ABSTRACT
Obesity is closely associated with an increased risk for metabolic and cardiovascular diseases. Adipose tissue produces a number of secretory bioactive substances, also known as adipocytokines or adipokines, which directly affect adjacent or distant organs. Most adipocytokines are pro-inflammatory, thereby promoting the obesity-linked disorders. In contrast, there are a small number of adipocytokines that exhibit anti-inflammatory properties. It is now recognized that dysregulated production or secretion of adipocytokines caused by adipocyte dysfunction leads to the development of obesity-linked complications. In this review, we focus on the functional role of several adipocytokines in metabolic and cardiovascular diseases.
Key Words: adipocytokine, adiponectin, Sfrp5, adipolin, inflammation, cardiovascular disease
INTRODUCTION
The prevalence of obesity has been increasing in the Western countries during the last decades, and obesity has become the major health problems. Obesity, in particular, excess visceral adiposity, is strongly associated with a cluster of type 2 diabetes, hypertension and dyslipidemia, also known as metabolic syndrome, and it is associated with increased cardiovascular mortality and morbidity1,2). It is estimated that obesity increases the risk of disease as much as 20 years of aging3). Accumulating evidence indicates that obesity contributes to chronic inflammation, thereby leading to the development of insulin resistance and the metabolic syndrome4-6). Recent findings indicate that fat tissue is an endocrine organ, which produces and secretes a variety of bioactive substances, referred to as adipocytokines or adipokines7-9). Furthermore, it is recognized that dysregulation of adipocytokines caused by dysfunctional adipocytes (e.g. excess adiposity) could contribute to the pathogenesis of various obese complications. This review article focuses on the functional significance of key adipocytokines that display favorable effects on obesity-linked disorders.
ADIPOSE TISSUE AS AN ENDOCRINE ORGAN
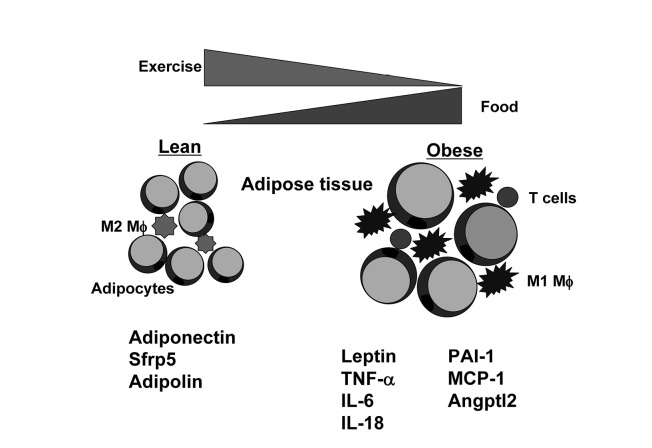
Adipose tissue has been considered as an energy storage organ with less attention, but it is now recognized that it functions as an endocrine organ by producing numerous adipocytokines7,8,10). Adipsin/compliment factor D was initially identified as an adipocytokine that is primarily produced by adipocytes in 198711). In 1993, tumor necrosis factor-α (TNF-α) was identified as a pro-inflammatory adipocytokine that is potentially involved in the pathogenesis of insulin resistance12). In 1994, leptin was identified as a fat-specific adipocytokine that plays an important role in regulation of food intake and energy expenditure13). Furthermore, plasminogen activator inhibitor-1 (PAI-1) was identified as an adipocytokine that is highly expressed in adipose tissue10). PAI-1 is robustly upregulated in visceral adipose depots during the development of fat accumulation in a rat model of obesity. Plasma levels of PAI-1 positively correlate with visceral adiposity in humans, indicating that PAI-1 can serve as a thrombogenic adipocytokine that potentially contributes to the pathogenesis of obesity-linked thrombotic disorders. About the same time, adiponectin/ACRP30 was found as an adipose-specific adipocytokine14-16), and its expression is unexpectedly decreased in obesity (Figure 1). Adiponectin is now recognized as a key factor that modulates various diseases usually found in obesity, and this adipocytokine is protective against a number of metabolic and cardiovascular disorders. It has also been shown that several inflammatory mediators including IL-6, IL-18 and MCP-1 induce the metabolic dysfunction9) (Figure 1). A recent report showed that angiopoietin-like protein 2 (Angptl2) acts as an adipocytokine that promotes inflammation and insulin resistance17) (Figure 1).
