Abstract
This article reviews the pulmonary route of administration, aerosol delivery devices, characterization of pulmonary drug delivery systems, and discusses the rationale for inhaled delivery of siRNA. Diseases with known protein malfunctions may be mitigated through the use of siRNA therapeutics. The inhalation route of administration provides local delivery of siRNA therapeutics for the treatment of various pulmonary diseases, however barriers to pulmonary delivery and intracellular delivery of siRNA exists. siRNA loaded nanocarriers can be used to overcome the barriers associated with the pulmonary route, such as anatomical barriers, mucociliary clearance, and alveolar macrophage clearance. Apart from naked siRNA aerosol delivery, previously studied siRNA carrier systems comprise of lipidic, polymeric, peptide, or inorganic origin. Such siRNA delivery systems formulated as aerosols can be successfully delivered via an inhaler or nebulizer to the pulmonary region. Preclinical animal investigations of inhaled siRNA therapeutics rely on intratracheal and intranasal siRNA and siRNA nanocarrier delivery. Aerosolized siRNA delivery systems may be characterized using in vitro techniques, such as dissolution test, inertial cascade impaction, delivered dose uniformity assay, laser diffraction, and laser Doppler velocimetry. The ex vivo techniques used to characterize pulmonary administered formulations include the isolated perfused lung model. In vivo techniques like gamma scintigraphy, 3D SPECT, PET, MRI, fluorescence imaging and pharmacokinetic/pharmacodynamics analysis may be used for evaluation of aerosolized siRNA delivery systems. The use of inhalable siRNA delivery systems encounters barriers to their delivery, however overcoming the barriers while formulating a safe and effective delivery system will offer unique advances to the field of inhaled medicine.
Keywords: siRNA, nanocarrier, nanoparticle, aerosolization, pulmonary, lung
Graphical Abstract
1. Introduction
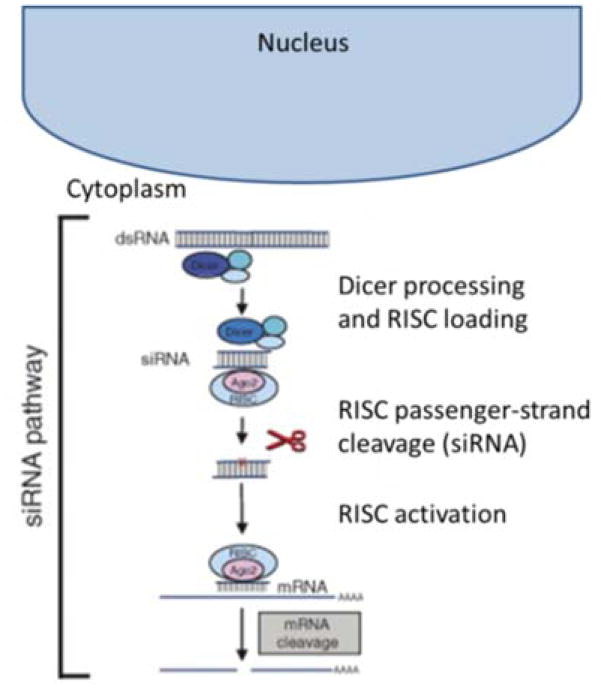
RNA interference (RNAi) is a process in which RNA molecules inhibit gene expression by causing the destruction of specific messenger RNA (mRNA). Small interfering RNA (siRNA) are double stranded RNA molecules containing 20–25 nucleotides that are involved in the RNAi pathway and interfere with the expression of a specific gene with complementary nucleotide sequences (Agrawal N. et al., 2003). As shown in Figure 1, siRNA degrades mRNA after transcription, thereby preventing translation, or plays a role in RNAi-related pathways (Agrawal N. et al., 2003). siRNA has potential therapeutic applications in treating ‘undruggable’ diseases by downregulating the expression of a target gene in a post-transcriptional manner. Since the discovery of siRNA in 1998 by Fire and Mello et al., the mechanisms of RNAi have been extensively reviewed (Fellmann C. and Lowe S.W., 2014, Fire A. et al., 1998, Hannon G.J., 2002, Kim D.H. and Rossi J.J., 2007). The siRNA possess a specific sequence that is complementary with its target mRNA that induces site-specific cleavage and subsequent inhibition of intracellular protein synthesis. The siRNA, once in the cytoplasm of the cell, can incorporate within the RNA-induced silencing complex (RISC) and activates this complex. The sense strand is then removed from the duplex and degraded by nucleases in the activated RISC complex. At the same time, the antisense strand directs the RISC to the base-complementary sequence of the target mRNA located within the cell cytoplasm. Binding of mRNA to the antisense strand in the activated RISC eventually induces cleavage by the endonuclease Argonaute and post-transcriptional silencing of the target gene expression. Major advantages of siRNA over small molecule drugs or protein therapeutics are that the sequences can be rapidly designed for highly specific inhibition of the target of interest and that the synthesis of siRNAs is relatively simple because it does not require a cellular expression system, complex protein purification, or refolding schemes (Amarzguioui M. et al., 2005).
Figure 1.
siRNA pathway schematic. Reprinted with permission from Ref. (De Fougerolles A. and Novobrantseva T., 2008, De Fougerolles A. et al., 2007). Copyright: (2007) Nature Publishing Group.
Pulmonary diseases such as lung cancer, cystic fibrosis, pulmonary hypertension, asthma, and chronic obstructive pulmonary disorder (COPD) have potential siRNA therapeutic targets (Amarzguioui M. et al., 2005, Burnett J.C. and Rossi J.J., 2012, Kanasty R. et al., 2013). Nevertheless, siRNA delivery systems are likely to have instability issues that cause premature release of the nucleic acids, especially with systems that incorporate their cargo through electrostatic interactions. Due to the high negative charge density and relatively large size of the siRNA molecules, naked siRNA molecules are not able to enter cells efficiently (Reischl D. and Zimmer A., 2009). Pulmonary delivery of siRNA faces major challenges that involve a lack of correlation between in vitro and in vivo experiments, difficulty in translation from animal models to humans, and non-applicable administration routes used in animal studies for human use (Lam J.K.-W. et al., 2012). In Part I, we review the modes of pulmonary delivery of siRNA, the evaluation of aerosol drug delivery systems, and the rationale for the use of nanocarriers to overcome the barriers of pulmonary delivery and cellular uptake of siRNA. Part II focuses on the siRNA loaded non-viral particulates for aerosolized delivery systems, and preparation and characterization techniques for siRNA loaded nanoparticles.
2. Pulmonary Route of Administration
To achieve pulmonary delivery, inhalable aerosols generated by an inhaler or nebulizer are the preferred option. Before entering clinical trials, new therapeutic agents must demonstrate preclinical efficacy in appropriate animal models that are translatable to humans (Laube B.L., 2014). Pulmonary aerosols are usually administered via the inhalation, intratracheal, or intranasal routes. Intratracheal and intranasal routes of administration are commonly used to deliver therapeutic siRNA or other therapeutic agents to the lungs of animals due to ease of experimental setup and control (Driscoll K.E. et al., 2000). In preclinical studies, the very different lung anatomy of mice and humans needs to be considered while selecting the route of administration to assess delivery and efficacy. Formulations administered via the pulmonary route of administration are required to be nonirritating to reduce risk of pharyngeal edema, bronchial spasm, anaphylaxis, peracute death, and chronic pulmonary fibrosis (Turner P.V. et al., 2011). These factors are vital to the successful development of an orally or intranasally inhaled siRNA delivery system.
2.1 Inhalation Route
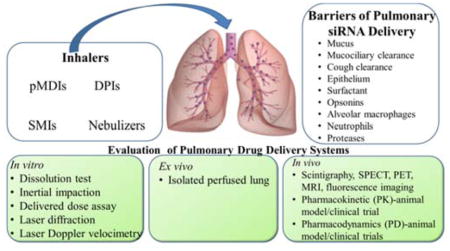
The most non-invasive way to locally deliver therapeutics to the lungs is through inhalation. Four types of inhalation devices are currently available including pressurized metered dose inhalers (pMDIs), dry powder inhalers (DPIs), nebulizers, and soft mist inhalers (SMIs). With appropriate developmental optimization, these devices may deliver siRNA to the lungs. During development, key parameters should be considered for an optimum inhaler system, as shown in Table 1.
Table 1.
Parameters to consider in siRNA formulation design for inhalation.
| Property | Parameters | |
|---|---|---|
| Aerosol properties | Mass median aerodynamic diameter | |
| Geometric standard deviation | ||
| Fine particle fraction | ||
| Air/particle velocity | ||
|
| ||
| Particle properties | Volume diameter | |
| Bulk density | ||
| Tap density | ||
| Shape | ||
| Charge | ||
|
| ||
| Physiochemical properties | Solubility | |
| Hygroscopicity | ||
|
| ||
| Lung properties | Disease state on airway structure and breathing pattern | |
| Disease state on surfactant production | ||
| Disease state on mucus production and mucociliary clearance | ||
| Age of patient, pulmonary or nasal breathing | ||
| Airway structure and diameter | ||
2.1.1 Inhalation Aerosol Delivery Devices
pMDIs are currently the most commonly used inhalers. The therapeutic agents within a pMDI are in either a suspended particulate state or dissolved within propellants, such as chlorofluorocarbons (CFCs) and hydrofluoroalkanes (HFAs) (Lam J.K.-W. et al., 2012). The propellants are an indispensable part of pMDIs as they supply the energy required to aerosolize the drug for inhalation. siRNA or siRNA loaded nanocarriers may not be compatible with propellant vehicles which limit the formulation of siRNA into the pMDIs (Lam J.K.-W. et al., 2012). However, crosslinked chitosan-PEG1000 based nanocarriers with particle size of less than 230 nm were found to be physically stable within HFA-227, highly dispersible, and successfully delivered to the deep lung airways using pMDIs (Sharma K. et al., 2012). Selecting a stable and appropriate formulation for use with the pMDI devices will allow for the development of siRNA containing pMDIs.
DPIs and their formulations allow for the inhalation of aerosol clouds of dry particles. DPI device design has a major impact on their performance. Advantages of DPIs are that they have improved sterility and chemical stability of biomolecules compared to liquid aerosols (Lam J.K.-W. et al., 2012). Drug deposition from inhaled DPIs is dependent on the inspiration flow rate of the patient, and often times the illness that warrants their use of the inhalation therapy causes their inspiration flow rates to be abnormally low (Feddah M.R. et al., 2000, Ross D.L. and Schultz R.K., 1996). The device must be designed to minimize these variation with respect to patient disease state (Shoyele S.A. and Slowey A., 2006). Spray drying techniques are often used to prepare protein and peptide inhalable DPI formulations that have demonstrated successful in vivo delivery of therapeutic macromolecules (Bai S. et al., 2010, Codrons V. et al., 2003, Mastrandrea L.D. and Quattrin T., 2006, Rawat A. et al., 2008). The DPIs formulation can be used for pulmonary delivery of siRNA. The issues and challenges that have risen from the formulation of biological macromolecules, such as flowability, dispersibility, and biochemical stability needs to be also considered for development of siRNA based DPIs (Chan H.-K., 2003). Considerations for formulation of proteins as powder aerosols have been reviewed previously (Chan H.-K., 2003). Maintaining biochemical stability of siRNA during formulation limits the processes that may be used to prepare siRNA based dry powder formulations.
Nebulizers generate liquid aerosols that can deliver saline-based solutions or suspensions of drug product at large volumes via inhalation (Sharma K. et al., 2013). Nebulizer treatments usually last over several minutes, rather than single inhalation. Generated droplets have aerodynamic diameters between 1–10 μm, depending on the formulation and the type of nebulizer device. The four major categories of nebulizers are jet (pneumatic), vibrating mesh/membrane, smart, and ultrasonic nebulizers (Ari A., 2014, Dailey L.A. et al., 2003).
A jet, or pneumatic nebulizer, can be used to deliver suspension formulation which makes them suitable for delivering siRNA or siRNA carrier delivery systems. Breath-enhanced jet nebulizers release more aerosol during inhalation, whereas breath-actuated jet nebulizers sense the patient’s inspiratory flow and deliver aerosol only during inhalation (Arunthari V. et al., 2012, Haynes J.M., 2012, Ho S.L. et al., 2001). In an air jet nebulizer, compressed gas draws up bulk liquid to a jet by the Bernoulli effect and atomization takes place where the liquid emerging from the jet interacts with the shear force set up by the gas flow. Rayleigh dispersion of bulk liquid into droplets occurs and baffles remove the coarse droplets, which fall back into the reservoir, whereas droplets with aerodynamic diameters of < 10 μm are available for inhalation. It has been estimated that 99% of the generated aerosols are recycled back to the reservoir, thus the shear stress is exerted multiple times on the formulation and hence, the possible degradation of naked siRNA during this process needs to be evaluated (Agu R.U. et al., 2001).
Vibrating mesh nebulizers use micropumps to force liquid through a mesh or aperture plate for aerosol generation. Suspensions, nanocarriers, and nucleic acids have been successfully delivered via mesh nebulizers (Elhissi A. et al., 2006, Elhissi A. and Taylor K., 2005, Hibbitts A. et al., 2014, Lentz Y.K. et al., 2006, Luo Y. et al., 2012, Wagner A. et al., 2006, Yoshiyama Y. et al., 2002). Mesh nebulizers have consistent and improved aerosol generation efficiency, large fine-particle fraction, low residual volume, and the ability to nebulize low volumes (Ari A., 2014, Dhand R., 2002, Dolovich M.B. and Dhand R., 2011, Waldrep J. and Dhand R., 2008). Vibrating mesh/membrane nebulizers are more expensive than jet nebulizers because of the electronics involved with controlling the vibration process. In addition, mesh nebulizers are difficult to clean. However, mesh nebulizers are more efficient than jet nebulizers at providing higher drug doses to patients (Ari A., 2014). The limitations of mesh nebulizers include the blocking of the mesh or apertures during the nebulization of viscous suspensions or the precipitation and crystallization of drug or excipients (Ari A., 2014, Najlah M. et al., 2014). This should be a consideration when formulating a siRNA nanocarrier suspension for nebulization.
There are two types of vibrating mesh nebulizers, termed active and passive. Active mesh nebulizers utilize a piezoelectrical element that expands and contracts upon application of electrical currents which then vibrates a mesh in contact with the medication to generate the aerosol. Passive mesh nebulizers use a transducer horn that induces passive vibrations on the mesh plate for aerosol generation (Ari A., 2014).
