Abstract
The five senses have specific ways to receive environmental information and lead to central nervous system. The perception of time is the sum of stimuli associated with cognitive processes and environmental changes. Thus, the perception of time requires a complex neural mechanism and may be changed by emotional state, level of attention, memory and diseases. Despite this knowledge, the neural mechanisms of time perception are not yet fully understood. The objective is to relate the mechanisms involved the neurofunctional aspects, theories, executive functions and pathologies that contribute the understanding of temporal perception. Articles form 1980 to 2015 were searched by using the key themes: neuroanatomy, neurophysiology, theories, time cells, memory, schizophrenia, depression, attention-deficit hyperactivity disorder and Parkinson’s disease combined with the term perception of time. We evaluated 158 articles within the inclusion criteria for the purpose of the study. We conclude that research about the holdings of the frontal cortex, parietal, basal ganglia, cerebellum and hippocampus have provided advances in the understanding of the regions related to the perception of time. In neurological and psychiatric disorders, the understanding of time depends on the severity of the diseases and the type of tasks.
Key words: Time perception, time cells, memory, psychiatric diseases
Introduction
Time perception is a concept that describes the subjective experience of time and how an individual interprets the duration of an event.1 Depending on the occasion, people may feel that time passes quickly or slowly. In addition to being related to several cognitive and behavioral actions, it is also due to the way in which our central nervous system processes environmental information (Figure 1).2 Distortions of time interpretation are also associated with some psychiatric and neurologic diseases.3 Time perception has attracted considerable attention from researchers who aim to develop an understanding of the neural functionality of time perception and its relation to some diseases.4,5 There is a consensus that individuals who suffer from impairments of time perception lack a specific pathway that carries key information about the passage of time from the external environment to the brain.6 Temporal perception includes all sensory channels; however, it is not clear as to the extent to which these representations are mediated by neural structures.4 Moreover, the diverse brain regions associated with the sense of time (frontal cortex, basal ganglia, parietal cortex, cerebellum, and hippocampus) are responsible for receiving, associating and interpreting information in fractions of milliseconds, seconds and minutes.7 These neural processes are only completely perceived through the participation of memory, attention, and other emotional states. However, on many occasions, time can be hyper or hypo estimated.8 For instance, when we are looking forward to an important event, such as the day we are going on vacation, time seems to pass more slowly than when the vacation is coming to an end and we are close to return to work.
Figure 1.

The central nervous system has a critical role in high hierarchy timing process and executive functions such as memory (freeimages.com/Adrian Boca), decision-making (picjumbo.com) and attention (freeimages.com/Steve Knight).2
Different time perceptions can be associated with differences in the way we perceive daily activities as well as being influenced by psychiatric and neurological diseases. Studies involving individuals who suffer from attention deficit hyperactivity disorder, depression, schizophrenia and/or Parkinson’s disease (PD) have revealed that individuals with such conditions often have an impaired time perception.9 Interest in this area has resulted in the development of several models that were specifically designed to define how the central nervous system analyzes and encodes time perception. These models enable a better understanding of some of the phenomena associated with time, such as those relating to memory and attention. Some of these models are more widely accepted by the scientific community than others, and a universally accepted, precise mode that defines the relationship between the central nervous system and time perception has yet to be developed.10 With this in mind, this paper aim to review the fundamental theories and ideas that are considered to be of strategic importance in the development of an understanding of time perception. We will discuss the different models of time perception that have been developed and will describe the main theories that have emerged in relation to the brain regions, memory participation and neurological diseases associated with time perception. The first part of the paper describes the neuroanatomy involved in temporal processing and the second part describes how memory is related to the perception of time, as well as outlining some of the pathologies that distort time perception.
Materials and Methods
This study consisted of a literature review that involved English language research articles about time perception that were published between 1980 and 2015. Case reports, original papers, and reviews were included in this integrative review. Relevant articles were identified by performing a database search on the terms neuroanatomy, time cells, neurophysiology, theories, memory, schizophrenia, depression, Parkinson’s disease and attention deficit hyperactivity disorder in combination with the phrase time perception. The results were analyzed and articles that were deemed to be relevant and of an acceptable global quality were included in the analysis.
Selection
We selected 10 articles for introduction, 90 articles matching terms time perception and neuroanatomy, 22 with hippocampus and time cells, 16 with time perception and memory, 20 about psychiatric diseases and time perception. After the selection, 158 articles fulfilled the goal and were included in this integrative review.
Time perception theories
The neural mechanisms involved in time count and codification are not clear yet fully understood.11 Diverse models of time perception have been presented, some of which include neurobiological internal clocks; spectral time; state dependent; and linear and non-linear network models that are able to identify mistakes, learn and change strategies.12 Of these, perhaps the best know is the internal clock, which is based on scalar expectancy theory.13 Studies in this area often incorporate a pacemaker-switch-accumulator mechanism. The switch turns on the pacemaker, which is controlled by attention;8 that is, when attention is focused on a stimulus that needs to be temporized, the switch closes, allowing the impulses sent by the pacemaker to flow into the accumulator.14 On stimulus displacement, the switch reopens and interrupts the flow of the impulses.15 Thus, time is estimated according to the numbers of impulses accumulated during the interval of time (Figure 2).16
Figure 2.
