Abstract
Glioblastomas (GBM) are one of the most recalcitrant brain tumors because of their aggressive invasive growth and resistance to therapy. They are highly heterogeneous malignancies at both the molecular and histological levels. Specific histological hallmarks including pseudopalisading necrosis and microvascular proliferation distinguish GBM from lower-grade gliomas, and make GBM one of the most hypoxic as well as angiogenic tumors. These microanatomical compartments present specific niches within the tumor microenvironment that regulate metabolic needs, immune surveillance, survival, invasion as well as cancer stem cell maintenance. Here we review features and functions of the distinct GBM niches, detail the different cell constituents and the functional status of the vasculature, and discuss prospects of therapeutically targeting GBM niche constituents.
Keywords: GBM, cancer stem cells, tumor microenvironment, tumor vasculature, macrophages, microglial cells, astrocytes, tumor niches, therapy
Cellular heterogeneity of GBM
Glioblastoma (GBM) is the most common and malignant primary brain tumor in human adults. The capacity of GBM to aggressively invade and infiltrate normal surrounding brain tissue makes complete surgical resection impossible. In addition, GBM tumors are very resistant to radiation and cytotoxic chemotherapy. Recurrence is therefore inevitable despite an advanced multimodal standard therapy, resulting in a dismal median survival of just beyond one year [1]. Our current understanding of the complex biology of gliomas is largely derived from studies of genetic and molecular changes within cancer cells. Specifically, recent characterization of the genome, epigenome and transcriptome of GBM has provided a higher-resolution picture of their alterations, revealing different subtypes with distinct molecular signatures [2-7]. In addition, single cell RNA sequencing revealed that multiple subtypes can exist within a single tumor, underscoring the substantial inter- and intra-tumor heterogeneity of GBM [8].
Microenvironmental heterogeneity of GBM
A further layer of complexity in GBM arose from the discovery of cancer stem cells (CSCs) in GBM. CSCs play a crucial role in tumor initiation and maintenance, and provide evidence for stem cell-driven hierarchies within the tumor that also affect tumor heterogeneity [9, 10] (Box1). Moreover, the tumor microenvironment in which these tumor cells develop and grow display heterogeneous phenotypes of which some are characteristic hallmarks in GBM. In fact, not only is the glioma microenvironment composed of a wide variety of non-neoplastic stromal cells, including the vasculature, the various infiltrating and resident immune cells and other glial cell types, but it is also compartmentalized in anatomically distinct regions, referred to as tumor niches that besides tumor cells also entail cancer stem cells. These microenvironmental niches do not just harbor stem cells but rather they exhibit communication centers in which tumor and host cell populations dynamically interact via direct cell contact or paracrine signaling cues to ensure maintenance, growth and protection of tumor cells and CSC from immune surveillance and therapeutic threats. Although tumor niches have been portrayed as conceptually similar in a variety of tumor types, their regulation is probably tissue specific. Moreover, it is important to note that a single tumor can also display morphologically and functionally distinct tumor niches. This phenomenon is most prominent in GBM in which at least three specialized tumor niches exist with the vasculature as an integral regulatory part (Fig.1). In the perivascular tumor niche tumor stem cells nestle in close juxtaposition with the abnormal angiogenic vasculature (Fig.1A) while in the vascular-invasive tumor niche tumor cells co-opt normal blood vessels enabling migration deep into the brain parenchyma (Fig.1C). In contrast, the vasculature in the hypoxic tumor niche is either non-functional or regressed leading to necrotic areas that are surrounded by a row of hypoxic palisading tumor cells (Fig.1B). All three niches elicit specific features and functions that go beyond CSC maintenance as evidenced by the differing composition of host-cell constituents and functional status of the vasculature. Here, we will discuss the nature and tasks of the three aforementioned niches in facilitating tumor growth, angiogenesis and tumor invasion with a focus on the main residents, the vasculature, the immune cells and the tumor cells/CSC. Finally, we will highlight advantages as well as challenges of strategies that employ tumor niche constituents as therapeutic targets in order to improve and prolong survival of GBM patients.
Box 1. Models of tumor heterogeneity.
There are two prominent models that explain tumor heterogeneity and resistance to therapy. Clonal evolution or the stochastic model: The clonal evolution hypothesis is based on the idea that most cancers arise from a single altered cell, and every cell within a tumor is equally likely to be the cell of origin. The cell of origin facilitates tumor initiation and progression as the progeny of that cell expand as a neoplastic “clone.” Neoplastic cells are genetically unstable compared to non-neoplastic cells. Enhanced genetic instability within the original clone over time results in the appearance of new genetic variants, most of which do not survive. However, those few variants with a selective growth advantage expand to become the predominant subpopulations within the tumor, resulting in tumor progression over time. The continued presence of multiple subpopulations within the tumor provides the basis for heterogeneity. This model accounts for both genetic and epigenetic heterogeneity in a given tumor, and suggests that a tumor cell can become progressively more malignant through selective evolutionary processes [109, 110]. This theory was further supported by genome-wide studies that revealed that GBMs have many driver and passenger mutations, which reflects the genetic landscape of subclonal heterogeneity and intraclonal genetic diversity within GBM [2, 8]. Under selective pressures such as chemo- and radiotherapy, some tumor subpopulations survive, because they have acquired more resistant properties, a protective location, or a combination of these factors. These resistant subpopulations are responsible for tumor recurrence.
The cancer stem cell (CSC) or hierarchical model: The CSC hypothesis suggests that regardless of cell-of-origin, many cancers are hierarchical organizations in which the apex of the hierarchy is composed of CSCs, which possess the distinct properties of self-renewal, multipotency, and tumor-initiating ability upon transplantation into mice. CSCs are a small subpopulation that gives rise to phenotypically diverse and partially differentiated non- or less-tumorigenic cancer cells that compose the bulk of cells in the tumor. CSCs reside in specialized niches where the microenvironment regulates their stem cell behavior. Niches are composed of different non-cancerous cell populations that emanate signals to maintain CSCs. CSCs are resistant to conventional therapies and are thought to be responsible for tumor recurrence after therapy [9, 10].
