Abstract
Development of a subunit vaccine targeting liver-stage Plasmodium parasites requires the identification of antigens capable of inducing protective T cell responses. However, traditional methods of antigen identification are incapable of evaluating T cell responses against large numbers of proteins expressed by these parasites. This bottleneck has limited development of subunit vaccines against Plasmodium and other complex intracellular pathogens. To address this bottleneck, we are developing a synthetic minigene technology for multi-antigen DNA vaccines. In an initial test of this approach, pools of long (150 bp) antigen-encoding oligonucleotides were synthesized and recombined into vectors by ligation-independent cloning to produce two DNA minigene library vaccines. Each vaccine encoded peptides derived from 36 (vaccine 1) and 53 (vaccine 2) secreted or transmembrane pre-erythrocytic P. yoelii proteins. BALB/cj mice were vaccinated three times with a single vaccine by biolistic particle delivery (gene gun) and screened for interferon-γ-producing T cell responses by ELISPOT. Library vaccination induced responses against four novel antigens. Naïve mice exposed to radiation-attenuated sporozoites mounted a response against only one of the four novel targets (PyMDH, malate dehydrogenase). The response to PyMDH could not be recalled by additional homologous sporozoite immunizations but could be partially recalled by heterologous cross-species sporozoite exposure. Vaccination against the dominant PyMDH epitope by DNA priming and recombinant Listeria boosting did not protect against sporozoite challenge. Improvements in library design and delivery, combined with methods promoting an increase in screening sensitivity, may enable complex minigene screening to serve as a high-throughput system for discovery of novel T cell antigens.
Introduction
Plasmodium parasites cause malaria, a mosquito-borne disease responsible for hundreds of thousands of deaths and hundreds of millions of clinical cases annually [1]. After transmission from feeding Anopheline mosquitoes, injected sporozoite-stage parasites travel through the skin and eventually make their way to the liver where they initiate an asymptomatic hepatocyte infection that culminates days later in release of erythrocyte-stage merozoites. The merozoites initiate a cyclical infection of erythrocytes that leads to all the clinical manifestations of malaria, including fevers and chills and sometimes progressing to severe anemia, coma and death. The single greatest advance in the fight against malaria would be the production of a safe and effective vaccine that induces complete protection against infection with Plasmodium sporozoites. To achieve this goal, a major focus has been placed on vaccines targeting the pre-erythrocytic stages of development (the transmitted sporozoite stage and the subsequent liver stage) [2]. Experimental vaccination of humans and mice with attenuated sporozoites protects against challenge with wild-type sporozoites [3–8]. Antibodies can block hepatocyte invasion [9], while CD8+ cytotoxic T cells recognize parasite-infected hepatocytes [10–12] and can provide sterile protection in several mouse models [13]. Furthermore, CD8+ T cells play a role in protection in primates [14] and likely in humans as well [15] and are induced in humans experimentally immunized with attenuated sporozoites [16, 17]. Parasite-specific CD8+ T cells may kill infected cells by one or more proposed mechanisms (reviewed in [18]). Thus, inclusion of CD8+ T cell target antigens is likely to be critical for any sterile protective malaria vaccine.
There are two basic approaches to pre-erythrocytic malaria vaccine development–manufacture of an attenuated ‘whole organism’ vaccine or identification and manufacture of a single subunit antigen or set of antigens that can provide complete protection [19, 20]. However, the extremely large number of genes expressed by Plasmodium has thus far prevented the efficient identification of broadly protective antigens suitable either for inclusion within a subunit vaccine or for targeting by transgenic parasite vaccines with improved protective efficacy.
For subunit vaccines, there are two sequential phases of development: antigen discovery and formulation testing. Antigen discovery typically involves screening of immune cells from pathogen-exposed subjects for responses against small numbers of laboriously cloned, pathogen-derived gene products or predicted peptide epitopes. Antigen discovery is then followed by an equally laborious process of production and evaluation of experimental vaccines targeting the newly discovered antigenic proteins–many vaccines fail here because the antigens discovered simply do not induce protective responses. As the number of proteins increases, each step requires considerable investment of time and money, with no guarantee that the antigens discovered will ultimately confer protection when formulated as a vaccine.
Plasmodium species each encode ~5,300 proteins but only a few pre-erythrocytic Plasmodium proteins (i.e., CSP, TRAP, LSA1, Exp1/Hep17, CelTOS, L3, Pf16, STARP) have been studied as T cell antigens [21–31]. Amongst those tested in humans (CSP, LSA-1, Exp-1, TRAP, CelTOS), most have not reliably induced complete protection (reviewed in [32]), although CSP protected 85% of subjects in one study [33]. Because it is nearly impossible to systematically study all potential T cell antigens using conventional methods, a number of higher throughput approaches have been applied to malaria T cell antigen discovery. Synthetic Plasmodium peptides [34–37] or antigen presenting cells (APCs) transfected with Plasmodium protein-expressing plasmids [38] have all been used to screen for T cell interferon-γ (IFNγ) responses in vitro, but such approaches are limited by the cost of large peptide libraries and the complexity of cloning A/T-rich Plasmodial genes, respectively. Recently, a peptide screening approach identified multiple new antigens targeted by T cell responses from RAS-immunized humans [37]—protection afforded by these antigens is not yet known. We previously utilized APCs transfected with minigene libraries encoding long Plasmodium peptides in an effort to capture a larger portion of the proteome using synthetic biology techniques [26].
New methods that accelerate discovery of vaccine subunits for pathogens with thousands of genes would be a major step forward in the fight against Plasmodium and other complex intracellular pathogens. DNA vaccination and screening is ideally suited for such higher-complexity evaluation of vaccine candidates. In malaria, DNA vaccination against P. yoelii CSP achieves CD8+ T cell-dependent protection in the rodent model [39]. In addition, DNA vaccination of humans against P. falciparum CSP can induce CD8+ T cell responses [40]. Finally, small numbers of Plasmodium protein-coding genes have been shown to be immunogenic when used in a four-gene DNA vaccine in mice or non-human primates [41, 42]. A five-gene DNA vaccine administered to humans was also able to induce CD8+ T cell responses that could be further boosted by exposure to sporozoites [43]. Even though there was no evidence of protection in this human study, the study showed that a DNA prime / Plasmodium boost protocol can increase responses. Studies from outside the malaria field further indicate that complex DNA vaccines delivered by biolistic (gene gun) delivery can be physically segregated onto distinct gold beads and that this serves to maintain diversity of both antibody and T cell responses [44–46].
We previously used a high-throughput synthetic minigene technology for in vitro detection of murine CD8+ T cells primed with live Plasmodium parasites in vivo [26]. This work led us to hypothesize that these same minigenes could be used to both initiate specific immune responses in vivo as a DNA-based vaccine, and to subsequently screen DNA vaccine-induced T cell responses to identify antigenic targets. This approach potentially combines the antigenic complexity achieved by whole organism vaccines with the feasibility of multi-subunit vaccination. Using a microarray-based oligonucleotide synthesis technology, we rapidly produced two complex minigene vaccines, each encoding over >1,000 peptides derived from 36 (vaccine 1) and 53 (vaccine 2) liver-stage P. yoelii proteins. Targets were a set of pre-erythrocytic proteins containing signal peptides and/or transmembrane domains. Putatively secreted or transmembrane proteins were selected based on their potential to cross both the parasite membrane and the parasitophorous vacuolar membrane to enter the hepatocyte MHC class I pathway. The assumption that such parasite proteins may be exported into the hepatocyte is based on the observation that exported erythrocyte-stage PEXEL/HT domain-containing proteins also contain a signal peptide or a transmembrane domain [47, 48].
Following vaccine production, mice were repeatedly gene gun vaccinated with the minigene libraries followed, in some mice, by sporozoite exposures. T cell IFNγ responses were evaluated and several novel responses were identified including a strong response to P. yoelii malate dehydrogenase (PyMDH), which was further characterized and found to bear similarities to another recently described Plasmodium liver-stage T cell response.
This preliminary study indicates that T cell responses against genuine Plasmodium antigens can be induced and detected using highly complex DNA minigene vaccines. As described in the discussion, additional improvements designed to increase the breadth and magnitude of responses, coupled with improved screening sensitivity may allow minigene vaccination/screening technology to be used for high-throughput identification of protective T cell antigens.