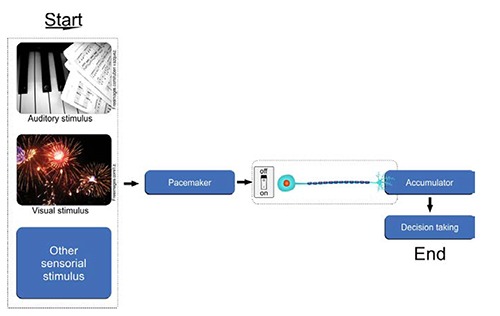
The internal clock model is defined by three main components: a time processor (pacemaker); a switch; an (accumulator). The internal clock has been associated with sensorial stimulis receivers by SNC (pacemaker) which may either acumulation or not in long term memory. Finally, the internal clock theory show the decision making.12
The information processing model in relation to the time scalar theory has been studied in a range of contexts covering periods of time that range from seconds to minutes with the objective of characterizing the relationship between judgments of duration, deceleration of the internal clock and internal attention, and memory deficits.17 Within these studies, different groups of participants have been involved in different tasks relating to the reproduction and production of time, time reaction, attention and memory. Many of the studies have demonstrated a relationship between time estimation and cognitive functions (processing and memory speed) and task period and age.18 According Tse et al.19 the brain only has access to a ratio of all the information you have processed, and this is distorted due to subjective expansion of time. In this case, a meter monitors the number of time information units. On the other hand, according to the state dependent model, temporal processing is codified on neural networks,20 and can be explained by a complex nonlinear function of the stimulus interaction.21
A neural network can include continuous activity (active state) and dependent properties of neural time (hidden state).22 This model can be regarded as intrinsic of time, insofar as it is not based on the mechanisms that are considered to represent specialized timing.23 Independent of the models, human beings estimate and distort time.24 Thereby, time notion is dependent on intrinsic (emotional state) and extrinsic context (sensitive information),25 in which relations between emotion and time do not distort the function of the internal clock but change how the clock adapts to events.13 This indicates that there is no such thing as homogenous time, but rather multiple experiences of time,26 and these reflect the way the brain adapts to diverse temporal scales.27 In this way, the different models proposed are somewhat subjective and are limited in that they only demonstrate that differences in perceptions of time are linked to the quantity and characteristic of the oscillators.12
Frontal cortex activity on time perception
The human being is able to process time duration as a result of adaptive functions involving neural regions (Figure 1)28 that are arranged according to the duration of the stimulus received.29 Thereby, time perception depends on the interaction between the cortical structures linked to the internal clock and the areas involved with a specific task.30,31 In this respect, the frontal cortex (Figure 3) has been widely associated with temporal information processing in the short- and long-term memory.32,33 Specifically, the role of the prefrontal cortex in terms of an individual’s estimation of a given time period relates to the storage and recovery of memory.6,34
Figure 3.
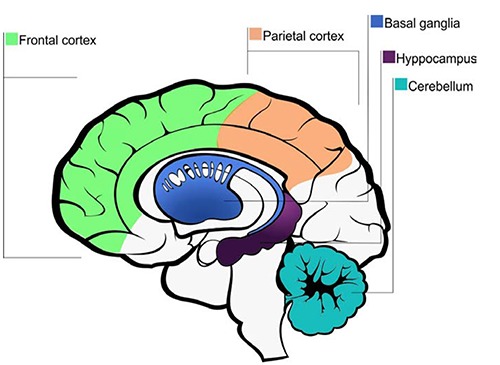
Cortical and subcortical areas involved in the time perception cerebral mechanisms.1
When it is necessary associate attention among tasks, we do and tasks we left, we have to modify attention subjectively, and then time perception is triggered. In this moment, frontal lobe takes part due its relation with prospective memory activation to predict and monitor the accuracy of the time estimation.35 In this context, frontal cortex is well developed and its relation to the memory storage has an important participation in detailed time duration.36 Moreover, the modulation by brain neurochemistry and integration with other brain areas such as the cerebellum and basal ganglia have been highlighted by dopamine,37 which appears to be related with perception of seconds to minutes, and associated to the frontostriatal circuitry.38 On the other hand, the acetylcholine is related to memory and attention on tasks involving time perception, being also present on frontal cortex and parietal relations.39 The role the frontal lobe plays in terms of time perception seems to differ according to the activities of the left and right hemisphere. Some authors support the theory that the activity of the right frontal lobe ceases when task duration is memorized, while frontal left activity helps to maintain attention until this point.40 Dorsolateral prefrontal right cortex is considered as the region most involved in time perception. This have been observed in patients with lesions in the dorsolateral prefrontal right cortex, showing changes in the performance of temporal discrimination tasks.41 The findings revealed that verbal estimation of time seems to be associated with the motor supplementary area, and that the presence of lesions in this region leads to modifications of the production of rhythm and the perception of the duration of tasks. Furthermore, models of dominant time associate the motor supplementary area with the specific region between the attention and the time accumulator.42 On the other hand, Coull et al.43 attested that the activities of the dorsolateral prefrontal cortex and supplementary motor areas are linked to the cognitive difficulties of a task, not to time perception. Moreover, Meck and Malapani analyzed the temporization of minutes and seconds and observed frontal bilateral activity in tasks involving memory work.44
Time perception in basal ganglia
The basal ganglia (BG) (Figure 3) facilitates the execution of motor control.45,46 The BG is also associated with emotions, motivation and cognition (Figure 1),47-49 learning, procedural memory, reward, and reinforcement, addictive behavior development, formation of habits and time perception.4 Specifically, some researchers have investigated the role the BG plays in terms of time perception.50 Existing studies have compared the nucleus accumbens, putamen and caudate and dopamine mediation in time perception tasks performed by healthy subjects with those suffering from Parkinson’s disease.51,52 Haber described the involvement of the BG on period time, particularly the dorsal striatum.53 Representation of time is influenced by the striatum’s ability to detect similar patterns of cortical and thalamic oscillations, and then synchronize neural firing in response to different requirements of time perception.54 This complies with the findings of Jones et al.,55 who verified the involvement of BG in temporal processes of milliseconds and seconds, and also the role of dopamine in its modulation. They investigated 12 Parkinson’s disease patients with on and off dopamine medication together with 20 healthy subjects as they performed three tasks involving time perception. The results of this research suggested that BG integrity is necessary to the production of time in seconds, as well as time reproduction in short periods. Moreover, Coull et al.56 observed that an individual’s accuracy of time perception is damaged by changes of dopamine on putamen, leading subjects to hyper or hypo estimate the passage of time. These effects were also noted in studies involving dopaminergic agonist and antagonist and on Parkinson’s disease patients.55 As such, frontostriatal circuitry allows the representation of time period that contributes to the process by which the duration of motor acts is coded. The influence of BG on time perception seems to be related to adjustments to the motor component of time perception.57
Organization of parietal cortex on time perception
The parietal cortex (Figure 3) is known as a center of integration of sensory information,58-60 and is related to a variety of cognitive functions.61-63 Its anatomic-functional relations with the temporal and dorsolateral prefrontal cortex are also associated with action control and spatial reference.64-66 In this context, parietal cortex is essential in planning movements based on sensory informations and codification of cognitive functions (Figure 1).67,68 Thus, the perception of external stimuli is integrated by parietal cortex to the time scales for a count of milliseconds and seconds intervals.69
