Abstract
Delayed sleep phase disorder (DSPD) is common among adolescents and further increases their susceptibility to chronic sleep restriction and associated detrimental outcomes, including increased risk of depression, drug and alcohol use, behavioral problems, and poor scholastic performance. DSPD is characterized by sleep onset that occurs significantly later than desired bedtimes and societal norms. Individuals with DSPD exhibit long sleep latencies when attempting to sleep at conventional bedtimes. Circadian sleep disorders such as DSPD can occur when there is misalignment between sleep timing and societal norms. This review discusses studies using light therapy to advance the timing of sleep in adolescents and college students, in particular on those suffering from DSPD. A discussion on how to increase effectiveness of light therapy in the field will also be provided.
Keywords: circadian, melatonin, light, sleep, sleep phase disorder, adolescents
Background
Biological rhythms that repeat approximately every 24 hours are known as circadian rhythms. Light–dark patterns reaching the back of the eye synchronize our circadian rhythms, such as the sleep–wake cycle, to local time on Earth. Lack of synchronization resulting from irregular or insufficient light–dark exposures may lead to decrements in health and well-being.1,2
The type of light that maximally impacts the circadian system differs from those that maximally impact visibility. In terms of absolute sensitivity, it has been demonstrated that less light than originally shown by Lewy et al3 in the 1980s can affect melatonin; however, the amount of light needed to suppress melatonin is still higher than the amount of light needed for visibility of large objects of high contrast.4 For example, a person can safely navigate in a space illuminated with a warm color nightlight delivering less than 1 lux at the eye, but this same light will not cease nocturnal melatonin production in humans. It is important to note, however, that the absolute sensitivity of the circadian system will vary depending on previous light exposures.5,6
Humans are “blue sky detectors” when it comes to modulating melatonin levels; the peak sensitivity for acute melatonin suppression and phase shifting of dim light melatonin onset (DLMO) is close to 460 nm.7–9 While light signals exiting the retina reach the suprachiasmatic nuclei, where the biological clock is located, via the intrinsically photosensitive retinal ganglion cells (ipRGCs),10 these photoreceptors are not the only photoreceptors involved in circadian phototransduction, which is how the retina converts light signals into neural signals for the circadian system. Studies have shown that the ipRGCs receive neural input from classical photoreceptors, the rods and cones.11,12
The effects of light on the circadian system vary over the course of the 24-hour day. Morning light, given after the nadir of core body temperature (CBT) that typically occurs in the second half of the night (~2–3 hours prior to natural waking), will advance the timing of sleep in the following cycle, while evening light, given prior to the nadir of CBT, will delay the timing of sleep.13
As discussed in this review, successful application of light to phase shift the timing of the circadian clock is highly dependent on measurement of light exposures over the 24-hour day, as opposed to taking just a “snapshot” measurement of light exposure at one circadian time.14,15 The circadian system seems to keep track of light exposure, and therefore, knowing an individual’s light exposure history over the past 24 hours can help determine the best light prescription for the next 24 hours.16 Therefore, it would be best if a light treatment designed to promote earlier bedtimes is not limited to prescribing morning light exposure;14,17,18 rather, it is recommended that a light treatment be prescribed so that the total circadian light exposure during waking hours is monitored to increase morning light exposures and reduce evening exposures.14,16
A person is more likely to experience a good night of sleep when the circadian and homeostatic systems, both of which influence the sleep–wake cycle, are aligned. Sleep homeostasis increases with time awake, contributing to high sleep pressure at night. The circadian system sends an alerting signal to the body during the day, counteracting the increase of sleep pressure with time awake, and a sleeping signal during the night, promoting a consolidated night of sleep. Circadian sleep disorders, such as delayed sleep phase disorder (DSPD) or advanced sleep phase disorder, can occur when there is misalignment between the timing and socially imposed demands according to external clock time. This review will discuss studies using light therapy to advance the timing of sleep in adolescents, in particular, on those suffering from DSPD. While the use of light therapy in combination with cognitive behavior therapy or pharmacological therapy has been shown to be an effective treatment, these combined therapies will not be discussed here.19,20
Sleep in adolescents and college students
Pubertal development is associated with a delay in the preferred timing of sleep and wakefulness and contributes to significant reductions in total sleep time during adolescence.21–29 A report by the National Sleep Foundation states that, even though adolescents require as much sleep as they did as preadolescents (~8.5–9.25 hours per night), they tend to fall asleep later (~11 pm) and their average sleep time is only 7 hours and 42 minutes per night when they are 13 years old and 7 hours and 4 minutes when they are 19 years old.30 In fact, the report states that less than 10% of older adolescents sleep 9 hours or more per night during school nights. The numerous adverse consequences associated with chronic sleep restriction have led to the characterization of adolescents as high risk for problematic sleepiness by the National Institutes of Health.31 High school students with below-average grades typically go to bed later or at irregular times, and sleep fewer hours at night, compared to students with higher grades.29 Such sleep complaints have also been associated with increased use of stimulants and alcohol, symptoms of depression, and a higher frequency of behavioral problems than those without complaints.29,32–35
This pattern of late sleep onset continues through their college years.36–40 One study indicated that 73% of college students reported at least occasional sleep problems,37 while another showed that more than 48% of students suffer from sleep debt. Student stress, social habits, and educational demands may contribute to these problems. One survey found that 40% of US college students feel well-rested only 2 days per week or less.41 The tendency to delay bedtimes and extend rise times seems to extend across the college population,34,42,43 leaving this population at risk for serious consequences of sleep debt. Sleep quality is closely linked with mental and physical health and well-being, thus intervention programs aimed at improving sleep in this population are recommended.36,39,40,43,44
DSPD is common among adolescents45–47 and further increases their susceptibility to chronic sleep restriction and associated detrimental outcomes.48 DSPD is characterized by the American Academy of Sleep Medicine as a circadian rhythm sleep–wake disorder. It is characterized by sleep onset that occurs significantly later than desired bedtimes and societal norms.49 Individuals with DSPD also exhibit long sleep latencies when attempting to sleep at conventional bedtimes. Patients are diagnosed with DSPD if these symptoms cause distress and impairment and if they persist for at least 3 months. DSPD accounts for approximately 0.2%–10% of insomnia patients presenting to sleep medicine clinics, and adolescent prevalence estimates exceed 7%.48 Studies involving 22 adolescents with DSPD showed that 59% of the participants demonstrated poor scholastic performance and 45% displayed various behavioral problems.45
Several biological and behavioral factors likely contribute to the development of DSPD, including misalignment between the timing of circadian phase (eg, CBT nadir) and timing of sleep, longer circadian tau, increased sensitivity to light in the phase-delay portion of the phase response curve (PRC), and slower buildup of homeostatic sleep drive compared to normal controls. A more detailed discussion of these factors is beyond the scope of this paper and can be found in the work of Micic et al50 and Gradisar and Crowley.51
The effects of light on circadian phase, melatonin levels, and sleep
Auger et al52 prospectively compared light exposures in 16 adolescents diagnosed with DSPD and 22 age-matched controls. The authors hypothesized that DSPD patients would receive significantly more light in the evening hours (ie, in the delay portion of the PRC) because both groups of adolescents had a fixed wake time, but DSPD patients would stay awake longer and have more opportunities to receive evening light. Participants were asked to wear actiwatches continually for 386 days to evaluate light exposures during winter, summer, fall, and spring. Evening hours were defined as between 8 pm and 5 am, while morning hours were defined as between 5 am and 2 pm. Participants with DSPD received more evening light and less morning light than controls, but also seemed to receive less light prior to sleep, once the sleep onset times were taken into account. The authors did not find significant differences in regard to sleep offset times. After adjusting for sleep timing, the authors concluded that exposure to light directly prior to falling asleep and upon waking did not contribute to the phase delay observed in adolescents with DSPD and hypothesized that these individuals may be less sensitive to morning light.
Peixoto et al53 compared sleep onset time of adolescents with and without electricity at home, evaluating 37 adolescents in southern Brazil for 1 week during the autumn (sunrise at 7 am; sunset at 5.30 pm). The students who lived in homes without electricity experienced light levels of <3 lux in all rooms after sunset. The students who lived in homes with electricity experienced varying light levels from 16 to 90 lux. None had access to a computer, cell phone, video games, or other electronic media. The data showed that significantly earlier sleep onset occurred on school days for adolescents living without electricity; adolescents with electric lighting at home typically experienced delayed sleep onset and shorter sleep duration. Overall, sleep duration was reduced by approximately 1 hour in adolescents with electricity at home.