Smart nebulizers incorporate adaptive aerosol delivery (AAD®) technology that analyzes the patient’s breathing pattern in order to determine the timing of aerosol drug delivery during inhalation (Denyer J. and Dyche T., 2010). The device is able to adapt to the patients breathing pattern, therefore it is able to reduce the aerosol losses and the variation in drug delivery. Smart nebulizers can provide effectiveness and end of dose feedback to the patient during therapy, which can increase patient compliance (Denyer J. and Dyche T., 2010, Denyer J. et al., 2004).
Ultrasonic nebulizers utilize a piezoelectric crystal vibrating at high frequencies of 1–3 MHz to produce aerosols. These types of nebulizers have the limitations of large residual volumes, inability to aerosolize viscous solutions, and degradation of heat sensitive materials (Ari A., 2014, Ari A. et al., 2009, Ari A. and Restrepo R.D., 2012). Therefore, ultrasonic nebulizers may not be useful for suspensions of naked siRNA or siRNA nanocarriers (Taylor K.M. and Mccallion O.N., 1997, Watts A.B. et al., 2008).
Chemical and physical stability of the naked siRNA during the nebulization process is of high concern. Therefore, delivery of siRNA using nebulizers should involve the development of a formulation which protects the siRNA from physical degradation, shear stress, and chemical degradation mediated via hydrolysis and endogenous enzymes (Lam J.K.-W. et al., 2012).
SMIs are a recently introduced category of inhaler delivery system marketed as Respimat ® produced by Boehringer-Ingelhem (Dalby R. et al., 2004). This technology provides a metered dose to the user who activates the inhaler and energy from a spring imposes pressure on the liquid container. The SMI generates the aerosol by impinging opposing droplet streams emerging from a uniblock nozzle created using silicon wafer technology (Dalby R. et al., 2004). The advantages of the SMI are that it has high lung deposition due to the low velocity of the aerosol cloud and thus minimizing the deposition in the mouth and throat (Dalby R. et al., 2004, Hochrainer D. et al., 2005). In addition, the patient is not required to exert excessive inspiratory force in generating the aerosol clouds associated with DPIs. The effect on physical and chemical stability of siRNA must be considered while delivering a formulation via SMIs (Merkel O.M. et al., 2014, Sharma K. et al., 2013). The feasibility of SMIs as delivery devices for non-viral siRNA delivery has not been explored, but it may be a viable option.
2.2 Intratracheal Route
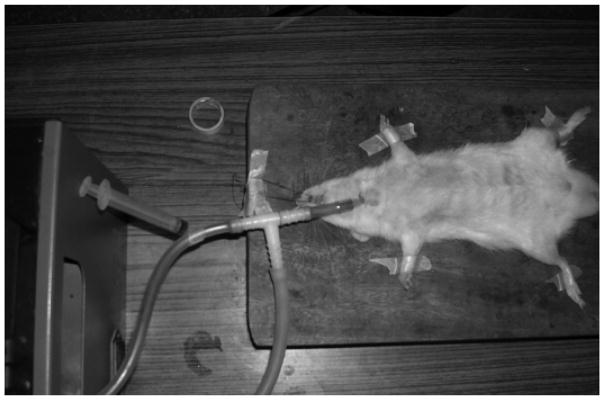
The intratracheal route of administration is used most often in animal studies for assessing the inhalation delivery of various drugs. However, the clinical application of this route is limited due to its invasive setup and uncomfortable delivery technique (Driscoll K.E. et al., 2000, Sakagami M., 2006). The intratracheal route is not feasible for human clinical studies due to method invasiveness that involves inserting a tube to dispense the formulation within a surgical incision made within the tracheal rings (Lam J.K.-W. et al., 2012). The traditional method involves the animal to be anesthetized, undergo surgical tracheotomy, and after the trachea is exposed, an endotracheal tube or needle is inserted into the incision between the tracheal cartilaginous rings, with the tip projected at a defined position before the tracheal bifurcation, as shown in Figure 2. The drug solution or suspension formulation is administered through the tube using a microsyringe and instilled into the airways. Bivas-Benita et al. described a non-invasive intratracheal instillation that did not involve surgical resection of the trachea that is also known as the oro-tracheal route (Bivas-Benita M. et al., 2005). Aerosol is delivered to the lungs by placing a microsprayer over the anesthetized animals tongue and down to the trachea or the animal is intubated and the drug is instilled in solution or suspension form. This method has the drawbacks of difficulty in accurate placement of the microsprayer catheter and reduced mucociliary clearance due to anesthesia.
Figure 2.
Intratracheal instillation and bronchoalveolar lavage on rat. Reprinted with permission from Ref. (Patel G. et al., 2009).
There have been a number of studies that utilized the intratracheal route for siRNA delivery to the lungs (Conde J. et al., 2013, Garbuzenko O.B. et al., 2009, Merkel O.M. et al., 2009, Moschos S.A. et al., 2007, Perl M. et al., 2005, Rosas-Taraco A.G. et al., 2009, Wang J.-C. et al., 2010). Drug deposition by this route is less uniform than by the inhalation route of administration (Sakagami M., 2006). Since this route avoids oropharynx deposition, the amount of drug loss is lower compared to the inhalation route. However, this route of delivery does not allow for determination of the effect of aerodynamic particle size on the lung deposition, therefore the in vivo intratracheal instillation studies in the animals do not reflect the intricacies of lung deposition. The intratracheal instillation route of delivery is suitable for providing proof-of-concept for local lung delivery studies in animal models.
2.2.1 Intratracheal Aerosol Delivery Devices
Intratracheal aerosolization devices consists of an atomizer located in the distal tip of a long, narrow, stainless steel or plastic tube used to deliver an aerosol directly to the lungs when inserted down the trachea, above the carina or further down, in an anesthetized animal. Devices for preclinical studies include commercially available technologies for administration of solution and for dry powder aerosol formulations. Intratracheal delivery can be achieved through devices, such as the Penn-Century microsprayer (Gutbier B. et al., 2010, Rosas-Taraco A.G. et al., 2009), the Micro-Mist nebulizer (Zamora-Avila D. et al., 2009), or the Aeroprobe nebulizing catheter (Nielsen E.J. et al., 2010). The Penn-Century, Inc. (Wyndmoor, PA, USA) offers an air-free liquid device and an air-driven dry powder device for preclinical use (Bivas-Benita M. et al., 2005, Morello M. et al., 2009). The miniaturized nebulizing catheter system has shown success for targeted gene delivery to the lungs (Koping-Hoggard M. et al., 2005, Tronde A. et al., 2002). The nebulizing catheter device (NCD) was first adapted for the delivery of peptide therapeutics to the rat lungs (Aeroprobe ™, Trudell Medical International, London, Ontario, Canada) (Tronde A. et al., 2002). This NCD delivers a liquid, which is transported down a tube through a central lumen and pressurized gas is pushed through the peripheral lumens from a compressed airway source at 50 psi. The close proximity of the liquid and gas lumens at the distal tip produces an aerosol in the particle size range of 20–40 μm (Tronde A. et al., 2002).
2.3 Intranasal Route
The intranasal route provides a straight forward animal model experimental setup for delivery of siRNA to the lungs, therefore it is another common siRNA delivery route (Bitko V. et al., 2005, Fulton A. et al., 2009, Ghosn B. et al., 2010, Gutbier B. et al., 2010, Hosoya K. et al., 2011, Howard K.A. et al., 2006, Li B.-J. et al., 2005, Massaro D. et al., 2004, Senoo T. et al., 2010, Tompkins S.M. et al., 2004, Zhang X. et al., 2004). The animals must be anesthetized and siRNA formulations are then instilled into the nasal cavity to be breathed in. Although this route has shown success in siRNA delivery to the lungs in mouse animal models, the translation of these studies to humans has been limited. Humans are not obligate nose breathers and have a nasal cavity that filters out the majority of particulates, therefore human intranasal studies do not see such a high lung deposition (Hickey A.J. and Garcia-Contreras L., 2001). Additionally, anesthetics cause the animals to have reduced mucociliary action, therefore the reduced mucociliary clearance overestimates the transfection efficiency of the formulations (Hinchcliffe M. and Illum L., 1999). A study by Heyder et al. found that only 3% of monodisperse powder particles with aerodynamic diameters of 1–5 μm were deposited within human bronchial airways after intranasal administration (Heyder J. et al., 1986). Since approximately 97% of the monodisperse particles were deposited within the nasopharynx area, the intranasal route of delivery is ideal for targeting this site in humans. The ALN-RSV01 siRNA, which targets the mRNA encoding the N-protein of respiratory syncytial virus (RSV), developed by Alnylam Pharmaceuticals (Cambridge, MA, USA), has completed phase II clinical trials for the treatment of human RSB infections using the intranasal route of administration (Devincenzo J. et al., 2008, Devincenzo J. et al., 2010, Zamora M.R. et al., 2011). This naked siRNA was delivered via a nasal spray to the upper respiratory tract.
The intranasal route may also be used to allow for systemic or central nervous system (CNS) delivery of siRNA. Protein and peptide biological macromolecules have been previously administered via the intranasal route to access the systemic circulation (Hinchcliffe M. and Illum L., 1999). The nasal cavity has a relatively large surface area and vascularization for facilitating rapid absorption. The intranasal route can access the CNS by bypassing the blood-brain barrier (Hanson L.R. and Frey W.H., 2nd, 2008, Kamei N. and Takeda-Morishita M., 2015).
2.3.1 Intranasal Aerosol Delivery Devices
Intranasal delivery devices for liquid solution or suspension include the rhynyle catheter and instillation tube. This simple method, often used on anesthetized or sedated animals, involves the intranasal insertion of a fine catheter or micropipette followed by instilling the liquid into the desired area to be aspirated into the airways during breathing (Fernandes C.A. and Vanbever R., 2009). The use of small volumes of 5 μL per nostril or 10 μL total limited drug deposition to the nasal cavity, whereas larger volumes of 25 μL per nostril or 50 μL total allowed for deposition with the lung upper airways (Minne A. et al., 2007). Intranasal instillation is limited due to its hindered ability to reach the deep lung.
For the intranasal delivery of dry powders to the pulmonary route of administration, the Dry Powder Insufflator™ for mice can be used to aerosolize the powder dose (Sinsuebpol C. et al., 2013). This device is actuated using either an air syringe or a Penn-Century air pump, which generates 200 μL puffs of air that generates the powder dose and carries it into the deep lung.
2.4 Passive Inhalation Exposure Chambers for Animal Studies
Aerosol exposure chambers may be classified as nose-only, head-only, and whole-body exposure chambers. These systems are commonly used to deliver nebulized formulations (Cryan S.-A. et al., 2007). An example of a nose-only exposure chamber is the InExpose™ (SCIREQ®) model as shown in Figure 3. This device has been evaluated for use with inhalable drug carrier systems containing antisense oligonucleotides and siRNA for the treatment of lung cancer (Mainelis G. et al., 2013). Liposomes with particle size and mass median diameter of 130 nm and 270 nm respectively, aerosolized by a jet Collison nebulizer were found to be stable over the continuous aerosolization and a higher lung dose and retention time compared to intravenous route was observed (Mainelis G. et al., 2013). Other examples of nose-only exposure chambers include the Oro-Nasal and Respiratory Exposure Systems (CH Technologies, Westwood, NJ) and the In-Tox Products Small Animal Exposure System (Intox Products, Edgewood, NM). Nose-only and head-only exposure chambers avoid exposure of the aerosol to other parts of body. These set-ups can be stressful for the animal due to the restraint and exposure facemasks placed on the animals face and neck (Cryan S.-A. et al., 2007).
Figure 3.
InExpose™ (SCIREQ®) nose-only exposure system.
The whole-body exposure setup represents a less invasive exposure chamber because rodents are free to move during the passive aerosol delivery (Cryan S.-A. et al., 2007). Whole-body exposure can also simulate environmental exposures to aerosols (Mühlfeld C. et al., 2008). These exposure chambers are usually composed of a chamber where the animal resides during the aerosol therapy, and a port where aerosol can be delivered to the inside of the chamber (Adamcakova-Dodd A. et al., 2014, O’shaughnessy P.T. et al., 2003, Pettis R.J. et al., 2000). When delivering a therapeutic aerosol via a whole-body exposure chamber, there is possible administration of significant amount of formulation to other routes, such as the oral route (Cryan S.-A. et al., 2007). Parameters that impact the variation of the study may include the size of the chamber, the animals size, lung capacity, the inspiratory flow rate, and the formulation characteristics (Cryan S.-A. et al., 2007).
3. Evaluation of Pulmonary Drug Delivery Systems
Aerosolized siRNA formulations are characterized using various in vitro, ex vivo, and in vivo techniques that are shown in Table 2 (Mansour H.M. et al., 2009, Newman S.P. et al., 2000). The purpose of these performance tests is primarily to establish the efficacy of the drug product and device delivery platform have been addressed. These tests were elaborated in Chapter <601> of the USP (Chapters U.G., 2009).
Table 2.
Techniques for evaluating pulmonary drug delivery systems.
| Type | Technique | Measurement determined |
|---|---|---|
| In vitro | Dissolution test | Dissolution rate |
| Inertial impaction using cascade impactor | Aerodynamic particle size distribution (APSD) | |
| Delivered dose assay | Total delivered dose uniformity | |
| Laser diffraction | Particle size and particle size distribution | |
| Laser Doppler velocimetry | Aerosol velocity | |
| Ex vivo | Isolated perfused lung | Mechanisms of drug transport and deposition |
| In vivo | Scintigraphy | Visualization and quantification of aerosol deposition in respiratory tract |
| Pharmacokinetic (PK)-animal model/clinical trial | PK parameters | |
| Pharmacodynamics (PD) | Biochemical and physiological effects of a drug |
3.1 In vitro Characterization
Aerosolized siRNA formulation dissolution tests may be performed by adding the formulation within dissolution medium vessel under constant agitation (Shah V.P. et al., 2008, Son Y.J. and Mcconville J.T., 2009). The solution is then sampled at pre-determined time intervals to measure the amount of siRNA released from the nanoparticles. Since there is not a standard method developed for determining siRNA dissolution rate, various media, such as distilled water (Dickinson P.A. et al., 2001), phosphate buffer (Murata N. et al., 2008), simulated lung fluid (SLF) and modified SLF have been used. In addition, various apparatus, such as the flow-through cell (Davies N.M. and Feddah M.R., 2003), standard USP dissolution apparatus, and stirring vessels have been used. Test conditions, such as sink, and particle introduction into the medium being either direct or by aerosolization have been used to characterize the dissolution rate of inhaled drugs.