Parietal cortex is also associated to the magnitude theory, which proposes similarities among space, size, number, velocity and time.1,70 The participation of the parietal cortex on time estimation and spatial orientation is difficult to be delimited. Although spatial regulation is related both to a static component and intervals of time perception (dynamic components), they are considered equivalent. In this context, a study evaluated these components and identified activation at left inferior parietal cortex.71 Particularly, lateral intraparietal area (LIA) was associated to time perception.72 Moreover, Maimon and Assad demonstrated a wider participation of neurons on LIA in timing the execution of movements in response to external stimuli.73 They support the idea that activity of the LIA has a probability in determining if an event is about to occur.69
Studies involving transcranial magnetic stimulation (TMS) have demonstrated changes in the right posterior parietal cortex during tasks involving time perception,74 with the posterior parietal cortex functioning to mediate the adaptation of time processing.75 Hayashi et al.76 used functional magnetic resonance imaging and TMS in tasks that involved numerical discrimination and observed the simultaneous activation of the right intraparietal cortex (RIC) and inferior frontal gyrus (IFG). Their results demonstrated that the RIC modulates the degree of influence of interaction of numerosity and damage of precise time estimation. Besides, subjects who have suffered a right temporoparietal stroke are unable to discriminate sub-second temporal durations between two successive events. Thus, their perception of time is impaired due to the refractory period of stimuli.77
Cerebellar activity on time perception
The cerebellum (Figure 3) has connections with almost all central nervous system, directly or indirectly.78 For a long time, the cerebellum was exclusively associated with motor functions, however it is involved en different processes motivation, attentional (Figure 1), associative learning and proprioceptive.13,79-82 Specifically, the participation of the cerebellum on the biological basis of time perception has been highlighted,13 but its function is yet not well established.83 It is believed that there are two systems of timing. The first, automatic, acts on motor circuits of the cerebellum is responsible by events of milliseconds.6,84 The second, controlled cognitively, is formed by parietal and prefrontal areas linked to attention and memory, being responsible by periods of minutes.85 A research analyzed patients with cerebellar lesion in tasks of discriminate time with intervals of 400 ms and 4s and noted damage into perception of milliseconds and seconds.28
The cerebellum and BG integrate proprioceptive informations during the motor task and the time perception mechanisms.86 The processes of time synchronization seem to be related with the lateral cerebellum, while the mechanisms of time aceleration with the BG.87 In this sense, the cerebellum encodesdiscrete periods of time, whilst the BG take part on the perception of rhythms more regular.88,89 Specifically, it has been observed that lateral cerebellar hemispheres have a wide participation on time perception.90 Moreover, Purkinje cells are broadly active when the time is determined by the interval between the conditional and unconditional stimulus.91 Gooch et al.92 conducted a study with patients who had cerebellar lesions and observed a biggest effect on activities related to milliseconds. Their findings suggesting the damage on left hemisphere represents changes on perception of milliseconds and minutes of perceptual tasks.11 A probable explanation is that lesions on this region make the clock mechanism be executed slowly and accumulate fewer beats (Figure 2).
Moreover, the cerebellum participates in feedback control of motor activities, which commonly involve sub- and supra-second intervals reflecting changes occurring during a task. The examples of such changes are those occurring over sub-second intervals in the activity of muscles to produce a change in the direction of movements of the limbs, hands and fingers. Thus, the circuits associated with feedback activities within the cerebellum represent time information in sub- to supra-second range resulting from its role in successful motor interactions involving external physical time parameters, such as the speed and duration. After a successful execution of a task, the time information, represented within the cerebellar circuits, is transferred to inbuilt oscillators via modular connections,93 which would help to calibrate the inbuilt neuronal clock mechanisms associated with various tasks. The role of the feedback processes in the interval timing functions of the cerebellum is supported by a study that showed increased variability in subjects with cerebellar lesions, as one of the main roles of a feedback process is to maintain a normal range.94,95 The unipolar brush cells can represents intervals of time on cerebellar cortex.96 These cells are involved on excitatory synaptic input delayed in response to cerebellum presynaptic stimulation, it is believed that the temporal codification depends on the stimulation frequency and can cause delays that range from zero until hundred of milliseconds.97 In this way, computational models have suggested that the mechanisms of time on behavioral tasks dependents of the cerebellum are calculated specifically on the cerebellar cortex.98 However, some researchers have defended the idea that the cerebellum is not the focus of an internal clock, it only provide signals about events. In this case, the cerebellum and cortical regions are associated, as the cerebellum regulating temporally the neurons activity on these regions.99,100 Besides, O’Reilly et al.101 noticed a bigger interaction between the cerebellum and the intraparietal region when a temporal aspect is added to a perceptual prevision.
Hippocampus and time cells
The hippocampus (Figure 3) is a structure of the CNS which is associated with memory formation (Figure 1),102 environmental exploratory process and the initial storage and transition of the ability to acquire, retain and recall, information relevant to the long-term memory.103-105 The types of memory (e.g., episodic and working) require a temporal sequence of successful encodings between events to consolidate and evoke memories.106 The memory acquisition corresponding to the input informations by means of external sensors are directed to neural systems to be stored and are selected according to the extent to which an individual perceives an event to be important (e.g., emotional situations such as the wedding of a child) or the frequency with which the event takes place (e.g., repetitive tasks such as training animals). Once the information is retained for a long period or permanently occurs, it is consolidated in the memory and can be evoked later.107,108 To evoke memory, time perception is essential in the processing of sequential events,109 and includes the participation of the hippocampus for organization and recruitment of episodic memory.108,110