These results are consistent with those from Wright et al54 who studied eight individuals who went camping in the Colorado Rocky Mountains for 2 weeks. During the camping trip, the group experienced robust 24-hour light–dark patterns including daytime light exposures of at least four times greater than what they typically received prior to the trip. Participants showed an advance in DLMO by approximately 2 hours when camping compared to prior to the trip, although they did not observe a significant change in sleep duration.
More recently, Piosczyk et al55 reported on sleep parameters in five adults who lived in a Stone Age-type environment for 2.5 months. On average, the study participants experienced 2-hour phase advances in sleep onset, 0.5-hour advances in wake times, and 1.5-hour increases in sleep duration. Together, these studies underscore the importance of monitoring both daytime and evening light exposures for sleep health. Additionally, these studies suggest that electric lighting has contributed to the delayed sleep onset and increased sleep debt typically found in patients with DSPD.
Another concern is whether adolescents and DSPD patients are more sensitive to evening light. Aoki et al56 investigated this question by comparing nocturnal melatonin suppression by light in 15 DSPD patients and 15 age-matched controls. The experiment occurred over two nighttime sessions. In the first session, participants remained in darkness and the peak melatonin time was determined. In the second session, participants slept in dim light (<10 lux) until 2 hours prior to their peak melatonin concentration, at which time they were awakened and received 1,000 lux of light for 2 hours. Results showed that DSPD patients had significantly later peak melatonin than controls and suppressed significantly more melatonin after light exposure, suggesting a hypersensitivity to light in the phase delay portion of the PRC in those with DSPD.
In a recent study, Crowley et al57 investigated whether sensitivity to light differs between stages of adolescent puberty. Melatonin suppression after a 1-hour exposure to three light levels (15, 150, and 500 lux) was measured in 38 pre- to mid-pubertal (9.1–14.7 years) and 29 late- to post-pubertal (11.5–15.9 years) adolescents. Melatonin suppression was calculated relative to melatonin levels obtained in a dark, control night. One group received the light in the morning and the other received the light in the evening. The younger pubertal groups showed significantly greater melatonin suppression to all three lighting conditions during the evening light exposure, but no significant differences between the groups were observed in the morning melatonin suppression. Authors suggest that evening light exposures may be particularly disruptive of sleep in younger adolescents.
Figueiro et al58 investigated the impact of morning and evening light on DLMO in eighth grade students at a North Carolina school with plentiful daylight availability. In the study, eleven teenage participants wore, from their wake times until 3 pm for 5 consecutive school days, special orange-tinted glasses that block optical radiation below 525 nm. They found that the students’ DLMO was delayed by approximately 30 minutes compared to the previous week, when the orange-tinted glasses were not worn. They repeated the same protocol in a between-subjects study, where eleven teenage participants in the same school wore, for 5 consecutive school days, the orange-tinted glasses while another eleven age-matched participants did not. DLMO was delayed by 30 minutes in those who wore the orange-tinted glasses compared to those who did not. No significant differences in short-term performance (reaction times) and mood scales were observed between the two groups.59
In a third study, Figueiro et al60 measured DLMO of 16 teenage students in upstate New York in winter and spring. DLMO was delayed by approximately 20 minutes in spring, relative to winter. Sleep onset was delayed by an average of 16 minutes in the spring, relative to winter. The authors found that adolescents sleep less in spring than in winter. Evening daylight can delay sleep onset; in combination with a fixed time to rise in the morning, this can lead to sleep deprivation.
The delays in DLMO and sleep onset shown in these studies were consistent with the differential circadian light exposures measured by the Daysimeter, a calibrated personal light and activity meter.15,61 Restricting circadian light exposure in the morning and extending it in the evening will delay evening DLMO and sleep onset. Controlling light exposure only in the morning or only in the evening may not result in the predicted or desired effect because light exposure at any time over the course of the 24-hour day will have an effect on the circadian clock.
Recently, Hersh et al62 examined the relationship between 100 adolescent high school students’ sleep patterns and behavior and their urinary 6-sulfatoxymelatonin (aMT6s). They asked adolescents to fill out questionnaires probing their nighttime habits, sleep patterns, chronotype, and ambient light exposure. Participants were also asked to collect first-morning urine samples in their homes. Results showed no significant association between any self-reported measure of ambient light in and around the bedroom and the aMT6s levels. Participants who reported the latest bedtimes on weekends had the lowest midweek melatonin levels. Authors explained their results by hypothesizing that those who slept latest on weekends had the latest circadian phase, and thus their first morning urine sample reflected an incomplete overnight secretion of melatonin.
The effects of self-luminous devices on circadian phase, melatonin levels, and sleep
The US Census Bureau reports that in 2011, more than 83% of children and adolescents (age 3–17 years) in the US live in a home with at least one computer.63 In addition to affecting children and adolescents, the use of computer screens at home can impact adults as well. In 2003, 62% of US households had a computer in the home, with 55% having Internet access. Today, more than 75% of all households have a computer and 72% of households report accessing the Internet.63 According to the 2011 Sleep in America Poll, approximately six in ten people use a computer within the hour before bedtime at least several nights per week.64 According to the 2014 Sleep in America Poll, 16% of children reported reading or sending emails or text messages after initiating sleep.65
Recently, Hysing et al66 surveyed nearly 10,000 Norwegian adolescents aged 16–19. Their study found that adolescents who use self-luminous devices during the day and at bedtime are more likely to experience a delay in the timing of sleep onset, abbreviated sleep duration, and greater sleep deficiency. Results showed a dose–response relationship between sleep duration and self-luminous device usage. It is important to note that associations are not the same as causality.
In 2015, Hale and Guan67 reviewed the literature on sleep and “screen time” and found that screen time is strongly associated with a delay in the timing of sleep onset and abbreviated sleep duration in children and adolescents. The authors recommend that screen time should be limited to minimize the deleterious effects on sleep and overall well-being.
It is reasonable to expect that those who go to bed later and have a fixed wake time will experience shorter sleep durations, even if they do not use self-luminous devices before bed. The direct effects of self-luminous displays on sleep in adolescents may be related to the increase in evening light exposures from these devices. More importantly, the newer devices use light emitting diodes (LEDs), which emit more short-wavelength radiation. Cajochen et al68 examined the effects of LED-backlit computer screens on circadian rhythms and cognitive performance in a study of 13 participants. Two 5-hour experimental sessions were held with one of two computer screen types: white LED-backlit computer screen or non-LED-backlit screen (cathode fluorescent lamp). The screens had similar illuminance and visual comfort ratings, but different spectral composition. The LED-backlit screen emitted more short-wavelength radiation. Results showed that a 5-hour exposure to a LED-backlit computer screen significantly suppressed melatonin, reduced sleepiness levels, and enhanced cognitive performance (eg, attention, memory) compared to a non-LED-backlit screen. Although melatonin levels still rose during the night, they did not rise as steeply as when participants experienced the non-LED-backlit computer screen condition.
Wood et al69 investigated the effects of 1- and 2-hour tablet use (iPad) on acute melatonin suppression in adolescents and college students. Three experimental conditions were employed in a within-subjects study: 1) tablet use at full brightness; 2) tablet use while participants were wearing orange-tinted glasses that block optical radiation below 525 nm; and 3) tablet use while participants were wearing light goggles delivering 40 lux of a 470 nm light. Condition 2 was used as a “true negative control” because it was not expected to suppress melatonin while condition 3 was used as a “true positive control” because the 470 nm light was expected to suppress melatonin. Melatonin suppression from the tablet-only condition was 3% after 1-hour use and 22% after 2-hour use. To place these numbers in perspective, Rea et al9 showed, based on a series of previously published studies, that the half maximum saturation for acute melatonin suppression after 1 hour exposure is approximately 35% and the maximum suppression is 70%.
More recently, Chang et al70 confirmed and extended these results by showing that participants using light-emitting e-readers delayed DLMO by more than 1.5 hours, acutely suppressed melatonin by approximately 55%, delayed sleep by approximately 10 minutes, and significantly reduced the occurrence of rapid eye movement sleep compared to reading a print book in near darkness for 4 hours prior to bed. Light levels while using the e-readers were between 30 and 50 lux at eye level and light levels in near darkness, while participants were reading the print book, were not presented in lux. Given that participants remained in very dim light while reading the print book, it cannot be ruled out that it was the lack of evening light that advanced DLMO.