Inertial impaction is a standard in vitro technique used to predict aerosol deposition in the lungs (Padhi B.K. et al., 2009). The cascade impactor is the equipment of choice for particle size analysis of most inhaled aerosols because it allow assay for mass of drug, determined aerodynamic size directly, and provide size information that be indicative of lung deposition. The inertial size-separation and fractionation in a cascade or sequence of single stages within the cascade impactor apparatus measures aerodynamic particle size distribution (APSD) (Chapters U.G., 2009, Hickey A.J., 2003). The jet stream is aimed towards a flat impaction plate, upon which the flow of particles having high inertia is disrupted and impact on the plate. Meanwhile, particles with lower inertia remain within the streamline airflow and do not impact onto the plate (Chapters U.G., 2009, Hickey A.J., 2003, Merkus H.G., 2009). Several impactor plates separate aerosol particles into size ranges according to their aerodynamic size. The larger particles will impact on the plate, whereas the smaller ones will remain airborne. The Stokes number, which is defined as the ratio of the stopping distance to the orifice diameter, can be used to estimate the impaction efficiency (Mitchell J.P. and Nagel M.W., 2003).
The available cascade impactors accepted by the United States Pharmacopeia (USP) and the European Pharmacopeia (Ph.Eur.) are listed in Table 3 (Mitchell J.P. et al., 2010, Nichols S.C. et al., 2013). The Andersen Cascade Impactor (ACI) is a pharmacopeial method for pMDIs and DPIs and is the most commonly used inertial impactor instrument (Figure 4A). Other cascade impactors include the Marple-Miller model 160 (Figure 4B) and the multistage liquid impinger (Figure 4C) for DPIs. Nebulizers are suggested to be characterized by the USP <601>/Ph.Eur. 2.9.44 using the Next-Generation Impactor (NGI) at 15 L/min or with a suitable validated cascade impactor that operates at ≤15 L/min (Nichols S.C. et al., 2013).
Table 3.
Ph.Eur. and USP Cascade Impactors for orally inhaled dosage forms.
| Dosage Form | USP | Ph.Eur. |
|---|---|---|
| pMDI |
|
|
| DPI |
|
|
| Nebulizer* |
|
|
operated at a flow rate of 15 L/min. Ph.Eur. 2.9.44 includes the NGI and is harmonized with <1601> of the USP
Figure 4.
Schematic representation of the (A) Andersen 8-stage cascade impactor, (B) Marple- Miller impactor, and (C) Multi-stage liquid impinger. Reprinted with permission from Ref. (Nahar K. et al., 2013). Copyright 2013.
Cascade impactors are apparatuses for measuring APSD of inhaled aerosols and are not lung simulators or models (Mitchell J.P. and Nagel M.W., 2003). Since cascade impactors operate at a constant flow rate, they do not simulate the environment of the respiratory tract, such as temperature, relative humidity, and the size-selectivity of the various processes that govern the deposition in the human respiratory tract. The constant particle velocity within a cascade impactor contrasts with the decreasing air velocity with increasing airway generation number in the lung. The cascade impactor stage selectivity is much steeper than selectivity for regional deposition (Dunbar C. and Mitchell J., 2005).
Non-aerodynamic droplet or particle size distribution was determined using laser diffraction (Chougule M. et al., 2008, De Boer A.H. et al., 2002, Padhi B.K. et al., 2009). This technique measures the low angle scattering of monochromatic coherent light from every particle in the measurement zone. These simultaneous measurements are combined to contribute to the angular scattered light intensity profile. Laser diffraction has the advantages of that it is non-invasive, rapid, has high resolution, wide-dynamic range, can measure volume weighted particle size distribution directly, and is an absolute technique that does not require calibration or verification using optical reticles (Lee Black D. et al., 1996). During a laser diffraction particle size determination measurement, particles of the same size scatter light to the same position in the detector array, where smaller particles scatter light to wider angles (De Boer A.H. et al., 2002). Efficient data inversion algorithms ensure rapid convergence to a valid solution. Laser diffraction method utilizes the interpretation of the relationship between scattered light intensity and scattering angle, which is the distance from the central axis of the photodetector. The Fraunhofer approximation is a simplified description assuming particles absorb light completely (Eshel G. et al., 2004). In this case, only scattering at contour is considered and the particle refractive index (Np) is not needed. The Fraunhofer model works best when particle size is much larger than the wavelength of light, and when the particles are opaque, spherical, and when Np is different to the refractive index of the surrounding medium (e.g. air) (Newman S.P. et al., 2001). The Lorenz-Mie theory is a complete description of angular light scattering process that requires a full description of complex particle refractive index, which at times is unknown (Eshel G. et al., 2004). The Lorenz-Mie model applies to all sizes of particles, but assumes that particles are homogeneous, isotropic, spherical, and has a known complex Np that incorporates both refraction and absorption components. A time-averaged data presentation mode is useful to capture overall performance of a pulmonary inhaled product. The size distribution can be presented in both differential and cumulative formats and the key metrics like particle size of the 10th, 50th, and 90th volume percentiles can be obtained (De Boer A.H. et al., 2002). Laser diffraction equipment can either be an open bench or a close cell. Open bench is simple to perform and is widely used where the aerosol can be directed across the measurement zone. Closed sampling is necessary for DPI testing and may be needed to control evaporation for some measurements, especially with continuous nebulizers. The limitations for laser diffraction are that the method has no assay for drug mass, particles are assumed to be spherical, constant droplet density versus size, vignetting, beam splitting, and droplet evaporation (De Boer A.H. et al., 2002).
The delivered dose uniformity assay is used to quantitatively determine the delivered dose and dose uniformity using a dose uniformity sampling apparatus (DUSA) (Chapters U.G., 2009). The apparatus is connected to an aerosol delivery system through a mouthpiece adapter and a vacuum pump is connected at the other end to provide the desired air flow. The aerosol released into the sampling apparatus is captured onto a filter that is then assayed to quantify the delivered dose. The dose uniformity is determined from variability of the measured delivered dose by the collection of the total mass of contents for the actuation of the clinical dose on the label, excluding volatile propellant and low-volatile species.
3.2 Ex vivo Characterization
Ex vivo studies are used to identify lung-specific PK without interference from systemic absorption, distribution, metabolism, and excretion (ADME) that occur during in vivo studies (Sakagami M., 2006). The isolated perfused lung method includes the isolation of a lung from the body and keeping it within an artificial system under certain experimental conditions. These tests utilize isolated perfused lung models that maintain lung tissue functionality and architecture (Cryan S.-A. et al., 2007, Sakagami M., 2006). The isolated perfused lung model is often used to establish the mechanisms of drug absorption and deposition in the lungs. The shortcoming of ex vivo methods include relatively shortened timeframes for data collections due to the viability of the perfused lungs, complex experimental set up, and the absence of tracheo-bronchial circulation and mucociliary clearance (Cryan S.-A. et al., 2007).
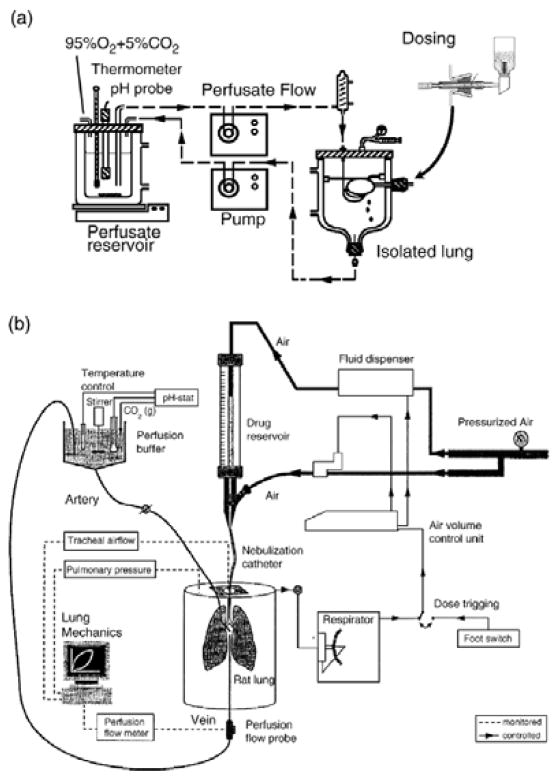
The isolated perfused lung preparation has been used in rats, guinea pigs, rabbits, dogs, and monkeys (Sakagami M., 2006). The preparation, as shown in Figure 5A–B, consists of peristaltic pumps and a tubing assembly to carry the perfusate to and from the lung, and a double-jacketed artificial thorax to house the isolated perfused lung at 37°C. Perfusate flow, which is normally at 12–15 mL/min in rat lungs, is pumped from the reservoir to the isolated perfused lung through a central porthole of the thorax lid. The bottom of the thorax has an opening, where the perfusate can return to the reservoir or be collected. The thorax is sealed, which enables negative or positive pressure ventilation or the maintenance of pressure (Sakagami M., 2006). While the animal is under anesthesia, the pulmonary circulation in cannulated via the pulmonary artery and the lung is perfused with autologous whole blood or a buffered artificial medium at pH 7.4 (Sakagami M., 2006). The surgical procedures involved in preparing the isolated perfused lung model was discussed previously (Sakagami M., 2006). Following perfusion, the formulations are administered via the tracheal port and the perfusate samples may be collected at predetermined time points to establish absorption profiles (Sakagami M., 2006).
Figure 5.
Isolated perfused rat lung preparations: (a) horizontally positioned isolated perfused rat lung with a scheme of forced solution instillation, (b) vertically positioned isolated perfused rat lung with a scheme of nebulization catheter dosing. Reprinted with permission from Ref. (Sakagami M., 2006). Copyright 2003.
3.3 In vivo Characterization
Gamma scintigraphy is a non-invasive imaging technique that allows for visualization of drug deposition following pulmonary delivery in the respiratory tract, predicts in vivo efficacy, and estimates mucociliary clearance (Newman S. and Wilding I., 1998, Newman S.P. and Wilding I.R., 1999, Snell N. and Ganderton D., 1999). Prior to pulmonary delivery, the drug formulation is radio-labelled with gamma-ray-emitting radiotracer, such as Technetium 99m (99mTc). Alternatively, the radiolabel can be incorporated or attached to the microparticle or nanocarrier instead of the drug (Nahar K. et al., 2013). Radiolabeling could be simply achieved by mixing a radiotracer solution with the formulation (Newman S.P. et al., 2003). An appropriate in vitro validation is required to ensure distribution of the radiotracer across particle size range and that the radiotracer is not affecting the particle size distribution (Newman S. and Wilding I., 1998). A gamma camera is used to visualize and quantify the drug deposition in regions of interest within the respiratory tract following inhalation of the radiolabeled formulation. Scintigraphy data are considered an equivalency assessment tool since the data have been demonstrated to correlate well with clinical efficacy data (Bondesson E. et al., 2003, Newman S. and Wilding I., 1998). After pulmonary delivery of the formulation, a gamma camera scans the thorax and radioactive counts can be digitalized to get a 2D image of the lungs, oropharynx, stomach, the inhalation device, and the exhalation filter (Majoral C. et al., 2014, Nahar K. et al., 2013). The periphery of the lungs is imaged by 80mKr ventilation or transmission scans, and is superimposed to quantify the gamma-labelled particles to establish distribution and permeation (Conway J., 2012, Nahar K. et al., 2013). However, the two dimensional (2D) nature of gamma scintigraphy images make it difficult to differentiate the overlaid anatomical structures. The ratio of peripheral to central deposition, known as the penetration index (PI), measures the extent to which an aerosol reached the lower airways in the lung periphery (Conway J., 2012, Nahar K. et al., 2013).
Three dimensional (3D) imaging methods, such as single photon emission computed tomography (SPECT) overcome 2D imaging issues in distinguishing physiological landmarks (Perring S. et al., 1994). Radiolabeled formulations are tracked by a gamma camera that rotates completely around the subject to form a topographical image in all planes (Conway J., 2012, Fleming J. et al., 2011, Nahar K. et al., 2013). 3D images can be formed using computer software (Snell N. and Ganderton D., 1999). A limitation of SPECT is the possible occurrence of regional deposition, redistribution by mucociliary clearance, coughing, and absorption to the bloodstream during the 30 min method timeframe (Huchon G.J. et al., 1987, Nahar K. et al., 2013). Other limitations are the requirement of a high dose and the use of radiation. Other in vivo imaging methods have been applied to overcome these issues, nevertheless the relatively lower cost and technical skills required are much lower for planar gamma scintigraphy or SPECT imaging (Everard M.L., 1994, Nahar K. et al., 2013). SPECT 3D imaging is suitable for correlating in vitro and in vivo data (Chan H.-K. et al., 2007, Eberl S. et al., 2006, Snell N. and Ganderton D., 1999).
Positron emission tomography (PET) utilizes positron labeled drugs that emits two high-energy photons through electron annihilation (Cherry S.R. and Gambhir S.S., 2001, Dolovich M.B., 2009, Nahar K. et al., 2013, Phelps M.E., 2000). These photons are concurrently emitted at 180° from one another, then detected and counted as a single event (Nahar K. et al., 2013). Accurate delineation regions of interest are obtained by combining the 3D PET image with MRI or spiral CT scan of the thorax (Frauenfelder T. et al., 2015, Yoon S.H. et al., 2014). This allows for the calculation of the percentage of the inhaled dose deposited in these regions (Nahar K. et al., 2013). The advantage of PET is that the drug can act as the radioactive tracer by incorporating a positron emitting isotope (11C, 15O, 13N, 18F, 64Cu, and 124I) by isotopic substitution at trace amounts (Carvalho T.C. et al., 2011, Chakravarty R. et al., 2014, Conway J., 2012, Park C.-W. et al., 2012). PET has been employed to analyze lung pathology, biochemistry, inflammation, transgene expression, and cellular responses in vivo (Park C.-W. et al., 2012). The utility of the method is limited by the short half-life (11C t1/2 ~20 min) of radionuclides that necessitates the need to be close to a cyclotron during the study (Saha G.B. et al., 1992). PET has been suggested to have no advantage over planar gamma scintigraphy and PK studies for the calculation of total lung deposition of pulmonary inhaled formulations (Nahar K. et al., 2013, Snell N. and Ganderton D., 1999).