Materials and Methods
Vaccine design
Eighty-nine liver-stage P. yoelii 17XNL proteins predicted to contain a signal peptide and/or have transmembrane domains were selected using filters built into PlasmoDB. Coding sequences were downloaded and broken into sequential 33 codon segments overlapping by 14 codons. In regions of variability, all permutations of closely-spaced variations were included as alternate minigenes. A pool-specific primer unique to groups of 10 minigenes followed by a start codon were appended onto the 5’ end of each segment, while a common primer sequence was appended onto the 3’ end. The reverse compliment of each 150 bp minigene template was ordered as a single oligo-pool synthesis (CustomArrays, Inc., Bothell, WA). Gene identifiers and product descriptions from PlasmoDB.org as well as pool-specific primers, minigene sequences and encoded peptides are listed in S1 Table.
Vaccine assembly
Each pool of 10 minigenes was amplified using individual pool-specific primers plus the common primer. Pool-specific primers included a T7 promoter sequence on the 5’ end. Equivalent amounts of each pool were then combined and subjected to dial-out error correction as described [49]. Briefly, random tags were added by amplifying the combined library using stepout primers hybridizing to the T7 and common primer sequences. The tagged library was sequenced using base paired-end reads on an Illumina miSeq by a commercial vendor. Reads were aligned with the original library design and dialout primer pairs flanking accurate minigenes were selected. Dialout primer pairs for each minigene were ordered from IDT, Inc. (Coralville, IA). Primers could not be adequately designed for some minigenes and such minigenes were omitted from the final library post-dial out. Error-corrected minigenes were amplified using the selected dial-out primers. Each minigene was individually recombined as an amino-terminal fusion with the mouse LC3 coding sequence in a modified pNGVL3 vector using SLiCE ligation-independent cloning [50]. Recombined plasmids were transformed into DH10G E. coli hosts by electroporation using an AMAXA 96-well shuttle device (Lonza, Walkersville, MD) and cultured individually in 1.2 mL LB broth in deep-well 96-well plates. Groups of 10 cultures were pooled, centrifuged and frozen until purification. Bacterial pellets were processed using a 96-Plus Endotoxin-free kit (Qiagen, Valencia, CA) according to manufacturer instructions. Typical yields were between 100 and 300 ng/μL for each pool.
Loading of gene gun cartridges
Nine μL of each plasmid pool, each containing 10 plasmids, were combined with 1 μL of 200 ng LT adjuvant plasmid [51] in 96-well V-bottom plates. Each well was diluted with 10 μL 50 mM spermidine and 1 mg of 1 μm gold beads (Inbios Gold, Hurstbridge, Australia). Plates were agitated on a horizontal shaker while 10 μL 10% CaCl2 was added to each well. DNA-coated gold particles were centrifuged and washed thrice with 100% ethanol. Gold particles were suspended in 10 μL 100% ethanol with 50 μg/mL polyvinylpyrrolidone. One-quarter of all pools in a vaccine (the equivalent of twelve 40-kD proteins) were combined and loaded into Tefzel cartridges using a custom-made tube turner. Gene gun cartridge tubes were dried, sliced and stored desiccated at 4°C until use.
Mice
All animal studies were approved by the University of Washington Institutional Animal Care and Use Committee (protocol 4317–01). BALB/cj mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in approved facilities at the University of Washington. Thy1.1+ BALB/c mice were bred at the University of Washington from a pair originally obtained from Jackson Laboratories. Humane sacrifice was performed by flow-metered carbon dioxide overdose.
Gene gun vaccinations
Female Balb/cj (6–8 wk old) mice were shaved on the abdomen and administered the DNA vaccine corresponding to 0.5–1.0 μg total DNA using a PowderJect XR1 research device [52]. On Days 0, 21 and 49, each mouse received four separate cartridges, one cartridge corresponding to each quarter of the vaccine. At later time points, vaccinated mice were humanely sacrificed and splenocytes harvested and pooled for screening as described later.
Generation of screening templates
One μL of each vaccine pool was diluted 1:100 and further amplified using Rolling Circle amplification using a TempliPhi kit (GE BioSciences, Pittsburgh, PA). Rolling circle-amplified DNA from each minigene pool was used as a template to amplify short linear expression cassettes from the CMV promoter through the human beta-globin 3’UTR/polyA sequence. PCR reactions utilized 20 ng template combined with a CMV promoter-specific primer and a 3’ human beta-globin UTR antisense primer and Phusion polymerase. Cycling conditions were 35 cycles of 98°C (15 sec), 55°C (15 sec) and 72°C (2 min) each. Products were purified using 96-well filter plates (Qiagen, Valencia, CA) and resuspended in 20 μL 10 mM Tris (pH 7.8).
Minigene library ELISPOT screening of sensitized splenocytes
Ready-Set-Go mouse IFNγ ELISPOT kits (eBioscience, San Diego, CA) were used as previously described [26]. Briefly, 2 μL of PCR-amplified expression cassettes from each minigene pool in each vaccine were transfected into 1x106 freshly-dividing P815 cells using the RAW264.7 program of an AMAXA 96-well shuttle. Splenocytes (1x106/well) were added as responders, and plates were incubated overnight. ConA was included as positive control and mock-transfected P815 cells were used as negative controls. ELISPOT plates were developed according to the manufacturer’s instructions and scanned and analyzed using an ImmunoSPOT counter (Cellular Technology Limited, Shaker Heights, OH). Transfection efficiency was also controlled by checking GFP expression and viability by flow cytometry of separate wells of P815 cells transfected on each plate of wells.
Sporozoite immunizations
Where indicated, BALB/cj mice were singly or doubly immunized with 1x104 wild-type P. yoelii sporozoites and were then administered azithromycin i.p. (0.8 mg/d) on Days 1–3 post-immunization. In other experiments, mice were immunized once or twice with combinations of PyRAS and/or genetically-attenuated Pyfabb/f- sporozoites [53] as described [26]. Sporozoites were obtained from the Center for Mosquito Production and Malaria Infection Research (CeMPMIR, Center for Infectious Disease Research, Seattle, WA).
MHC binding assay
RMA/S cells expressing H2-Kd were used to test MHC binding as described [26, 54].
In vivo cellular cytotoxicity assay
The in vivo killing assay was performed to measure cellular cytotoxicity as previously reported [26] although three populations of target cells were differentially labeled here with Cell Proliferation Dye eFluor® 670 (eBioscience) at 5 μM (PyMDH), 0.75 μM (PyCSP) and 0.1 μM (mock).
Immunization-challenge experiments
To generate DNA and Listeria-based vaccines for single antigen immunization, minigene-encoded antigens were isolated and further cloned into the delivery vectors. Briefly, minigene expression constructs encoding the dominant PyCSP epitope SYVPSAEQI and the dominant PyMDH epitope SYQKSINNI were cloned separately into the pNGVL3.LC3 vector for use as DNA vaccines. Recombinant PyCSP-expressing actA-/inlB- L. monocytogenes was a kind gift from Aduro Biotech. PyMDH-expressing actA- L. monocytogenes was constructed by cloning a minigene encoding the PyMDH epitope into the pPL2-N4 vector. The resulting pPL2-N4.MDH plasmid was transformed into SM10 hosts and conjugated into actA- Lm10403S. Using these materials, a gene gun DNA prime/recombinant Listeria boost protocol was used to vaccinate mice against single antigens prior to sporozoite challenge. For each arm of the experiment, mice were primed against either PyCSP or PyMDH using two gene gun cartridges on Days 0 and 2. Three weeks later, animals were boosted i.v. with 5x106 CFU recombinant Lm expressing the cognate antigen and treated with 2 mg/mL ampicillin in the drinking water for 3 days thereafter. Animals were challenged three weeks later with 1x104 wild-type P. yoelii sporozoites i.v. Forty-four hr later, animals were humanely sacrificed and livers perfused with PBS. Livers were harvested and emulsified in a bead beater with NucliSens lysis buffer (bioMérieux, Durham, NC) to preserve RNA. Total nucleic acid was extracted using a NucliSens EasyMag (bioMérieux) and Plasmodium 18S rRNA and murine GAPDH were measured by RT-PCR as described [26]. Liver burden is reported as log10 changes between mice for Plasmodium 18S rRNA copy number normalized to mouse GAPDH. At the time of humane sacrifice, spleens were also harvested for ELISPOT analysis as a measure of T cell frequency at the time of challenge (no T cell expansion during 0–44 hr post-challenge).
Results
Synthetic biology methods enable rapid production of multi-protein synthetic minigene libraries suitable for T cell vaccination and subsequent screening
Two minigene libraries encoding peptides from 36 (Vaccine 1) or 53 (Vaccine 2) P. yoelii proteins each were synthesized (S1 Table). Error-correction including Dial-out tagging, sequencing, analysis and re-amplification was completed in 4 weeks. The libraries were designed to contain the complete peptide compliment of all included proteins, but after error correction by dial-out PCR, a fraction of minigenes could not be recovered as error-free sequences. Vaccine 1 contained 91.9% of the intended proteome (95%CI: 87.4–96.4%) and Vaccine 2 contained 91.1% (95%CI: 87.0–95.3%) as indicated in S1 Table. Plasmid insertion and plasmid culture/isolation were accomplished in three weeks. Spot-checking individual cultures by sequencing showed that >90% of clones encoded the intended sequence. The proteins included in the libraries are listed in S1 Table. Minigenes were adjuvanted with the LT-encoding plasmid [51] and loaded onto individual aliquots of gold particles for biolistic delivery.