Following the Wood et al69 study, Figueiro and Overington71 investigated the impact of self-luminous devices on acute melatonin suppression in adolescents only. Twenty high school students (aged 14–18 years; 13 females) participated in this field study on 2 consecutive nights at home. On the first night, participants were asked to wear the orange-tinted glasses for 3 hours prior to their normal bedtimes and to work with self-luminous devices (computers, tablets, phones) for the duration of the study. All participants wore a calibrated light meter as a pendant. They were asked to collect saliva samples at 60, 90, and 120 minutes after the start of the experiment. For the second day of testing, participants took the saliva tests at the same times as on the first night, but only wore the orange-tinted glasses for the first hour of the data collection period in which they were using the self-luminous devices. Results showed that 1-hour exposure to light from the self-luminous devices significantly suppressed melatonin by 23%, whereas 2-hour exposure suppressed melatonin by 38%, which was greater than the suppression obtained in the Wood et al69 study. Given that measured light exposures were similar between studies, these data suggest that adolescents are more sensitive to light for evening acute melatonin suppression than those in their mid-20s and 30s.
These studies suggest that social activities combined with evening exposure to light from self-luminous devices, may be delaying the onset of sleep and curtailing the total sleep time during work/school days. One recent study, however, disputes this notion showing that sleep in industrial society is not significantly less than in those still living in preindustrial societies in Tanzania, Bolivia, and Namibia.72 Additional field and epidemiological studies would increase the understanding of the relationship between sleep, evening light, and social activities, and whether controlling behavior and light would increase sleep duration in DSPD patients.
Advancing sleep and circadian phase with light
Light therapy, if given and removed at the appropriate times, has the potential to be a powerful nonpharmacological tool to advance the timing of sleep in adolescents, college students, and persons with DSPD. However, too few field studies have been performed, and results demonstrating that light treatment is beneficial are mixed, as will be summarized below. In general, results are positive when studies are performed under controlled conditions (ie, laboratory settings), while studies that were performed in the field showed inconsistent results. Some reasons as to why this occurs are also discussed.
Laboratory studies
Rosenthal et al73 published one of the first studies investigating the phase shifting effects of 2-hour morning bright light exposure as a treatment for DSPD. The study was designed to be a crossover between an “active” treatment and a “control” treatment. The active treatment consisted of 2,500-lux full-spectrum light treatment for 2 hours in the morning between 6 and 9 am and dark goggles worn from 4 pm until dusk. The control treatment consisted of 300-lux full-spectrum light for 2 hours between 6 and 9 am and clear goggles, worn from 4 pm until dusk. After dusk, light was restricted to one or two bedside lamps for both conditions. The timing of awakening was kept constant. The CBT was measured every 5 minutes for a 24-hour period using a Vitalog monitor and thermistor. Multiple sleep latency tests were performed before and after each treatment condition. There was no significant difference in the expectation questionnaire responses. Participants rated the active intervention as being better than the control. Greater alertness and earlier sleep times during the active intervention were also reported. The active intervention significantly phase advanced circadian rhythms of CBT by approximately 1 hour and 25 minutes compared to 10 minutes in the control condition. A significant increase in multiple sleep latencies was observed at 9 and 11 am during the active intervention compared to the control intervention. The authors did not specify whether the light levels reported were corneal or horizontal illuminances, but if the light levels reported were measured at eye level, the control condition delivered fairly high light levels, and yet, the observed phase shifting of CBT was very modest, suggesting a reduced sensitivity to morning light in this population.
In a laboratory setting, Watanabe et al74 administered 5 days of light therapy to a group of individuals with DSPD. Polysomnography (PSG) was used each night starting at each patient’s bedtime and ending with their typical wake time. Light therapy was administered to each patient for 3 hours in the morning, starting 1.5 hours after CBT nadir. CBT was sampled every 5 minutes using a rectal temperature probe and ambulatory temperature monitor for 10 days which included the periods of light therapy application and PSG measurements. Sleep onset time was significantly advanced by more than 2 hours, and sleep offset time was significantly advanced by more than 3 hours. Total sleep time and amounts of stage 2 and rapid eye movement from the PSG were reduced after light therapy. The timing of CBT nadir was advanced in all patients after the light therapy.
These studies suggest that, in controlled laboratory conditions, light can be used to phase advance the timing of sleep and circadian markers in those suffering from DSPD.
Field studies
Addressing the concerns of adolescents losing sleep during the week due to school schedules, Crowley and Carskadon75 asked 12 adolescents to maintain a regular sleep schedule of 7.5 hours during weekdays, while allowing them to sleep for 9 hours during weekends, with bedtimes delayed by 1.5 hours. Participants were randomly either woken up 3 hours later than the weekday schedule (“typical” condition) or were woken up 1 hour later than the usual schedule, and given a chance to take a 2-hour nap on Saturday and Sunday (“nap” condition). All 12 adolescents continued on to a second experiment with 33 participants, repeating the same sleep schedule during weekdays, but were assigned a “light” condition instead of a nap. In this condition, participants sat 24 cm in front of a short-wavelength LED light box (464–484 nm) at either 30% or 100% intensity for at least 30 minutes. The results showed that DLMO occurred later on the weekends, with a mean shift of 45 minutes for the “typical” condition and 41 minutes for the “nap” condition. During the second experiment, DLMO shifted 46 minutes for a “typical” condition and 38 minutes on a “light” condition weekend. The delays resulting from the “nap” and “light” conditions were similar, which was not expected, causing the authors to suggest a combination of those conditions to compensate for sleep recovery. A factor to consider is that the morning light may have been administered at the wrong time in their PRC to promote a phase advance. Another main issue with the study was that the 24-hour light exposure was not monitored; therefore, participants who received the morning short-wavelength light upon waking may have received too much evening light that counteracted their morning light treatment. On the other hand, the control condition may have received enough outdoor morning light that would have shifted DLMO similar to the short-wavelength light treatment. Overall, sleep start and end times were later on the experimental weekends, and participants generally had less sleep duration on weekends when they napped. Without monitoring and control of the total light exposures over the waking period, it is difficult to interpret the negative results observed by the authors.
Consistent with the idea that controlling the entire 24-hour light–dark exposure is important to determine the net effect of a light treatment on circadian phase, Sharkey et al17 placed two groups of young adults who were “late types” on an advanced sleep–wake schedule. After a baseline week, participants kept individualized, fixed, advanced 7.5-hour sleep schedules for 6 days. During the intervention week, all participants were scheduled to wake up between 1 and 2.5 hours earlier than the baseline weekday schedule. Participants were randomly assigned to groups to receive “blue” (470 nm, ∼225 lux, n=12) or “dim” (<1 lux, n=13) light for 1 hour after waking each day. No evening light intervention was performed. Participants also filled out questionnaires probing their mood, depression, anxiety, and stress.
After 1 week on the advanced sleep–wake schedule, both groups (control and intervention) experienced similar circadian phase advances. The average DLMO advances (mean ± SD) were 1.5±1.1 hours in the dim light group and 1.4±0.7 hours in the blue light group. The total 24-hour light exposures, as measured by the Daysimeter placed near the cornea, were similar for both groups, even though the amount of circadian light experienced by the intervention group in the first hour after waking was significantly greater than those experienced by the control group; thus the daily 24-hour light exposures associated with the advanced sleep–wake schedule were sufficient to advance circadian phase, with or without the morning light intervention. No significant changes in mood, depression, anxiety, and stress were observed before and after the intervention.
Appleman et al14 placed study participants on an advanced sleep–wake schedule; both groups were woken up 1.5 hours earlier than their typical wake times. One group received a lighting intervention designed to advance circadian phase: short-wavelength light in the morning and orange-tinted glasses that block optical radiation below 525 nm in the evening. The other group received a light intervention designed to delay circadian phase: orange-tinted glasses in the morning and short-wavelength light in the evening. The advancing light intervention group advanced their circadian phase (132±19 minutes), while the delaying light intervention group delayed their circadian phase (59±7.5 minutes), showing that the light–dark pattern can override the phase advancing effect of an advanced sleep–wake schedule. These results were similar to those by Mitchell et al76 who showed that coordinating timing of bright light exposures and the sleep–wake schedules promotes circadian adaptation to a night shift schedule. Together, these studies show the importance of coordinating the sleep–wake schedule with the timing of light exposure and the importance of controlling both morning and evening light exposures to achieve the desired circadian phase shift.