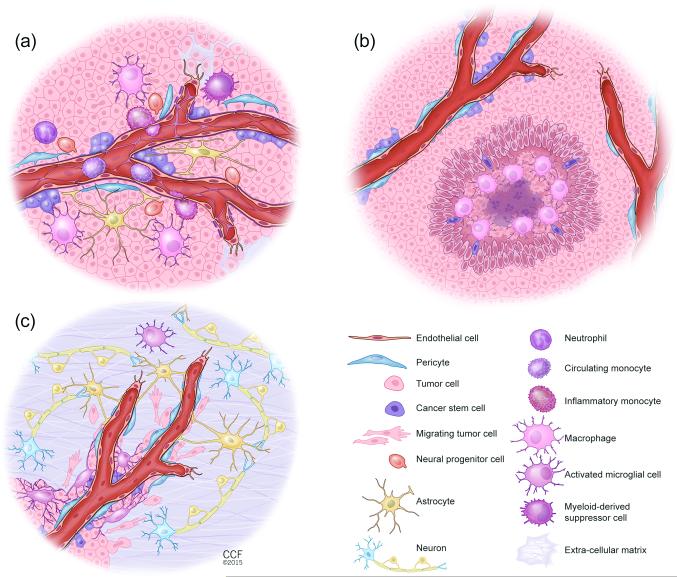
Figure 1. Illustrations of the different Glioblastoma (GBM) niches.
(a) The perivascular GBM niche (PVN). Non-neoplastic cell and glioma cell interactions create a specialized vascular niche, which provides a supportive environment for cancer stem cell (CSC) growth, maintenance, and survival. The PVN niche is a multi-cellular structure composed of non-neoplastic cells, including endothelial cells, pericytes, macrophages, neutrophils, myeloid-derived suppressor cells (MDSCs), reactive astrocytes, and infiltrating neural progenitor cells (NPCs), as well as neoplastic cells including tumor bulk (TB) and CSCs. Macrophages and other non-neoplastic cells are recruited to the tumor by TB and CSCs. These recruited and reprogrammed non-neoplastic cells secrete soluble factors that expand the TB and CSCs and establish a supportive niche for CSCs. In addition, interactions between reactive astrocytes and TB and CSCs can lead to further tumor growth. Pericytes interact with CSCs and TB to promote tumor growth and to contribute to leakiness of the blood brain barrier (BBB). In contrast to normal brain microvessels, both astrocyte and pericyte coverage is incomplete in GBM vessels. Lastly, each cellular component has the capacity to change the extracellular matrix composition that impacts glioma proliferation, survival, and expansion.
(b) The hypoxic GBM niche. The pseudopalisading areas with a necrotic core are hallmarks of GBM and create the hypoxic niche for CSCs. Hypoxia through induction of hypoxia inducible factor 1 alpha (HIF-1α) and of hypoxia inducible factor 2 alpha (HIF-2α) promotes the expansion of cancer stem cells (CSCs) and recruits innate immune cells including macrophages. Hypoxia generally occurs when tumor growth exceeds neovascularization.
(c) The invasive GBM niche. Glioma cells migrate along blood vessels at the invasive edge that is defined as perivascular invasion. Major cell types that constitute the microenvironment at the invasive edge of GBM include endothelial cells, pericytes, activated microglia, reactive astrocytes and neurons. Invading glioma cells make surgical resection incomplete and are partially responsible for tumor recurrence.
The perivascular GBM niche
One feature of GBM is the vigorous and abnormal angiogenesis leading to disorganized and leaky blood vessels that is predominantly induced by the substantial elevation of vascular endothelial growth factor (VEGF) activity. VEGF and other angiogenic factors like fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) are largely produced by tumor cells. Specifically CD133-positive CSCs, which are closely aligned to the tumor vasculature [11], produce high levels of VEGF [12]. VEGF causes pericyte detachment and vascular basement membrane degradation resulting in abnormal, greatly enlarged vessels (coined “mother vessels”) that are susceptible to leakiness and microhemorrhages, a condition known as chronic vascular hyperplasia (CVH) [13]. These blood vessels can evolve into a vascular phenotype of glomeruloid microvascular proliferation (GMP), a common hallmark in GBM, in which endothelial cells and pericytes form poorly organized and dysfunctional vascular structures reminiscent of kidney glomeruli [13]. The expansion of the GBM vasculature can occur by different mechanisms. In addition to angiogenesis, which involves proliferation of existing endothelial cells, bone marrow-derived endothelial and pericyte progenitors have been reported to be recruited and incorporated into growing vessels in several mouse models of glioma, but it appears that this vasculogenic mechanism plays a minor role [14, 15]. More recently, lineage tracing experiments in mouse GBM models and genetic mutational analysis of endothelial cells in human GBM tumors have revealed that CSCs, which are closely associated with tumor vessels, can apparently directly participate in GBM vessel formation by transdifferentiating into endothelial cells or pericytes, the mural support cells of the microvasculature [16-19]. These findings, however, are a matter of debate due to the differing and even opposing results among research groups which question both the extent to which CSC produce functional perivascular cells, and if endothelial cells or pericytes convey the dominant progeny [20-22].
The aforementioned vascular abnormalities in GBM have severe consequences as they can cause the disruption of the blood-brain barrier (BBB, Box 2). The BBB is comprised of endothelial cells, pericytes and astrocytes forming a neurovascular unit that tightly regulates the transfer of ions and molecules between the blood and the brain and ensures that the brain is an immune-privileged organ [23]. In GBM, tumor cells displace non-neoplastic astrocytes, which together with the vascular abnormalities lead to a failure in barrier properties, inducing vessel permeability that allows plasma and fluid to leak into the tumor tissue and induce cerebral edema and interstitial pressure [24]. As a result of the BBB breakdown and the production of tumor-derived chemokines and cytokines, circulating immune cells enter the brain. Monocytes [25], neutrophils [26] and myeloid-derived suppressor cells (MDSC) [27] are all commonly found in the perivascular tumor niche. These cells serve as another source of angiogenic factors, convey immune-suppressive functions and interact with tumor cells and CSC, thereby promoting tumor propagation and progression [25, 26].
Box 2. The blood-brain barrier (BBB).
The blood–brain barrier (BBB) is a highly selective permeability barrier of the central nervous system (CNS) vasculature that tightly regulates the movement of ions, molecules, and cells between the blood and the CNS [23]. The BBB protects the brain from toxins and pathogens, delivers nutrients and oxygen and creates the microenvironment necessary for proper function of neurons. An intact BBB is also a major obstacle for the entry of therapeutic drugs into the brain. The different cell constituents that form the BBB are defined as a neurovascular unit. The central structure of the BBB are capillaries that are composed of endothelial cells interlinked by tight junctions (TJs) creating the inner lining of the vessel wall while pericytes (mural cells) cover the abluminal surface. They together produce the vascular basement membrane. Although the major properties of the BBB are maintained by endothelial cells, its proper function and maintenance also requires pericytes [111]. Astrocytes, which are a major class of glial cells, are another critical component of the BBB. Astrocytic processes that form endfeet cover around 99% surface of CNS vessel walls. Astrocytes have been suggested to control the exchange of water and solutions between blood and brain [77]. The extended processes of microglia, which are resident macrophages of the CNS, were found in a few limited sites where astrocytic endfeet were disconnected, providing immune surveillance [77]. The BBB is formed during embryogenesis when endothelial cells invade the CNS and pericytes are recruited to the nascent vessels–events that occur one week before astrocytes are formed [111]. BBB dysfunction exists in many CNS disorders, including GBM. BBB damage is often associated with neuroinflammation, which seems to occur earlier than direct neuronal pathology and may play either a causative or exacerbating role in neuronal dysfunction or loss [23].