Library vaccination and minigene screening enables identification of library vaccine-induced IFNγ-producing T cell responses
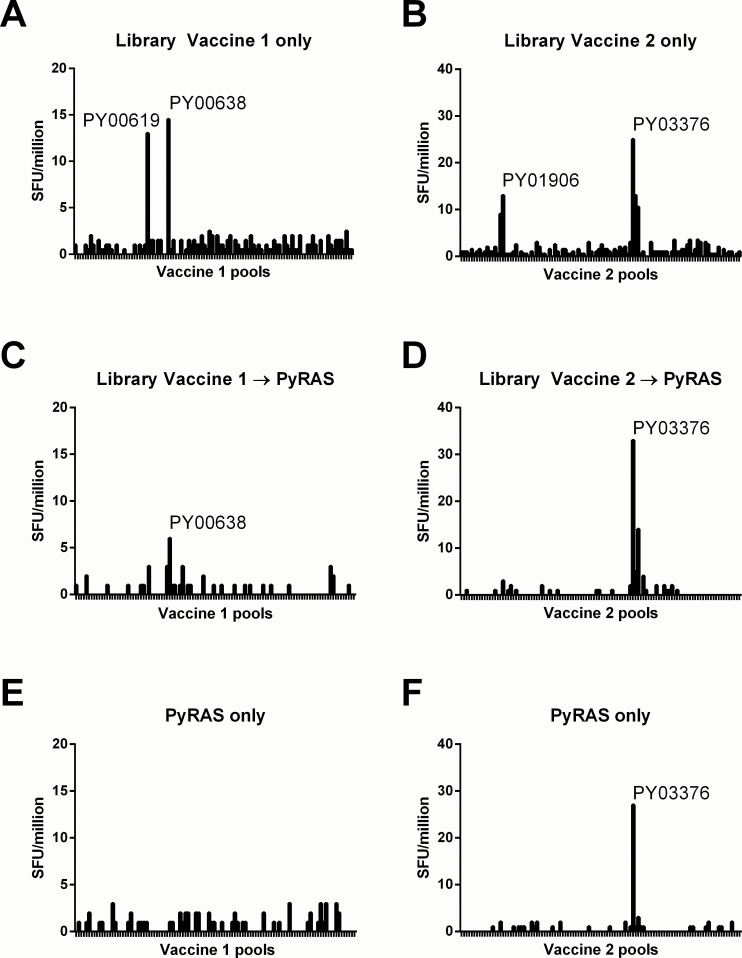
BALB/cj mice (n = 5/group) were vaccinated with Vaccine 1 or Vaccine 2 on Days 0, 21 and 49 using a PowderJect research device. Aside from mild erythema at the gene gun vaccination site for 1–2 days post-vaccination, the mice tolerated the DNA vaccination without any obvious signs of distress. IFNγ responses were evaluated usingpooled splenocytes harvested six days after the final gene gun vaccination. To evaluate the >100 minigene pools per vaccine, duplicate wells of target cells (P815) were each transfected with PCR-amplified expression cassettes corresponding to each minigene pool in a vaccine (10 minigenes/pool). The transfected target cells (1x106/well) were combined with 1x106 splenocytes from library-vaccinated mice in overnight IFNγ ELISPOT assays as previously reported [26]. Compared to previous minigene-transfected ELISPOT screens of sporozoite-immunized mice [26], background production of IFNγ in these experiments on DNA minigene library-vaccinated mice was extremely low (<3 SFU/million splenocytes). Such low background signal is critical for detection of responses in the setting of multi-protein vaccination. Two antigens in each vaccine induced responses significantly above baseline (Fig 1A and 1B). Each of the antigenic pools contained minigenes from a single protein such that the initial screen identified responses to the products of genes PY00619 and PY00638 in Vaccine 1 and PY01906 and PY03376 in Vaccine 2.
Fig 1.
ELISPOT IFNγ responses in mice immunized with minigene library vaccines alone (A-B), library vaccines followed by sporozoites (C-D) or sporozoites alone (E-F) identify novel responses. ELISPOT results for Vaccine 1 (A,C,E) and Vaccine 2 (B,D,F), as indicated. Responses to Library vaccination alone (A-B), Library vaccine plus Sporozoites (C-D) or Sporozoites only (E-F). Minigene pools are indicated along the x-axis (Vaccine 1 = 103 pools; Vaccine 2 = 105 pools) with the y-axis indicating SFU/million splenocytes.
In Vaccine 2, the known epitope from the circumsporozoite protein (CSP, pool 55) did not yield detectable responses. There are several possible explanations for this result. First, a particular minigene may not express in an optimal manner for several reasons including failure to clone, presence of a PCR-induced mutation, inclusion of a suboptimal 5’ UTR which is dictated by the pool-specific primer, efficient shunting into the autophagosome via the LC3 tag [55], and/or poor representation relative to other minigenes in that pool. Another possible explanation is that ELISPOT screening using minigene-transfected P815 cells as APCs is less sensitive than peptide ELISPOTs since the minigene-transfected APCs express lower quantities of antigen, typically yielding spots of smaller diameter and lower intensity. To evaluate these possibilities, we subcloned the CSP epitope-containing minigene from Vaccine 2 using minigene specific primers and verified its presence and primary sequence in the library (data not shown). In a mixed minigene vaccination experiment using immunization with a single pool consisting of 20 linear minigenes with and without the library-derived LC3-tagged CSP minigene, we observed relatively low frequency responses (20–30 spots/million splenocytes) using CSP peptide as a recall antigen. When the CSP epitope was tested as a single minigene vaccine with a ubiquitin tag, this minigene could elicit strong CSP responses in IFNγ ELISPOTs (500–1000 spots/million splenocytes). Thus, it appears that the minigene encoding the major CSP epitope as a fusion to LC3 was functional but was unable to induce a response when formulated to at extremely high complexities. In addition, since we were unable to detect CSP responses in these experiments, we do not regard the lack of response against any library-encoded peptide as evidence that the target is not immunogenic or protective. Optimized delivery and increased dose are likely to induce responses against a larger percentage of the proteins targeted in these vaccines.
A subset of library vaccine-induced responses are recalled by immunization with irradiated P. yoelii sporozoites but only one such response is primed by sporozoites alone
A subset of the minigene library vaccinated animals were administered 2x104 irradiated P. yoelii sporozoites (PyRAS) 16 days after the final gene gun vaccination and splenocytes were harvested 7 days after RAS exposure. Minigene-transfected P815 ELISPOT screening was again conducted to identify vaccine-primed, RAS-recalled responses. Recall responses were observed against pools encoding PY00619, PY00638 and PY03376 whereas responses against the PY01906 pool were undetectable (Fig 1C and 1D). To determine if RAS alone could induce these responses, completely naïve BALB/cj mice were administered 2x104 PyRAS and evaluated by minigene-transfected P815 ELISPOT one week later. In mice exposed only to PyRAS, the only detectable response was to the pool for PY03376, which encodes P. yoelii malate dehydrogenase (PyMDH) (Fig 1E and 1F).