In a follow-up study, Figueiro et al18 placed 12 early types and eleven late types on a 2-week, advanced sleep–wake schedule, twice in a mixed design. During the intervention weeks, half of the participants were randomly assigned to receive an advancing light pattern (2 hours of 470 nm light goggles in the morning and 3 hours of orange-tinted goggles in the evening) and the others received a delaying light pattern (3 hours of 470 nm light goggles in the evening and 2 hours of orange-tinted goggles in the morning). After a 3-week washout period, they were placed on the opposite protocol. The sleep–wake schedule was advanced by 1.5 hours on the intervention weeks. DLMO was collected at the end of baseline and intervention weeks. Daily 24-hour light–dark patterns were monitored with the Daysimeter.15,61 DLMO was significantly delayed after the delaying light exposure pattern and significantly advanced after the advancing light exposure pattern for both early and late types, compared to the baseline week. For the 12 participants in the Early Group, the mean ± SD phase advance was 122 ± 34 minutes when participants were in the advancing light intervention and the mean ± SD phase delay was −37 ± 38 minutes when participants experienced the delaying light intervention. For the 11 participants in the Late Group, the mean ± SD phase advance was 108 ± 28 minutes and the mean ± SD phase delay was −59 ± 28 minutes when participants experienced the advancing and the delaying light interventions, respectively. The research team found no significant difference in how early and late types respond to light–dark exposure patterns.
Using the knowledge that short-wavelength light is very effective for phase shifting DLMO, Lack et al77 examined the effects of utilizing blue light to phase advance sleep and melatonin rhythms in those suffering from mild DSPD living outside laboratory conditions; 12 participants were assigned to a blue light group and six participants were assigned to a control group. During the treatment week, each morning upon waking, participants in the blue light group received 2 hours of light via light goggles delivering 470 nm (blue) light. Wake times and exposure to blue light were gradually advanced each morning over the treatment week, starting from each participant’s typical wake time and finishing at the target wake time of 6 am. The control group followed the same protocol, but they did not receive the blue light intervention in the morning. DLMO was significantly advanced by 2.5 hours in the treatment group but remained the same in the control group. However, mean sleep onset time, wake time, and total sleep time were not significantly changed with the treatment. Although wake times were advanced by 3 hours during the intervention week, the sleep onset times were only advanced by 70 minutes in the treatment group. In that study, participants were not given instructions about maintaining an earlier sleep–wake schedule, and so it is possible that the delayed sleep habits may have overridden the effects of the observed DLMO phase advance.
In all of these field studies, compliance to the light treatment may have played a role in reducing the response. One way to overcome compliance issues and perhaps increase efficacy of light therapy in this population is to deliver light through closed eyelids, while patients are asleep. Robinson et al78 measured light transmission through human eyelids. The results suggest that the eyelids function as a red-pass filter, with transmission of 14.5% of 700 nm light, but only 3% or less transmission of light at 580 nm and below. Similarly, Ando et al79 showed that red light (615–630 nm) was attenuated by 1/20, while blue (400–510 nm), and green (540–565 nm) light were attenuated by 1/100.
More recently, Bierman et al80 measured and proposed a model for eyelid transmittance (Figure 1). They acquired data using a novel technique developed to provide accurate measurements of eyelid transmittance across the visible spectrum. Eyelid transmittances of 27 participants were measured using a phosphor-converted, white, LED placed under the eyelid and a spectrometer with a fiber optic input that recorded the transmitted light. The input end of the spectrometer fiber optic and the white LED were mounted to a handheld wand that fixed the relative positions of the source and the detector. The mean ± SD optical density of the eyelid from 450 to 650 nm was 2.1±0.3, with a range of approximately 1.0. Results showed that transmittance in the 470 nm region are 0.2%, while transmittance near 530 nm is 0.5%.
Figure 1.
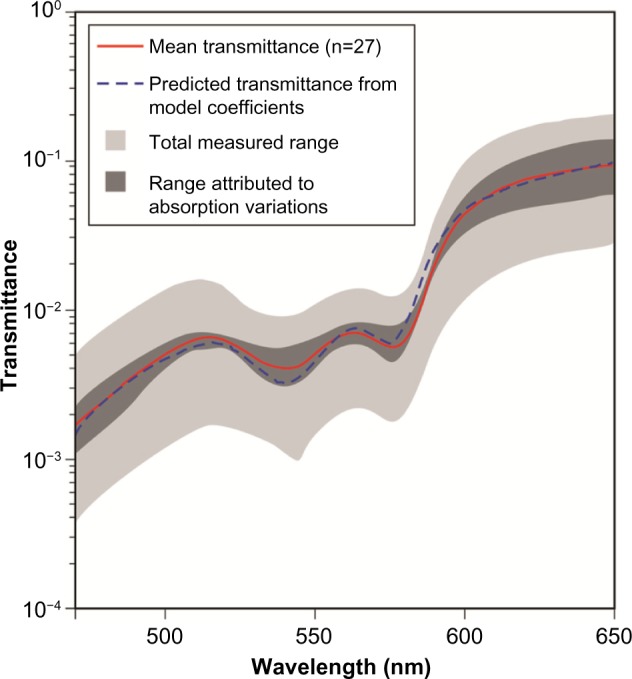
Model for eyelid transmittance proposed by Bierman et al. Figure adapted from Bierman A, Figueiro MG, Rea MS. Measuring and predicting eyelid spectral transmittance. J Biomed Optics. 2011;16(6):067011.80 Copyright 2011 Society of Photo Optical Instrumentation Engineers. One print or electronic copy may be made for personal use only. Systematic electronic or print reproduction and distribution, duplication of any material in this paper for a fee or for commercial purposes, or modification of the content of the paper are prohibited. http://dx.doi.org/10.1117/1.3593151.
Earlier studies investigated whether light delivered through closed eyelids could phase advance body temperature or melatonin rhythms. Ando et al81 exposed five participants to 500 lux of a white light delivered through closed eyelids for 3 hours prior to waking during 12 days. Five participants received a placebo light delivering 0.1 lux at the same time. Although not statistically significant, there was a small advance in the CBT rhythm and a delay in the melatonin rhythms in those receiving the active light. Authors pointed out some methodological issues that could have explained the negative results, including that the timing of light administration could have been too early and the light levels were too low to be effective through the eyelids.
In the study by Cole et al,82 participants diagnosed with DSPD received either bright light (2,700 lux) or dim red light (0.1 lux) through closed eyelids for 26 days. Light masks remained on at full brightness for 3 hours prior to waking. Participants were also asked to advance sleep time (equivalent to 1 hour per week), reduce evening light exposure, and avoid daytime naps during the study. Authors found that while the acrophase of the melatonin rhythms advanced in the bright light group, it was significantly different from the control group only when the analyses included data from participants who had melatonin acrophase after 6 am. These results imply that the treatment was only effective in those who tended to be more delayed. It is not clear from the study, however, whether it was the light treatment or the removal of evening light that resulted in the observed effect, given that the light levels transmitted through closed eyelids were still small.
Figueiro et al83,84 showed that, if light levels are adjusted to account for eyelid transmittances based on the Bierman et al80 model, light through closed eyelids delivered before predicted CBT nadir acutely suppressed melatonin and phase delayed DLMO by approximately 20 minutes after 1 night of wearing the mask during sleep. The downside of delivering continuous light through closed eyelids is the heat buildup, because light levels needed to account for eyelid transmittance are as high as 50,000 lux at the eyelid. The same group later showed that flashing 480-nm (blue) light (2-second flash every 30 seconds for a total of 1 hour) phase delayed DLMO by approximately 32 minutes in just 1 night in the laboratory.85 The advantage of delivering a train of brief flashes is the lack of heat buildup from the light source, which makes it possible to use the light mask at home. These results are consistent with those from Zeitzer et al86 who published a laboratory study involving 13 participants, where six participants received a flashing light pulse via closed eyelids. Their results showed that, compared to a dark night, those who received the flashing light for one night phase delayed DLMO by approximately 30 minutes.
Following the laboratory study, Figueiro87 collected field data from 28 participants (nine early awakening insomniacs) in an 8-week, within-subjects study. Twice, participants collected data during 2 baseline weeks and 1 intervention week. During the intervention week, participants wore a flashing blue (active) or a flashing red (control) light mask during sleep (Figure 2). Light was expected to delay circadian phase. Saliva samples for DLMO were collected at the end of each baseline and intervention week. Wrist actigraphy and Daysimeter15,61 data were collected during the entire study. Results showed that, compared to baseline, flashing blue light, but not flashing red light, significantly delayed DLMO (by approximately 34 minutes). Compared to day 1, sleep start times were significantly delayed (by approximately 46 minutes) at day 7 after the flashing blue light. More importantly, the light intervention did not affect sleep efficiency. Future work should investigate how effective a light mask can be at delivering phase advancing light to adolescents and DSPD patients while they are asleep. The challenge would be to determine the timing of CBT nadir, so that light can be applied at the correct portion of the PRC. A recommended way to accomplish this is to determine DLMO,88 which has been shown to occur approximately 6–7 hours prior to CBT nadir. Data collection to determine DLMO can be done in participants’ homes.89,90
Figure 2.