The role of the hippocampus in time perception was explored in 1984 through experiments in mice (control and injuries fimbirafornix groups) which consisted in carrying out training tasks in radial arm maze to discriminate auditory signals that differed in duration (2 or 8s) and peak range with visual signal 5s. The results revealed that the precision of the rate and duration of auditory discrimination were not affected by the injury, however, the point of subjective equality was shifted to a shorter duration. From the peak interval, the injured rats had a shift to the right in relation to the objective time of 5s, meaning that lesions in this region impair working memory.111 Thus, various studies started with the objective of understanding the involvement of the hippocampus in time perception. For this proposition, the researchers performed various types of intervention, among them, injuring the medial septal area, resection of the temporal lobe, selective dorsal hippocampus injuries and total destruction of the hippocampus.112 Meck, Matell and Church isolated the effects of the hippocampus in specific phases of the temporal memory processing,113 providing an analysis of the factors that contributed to the hippocampal influence. In that proposition, an injury to the fimbria-fornix and observed change in information retention time in the working memory and distortion in the content of the reference memory is carried out. This means that an injury to the fornix may cause difficulty remembering long-term information. In addition, neuroimaging studies show that the formation and maintenance of memory are performed with adjuvant action of the hippocampus, associated with the connections of cortical structures such as the frontal and parietal cortex.114 Gorchetchnikov and Grossberg propose that the hippocampus on the temporal processing is performed by the entorhinal cortex circuits, dentate gyrus and CA1 areas, CA2, and CA3 corresponding to the hippocampal circuit regions that act at the gateway to the entorhinal cortex.108,115 In particular, the interaction between these regions of the brain transform temporal scales and stimuli sequences in a set of codes that may be consolidated in memory. The adaptive learning models showed a spectrum of different hippocampal cells in synchronization and modulation on learning daily or conditional events.116
Timing of activities and organization of events, for example, everyday tasks like remembering a stored object, receive acting hippocampal neurons called time cells.108 The time cells represent the temporal processing of recruitment events memory such as fear conditioning task.117-119 Eichenbaum in their review article demonstrated the activities of time cells in studies involving physiological and behavioral approaches in animals and humans.108 Similarly, neurophysiological studies using classical conditioning, which corresponds to the basic form of learning involving a simple response or a complex series of responses to certain stimuli, suggesting that occur a time series involved in evoking memories consolidated resulting from repetitive tasks.120,121 Moreover, another study suggested the involvement of the hippocampus in standard separation time using experiments in which rats learned to associate different durations of time intervals with odor stimuli. The researchers found that the hippocampus played an essential role in the behavior of rats in terms of their ability to explore a maze based on odors and to keep track of time elapsed over a course of several minutes.122
The performance of the time cells have demonstrated temporal organization of sequential events that compose a lived experience; for example, something traumatic or pleasurable. Kraus et al.119 observed this in a study, which time cells were deemed to have an influence on a rat’s perception of spatial location in mazes. The timekeeping on treadmill tasks was observed concomitantly with neuroimaging to record the neuronal firing activity that occurred when the tasks were performed. The researchers observed neuronal firing in large parts of hippocampal neurons at the moment which the rat performed the task. This result demonstrated that neuronal activation could not be attributed to residual odors; for example, something that could guide the rat to perform better in the task, thereby proving that the rats’ behavior was strongly influenced by time and distance. This finding suggests that experience memories are organized through active participation of the hippocampus in terms of order of occurrence and the frequency or importance of events.108,123
Time perception and memory
All people are continually involved in temporal activities, such as controlling the timing of a movement, expressing general knowledge, representing events and remembering past episodes.2,124 This information is filed into a system of storage (memory) and can be recovered when requested.125 In this context, human memory plays an important role in terms of our perceptions.126 Specifically, four systems of memory are involved to a greater or lesser extent in different experiences.124 Namely, the semantic memory (responsible for processing information, like concepts, linguistic expressions and facts); the procedural memory (involved in the performance of relatively automatic movements and of learned movements); the working memory (responsible for processing information about current or recent past events) and the episodic memory (responsible for processing past personal information).127,128
Pan and Luo observerd that working memory is involved in the perception of time.129 This fact was noted in tasks that required planning and time control of the movement to timing the intervals referred to the sequences of automated movements.130 Moreover, time perception is involved with diverse cognitive processes.131,132 Existing studies have noted that the less attention is paid to task, the greater the reduction in subjective time perception.2,133 Studies on patients with amnesia demonstrated that individuals who suffer from this condition are less able to precisely assess temporal judgments of short duration (less than 10 seconds) and more likely to underestimate longer temporal durations (more than 10 or 20 seconds); however, these studies linked the deficits only to a dysfunction of the long-term memory.134-136 Based on this notion, Pouthas and Perbal conducted further research using tasks which involved the reproduction of time and production to assess the capacities of distribution that a patient with amnesia showns in terms of selective deficit on episodic memory.18 Some studies on time perception dysfunctions in patients with PD have explained such impairment in terms of an internal timing mechanism.137 In this way, memory is associated due to the difficulty presented by the patient with PD on the interpretation of time.57,132,138 Similarly, the performance of patients with PD was assessed in a time reproduction task which was dependent on memory and during a time production task which required the participants to identify timing internal periods.132 During the reproduction task, judgments relating to duration varied more in patients with than without PD, and this correlated with the gravity of the illness and the extent of the memory impairment.
Diseases evoke distortions on time perception
Despite the diverse components that are involved in the interpretation of reality, it is well known that time is essential to information processing because it allows individuals an opportunity to perceive their surrounding environment and is related to the detection of many events.13,38 The term time has also been used to refer to an estimation of the duration of an event.6 The ability of a human being to estimate time is considered a stable function that may vary as a result of the development of some diseases, toxic situations or psychiatric disorders.12 Time is subjectively estimated by a subject and involves the participation of an internal clock responsible for measuring the objective time without the influence of external stimuli.44 This section describes some of the common illnesses associated with distortions of the time perception.
Depression is a common affective disorder that is characterized by a sensation of emptiness or sadness. For some people, depression is associated with the perception that time is passing very slowly; i.e., depression can alter an individual’s subjective experience of time.14 Some patients with depression report that time passes slower than normal or even stops completely (fewer pulses are accumulated by time units).14,139,140 However, this subjective sensation does not indicate an intrinsic change in time perception; that is to say that the affected individuals experience time in the same way as others, but with a kind of desynchronization.8 This fact was observed in a study in which the participants classified a signal between 400 and 1600 milliseconds as being short or long. The results showed that the higher the depression, minor is the duration of time perceived.141 Furthermore, Oberfeld et al.142 studied interval times (verbal estimation, production, and reproduction of time) in patients with depression but did not identify any changes in time intervals.