Identification of a Class I epitope in P. yoelii malate dehydrogenase
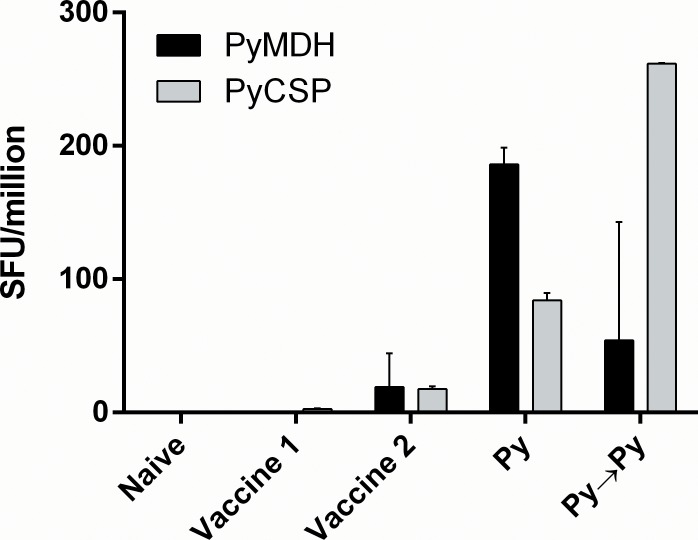
Three adjacent minigene pools from PyMDH were targeted in the primary screens. These pools included a total of 23 overlapping minigenes that were required to encode all permutations of four closely-spaced, non-synonymous sequence variants reported in the genome of P. yoelii strain 17XNL. It is unknown whether these reported variations are genuine or are the result of sequencing errors. In an attempt to identify a targeted epitope, the translated sequence of each variant minigene was evaluated for predicted Class I MHC binding using the NetMHC Pred 3.4 tool [56] (available at http://www.cbs.dtu.dk/services/NetMHC-3.4/). Prediction was limited to MHC Class I H2d molecules since mastocytoma P815 targets used in the screens do not express MHC class II. The peptide SYQKSINNI yielded strong predicted binding characteristics for H2-Kd and corresponded to a homologous, invariant sequence reported for P. berghei (PBANKA_1117700) MDH. This peptide was synthesized and tested by ELISPOT using frozen splenocytes from animals vaccinated with Vaccine 2 and from animals exposed to one or two doses of 1x104 Py wild-type sporozoites with concurrent prophylactic azithromycin drug treatment to prevent onset of erythrocyte-stage infection. Splenocytes from all such mice responded to the PyMDH peptide, although the response in animals exposed to two doses of Py sporozoites was markedly reduced compared to a single dose of sporozoites (Fig 2). Sequencing of a genomic PCR product amplified from the P. yoelii 17XNL isolate used for PyRAS production confirmed that SYQKSINNI was the encoded peptide. Despite the aforementioned ambiguity in the Plasmodb database, sequencing revealed no evidence of non-synonymous variation within the SYQKINNI-coding region.
Fig 2. Identification of SYQKSINNI as a dominant natural epitope in P. yoelii malate dehydrogenase.
Cryopreserved splenocytes were obtained from library-vaccinated mice (Vaccine 1 or Vaccine 2) or from mice singly (Py) or doubly (Py→Py) with doses of 1x104 wild-type Py sporozoites administered with azithromycin (0.8 mg/d) on Days 1–3 post-immunization); for Py→Py, immunizations were spaced 3 wks apart. In all situations, splenocytes were harvested 6 d after the final immunization. Cells were thawed and tested by IFNγ ELISPOT against purified peptides corresponding to PyMDH (SYQKSINNI), PyCSP (SYVPSAEQI) and PyL3. Bars indicate mean and 95% confidence interval.
The same approach was used to bioinformatically predict H2d and H2b binders for PY00619, PY00638, and PY01906. However to date we have not identified any specific peptide epitopes from these proteins by ELISPOT screening (data not shown). Nonetheless, the PY00619, PY00638 and PY01906 minigene pools were indeed antigenic as they were each able to induce >100 SFU/million when administered to mice as a single pool gene gun immunization (data not shown). It is possible that some of the minigene-induced responses are directed at fusion peptides formed by the junction of the pathogen-derived peptide and the C-terminal LC3 fusion partner. Such antigens could not be present in the parasite itself and would therefore not be boosted upon challenge, however fusion peptides of this nature have not been formally tested. These data highlight the complexity of epitope prediction and suggest that the reactive peptide in the minigene screening stage was a different peptide than that predicted bioinformatically. For the purposes of this study, the peptides from PY00619, PY00638 and PY01906 were not pursued further.
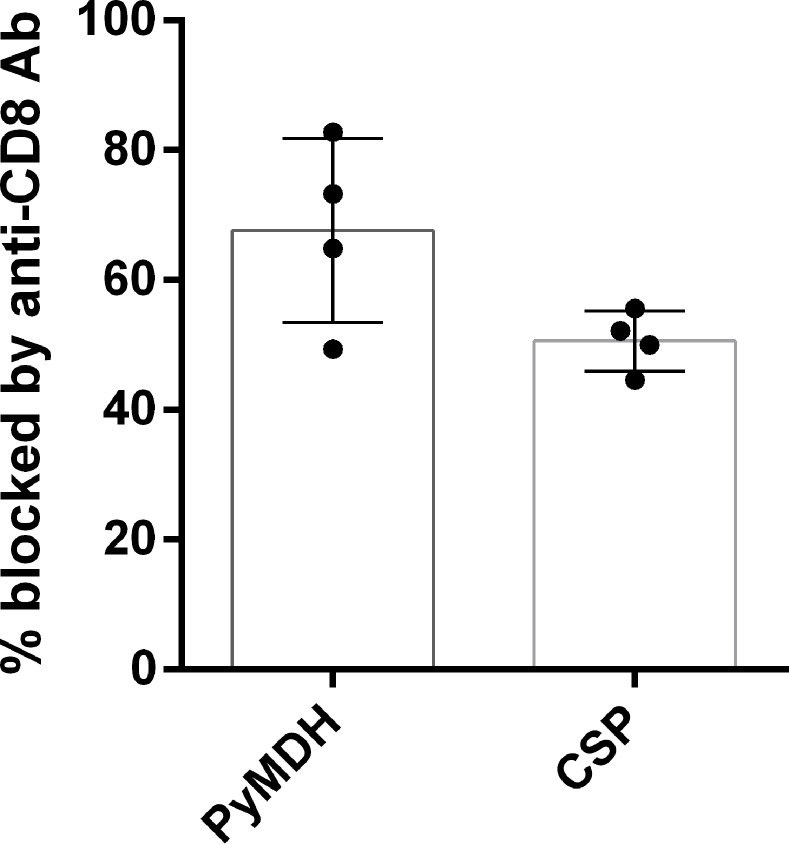
When ELISPOT assays were conducted in the presence or absence of anti-CD8a antibodies, PyCSP- and PyMDH-specific T cell responses were comparably reduced, indicating IFNγ production in both antigen-specific responses was CD8+ T cell-dependent (Fig 3).
Fig 3. The PyMDH-specific response is CD8 T cell-dependent.
ELISPOT wells were treated with anti-CD8 blocking antibody (clone 2.43, final 10 μg/mL) and compared to isotype-control treated wells. The y-axis shows the percentage reduction in SFU/million splenocytes for antibody-treated versus untreated wells. Splenocytes were from BALB/cj mice immunized with 5x104 wild-type P. yoelii sporozoites administered with azithromycin prophylaxis on Days 0–2 post-challenge. Bars indicate mean and 95% confidence interval.
The MDH epitope SYQKINNI binds to murine H2-Kd
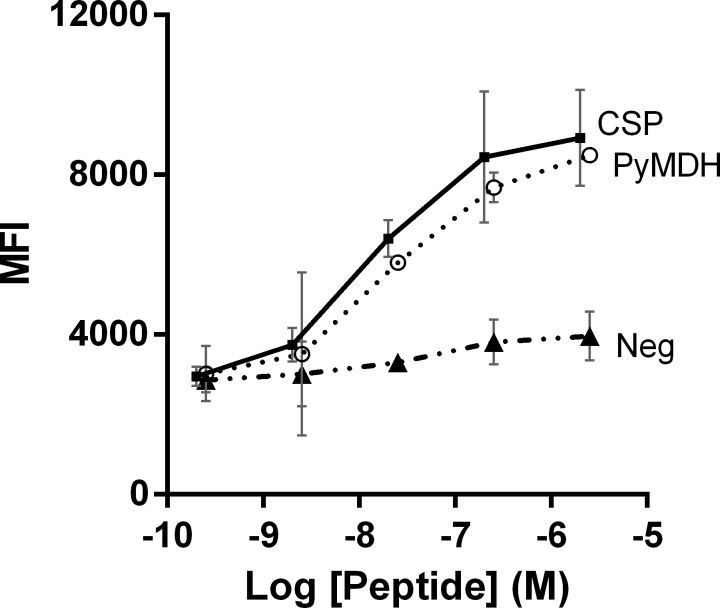
The affinity of SYQKSINNI for H2-Kd was determined using an RMA/S lymphoma cell line expressing H2-Kd as previously described [26, 54]. H2-Kd was stabilized by a known binder (PyCSP-derived SYVPSAEQI) and with similar affinity by PyMDH-derived SYQKSINNI. The apparent Kd for the peptides was 11.6 nM (PyMDH) compared to 9.6 nM (PyCSP) (Fig 4).
Fig 4. The PyMDH epitope binds to murine H2-Kd.
RMA/S cells expressing H2-Kd were incubated with peptides for PyCSP (squares), PyMDH (circles) or non-specific peptide. PyCSP and PyMDH peptides stabilized H2-Kd, indicative of specific MHC binding. Error bars indicate the 95% confidence interval.