Tumor-associated macrophages (TAMs) are the dominant infiltrating immune cell population in GBM. Historically, TAMs have been defined as either anti-tumoral M1/Th1-skewed, exhibiting features similar to lipopolysaccharide (LPS) and interferon-γ (IFN-γ) ‘classically’ activated macrophages, while pro-tumorally skewed M2/Th2-macrophages have properties similar to interleukin (IL)-4 and IL-13 ‘alternatively’ activated macrophages [28,29], It has become increasingly evident, however, that the M1/M2 polarization model appears too simplistic to appropriately describe the heterogeneous macrophage phenotypes in tumors including GBM, and therefore it may be more appropriate to define myeloid cells by their phenotype, function, and context [30]. Bone marrow-derived macrophages are closely related to microglial cells, the resident brain macrophages, which are the immune gatekeepers of the brain. TAMs and microglial cells together constitute ~30-40% of cells in GBM [31, 32]. Recent fate-mapping and lineage-tracing studies have revealed that microglial cells are derived from immature yolk sac progenitors during development, and maintain themselves in the brain by virtue of their longevity and limited self-renewal [33-35]. Bone marrow-derived granulocytes and monocytes only enter the brain during central nervous system (CNS) injury and tumorigenesis (in details discussed in [36]) (Figure 2). In mice, CD11b+ monocytes in the blood circulation can be subdivided into LY6CHigh; CX3CR1Low; CCR2+ inflammatory monocytes and LY6CLow; CX3CR1High; CCR2− circulating monocytes (Figure 2) [37, 38]. Upon glioma infiltration, monocytes differentiate into macrophages, which until now have been histologically indistinguishable from activated microglia cells by molecular analysis due to similar expression profiles and levels of common cell surface markers like MHCII, CD45, CD11b, CD68, F4/80 Iba1, CXC3R1 and CSF1R. Recently, CCR2 has been reported to be a potential marker to distinguish infiltrating myeloid cells from microglial cells, which lack CCR2 expression even after activation (as seen in a mouse model of experimental autoimmune encephalomyelitis (EAE)) [39-41]. Future studies may now be able to reveal the extent to which tumor-associated macrophages and microglial cells differ in their effects and functions in GBM compared to EAE [39, 40]. In support of this notion is a recent observation that the fractalkine/CX3CR1 receptor, which is expressed in both microglial and infiltrating monocytes/macrophages, has differing functions in these two populations in response to glioma growth [25]. While genetic deletion of CX3CR1 did not affect accumulation or migration of microglia cells, it indirectly promoted the trafficking of circulating Ly6CHigh, CX3CR1-positive inflammatory monocytes into gliomas, resulting in their increased accumulation in the perivascular niche concomitant with interleukin 1 beta (IL1β) upregulation. IL1β activated the p38 mitogen-activated protein kinase (MAPK) signaling pathway and expression of CCL2/monocyte chemoattractant protein (MCP-1) by tumor cells, thereby further enhancing the retention of CCR2-positive monocytes. Interestingly, IL1β also promoted tumor growth of a proneural GBM model, in part by increasing their CSC population [25].
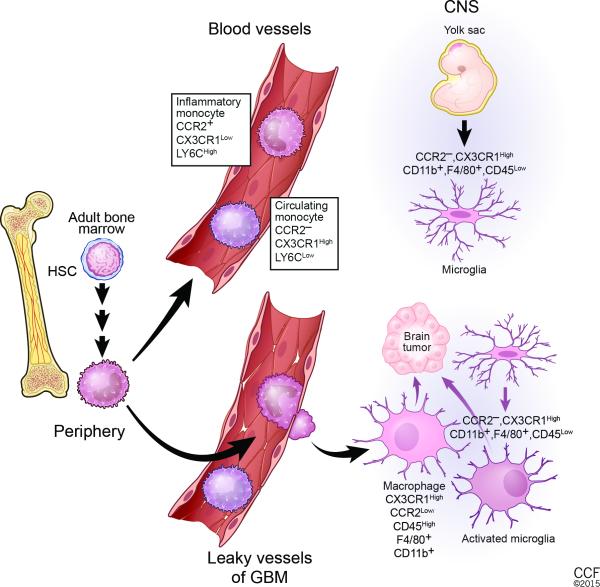
Fig. 2. Cellular origin of GBM-associated microglia and monocytes.
Under steady-state conditions, microglia and monocytes reside in separate locations. In adult life, monocytes are generated from hematopoietic stem cells (HSCs) via a series of intermediate progenitors. Ly6CLow, CCR2−, CX3CR1High circulating monocytes and Ly6CHigh, CCR2+, CX3CR1Low inflammatory monocytes are released into the blood circulation. Inflammatory monocytes are recruited to sites of pathological conditions like GBM. Once within the central nervous system (CNS), they can differentiate into tumor-associated macrophages and become phenotypically nearly indistinguishable from activated resident microglia. Microglia are resident macrophages in the brain that originate from yolk sac progenitors in the neuroepithelium beginning around embryonic day 8.5 in the mouse. In the adult brain, they are negative for CCR2 but express high levels of CX3CR1, CD11b, F4/80, and low levels of CD45. GBM cells induce local inflammation that compromises the integrity of the BBB and results in tumor infiltration of inflammatory monocytes and attracts brain-resident microglial cells to glioma margins.
Congruent with these observations, TAMs are commonly, but not solely, found in the perivascular tumor niche in the vicinity of CSCs [25]. Because TAM numbers appear to correlate with CSC density in human tumors, it has been suggested that CSCs may recruit TAMs more efficiently than their more differentiated neoplastic counterparts, perhaps due to higher expression of chemoattractants such as VEGF, colony stimulating factor 1 (CSF1), stromal cell-derived factor 1a (SDF1a), interleukin 6 (IL6) and IL1β [25, 42]. These factors polarize macrophages and immature monocytes to an immunesuppressive and angiogenic phenotype that enables tumor cells to escape from immune surveillance and promotes their maintenance and growth.
TAMs also release transforming growth factor beta (TGF-ß) that induces matrix metalloproteinase 9 (MMP9) expression and thereby can increase CSC invasiveness [43]. Furthermore, CSCs release the extracellular matrix protein periostin that accumulates in the perivascular tumor niche and acts as another chemoattractant for TAMs via integrin receptor αvβ3 signaling, facilitating the migration of macrophages to the niche [44]. Congruent with the observation that the number of TAMs relates to tumor grade, periostin is highly upregulated in GBM, and its levels directly correlate with tumor grade and recurrence [45].