Fig. 1.
Adipocytokines during the development of obesity.
Adiposity is influenced by the degrees of food intake and exercise activity. Lean fat expresses markers of the M2 or “alternatively activated” macrophages. Obesity leads to recruitment and accumulation of the M1 or “classically activated” macrophages and T cells in adipose tissue. Adipocytokines including adiponectin, secreted frizzled-related protein 5 (Sfrp5) and adipolin are highly produced by lean adipose tissue. Conversely, obese fat generates a large amount of adipocytokines including leptin, TNF-α, IL-6, IL-18, PAI-1, MCP-1, angiopoietin-like protein 2 (Angptl2).
Most of adipocytokines such as TNF-α, IL-6 and PAI-1 are upregulated in obese states and promote obesity-inducible metabolic and cardiovascular diseases (Figure 1). In contrast, there are a smaller number of adipocytokines that exerts beneficial actions on obese complications with anti-inflammatory properties. Thus, it is conceivable that the imbalance in the production of pro-inflammatory and anti-inflammatory adipocytokines under conditions of obesity contributes to the development of obesity-linked disorders.
ADIPONECTIN
Adiponectin overview
Adiponectin is abundantly present in human blood stream, and its plasma levels range 3 to 30 µg/ml, which accounts for approximately 0.01% of total plasma protein7,18). Adiponectin is a 244-amino acid protein that contains a putative signal sequence and a collagen-like domain followed by a globular domain similar to collagens VIII and X and compliment factor C1q. It forms trimers through a collagen domain and further combines to make multimeric oligomers. Adiponectin exists in blood stream as three major oligomeric complexes: trimers, hexamers and high-molecular weight form7,18). Of importance, plasma adiponectin levels are paradoxically decreased in obese subjects18). Large adipocytes, found in obese subjects, produce lower levels of adiponectin but higher levels of pro-inflammatory adipocytokines such as TNF-α, and IL-6, which, in turn, inhibit the production of adiponectin in adipocytes 7, 8. PPAR-γ agonists, which promote adipocyte differentiation, increase adiponectin expression in vitro and in vivo19). Other factors that negatively regulate adiponectin expression include hypoxia and oxidative stress20,21). In addition, low plasma adiponectin levels are closely associated with obesity-linked complications including type 2 diabetes, coronary heart disease and hypertension.
Insulin-sensitizing actions of adiponectin
A large number of evidence from experimental models indicates that adiponectin acts as a protective adipocytokine against obesity-linked metabolic dysfunction. Systemic delivery of adiponectin has been shown to reduce hyperglycemia in diabetic mice via enhancement of insulin action22). Administration of adiponectin increases fatty acid oxidation in muscle and reduces plasma levels of glucose, free fatty acids and triglycerides23). Consistent with these observations, adiponectin-deficient (APN-KO) mice develop severe diet-induced insulin resistance on a high caloric diet24-26).
Adiponectin promotes insulin sensitivity by its ability to activate AMP-activated protein kinase (AMPK) in skeletal muscle27,28) and liver28). Adenovirus-mediated delivery of adiponectin is reported to enhance AMPK activation in skeletal muscle and increase insulin sensitivity in rats29). Adiponectin transgenic mice also show improved insulin sensitivity and increased AMPK activation in liver30). Adiponectin is supposed to stimulate AMPK activation through interactions with its cell surface receptors AdipoR1 and AdipoR231). AdipoR1 is expressed ubiquitously, but most abundantly on skeletal muscle, whereas AdipoR2 is mainly expressed in liver. AdipoR1-deficiency causes reduced adiponectin-induced AMPK activation, increased glucose production, and impaired insulin seisitivity32). In contrast, AdipoR2-deficiency causes decreased activity of PPAR-α signaling pathways and enhanced insulin resistance. Disruption of both AdipoR1 and AdipoR2 abolishes adiponectin binding and actions, thereby leading to exacerbation of insulin resistance and glucose intolerance. These data suggest that adiponectin acts as an insulin-sensitizing adipocytokine.