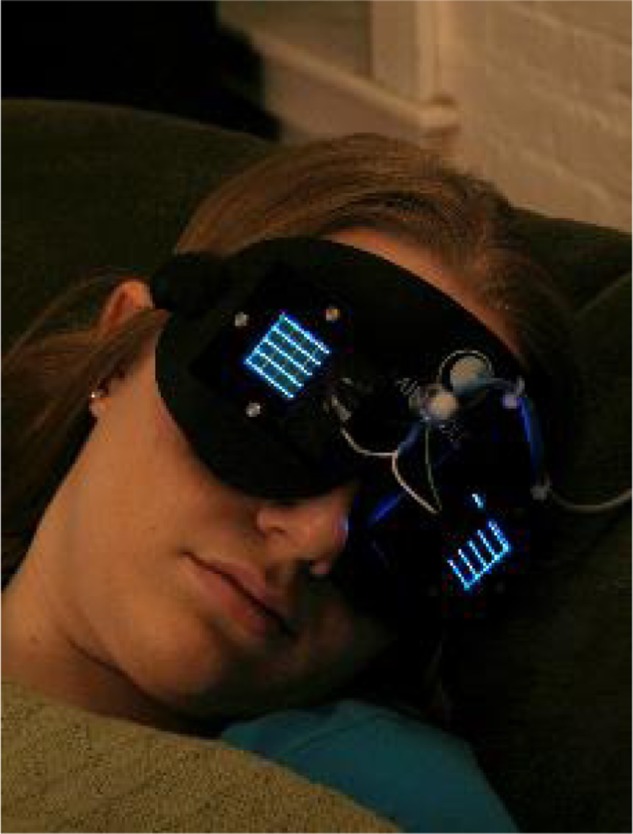
Flashing blue light mask used by older adults living at home.
Notes: Flashing light was delivered through closed eyelids during sleep. The blue light mask shown here contains two blue LED arrays (λmax =480 nm, FWHM =24 nm), one for each eyelid. In the study, the light-stimulus condition was a train of blue or red light pulses: 2-second duration light pulses spaced apart 30 seconds, for no more than 3 hours, delivered before predicted minimum core body temperature. Photo courtesy of Lighting Research Center, Rensselaer Polytechnic Institute.95
Abbreviations: LED, light emitting diode; FWHM, full width at half maximum.
Summary
Table 1 summarizes the findings of the studies investigating how light can be used to treat DSPD symptoms in the lab and in the field. In summary, the literature suggests that a tailored lighting intervention has the potential to be used as a nonpharmacological treatment to help advance circadian phase in those suffering from DSPD, but results are still mixed. Light therapy also has the potential to improve mood and depression in this population. However, as expected, the effect of light on phase advancing the timing of the circadian clock outside laboratory conditions does not seem to be as strong as when participants are placed under controlled laboratory conditions. Data from Figueiro et al18 and Appleman et al14 suggest that a phase advance of approximately 2 hours can be obtained after 1 week of light treatment that includes morning short-wavelength light and evening orange-tinted glasses. These results were consistent with those by Burgess et al91 and Revell et al92 who showed that 3 days of morning bright light and no control of evening light resulted in a phase advance of approximately 2 hours. Therefore, while the potential for successful treatment of DSPD symptoms with light therapy is considerable, it perhaps requires some patience and dedication on the part of the patients and families.
Table 1.
Studies investigating how light can be used to treat DSPD symptoms in the lab and in the field
| Author | Year | Title | Objective | Participants | Methods/protocol | Results |
|---|---|---|---|---|---|---|
| Ando et al81 | 1999 | Light mask 500 lux treatment for delayed sleep phase syndrome | To test, in the field, whether a light mask treatment, delivering light through eyelids during sleep advanced circadian markers in patients diagnosed with DSPD | Five participants meeting the criteria for DSPD experienced the active light and five experienced the placebo light | Participants were divided into two groups by bedtime before or after 2 am, based on the 2-week baseline sleep log data. Participants were then randomly assigned to the active (500 lux for 3 hours prior to awakening for 12 days) or the placebo light condition (0.1 lux light with the same timing). CBT and urinary 6-sulfatoxymelatonin were measured. Mood was assessed with the SIGH-SAD at baseline, during treatment, and immediately after treatment. Sleep logs were kept at all times | Slight, albeit not statistically significant phase advance of the body temperature rhythm and a slight phase delay of the melatonin rhythm. Both groups reported significant mood improvement |
| Appleman et al14 | 2013 | Controlling light–dark exposure patterns, rather than sleep schedules, determines circadian phase | To study, in the field, the effects of differing light exposure timing on early and late sleepers, while placing participants on an advanced sleep schedule, and a delayed sleep schedule | 21 participants who normally woke between 6.30 and 8 am, and who went to bed between 11 pm and 1.30 am | Participants collected baseline data for 5 days after which half of the participants received an advancing light pattern (2 hours of 470-nm light goggles in the morning and 3 hours of orange-tinted goggles in the evening), while the remaining participants received a delaying light pattern (3 hours of 470-nm light goggles in the evening and 2 hours of orange-tinted goggles in the morning) for 7 days. Participants were placed on a 1.5-hours advanced sleep–wake schedule. DLMO was collected at the end of baseline and intervention weeks | After 7 days of the light and sleep intervention, DLMO was significantly delayed and advanced respectively following the corresponding light treatment |
| Cole et al82 | 2002 | Bright light mask treatment of delayed sleep phase syndrome | To test, in the field, whether a light mask treatment delivering light through closed eyelids during sleep would phase advance circadian rhythms in DSPD patients | 54 participants. 28 were randomly assigned to the bright light group and 26 to the dim light group | Light masks delivering 2,700 lux at the surface of the closed eyelid (estimated corneal illuminance was 57 lux). For dim light treatment, corneal illuminance was 0.1 lux. Lights were turned on 4 hours prior to waking, ramped up for 1 hour, and stayed on at full brightness until participants got up | Intervention produced significantly earlier phases and earlier sleep onsets only among participants whose baseline 6-sulfatoxymelatonin acrophase was later than 6.02 am. Despite equal expectations at baseline, participants rated bright treatment as more effective than dim treatment |
| Crowley and Carskadon75 | 2010 | Modifications to weekend recovery sleep delay circadian phase in older adolescents | To study, in the field, the effects of an extended weekend sleep period on the sleep–wake cycle in adolescents, (experiment 1) and to test whether a modified weekend sleep schedule or lighting treatment and extended weekend sleep could adjust and improve the sleep–wake cycle (experiment 2) | 12 participants were included in experiment 1, and 33 participants were included in experiment 2 | Experiment 1 was a 4-week, within-subjects counterbalanced design, which compared a “typical” weekend to a “nap” weekend. Each participant wore an actigraph. Experiment 2 was a follow-up to experiment 1, and was a 2-week between-subjects protocol with the two groups maintaining the identical sleep schedules and differing morning light exposure. The light group sat in front of an LED short-wavelength (454–484 nm) light box for 1 hour after awakening on Saturday and Sunday. Before Friday, and after Sunday, saliva samples were collected for DLMO for both experiments | Adolescents experienced a phase delay after keeping an extended weekend sleep schedule. Changing wake time or exposing participants to short-wavelength light therapy did not allow the adolescents’ circadian rhythm to stabilize in this study |
| Figueiro et al18 | 2014 | The effects of chronotype, sleep schedule, and light–dark pattern exposures on circadian phase | To study, in the field, the effects of differing light exposure timing on early and late sleepers, while placing participants on a fixed sleep schedule | 23 participants divided between self-reported early (n=12) and late (n=11) sleepers | Participants collected baseline data for 6 days, where they wore a light sensing device on their wrists. During the intervention weeks, half of the participants were randomly assigned to receive an advancing light pattern (2 hours of 470 nm light goggles in the morning and 3 hours of orange-tinted goggles in the evening) and the others received a delaying light pattern (3 hours of 470 nm light goggles in the evening and 2 hours of orange-tinted goggles in the morning). After a 3-week washout period, they were placed on the opposite protocol. The sleep–wake schedule was advanced by 1.5 hours on the intervention weeks. DLMO was collected at the end of baseline and intervention weeks | DLMO was significantly delayed and advanced respectively, following the corresponding light treatment. There were no significant differences in DLMO times between groups, but later chronotypes tended to delay more than earlier ones with the delaying light and earlier chronotypes tended to advance more than later chronotypes with the advancing light |
