darrah J, Maguire T, Byrne PJ. Construction and validation of the Alberta Infant Motor Scale (AIMS) Canadian Journal of Public Health. 1992;83(Suppl 2):S46–S50. [PubMed] [Google Scholar]
- Pistorio AL, Vintch B, Wang X. Acoustic analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus) Journal of the Acoustical Society of America. 2006;120(3):1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Power ML, Ross CN, Schulkin J, Tardif SD. The development of obesity begins at an early age in captive common marmosets (Callithrix jacchus) American Journal of Primatology. 2012;74(3):261–269. doi: 10.1002/ajp.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power ML, Ross CN, Schulkin J, Ziegler TE, Tardif SD. Metabolic consequences of the early onset of obesity in common marmoset monkeys. Obesity (Silver Spring, Md) 2013;21(12) doi: 10.1002/oby.20462. 10.1002/oby.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley RM, Hardy A, Maclean CJ, Baker HF. Non-spatial acquisition and retention deficits following small excitotoxic lesions within the hippocampus in monkeys. Neuroscience. 2001;107(2):239–248. doi: 10.1016/s0306-4522(01)00358-x. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Stafford D, Ward JP. Head cocking in galagos. Animal Behaviour45(5):943–952. doi: 10.1006/anbe.1993.1113. [DOI] [Google Scholar]
- Ross CN, Davis K, Dobek G, Tardif SD. Aging Phenotypes of Common Marmosets (Callithrix jacchus) Journal of Aging Research. 2012 doi: 10.1155/2012/567143. 567143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN, Power ML, Artavia JM, Tardif SD. Relation of food intake behaviors and obesity development in young common marmoset monkeys. Obesity. 2013;21(9):1891–1899. doi: 10.1002/oby.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe H. Observations on the delivery behavior in the common marmoset (Callithrix jacchus Erxleben, 1777) Folia Primatologica. 1973;19:257–285. doi: 10.1159/000155543. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, et al. Kamioka M. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459(7246):523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Coe CL. Repeated social stress during pregnancy impairs neuromotor development of the primate infant. Journal of Developmental and Behavioral Pediatrics. 1993;14(2):81–87. [PubMed] [Google Scholar]
- Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behavior and Development. 1992;15(2):155–177. doi: 10.1016/0163-6383(92)80021-L. [DOI] [Google Scholar]
- Schneider ML, Roughton EC, Lubach GR. Moderate alcohol consumption and psychological stress during pregnancy induce attention and neuromotor impairments in primate infants. Child Development. 1997;68(5):747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Development. 1999;70(2):263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Soini P. Ecology and population dynamics of the pygmy marmoset Cebuella pygmaea. Folia Primatologica (Basel) 1982;39(1-2):1–21. doi: 10.1159/000156066. [DOI] [PubMed] [Google Scholar]
- Springer AD, Troilo D, Possin D, Hendrickson AE. Foveal cone density shows a rapid postnatal maturation in the marmoset monkey. Visual Neuroscience. 2011;28(6):473–484. doi: 10.1017/S0952523811000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson MF, Poole TB. An ethogram of the common marmoset (Calithrix jacchus jacchus): general behavioural repertoire. Animal Behavior. 1976;24(2):428–451. doi: 10.1016/s0003-3472(76)80053-x. [DOI] [PubMed] [Google Scholar]
- Stevenson MF, Rylands AB. The marmosets, genus Callithrix. In: Mittermeir RA, Rylands AB, Coimbra-Filho AF, da Fonseca GAB, editors. Ecology and behavior of neotropical primates. Vol. 2. Washington, DC: World Wildlife Fund; 1988. pp. 131–222. [Google Scholar]
- Takemoto A, Izumi A, Miwa M, Nakamura K. Development of a compact and general-purpose experimental apparatus with a touch-sensitive screen for use in evaluating cognitive functions in common marmosets. Journal of Neuroscience Methods. 2011;199(1):82–86. doi: 10.1016/j.jneumeth.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Tardif S, Jaquish C, Layne D, Bales K, Power M, Power R, Oftedal O. Growth variation in common marmoset monkeys (Callithrix jacchus) fed a purified diet: relation to care-giving and weaning behaviors. Laboratory Animal Science. 1998;48(3):264–269. [PubMed] [Google Scholar]
- Tardif S, Power M, Oftedal O, Power R, Layne D. Lactation, maternal behavior and infant growth in common marmoset monkeys (Callithrix jacchus): effects of maternal size and litter size. Behavioral Ecology and Sociobiology. 2001;51(1):17–25. doi: 10.1007/s002650100400. [DOI] [Google Scholar]
- Tardif SD, Layne DG, Cancino L, Smucny DA. Neonatal behavioral scoring of common marmosets (Callithrix jacchus): relation to physical condition and survival. Journal of Medical Primatology. 2002;31(3):147–151. doi: 10.1034/j.1600-0684.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Abee CR, Mansfield KG. Workshop Summary: Neotropical Primates in Biomedical Research. Institute of Laboratory Animal Resources. 2011;52(3):386–392. doi: 10.1093/ilar.52.3.386. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. Institute of Laboratory Animal Resources. 2011;52(1):54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biology of Reproduction. 1996;55(2):254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- Tomioka I, Maeda T, Shimada H, Kawai K, Okada Y, Igarashi H, et al. Okano H. Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes to Cells. 2010;15(9):959–969. doi: 10.1111/j.1365-2443.2010.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhave PS, Vanwersch RA, van Helden HP, Smit AB, Philippens IH. Two new test methods to quantify motor deficits in a marmoset model for Parkinson's disease. Behavioural Brain Research. 2009;200(1):214–219. doi: 10.1016/j.bbr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fang Q, Gong N. Motor assessment of developing common marmosets. Neuroscience Bulletin. 2014;30(3):387–393. doi: 10.1007/s12264-013-1395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes MFF, Sousa MBC. Family composition and the characteristics of parental care during the nursing phase of captive common marmosets (Callithrix jacchus) Primates. 1996;37(2):175–183. doi: 10.1007/bf02381405. [DOI] [Google Scholar]
- Yamamoto M. Marmosets and tamarins: systematics, behaviour and ecology. Oxford; Oxford University Press; 1993. From dependence to sexual maturity: the behavioural ontogeny of Callitrichidae; pp. 235–254. [Google Scholar]