Regulation of macrophage function by adiponectin
Increasing evidence documents that adiponectin modulates macrophage function and phenotype, leading to resolution of inflammation. The accumulation of lipid-laden foam cells and macrophage-related inflammation are key features of atherosclerotic lesion progression. Adiponectin inhibits macrophage-to-foam cell transformation and reduces intracellular cholesteryl ester content in human macrophages by suppressing expression of class A scavenger receptor (SR-A)33) (Figure 2). Adiponectin suppresses lipopolysaccharide (LPS)-stimulated TNF-α production in macrophages34,35) (Figure 2). Furthermore, globular adiponectin reduces TNF-α production from leptin-stimulated macrophages36). Adiponectin treatment also inhibits Toll-like receptor-mediated NF-κB activation in mouse macrophages37) (Figure 2). Adiponectin stimulates the production of IL-10, an anti-inflammatory cytokine in porcine macrophages35), and it increases the production of tissue inhibitor of metalloproteinase-1 in human macrophages through its ability to stimulate IL-10 expression38).
Fig. 2.
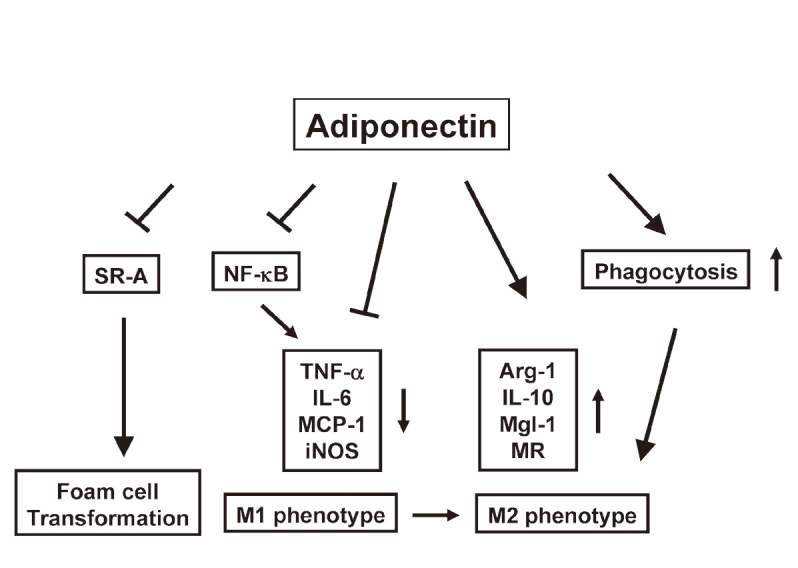
Effects of adiponectin on macrophage function and phenotype.
Adiponectin suppresses macrophage-to-foam cell transformation via inhibition of class A scavenger receptor (SR-A) expression. Adiponectin reduces expression of M1 macrophage markers including TNF-α, IL-6, MCP-1 and inducible nitric oxide synthase (iNOS), which are involved in activation of NF-κB. Conversely, adiponectin promotes expression of M2 macrophage markers including arginese-1 (Arg-1), IL-10, macrophage galactose N-acetyl-galactosamine specific lectin-1 (Mgl-1) and mannose receptor (MR), contributing to an anti-inflammatory phenotype. Adiponectin can facilitate the phagocytosis of early apoptotic cells by macrophages, which confers the M2 phenotype. Thus, adiponectin promotes the polarization of macrophages towards anti-inflammatory phenotype.