The attention-deficit hyperactivity disorder (ADHD) is a neurological disturb characterized by impairment of the executive function.4 Considering time perception an executive function, can be established relations with changes on this ability in patients with ADHD.143 Moreover, time perception frequently involves the presentation of stimuli pairs with duration relatively short (usually in milliseconds) to the subject who should assess the differences in the duration of these intervals.144 A study involving children with ADHD reported temporal discrimination deficit of periods of time too short (between 1000 and 1300 milliseconds).144 By the other side, some studies confirmed that occur difference on time duration discrimination in subjects with ADHD, but in this case the subjects were less precise in discriminate longer time duration.145,146
The schizophrenia is considered a complex and serious psychiatric disturb, characterized by symptoms of hallucinations and delusions associated to thought disorganization. Its pathogenesis remains unknown, but can generate deficits on some attention processes, memory, cognition, executive functions and perception.147,148 Researches pointed that schizophrenia can be related with change on time’s processing. This affirmative considers clinic symptoms like hallucinations, psychomotor poverty, delirium and poverty of speech.12,149,150 Clinical and experimental findings indicate that patients who suffer from schizophrenia are able to estimate time less accurately than healthy subjects. In addition to attention deficits, schizophrenia is also associated with the impairment of working memory.148 However, studies of episodic memory have suggested that patients who suffer from schizophrenia can remember that an event occurred, but do not know when it occurred. These results indicate that patients do not lose memory, but experience a disorganization of time perception.151,152 Thus, to better understand the changes that emerge as a result of schizophrenia, researchers should assess time perception,148 due to relationship between schizophrenia and time perception brain regions.90 The PD is accompanied by cognitive and motor changes, including disorderly movement time, usually expressed as bradykinesia and/or akinesia and by longer time to processes an information, named as bradyphrenia.153 The PD is characterized by dysfunction of the BG circuitry due a degenerative process on the nigrostriatal pathway that causes progressive death of cells on the compact part of substantia nigra. It causes less dopamine on striado and leads to an indirect form of time perception’s dysfunction.12 Patients with PD present increased reaction time, attenuation of movement and of the information processing. They also show speech impairment and decreased ability of keeping fixed rhythms on motor tasks.154-156 Studies involving patients with PD are frequently performed within scalar theory to observe temporal processing on seconds and minutes.157,158 The frontostriatal circuitries may participate in the estimation of long intervals of time and this consists in one of the theories to explain the changes on time perception in patients with PD.153
Conclusions
Regardless of being taken by emotion, relaxed, hurried or talking on the phone, time is part of our day to day and is present in all moments. In this context, the theories of time perception and its modeling support the existence of multiple clocks, but without a conclusive form of its functionality. Moreover, subjects with neurological and psychiatric damages have difficult on perceive and organize the time, frequently due disorders on attention, memory and neurotransmitters action as dopamine and acetylcholine, thence the difficulties of perceive time and related it to actions of present and future, affecting cognitive and motor resources. Furthermore, manifestations of disorders resulting of frontal brain lesions, BG, cerebellum, hippocampus and parietal cortex become more investigated in order to answer which models are involved and its neural functional relations with time perception.
References
- 1.Walsh V. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn Sci 2003;7:483-8. [DOI] [PubMed] [Google Scholar]
- 2.Block RA, Gruber RP. Time perception, attention, and memory: a selective review. Acta Psychol (Amst) 2014;149:129-33. [DOI] [PubMed] [Google Scholar]
- 3.Lucas M, Chaves F, Teixeira S, et al. Time perception impairs sensory-motor integration in Parkinson’s disease. Int Arch Med 2013;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain 2012;135:656-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr D, Morrone C. Time perception: space-time in the brain. Curr Biol 2006;16:R171-3. [DOI] [PubMed] [Google Scholar]
- 6.Coull JT, Cheng R-K, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 2011;36:3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 2005;6:755-65. [DOI] [PubMed] [Google Scholar]
- 8.Droit-Volet S. Time perception, emotions and mood disorders. J Physiol Paris 2013;107:255-64. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Jia L, Ren W. Time changes with feeling of speed: an embodied perspective. Front Neurorobot. 2014;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews WJ, Meck WH. Time perception: the bad news and the good. Wiley Interdiscip Rev Cogn Sci 2014;5:429-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivry RB, Spencer RMC. The neural representation of time. Curr Opin Neurobiol 2004;14:225-32. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira S, Machado S, Paes F, et al. Time perception distortion in neuropsychiatric and neurological disorders. CNS Neurol Disord Drug Targets 2013;12:567-82. [DOI] [PubMed] [Google Scholar]
- 13.Grondin S. Timing and time perception: a review of recent behavioral and neuroscience findings and theoretical directions. Atten Percept Psychophys 2010;72:561-82. [DOI] [PubMed] [Google Scholar]
- 14.Kornbrot DE, Msetfi RM, Grimwood MJ. Time perception and depressive realism: judgment type, psychophysical functions and bias. PLoS One 2013;8:e71585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effron DA, Niedenthal PM, Gil S, Droit-Volet S. Embodied temporal perception of emotion. Emotion 2006;6:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko S, Murakami I. Perceived duration of visual motion increases with speed. J Vis 2009;9:14. [DOI] [PubMed] [Google Scholar]
- 17.Staddon JE, Higa JJ. Time and memory: towards a pacemaker-free theory of interval timing. J Exp Anal Behav 1999;71:215-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouthas V, Perbal S. Time perception depends on accurate clock mechanisms as well as unimpaired attention and memory processes. Acta Neurobiol Exp (Wars) 2004;64:367-85. [DOI] [PubMed] [Google Scholar]
- 19.Tse PU, Intriligator J, Rivest J, Cavanagh P. Attention and the subjective expansion of time. Percept Psychophys 2004;66:1171-89. [DOI] [PubMed] [Google Scholar]
- 20.Jantzen KJ, Steinberg FL, Kelso JAS. Functional MRI reveals the existence of modality and coordination-dependent timing networks. Neuroimage 2005;25:1031-42. [DOI] [PubMed] [Google Scholar]