PyMDH-specific CD8+ T cells are functionally cytotoxic
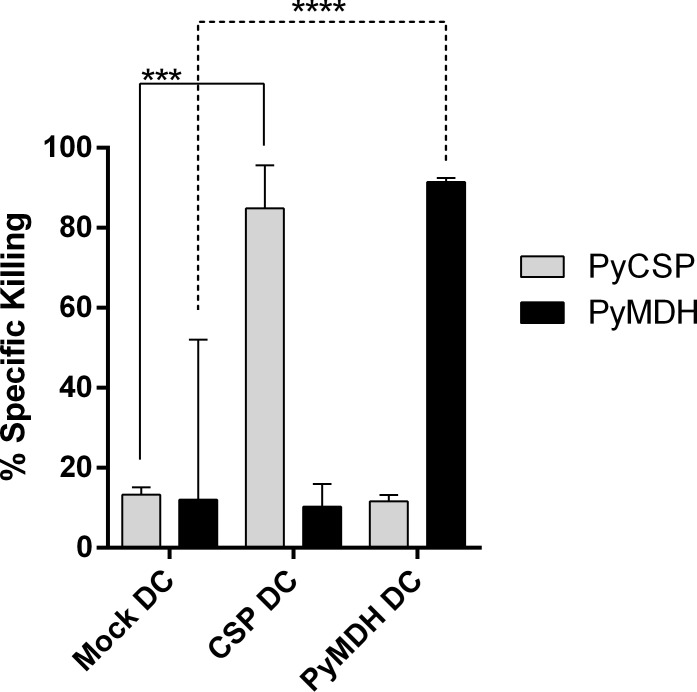
To evaluate the functional capacity of PyMDH-specific T cells, BALB/cj (Thy1.2+) mice were primed i.v. with 2x106 GM-CSF-bone marrow-derived dendritic cells activated and pulsed overnight with lipopolysaccharide (0.1 μg/mL) and either 1 μg/mL PyCSP or PyMDH or no peptide. Six days later, splenocytes from Thy1.1 BALB/c mice were pulsed with PyCSP or PyMDH peptides or with no peptide and were differentially stained, mixed and injected into animals primed against individual peptides. Eighteen hr later, spleens were harvested and peptide-specific killing measured by flow cytometry. Animals immunized against the PyCSP peptide specifically killed 84% of PyCSP-pulsed targets, and animals sensitized to the PyMDH peptide killed 91% of PyMDH-pulsed targets (Fig 5).
Fig 5. PyMDH-specific T cells are capable of antigen-specific cytotoxic killing.
Mice were previously immunized with 1x106 mature DCs pulsed with 1 μg/mL pf the antigenic peptide (PyCSP DC or PyMDH DC). Six days later mice were administered equal numbers of PyCSP peptide-coated CFSEHI and PyMDH-peptide coated CFSELO target splenocytes; antigen-specific killing was monitored 18 hr later by flow cytometry. Like cytotoxic PyCSP-specific T cells, PyMDH-specific T cells demonstrate a high rate of antigen-specific killing. Bars indicate mean and 95% confidence interval; ***p<0.001; ****p<0.0001 (Student’s t test).
High-frequency PyMDH-specific CD8+ T cells do not protect mice from P. yoelii sporozoite challenge
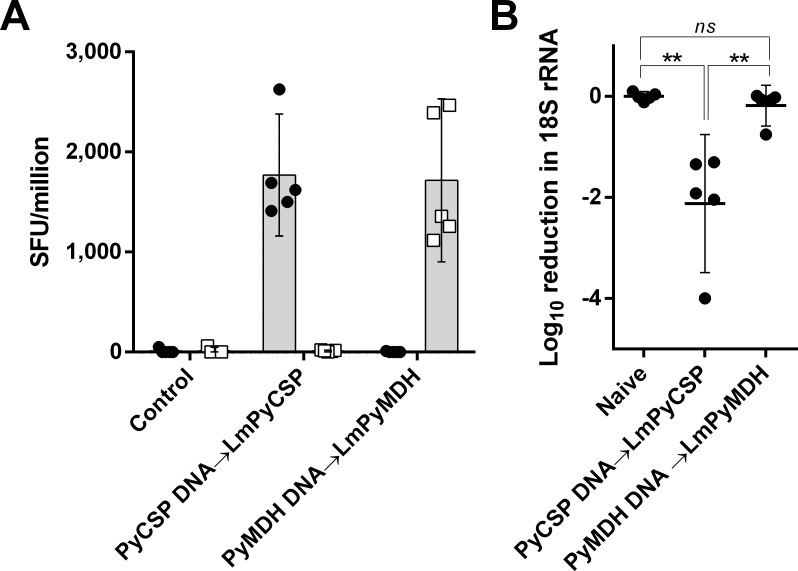
Five animals per group were vaccinated with either PyCSP or PyMDH using a DNA prime/Listeria boost protocol. At a memory time point, all vaccinated mice plus five naïve infectivity control mice were challenged with 1x104 wild-type P. yoelii sporozoites. ELISPOT performed at the time of liver harvest demonstrated antigen-specific, high frequency IFNγ-producing responses to PyCSP and PyMDH in appropriately immunized mice (Fig 6A). Compared to infectivity control mice, 5/5 PyCSP-vaccinated mice showed significant vaccine-induced protection, with one animal showing undetectable Py 18S RNA in the liver indicating sterile protection (Fig 6B). In contrast, the Py 18S rRNA concentration in animals vaccinated with PyMDH was indistinguishable from that of the infectivity controls (Fig 6B), indicating no protection despite high frequency PyMDH-specific T cell responses.
Fig 6. High frequency IFNγ-producing PyMDH-specific T cells do not protect against sporozoite challenge.
BALB/cj mice were DNA gene gun vaccinated against Class I epitopes from PyCSP or PyMDH and boosted 21 d later with 5x106 cfu attenuated Listeria monocytogenes expressing the same epitope. Mice were challenged with 1x104 PyWT sporozoites i.v. and sacrificed for spleen and liver harvest 44 hr post-challenge. (A). ELISPOT performed on splenocytes at the 44 hr time point reflects the frequency of antigen-specific T cells at the time of challenge. Circles, PyCSP peptide; squares, PyMDH peptide; bars indicate mean and 95% confidence interval. (B). Liver stage infection was monitored by Plasmodium 18S rRNA RT-PCR. Bars indicate mean and 95% CI for Plasmodium 18S rRNA normalized to mouse GAPDH mRNA content; **p < 0.01 (Student’s t-test).
Heterologous cross-species immunization with a late-arresting sporozoite re-expands the PyMDH-specific T cell population more than homologous immunizations
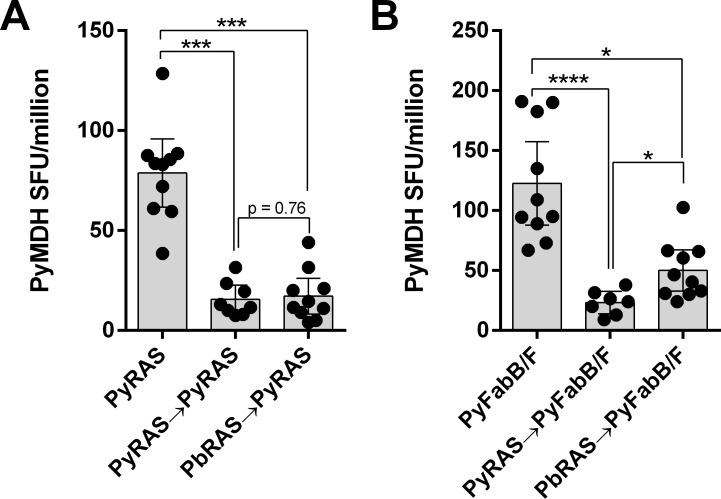
Previous studies showed that T cell responses to pre-erythrocytic antigens could either be expanded by repeated homologous (same-species) sporozoite immunization (e.g., CSP-specific CD8 T cells) or failed to re-expand (e.g., L3-specific CD8 T cells) [26]. Recent unpublished work has shown that immunization with two different murine-infecting Plasmodium species (P. yoelii 17XNL and P. berghei ANKA) could recall the L3-specific response to the epitope shared between species if the secondary immunization was with a late-arresting, genetically-attenuated sporozoite (S. Murphy, pers. comm.–manuscript in review). Since the PyMDH epitope was also conserved between P. yoelii and P. berghei, mice underwent homologous or heterologous immunizations with combinations of PyRAS, PbRAS and Pyfabb/f- parasites. As observed for L3, when RAS parasites were used, neither homologous (PyRAS→PyRAS) nor heterologous (PbRAS→PyRAS) regimens re-expanded the PyMDH-specific cells (Fig 7A). However, when the secondary immunization was switched to the less-attenuated Pyfabb/f- strain, the heterologous regimen (PbRAS→Pyfabb/f-) expanded PyMDH-specific responses more so than the homologous regimen (PyRAS→Pyfabb/f-), although not to the level observed with a single Pyfabb/f- immunization (Fig 7B). This finding suggests that PyMDH-specific responses and those against other proteins with similar protein expression kinetics may be boosted by heterologous more so than homologous species immunizations.