Adiponectin is structurally similar to the members of collectin family including C1q, surfactant protein A and surfactant protein D34), which are abundantly expressed in serum and form stable multimers like adiponectin39). This collectin proteins promote the rapid removal of apoptotic debris from the body which is critical in preventing inflammation and immune system dysfunction40). We have shown that adiponectin is functionally similar to collectin family proteins. Adiponectin preferentially binds to apoptotic cells and facilitates the phagocytosis of early apoptotic cells by macrophages41) (Figure 2). Of importance, adiponectin promotes the uptake of dead cells by macrophages through interactions with calreticulin and its adaptor protein CD91 on the cell surface. APN-KO mice display the reduced ability of macrophages to clear early apoptotic cells in the peritoneal cavity. Conversely, adiponectin administration promotes the efficient clearance of apoptotic cells by macrophages in both APN-KO and wild-type mice. Adiponectin overexpression also promotes macrophage ingestion of apoptotic cells and reduces features of autoimmunity in lpr mice, which possess a mutation in the Fas gene and exhibit impaired clearance of dying cells, systemic inflammation and lymphadenopathy. Furthermore, adiponectin-deficiency in lpr mice exacerbates chronic inflammatory phenotypes, which are associated with further impairment of apoptotic cell clearance. Taken together, these data suggest that adiponectin protects the organism from systemic inflammation, at least in part, through its ability to promote the phagocytosis of early apoptotic cells by macrophages via a receptor-dependent pathway involving calreticulin/CD91 co-receptor system (Figure 2).
Macrophages that infiltrate into adipose tissue of obese mice predominantly express the genes related to the M1 or “classically activated” macrophage, whereas macrophages from adipose tissue of lean mice express markers of the M2 or “alternatively activated” macrophages42). A recent study has shown that adiponectin can switch the macrophage polarization towards anti-inflammatory phenotype43) (Figure 2). APN-KO mice show increased expression of pro-inflammatory M1 markers and decreased expression of anti-inflammatory M2 markers in peritoneal macrophages and the stromal vascular fraction (SVF) cells of adipose tissue. Systemic delivery of adiponectin stimulates the expression of M2 markers in peritoneal macrophages and SVF cells in both wild-type and APN-KO mice. Stimulation with recombinant adiponectin protein results in an increase in the levels of M2 markers and a reduction of reactive oxygen species (ROS) generation in cultured macrophages. These data suggest that adiponectin functions as a regulator of macrophage polarization. Phagocytosis of early apoptotic debris by macrophages can favor the M2 phenotype40). Collectively, high levels of adiponectin can confer an anti-inflammatory phenotype in macrophages, thereby protecting against obesity-linked diseases.
Vascular protection by adiponectin
Increasing evidence from experimental studies indicates that adiponectin is protective against various obesity-linked cardiovascular diseases. Initial observations demonstrate that adiponectin reduces TNF-α-stimulated expression of endothelial adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and IL-8 in human aortic endothelial cells as well as monocyte attachment to TNF-α-stimulated endothelial cells via suppression of NF-κB activation44-46) (Figure 3). The inhibitory effect of adiponectin on NF-κB pathway is mediated, at least in part, by its ability to promote signaling through cyclicAMP (cAMP)/protein kinase A (PKA). Similarly, adiponectin attenuates high glucose-induced production of ROS in endothelial cells through a cAMP-PKA-dependent mechanism47). In line with these in vitro findings, adenovirus-mediated overexpression of adiponectin attenuates the atherosclerotic lesion formation and decreases the expression of SR-A, TNF-α and VCAM-1 in the vascular wall in a model of atherosclerosis48,49). These data indicate the ability of adiponectin to attenuate atherogenesis through anti-inflammatory actions on macrophages and vascular endothelial cells.
