Abstract
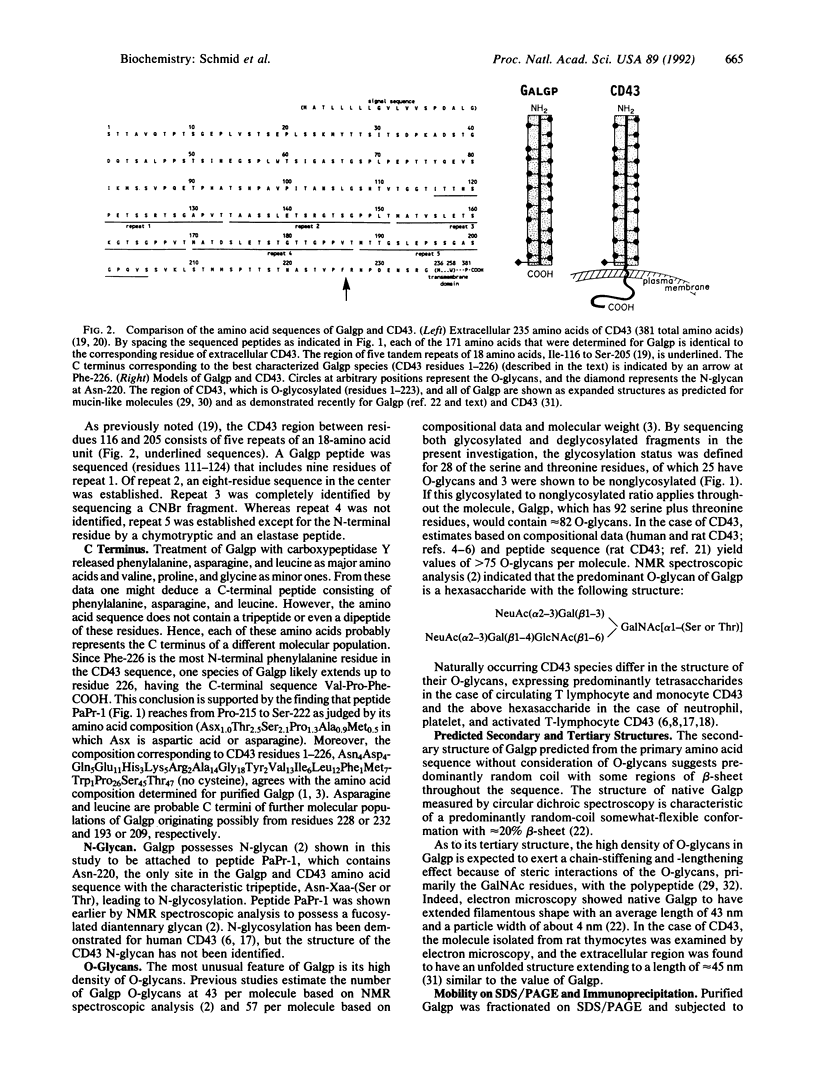
The amino acid sequence of galactoglycoprotein purified from human plasma was elucidated to 75% completeness by using chemical degradation of peptides and glycopeptides derived from digests of the protein with seven specific proteases. This sequence represents a polypeptide chain of approximately 220 amino acid residues including a high content of serine, threonine, alanine, and proline with one N-linked and multiple O-linked glycans. Comparison of peptide sequences from the native galactoglycoprotein and the deglycosylated derivative demonstrated the locations of 25 sites of O-glycosylation and three serine sites that are not glycosylated. The homogeneous N terminus was established as serine. C-terminal analysis revealed multiple C-terminal residues, suggesting that galactoglycoprotein molecules are of varying lengths. A search of a protein data base revealed that the galactoglycoprotein polypeptide is identical to the N-terminal (extracellular) polypeptide region of the blood-cell surface molecule CD43 (sialophorin, leukosialin). Further support of the relatedness of these molecules was obtained by immunoprecipitation of 125I-labeled galactoglycoprotein by monoclonal anti-CD43 antibodies. The composition and properties of the molecules together with the known structure of the gene encoding CD43 suggest that galactoprotein is derived by proteolytic cleavage from transmembrane "hexasaccharide CD43," known to be expressed on neutrophils, activated T lymphocytes, and platelets.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama K., Simons E. R., Bernasconi P., Schmid K., van Halbeek H., Vliegenthart J. F., Haupt H., Schwick H. G. The structure of the carbohydrate units of human plasma galactoglycoprotein determined by 500-megahertz 1H NMR spectroscopy. J Biol Chem. 1984 Jun 10;259(11):7151–7154. [PubMed] [Google Scholar]
- Araki T., Gejyo F., Takagaki K., Haupt H., Schwick H. G., Bürgi W., Marti T., Schaller J., Rickli E., Brossmer R. Complete amino acid sequence of human plasma Zn-alpha 2-glycoprotein and its homology to histocompatibility antigens. Proc Natl Acad Sci U S A. 1988 Feb;85(3):679–683. doi: 10.1073/pnas.85.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardman B., Sikorski M. A., Settles M., Staunton D. E. Human immunodeficiency virus type 1-infected individuals make autoantibodies that bind to CD43 on normal thymic lymphocytes. J Exp Med. 1990 Oct 1;172(4):1151–1158. doi: 10.1084/jem.172.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson B., Hammarström S., Finne J., Perlmann P. The large sialoglycoprotein of human lymphocytes. II. Biochemical features. Eur J Immunol. 1985 May;15(5):427–433. doi: 10.1002/eji.1830150503. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Carraway K. L., Hull S. R. Cell surface mucin-type glycoproteins and mucin-like domains. Glycobiology. 1991 Mar;1(2):131–138. doi: 10.1093/glycob/1.2.131. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Theibert J. L., Francois J. M., Ashley C. C., Potter J. D. Amino acid sequences and Ca2(+)-binding properties of two isoforms of barnacle troponin C. Biochemistry. 