The hypoxic GBM niche
The compromised vascular function in GBM causes sluggish blood flow and inconsistent oxygen delivery within the tumor. Consequently, local regions of hypoxia develop that can turn into pseudopalisading necrosis when tumor vessels become obstructed. Several mechanisms have been proposed for the generation of pseudopalisading necrosis in addition to edema-enhanced vessel collapse, including vasoocclusion from angiopoietin-2-mediated endothelial cell apoptosis, vascular regression, and intravascular thrombosis due to the elaboration of procoagulation factors [46, 47]. The latter hypothesis is based on the observation that more than 50% pseudopalisades display thrombosed vessels in their necrotic areas. Regardless of the mechanism, glioma cells respond to this cell death in a very specific manner by elongating their nuclei and aligning like palisades in neat rows around centers of tumor necrosis–a feature which has been recognized as a morphological hallmark of GBM [48] (Fig. 1B). These cells have a low proliferation rate, suggesting that pseudopalisades represent a row of hypoxic tumor cells that actively migrate away from central necrosis in response to the vascular insult [49].
Although one would expect that the occurrence of impaired oxygen delivery, apoptosis, and necrosis should slow down tumor growth, in fact pseudopalisading necrosis and microvascular hyperplasia are the two most powerful predictors of poor prognosis among diffuse gliomas, and characterize the transition from a high-grade astrocytoma to glioblastoma. This correlation strongly supports the functional involvement of hypoxic and necrotic regions in tumor progression and aggressiveness. Over the years, a plethora of evidence has been accumulated pointing to hypoxia as a crucial regulator of tumor cell survival, stemness and immune surveillance in these niches [50-53]. Molecular responses to hypoxia are predominantly mediated by the hypoxia-inducible factors (HIF-1 and HIF-2 [54, 55]. HIF proteins are composed of an α- and a β-subunit, and become stabilized and active under low oxygen tension [56]. HIF proteins are upregulated in pseudopalisading glioma cells and induce the expression of factors such as VEGF and interleukin 8 (IL8), which are implicated in different aspects of cellular homeostasis including survival, metabolism, invasion, and angiogenesis [50, 54, 57, 58]. Several studies from GBM and other tumor types have provided evidence that CSCs are enriched in hypoxic tumor niches including CSC in perinecrotic regions of human glioblastoma biopsies [51]. Hypoxia promotes stemness through the activation of genes implicated in self-renewal and dedifferentiation, and protects tumor cells and CSCs from chemo- and radiotherapy. Therefore, hypoxia increases stem cell properties of tumors, as measured by an increase in side population and neurosphere forming ability of CSC concomitant with increased expression of several CSC markers, including CD133, Sox2, Oct4, nestin and Klf4 [51-53]. HIF1α [52, 53] or HIF2α [51, 59, 60] upregulation is a crucial step in maintaining stemness as knockdown of either isoform reduced GSC self-renewal and tumor formation in mice.
The necrotic cell death in the center of the hypoxic niche releases proinflammatory signals into the surrounding tissue microenvironment that turns inflammatory cells like TAMs and neutrophils into immune-suppressive and angiogenesis-promoting cells while losing their original function of removing necrotic debris [61-64]. In addition, hypoxia appears to enhance transdifferentiation of CSC into endothelial-like cells, and promote their incorporation into tumor vessels, which were more abundantly found in hypoxic areas of GBM derived from spontaneous glioma mouse models [17]. It is therefore not surprising that microvascular hyperplasia is often observed in close proximity to pseudopalisading necrosis, enabling tumor cells and CSC to grow outward towards ingrowing newly formed blood vessels and thus contributing to the creation of a vicious cycle (Fig. 1B).
The invasive GBM niche
GBMs not only migrate away from hypoxic regions within the tumor bulk, but like all diffuse gliomas, have the propensity to invade normal tissue. GBM infiltrate as single cells or move along white matter tracts and basement membranes including those of blood vessels [65]. Indeed, invading GBM cells often co-opt normal blood vessels for use as a highway to invade normal brain parenchyma [66]. It is notable that several MMPs have been associated with GBM infiltration that does not reflect the perivascular invasive mode of GBM [67]. Indeed, complete genetic deletion of MMP-2 and MMP-9 in the GBM and host cell compartment rather enhanced perivascular invasiveness in vivo in GBM mouse models concomitant with reduction in angiogenesis which underscores the distinct mechanisms underlying tumor cell infiltration and perivascular invasion [14, 68]. These results are also congruent with the observation that GBM utilize such a perivascular invasive mode in response to antiangiogenic therapy, in part due to enhanced activity of the receptor tyrosine kinase Met by both hypoxia-driven and hypoxia-independent mechanisms [69, 70]. Hypoxia induces Met expression in GBM cells and in GBM patient samples in a HIF-dependent manner, while VEGF ablation enhances Met phosphorylation and subsequent invasion in murine and human GBM through a VEGFR2:Met complex in tumor cells [71-74]. Importantly, such induced tumor invasiveness is associated with the induction of EMT that can induce some of the defining features of stem cells including self-renewal ability, and has been associated with an increase in CSC in other tumor types [75, 76]. In concordance with these observations, triggering EMT in tumor cells in the niche not only induces stem cell phenotypes that likely are transiently and dynamically regulated, but also maintains the stemness of cancer cells [76].