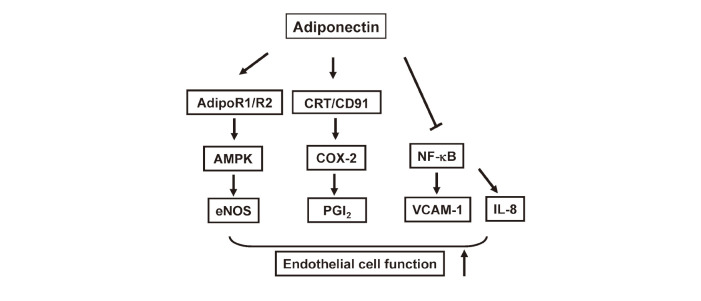
Fig. 3.
Effects of adiponectin on endothelial cell function.
Adiponectin stimulates eNOS activity in endothelial cells through its ability to activate AMPK, which is mediated by AdipoR1/R2. Adiponectin stimulates cyclooxygenase-2 (COX-2) expression and prostaglandin I2 (PGI2) production through calreticulin (CRT)/CD91 co-receptor system. Adiponectin reduces expression of vascular cell adhesion molecule-1 (VCAM-1) and IL-8 via suppression of NF-κB activation.
Several studies have shown that adiponectin also exerts beneficial actions on endothelial function. Adiponectin acts as an important regulator of endothelial nitric oxide synthase (eNOS), a key determinant of endothelial cell function. Adiponectin promotes eNOS phosphorylation in endothelial cells through its ability to activate AMPK50,51) (Figure 3). Furthermore, adiponectin stimulates endothelial cell migration and differentiation into capillary-like structures, and prevents apoptosis of endothelial cells through activation of AMPK signaling50,52-54). These in vitro observations were supported by the in vivo findings with mouse genetic models. APN-KO mice show impaired endothelium-dependent vasodilation on an atherogenic diet55) and salt-induced hypertension with reduced eNOS expression in aorta56). Furthermore, adiponectin protects against cerebral ischemia-reperfusion injury through eNOS-dependent mechanisms57). Adiponectin also stimulates ischemia-induced revascularization partly via activation of AMPK signaling58). Of note, caloric restriction promotes revascularization in response to tissue ischemia through an adiponectin-mediated activation of eNOS59). These studies suggest that adiponectin plays a crucial role in retaining vascular tone and function, at least in part, by its ability to promote AMPK/eNOS signaling pathways (Figure 3).
A recent report demonstrates that adiponectin promotes ischemia-induced revascularization in muscle through cyclooxygenese-2 (COX-2)-dependent mechanism60). Ablation of COX-2 in an endothelial specific manner in mice results in attenuation of adiponectin-mediated revascularization response in ischemic muscle. Adiponectin stimulates COX-2 expression and its metabolites prostaglandin I2 (PGI2) production, and promotes endothelial cell function via activation of COX-2 signaling pathway within endothelial cells (Figure 3). Importantly, adiponectin promotes the COX-2 regulatory pathways and endothelial cell function via CRT/CD91 co-receptor systems on the surface of endothelial cells60). Therefore, adiponectin can regulate endothelial cell function, at least in part, through COX-2-PGI2-dependent pathway. Taken together, adiponectin regulates vascular homeostasis via at least two regulatory pathways involving AMPK-eNOS and COX-2-PGI2 within endothelial cells.
Cardioprotection by adiponectin
A number of experimental findings have shown that adiponectin exerts beneficial actions on the heart under pathological conditions. Lack of adiponectin results in enhancement of myocardial ischemia-reperfusion injury, which is associated with increased myocardial cell apoptosis and TNF-α production61). Adiponectin stimulates COX-2 expression and prostaglandin E2 (PGE2) synthesis in cardiac cells, thereby preventing LPS-induced secretion of TNF-α from cardiac cells. Adiponectin also inhibits apoptosis of cardiac cells in response to hypoxia-reoxygenation via AMPK-dependent pathways61). Collectively, adiponectin protects against myocardial ischemia-reperfusion injury through COX-2-mediated anti-inflammatory and AMPK-mediated anti-apoptotic mechanisms.
