Abstract
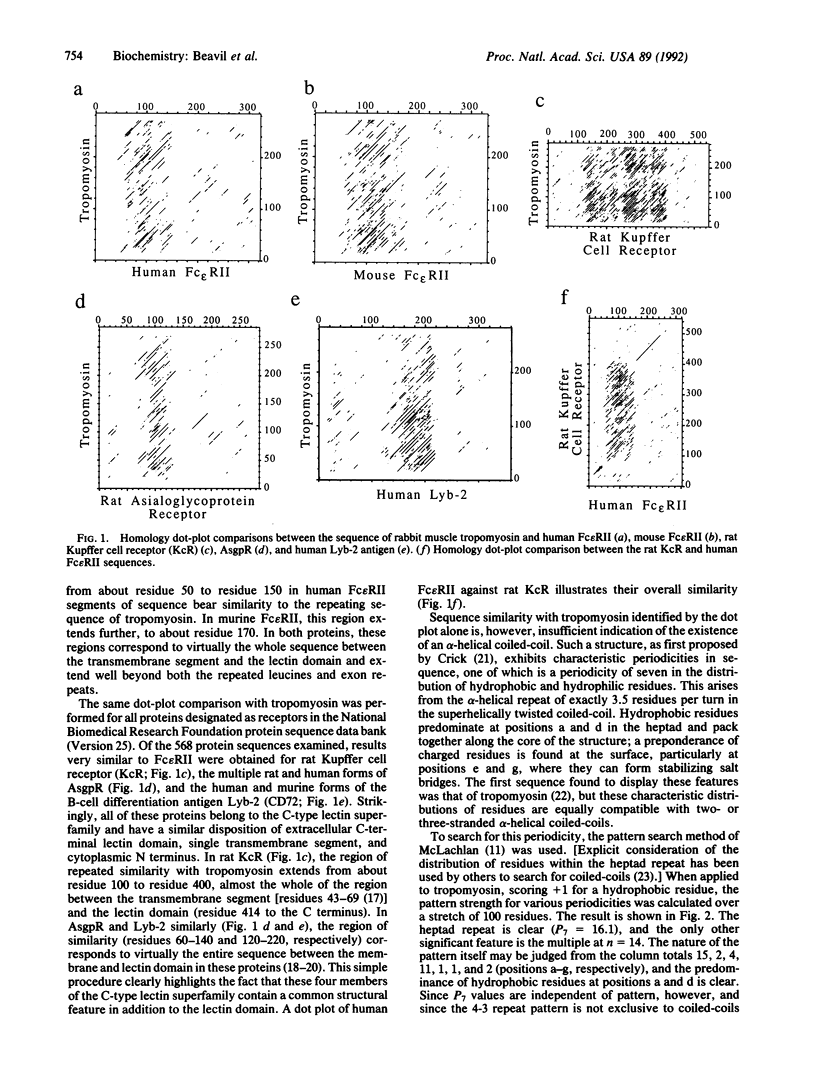
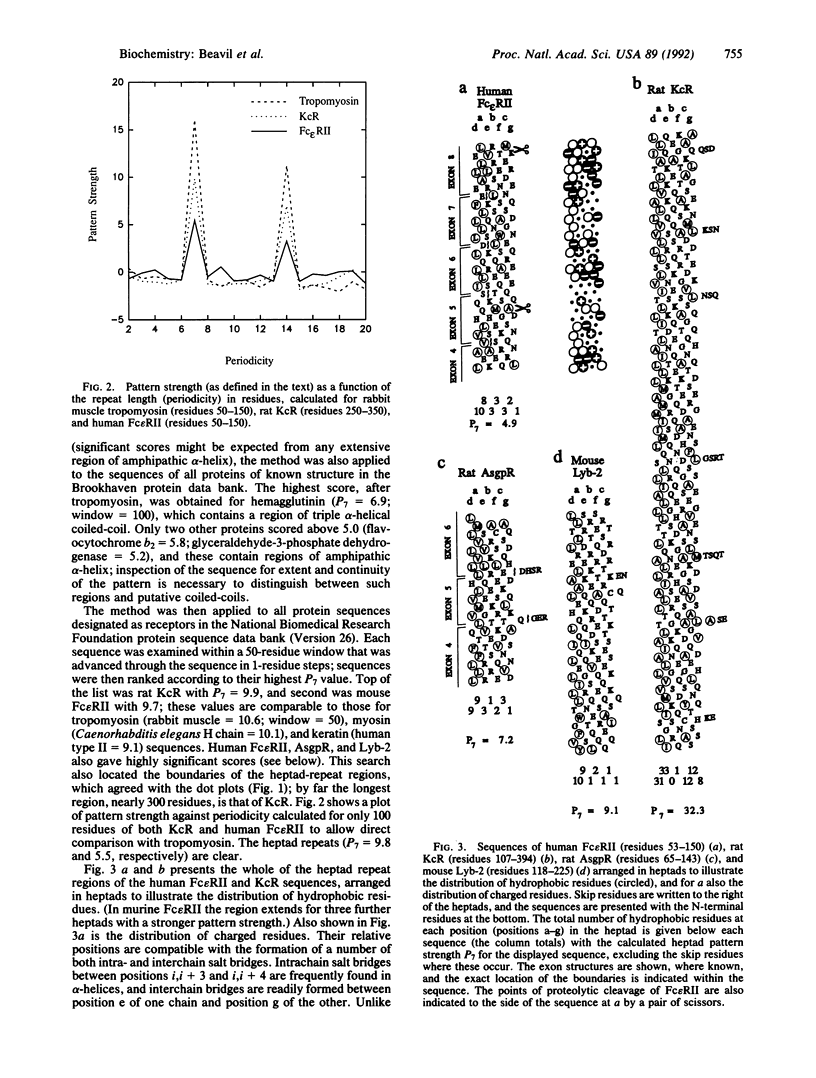
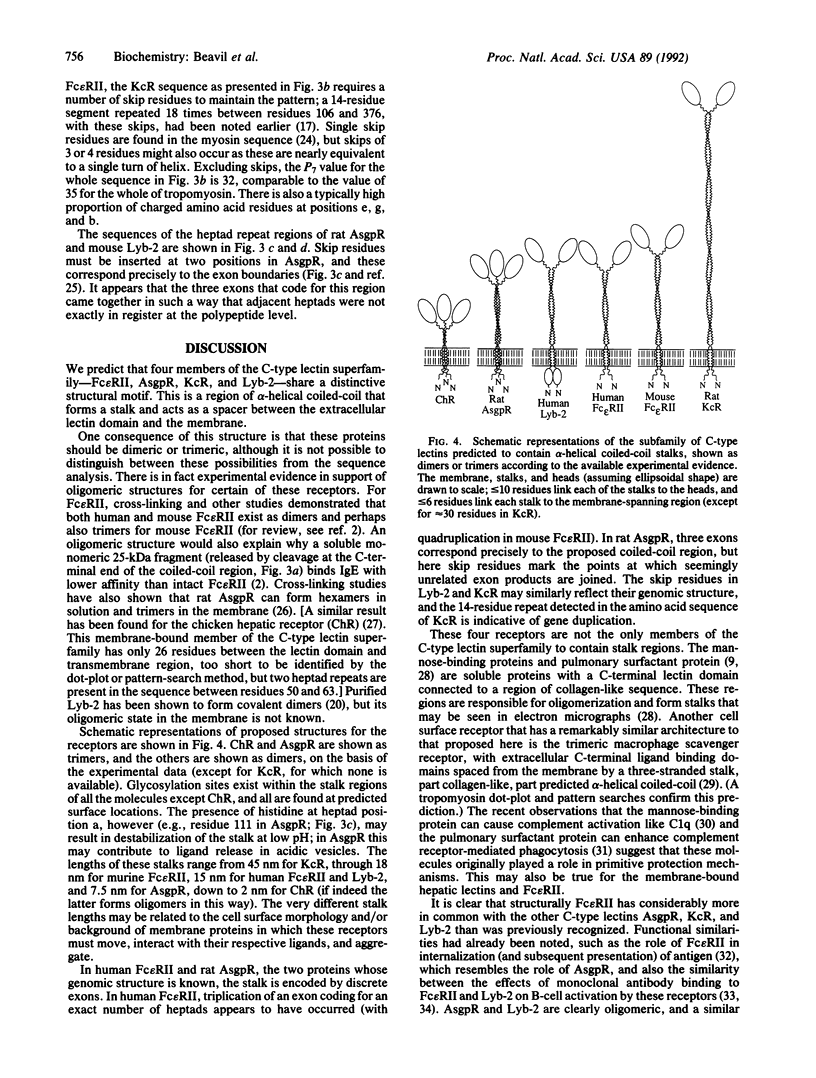
The low-affinity receptor for IgE (Fc epsilon RII/CD23) is a cell surface glycoprotein that plays a role in both cellular immunity and allergic inflammation. Its extracellular IgE-binding domain bears homology to C-type animal lectins, and the protein is, therefore, classified as a member of this superfamily. We predict that this lectin-like domain is separated from the cell membrane by an extensive region of alpha-helical coiled-coil structure, based upon sequence comparisons with tropomyosin, the archetypal alpha-helical coiled-coil structure, and detection of characteristic heptad repeats. Analysis of other receptor protein sequences identified a similar structural motif in other membrane-bound members of the C-type lectin superfamily, including the asialoglycoprotein receptor, the Kupffer cell receptor, and the B-cell differentiation antigen Lyb-2 (CD72). It appears that within the C-type lectin superfamily, there is a subfamily of structurally related membrane-bound receptor proteins that contain alpha-helical coiled-coil stalks of various lengths.