- 21.Buonomano DV, Bramen J, Khodadadifar M. Influence of the interstimulus interval on temporal processing and learning: testing the state-dependent network model. Philos Trans R Soc Lond B Biol Sci 2009;364:1865-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laje R, Buonomano DV. Robust timing and motor patterns by taming chaos in recurrent neural networks. Nat Neurosci 2013;16:925-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buonomano DV, Laje R. Population clocks: motor timing with neural dynamics. Trends Cogn Sci 2010;14:520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci 2004;27:307-40. [DOI] [PubMed] [Google Scholar]
- 25.García-Pérez MA. Does time ever fly or slow down? The difficult interpretation of psychophysical data on time perception. Front Hum Neurosci. 2014;8:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teki S, Grube M, Griffiths T. A unified model of time perception accounts for duration-based and beat-based timing mechanisms. Front Integr Neurosci 2012;5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Droit-Volet S, Gil S. The time-emotion paradox. Philos Trans R Soc Lond B Biol Sci 2009;364:1943-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Cogn Brain Res 1998;7:15-39. [DOI] [PubMed] [Google Scholar]
- 29.Onoe H, Komori M, Onoe K, et al. Cortical networks recruited for time perception: a monkey positron emission tomography (PET) study. Neuroimage 2001;13:37-45. [DOI] [PubMed] [Google Scholar]
- 30.Merchant H, Pérez O, Zarco W, Gámez J. Interval tuning in the primate medial premotor cortex as a general timing mechanism. J Neurosci 2013;33:9082-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington DL, Zimbelman JL, Hinton SC, Rao SM. Neural modulation of temporal encoding, maintenance, and decision processes. Cereb Cortex 2010;20:1274-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genovesio A, Tsujimoto S, Wise SP. Feature- and order-based timing representations in the frontal cortex. Neuron 2009;63:254-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charles DP, Gaffan D, Buckley MJ. Impaired recency judgments and intact novelty judgments after fornix transection in monkeys. J Neurosci 2004;24:2037-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001;24:167-202. [DOI] [PubMed] [Google Scholar]
- 35.McFarland CP, Glisky EL. Frontal lobe involvement in a task of time-based prospective memory. Neuropsychologia 2009;47:1660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genovesio A, Tsujimoto S, Wise SP. Neuronal activity related to elapsed time in prefrontal cortex. J Neurophysiol 2006;95:3281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meck WH. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res 2006;1108:157-67. [DOI] [PubMed] [Google Scholar]
- 38.Meck WH. Neuropharmacology of timing and time perception. Cogn Brain Res 1996;3:227-42. [DOI] [PubMed] [Google Scholar]
- 39.Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn 2002;48:195-211. [DOI] [PubMed] [Google Scholar]
- 40.Pfeuty M, Ragot R, Pouthas V. When time is up: CNV time course differentiates the roles of the hemispheres in the discrimination of short tone durations. Exp Brain Res 2003;151:372-9. [DOI] [PubMed] [Google Scholar]
- 41.Casini L, MacAr F. Multiple approaches to investigate the existence of an internal clock using attentional resources. Behav Processes 1999;45:73-85. [DOI] [PubMed] [Google Scholar]
- 42.Lambrechts A, Mella N, Pouthas V, Noulhiane M. Subjectivity of time perception: a visual emotional orchestration. Front Integr Neurosci 2011;5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coull JT. fMRI studies of temporal attention: allocating attention within, or towards, time. Cogn Brain Res 2004;21:216-26. [DOI] [PubMed] [Google Scholar]
- 44.Meck WH, Malapani C. Neuroimaging of interval timing. Brain Res Cogn Brain Res 2004;21:133-7. [DOI] [PubMed] [Google Scholar]
- 45.Hikosaka O, Miyashita K, Miyachi S, et al. Differential roles of the frontal cortex, basal ganglia, and cerebellum in visuomotor sequence learning. Neurobiol Learn Mem 1998;70:137-49. [DOI] [PubMed] [Google Scholar]
- 46.Gershman SJ, Moustafa A, Ludvig E. Time representation in reinforcement learning models of the basal ganglia. Front Comput Neurosci 2014;7:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science 1994;265:1826-31. [DOI] [PubMed] [Google Scholar]
- 48.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev 2001;36:129-38. [DOI] [PubMed] [Google Scholar]
- 49.Hadders-Algra M. Developmental coordination disorder: is clumsy motor behavior caused by a lesion of the brain at early age? Neural Plast 2003;10:39-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helie S, Chakravarthy S, Moustafa A. Exploring the cognitive and motor functions of the basal ganglia: an integrative review of computational cognitive neuroscience models. Front Comput Neurosci 2013;7:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroll H, Hamker FH. Computational models of basal-ganglia pathway functions: focus on functional neuroanatomy. Front Syst Neurosci 2013;7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones C, Jahanshahi M. Dopamine modulates striato-frontal functioning during temporal processing. Front Integr Neurosci 2011. 25;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 2003;26:317-30. [DOI] [PubMed] [Google Scholar]
- 54.Matell MS, Meck WH, Nicolelis MAL. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci 2003;117:760-73. [DOI] [PubMed] [Google Scholar]
- 55.Jones CRG, Malone TJL, Dirnberger G, et al. Basal ganglia, dopamine and temporal processing: Performance on three timing tasks on and off medication in Parkinson’s disease. Brain Cogn 2008;68:30-41. [DOI] [PubMed] [Google Scholar]
- 56.Coull JT, Hwang HJ, Leyton M, Dagher A. Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. J Neurosci 2012;32:16704-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rammsayer T, Classen W. Impaired temporal discrimination in Parkinson’s disease: temporal processing of brief durations as an indicator of degeneration of dopaminergic neurons in the basal ganglia. Int J Neurosci 1997;91:45-55. [DOI] [PubMed] [Google Scholar]
- 58.Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci 2007;11:30-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bueti D, Walsh V. The parietal cortex and the representation of time, space, number and other magnitudes. Philos Trans R Soc Lond B Biol Sci 2009;364:1831-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol 2006;16:205-12. [DOI] [PubMed] [Google Scholar]
- 61.Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci 2008;9:613-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sereno MI, Huang RS. Multisensory maps in parietal cortex. Curr Opin Neurobiol 2014;24:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teixeira S, Machado S, Velasques B, et al. Integrative parietal cortex processes: neurological and psychiatric aspects. J Neurol Sci 2014;338:12-22. [DOI] [PubMed] [Google Scholar]
- 64.Rockland KS, Van Hoesen GW. Some temporal and parietal cortical connections converge in CA1 of the primate hippocampus. Cereb Cortex 1999;9:232-7. [DOI] [PubMed] [Google Scholar]
- 65.Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron 2007;53:9-16. [DOI] [PubMed] [Google Scholar]