Adiponectin also prevents pathological cardiac remodeling following pressure overload or angiotensin II infusion in vivo, in part, through activation of AMPK signaling62,63). Furthermore, adiponectin protects against detrimental cardiac remodeling (e.g. myocardial fibrosis, systolic dysfunction) after myocardial infarction64). APN-KO mice following aldosterone infusion also show increased left ventricular hypertrophy and pulmonary congestion, which are accompanied by severe diastolic dysfunction65). A recent study has demonstrated that adiponectin attenuates doxorubicin-induced cardiotoxicity through modulation of cardiomyocyte survival66). Overall, adiponectin serves as a protective adipocytokine against the development of various obesity-linked heart diseases.
SFRP5
Recently we identified secreted frizzled-related protein 5 (Sfrp5) as a novel adipocytokine that exerts salutary effects on metabolic function with anti-inflammatory properties67). Sfrp proteins act as soluble modulators that sequester Wnt proteins in the extracellular space between cells and prevents their binding to receptors68). Sfrp5 is expressed abundantly in white adipose tissue among various adult mouse tissues. Sfrp5 expression is down-regulated in fat tissue of obese rodents such as ob/ob mice, wild-type obese mice after long-term treatment with high caloric diet, and Zucker diabetic fatty rats (Figure 1). Wnt5a protein, which is antagonized by Sfrp5, is upregulated in fat tissues of obese rodents, and the Wnt5a/Sfrp5 protein expression ratio in adipose tissue is also increased in obese states.
Sfrp5-knockout (Sfrp5-KO) mice show normal glucose tolerance on a regular diet, but exhibit impaired insulin sensitivity, severe glucose intolerance and severe hepatic steatosis on a high calorie diet compared with control mice67). Sfrp5-deficiency also causes increased accumulation of macrophages in white adipose tissue, which is associated with enhanced production of pro-inflammatory adipocytokines including TNF-α and IL-6. Of note, the signaling of c-Jun N-terminal kinase (JNK), a downstream target of the non-canonical Wnt signaling, is activated in fat tissue in Sfrp5-KO mice on a high calorie diet. A number of in vitro experiments indicate that Sfrp5 reduces Wnt5a-stumulated phosphorylation of JNK in adipocytes and macrophages, and that Sfrp5 blocks Wnt5a-induced production of pro-inflammatory mediators in macrophages. JNK1 plays a key role in regulation of insulin resistance and inflammation69-72). Therefore, Sfrp-5 deficiency exacerbates fat inflammation and insulin resistance under conditions of obesity through enhancement of JNK1 activation in fat tissue. Sfrp5 may represent a potential target for manipulation of obesity-linked metabolic disease73,74).
ADIPOLIN
More recently we identified C1q domain containing 2 (C1qdc2)/C1q/TNF-related protein 12 (CTRP12) as a novel adipocytokine and designated this adipocytokine as adipolin (adipose-derived insulin-sensitizing factor) to indicate its potential function75). Adipolin belongs to the CTRP family proteins, which are conserved adiponectin paralogs. Adipolin is predominantly expressed in adipocytes, and its expression in adipose tissue and plasma is decreased in mouse models of obesity (Figure 1). Of importance, systemic administration of adipolin to diet-induced obese mice leads to improvement of glucose intolerance and insulin resistance, which is associated with reduced macrophage infiltration and attenuated expression of pro-inflammatory adipocytokines in fat tissue. In cultured macrophages, conditioned media from adipolin-expressing cells suppresses the expression of pro-inflammatory mediators following inflammatory stimuli. These findings suggest that adipolin functions as an anti-inflammatory adipocytokine that promotes insulin sensitivity, at least in part, through suppression of macrophage activation. Therefore, adipolin can represent a target molecule for the treatment of insulin resistance.
CONCLUSION
Adipocytokines include both pro-inflammatory and anti-inflammatory molecules, and the balance of production of these adipocytokines can be a crucial determinant for homeostasis related to nutritional status. Adipocyte dysfunction induced by obese states contributes to dysregulation of adipocytokine production, leading to the initiation and progression of obesity-induced metabolic and cardiovascular disorders. In particular, the reduced production of the beneficial adipocytokines with anti-inflammatory properties including adiponectin, Sfrp5 and adipolin, causes the development of obese complications. Thus, further elucidation of the function and regulation of adipocytokines will lead to better understanding for the pathogenesis of obesity-linked disorders.