In contrast to the perivascular tumor niche, the invasive tumor niche displays a more functional vasculature and is associated with a different and more varied set of host cell constituents given its location at the border of the normal brain parenchyma. Astrocytes, which regulate metabolic and fluid homeostasis as well as vascular blood flow, contact endothelial cells and pericytes with their astrocytic endfeet covering more than 99% of the cerebrovascular surface [77]. This is an important mechanism to exchange ions and metabolites from the blood to the brain. However, in the invasive tumor niche, glioma cells displace astrocytes from the vessel to a substantial degree, sometimes completely encasing the blood vessel. In human GBM xenograft models, GBM cells that disrupted the astrocyte-vascular interactions in the invading tumor areas were found to seize control over the regulation of the vascular tone, and were sufficient to rupture the BBB albeit likely to a lower extent than glioma cells within the tumor bulk [78]. Notably, astrocytes can also be engulfed from the growing tumor, but the distribution of astrocytes within the tumor bulk can vary widely. Moreover, astrocytes surrounding GBM undergo reactive astrogliosis similar to that observed during CNS injury, given that tumors resemble various aspects of a wound. Thereby, astrocytes become proliferative and migratory by activating signaling pathways that produce growth factors, metabolites and cytokines. These molecules in turn likely affect gliomagenesis. The first insight into the paracrine interaction between astrocytes and glioma cells was obtained from coculture experiments, demonstrating that astrocytes can enhance proliferation and MMP-2 dependent invasion of glioma cells in vitro. Furthermore, reactive astrocytes produce connective tissue growth factor (CTGF) that binds to tyrosine kinase receptor type A (TrkA) and integrin beta 1 on CSCs thereby activating nuclear factor kappa B (NF-kB) and inducing zinc finger E-box binding homeobox 1 (ZEB1), an epithelial-mesenchymal transition (EMT) transcription factor that facilitated tumor cell infiltration [79]. In GBM, reactive astrocytes, like brain endothelial cells, were found to express sonic hedgehog (SHH) [80]. SHH binds to the membrane proteins patched-1 and smoothened on glioma cells, leading to the activation of GLI transcription factors that foster glioma growth in part by promoting glioma stemness/self-renewal [80, 81]. Taken together, these studies provide evidence that astrocytes can be intimately involved in CSC maintenance and tumor infiltration in the invasive niche. As astrocytes were also identified in the perivascular tumor niche of PDGFB-driven GBM in mice, it is conceivable that intratumoral astrocytes could support tumor and tumor stem cells in the perivascular tumor niche [80] (Fig. 1A).
Microglial cells are also closely associated with invading glioma cells. These immune cells are distributed through the brain and display a typical ramified morphology with a small cell body and fine, long cellular processes for scanning and monitoring neighboring cells and the extracellular space [82]. As soon as microglia experience any disturbances of CNS homeostasis, they become activated concomitant with profound alterations in cell shape, gene expression and functional behavior [82]. Microglial cells thus move to and accumulate at the glioma margins, but they do not always exhibit an amoeboid shape as observed in other CNS insults. This may be due to the fact that glioma-conditioned medium appears to activate microglial cells in a way that is distinct from the inflammatory phenotype. Indeed, glioma-directed microglial activation does not trigger the release of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), but leads to alterations that are associated with promotion of glioma growth and invasion. Many glioma cell lines established from patient samples express high levels of CSF1 and interleukin 34 (IL34), the ligands for CSF1 receptor (CSF1R), as well as stem cell factor (SCF), the ligand for KIT, which attract and activate surrounding microglia. These changes have been shown to be concomitant with an upregulation of the metalloproteinases MMP14 and MMP2 and suggested a role in the enhanced tumor invasiveness [83]. CSF1 differentiates recruited intratumoral blood monocytes into immune-suppressive and angiogenic macrophages as first demonstrated in a mammary carcinoma model [84]. Similar to studies that revealed a paracrine loop between macrophages and intravasating breast cancer cells, microglia were found to produce epidermal growth factor (EGF) to promote GL261 glioblastoma migration, while GBM cells secreted CSF1 that attracted microglial cells, demonstrating a synergistic interaction between these two cell types [85]. In line with these observations, a recent study revealed that naïve microglia can reduce the sphere-forming ability of human CSCs to suppress glioma growth, while microglia or monocytes cultured from glioma patients have lost this anti-tumorigenic potential [86]. Supernatants from CSCs likewise inhibited the phagocytic activity of TAMs and induced secretion of the immunesuppressive molecules interleukin 10 and TGF-β1 [87].
As mentioned above, the terms macrophages and microglial cells in brain tumors have been interchangeably used in many studies because of the technical challenges of distinguishing the two cell types by molecular markers. So far, only studies involving co-culture experiments with glioma and microglial cells in vitro or fluorescently labeled bone marrow (BM) transplantation experiments in mice are able to distinguish between BM-born macrophages and brain-resident microglial cells in the tumor setting. The later often involves total body lethal irradiation (TBI) that can potentially lead to enhanced and abnormal mobilization of BM cells into the circulation, resulting in more immune cell infiltrates compared to non-irradiated GBM [34, 88].
The studies mentioned above provide evidence that microglial cells, like astrocytes, can be implicated in tumor propagation and infiltration in the invasive niche. It is noteworthy that tumor cells, besides moving along blood vessels, can also cluster around neuronal somata described as perineuronal satellitosis, A recent elegant study revealed that activated neurons promote glioma growth by secreting the synaptic protein neuroligin-3 (NLGN-3) which induced the PI3K-mTOR pathway and feed-forward expression of NLGN3 in glioma cells [89]. Further, the neurotransmitter glutamate secreted from glioma cells was found to enhance their own proliferation and invasion through autocrine as well as paracrine signaling and increase the excitability of affected cortical [90].
As tumors grow, it is important to keep in mind that invasive tumor niches can be engulfed and become part of the expanding tumor mass. This will then trigger hypoxia and subsequent neovascularization, transitioning these niches into hypoxic and perivascular tumor niches. Therefore tumor niches undergo dynamic alterations in a temporal and spatial manner that create a succession of tumor microenvironments to accommodate the aggressive growth of a GBM into normal tissue, during tumor progression as well as in response to therapeutic agents.
Targeting glioma niches
Since GBM niches support and protect CSCs and their progeny, targeting non-neoplastic niche components or the molecules they release to support tumor cell growth may corroborate a more effective tactic to overcome the extensive plasticity that is associated with therapeutic resistance of GBM. Reducing and pruning tumor vessel growth by blocking VEGF/VEGFR signaling in GBM emerged as the first promising treatment strategy because it impaired CSC maintenance in the perivascular niche in brain tumor mouse models thereby reducing tumor growth [11, 12], and resulted in improvements in radiographic response, progression-free survival, and quality of life of GBM patients [91]. Consequently, bevacizumab, a humanized monoclonal antibody directed against VEGF-A, became the third drug approved by the United States Food and Drug Administration (FDA) for use in recurrent GBM in 2009 [91].
To date however, anti-VEGF therapy has aided only a subset of GBM patients, and those patients have demonstrated only transitory improvements without eliciting benefits in overall survival. Recent results now revealed that the effects of anti-VEGF therapy maybe GBM subtype-specific. Two randomized placebo-controlled phase III trials in newly diagnosed GBM AVAglio [92] and RTOG-0825 [93]) reported prolonged progression-free survival (PFS), but not overall survival (OS), with the addition of bevacizumab to radiotherapy plus temozolomide. A multivariable analysis, however, revealed that bevacizumab conferred a significant OS advantage versus placebo for patients with proneural isocitrate dehydrogenase 1 (IDH1) wild-type tumors [94]. These results, together with the observation that patients who experience enhanced tumor blood perfusion with bevacizumab have a longer survival benefit than those without vascular changes, suggest that subtype stratification of GBM patients with early imaging perfusion markers could help to identify the subset of patients who will benefit the most from anti-VEGF agents [95].