Magnetic resonance imaging (MRI) does not require the radiolabeling of an aerosol to determine qualitative and quantitative deposition of particles in the lungs, but rather is based on the Nuclear Magnetic Resonance phenomenon. This method utilizes non-ionizable radiation to generate images that can be augmented through the use of soft tissue contrast mechanisms and orientations of 2D and 3D images (Nahar K. et al., 2013). The interaction of nuclear magnetic moments of the experimental sample tissue with an external magnetic and electromagnetic fields form spin density, T1 and T2 relaxation times, and motion which ranges from diffusion to rapid produce the signals the MRI equipment utilized to form images. T1 and T2 depend on the local nuclei environment which is subject to disease state of the tissues, where T1 is the longitudinal relaxation time constant and T2 is the transverse relaxation time constant (Nahar K. et al., 2013, Thompson R.B. and Finlay W.H., 2012). Signals measured from the nuclei are either full or half integer values of spin in proportion to the angular momentum that results from an odd number of protons or neutrons. The majority of MRIs measure the signal from the ubiquitous hydrogen (1H) atom, however lung imaging performed using helium-based (3He) methods is now well established because the non-lipid soluble properties of helium allow it to stay within the airspace (Komlosi P. et al., 2015, Kruger S.J. et al., 2014, Nahar K. et al., 2013). Hyperpolarized 3He or 129Xe gas MRI produce more polarization, which leads to better imaging (Mosbah K. et al., 2008, Taratula O. and Dmochowski I.J., 2010, Thompson R.B. and Finlay W.H., 2012). MRI has been employed within in vivo animal studies and within in vitro human lung airway replicas (Martin A.R. et al., 2008, Sood B.G. et al., 2008, Sood B.G. et al., 2010, Thompson R.B. and Finlay W.H., 2012, Wang H. et al., 2015).
Fluorescence imaging tracks fluorescently tagged particles within an aerosol to monitor their deposition in the lungs (Nahar K. et al., 2013, Ntziachristos V. et al., 2008). This method is limited due to the issues of the excitation and emission fluorescence signals are scattered, reflected, and absorbed by the tissues (Nahar K. et al., 2013, Yi D. et al., 2010). This affects the light captured by the detector and the actual number of molecules. In addition, a heterogeneous background signal exists because biological tissue has the capability to auto-fluoresce (Yi D. et al., 2010). The autofluorescence background can be measured using untreated tissue and subtracted from the experimental background or the use of near-infrared wavelengths can be used to overcome this issue (Adams K.E. et al., 2007, Comsa D.C. et al., 2008, Kovar J.L. et al., 2007, Murata M. et al., 2014). Fluorescent dyes are aerosolized, delivered to the lungs via inhalation, intracheal, or intranasal routes, and imaged using an in vivo imaging system (IVIS). The particle deposition in the lobes of the lungs can be visualized and quantified using image analyzing software (Nahar K. et al., 2013). Fluorescence imaging eliminates the need for radioactive tags, therefore it may be used to image deposition patterns in live animals (Ntziachristos V. et al., 2008).
PK studies investigate ADME of drugs by collecting lung deposition and plasma concentration data from either animal or human test subjects to determine the PK parameters. These parameters include drug absorption parameters such as the peak drug concentration (Cmax), the time Cmax occurs (tmax), and the absorption rate constant (ka) to determine the absorption rate and the area under curve (AUC) to estimate bioavailability. Also, drug distribution and elimination parameters such as clearance (CL), volume of distribution (Vd), and half-life of the drug (t1/2) can be determined. Mice and rat animal models are commonly used in animal PK studies because of their development in modeling various respiratory diseases, smaller drug requirement, and low cost (Cryan S.-A. et al., 2007, Sakagami M., 2006). The drug can be administered through intranasal or intratracheal routes or by using passive inhalation (Cryan S.-A. et al., 2007, Sakagami M., 2006, Zhang J. et al., 2011). Intratracheal or intranasal direct administration avoids oropharyngeal deposition, enabling accurate and reproducible dosing (Chiang P.-C. et al., 2009).
4. siRNA Delivery to the Lungs
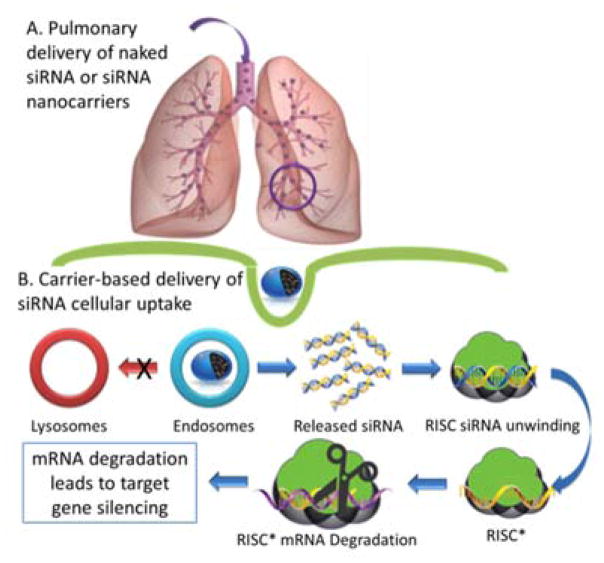
For clinical use, siRNA needs to be delivered to the target region of the lung and be released within the cytoplasm after entering the target cells as shown in Figure 6. Successful gene therapy requires the effective levels of transgene expression in specific cell types to enhance the treatment efficacy and to avoid adverse side effects caused by the expression in inappropriate cell types.
Figure 6.
Aerosol siRNA delivery to the lungs. (A) pulmonary delivery of naked or siRNA nanocarriers, (B) Carrier-based delivery of siRNA cellular uptake. The aerosolized formulation of siRNA and use of appropriate device delivers siRNA by inhalation, intratracheal, or intranasal delivery to the human or animal lung. Once the siRNA carriers reach the lower respiratory tract, they may reach target cells and become internalized through endocytosis. Carriers capable of endosomal escape avoid degradation in the lysosome and release free siRNA within the cytoplasm. The RNAi pathway may then be initiated, ultimately leading to mRNA degradation and gene silencing.
siRNA is a highly negatively charged, hydrophilic, and large sized (approximately 13.3 kDa) macromolecule that cannot cross biological membranes to reach their target sites. Viral vectors have previously demonstrated cell uptake and siRNA efficacy, however, major limitations to human therapeutic delivery exist, such as uncontrolled viral replication, immunogenicity, tumorigenicity, and toxicity (Thomas C.E. et al., 2003). Due to these concerns, non-viral delivery systems have been developed and successfully used to deliver siRNA. An ideal siRNA delivery system should (1) condense siRNA into nanosized particles, (2) protect siRNA from enzymatic degradation, (3) facilitate cellular uptake, (4) promote endosomal escape to release siRNA to the cytoplasm where the RISC is located, (5) have negligible effects on gene silencing activity or specificity and (6) have negligible toxicity (Lam J.K.-W. et al., 2012, Merkel O.M. et al., 2012). Non-viral delivery systems include naked siRNA delivery, and delivery vectors such as lipids, polymers, peptides, and inorganic materials.
4.1 Barriers of Pulmonary Delivery of siRNA
Delivering siRNA efficiently to the lungs via the pulmonary route requires understanding of the anatomical and physiological characteristics of the respiratory tract. The human respiratory tract has a highly efficient gas exchange capacity and to keep particulates out (Table 4) as discussed previously (Merkel O.M. et al., 2014). Therefore, the development of siRNA aerosol delivery systems should consider careful control of particle or droplet size to bypass the lungs natural defense mechanisms.
Table 4.
Pulmonary barriers to lung siRNA delivery (Merkel O.M. et al., 2014).
| Airway Defenses | Alveoli Defenses |
|---|---|
| Upper airway (nose, throat, and trachea) | Degradative Enzymes e.g. proteases, RNase |
| Bifurcations | Neutrophils |
| Mucus | Opsonins |
| Mucociliary Clearance | Surfactant |
| Cough Clearance | Complement |
| Airway epithelium | Alveolar Macrophages |
The respiratory tract is divided into two regions, (1) the conducting airways and (2) the respiratory region. The conducting airways encompass the nasal cavity, pharynx, trachea, bronchi, and bronchioles and the respiratory region consists of the respiratory bronchioles and alveoli (Lam J.K.-W. et al., 2012). The human respiratory tract has evolved to be a size selective fractionator that prevents particulates from reaching the deep lung. For example, the largest particles are removed at the naso- or oropharynx and progressively finer particles deposit in the proximal, intermediate, and distal airways. The main feature of the respiratory tract is the high degree of bifurcations that have been described according to Weibel’s lung model to have 24 generation in total. These gradually narrowing airways from the carina to the alveolar sacs cause increased particle velocity, which causes particles to impact the walls of airways instead of reaching the lower regions of the lungs. Therefore, for a therapeutic agent to reach the target site of action, it must possess aerodynamic properties to allow it to pass through the branched airways to the deep lung.
Aerodynamic particle size distribution is an important influence on the location of lung deposition of a formulation (Bisgaard H. et al., 2001). Three forces dominate particle motion within an aerosol cloud: gravitational sedimentation, inertial impaction, and Brownian diffusion. Gravitational sedimentation and inertial impaction are dominant when the aerodynamic diameter is > 2 μm. Large particles with aerodynamic particle size of > 6 μm deposit mostly on the airway walls at bifurcations in the upper airways due to their high momentum. Smaller particles of aerodynamic diameters of < 1 μm deposit due to Brownian diffusion (Morén F., 1993). If submicron sized particles are delivered without a larger carrier particle or droplet, then they would be mostly exhaled in the breath. A breath hold method may be used to increase deposition within the lower respiratory tract, however, it has lower efficiency. The most optimum aerodynamic particle sizes for deposition within the lower airways are between 1 to 5 μm (Agu R.U. et al., 2001, Sakagami M., 2006). As particle size decreases below 1 μm, lung deposition increases due to increased diffusional mobility (Yang W. et al., 2008). For particles less than 100 nm in size, approximately 50% of the nanoparticles will deposit within the alveolar region of the lungs. These nanoparticles often enter the lungs as large agglomerates that break apart upon deposition.
The elimination pathways for nanoparticles within the lungs involve coughing, dissolution, clearance via the mucociliary escalator, translocation from the airways, phagocytosis by macrophages and neuronal uptake (Roy I. and Vij N., 2010, Sanders N. et al., 2009, Zhang J. et al., 2011). Nanoparticle degradation is dependent on the composition of the delivery system and mediated through hydrolysis, oxidation, and reduction pathways (Roy I. and Vij N., 2010, Rytting E. et al., 2008). After deposition in the lower airways following inhalation, free siRNA will be susceptible to degradation from RNase, whose enzymatic activity is very high in the lungs (Amatngalim G.D. et al., 2015, Morita T. et al., 1986, Sorrentino S., 1998).
A major pulmonary delivery barrier is the mucociliary clearance driven by the physical action of the ciliated epithelial cells, which move the mucus and alveolar fluid towards the upper airways, and eventually, the throat. Mucociliary clearance also includes the alveolar macrophages along the airways, which phagocytose foreign particles and deposits their remains to be removed by the mucociliary escalator. Mucus is present on the respiratory epithelium from the nasal cavity to the terminal bronchioles (Knowles M.R. and Boucher R.C., 2002). Types of glycosylated protein, known as mucins, are the main component of mucus (Rose M.C. et al., 2001). Mucus is a physical barrier as it increases in viscosity of the moist surface of the lung epithelial cells, thereby reducing drug penetration and diffusion rate (Hill D.B. et al., 2014, Mellnik J. et al., 2014). Particles that are deposited on the mucosal layer above the ciliated epithelial cells are rapidly removed by mucociliary clearance and are eventually either coughed out or swallowed into the gastrointestinal tract (Knowles M.R. and Boucher R.C., 2002, Rubin B.K., 2002). The alveolar fluid is found on the surface of alveoli epithelium as a thin layer of pulmonary surfactant which is composed of phospholipids and surfactant-associated proteins known as SP-A, SP-B, SP-C and SP-D (Bastacky J. et al., 1995, Possmayer F. et al., 2001). Following delivery of foreign antigen or nanocarrier, pulmonary surfactant proteins SP-A and SP-D have been shown to play a role in macrophage uptake by acting as opsonins and scavenger molecules (Kishore U. et al., 2006). These pulmonary surfactants are located at the air-liquid interface of the alveoli, therefore they can interact with particulate aerosols that reach the lower airways (Seaton B.A. et al., 2010). Other major opsonins of the airways and alveoli of the lungs include immunoglobulins (IgG or IgM), complement fragments (C3, C4, C5), fibronectin, and laminin (Kuroki Y. et al., 2007, Patel B. et al., 2015). The alveolar macrophages located within the alveoli rapidly engulf foreign particles by phagocytosis as a defense mechanism (Patton J.S., 1996, Weinberger B. et al., 2014, Zhao F. et al., 2011).
In addition to macrophages, the other major phagocytes are the neutrophils, or polymorphonuclear neutrophilic leukocytes (PMNs or polys), that are abundant in the blood but are not present in normal healthy tissue (Kolaczkowska E. and Kubes P., 2013). Like macrophages, neutrophils are phagocytic cells that have a role in the innate immunity because they recognize, ingest, and destroy many pathogens without the adaptive immune response (Kolaczkowska E. and Kubes P., 2013). When macrophages first encounter a pathogen within the lung, their action is augmented by the recruitment of large number of neutrophils to the infection site (Burns A.R. et al., 2003, Kolaczkowska E. and Kubes P., 2013). Pulmonary administered naked siRNA is susceptible to alveolar macrophage or neutrophil uptake and are subsequently degraded within these cells, thus reducing their therapeutic efficacy.
Considering disease state is an important aspect of pulmonary clearance since the physiological conditions of the airways are altered and pose a threat on the efficiency of an aerosolized siRNA delivery system. During infection and inflammation, the airways become congested because there is increased mucus secretion and decreased mucociliary clearance (Groneberg D. et al., 2002, Patton J.S. et al., 2010). The thickness, viscosity, viscoelasticity, and the composition of the mucus layer depend on the pathological condition and have inter-patient variability (Sanders N. et al., 2009). For different therapeutic applications, disease state of the patient should be considered in order to design an effective aerosolized delivery system. In addition to using particles with small aerodynamic diameter suitable for deposition in the lower airways, it has been reported that the use of large porous particles can prolong retention time in the lungs by effectively avoiding alveolar macrophage phagocytosis (Arnold M.M. et al., 2007, Ben-Jebria A. et al., 2000, Edwards D.A. et al., 1997). Porous particles over 10 μm in geometric diameter have a smaller aerodynamic diameter due to their mass-inertia relationship. Their smaller aerodynamic diameters of 1–3 μm fall within the ideal aerodynamic size range for effective lung deposition, while their actual geometric size is larger than the size range of alveolar macrophage uptake and removal.