Fig 7. PyMDH-specific T cells are not recalled with repeated homologous sporozoite immunization but can be recalled by heterologous cross-species immunization.
(A) BALB/cj mice were immunized with nothing or with 1x104 PyRAS or PbRAS and three wks later all groups received 1.5x105 purified PyRAS. IFNγ ELISPOT was performed on splenocytes obtained 6 days after the final vaccination. (B) BALB/cj mice were immunized with nothing or with 1x104 PyRAS or PbRAS and three wks later all groups received 1.5x105 purified, genetically-attenuated Pyfabb/f- sporozoites. IFNγ ELISPOT was performed as in (A). Bars show mean +/- 95% confidence interval; * p<0.05, ***p<0.001; ****p<0.0001 (Student’s t-test).
Discussion
Development of antibody-based subunit vaccines has been aided by the inherent stability, sensitivity and soluble nature of antibodies in serum. In contrast, the development of T cell-based vaccines requires evaluation of live cellular MHC-restricted interactions between T cells and MHC-matched APCs displaying the cognate target antigen. For intracellular pathogens expressing thousands of potential targets such as malaria, this requirement has inhibited discovery of protective vaccine subunits. The synthetic minigene vaccine technology described here is designed to enable the rapid evaluation of T-cell responses against large numbers of pathogen-derived proteins, in order to identify both dominant and sub-dominant antigens.
In these proof-of-principle studies, minigene vaccination resulted in the identification of four novel T cell antigens from among 89 pre-erythrocytic stage P. yoelii proteins. These included one dominant antigen encoded by PY03376 (PyMDH), and three antigens encoded by PY00619, PY0638 and PY01906 minigene pools. We identified the peptide SYQKSINNI as a major PyMDH epitope. Robust responses against PyMDH were detected in animals receiving the vaccine alone and in response to sporozoite immunization in the absence of DNA vaccination. PyMDH-specific responses in DNA-vaccinated animals could be recalled by sporozoites. However, despite recent reports of a protective T cell response against Toxoplasma gondii malate dehydrogenase [57], no protection against PyMDH was observed following DNA prime/Listeria boost vaccination despite induction of extremely high frequency, cytotoxic PyMDH-specific CD8+ T cell responses. The PyMDH response was also evaluated in mice exposed to one or two rounds of sporozoite immunizations. The PyMDH-specific response was not potently recalled by homologous sporozoite immunizations, although the epitope is shared with P. berghei ANKA MDH and recall could be partially augmented by immunization with two different species of murine-infecting Plasmodium sporozoites. Thus, PyMDH appears to be an antigen in the same category as that of the recently described pre-erythrocytic P. yoelii ribosomal protein L3, which also displayed robust pre-erythrocytic immunogenicity but no protective efficacy and poor recall by repeated homologous sporozoite exposures [26].
The other antigenic minigene pools have not been characterized to the same extent as PyMDH. Animals receiving PyRAS 16 days after the final DNA library vaccine exhibited low level responses against PY00619 and PY00638 minigene pools but not to PY01906, and additional studies will be required to determine whether these responses represent true parasite recall or simply residual, declining responses from the initial DNA vaccination. Each of these three minigene pools was able to induce IFNγ responses in mice vaccinated with single pool DNA vaccines. The antigenic epitope(s) for PY00619, PY00638 and PY01906 are currently unknown and additional studies are ongoing to evaluate these antigens further.
The fact that the robust responses against PyMDH are not protective suggests that the epitope may not be displayed on the surface of an infected hepatocyte harboring live proliferating parasites, or may be presented by the hepatocyte at a time point that is too late to provide protection. To be displayed on the surface of the hepatocyte, PyMDH would probably need to be exported from the parasite and across the hepatocyte-derived vacuole membrane to the hepatocyte cytoplasm. However unlike erythrocyte-stage parasites [47, 48], there is currently no known consensus motif that directs pre-erythrocytic protein export from the vacuolar parasite to the hepatocyte cytoplasm. Responses to PyMDH-like epitopes may be driven either entirely or in part by cross-presentation of dead or dying parasites. We [26] and others [58, 59] have identified other antigens with similar response profiles. This type of antigen may represent an immune evasion strategy employed by the parasite whereby epitopes derived from proteins that are not protective serve as “decoys”, directing immune system resources toward non-protective targets. There is increasing evidence that this decoy antigen phenomena also occurs in bacterial and viral infections [60, 61]. Such antigens may be under selective pressure to remain invariant as T cell responses against them could promote survival of the parasite. In contrast, T cell epitopes from proteins that are protective are likely to be expressed and presented by hepatocytes during liver-stage infection and therefore may be under selective pressure to alter their primary sequence to avoid immune detection. Separating antigenic protective antigens (the “wheat”) from dispensable antigens such as PyMDH and P. yoelii ribosomal protein L3 (the “chaff”) represents a major hurdle to our understanding of the Plasmodium-specific T cell repertoire and to development of effective subunit vaccines for malaria.
Both multi-gene DNA library vaccines elicited responses against just two novel antigens each. Thus, a major question concerning our ‘highly parallel immunization’ strategy is whether immunization with highly complex DNA vaccines is capable of stimulating responses against a highly diverse set of targets, even when small subsets of minigenes are segregated and delivered to different APCs via different gold bead carriers. The malaria literature indicates that mixtures of small numbers of Plasmodium genes encoding bona fide antigens are immunogenic in mice and non-human primates [41, 42] and humans [43], although the quantity of DNA and the administration methods differ from the gene gun approach reported here. In one mouse study, administration of a mixture of five P. falciparum antigen-coding plasmids led to alterations (CSP and Exp1 decrease and LSA1 increase) in T cell immunogenicity compared to immunization with single gene plasmids only [62]. During construction of the vaccines reported here, each of the more than 100 minigene pools (10 cloned minigenes/pool) in each vaccine were individually precipitated onto separate aliquots of gold beads in parallel in an effort to promote diverse responses against multiple vaccine-encoded antigens. Such physical segregation of antigens onto different pools of gold beads is known to promote both antibody and T cell response diversity in other studies of gene gun-delivered antigens [44–46]. However, the minigene library vaccine represents a much larger set of antigens than studied in the aforementioned references, and we are uncertain how the degree of diversity and the absolute quantity of any individual antigen-encoding DNA ultimately affect immune response outcomes. In addition, a small portion of some proteins were not included in the library because correct sequences were not recovered following dial-out error correction. Therefore, some bona fide antigens could also have been encoded in the omitted sequences. Given these caveats, the library vaccination-screening strategy described herein can rule in T cell responses, but is not sufficiently sensitive in its current form to rule out immunogenicity or protective effects of targets where responses are not detected or for peptides not covered for specific proteins.
Given the suboptimal performance of the CSP control, we are currently exploring several methods to increase the intensity and breadth of responses induced by complex DNA library vaccines. First, as mentioned earlier, we have adopted a standard 5’ UTR and a ubiquitin fusion design that have proven superior to the design used in the LC3-based vaccines. Ubiquitin-tagged proteins have shown increased CD8+ T cell immunogenicity in many systems [63–67] including for some malaria DNA vaccine antigens [68]. Simply increasing the dose of DNA on gold beads is unlikely to yield a significant effect as previous titration studies with the gene gun [52] suggest that the dose of DNA required to stimulate a response is well within the range of each construct achieved in this protocol. In contrast, increasing the number of DNA vaccination cartridges administered per animal would result in an increase in the number of DNA-coated particles delivered to dermal APCs, potentially boosting the number of responders induced against multiple antigens. In addition, using the sub-optimal CSP minigene, we have tested novel adjuvants targeting B7 family inhibitory receptors and have observed a significant increase in the number of responders induced (B. Stone, S. Murphy, unpublished observation). Furthermore, we tested a P815 cell line that overexpresses the co-stimulatory molecule B7.1 [69] as APCs in minigene-transfected ELISPOT screening assays and observed five-fold higher sensitivity for IFNγ producing cells, indicating that additional antigens may be discoverable by ELISPOT with optimized transfection-compatible APCs. Vaccination studies testing these improvements using these and additional minigene libraries are currently underway.