REFERENCES
- 1).Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004; 24: 29–33. [DOI] [PubMed]
- 2).Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003; 108: 1546–1551. [DOI] [PubMed]
- 3).Sturm R. The effects of obesity, smoking, and drinking on medical problems and costs. Health Aff (Millwood). 2002; 21 (2): 245–253. [DOI] [PubMed]
- 4).Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003; 112 (12): 1785–1788. [DOI] [PMC free article] [PubMed]
- 5).Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444 (7121): 860–867. [DOI] [PubMed]
- 6).Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006; 116 (7): 1793–1801. [DOI] [PMC free article] [PubMed]
- 7).Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003; 14: 561–566. [DOI] [PubMed]
- 8).Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005; 96 (9): 939–949. [DOI] [PubMed]
- 9).Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011; 11 (2): 85–97. [DOI] [PMC free article] [PubMed]
- 10).Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat. Med. 1996; 2: 800–803. [DOI] [PubMed]
- 11).Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987; 237 (4813): 402–405. [DOI] [PubMed]
- 12).Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259: 87–91. [DOI] [PubMed]
- 13).Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372: 425–432 Erratum in: Nature 1995 Mar 1930; 1374: 1479. [DOI] [PubMed]
- 14).Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996; 271: 10697–10703. [DOI] [PubMed]
- 15).Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 1996; 221: 286–289. [DOI] [PubMed]
- 16).Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995; 270: 26746–26749. [DOI] [PubMed]
- 17).Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009; 10 (3): 178–188. [DOI] [PubMed]
- 18).Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999; 257: 79–83. [DOI] [PubMed]
- 19).Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001; 50 (9): 2094–2099. [DOI] [PubMed]
- 20).Hattori Y, Akimoto K, Gross SS, Hattori S, Kasai K. Angiotensin-II-induced oxidative stress elicits hypoadiponectinaemia in rats. Diabetologia. 2005; 48 (6): 1066–1074. [DOI] [PubMed]
- 21).Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007; 56 (4): 901–911. [DOI] [PubMed]
- 22).Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001; 7: 947–953. [DOI] [PubMed]
- 23).Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA. 2001; 98: 2005–2010. [DOI] [PMC free article] [PubMed]
- 24).Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002; 8: 731–737. [DOI] [PubMed]
- 25).Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 2006; 281: 2654–2660. [DOI] [PubMed]
- 26).Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002; 277: 25863–25866. [DOI] [PubMed]
- 27).Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. USA. 2002; 99: 16309–16313. [DOI] [PMC free article] [PubMed]
- 28).Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002; 8: 1288–1295. [DOI] [PubMed]
- 29).Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005; 54 (5): 1304–1313. [DOI] [PubMed]
- 30).Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004; 145 (1): 367–383. [DOI] [PubMed]
- 31).Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003; 423: 762–769. [DOI] [PubMed]
- 32).Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007; 13 (3): 332–339. [DOI] [PubMed]
- 33).Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001; 103: 1057–1063. [DOI] [PubMed]
- 34).Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000; 96: 1723–1732. [PubMed]
- 35).Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004; 316 (3): 924–929. [DOI] [PubMed]
- 36).Zhao T, Hou M, Xia M, Wang Q, Zhu H, Xiao Y, Tang Z, Ma J, Ling W. Globular adiponectin decreases leptin-induced tumor necrosis factor-alpha expression by murine macrophages: involvement of cAMP-PKA and MAPK pathways. Cell Immunol. 2005; 238 (1): 19–30. [DOI] [PubMed]
- 37).Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S, Yamashita Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005; 579 (30): 6821–6826. [DOI] [PubMed]
- 38).Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004; 109 (17): 2046–2049. [DOI] [PubMed]
- 39).Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc. Am. Thorac. Soc. 2007; 4 (3): 252–257. [DOI] [PMC free article] [PubMed]
- 40).Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002; 2: 965-975. [DOI] [PubMed]