The mucus layer may be partially deteriorated using mucolytic agents that break down the three dimensional gel network of mucus. A mucus inhibitor, such as glucopyrrolate, may also be used to overcome the mucus barrier (Ferrari S. et al., 2001). In patients with bronchiectasis or cystic fibrosis, mannitol has been clinically proven to improve the hydration and viscoelastic surface properties of the sputum, which increased mucus clearance (Daviskas E. et al., 2010). The deposition site of nucleic acid delivery systems in the airways has been previously manipulated and directed through the use of ultrasound and magnetic field technologies (Dames P. et al., 2007, Xenariou S. et al., 2006, Xenariou S. et al., 2007). Another less-invasive option to enhance the transport across the mucus layer is to coat the surface of nanoparticles with neutrally charged molecules, like polyethylene glycol (PEG) (Schuster B.S. et al., 2013).
Lung disease involving chronic lung inflammation, like cystic fibrosis, emphysema, or asthma, are characterized by increased levels of pulmonary proteases (Greene C.M. and Mcelvaney N.G., 2009). These proteases may degrade peptide conjugated siRNA or polymeric nanocarriers of siRNA before the carrier gets to target specific cells of interest.
4.2 Barriers of Intracellular siRNA Delivery
To exert their effects, the siRNA must cross the cellular membrane and be released within the cytoplasm of the target cells of the respiratory tract, and it must access the RISC, where their intracellular interaction takes place. Due to the size of 13 kDa and a negative charge, the siRNA do not readily cross biological membranes. Therefore, an appropriate delivery system should facilitate siRNA cellular uptake.
The major cellular uptake pathway for non-viral siRNA delivery systems that are under 150 nm in size is known as endocytosis (Khalil I.A. et al., 2006). Endocytosis is the mechanism by which cells engulf polar molecules or nanocarriers that cannot readily pass through the hydrophobic cellular membrane. The four endocytosis pathways are clathrin-mediated endocytosis, caveolae-mediated endocytosis, micropinocytosis, and phagocytosis (Marsh M., 2001). For the inhalation route of administration, a particle size of less than 150 nm also avoids macrophage uptake, thus slowing lung clearance (Lebhardt T. et al., 2010). Clathrin-mediated endocytosis is the major pathway that allows for particle uptake through enclosed clathrin-coated vesicles (Khalil I.A. et al., 2006). These vesicles then fuse with early endosomes to form late endosomes, which subsequently form into lysosomes. As this process progresses from early endosome to lysosome, the pH within the vesicles drop down to approximately pH 5.0 and degradative DNAse and RNase are present within the lysosome (Bally M.B. et al., 1999, Khalil I.A. et al., 2006, Maxfield F.R. and Mcgraw T.E., 2004). For a siRNA to elicit therapeutic effects within the cell, it must escape from the endosome and be released within the cytoplasm to avoid degradation by degradative enzymes. To promote endosomal escape, the “proton sponge hypothesis” can be practiced by using a high buffering capacity polymer with a large pH range, such as polyethylenimine (PEI), which will become protonated as the pH of the endosome drops (Boussif O. et al., 1995, Gunther M. et al., 2011). This protonation of the polymer causes an influx of chloride ions, protons and water into the endosomes. The building osmotic pressure causes the endosomes to burst, thus releasing its contents into the cytoplasm (Gunther M. et al., 2011). However, the use of PEI has been associated with toxicity issues (Seow W.Y. et al., 2013). An alternative strategy to promote endosomal escape is through the use of pH sensitive fusogenic peptides. These peptides undergo pH-dependent conformation changes at low pH which cause a membrane disrupting conformational change that destabilizes endosomal membranes (Choi S.W. et al., 2010, Kusumoto K. et al., 2013, Lee S.H. et al., 2007, Oliveira S. et al., 2007).
Another cellular uptake mechanism of siRNA delivery systems is the caveolae-mediated endocytosis pathway (Khalil I.A. et al., 2006). The delivery system is internalized through a caveolin-coated vesicle known as a caveosome. The caveosomes are void of nucleases, degradative enzymes, and have a non-acidic pH. The delivery systems avoid lysosomal degradation because the contents of the caveosomes are transported to the Golgi or endoplasmic reticulum. Caveolin is expressed within lung tissues, therefore it may be a more efficient route for inhalation siRNA delivery systems compared to clathrin-mediated endocytosis, especially if the delivery system doesn’t possess endosomal escape properties (Lam J.K.-W. et al., 2012, Parton R.G. and Richards A.A., 2003, Yacobi N.R. et al., 2010).
Another cellular uptake mechanism of cells of the lungs is phagocytosis (Khalil I.A. et al., 2006). This route can only be performed by alveolar macrophages and other specialized cells. Unless the target cell type is alveolar macrophages, then uptake of delivery systems by this pathway should be avoided since siRNA molecules taken up are eventually degraded by the phagolysosomes of the cells.
5. Conclusion
Lower airway pulmonary delivery can be achieved through the inhalation of an aerosol from delivery devices such as pMDIs, DPIs, nebulizers, and SMIs. Preclinical safety and efficacy studies using animal models often deliver pulmonary aerosols via the inhalation, intratracheal, and intranasal routes. The lung physiological and anatomical differences between human and animals are an integral consideration when selecting the route of administration to assess the efficacy of an aerosol. Intratracheal administration involves an invasive surgical setup and anesthesia. The intranasal route of administration can be used to reach the airways in mouse models by instilling and aspirating the liquid solution or suspension into the nasal cavity of the animal. Dry powder formulations can be delivered using a microsprayer that when actuated, generates the aerosol and propels it into the airways. The nose-only exposure chamber used in preclinical studies has provided a robust means of delivering aerosols to the deep lung. Aerosolized siRNA formulations are characterized using in vitro, ex vivo, and in vivo techniques. In vitro techniques measure the dissolution rate, aerodynamic particle size distribution, total delivery dose uniformity, particle size, and aerosol velocity. Ex vivo techniques analyze the mechanisms of drug transport, deposition, and absorption. In vivo techniques provide visualization and quantification of aerosol deposition in the respiratory tract, PK parameters, and biochemical and physiological effects of the pulmonary delivered drug. Barriers for pulmonary delivery of siRNA exist due to the natural defenses of the lungs to keep exogenous particulates and substances out. Delivering aerosolized siRNA effectively to the lungs and to their target site-of-action involves the understanding of the anatomical and physiological characteristics of the respiratory tract. The barriers for aerosolized delivery of siRNA include degradation by RNase and mucociliary clearance. Intracellular naked siRNA delivery is impeded due to their large size, negative charge, and susceptibility to degradation. These intracellular delivery constraints may be mitigated through the use of siRNA nanocarrier systems for aerosol delivery including those of lipidic, polymeric, peptide, or inorganic origin. Part II of this review article will discuss the preparation methods of various siRNA nanocarrier systems, accompanied by a series of examples.
Acknowledgments
The authors acknowledge the support of the National Institute of General Medical Science of the National Institutes of Health under award number SC3GM109873. The authors acknowledge Hawai’i Community Foundation, Honolulu, HI 96813, USA, for research support on lung cancer, mesothelioma, and asthma projects (Leahi Fund) in 2015, 2013, and 2011, respectively. The authors would like to acknowledge the 2013 George F. Straub Trust and Robert C. Perry Fund of the Hawai’i Community Foundation, Honolulu, HI 96813, USA, for research support on lung cancer. The authors also acknowledge a seed grant from the Research Corporation of the University of Hawai’i at Hilo, Hilo, HI 96720, USA, and The Daniel K. Inouye College of Pharmacy, University of Hawai’i at Hilo, Hilo, HI 96720, USA, for providing start-up financial support to their research group. The authors acknowledge the donation from Dr. Robert S. Shapiro, MD, Dermatologist, Hilo, HI, USA in support of development of nanotechnology based medicines.
Biographies

Susanne R. Youngren-Ortiz is a Ph.D. candidate the Daniel K. Inouye College of Pharmacy of the University of Hawai’i at Hilo. She obtained her Bachelor of Science Degree in Pharmaceutical Sciences with specialization in Industrial and Physical Pharmacy and minors in Chemistry and Statistics from Purdue University. Her dissertation research project investigates the optimal delivery and the evaluation of cellular effects of therapeutic nanocarriers for the treatment of asthma. She has authored original research and review articles. She is an active member of the American Association of Pharmaceutical Scientists and has chaired the University of Hawaii Student Chapter.

Nishant S. Gandhi is a Ph.D. candidate at the Daniel K. Inouye College of Pharmacy UH Hilo. He has completed his B.Pharm and M.Pharm from University of Pune in India. He has also worked as a chemist at Aristo Pharmaceuticals in Bhopal, India. He is currently working on developing novel nanotechnology based drug delivery systems for the treatment of Lung Cancer. He has authored publications including original research articles, review articles and book chapters. He is currently serving as chair of American Association of Pharmaceutical Scientists (DKICP chapter) and is currently a member of professional pharmacy fraternity “Phi Delta Chi”.

Dr. Laura España-Serrano is a Postdoctoral researcher in the Translational Drug Delivery Research laboratory in the department of Pharmaceutical Sciences of the Daniel K. Inouye College of Pharmacy at the UH Hilo. She obtained her PhD in Biochemistry and Molecular Biology from the Complutense University of Madrid, Spain. She has nine years of experience in the fields of Cellular and Molecular Biology of Cancer and Psychiatric diseases. She has authored original research publications and review articles, and she is a member of the National Postdoctoral Association and the American Association of Pharmaceutical Scientists.

Mahavir B. Chougule, Associate Professor of the Department of Pharmaceutical Sciences, investigates the use of therapeutic agent, siRNA, and targeted nanotechnology based formulations for treatment of lung cancer and asthma. Chougule has 25 publications, 8 patents, 3 review articles, 5 book chapters. He delivered 16 invited talks and 63 scientific presentations at the international conferences. He served as a grant reviewer on the NIH, DOD, and international review panels. He is also serving as a member of editorial board on 5 international journals. Dr. Chougule is a recipient of several awards including recent American Association of Cancer Research Minority-Serving Institution Faculty Scholar in Cancer Research Award. He is an active member of American Association of Cancer Research and American Association of Pharmaceutical Scientists.