| Lack et al77 | 2007 | Morning blue light can advance the melatonin rhythm in mild delayed sleep phase syndrome | To test, in the field, whether morning blue light exposure,administered via LEDs, can promote an advance of the melatonin rhythm and the sleep period in participants with mild DSPD | 18 participants meeting the criteria for DSPD were recruited from a university population | The participants were randomly selected to receive 2 hours of 470 nm light upon awakening or a control intervention. Each participant kept a sleep–wake diary during baseline week. Wake times and exposure to blue light were gradually advanced each morning over the treatment week. The control group followed the same wake protocol, but they did not receive the morning blue light intervention. Participants self-selected their bedtimes. At the end of the week, saliva samples were collected to determine DLMO | DLMO was significantly advanced by 2.5 hours in the treatment group and remained the same in the control group. However, mean sleep onset time, wake time, and total sleep time were not significantly changed with the treatment. Sleep onset times were advanced by 70 minutes in the treatment group. Authors state that effective DSPD treatment may require adjunct behavioral instructions |
| Rosenthal et al73 | 1990 | Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome | To test, in the lab, whether patients with DSPD can have their circadian rhythms successfully phase advanced by a combination of morning bright light and late afternoon/evening light restriction | 20 participants who met DSPD criteria: sleep onset after 1 am at least 4 nights per week, significant disruption of work/social relationships due to sleep patterns, and inability to be alert in the morning | In a crossover study, participants experienced an active treatment (2,500-lux full-spectrum light treatment for 2 hours between 6 and 9 am and dark goggles from 4 pm until dusk) and a control treatment (300-lux full-spectrum light for 2 hours between 6 and 9 am and clear goggles from 4 pm until dusk). After dusk, light was restricted to one or two bedside lamps for both conditions. The timing of awakening was kept constant. The CBT was measured every 5 minutes for a 24-hour period using a Vitalog monitor and thermistor. Multiple sleep latency tests were performed before and after each treatment condition | Participants rated the active intervention as being better, resulting in greater alertness and earlier sleep times than the control intervention. The active intervention significantly phase advanced circadian rhythms of CBT by approximately 1 hour 25 minutes compared to 10 minutes in the control condition. A significant increase in multiple sleep latencies were observed at 9 and 11 am during the active intervention compared to the control intervention |
| Sharkey et al17 | 2011 | Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules | To examine the effects of an advanced sleep/wake schedule and morning short wavelength (blue) light in 25 adults with late sleep schedules and subclinical features of DSPD | 25 participants with late sleep schedules and subclinical features of DSPD | After a baseline week, participants kept individualized, 1- to 2.5-hour advanced, 7.5-hour sleep schedules for 6 days. Participants were randomly assigned to groups to receive “blue” (470 nm, ∼225 lux) or “dim” (<1 lux) light for 1 hour after waking each day. Head-worn Daysimeters measured light exposure; actigraphs and sleep diaries confirmed schedule compliance. At the end of the baseline and intervention weeks, participants returned to the lab to collect saliva samples for DLMO. Participants also filled out questionnaires probing their mood, depression, anxiety, and stress | After 6 days, both groups showed significant circadian phase advances, but morning blue light was not associated with larger phase shifts than dim-light exposure. No significant changes in mood, depression, anxiety, and stress were observed before and after the intervention. Authors conclude that a fixed, advanced schedule should be effective at advancing sleep and circadian phase in those suffering from DSPD |
| Watanabe et al74 | 1999 | Effects of phototherapy in patients with delayed sleep phase syndrome | To examine, in the lab, the effects of short-term exposure to light therapy on PSG and CBT of DSPD patients | Six DSPD patients participated in the study | PSG was performed between bedtimes and natural awakening for 2 consecutive nights before light administration. After the baseline PSG, participants were given light therapy for 5 consecutive days. Light treatment was administered to each participant for 3 hours in the morning, starting 1.5 hours after the time of CBT nadir. CBT was sampled every 5 minutes using a rectal temperature probe and ambulatory temperature monitor for 10 days which included the periods of light therapy application and PSG measurements | Sleep onset time was significantly advanced by more than 2 hours and sleep offset time was significantly advanced by more than 3 hours. Total sleep time and amounts of stage 2 and rapid eye movement sleep from the PSG were reduced after light therapy. The timing of minimum CBT was advanced in all participants after the light therapy |
Abbreviations: CBT, core body temperature; DLMO, dim light melatonin onset; DSPD, delayed sleep phase disorder; h, hour(s); LED, light-emitting diode; min, minute(s); PSG, polysomnography; SIGH-SAD, Structured Interview Guide for Hamilton Depression Rating Scale – Seasonal Affective Disorder version.
Two reasons may explain the less robust effects shown in field studies. As mentioned earlier, the lack of compliance by participants may have impacted the results. In all of the field studies, participants were asked to expose themselves to the light treatment upon waking for at least 1 hour, which forces them to get up at least 1 hour earlier to comply with the experimental protocol. In real-life situations, it will be difficult to assure that DSPD patients or adolescents who have late sleep habits will comply with this requirement over a long period of time. One strategy to address this issue would be to adjust patients’ behaviors, and start the light therapy after the usual wake times for a few days and gradually shift the timing of light therapy to earlier times. Another strategy, as discussed, would be to deliver light through closed eyelids during sleep.
A second reason for the reduced effect of light therapy in the field is the fact that most studies control only the treatment light exposure, rather than controlling the total waking light exposures. One of the most interesting insights from the data from Sharkey et al,17 Appleman et al,14 and Figueiro et al18 is described in Rea et al.16 Using the modified model of the human circadian oscillator93 that allows for quantitative predictions of circadian phase changes resulting from light exposure, Rea et al16 showed that light exposures during total waking hours need to be monitored and controlled in order to predict circadian phase change resulting from a light treatment. The model by Kronauer et al93 consists of a light-stimulus phototransduction process (L) driving an oscillator-based pacemaker process (P). In the proposed model by Rea et al,16 circadian stimulus (CS) is used as input to process L instead of photopic illuminance (lux). CS is calculated using a spectral sensitivity function that matches the response by the circadian system. Parameters of the process P were revised based upon data from field studies where light exposures and circadian phase changes were measured.14,17,18 More specifically, two parameters in the process P were adjusted (k and q) and a time-dependent sensitivity modulation factor was removed. Overall, the proposed model by Rea et al16 improves upon the existing model by incorporating new knowledge of human circadian phototransduction; thus the model can provide more accurate quantitative predictions of circadian phase changes resulting from light exposures.
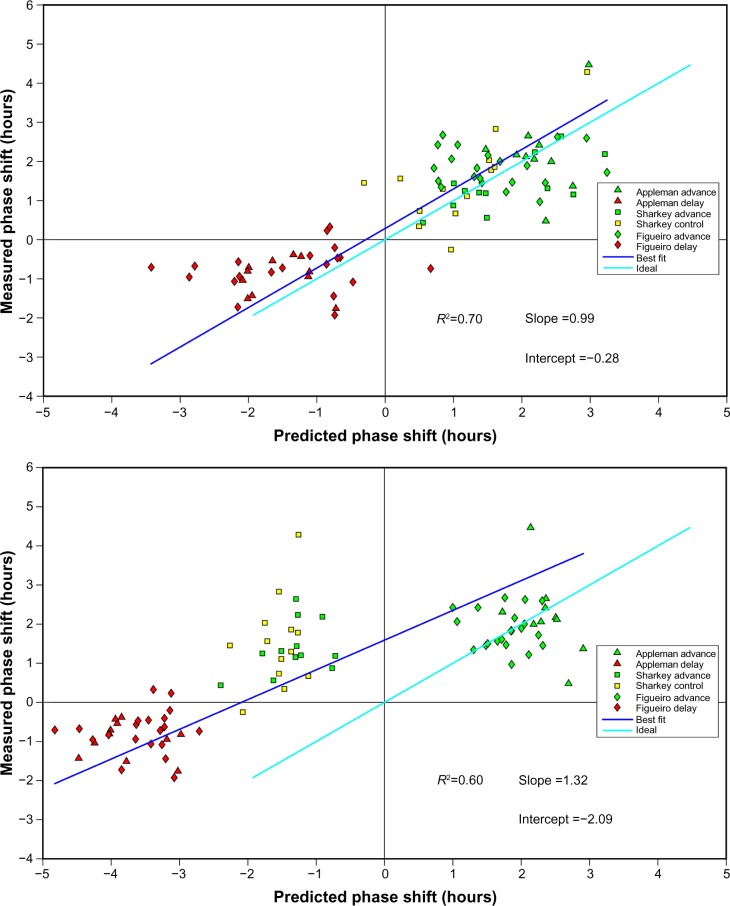
Model predictions of circadian phase changes based upon continuously measured 24-hour light exposures were compared to measured phase changes (DLMO) from the three field studies.14,17,18 Figure 3A shows the correlation between DLMO and the predicted phase changes calculated from the Daysimeter15,61 data and the modified Kronauer model16,93 which was statistically significant (R2=0.70; P<0.0001) with a prediction uncertainty of 1.75 hours (95% confidence). Interesting too, and shown in Figure 3B, when only the treatment light exposure, and not the total light exposures measured over the entire waking period, are included in the model, predictions of circadian phase change are not as good, as shown by the large deviation of the best fit from the ideal fit. Future field studies should test a priori predictions generated by this proposed model.