- 66.Vingerhoets G. Contribution of the posterior parietal cortex in reaching, grasping, and using objects and tools. Front Psychol 2014;5:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci 2004;24:1833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scherberger H, Andersen RA. Target selection signals for arm reaching in the posterior parietal cortex. J Neurosci 2007;27:2001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cook EP, Pack CC. Parietal cortex signals come unstuck in time. PLoS Biol 2012;10:e1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nachev P, Husain M. Disorders of visual attention and the posterior parietal cortex. Cortex 2006;42:766-73. [DOI] [PubMed] [Google Scholar]
- 71.Assmus A, Marshall JC, Ritzl A, et al. Left inferior parietal cortex integrates time and space during collision judgments. Neuroimage 2003;20:S82-8. [DOI] [PubMed] [Google Scholar]
- 72.Schneider BA, Ghose GM. Temporal production signals in parietal cortex. PLoS Biol. 2012;10:e1001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maimon G, Assad JA. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci 2006;9:948-55. [DOI] [PubMed] [Google Scholar]
- 74.Battelli L, Walsh V, Pascual-Leone A, Cavanagh P. The when parietal pathway explored by lesion studies. Curr Opin Neurobiol 2008;18:120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magnani B, Oliveri M, Renata Mangano G, Frassinetti F. The role of posterior parietal cortex in spatial representation of time: a TMS study. Behav Neurol 2010;23:213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hayashi MJ, Kanai R, Tanabe HC, et al. Interaction of numerosity and time in prefrontal and parietal cortex. J Neurosci 2013;33:883-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snyder JJ, Chatterjee A. Spatial-temporal anisometries following right parietal damage. Neuropsychologia 2004;42:1703-8. [DOI] [PubMed] [Google Scholar]
- 78.Bareš M, Husárová I, Lungu O. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov (N Y) 2012;2:tre-02-93-653-1. [PMC free article] [PubMed] [Google Scholar]
- 79.Bares M, Lungu O, Liu T, et al. Impaired predictive motor timing in patients with cerebellar disorders. Exp Brain Res 2007;180:355-65. [DOI] [PubMed] [Google Scholar]
- 80.Bledsoe JC, Semrud-Clikeman M, Pliszka SR. Neuroanatomical and neuropsychological correlates of the cerebellum in children with attention-deficit/hyperactivity disorder-combined type. J Am Acad Child Adolesc Psychiatry 2011;50:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Proske U, Gandevia SC. The kinaesthetic senses. J Physiol 2009;587:4139-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci 2013;33:14301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harrington DL, Lee RR, Boyd LA, et al. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain 2004;127:561-74. [DOI] [PubMed] [Google Scholar]
- 84.Koch G, Oliveri M, Torriero S, et al. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp Brain Res 2007;179:291-9. [DOI] [PubMed] [Google Scholar]
- 85.Lewis PA, Miall RC. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia 2003;41:1583-92. [DOI] [PubMed] [Google Scholar]
- 86.Mioni G, Mattalia G, Stablum F. Time perception in severe traumatic brain injury patients: a study comparing different methodologies. Brain Cogn 2013;81:305-12. [DOI] [PubMed] [Google Scholar]
- 87.Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol 2007;98:145-52. [DOI] [PubMed] [Google Scholar]
- 88.Grahn JA, Rowe JB. Feeling the beat: premotor and striatal interactions in musicians and nonmusicians during beat perception. J Neurosci 2009;29:7540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grube M, Cooper FE, Chinnery PF, Griffiths TD. Dissociation of duration-based and beat-based auditory timing in cerebellar degeneration. Proc Natl Acad Sci USA 2010;107:11597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp (Wars) 2004;64:329-40. [DOI] [PubMed] [Google Scholar]
- 91.Jirenhed DA, Hesslow G. Learning stimulus intervals-adaptive timing of conditioned purkinje cell responses. Cerebellum 2011;10:523-35. [DOI] [PubMed] [Google Scholar]
- 92.Gooch CM, Wiener M, Hamilton AC, Coslett HB. Temporal discrimination of sub- and suprasecond time intervals: a voxel-based lesion mapping analysis. Front Integr Neurosci 2011;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta DS. Processing of sub- and supra-second intervals in the primate brain results from the calibration of neuronal oscillators via sensory, motor, and feedback processes. Front Psychol 2014;5:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gooch CM, Wiener M, Wencil EB, Coslett HB. Interval timing disruptions in subjects with cerebellar lesions. Neuropsychologia 2010;48:1022-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bartolo R, Merchant H. Beta oscillations are linked to the initiation of sensory-cued movement sequences and the internal guidance of regular tapping in the monkey. J Neurosci 2015;35:4635-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arenz A, Silver RA, Schaefer AT, Margrie TW. The contribution of single synapses to sensory representation in vivo. Science 2008;321:977-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D’Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci 2009;32:30-40. [DOI] [PubMed] [Google Scholar]
- 98.Van Dorp S, De Zeeuw CI. Variable timing of synaptic transmission in cerebellar unipolar brush cells. Proc Natl Acad Sci USA 2014;111:5403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coull JT, Vidal F, Nazarian B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science 2004;303:1506-8. [DOI] [PubMed] [Google Scholar]
- 100.Lustig C, Matell MS, Meck WH. Not just a coincidence: frontal-striatal interactions in working memory and interval timing. Memory 2005;13:441-8. [DOI] [PubMed] [Google Scholar]
- 101.O’Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci 2008;28:2252-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiltgen BJ, Zhou M, Cai Y, et al. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol 2010;20:1336-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holscher C. Time, space and hippocampal functions. Rev Neurosci 2003;14:253-84. [DOI] [PubMed] [Google Scholar]
- 104.Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb Cortex 2011;21:272-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eichenbaum H. Memory on time. Trends Cogn Sci 2013;17:81-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MacDonald CJ. Prospective and retrospective duration memory in the hippocampus: is time in the foreground or background?. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20120463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nielson DM, Smith TA, Sreekumar V, Dennis S, Sederberg PB. Human hippocampus represents space and time during retrieval of real-world memories. Proc Natl Acad Sci 2015;112:11078-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci 2014;15:732-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herai T, Mogi K. Perception of temporal duration affected by automatic and controlled movements. Conscious Cogn 2014;29:23-35. [DOI] [PubMed] [Google Scholar]
- 110.Nakazono T, Sano T, Takahashi S, Sakurai Y. Theta oscillation and neuronal activity in rat hippocampus are involved in temporal discrimination of time in seconds. Front Syst Neurosci 2015;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behav Neurosci 1984;98:3-22. [DOI] [PubMed] [Google Scholar]
- 112.Balci F, Meck WH, Moore H, Brunner D. Timing deficits in aging and neuropathology. Bizon JL, Woods AG, eds. Animal models of human cognitive aging. New York: Humana Press; 2009. pp 1-41. [Google Scholar]