References
- Adamcakova-Dodd A, Stebounova LV, Kim JS, Vorrink SU, Ault AP, O’shaughnessy PT, Grassian VH, Thorne PS. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:b1. doi: 10.1186/1743-8977-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams KE, Ke S, Kwon S, Liang F, Fan Z, Lu Y, Hirschi K, Mawad ME, Barry MA, Sevick-Muraca EM. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. Journal of biomedical optics. 2007;12:024017-024017-024019. doi: 10.1117/1.2717137. [DOI] [PubMed] [Google Scholar]
- Agrawal N, Dasaradhi P, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK. Rna interference: Biology, mechanism, and applications. Microbiology and molecular biology reviews. 2003;67:657–685. doi: 10.1128/MMBR.67.4.657-685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agu RU, Ugwoke MI, Armand M, Kinget R, Verbeke N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respiratory research. 2001;2:198. doi: 10.1186/rr58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarzguioui M, Rossi JJ, Kim D. Approaches for chemically synthesized sirna and vector-mediated rnai. FEBS Letters. 2005;579:5974–5981. doi: 10.1016/j.febslet.2005.08.070. [DOI] [PubMed] [Google Scholar]
- Amatngalim GD, Van Wijck Y, De Mooij-Eijk Y, Verhoosel RM, Harder J, Lekkerkerker AN, Janssen RA, Hiemstra PS. Basal cells contribute to innate immunity of the airway epithelium through production of the antimicrobial protein rnase 7. J Immunol. 2015;194:3340–3350. doi: 10.4049/jimmunol.1402169. [DOI] [PubMed] [Google Scholar]
- Ari A. Jet, ultrasonic, and mesh nebulizers: An evaluation of nebulizers for better clinical outcomes. 2014. [Google Scholar]
- Ari A, Hess D, Myers T, Rau J. A guide to aerosol delivery devices for respiratory therapists. Dallas, Texas: American Association for Respiratory Care; 2009. [Google Scholar]
- Ari A, Restrepo RD. Aerosol delivery device selection for spontaneously breathing patients: 2012. Respiratory care. 2012;57:613–626. doi: 10.4187/respcare.01756. [DOI] [PubMed] [Google Scholar]
- Arnold MM, Gorman EM, Schieber LJ, Munson EJ, Berkland C. Nanocipro encapsulation in monodisperse large porous plga microparticles. Journal of Controlled Release. 2007;121:100–109. doi: 10.1016/j.jconrel.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunthari V, Bruinsma RS, Lee AS, Johnson MM. A prospective, comparative trial of standard and breath-actuated nebulizer: Efficacy, safety, and satisfaction. Respiratory care. 2012;57:1242–1247. doi: 10.4187/respcare.01450. [DOI] [PubMed] [Google Scholar]
- Bai S, Gupta V, Ahsan F. Inhalable lactose-based dry powder formulations of low molecular weight heparin. Journal of aerosol medicine and pulmonary drug delivery. 2010;23:97–104. doi: 10.1089/jamp.2009.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally MB, Harvie P, Wong FM, Kong S, Wasan EK, Reimer DL. Biological barriers to cellular delivery of lipid-based DNA carriers. Advanced drug delivery reviews. 1999;38:291–315. doi: 10.1016/s0169-409x(99)00034-4. [DOI] [PubMed] [Google Scholar]
- Bastacky J, Lee C, Goerke J, Koushafar H, Yager D, Kenaga L, Speed TP, Chen Y, Clements JA. Alveolar lining layer is thin and continuous: Low-temperature scanning electron microscopy of rat lung. Journal of Applied Physiology. 1995;79:1615–1628. doi: 10.1152/jappl.1995.79.5.1615. [DOI] [PubMed] [Google Scholar]
- Ben-Jebria A, Eskew ML, Edwards DA. Inhalation system for pulmonary aerosol drug delivery in rodents using large porous particles. Aerosol Science & Technology. 2000;32:421–433. [Google Scholar]
- Bisgaard H, O’callaghan C, Smaldone GC. Drug delivery to the lung. CRC Press; 2001. [Google Scholar]
- Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered sirna. Nature medicine. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- Bivas-Benita M, Zwier R, Junginger HE, Borchard G. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. European Journal of Pharmaceutics and Biopharmaceutics. 2005;61:214–218. doi: 10.1016/j.ejpb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Bondesson E, Bengtsson T, Borgström L, Nilsson LE, Norrgren K, Trofast E, Wollmer P. Planar gamma scintigraphy—points to consider when quantifying pulmonary dry powder aerosol deposition. International journal of pharmaceutics. 2003;258:227–240. doi: 10.1016/s0378-5173(03)00148-0. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proceedings of the National Academy of Sciences. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JC, Rossi JJ. Rna-based therapeutics: Current progress and future prospects. Chemistry & biology. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. 2003. [DOI] [PubMed] [Google Scholar]
- Carvalho TC, Peters JI, Williams RO., III Influence of particle size on regional lung deposition – what evidence is there? International Journal of Pharmaceutics. 2011;406:1–10. doi: 10.1016/j.ijpharm.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Chakravarty R, Hong H, Cai W. Positron emission tomography image-guided drug delivery: Current status and future perspectives. Molecular pharmaceutics. 2014;11:3777–3797. doi: 10.1021/mp500173s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HK. Formulation challenges: Protein powders for inhalation. DRUGS AND THE PHARMACEUTICAL SCIENCES. 2003;126:879–890. [Google Scholar]
- Chan H-K, Eberl S, Glover W. Encyclopedia of Pharmaceutical Technology. 3. Informa Healthcare; New York: 2007. Radiolabeling of pharmaceutical aerosols and gamma scintigraphic imaging for lung deposition; pp. 3094–3107. [Google Scholar]
- Chapters UG. Usp< 601> aerosols, nasal sprays, metered-dose inhalers and dry powder inhalers. USP 32-NF. 2009 [Google Scholar]
- Cherry SR, Gambhir SS. Use of positron emission tomography in animal research. ILAR journal. 2001;42:219–232. doi: 10.1093/ilar.42.3.219. [DOI] [PubMed] [Google Scholar]
- Chiang PC, Alsup JW, Lai Y, Hu Y, Heyde BR, Tung D. Evaluation of aerosol delivery of nanosuspension for pre-clinical pulmonary drug delivery. Nanoscale research letters. 2009;4:254–261. doi: 10.1007/s11671-008-9234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Lee SH, Mok H, Park TG. Multifunctional sirna delivery system: Polyelectrolyte complex micelles of six-arm peg conjugate of sirna and cell penetrating peptide with crosslinked fusogenic peptide. Biotechnology progress. 2010;26:57–63. doi: 10.1002/btpr.310. [DOI] [PubMed] [Google Scholar]
- Chougule M, Padhi B, Misra A. Development of spray dried liposomal dry powder inhaler of dapsone. AAPS PharmSciTech. 2008;9:47–53. doi: 10.1208/s12249-007-9024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codrons V, Vanderbist F, Verbeeck RK, Arras M, Lison D, Préat V, Vanbever R. Systemic delivery of parathyroid hormone (1–34) using inhalation dry powders in rats. Journal of pharmaceutical sciences. 2003;92:938–950. doi: 10.1002/jps.10346. [DOI] [PubMed] [Google Scholar]
- Comsa DC, Farrell TJ, Patterson MS. Quantitative fluorescence imaging of point-like sources in small animals. Physics in medicine and biology. 2008;53:5797. doi: 10.1088/0031-9155/53/20/016. [DOI] [PubMed] [Google Scholar]
- Conde J, Tian F, Hernandez Y, Bao C, Cui D, Janssen KP, Ibarra MR, Baptista PV, Stoeger T, De La Fuente JM. In vivo tumor targeting via nanoparticle-mediated therapeutic sirna coupled to inflammatory response in lung cancer mouse models. Biomaterials. 2013;34:7744–7753. doi: 10.1016/j.biomaterials.2013.06.041. [DOI] [PubMed] [Google Scholar]
- Conway J. Lung imaging—two dimensional gamma scintigraphy, spect, ct and pet. Advanced drug delivery reviews. 2012;64:357–368. doi: 10.1016/j.addr.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Cryan SA, Sivadas N, Garcia-Contreras L. In vivo animal models for drug delivery across the lung mucosal barrier. Advanced drug delivery reviews. 2007;59:1133–1151. doi: 10.1016/j.addr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Dailey LA, Schmehl T, Gessler T, Wittmar M, Grimminger F, Seeger W, Kissel T. Nebulization of biodegradable nanoparticles: Impact of nebulizer technology and nanoparticle characteristics on aerosol features. Journal of Controlled Release. 2003;86:131–144. doi: 10.1016/s0168-3659(02)00370-x. [DOI] [PubMed] [Google Scholar]
- Dalby R, Spallek M, Voshaar T. A review of the development of respimat® soft mist™ inhaler. International journal of pharmaceutics. 2004;283:1–9. doi: 10.1016/j.ijpharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Dames P, Gleich B, Flemmer A, Hajek K, Seidl N, Wiekhorst F, Eberbeck D, Bittmann I, Bergemann C, Weyh T, Trahms L, Rosenecker J, Rudolph C. Targeted delivery of magnetic aerosol droplets to the lung. Nat Nanotechnol. 2007;2:495–499. doi: 10.1038/nnano.2007.217. [DOI] [PubMed] [Google Scholar]
- Davies NM, Feddah MR. A novel method for assessing dissolution of aerosol inhaler products. International journal of pharmaceutics. 2003;255:175–187. doi: 10.1016/s0378-5173(03)00091-7. [DOI] [PubMed] [Google Scholar]
- Daviskas E, Anderson SD, Jaques A, Charlton B. Inhaled mannitol improves the hydration and surface properties of sputum in patients with cystic fibrosis. CHEST Journal. 2010;137:861–868. doi: 10.1378/chest.09-2017. [DOI] [PubMed] [Google Scholar]
- De Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Characterization of inhalation aerosols: A critical evaluation of cascade impactor analysis and laser diffraction technique. Int J Pharm. 2002;249:219–231. doi: 10.1016/s0378-5173(02)00526-4. [DOI] [PubMed] [Google Scholar]
- De Fougerolles A, Novobrantseva T. Sirna and the lung: Research tool or therapeutic drug? Current opinion in pharmacology. 2008;8:280–285. doi: 10.1016/j.coph.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: A progress report on sirna-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer J, Dyche T. The adaptive aerosol delivery (aad) technology: Past, present, and future. Journal of aerosol medicine and pulmonary drug delivery. 2010;23:S-1–S-10. doi: 10.1089/jamp.2009.0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer J, Nikander K, Smith N. Adaptive aerosol delivery (aad®) technology. Expert opinion on drug delivery. 2004;1:165–176. doi: 10.1517/17425247.1.1.165. [DOI] [PubMed] [Google Scholar]
- Devincenzo J, Cehelsky JE, Alvarez R, Elbashir S, Harborth J, Toudjarska I, Nechev L, Murugaiah V, Van Vliet A, Vaishnaw AK. Evaluation of the safety, tolerability and pharmacokinetics of aln-rsv01, a novel rnai antiviral therapeutic directed against respiratory syncytial virus (rsv) Antiviral research. 2008;77:225–231. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Devincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, Meyers R, Gollob J, Vaishnaw A. A randomized, double-blind, placebo-controlled study of an rnai-based therapy directed against respiratory syncytial virus. Proceedings of the National Academy of Sciences. 2010;107:8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respiratory care. 2002;47:1406–1416. discussion 1416–1408. [PubMed] [Google Scholar]
- Dickinson PA, Howells SW, Kellaway IW. Novel nanoparticles for pulmonary drug administration. Journal of drug targeting. 2001;9:295–302. doi: 10.3109/10611860108997937. [DOI] [PubMed] [Google Scholar]
- Dolovich MB. 18f-fluorodeoxyglucose positron emission tomographic imaging of pulmonary functions, pathology, and drug delivery. Proceedings of the American Thoracic Society. 2009;6:477–485. doi: 10.1513/pats.200904-023AW. [DOI] [PubMed] [Google Scholar]
- Dolovich MB, Dhand R. Aerosol drug delivery: Developments in device design and clinical use. The Lancet. 2011;377:1032–1045. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, Schlesinger RB. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicological Sciences. 2000;55:24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- Dunbar C, Mitchell J. Analysis of cascade impactor mass distributions. Journal of Aerosol Medicine-Deposition Clearance and Effects in the Lung. 2005;18:439–451. doi: 10.1089/jam.2005.18.439. [DOI] [PubMed] [Google Scholar]
- Eberl S, Chan HK, Daviskas E. Spect imaging for radioaerosol deposition and clearance studies. Journal of aerosol medicine. 2006;19:8–20. doi: 10.1089/jam.2006.19.8. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, Mintzes J, Deaver D, Lotan N, Langer R. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–1872. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- Elhissi A, Karnam KK, Danesh-Azari MR, Gill HS, Taylor KM. Formulations generated from ethanol-based proliposomes for delivery via medical nebulizers. Journal of pharmacy and pharmacology. 2006;58:887–894. doi: 10.1211/jpp.58.7.0002. [DOI] [PubMed] [Google Scholar]
- Elhissi A, Taylor K. Delivery of liposomes generated from proliposomes using air-jet, ultrasonic, and vibrating-mesh nebulisers. Journal of drug delivery science and technology. 2005;15:261–265. [Google Scholar]
- Eshel G, Levy G, Mingelgrin U, Singer M. Critical evaluation of the use of laser diffraction for particle-size distribution analysis. Soil Science Society of America Journal. 2004;68:736–743. [Google Scholar]
- Everard ML. Studies using radiolabelled aerosols in children. Thorax. 1994;49:1259–1266. doi: 10.1136/thx.49.12.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feddah MR, Brown KF, Gipps EM, Davies NM. In-vitro characterisation of metered dose inhaler versus dry powder inhaler glucocorticoid products: Influence of inspiratory flow rates. J Pharm Pharm Sci. 2000;3:318–324. [PubMed] [Google Scholar]
- Fellmann C, Lowe SW. Stable rna interference rules for silencing. Nature cell biology. 2014;16:10–18. doi: 10.1038/ncb2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes CA, Vanbever R. Preclinical models for pulmonary drug delivery. Expert opinion on drug delivery. 2009;6:1231–1245. doi: 10.1517/17425240903241788. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Kitson C, Farley R, Steel R, Marriott C, Parkins D, Scarpa M, Wainwright B, Evans M, Colledge W. Mucus altering agents as adjuncts for nonviral gene transfer to airway epithelium. Gene therapy. 2001;8:1380–1386. doi: 10.1038/sj.gt.3301525. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded rna in caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Fleming J, Conway J, Majoral C, Tossici-Bolt L, Katz I, Caillibotte G, Perchet D, Pichelin M, Muellinger B, Martonen T. The use of combined single photon emission computed tomography and x-ray computed tomography to assess the fate of inhaled aerosol. Journal of aerosol medicine and pulmonary drug delivery. 2011;24:49–60. doi: 10.1089/jamp.2010.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder T, Kestenholz P, Hunziker R, Nguyen TDL, Fries M, Veit-Haibach P, Husmann L, Stahel R, Weder W, Opitz I. Use of computed tomography and positron emission tomography/computed tomography for staging of local extent in patients with malignant pleural mesothelioma. Journal of computer assisted tomography. 2015;39:160–165. doi: 10.1097/RCT.0000000000000174. [DOI] [PubMed] [Google Scholar]
- Fulton A, Peters ST, Perkins GA, Jarosinski KW, Damiani A, Brosnahan M, Buckles EL, Osterrieder N, Van De Walle GR. Effective treatment of respiratory alphaherpesvirus infection using rna interference. PLoS One. 2009;4:e4118. doi: 10.1371/journal.pone.0004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzenko OB, Saad M, Betigeri S, Zhang M, Vetcher AA, Soldatenkov VA, Reimer DC, Pozharov VP, Minko T. Intratracheal versus intravenous liposomal delivery of sirna, antisense oligonucleotides and anticancer drug. Pharmaceutical Research. 2009;26:382–394. doi: 10.1007/s11095-008-9755-4. [DOI] [PubMed] [Google Scholar]