Mechanistically, blocking VEGF/VEGFR signaling leaves behind a more mature and functional vasculature by selectively pruning immature blood vessels and enhancing pericyte attachment to tumor vessels. This results in reduction of vasogenic brain edema, enhanced vessel perfusion and subsequent oxygenation throughout the tumor concomitant with a decrease in immune suppression, thus creating conditions for better drug delivery and efficacy [95]. However, the inability to finely tune anti-VEGF/VEGFR therapy to create persistent normalization without further vessel pruning results in the recurrence of hypoxia and the emergence of acquired resistance [96]. Indeed, hypoxia induces several phenotypes associated with resistance that have been observed in relapsing tumors including GBM. Hypoxia promotes EMT and stem-like properties of tumor cells, upregulates pro-angiogenic and invasive factors, and drives the infiltration and polarization of angiogenic and immune-suppressive myeloid cells (reviewed in [97, 98]).
Radiographic and tissue studies from a subset of patients with recurrent GBM who were treated with bevacizumab or the angiokinase inhibitor cediranib support the results of enhanced tumor invasiveness and immune cell infiltration of TAMs and other CD11b+ myeloid cells observed in GBM mouse model systems [95, 99-101]. These innate immune cells have been shown to facilitate resistance to antiangiogenic therapy by reinitiating VEGF-independent angiogenesis and immunosuppression that makes tumors non-responsive to VEGF blockade [64, 102]. Congruently, targeting innate immune cells and their central pathways has been shown to sensitize various tumors to antiangiogenic therapy [102, 103].
In line with these studies, TAMs and other myeloid CD11b+ cells, which are abundant in GBM, and promote tumor growth by supporting vessel and tumor cell growth as well as suppressing tumor immunity, are another attractive target to impair niche function in GBM [36]. Two major strategies have been pursued so far to target TAMs/microglial cells in glioma: i) interfering with the activation and differentiation pathways responsible for their tumor supportive phenotype, and ii) disturbing the interaction of TAM with GSCs. For example, glioma conditioned medium was shown to activate the signal transducer and activator of transcription 3 (STAT3) or p38 MAPK pathways in microglial cells in vitro. Conversely, inhibition of these pathways resulted in extension of survival in glioma-bearing mice although one cannot exclude that the beneficial effects were conceived by additionally blocking these signaling circuits in the tumor cell population [104, 105]. In contrast, inhibition of the CSF-1 receptor targets only myeloid cells and resulted in increased survival and tumor shrinkage in a proneural murine GBM model [106]. Surprisingly, TAMs were not reduced in tumors because glioma cells secreted granulocyte-macrophage CSF (GM-CSF) and interferon-γ (IFN-γ) that apparently facilitated TAM survival in the context of CSF1R inhibition. Considering that CSF1R inhibition caused TAM reduction in other tumor types and resulted in elimination of ~99% of microglia in naïve mice [35], it raises the question whether GBM infiltrating TAMs respond differently to CSF1 receptor signaling.
In contrast to the promising effects in the proneural GBM model, a recent phase 2 study of the CSF1-receptor inhibitor PLX3397 in patients with recurrent GBM tissue did not reveal any significant improvements in PFS [107]. Whether the differing results may be a reflection of GBM subtype-specific responses to blocking CSF1R + myeloid cells, − as observed in recent anti-angiogenic trials-, remains to be determined. In line with the observation that tumors endorse immune suppressive features in myeloid cells, a recent study revealed that naïve human macrophages and microglial cells alleviated sphere-forming capacity of glioma-patient derived tumor initiating cells by inducing cell cycle arrest and differentiation while glioma-associated myeloid cells were unable to do so [86]. Interestingly, a drug screen identified amphotericin B, a common anti-fungal medication, to induce immune-stimulating activity in tumor-associated macrophages and microglial cells that was sufficient to impede growth of glioma stem cells in vitro and tumor growth in vivo [86]. Indeed, with the new encouraging results of immune checkpoint inhibitors in several tumor types such as melanomas and non-small cell lung cancer (NSCLC), therapies that promote an immune-stimulatory milieu may help to enhance the infiltration of cytotoxic T-cells into glioma niches to eradicate CSC and tumor cells.
Finally, signaling cues from reactive astrocytes in the tumor niche have become another promising target to restrain glioma growth. Astrocytes as well as endothelial cells express endothelins that have vasoconstrictive properties. A recent study revealed that endothelin (ET)-induced activation of its receptors ETAR and ETBR, on glioma cells protected the tumor cells from chemotherapy by upregulating expression of anti-apoptotic proteins in cancer cells. Conversely, blocking both ET receptors with Macitentan, an approved drug for pulmonary arterial hypertension, abolished the angiocrine and astrocyte-induced chemoprotection of glioblastoma cells in vitro and down-regulated the expression of proteins associated with cancer cell growth and survival in vivo in orthotopic models of glioblastoma [108]. In addition, it is intriguing to speculate that macitentan also promoted vessel normalization thereby contributing to more efficient drug delivery and subsequent anti-tumor effects.
The studies summarized above highlight new developments to target signaling cues of host cell constituents in the GBM niche. These are promising attempts to make tumors more responsive by interfering with niche signaling pathways directed at tumor cells/CSC that originate from normal cell constituents. As some of the drugs targeting those pathways have already been approved for other diseases, this approach may yield an attractive and more effective strategy in combination with standard chemotherapy to sensitize as well resensitize GBM.
Concluding Remarks
In line with the rising concept of tumor niche heterogeneity, it is conceivable that not only GBMs but also other tumor types with hypoxic and invasive features are compartmentalized in anatomically distinct regions that display morphologically and functionally distinct tumor niches. Further, it is important to keep in mind that these distinct tumor niches will undergo dynamic alterations in a temporal and spatial manner that create a succession of tumor microenvironments to accommodate the aggressive growth of a tumor such as GBM into normal tissue, during tumor progression as well as in response to therapeutic agents. For example, invasive tumor niches can be engulfed and become part of the expanding tumor mass. This will then trigger hypoxia and subsequent neovascularization, transitioning these niches into hypoxic and subsequent perivascular tumor niches. The prevailing view so far has focused on the signaling events in a generic “tumor niche” but given the diverse composition of cell constituents and functional differences of the vasculature in the distinct niches, it is conceivable that the dialogues of the tumor and non-tumor niche constituents are niche-type specific to better amend the needs of CSC and tumor cells to these specific locations. This may also affect response to therapies as one can envision that certain therapies may only target pathways used in a subset of tumor niches. Further, certain therapies could thereby convert a tumor niche into another niche type rather than eliminating it and thereby losing their effectiveness. It is likely, for instance, that therapies such as radiation and cytotoxic chemotherapies, which create hypoxia and necrosis, may enhance hypoxic niches that will transition into perivascular tumor niches during tumor relapse. In support of this notion, targeting niche pathways have revealed encouraging results so far but also have faced challenges in part by targeting only niche subtypes.