Figure 3.
Correlation between DLMO and the predicted phase changes calculated from the Daysimeter data and the modified Kronauer model.
Notes: (A) Measured changes in dim light melatonin onset (DLMO) from baseline to postintervention are plotted on the ordinate and circadian stimulus (CS)-oscillator model predictions based on actual measured light exposures during the intervention are plotted on the abscissa. (B) Measured changes in DLMO from baseline to postintervention are plotted on the ordinate and CS-oscillator model predictions based solely on the treatment light exposures (ie, not using light exposures measured throughout the day by the Daysimeter) are plotted on the abscissa. The ideal fit was determined using the least square method, where the difference between the measured DLMO and the predicted DLMO was calculated.
Conclusion
Light therapy, if properly delivered and controlled, can be a powerful nonpharmacological intervention to help advance the timing of sleep in those suffering from DSPD. New technologies are now available that can be used to facilitate light delivery at the appropriate times. More field research testing the effectiveness of these new lighting technologies and systems is warranted. Also warranted are field studies investigating how the control of the total waking light exposures increases the effectiveness of the light treatment. New phone applications that use similar model predictions as those proposed by Rea et al16 are now available and can provide light prescriptions to obtain circadian phase shifting more effectively.94 Future work should be designed to test these lighting prescriptions in real-life situations.
Acknowledgments
Rebekah Mullaney, Dennis Guyon, Greg Ward, Andrew Bierman, and Mark Rea of the Lighting Research Center are acknowledged for their technical and editorial support. Funding sources include the Office of Naval Research, National Institute on Aging (#R01AG042602), National Institute on Drug Abuse (#U01DA023822), and US Green Building Council.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewy A, Wehr T, Goodwin T, Newsome D, Markey S. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 4.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33(4):198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer W, Millam J, Bradley F. Photostimulation of Japanese quail by dim light depends upon photophase contrast, not light intensity. Biol Reprod. 1988;38(3):536–543. doi: 10.1095/biolreprod38.3.536. [DOI] [PubMed] [Google Scholar]
- 7.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535(1):261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50(2):213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Berson D, Dunn F, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 11.Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin ganglion cells receive bipolar and amacrine cell synapse. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 13.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appleman K, Figueiro MG, Rea MS. Controlling light-dark exposure patterns rather than sleep schedules determines circadian phase. Sleep Med. 2013;14(5):456–461. doi: 10.1016/j.sleep.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45(4):421–434. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rea MS, Bierman A, Ward G, Figueiro MG. Field Tests of a Model of the Human Circadian Oscillator. Minneapolis, MN: SLEEP; 2014. [Google Scholar]
- 17.Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12(7):685–692. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueiro MG, Plitnick B, Rea MS. The effects of chronotype, sleep schedule and light/dark pattern exposures on circadian phase. Sleep Med. 2014;15(12):1554–1564. doi: 10.1016/j.sleep.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danielsson K, Jansson-Fröjmark M, Broman J, Markström A. Cognitive behaviour therapy: an additive treatment in delayed sleep phase disorder. Sleep Med. 2013;14:e104. doi: 10.1080/16506073.2016.1207096. [DOI] [PubMed] [Google Scholar]
- 20.Gradisar M, Dohnt H, Gardner G, et al. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep. 2011;34(12):1671–1680. doi: 10.5665/sleep.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade MM, Benedito-Silva AA, Domenice S, Arnhold IJ, Menna-Barreto L. Sleep characteristics of adolescents: a longitudinal study. J Adolesc Health. 1993;14(5):401–406. doi: 10.1016/s1054-139x(08)80016-x. [DOI] [PubMed] [Google Scholar]
- 22.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25(6):606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 23.Gau SF, Soong WT. The transition of sleep-wake patterns in early adolescence. Sleep. 2003;26(4):449–454. doi: 10.1093/sleep/26.4.449. [DOI] [PubMed] [Google Scholar]
- 24.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behavior in adolescence. J Sleep Res. 2002;11(3):191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 25.Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. J Sleep Res. 2001;10(1):59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 26.National Sleep Foundation . Sleep in America Poll. Washington DC: National Sleep Foundation; 2006. [Accessed January 12, 2016]. Available from: www.sleepfoundation.org. [Google Scholar]
- 27.Ouyang F, Lu BS, Wang B, et al. Sleep patterns among rural Chinese twin adolescents. Sleep Med. 2009;10(4):479–489. doi: 10.1016/j.sleep.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32(12):1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 30.National Sleep Foundation Adolescent Sleep Needs and Patterns Washington DC: Washington DC; National Sleep Foundation2000Available from: https://sleepfoundation.org/sites/default/files/sleep_and_teens_report1.pdfAccessed January 12, 2016 [Google Scholar]
- 31.National Sleep Foundation . Sleep in America Poll. Washington DC: National Sleep Foundation; 2005. [Accessed January 12, 2016]. Available from: www.sleepfoundation.org. [Google Scholar]
- 32.Noland H, Price JH, Dake J, Telljohann SK. Adolescents’ sleep behaviors and perceptions of sleep. J Sch Health. 2009;79(5):224–230. doi: 10.1111/j.1746-1561.2009.00402.x. [DOI] [PubMed] [Google Scholar]
- 33.Wolfson AR, Tzischinsky O, Brown C, Darley C, Acebo C, Carskadon MA. Sleep, behavior, and stress at the transition to senior high school [abstract] Sleep Res. 1995;24:115. [Google Scholar]
- 34.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17(1):5–12. [PubMed] [Google Scholar]
- 35.Pack AI, Pack AM, Rodgman D, Cucchiara A, Dinges DF, Schwab CW. Characteristics of crashes attributed to the driver having fallen asleep. Accid Anal Prev. 1995;27:769–775. doi: 10.1016/0001-4575(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 36.Gomes AA, Tavares J, Azevedo MH. Sleep-Wake Patterns and Academic Performance in University Students: European Conference on Educational Research; 11–12 September 2002; Lisbon, Portugal: University of Lisbon; [Google Scholar]
- 37.Buboltz WC, Jr, Brown F, Soper B. Sleep habits and patterns of college students: a preliminary study. J Am Coll Health. 2001;50(3):131–135. doi: 10.1080/07448480109596017. [DOI] [PubMed] [Google Scholar]
- 38.Eliasson AH, Lettieri CJ, Eliasson AH. Early to bed, early to rise! Sleep habits and academic performance in college students. Sleep Breath. 2010;14(1):71–75. doi: 10.1007/s11325-009-0282-2. [DOI] [PubMed] [Google Scholar]
- 39.Pilcher JJ, Walters AS. How sleep deprivation affects psychological variables related to college students’ cognitive performance. J Am Coll Health. 1997;46(3):121–126. doi: 10.1080/07448489709595597. [DOI] [PubMed] [Google Scholar]
- 40.Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students. J Psychosom Res. 1997;42(6):583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 41.American College Health Association . National College Health Assessment, Fall 2007 Report. Hanover, MD: American College Health Association; 2007. [Google Scholar]
- 42.Lack LC. Delayed sleep and sleep loss in university students. J Am Coll Health. 1986;35(3):105–110. doi: 10.1080/07448481.1986.9938970. [DOI] [PubMed] [Google Scholar]
- 43.Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46(2):124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Trockel MT, Barnes MD, Egget DL. Health-related variables and academic performance among first-year college students: implications for sleep and other behaviors. J Am Coll Health. 2000;49(3):125–131. doi: 10.1080/07448480009596294. [DOI] [PubMed] [Google Scholar]
- 45.Thorpy MJ, Korman E, Spielman AJ, Glovinsky PB. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1988;9(1):22–27. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 46.Dagan Y, Stein D, Steinbock M, Yovel I, Hallis D. Frequency of delayed sleep phase syndrome among hospitalized adolescent psychiatric patients. J Psychosom Res. 1998;45(1):15–20. doi: 10.1016/s0022-3999(97)00299-7. [DOI] [PubMed] [Google Scholar]
- 47.Dagan Y, Yovel I, Hallis D, Eisenstein M, Raichik I. Evaluating the role of melatonin in the long-term treatment of delayed sleep phase syndrome (DSPS) Chronobiol Int. 1998;15(2):181–190. doi: 10.3109/07420529808998682. [DOI] [PubMed] [Google Scholar]