- 113.Meck WH, Matell MS, Church RM. Hippocampus, time, and memory. A retrospective analysis. Behav Neurosci 2013;127:642-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paz R, Gelbard-Sagiv H, Mukamel R, et al. A neural substrate in the human hippocampus for linking successive events. Proc Natl Acad Sci USA 2010;107:6046-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gorchetchnikov A, Grossberg S. Space, time and learning in the hippocampus: how fine spatial and temporal scales are expanded into population codes for behavioral control. Neural Networks. 2007;20:182-93. [DOI] [PubMed] [Google Scholar]
- 116.Modi MN, Dhawale AK, Bhalla US. CA1 cell activity sequences emerge after reorganization of network correlation structure during associative learning. Elife 2014;3:e01982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gill PR, Mizumori SJY, Smith DM. Hippocampal episode fields develop with learning. Hippocampus 2011;21:1240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal time cells bridge the gap in memory for discontiguous events. Neuron 2011;71:737-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kraus BJ, Robinson RJ, White JA, et al. Hippocampal time cells: time versus path integration. Neuron 2013;78:1090-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J Neurosci 2003;23:1535-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yin B, Troger AB. Exploring the 4th dimension: hippocampus, time, and memory revisited. Front Integr Neurosci 2011;5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jacobs NS, Allen TA, Nguyen N, Fortin NJ. Critical role of the hippocampus in memory for elapsed time. J Neurosci 2013;33:13888-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lehn H, Steffenach HA, Van Strien NM, et al. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci 2009;29:3475-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hintzman DL. Memory strength and recency judgments. Psychon Bull Rev 2005;12:858-64. [DOI] [PubMed] [Google Scholar]
- 125.Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem 2004;82:171-7. [DOI] [PubMed] [Google Scholar]
- 126.Eichenbaum H, Cohen N. From conditioning to conscious recollection: memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- 127.Baddeley A. Human memory. Theory and practice. Hove: Psychology Press; 1997. [Google Scholar]
- 128.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 2004;30:161-79. [DOI] [PubMed] [Google Scholar]
- 129.Pan Y, Luo QY. Working memory modulates the perception of time. Psychon Bull Rev 2012;19:46-51. [DOI] [PubMed] [Google Scholar]
- 130.Gold JJ, Squire LR. The anatomy of amnesia: neurohistological analysis of three new cases. Learn Mem 2006;13:699-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Staddon JER. Interval timing: memory, not a clock. Trends Cogn Sci 2005;9:312-4. [DOI] [PubMed] [Google Scholar]
- 132.Perbal S, Deweer B, Pillon B, et al. Effects of internal clock and memory disorders on duration reproductions and duration productions in patients with Parkinson’s disease. Brain Cogn 2005;58:35-48. [DOI] [PubMed] [Google Scholar]
- 133.Hirst W, Pinner E. Memory and attention. Herrmann DJ, McEvoy C, Hertzog C, et al, eds. Basic and applied memory research. Vol. 1 Hove: Psychology Press; 2013. [Google Scholar]
- 134.Williams JM, Medwedeff CH, Haban G. Memory disorder and subjective time estimation. J Clin Exp Neuropsychol 1989;11:713-23. [DOI] [PubMed] [Google Scholar]
- 135.Kinsbourne M, Hicks R. Neuropsychological impairments of short-term memory. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 136.Mimura M, Kinsbourne M, O’Connor M. Time estimation by patients with frontal lesions and by Korsakoff amnesics. J Int Neuropsychol Soc 2000;6:517-28. [DOI] [PubMed] [Google Scholar]
- 137.Harrington DL, Haaland KY. Neural underpinnings of temporal processing: a review of focal lesion, pharmacological, and functional imaging research. Rev Neurosci 1999;10:91-116. [DOI] [PubMed] [Google Scholar]
- 138.Freeman JS, Cody FW, Schady W. The influence of external timing cues upon the rhythm of voluntary movements in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1993;56:1078-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cocenas-Silva R, Bueno JLO, Droit-Volet S. Temporal memory of emotional experience. Memory Cognition 2012;40:161-7. [DOI] [PubMed] [Google Scholar]
- 140.Gallagher S. Time, emotion, and depression. Emot Rev 2012:127-32. [Google Scholar]
- 141.Gil S, Rousset S, Droit-Volet S. How liked and disliked foods affect time perception. Emotion 2009;9:457-63. [DOI] [PubMed] [Google Scholar]
- 142.Oberfeld D, Thönes S, Palayoor BJ, Hecht H. Depression does not affect time perception and time-to-contact estimation. Front Psychol 2014;5:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997;121:65-94. [DOI] [PubMed] [Google Scholar]
- 144.Graham LN, Smith PA, Stoker JB, et al. Time course of sympathetic neural hyperactivity after uncomplicated acute myocardial infarction. Circulation 2002;106:793-7. [DOI] [PubMed] [Google Scholar]
- 145.Barkley RA, Murphy KR, Bush T. Time perception and reproduction in young adults with attention deficit hyperactivity disorder. Neuropsychology 2001;15:351-60. [DOI] [PubMed] [Google Scholar]
- 146.Toplak ME, Rucklidge JJ, Hetherington R, et al. Time perception deficits in attention-deficit/ hyperactivity disorder and comorbid reading difficulties in child and adolescent samples. J Child Psychol Psychiatry 2003;44:888-903. [DOI] [PubMed] [Google Scholar]
- 147.Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res 2004;70:117-45. [DOI] [PubMed] [Google Scholar]
- 148.Bonnot O, de Montalembert M, Kermarrec S, et al. Are impairments of time perception in schizophrenia a neglected phenomenon? J Physiol Paris 2011;105:164-9. [DOI] [PubMed] [Google Scholar]
- 149.Carroll CA, Boggs J, O’Donnell BF, et al. Temporal processing dysfunction in schizophrenia. Brain Cogn 2008;67:150-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn 2009;70:181-90. [DOI] [PubMed] [Google Scholar]
- 151.Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med 2009;39:889-905. [DOI] [PubMed] [Google Scholar]
- 152.Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry 2008;64:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith JG, Harper DN, Gittings D, Abernethy D. The effect of Parkinson’s disease on time estimation as a function of stimulus duration range and modality. Brain Cogn 2007;64:130-43. [DOI] [PubMed] [Google Scholar]
- 154.Bloxham C, Dick D, Moore M. Reaction times and attention in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1987;50:1178-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.O’Boyle DJ, Freeman JS, Cody FWJ. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain 1996;119:51-70. [DOI] [PubMed] [Google Scholar]
- 156.Elsinger CL, Rao SM, Zimbelman JL, et al. Neural basis for impaired time reproduction in Parkinson’s disease: an fMRI study. J Int Neuropsychol Soc 2003;9:1088-98. [DOI] [PubMed] [Google Scholar]
- 157.Lange KW, Tucha O, Steup A, et al. Subjective time estimation in Parkinson’s disease. J Neural Transm Suppl 1995;46:433-8. [PubMed] [Google Scholar]
- 158.Malapani C, Deweer B, Gibbon J. Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. J Cogn Neurosci 2002;14:311-22. [DOI] [PubMed] [Google Scholar]