Ongoing and future studies will be pivotal to more rigorously dissect signaling circuits tailored to the specific tumor niche type and to decipher potential common pathways to most efficiently target all of the three tumor niches. Another layer of complexity is caused by the substantial genetic and epigenetic heterogeneity of GBMs and within a GBM that appears to regulate the tumor's response to microenvironmental therapies. This further suggests that the formation of specific GBM niches is dependent not only on normal cell constituents in the tumor microenvironment but also on the genetic and epigenetic backbone of cancer stem cells and their progeny. This raises several questions about the critical dialogues between these two compartments that generate tumor niches, how they determine which type of tumor niche will be generated, and how the distinct niches react to cancer therapies (Outstanding Questions). The knowledge forthcoming will provide pivotal information about the nature, regulation and function of the distinct tumor niches and may identify new opportunities that can be exploited for therapeutic strategies aimed at all tumor niches in GBMs as well as potentially other cancers to enhance survival of cancer patients.
Box 3. Outstanding questions.
As much as normal cell constituents in the tumor microenvironment contribute to the formation of tumor niches so do cancer stem cells and their progeny. What are the critical dialogues between these two compartments that generate tumor niches, and how do they determine which type of tumor niche will be generated?
Because GBMs are molecularly heterogeneous tumors, are there differences in the composition of the distinct tumor niches among the different GBM subtypes?
Does the genetic and epigenetic heterogeneity within a GBM affect which type of tumor niche will be formed?
To which degree do “niche” signals emanate from the tumor cells/CSC or non-cancerous cell types (e.g. immune cells, astrocytes) to amend the tumor vasculature to a specific niche type?
What are common and distinct signaling circuits among the different tumor niches? Are signals underlying maintenance and protection of cancer stem cells dependent on the kind of tumor niche in GBM?
How do therapies affect the distinct tumor niches in GBM? To which extent impact tumor niches GBM recurrence? Do certain therapies convert a tumor niche into another niche type rather than eliminating it? For example, one can envision that radiation and chemotherapies may turn perivascular tumor niches into hypoxic niches that will transition back into perivascular tumor niches during tumor relapse.
How can one most efficiently target the three tumor niches?
Can vascular and immune modulatory strategies help to promote an immune-stimulatory environment that enhances cytotoxic T-cell infiltration into GBM?
Trends box.
Tumor niches are microanatomical structures where CSCs are maintained and protected from therapy.
GBM contain functionally distinct tumor niches that are interchangeable.
Targeting host cell-derived niche factors show promise and challenges for GBM therapy.
Acknowledgements
The authors would like to thank Dave Schumick, BS, CMI for his illustrations and Rebecca Bish-Cornelissen for editorial assistance. Reprinted with the permission of the Cleveland Clinic Center for Medical Art & Photography © 2015. All Rights Reserved. Due to the journal's space limitations, we apologize to all the investigators whose research could not be appropriately cited. The study was supported by funds from the National Cancer Institute to GB (U54 CA163155 and R01 CA188404) and DH (U01 CA160882 and seed money from Aflac Cancer and Blood Disorders Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Brennan CW, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research, N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhaak RG, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snuderl M, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20:810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Szerlip NJ, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A. 2012;109:3041–3046. doi: 10.1073/pnas.1114033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sottoriva A, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 10.Lathia JD, et al. Cancer stem cells in glioblastoma. Genes & development. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Bao S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 13.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3:1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du R, et al. HIF1alpha Induces the Recruitment of Bone Marrow-Derived Vascular Modulatory Cells to Regulate Tumor Angiogenesis and Invasion. Cancer cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergers G. Bone Marrow-Derived Cells in GBM Neovascularization. CNS Cancer Models, Markers, Prognostic Factors, Targets, and Therapeutic Approaches. 2009 [Google Scholar]
- 16.Wang R, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 17.Soda Y, et al. Trans differentiation of glioblastoma cells into vascular endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci-Vitiani L, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 19.Cheng L, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulla A, et al. Analysis of the TP53 gene in laser-microdissected glioblastoma vasculature. Acta Neuropathol. 2003;105:328–332. doi: 10.1007/s00401-003-0681-6. [DOI] [PubMed] [Google Scholar]
- 21.Liu XM, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol. 2011;105:173–179. doi: 10.1007/s11060-011-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez FJ, et al. Neoplastic cells are a rare component in human glioblastoma microvasculature. Oncotarget. 2012;3:98–106. doi: 10.18632/oncotarget.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. Journal of inherited metabolic disease. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 24.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng X, et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015 doi: 10.18632/oncotarget.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang J, et al. Neutrophils promote the malignant glioma phenotype through S100A4. Clin Cancer Res. 2014;20:187–198. doi: 10.1158/1078-0432.CCR-13-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohanbash G, Okada H. Myeloid-derived suppressor cells (MDSCs) in gliomas and glioma-development. Immunol Invest. 2012;41:658–679. doi: 10.3109/08820139.2012.689591. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy BC, et al. Tumor-associated macrophages in glioma: friend or foe? Journal of oncology. 2013;2013:486912. doi: 10.1155/2013/486912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szulzewsky F, et al. Glioma-Associated Microglia/Macrophages Display an Expression Profile Different from M1 and M2 Polarization and Highly Express Gpnmb and Spp1. PloS one. 2015;10:e0116644. doi: 10.1371/journal.pone.0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler JR, et al. Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS One. 2012;7:e43339. doi: 10.1371/journal.pone.0043339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charles NA, et al. The brain tumor microenvironment. Glia. 2012;60:502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 33.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ajami B, et al. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature neuroscience. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 35.Elmore MR, et al. Colony-stimulating factor. 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hambardzumyan D, et al. The role of microglia/macrophages in glioma maintenance and progression. Nature Neuroscience. 2015 doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geissmann F, et al. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 38.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saederup N, et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizutani M, et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J Immunol. 2012;188:29–36. doi: 10.4049/jimmunol.1100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi L, et al. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. Journal of neuroimmunology. 2011;232:75–82. doi: 10.1016/j.jneuroim.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Ye XZ, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. Journal of immunology. 2012;189:444–453. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 44.Zhou W, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nature cell biology. 2015 doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikheev AM, et al. Periostin is a novel therapeutic target that predicts and regulates glioma malignancy. Neuro Oncol. 2015;17:372–382. doi: 10.1093/neuonc/nou161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brat DJ, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer research. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 47.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 48.Wippold FJ, 2nd, et al. Neuropathology for the neuroradiologist: palisades and pseudopalisades. AJNR Am J Neuroradiol. 2006;27:2037–2041. [PMC free article] [PubMed] [Google Scholar]