1991 Jan 22;30(3):702–707. doi: 10.1021/bi00217a017. [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Shotton D. M., Williams A. F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991 Apr;10(4):893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejyo F., Chang J. L., Bürgi W., Schmid K., Offner G. D., Troxler R. F., Van Halbeek H., Dorland L., Gerwig G. J., Vliegenthart J. F. Characterization of the B-chain of human plasma alpha 2HS-glycoprotein. The complete amino acid sequence and primary structure of its heteroglycan. J Biol Chem. 1983 Apr 25;258(8):4966–4971. [PubMed] [Google Scholar]
- Gerken T. A., Butenhof K. J., Shogren R. Effects of glycosylation on the conformation and dynamics of O-linked glycoproteins: carbon-13 NMR studies of ovine submaxillary mucin. Biochemistry. 1989 Jun 27;28(13):5536–5543. doi: 10.1021/bi00439a030. [DOI] [PubMed] [Google Scholar]
- Higuchi K., Hospattankar A. V., Law S. W., Meglin N., Cortright J., Brewer H. B., Jr Human apolipoprotein B (apoB) mRNA: identification of two distinct apoB mRNAs, an mRNA with the apoB-100 sequence and an apoB mRNA containing a premature in-frame translational stop codon, in both liver and intestine. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1772–1776. doi: 10.1073/pnas.85.6.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong Y. H., Remold-O'Donnell E., LeBien T. W., Remold H. G. A monoclonal antibody to sialophorin (CD43) induces homotypic adhesion and activation of human monocytes. J Exp Med. 1989 Jul 1;170(1):259–267. doi: 10.1084/jem.170.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallant A., Eskenazi A., Mattei M. G., Fournier R. E., Carlsson S. R., Fukuda M., Frelinger J. G. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1328–1332. doi: 10.1073/pnas.86.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman R., Remold-O'Donnell E., Kenney D. M., Perrine S., Rosen F. S. Surface protein abnormalities in lymphocytes and platelets from patients with Wiskott-Aldrich syndrome. Lancet. 1981 Dec 19;2(8260-61):1387–1389. doi: 10.1016/s0140-6736(81)92802-6. [DOI] [PubMed] [Google Scholar]
- Piller F., Piller V., Fox R. I., Fukuda M. Human T-lymphocyte activation is associated with changes in O-glycan biosynthesis. J Biol Chem. 1988 Oct 15;263(29):15146–15150. [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Davis A. E., 3rd, Kenney D., Bhaskar K. R., Rosen F. S. Purification and chemical composition of gpL115, the human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Biol Chem. 1986 Jun 5;261(16):7526–7530. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D. M., Parkman R., Cairns L., Savage B., Rosen F. S. Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med. 1984 Jun 1;159(6):1705–1723. doi: 10.1084/jem.159.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D., Rosen F. S. Biosynthesis of human sialophorins and analysis of the polypeptide core. Biochemistry. 1987 Jun 30;26(13):3908–3913. doi: 10.1021/bi00387a025. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Rosen F. S. Proteolytic fragmentation of sialophorin (CD43). Localization of the activation-inducing site and examination of the role of sialic acid. J Immunol. 1990 Nov 15;145(10):3372–3378. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Rosen F. S. Sialophorin (CD43) and the Wiskott-Aldrich syndrome. Immunodefic Rev. 1990;2(2):151–174. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Savage B. Characterization of macrophage adhesion molecule. Biochemistry. 1988 Jan 12;27(1):37–41. doi: 10.1021/bi00401a007. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Zimmerman C., Kenney D., Rosen F. S. Expression on blood cells of sialophorin, the surface glycoprotein that is defective in Wiskott-Aldrich syndrome. Blood. 1987 Jul;70(1):104–109. [PubMed] [Google Scholar]
- Schmid K., Mao S. K., Kimura A., Hayashi S., Binette J. P. Isolation and characterization of a serine-threonine-rich galactoglycoprotein from normal human plasma. J Biol Chem. 1980 Apr 10;255(7):3221–3226. [PubMed] [Google Scholar]
- Shelley C. S., Remold-O'Donnell E., Davis A. E., 3rd, Bruns G. A., Rosen F. S., Carroll M. C., Whitehead A. S. Molecular characterization of sialophorin (CD43), the lymphocyte surface sialoglycoprotein defective in Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2819–2823. doi: 10.1073/pnas.86.8.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley C. S., Remold-O'Donnell E., Rosen F. S., Whitehead A. S. Structure of the human sialophorin (CD43) gene. Identification of features atypical of genes encoding integral membrane proteins. Biochem J. 1990 Sep 15;270(3):569–576. doi: 10.1042/bj2700569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogren R., Gerken T. A., Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry. 1989 Jun 27;28(13):5525–5536. doi: 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- Silverman L. B., Wong R. C., Remold-O'Donnell E., Vercelli D., Sancho J., Terhorst C., Rosen F., Geha R., Chatila T. Mechanism of mononuclear cell activation by an anti-CD43 (sialophorin) agonistic antibody. J Immunol. 1989 Jun 15;142(12):4194–4200. [PubMed] [Google Scholar]
- Stross W. P., Warnke R. A., Flavell D. J., Flavell S. U., Simmons D., Gatter K. C., Mason D. Y. Molecule detected in formalin fixed tissue by antibodies MT1, DF-T1, and L60 (Leu-22) corresponds to CD43 antigen. J Clin Pathol. 1989 Sep;42(9):953–961. doi: 10.1136/jcp.42.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Cortes M., Axelsson B., Larsson A., Berzins T., Perlmann P. Enhancement of human spontaneous cell-mediated cytotoxicity by a monoclonal antibody against the large sialoglycoprotein (CD 43) on peripheral blood lymphocytes. Scand J Immunol. 1988 Jun;27(6):661–671. doi: 10.1111/j.1365-3083.1988.tb02399.x. [DOI] [PubMed] [Google Scholar]
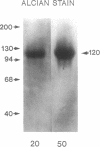
- Wardi A. H., Michos G. A. Alcian blue staining of glycoproteins in acrylamide disc electrophoresis. Anal Biochem. 1972 Oct;49(2):607–609. doi: 10.1016/0003-2697(72)90472-1. [DOI] [PubMed] [Google Scholar]
- Watzlawick H., Walsh M. T., Ehrhard I., Slayter H. S., Haupt H., Schwick H. G., Jourdian G. W., Hase S., Schmid K., Brossmer R. The effect of the carbohydrate moiety upon the size and conformation of human plasma galactoglycoprotein as judged by electron microscopy and circular dichroism. Structural studies of a glycoprotein after stepwise enzymic carbohydrate removal. Biochem J. 1991 Aug 1;277(Pt 3):753–758. doi: 10.1042/bj2770753. [DOI] [PMC free article] [PubMed] [Google Scholar]