Supporting Information
Microsoft Excel formatted spreadsheet with two worksheets (Vaccine 1 and Vaccine 2) listing the Vaccine (column A), gene ID (column B), product description (column C), minigene name (column D), vaccine-specific pool number (column E), minigene sequence (column F), encoded peptide (column G), common and pool-specific primers (columns H-I) and percentage coverage for each protein in the final error-corrected library (column J).
(XLSX)
Acknowledgments
We thank Emma Fritzen, Heather Kain, Matt Fishbaugher, Will Betz and Stefan Kappe of CeMPMIR (CID Research) for sporozoite production. B.C.S. and S.C.M. have filed provisional patents through the University of Washington with the US Patent and Trademark Office for novel methods of multi-gene DNA vaccination using minigene libraries and for novel antigens.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was partially funded by the NIH (1K08AI097238 to S.C.M., https://www.niaid.nih.gov) and the Bill and Melinda Gates Foundation (OPP1069993 to B.C.S. and S.C.M., http://www.gatesfoundation.org). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- 1.WHO Global Malaria Programme. World Malaria Report 2014. Geneva: World Health Organization, 2014. [Google Scholar]
- 2.Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Vaccine. 2015;33 Suppl 4:D13–23. Epub 2015/09/02. 10.1016/j.vaccine.2015.07.091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216(5111):160–2. Epub 1967/10/14. . [DOI] [PubMed] [Google Scholar]
- 4.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. The American journal of the medical sciences. 1973;266(3):169–77. Epub 1973/09/01. . [DOI] [PubMed] [Google Scholar]
- 5.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–7. Epub 2004/12/08. 10.1038/nature03188 . [DOI] [PubMed] [Google Scholar]
- 6.Putrianti ED, Silvie O, Kordes M, Borrmann S, Matuschewski K. Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. The Journal of infectious diseases. 2009;199(6):899–903. Epub 2009/05/13. . [DOI] [PubMed] [Google Scholar]
- 7.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. The New England journal of medicine. 2009;361(5):468–77. Epub 2009/07/31. 10.1056/NEJMoa0805832 . [DOI] [PubMed] [Google Scholar]
- 8.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–65. Epub 2013/08/10. 10.1126/science.1241800 . [DOI] [PubMed] [Google Scholar]
- 9.Keitany GJ, Sack B, Smithers H, Chen L, Jang IK, Sebastian L, et al. Immunization of mice with live-attenuated late liver stage-arresting Plasmodium yoelii parasites generates protective antibody responses to preerythrocytic stages of malaria. Infect Immun. 2014;82(12):5143–53. 10.1128/IAI.02320-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165(3):1453–62. Epub 2000/07/21. . [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Miller LH, Quakyi IA, Keister DB, Houghten RA, Maloy WL, et al. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988;334(6179):258–60. Epub 1988/07/21. 10.1038/334258a0 . [DOI] [PubMed] [Google Scholar]
- 12.Weiss WR, Mellouk S, Houghten RA, Sedegah M, Kumar S, Good MF, et al. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. The Journal of experimental medicine. 1990;171(3):763–73. Epub 1990/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Braeckel-Budimir N, Harty JT. CD8 T-cell-mediated protection against liver-stage malaria: lessons from a mouse model. Front Microbiol. 2014;5:272 10.3389/fmicb.2014.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss WR, Jiang CG. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PloS one. 2012;7(2):e31247 Epub 2012/02/23. 10.1371/journal.pone.0031247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedegah M, Hollingdale MR, Farooq F, Ganeshan H, Belmonte M, Kim Y, et al. Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+T cells targeting AMA1 class I epitopes. PloS one. 2014;9(9):e106241 Epub 2014/09/12. 10.1371/journal.pone.0106241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011;334(6055):475–80. Epub 2011/09/10. 10.1126/science.1211548 . [DOI] [PubMed] [Google Scholar]
- 17.Spring M, Murphy J, Nielsen R, Dowler M, Bennett JW, Zarling S, et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine. 2013;31(43):4975–83. Epub 2013/09/14. 10.1016/j.vaccine.2013.08.007 . [DOI] [PubMed] [Google Scholar]
- 18.Frevert U, Krzych U. Plasmodium cellular effector mechanisms and the hepatic microenvironment. Front Microbiol. 2015;6:482 Epub 2015/06/16. 10.3389/fmicb.2015.00482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman SL, Vekemans J, Richie TL, Duffy PE. The March Toward Malaria Vaccines. Am J Prev Med. 2015;49(6 Suppl 4):S319–33. 10.1016/j.amepre.2015.09.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Seidlein L, Bejon P. Malaria vaccines: past, present and future. Archives of disease in childhood. 2013;98(12):981–5. Epub 2013/09/26. 10.1136/archdischild-2013-304173 . [DOI] [PubMed] [Google Scholar]
- 21.Khusmith S, Charoenvit Y, Kumar S, Sedegah M, Beaudoin RL, Hoffman SL. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science. 1991;252(5006):715–8. Epub 1991/05/03. . [DOI] [PubMed] [Google Scholar]
- 22.Robson KJ, Hall JR, Jennings MW, Harris TJ, Marsh K, Newbold CI, et al. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335(6185):79–82. Epub 1988/09/01. 10.1038/335079a0 . [DOI] [PubMed] [Google Scholar]
- 23.Sanchez GI, Rogers WO, Mellouk S, Hoffman SL. Plasmodium falciparum: exported protein-1, a blood stage antigen, is expressed in liver stage parasites. Experimental parasitology. 1994;79(1):59–62. Epub 1994/08/01. 10.1006/expr.1994.1060 . [DOI] [PubMed] [Google Scholar]
- 24.Moelans II, Meis JF, Kocken C, Konings RN, Schoenmakers JG. A novel protein antigen of the malaria parasite Plasmodium falciparum, located on the surface of gametes and sporozoites. Molecular and biochemical parasitology. 1991;45(2):193–204. Epub 1991/04/01. . [DOI] [PubMed] [Google Scholar]
- 25.Fidock DA, Bottius E, Brahimi K, Moelans II, Aikawa M, Konings RN, et al. Cloning and characterization of a novel Plasmodium falciparum sporozoite surface antigen, STARP. Molecular and biochemical parasitology. 1994;64(2):219–32. Epub 1994/04/01. . [DOI] [PubMed] [Google Scholar]
- 26.Murphy SC, Kas A, Stone BC, Bevan MJ. A T-cell response to a liver-stage Plasmodium antigen is not boosted by repeated sporozoite immunizations. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):6055–60. Epub 2013/03/27. 10.1073/pnas.1303834110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doolan DL, Sedegah M, Hedstrom RC, Hobart P, Charoenvit Y, Hoffman SL. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. The Journal of experimental medicine. 1996;183(4):1739–46. Epub 1996/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerin-Marchand C, Druilhe P, Galey B, Londono A, Patarapotikul J, Beaudoin RL, et al. A liver-stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature. 1987;329(6135):164–7. Epub 1987/09/10. 10.1038/329164a0 . [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Hollingdale MR. Structure of Plasmodium falciparum liver stage antigen-1. Molecular and biochemical parasitology. 1991;48(2):223–6. Epub 1991/10/01. . [DOI] [PubMed] [Google Scholar]
- 30.Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Molecular microbiology. 2006;59(5):1369–79. Epub 2006/02/14. 10.1111/j.1365-2958.2005.05024.x . [DOI] [PubMed] [Google Scholar]
- 31.Bergmann-Leitner ES, Mease RM, De La Vega P, Savranskaya T, Polhemus M, Ockenhouse C, et al. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PloS one. 2010;5(8):e12294 Epub 2010/09/03. 10.1371/journal.pone.0012294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy PE, Sahu T, Akue A, Milman N, Anderson C. Pre-erythrocytic malaria vaccines: identifying the targets. Expert review of vaccines. 2012;11(10):1261–80. Epub 2012/11/28. 10.1586/erv.12.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. The New England journal of medicine. 1997;336(2):86–91. Epub 1997/01/09. 10.1056/NEJM199701093360202 . [DOI] [PubMed] [Google Scholar]
- 34.Reyes-Sandoval A, Pearson FE, Todryk S, Ewer K. Potency assays for novel T-cell-inducing vaccines against malaria. Current opinion in molecular therapeutics. 2009;11(1):72–80. Epub 2009/01/27. . [PubMed] [Google Scholar]