- 49.Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest. 2004;84:397–405. doi: 10.1038/labinvest.3700070. [DOI] [PubMed] [Google Scholar]
- 50.Semenza GL. Defining the role of hypoxia-inducible factor. 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seidel S, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain. 2010;133:983–995. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 52.Soeda A, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 53.Bar EE, et al. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177:1491–1502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zagzag D, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 55.Bar EE. Glioblastoma, cancer stem cells and hypoxia. Brain Pathol. 2011;21:119–129. doi: 10.1111/j.1750-3639.2010.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brat DJ. Glioblastoma: biology, genetics, and behavior. Am Soc Clin Oncol Educ Book. 2012:102–107. doi: 10.14694/EdBook_AM.2012.32.48. [DOI] [PubMed] [Google Scholar]
- 58.Filatova A, et al. The cancer stem cell niche(s): the crosstalk between glioma stem cells and their microenvironment. Biochim Biophys Acta. 2013;1830:2496–2508. doi: 10.1016/j.bbagen.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Heddleston JM, et al. Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ. 2012;19:428–439. doi: 10.1038/cdd.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casazza A, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivera LB, Bergers G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol. 2015;36:240–249. doi: 10.1016/j.it.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cuddapah VA, et al. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15:455–465. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12:495–508. doi: 10.1038/nrn3060. [DOI] [PubMed] [Google Scholar]
- 67.Lakka SS, et al. Proteases and glioma angiogenesis. Brain Pathol. 2005;15:327–341. doi: 10.1111/j.1750-3639.2005.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Du R, et al. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro-oncology. 2008;10:254–264. doi: 10.1215/15228517-2008-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubenstein JL, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckerich C, et al. Hypoxia can induce c-Met expression in glioma cells and enhance SF/HGF-induced cell migration. International journal of cancer. Journal international du cancer. 2007;121:276–283. doi: 10.1002/ijc.22679. [DOI] [PubMed] [Google Scholar]
- 72.Rose SD, Aghi MK. Mechanisms of evasion to antiangiogenic therapy in glioblastoma. Clinical neurosurgery. 2010;57:123–128. [PubMed] [Google Scholar]
- 73.Iwamoto FM, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu KV, et al. VEGF Inhibits Tumor Cell Invasion and Mesenchymal Transition through a MET/VEGFR2 Complex. Cancer cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo M, et al. Epithelial-mesenchymal plasticity of breast cancer stem cells: implications for metastasis and therapeutic resistance. Curr Pharm Des. 2015;21:1301–1310. doi: 10.2174/1381612821666141211120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathiisen TM, et al. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 78.Watkins S, et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edwards LA, et al. Effect of brain- and tumor-derived connective tissue growth factor on glioma invasion. J Natl Cancer Inst. 2011;103:1162–1178. doi: 10.1093/jnci/djr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Becher OJ, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68:2241–2249. doi: 10.1158/0008-5472.CAN-07-6350. [DOI] [PubMed] [Google Scholar]
- 81.Clement V, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kettenmann H, et al. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 83.Sielska M, et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230:310–321. doi: 10.1002/path.4192. [DOI] [PubMed] [Google Scholar]
- 84.Lin EY, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. The Journal of experimental medicine. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coniglio SJ, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18:519–527. doi: 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarkar S, et al. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nature neuroscience. 2014;17:46–55. doi: 10.1038/nn.3597. [DOI] [PubMed] [Google Scholar]
- 87.Wu A, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muller A, et al. Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. International journal of cancer. Journal international du cancer. 2014 doi: 10.1002/ijc.29379. [DOI] [PubMed] [Google Scholar]
- 89.Venkatesh HS, et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 2015;161:803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sontheimer H. A role for glutamate in growth and invasion of primary brain tumors. J Neurochem. 2008;105:287–295. doi: 10.1111/j.1471-4159.2008.05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen MH, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. The oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 92.LaViolette PS, et al. Vascular change measured with independent component analysis of dynamic susceptibility contrast MRI predicts bevacizumab response in high-grade glioma. Neuro-oncology. 2013;15:442–450. doi: 10.1093/neuonc/nos323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilbert MR, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sandmann T, et al. Patients With Proneural Glioblastoma May Derive Overall Survival Benefit From the Addition of Bevacizumab to First-Line Radiotherapy and Temozolomide: Retrospective Analysis of the AVAglio Trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.61.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu-Emerson C, et al. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol. 2015;33:1197–1213. doi: 10.1200/JCO.2014.55.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rivera LB, Bergers G. CANCER. Tumor angiogenesis, from foe to friend. Science. 2015;349:694–695. doi: 10.1126/science.aad0862. [DOI] [PubMed] [Google Scholar]
- 97.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature reviews. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu KV, Bergers G. Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS oncology. 2013;2:49–65. doi: 10.2217/cns.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gerstner ER, et al. Infiltrative patterns of glioblastoma spread detected via diffusion MRI after treatment with cediranib. Neuro-oncology. 2010;12:466–472. doi: 10.1093/neuonc/nop051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerstner ER, et al. Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J Clin Oncol. 2010;28:e91–93. doi: 10.1200/JCO.2009.25.0233. [DOI] [PubMed] [Google Scholar]
- 101.Piao Y, et al. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neuro-oncology. 2012;14:1379–1392. doi: 10.1093/neuonc/nos158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rivera LB, et al. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep. 2015;11:577–591. doi: 10.1016/j.celrep.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shojaei F, Ferrara N. Role of the microenvironment in tumor growth and in refractoriness/resistance to anti-angiogenic therapies. Drug Resist Updat. 2008;11:219–230. doi: 10.1016/j.drup.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L, et al. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57:1458–1467. doi: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 105.Hu F, et al. Glioma-associated microglial MMP9 expression is upregulated by TLR2 signaling and sensitive to minocycline. Int J Cancer. 2014;135:2569–2578. doi: 10.1002/ijc.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature medicine. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Butowski N, et al. Phase 2 Study of Orally Administered CSF1R inhibitor PLX3397 in Recurrent Glioblastoma: An Ivy Foundation Early Phase Clinical Trials Consortium. Neuro-Oncology. 2015 doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim SJ, et al. Macitentan, a dual endothelin receptor antagonist, in combination with temozolomide leads to glioblastoma regression and long-term survival in mice. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nowell PC. Mechanisms of tumor progression. Cancer research. 1986;46:2203–2207. [PubMed] [Google Scholar]
- 110.Stratton MR, et al. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Daneman R, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]