- 48.Fernández-Mendoza J, Ilioudi C, Montes MI, et al. Circadian preference, nighttime sleep and daytime functioning in young adulthood. Sleep Biol Rhythms. 2010;8(1):52–62. [Google Scholar]
- 49.American Academy of Sleep Medicine . The International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 50.Micic G, Lovato N, Gradisar M, Ferguson SA, Burgess HJ, Lack LC. The etiology of delayed sleep phase disorder. Sleep Med Rev. 2015;27:29–38. doi: 10.1016/j.smrv.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Gradisar M, Crowley SJ. Delayed sleep phase disorder in youth. Curr Opin Psychiatr. 2013;26(6):580–585. doi: 10.1097/YCO.0b013e328365a1d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auger RR, Burgess HJ, Dierkhising RA, Sharma RG, Slocumb NL. Light exposure among adolescents with delayed sleep phase disorder: a prospective cohort study. Chronobiol Int. 2011;28(10):911–920. doi: 10.3109/07420528.2011.619906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peixoto CA, da Silva AG, Carskadon MA, Louzada FM. Adolescents living in homes without electric lighting have earlier sleep times. Behav Sleep Med. 2009;7(2):73–80. doi: 10.1080/15402000902762311. [DOI] [PubMed] [Google Scholar]
- 54.Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piosczyk H, Landmann N, Holz J, et al. Prolonged sleep under Stone Age conditions. J Clin Sleep Med. 2014;10(7):719–722. doi: 10.5664/jcsm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18(2):263–271. doi: 10.1081/cbi-100103190. [DOI] [PubMed] [Google Scholar]
- 57.Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocrinol Metab. 2015;100(11):4067–4073. doi: 10.1210/jc.2015-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Figueiro MG, Rea MS. Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuro Endocrinol Lett. 2010;31(1):4. [PMC free article] [PubMed] [Google Scholar]
- 59.Figueiro MG, Brons JA, Plitnick B, Donlan B, Leslie RP. Measuring circadian light and its impact on adolescents. Light Res Technol. 2011;43(2):201–215. doi: 10.1177/1477153510382853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Figueiro MG, Rea MS. Evening daylight may cause adolescents to sleep less in spring than in winter. Chronobiol Int. 2010;27(6):1242–1258. doi: 10.3109/07420528.2010.487965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bierman A, Klein TR, Rea MS. The Daysimeter: a device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol. 2005;16:2292–2299. [Google Scholar]
- 62.Hersh C, Sisti J, Richiutti V, Schernhammer E. The effects of sleep and light at night on melatonin in adolescents. Hormones (Athens) 2015;14(3):399–409. doi: 10.14310/horm.2002.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.File T. Computer and Internet Use in the United States, P20-569. Washington, DC: U.S. Census Bureau; 2013. [Google Scholar]
- 64.National Sleep Foundation . Sleep in America Poll. Washington DC: National Sleep Foundation; 2011. [Accessed January 12, 2016]. Available from: www.sleepfoundation.org. [Google Scholar]
- 65.National Sleep Foundation . Sleep in America poll. Washington DC: National Sleep Foundation; 2014. [Accessed on Feb 11, 2016]. Available from: https://sleepfoundation.org/sites/default/files/2014-NSF-Sleep-in-America-poll-summary-of-findings---FINAL-Updated-3-26-14-.pdf. [Google Scholar]
- 66.Hysing M, Pallesen S, Stormark KM, Jakobsen R, Lundervold AJ, Sivertsen B. Sleep and use of electronic devices in adolescence: results from a large population-based study. BMJ Open. 2015;5(1):e006748. doi: 10.1136/bmjopen-2014-006748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50–58. doi: 10.1016/j.smrv.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cajochen C, Frey S, Anders D, et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol. 2011;110(5):1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- 69.Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl Ergon. 2013;44(2):237–240. doi: 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Figueiro MG, Overington D. Self-luminous devices and melatonin suppression in adolescents. Light Res Technol. Published online before print May 6, 2015, doi: 10.1177/1477153515584979. Available from: http://lrt.sagepub.com/content/early/2015/05/06/1477153515584979.abstract.
- 72.Yetish G, Kaplan H, Gurven M, et al. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25(21):2862–2868. doi: 10.1016/j.cub.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13(4):354–361. [PubMed] [Google Scholar]
- 74.Watanabe T, Kajimura N, Kato M, Sekimoto M, Takahashi K. Effects of phototherapy in patients with delayed sleep phase syndrome. Psychiatry Clin Neurosci. 1999;53(2):231–233. doi: 10.1046/j.1440-1819.1999.00484.x. [DOI] [PubMed] [Google Scholar]
- 75.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27(7):1469–1492. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI. Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms. 1997;12(1):5–15. doi: 10.1177/074873049701200103. [DOI] [PubMed] [Google Scholar]
- 77.Lack L, Bramwell T, Wright H, Kemp K. Morning blue light can advance the melatonin rhythm in mild delayed sleep phase syndrome. Sleep Biol Rhythms. 2007;5(1):78–80. [Google Scholar]
- 78.Robinson J, Bayliss S, Fielder A. Transmission of light across the adult and neonatal eyelid in vivo. Vision Res. 1991;31(10):1837–1840. doi: 10.1016/0042-6989(91)90031-y. [DOI] [PubMed] [Google Scholar]
- 79.Ando K, Kripke DF. Light attenuation by the human eyelid. Biol Psychiatry. 1996;39(1):22–25. doi: 10.1016/0006-3223(95)00109-3. [DOI] [PubMed] [Google Scholar]
- 80.Bierman A, Figueiro MG, Rea MS. Measuring and predicting eyelid spectral transmittance. J Biomed Optics. 2011;16(6):067011. doi: 10.1117/1.3593151. [DOI] [PubMed] [Google Scholar]
- 81.Ando K, Kripke DF, Cole RJ, Elliott JA. Light mask 500 lux treatment for delayed sleep phase syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(1):15–24. doi: 10.1016/s0278-5846(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 82.Cole RJ, Smith JS, Alcala YC, Elliott JA, Kripke DF. Bright-light mask treatment of delayed sleep phase syndrome. J Biol Rhythms. 2002;17(1):89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- 83.Figueiro MG, Rea MS. Preliminary evidence that light through the eyelids can suppress melatonin and phase shift dim light melatonin onset. BMC Res Notes. 2012;5(1):221. doi: 10.1186/1756-0500-5-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Figueiro MG, Bierman A, Rea MS. A train of blue light pulses delivered through closed eyelids suppresses melatonin and phase shifts the human circadian system. Nat Sci Sleep. 2013;5:133–141. doi: 10.2147/NSS.S52203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Figueiro MG, Plitnick B, Rea MS. Pulsing blue light through closed eyelids: effects on phase shifting of dim light melatonin onset in older adults living in a home setting. Nat Sci Sleep. 2014;6:149–156. doi: 10.2147/NSS.S73856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeitzer JM, Fisicaro RA, Ruby NF, Heller HC. Millisecond flashes of light phase delay the human circadian clock during sleep. J Biol Rhythms. 2014;29(5):370–376. doi: 10.1177/0748730414546532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Figueiro MG. Individually tailored light intervention through closed eyelids to promote circadian alignment and sleep health. Sleep Health. 2015;1(1):75–82. doi: 10.1016/j.sleh.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keijzer H, Smits MG, Duffy JF, Curfs LMG. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev. 2014;18(4):333–339. doi: 10.1016/j.smrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Burgess HJ, Wyatt JK, Park M, Fogg LF. Home circadian phase assessments with measures of compliance yield accurate dim light melatonin onsets. Sleep. 2015;38(6):889–897. doi: 10.5665/sleep.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pullman RE, Roepke SE, Duffy JF. Laboratory validation of an in-home method for assessing circadian phase using dim light melatonin onset (DLMO) Sleep Med. 2012;13(6):703–706. doi: 10.1016/j.sleep.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18(4):318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91(1):54–59. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kronauer RE, Forger DB, Jewett ME. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the phototopic range. J Biol Rhythms. 1999;14(6):500–515. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- 94.Forger DB, Jewett ME, Kronauer RE. A simpler model of the human circadian pacemaker. J Biol Rhythms. 1999;14(6):532–537. doi: 10.1177/074873099129000867. [DOI] [PubMed] [Google Scholar]
- 95.Figueiro MG. Pulsing Blue Light During Sleep Phase Shifts DLMO. Lighting Research Center, Rensselaer Polytechnic Institute; 2014. [Accessed on March 11, 2016]. Available from http://www.lrc.rpi.edu/resources/newsroom/pdf/2014/SleepMask8511.pdf. [Google Scholar]