- Ghosn B, Singh A, Li M, Vlassov AV, Burnett C, Puri N, Roy K. Efficient gene silencing in lungs and liver using imidazole-modified chitosan as a nanocarrier for small interfering rna. Oligonucleotides. 2010;20:163–172. doi: 10.1089/oli.2010.0235. [DOI] [PubMed] [Google Scholar]
- Greene CM, Mcelvaney NG. Proteases and antiproteases in chronic neutrophilic lung disease–relevance to drug discovery. British journal of pharmacology. 2009;158:1048–1058. doi: 10.1111/j.1476-5381.2009.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg D, Eynott P, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, Mccann B, Nicholson A, Harrison B. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology. 2002;40:367–373. doi: 10.1046/j.1365-2559.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- Gunther M, Lipka J, Malek A, Gutsch D, Kreyling W, Aigner A. Polyethylenimines for rnai-mediated gene targeting in vivo and sirna delivery to the lung. Eur J Pharm Biopharm. 2011;77:438–449. doi: 10.1016/j.ejpb.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Gutbier B, Kube SM, Reppe K, Santel A, Lange C, Kaufmann J, Suttorp N, Witzenrath M. Rnai-mediated suppression of constitutive pulmonary gene expression by small interfering rna in mice. Pulmonary pharmacology & therapeutics. 2010;23:334–344. doi: 10.1016/j.pupt.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Hannon GJ. Rna interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JM. Randomized controlled trial of a breath-activated nebulizer in patients with exacerbation of copd. Respiratory care. 2012;57:1385–1390. doi: 10.4187/respcare.01609. [DOI] [PubMed] [Google Scholar]
- Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. Journal of Aerosol Science. 1986;17:811–825. [Google Scholar]
- Hibbitts A, O’mahony AM, Forde E, Nolan L, Ogier J, Desgranges S, Darcy R, Macloughlin R, O’driscoll CM, Cryan SA. Early-stage development of novel cyclodextrin-sirna nanocomplexes allows for successful postnebulization transfection of bronchial epithelial cells. J Aerosol Med Pulm Drug Deliv. 2014;27:466–477. doi: 10.1089/jamp.2013.1045. [DOI] [PubMed] [Google Scholar]
- Hickey AJ. Pharmaceutical inhalation aerosol technology. CRC Press; 2003. [Google Scholar]
- Hickey AJ, Garcia-Contreras L. Immunological and toxicological implications of short-term studies in animals of pharmaceutical aerosol delivery to the lungs: Relevance to humans. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2001;18 [PubMed] [Google Scholar]
- Hill DB, Vasquez PA, Mellnik J, Mckinley SA, Vose A, Mu F, Henderson AG, Donaldson SH, Alexis NE, Boucher RC. A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PloS one. 2014;9:e87681. doi: 10.1371/journal.pone.0087681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe M, Illum L. Intranasal insulin delivery and therapy. Adv Drug Deliv Rev. 1999;35:199–234. doi: 10.1016/s0169-409x(98)00073-8. [DOI] [PubMed] [Google Scholar]
- Ho SL, Kwong WJ, O’drowsky L, Coates AL. Evaluation of four breath-enhanced nebulizers for home use. Journal of aerosol medicine. 2001;14:467–475. doi: 10.1089/08942680152744677. [DOI] [PubMed] [Google Scholar]
- Hochrainer D, Hölz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of respimat® soft mist™ inhaler and pressurized metered dose inhalers. Journal of aerosol medicine. 2005;18:273–282. doi: 10.1089/jam.2005.18.273. [DOI] [PubMed] [Google Scholar]
- Hosoya K, Satoh T, Yamamoto Y, Saeki K, Igawa K, Okano M, Moriya T, Imamura O, Nemoto Y, Yokozeki H. Gene silencing of stat6 with sirna ameliorates contact hypersensitivity and allergic rhinitis. Allergy. 2011;66:124–131. doi: 10.1111/j.1398-9995.2010.02440.x. [DOI] [PubMed] [Google Scholar]
- Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, Kjems J. Rna interference in vitro and in vivo using a novel chitosan/sirna nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Huchon GJ, Montgomery AB, Lipavsky A, Hoeffel JM, Murray JF. Respiratory clearance of aerosolized radioactive solutes of varying molecular weight, Journal of nuclear medicine: official publication. Society of Nuclear Medicine. 1987;28:894–902. [PubMed] [Google Scholar]
- Kamei N, Takeda-Morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J Control Release. 2015;197:105–110. doi: 10.1016/j.jconrel.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for sirna therapeutics. Nature materials. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacological reviews. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- Kim DH, Rossi JJ. Strategies for silencing human disease using rna interference. Nature Reviews Genetics. 2007;8:173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins sp-a and sp-d: Structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. The Journal of clinical investigation. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Komlosi P, Altes TA, Qing K, Mooney KE, Miller GW, Mata JF, De Lange EE, Tobias WA, Cates GD, Brookeman JR. Regional anisotropy of airspace orientation in the lung as assessed with hyperpolarized helium-3 diffusion mri. Journal of Magnetic Resonance Imaging. 2015 doi: 10.1002/jmri.24950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koping-Hoggard M, Issa MM, Kohler T, Tronde A, Varum KM, Artursson P. A miniaturized nebulization catheter for improved gene delivery to the mouse lung. J Gene Med. 2005;7:1215–1222. doi: 10.1002/jgm.762. [DOI] [PubMed] [Google Scholar]
- Kovar JL, Simpson MA, Schutz-Geschwender A, Olive DM. A systematic approach to the development of fluorescent contrast agents for optical imaging of mouse cancer models. Analytical biochemistry. 2007;367:1–12. doi: 10.1016/j.ab.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Kruger SJ, Niles DJ, Dardzinski B, Harman A, Jarjour NN, Ruddy M, Nagle SK, Francois CJ, Sorkness RL, Burton RM. Hyperpolarized helium-3 mri of exercise-induced bronchoconstriction during challenge and therapy. Journal of Magnetic Resonance Imaging. 2014;39:1230–1237. doi: 10.1002/jmri.24272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cellular microbiology. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- Kusumoto K, Akita H, Ishitsuka T, Matsumoto Y, Nomoto T, Furukawa R, El-Sayed A, Hatakeyama H, Kajimoto K, Yamada Y, Kataoka K, Harashima H. Lipid envelope-type nanoparticle incorporating a multifunctional peptide for systemic sirna delivery to the pulmonary endothelium. ACS Nano. 2013;7:7534–7541. doi: 10.1021/nn401317t. [DOI] [PubMed] [Google Scholar]
- Lam JKW, Liang W, Chan HK. Pulmonary delivery of therapeutic sirna. Advanced drug delivery reviews. 2012;64:1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy and vaccination: An update. Translational Respiratory Medicine. 2014;2:1–12. doi: 10.1186/2213-0802-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebhardt T, Roesler S, Beck-Broichsitter M, Kissel T. Polymeric nanocarriers for drug delivery to the lung. Journal of Drug Delivery Science and Technology. 2010;20:171–180. [Google Scholar]
- Lee Black D, Mcquay MQ, Bonin MP. Laser-based techniques for particle-size measurement: A review of sizing methods and their industrial applications. Progress in Energy and Combustion Science. 1996;22:267–306. [Google Scholar]
- Lee SH, Kim SH, Park TG. Intracellular sirna delivery system using polyelectrolyte complex micelles prepared from vegf sirna-peg conjugate and cationic fusogenic peptide. Biochemical and biophysical research communications. 2007;357:511–516. doi: 10.1016/j.bbrc.2007.03.185. [DOI] [PubMed] [Google Scholar]
- Lentz YK, Anchordoquy TJ, Lengsfeld CS. Rationale for the selection of an aerosol delivery system for gene delivery. Journal of aerosol medicine. 2006;19:372–384. doi: 10.1089/jam.2006.19.372. [DOI] [PubMed] [Google Scholar]
- Li BJ, Tang Q, Cheng D, Qin C, Xie FY, Wei Q, Xu J, Liu Y, Zheng BJ, Woodle MC. Using sirna in prophylactic and therapeutic regimens against sars coronavirus in rhesus macaque. Nature medicine. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhai X, Ma C, Sun P, Fu Z, Liu W, Xu J. An inhalable beta(2)-adrenoceptor ligand-directed guanidinylated chitosan carrier for targeted delivery of sirna to lung. J Control Release. 2012;162:28–36. doi: 10.1016/j.jconrel.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Mainelis G, Seshadri S, Garbuzenko O, Han T, Wang Z, Minko T. Characterization and application of a nose-only exposure chamber for inhalation delivery of liposomal drugs and nucleic acids to mice. Journal of aerosol medicine and pulmonary drug delivery. 2013;26:345–354. doi: 10.1089/jamp.2011-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoral C, Fleming J, Conway J, Katz I, Tossici-Bolt L, Pichelin M, Montesantos S, Caillibotte G. Controlled, parametric, individualized, 2d and 3d imaging measurements of aerosol deposition in the respiratory tract of healthy human volunteers: In vivo data analysis. Journal of aerosol medicine and pulmonary drug delivery. 2014;27:349–362. doi: 10.1089/jamp.2013.1065. [DOI] [PubMed] [Google Scholar]
- Mansour HM, Rhee YS, Wu X. Nanomedicine in pulmonary delivery. International Journal of Nanomedicine. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. Endocytosis. 2001 [Google Scholar]
- Martin AR, Thompson RB, Finlay WH. Mri measurement of regional lung deposition in mice exposed nose-only to nebulized superparamagnetic iron oxide nanoparticles. Journal of aerosol medicine and pulmonary drug delivery. 2008;21:335–342. doi: 10.1089/jamp.2008.0698. [DOI] [PubMed] [Google Scholar]
- Massaro D, Massaro GD, Clerch LB. Noninvasive delivery of small inhibitory rna and other reagents to pulmonary alveoli in mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004;287:L1066–L1070. doi: 10.1152/ajplung.00067.2004. [DOI] [PubMed] [Google Scholar]
- Mastrandrea LD, Quattrin T. Clinical evaluation of inhaled insulin. Advanced drug delivery reviews. 2006;58:1061–1075. doi: 10.1016/j.addr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Mcgraw TE. Endocytic recycling. Nature reviews Molecular cell biology. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mellnik J, Vasquez PA, Mckinley SA, Witten J, Hill DB, Forest MG. Micro-heterogeneity metrics for diffusion in soft matter. Soft matter. 2014;10:7781–7796. doi: 10.1039/c4sm00676c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel OM, Beyerle A, Librizzi D, Pfestroff A, Behr TM, Sproat B, Barth PJ, Kissel T. Nonviral sirna delivery to the lung: Investigation of peg pei polyplexes and their in vivo performance. Molecular pharmaceutics. 2009;6:1246–1260. doi: 10.1021/mp900107v. [DOI] [PubMed] [Google Scholar]
- Merkel OM, Rubinstein I, Kissel T. Sirna delivery to the lung: What’s new? Adv Drug Deliv Rev. 2014;75:112–128. doi: 10.1016/j.addr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel OM, Zheng M, Debus H, Kissel T. Pulmonary gene delivery using polymeric nonviral vectors. Bioconjug Chem. 2012;23:3–20. doi: 10.1021/bc200296q. [DOI] [PubMed] [Google Scholar]
- Merkus HG. Particle size measurements: Fundamentals, practice, quality. Springer Science & Business Media; 2009. [Google Scholar]
- Minne A, Louahed J, Mehauden S, Baras B, Renauld JC, Vanbever R. The delivery site of a monovalent influenza vaccine within the respiratory tract impacts on the immune response. Immunology. 2007;122:316–325. doi: 10.1111/j.1365-2567.2007.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: Their uses and limitations. Journal of Aerosol Medicine-Deposition Clearance and Effects in the Lung. 2003;16:341. doi: 10.1089/089426803772455622. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Nagel MW, Doyle CC, Ali RS, Avvakoumova VI, Christopher JD, Quiroz J, Strickland H, Tougas T, Lyapustina S. Relative precision of inhaler aerodynamic particle size distribution (apsd) metrics by full resolution and abbreviated andersen cascade impactors (acis): Part 1. Aaps Pharmscitech. 2010;11:843–851. doi: 10.1208/s12249-010-9452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello M, Krone CL, Dickerson S, Howerth E, Germishuizen WA, Wong YL, Edwards D, Bloom BR, Hondalus MK. Dry-powder pulmonary insufflation in the mouse for application to vaccine or drug studies. Tuberculosis. 2009;89:371–377. doi: 10.1016/j.tube.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Morén F. Aerosols in medicine: Principles, diagnosis, and therapy. Elsevier Science Limited; 1993. [Google Scholar]
- Morita T, Niwata Y, Ohgi K, Ogawa M, Irie M. Distribution of two urinary ribonuclease-like enzymes in human organs and body fluids. J Biochem. 1986;99:17–25. doi: 10.1093/oxfordjournals.jbchem.a135456. [DOI] [PubMed] [Google Scholar]
- Mosbah K, Ruiz-Cabello J, Berthezene Y, Cremillieux Y. Aerosols and gaseous contrast agents for magnetic resonance imaging of the lung. Contrast media & molecular imaging. 2008;3:173–190. doi: 10.1002/cmmi.252. [DOI] [PubMed] [Google Scholar]
- Moschos SA, Jones SW, Perry MM, Williams AE, Erjefalt JS, Turner JJ, Barnes PJ, Sproat BS, Gait MJ, Lindsay MA. Lung delivery studies using sirna conjugated to tat (48–60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjugate chemistry. 2007;18:1450–1459. doi: 10.1021/bc070077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlfeld C, Rothen-Rutishauser B, Blank F, Vanhecke D, Ochs M, Gehr P. Interactions of nanoparticles with pulmonary structures and cellular responses. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;294:L817–L829. doi: 10.1152/ajplung.00442.2007. [DOI] [PubMed] [Google Scholar]
- Murata M, Tahara K, Takeuchi H. Real-time in vivo imaging of surface-modified liposomes to evaluate their behavior after pulmonary administration. European Journal of Pharmaceutics and Biopharmaceutics. 2014;86:115–119. doi: 10.1016/j.ejpb.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Murata N, Takashima Y, Toyoshima K, Yamamoto M, Okada H. Anti-tumor effects of anti-vegf sirna encapsulated with plga microspheres in mice. Journal of Controlled Release. 2008;126:246–254. doi: 10.1016/j.jconrel.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Nahar K, Gupta N, Gauvin R, Absar S, Patel B, Gupta V, Khademhosseini A, Ahsan F. In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. Eur J Pharm Sci. 2013;49:805–818. doi: 10.1016/j.ejps.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Najlah M, Parveen I, Alhnan MA, Ahmed W, Faheem A, Phoenix DA, Taylor KM, Elhissi A. The effects of suspension particle size on the performance of air-jet, ultrasonic and vibrating-mesh nebulisers. Int J Pharm. 2014;461:234–241. doi: 10.1016/j.ijpharm.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Newman S, Wilding I. Gamma scintigraphy: An in vivo technique for assessing the equivalence of inhaled products. International journal of pharmaceutics. 1998;170:1–9. [Google Scholar]
- Newman SP, Pitcairn GR, Hirst PH. A brief history of gamma scintigraphy. Journal of Aerosol Medicine. 2001;14:139–145. doi: 10.1089/08942680152484072. [DOI] [PubMed] [Google Scholar]
- Newman SP, Pitcairn GR, Hirst PH, Rankin L. Radionuclide imaging technologies and their use in evaluating asthma drug deposition in the lungs. Advanced drug delivery reviews. 2003;55:851–867. doi: 10.1016/s0169-409x(03)00081-4. [DOI] [PubMed] [Google Scholar]
- Newman SP, Wilding IR. Imaging techniques for assessing drug delivery in man. Pharmaceutical science & technology today. 1999;2:181–189. doi: 10.1016/s1461-5347(99)00152-2. [DOI] [PubMed] [Google Scholar]