- 41).Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007; 117 (2): 375–386. [DOI] [PMC free article] [PubMed]
- 42).Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117 (1): 175–184. [DOI] [PMC free article] [PubMed]
- 43).Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem.285 (9): 6153–6160. [DOI] [PMC free article] [PubMed]
- 44).Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999; 100: 2473–2476. [DOI] [PubMed]
- 45).Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005; 97 (12): 1245–1252. [DOI] [PubMed]
- 46).Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000; 102: 1296–1301. [DOI] [PubMed]
- 47).Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, Hough K, Scalia R, Goldstein BJ. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006; 55 (6): 1840–1846. [DOI] [PubMed]
- 48).Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002; 106: 2767–2770. [DOI] [PubMed]
- 49).Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J. Biol. Chem. 2003; 278: 2461–2468. [DOI] [PubMed]
- 50).Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004; 279: 1304–1309. [DOI] [PMC free article] [PubMed]
- 51).Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003; 278: 45021–45026. [DOI] [PubMed]
- 52).Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun. 2005; 332 (1): 200–205. [DOI] [PubMed]
- 53).Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ. Res. 2004; 94: e27-e31. [DOI] [PMC free article] [PubMed]
- 54).Lin LY, Lin CY, Su TC, Liau CS. Angiotensin II-induced apoptosis in human endothelial cells is inhibited by adiponectin through restoration of the association between endothelial nitric oxide synthase and heat shock protein 90. FEBS Lett. 2004; 574 (1-3): 106–110. [DOI] [PubMed]
- 55).Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003; 42: 231–234. [DOI] [PubMed]
- 56).Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin Replenishment Ameliorates Obesity-Related Hypertension. Hypertension. 2006. [DOI] [PubMed]
- 57).Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008; 117 (2): 216–223. [DOI] [PubMed]
- 58).Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 2004; 279: 28670–28674. [DOI] [PubMed]
- 59).Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009; 284 (3): 1718–1724. [DOI] [PMC free article] [PubMed]
- 60).Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, Kihara S, Walsh K. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009; 29 (13): 3487–3499. [DOI] [PMC free article] [PubMed]
- 61).Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 2005; 11 (10): 1096–1103. [DOI] [PMC free article] [PubMed]
- 62).Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 2004; 10 (12): 1384–1389. [DOI] [PMC free article] [PubMed]
- 63).Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc. Res. 2005; 67 (4): 705–713. [DOI] [PubMed]
- 64).Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007; 42 (6): 1065–1074. [DOI] [PMC free article] [PubMed]
- 65).Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, Higuchi A, De Silva DS, Qin F, Walsh K, Ouchi N. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology.151 (1): 322–331. [DOI] [PMC free article] [PubMed]
- 66).Maruyama S, Shibata R, Ohashi K, Ohashi T, Daida H, Walsh K, Murohara T, Ouchi N. Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism. J Biol Chem. 2011; 286 (37): 32790–32800. [DOI] [PMC free article] [PubMed]
- 67).Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010; 329 (5990): 454–457. [DOI] [PMC free article] [PubMed]
- 68).Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008; 121 (Pt 6): 737–746. [DOI] [PubMed]
- 69).Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007; 6 (5): 386–397. [DOI] [PubMed]
- 70).Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008; 322 (5907): 1539–1543. [DOI] [PMC free article] [PubMed]
- 71).Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008; 3 (9): e3151. [DOI] [PMC free article] [PubMed]
- 72).Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002; 420 (6913): 333–336. [DOI] [PubMed]
- 73).Oh DY, Olefsky JM. Medicine. Wnt fans the flames in obesity. Science. 2010; 329 (5990): 397–398. [DOI] [PubMed]
- 74).Bird L. Inflammation: The fat controller. Nat Rev Immunol.10 (8): 540. [DOI] [PubMed]
- 75).Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T, Ouchi N. Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J Biol Chem. 2011; 286 (40): 34552–34558. [DOI] [PMC free article] [PubMed]