- 35.Doolan DL, Southwood S, Freilich DA, Sidney J, Graber NL, Shatney L, et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(17):9952–7. Epub 2003/07/30. 10.1073/pnas.1633254100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra S, Rai U, Shiratsuchi T, Li X, Vanloubbeeck Y, Cohen J, et al. Identification of non-CSP antigens bearing CD8 epitopes in mice immunized with irradiated sporozoites. Vaccine. 2011;29(43):7335–42. Epub 2011/08/03. 10.1016/j.vaccine.2011.07.081 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguiar JC, Bolton J, Wanga J, Sacci JB, Iriko H, Mazeika JK, et al. Discovery of Novel Plasmodium falciparum Pre-Erythrocytic Antigens for Vaccine Development. PloS one. 2015;10(8):e0136109 Epub 2015/08/21. 10.1371/journal.pone.0136109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang R, Arevalo-Herrera M, Gardner MJ, Bonelo A, Carlton JM, Gomez A, et al. Immune responses to Plasmodium vivax pre-erythrocytic stage antigens in naturally exposed Duffy-negative humans: a potential model for identification of liver-stage antigens. European journal of immunology. 2005;35(6):1859–68. Epub 2005/05/03. 10.1002/eji.200425807 . [DOI] [PubMed] [Google Scholar]
- 39.Sedegah M, Hedstrom R, Hobart P, Hoffman SL. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(21):9866–70. Epub 1994/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282(5388):476–80. Epub 1998/10/17. . [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Doolan DL, Charoenvit Y, Hedstrom RC, Gardner MJ, Hobart P, et al. Simultaneous induction of multiple antigen-specific cytotoxic T lymphocytes in nonhuman primates by immunization with a mixture of four Plasmodium falciparum DNA plasmids. Infect Immun. 1998;66(9):4193–202. Epub 1998/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferraro B, Talbott KT, Balakrishnan A, Cisper N, Morrow MP, Hutnick NA, et al. Inducing humoral and cellular responses to multiple sporozoite and liver-stage malaria antigens using exogenous plasmid DNA. Infection and immunity. 2013;81(10):3709–20. Epub 2013/07/31. 10.1128/IAI.00180-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R, Richie TL, Baraceros MF, Rahardjo N, Gay T, Banania JG, et al. Boosting of DNA vaccine-elicited gamma interferon responses in humans by exposure to malaria parasites. Infect Immun. 2005;73(5):2863–72. Epub 2005/04/23. 10.1128/IAI.73.5.2863-2872.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266(2):329–39. Epub 2000/01/20. 10.1006/viro.1999.0096 . [DOI] [PubMed] [Google Scholar]
- 45.Hooper JW, Custer DM, Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306(1):181–95. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuller DH, Rajakumar P, Che JW, Narendran A, Nyaundi J, Michael H, et al. Therapeutic DNA vaccine induces broad T cell responses in the gut and sustained protection from viral rebound and AIDS in SIV-infected rhesus macaques. PloS one. 2012;7(3):e33715 10.1371/journal.pone.0033715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306(5703):1934–7. Epub 2004/12/14. 10.1126/science.1102737 . [DOI] [PubMed] [Google Scholar]
- 48.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306(5703):1930–3. Epub 2004/12/14. 10.1126/science.1102452 . [DOI] [PubMed] [Google Scholar]
- 49.Schwartz JJ, Lee C, Shendure J. Accurate gene synthesis with tag-directed retrieval of sequence-verified DNA molecules. Nature methods. 2012;9(9):913–5. Epub 2012/08/14. 10.1038/nmeth.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Werling U, Edelmann W. Seamless Ligation Cloning Extract (SLiCE) cloning method. Methods in molecular biology. 2014;1116:235–44. 10.1007/978-1-62703-764-8_16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arrington J, Braun RP, Dong L, Fuller DH, Macklin MD, Umlauf SW, et al. Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines. Journal of virology. 2002;76(9):4536–46. Epub 2002/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13(15):1427–30. Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 53.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11(3):506–20. 10.1111/j.1462-5822.2008.01270.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mullbacher A, Lobigs M, Kos FJ, Langman R. Alloreactive cytotoxic T-cell function, peptide nonspecific. Scandinavian journal of immunology. 1999;49(6):563–9. Epub 1999/06/03. . [DOI] [PubMed] [Google Scholar]
- 55.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. The Journal of pathology. 2010;221(2):117–24. Epub 2010/03/13. 10.1002/path.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lundegaard C, Lamberth K, Harndahl M, Buus S, Lund O, Nielsen M. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8–11. Nucleic Acids Res. 2008;36(Web Server issue):W509–12. 10.1093/nar/gkn202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassan IA, Wang S, Xu L, Yan R, Song X, XiangRui L. Immunological response and protection of mice immunized with plasmid encoding Toxoplasma gondii glycolytic enzyme malate dehydrogenase. Parasite Immunol. 2014;36(12):674–83. 10.1111/pim.12146 . [DOI] [PubMed] [Google Scholar]
- 58.Van Braeckel-Budimir N, Harty J. Highly focused TCR Vβ repertoire is associated with a large number of naive precursors and robust CD8 T cell responses specific for a Plasmodium antigen (IRM14P.450). The Journal of Immunology. 2015;194(1 Supplement):198.10. [Google Scholar]
- 59.Doll K, Pewe L, Harty J. Protective capacity of CD8 T cells targeting a spectrum of Plasmodium-specific epitopes (MPF6P.735). The Journal of Immunology. 2014;192(1 Supplement):195.4. [Google Scholar]
- 60.Novotny LA, Bakaletz LO. The fourth surface-exposed region of the outer membrane protein P5-homologous adhesin of nontypable Haemophilus influenzae is an immunodominant but nonprotective decoying epitope. J Immunol. 2003;171(4):1978–83. Epub 2003/08/07. . [DOI] [PubMed] [Google Scholar]
- 61.Guo H, Zhou EM, Sun ZF, Meng XJ. Immunodominant epitopes mapped by synthetic peptides on the capsid protein of avian hepatitis E virus are non-protective. Viral immunology. 2008;21(1):61–7. Epub 2008/03/22. 10.1089/vim.2007.0082 . [DOI] [PubMed] [Google Scholar]
- 62.Sedegah M, Charoenvit Y, Aguiar J, Sacci J, Hedstrom R, Kumar S, et al. Effect on antibody and T-cell responses of mixing five GMP-produced DNA plasmids and administration with plasmid expressing GM-CSF. Genes and immunity. 2004;5(7):553–61. Epub 2004/08/20. 10.1038/sj.gene.6364125 . [DOI] [PubMed] [Google Scholar]
- 63.Reguzova A, Antonets D, Karpenko L, Ilyichev A, Maksyutov R, Bazhan S. Design and evaluation of optimized artificial HIV-1 poly-T cell-epitope immunogens. PloS one. 2015;10(3):e0116412 10.1371/journal.pone.0116412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hospers GA, Meijer C, Dam WA, Roossink F, Mulder NH. Construction of a triple modified p53 containing DNA vaccine to enhance processing and presentation of the p53 antigen. Vaccine. 2009;28(2):386–91. 10.1016/j.vaccine.2009.10.036 . [DOI] [PubMed] [Google Scholar]
- 65.Imai T, Duan X, Hisaeda H, Himeno K. Antigen-specific CD8+ T cells induced by the ubiquitin fusion degradation pathway. Biochem Biophys Res Commun. 2008;365(4):758–63. 10.1016/j.bbrc.2007.11.034 . [DOI] [PubMed] [Google Scholar]
- 66.Goldwich A, Hahn SS, Schreiber S, Meier S, Kampgen E, Wagner R, et al. Targeting HIV-1 Gag into the defective ribosomal product pathway enhances MHC class I antigen presentation and CD8+ T cell activation. J Immunol. 2008;180(1):372–82. . [DOI] [PubMed] [Google Scholar]
- 67.Fu TM, Guan L, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Induction of MHC class I-restricted CTL response by DNA immunization with ubiquitin-influenza virus nucleoprotein fusion antigens. Vaccine. 1998;16(18):1711–7. . [DOI] [PubMed] [Google Scholar]
- 68.Dobano C, Rogers WO, Gowda K, Doolan DL. Targeting antigen to MHC Class I and Class II antigen presentation pathways for malaria DNA vaccines. Immunol Lett. 2007;111(2):92–102. 10.1016/j.imlet.2007.05.007 . [DOI] [PubMed] [Google Scholar]
- 69.La Motte RN, Sharpe AH, Bluestone JA, Mokyr MB. Importance of B7-1-expressing host antigen-presenting cells for the eradication of B7-2 transfected P815 tumor cells. J Immunol. 1998;161(12):6552–8. Epub 1998/12/23. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microsoft Excel formatted spreadsheet with two worksheets (Vaccine 1 and Vaccine 2) listing the Vaccine (column A), gene ID (column B), product description (column C), minigene name (column D), vaccine-specific pool number (column E), minigene sequence (column F), encoded peptide (column G), common and pool-specific primers (columns H-I) and percentage coverage for each protein in the final error-corrected library (column J).
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.