- Newman SP, Wilding IR, Hirst PH. Human lung deposition data: The bridge between in vitro and clinical evaluations for inhaled drug products? International journal of pharmaceutics. 2000;208:49–60. doi: 10.1016/s0378-5173(00)00538-x. [DOI] [PubMed] [Google Scholar]
- Nichols SC, Mitchell JP, Shelton CM, Roberts DL. Good cascade impactor practice (gcip) and considerations for “in-use” specifications. Aaps Pharmscitech. 2013;14:375–390. doi: 10.1208/s12249-012-9905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen EJ, Nielsen JM, Becker D, Karlas A, Prakash H, Glud SZ, Merrison J, Besenbacher F, Meyer TF, Kjems J, Howard KA. Pulmonary gene silencing in transgenic egfp mice using aerosolised chitosan/sirna nanoparticles. Pharm Res. 2010;27:2520–2527. doi: 10.1007/s11095-010-0255-y. [DOI] [PubMed] [Google Scholar]
- Ntziachristos V, Culver JP, Rice BW. Small-animal optical imaging. Journal of biomedical optics. 2008;13 doi: 10.1117/1.2890838. 011001-011001-011001. [DOI] [PubMed] [Google Scholar]
- O’shaughnessy PT, Achutan C, O’neill ME, Thorne PS. A small whole-body exposure chamber for laboratory use. Inhalation toxicology. 2003;15:251–263. doi: 10.1080/08958370304504. [DOI] [PubMed] [Google Scholar]
- Oliveira S, Van Rooy I, Kranenburg O, Storm G, Schiffelers RM. Fusogenic peptides enhance endosomal escape improving sirna-induced silencing of oncogenes. International journal of pharmaceutics. 2007;331:211–214. doi: 10.1016/j.ijpharm.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Padhi BK, Chougule MB, Misra A. Aerosol performance of large respirable particles of amikacin sulfate produced by spray and freeze drying techniques. Curr Drug Deliv. 2009;6:8–16. doi: 10.2174/156720109787048267. [DOI] [PubMed] [Google Scholar]
- Park CW, Rhee YS, Vogt FG, Hayes D, Zwischenberger JB, Deluca PP, Mansour HM. Advances in microscopy and complementary imaging techniques to assess the fate of drugs ex vivo in respiratory drug delivery: An invited paper. Advanced drug delivery reviews. 2012;64:344–356. doi: 10.1016/j.addr.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: New insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Patel B, Gupta N, Ahsan F. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. European Journal of Pharmaceutics and Biopharmaceutics. 2015;89:163–174. doi: 10.1016/j.ejpb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Patel G, Chougule M, Singh M, Misra A. Nanoliposomal dry powder formulations. Methods in enzymology. 2009;464:167–191. doi: 10.1016/S0076-6879(09)64009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JS. Mechanisms of macromolecule absorption by the lungs. Advanced Drug Delivery Reviews. 1996;19:3–36. [Google Scholar]
- Patton JS, Brain JD, Davies LA, Fiegel J, Gumbleton M, Kim KJ, Sakagami M, Vanbever R, Ehrhardt C. The particle has landed--characterizing the fate of inhaled pharmaceuticals. J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 2):S71–87. doi: 10.1089/jamp.2010.0836. [DOI] [PubMed] [Google Scholar]
- Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. The American journal of pathology. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perring S, Summers Q, Fleming J, Nassim M, Holgate S. A new method of quantification of the pulmonary regional distribution of aerosols using combined ct and spect and its application to nedocromil sodium administered by metered dose inhaler. The British journal of radiology. 1994;67:46–53. doi: 10.1259/0007-1285-67-793-46. [DOI] [PubMed] [Google Scholar]
- Pettis RJ, Hall I, Costa D, Hickey AJ. Aerosol delivery of muramyl dipeptide to rodent lungs. AAPS PharmSci. 2000;2:53–61. doi: 10.1208/ps020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ME. Positron emission tomography provides molecular imaging of biological processes. Proceedings of the National Academy of Sciences. 2000;97:9226–9233. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possmayer F, Nag K, Rodriguez K, Qanbar R, Schürch S. Surface activity in vitro: Role of surfactant proteins. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2001;129:209–220. doi: 10.1016/s1095-6433(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Rawat A, Majumder QH, Ahsan F. Inhalable large porous microspheres of low molecular weight heparin: In vitro and in vivo evaluation. Journal of Controlled Release. 2008;128:224–232. doi: 10.1016/j.jconrel.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischl D, Zimmer A. Drug delivery of sirna therapeutics: Potentials and limits of nanosystems. Nanomedicine: Nanotechnology, Biology and Medicine. 2009;5:8–20. doi: 10.1016/j.nano.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Rosas-Taraco AG, Higgins DM, Sánchez-Campillo J, Lee EJ, Orme IM, González-Juarrero M. Intrapulmonary delivery of xcl1-targeting small interfering rna in mice chronically infected with mycobacterium tuberculosis. American journal of respiratory cell and molecular biology. 2009;41:136–145. doi: 10.1165/rcmb.2008-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: Mucin glycoproteins, muc gene regulation and goblet cell hyperplasia. American Journal of Respiratory Cell and Molecular Biology. 2001;25:533–537. doi: 10.1165/ajrcmb.25.5.f218. [DOI] [PubMed] [Google Scholar]
- Ross DL, Schultz RK. Effect of inhalation flow rate on the dosing characteristics of dry powder inhaler (dpi) and metered dose inhaler (mdi) products. Journal of aerosol medicine. 1996;9:215–226. doi: 10.1089/jam.1996.9.215. [DOI] [PubMed] [Google Scholar]
- Roy I, Vij N. Nanodelivery in airway diseases: Challenges and therapeutic applications. Nanomedicine. 2010;6:237–244. doi: 10.1016/j.nano.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BK. Physiology of airway mucus clearance. Respir Care. 2002;47:761–768. [PubMed] [Google Scholar]
- Rytting E, Nguyen J, Wang X, Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin Drug Deliv. 2008;5:629–639. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- Saha GB, Macintyre WJ, Go RT. Seminars in nuclear medicine. Elsevier; 1992. Cyclotrons and positron emission tomography radiopharmaceuticals for clinical imaging. [DOI] [PubMed] [Google Scholar]
- Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Advanced drug delivery reviews. 2006;58:1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Sanders N, Rudolph C, Braeckmans K, De Smedt SC, Demeester J. Extracellular barriers in respiratory gene therapy. Advanced drug delivery reviews. 2009;61:115–127. doi: 10.1016/j.addr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster BS, Suk JS, Woodworth GF, Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013;34:3439–3446. doi: 10.1016/j.biomaterials.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton BA, Crouch EC, Mccormack FX, Head JF, Hartshorn KL, Mendelsohn R. Structural determinants of pattern recognition by lung collectins. Innate Immunity. 2010 doi: 10.1177/1753425910368716. [DOI] [PubMed] [Google Scholar]
- Senoo T, Hattori N, Tanimoto T, Furonaka M, Ishikawa N, Fujitaka K, Haruta Y, Murai H, Yokoyama A, Kohno N. Suppression of plasminogen activator inhibitor-1 by rna interference attenuates pulmonary fibrosis. Thorax. 2010;65:334–340. doi: 10.1136/thx.2009.119974. [DOI] [PubMed] [Google Scholar]
- Seow WY, Liang K, Kurisawa M, Hauser CA. Oxidation as a facile strategy to reduce the surface charge and toxicity of polyethyleneimine gene carriers. Biomacromolecules. 2013;14:2340–2346. doi: 10.1021/bm4004628. [DOI] [PubMed] [Google Scholar]
- Shah VP, Smurthwaite MJ, Veranth JM, Zaidi K. The inhalation ad hoc advisory panel for the usp performance tests of inhalation dosage forms. 2008 [Google Scholar]
- Sharma K, Somavarapu S, Colombani A, Govind N, Taylor KM. Nebulised sirna encapsulated crosslinked chitosan nanoparticles for pulmonary delivery. Int J Pharm. 2013;455:241–247. doi: 10.1016/j.ijpharm.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Sharma K, Somavarapu S, Colombani A, Govind N, Taylor KMG. Crosslinked chitosan nanoparticle formulations for delivery from pressurized metered dose inhalers. European Journal of Pharmaceutics and Biopharmaceutics. 2012;81:74–81. doi: 10.1016/j.ejpb.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Shoyele SA, Slowey A. Prospects of formulating proteins/peptides as aerosols for pulmonary drug delivery. International journal of pharmaceutics. 2006;314:1–8. doi: 10.1016/j.ijpharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Sinsuebpol C, Chatchawalsaisin J, Kulvanich P. Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery. Drug Design, Development and Therapy. 2013;7:861–873. doi: 10.2147/DDDT.S47681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell N, Ganderton D. Assessing lung deposition of inhaled medications: Consensus statement from a workshop of the british association for lung research, held at the institute of biology, london, uk on 17 april 1998. Respiratory medicine. 1999;93:123–133. doi: 10.1016/s0954-6111(99)90302-5. [DOI] [PubMed] [Google Scholar]
- Son YJ, Mcconville JT. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int J Pharm. 2009;382:15–22. doi: 10.1016/j.ijpharm.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Sood BG, Shen Y, Latif Z, Chen X, Sharp J, Neelavalli J, Joshi A, Slovis TL, Haacke E. Aerosol delivery in ventilated newborn pigs: An mri evaluation. Pediatric research. 2008;64:159–164. doi: 10.1203/PDR.0b013e3181761841. [DOI] [PubMed] [Google Scholar]
- Sood BG, Shen Y, Latif Z, Galli B, Dawe EJ, Haacke EM. Effective aerosol delivery during high-frequency ventilation in neonatal pigs. Respirology. 2010;15:551–555. doi: 10.1111/j.1440-1843.2010.01714.x. [DOI] [PubMed] [Google Scholar]
- Sorrentino S. Human extracellular ribonucleases: Multiplicity, molecular diversity and catalytic properties of the major rnase types. Cell Mol Life Sci. 1998;54:785–794. doi: 10.1007/s000180050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taratula O, Dmochowski IJ. Functionalized 129 xe contrast agents for magnetic resonance imaging. Current opinion in chemical biology. 2010;14:97–104. doi: 10.1016/j.cbpa.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KM, Mccallion ON. Ultrasonic nebulisers for pulmonary drug delivery. International journal of pharmaceutics. 1997;153:93–104. [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Thompson RB, Finlay WH. Using mri to measure aerosol deposition. Journal of aerosol medicine and pulmonary drug delivery. 2012;25:55–62. doi: 10.1089/jamp.2011.0897. [DOI] [PubMed] [Google Scholar]
- Tompkins SM, Lo CY, Tumpey TM, Epstein SL. Protection against lethal influenza virus challenge by rna interference in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronde A, Baran G, Eirefelt S, Lennernäs H, Bengtsson UH. Miniaturized nebulization catheters: A new approach for delivery of defined aerosol doses to the rat lung. Journal of aerosol medicine. 2002;15:283–296. doi: 10.1089/089426802760292627. [DOI] [PubMed] [Google Scholar]
- Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: Routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science : JAALAS. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Vorauer-Uhl K, Katinger H. Nebulization of liposomal rh-cu/zn–sod with a novel vibrating membrane nebulizer. Journal of liposome research. 2006;16:113–125. doi: 10.1080/08982100600680733. [DOI] [PubMed] [Google Scholar]
- Waldrep J, Dhand R. Advanced nebulizer designs employing vibrating mesh/aperture plate technologies for aerosol generation. Current Drug Delivery. 2008;5:114–119. doi: 10.2174/156720108783954815. [DOI] [PubMed] [Google Scholar]
- Wang H, Sebrié C, Ruaud JP, Guillot G, Bouazizi-Verdier K, Willoquet G, Maître X, Darrasse L, De Rochefort L. Aerosol deposition in the lungs of spontaneously breathing rats using gd-dota-based contrast agents and ultra-short echo time mri at 1.5 tesla. Magnetic Resonance in Medicine. 2015 doi: 10.1002/mrm.25617. [DOI] [PubMed] [Google Scholar]
- Wang JC, Lai S, Guo X, Zhang X, De Crombrugghe B, Sonnylal S, Arnett FC, Zhou X. Attenuation of fibrosis in vitro and in vivo with sparc sirna. Arthritis research & therapy. 2010;12:R60. doi: 10.1186/ar2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AB, Mcconville JT, Williams RO., III Current therapies and technological advances in aqueous aerosol drug delivery. Drug development and industrial pharmacy. 2008;34:913–922. doi: 10.1080/03639040802144211. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Sinko PJ, Laskin JD, Laskin DL. Interaction with lung macrophages. Nanoparticles in the Lung: Environmental Exposure and Drug Delivery. 2014:85. [Google Scholar]
- Xenariou S, Griesenbach U, Ferrari S, Dean P, Scheule R, Cheng S, Geddes D, Plank C, Alton E. Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene therapy. 2006;13:1545–1552. doi: 10.1038/sj.gt.3302803. [DOI] [PubMed] [Google Scholar]
- Xenariou S, Griesenbach U, Liang H, Zhu J, Farley R, Somerton L, Singh C, Jeffery P, Ferrari S, Scheule R. Use of ultrasound to enhance nonviral lung gene transfer in vivo. Gene therapy. 2007;14:768–774. doi: 10.1038/sj.gt.3302922. [DOI] [PubMed] [Google Scholar]
- Yacobi NR, Malmstadt N, Fazlollahi F, Demaio L, Marchelletta R, Hamm-Alvarez SF, Borok Z, Kim KJ, Crandall ED. Mechanisms of alveolar epithelial translocation of a defined population of nanoparticles. American journal of respiratory cell and molecular biology. 2010;42:604–614. doi: 10.1165/rcmb.2009-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Peters JI, Williams RO., 3rd Inhaled nanoparticles--a current review. Int J Pharm. 2008;356:239–247. doi: 10.1016/j.ijpharm.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Yi D, Price A, Panoskaltsis-Mortari A, Naqwi A, Wiedmann TS. Measurement of the distribution of aerosols among mouse lobes by fluorescent imaging. Analytical biochemistry. 2010;403:88–93. doi: 10.1016/j.ab.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH, Goo JM, Lee SM, Park CM, Seo HJ, Cheon GJ. Positron emission tomography/magnetic resonance imaging evaluation of lung cancer: Current status and future prospects. Journal of thoracic imaging. 2014;29:4–16. doi: 10.1097/RTI.0000000000000062. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Yazaki T, Arai M, Asai K, Kanke M. The nebulization of budesonide suspensions by a newly designed mesh nebulizer, Respiratory drug delivery VIII. Raleigh: Davis Horwood; 2002. pp. 487–489. [Google Scholar]
- Zamora-Avila D, Zapata-Benavides P, Franco-Molina M, Saavedra-Alonso S, Trejo-Avila L, Resendez-Perez D, Mendez-Vazquez J, Isaias-Badillo J, Rodriguez-Padilla C. Wt1 gene silencing by aerosol delivery of pei–rnai complexes inhibits b16-f10 lung metastases growth. Cancer gene therapy. 2009;16:892–899. doi: 10.1038/cgt.2009.35. [DOI] [PubMed] [Google Scholar]
- Zamora MR, Budev M, Rolfe M, Gottlieb J, Humar A, Devincenzo J, Vaishnaw A, Cehelsky J, Albert G, Nochur S, Gollob JA, Glanville AR. Rna interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2011;183:531–538. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu L, Chan HK, Watanabe W. Formation, characterization, and fate of inhaled drug nanoparticles. Advanced drug delivery reviews. 2011;63:441–455. doi: 10.1016/j.addr.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering rna targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. Journal of Biological Chemistry. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]