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettler B., Hofstetter H., Rao M., Yokoyama W. M., Kilchherr F., Conrad D. H. Molecular structure and expression of the murine lymphocyte low-affinity receptor for IgE (Fc epsilon RII). Proc Natl Acad Sci U S A. 1989 Oct;86(19):7566–7570. doi: 10.1073/pnas.86.19.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V., Karlin S. Too many leucine zippers? Nature. 1989 Oct 19;341(6243):574–575. doi: 10.1038/341574a0. [DOI] [PubMed] [Google Scholar]
- Conrad D. H. Fc epsilon RII/CD23: the low affinity receptor for IgE. Annu Rev Immunol. 1990;8:623–645. doi: 10.1146/annurev.iy.08.040190.003203. [DOI] [PubMed] [Google Scholar]
- Delespesse G., Hofstetter H., Sarfati M., Suter U., Nakajima T., Frost H., Letellier M., Peleman R., Kilchherr E. Human Fc epsilon RII. Molecular, biological and clinical aspects. Chem Immunol. 1989;47:79–105. [PubMed] [Google Scholar]
- Drickamer K., Mamon J. F., Binns G., Leung J. O. Primary structure of the rat liver asialoglycoprotein receptor. Structural evidence for multiple polypeptide species. J Biol Chem. 1984 Jan 25;259(2):770–778. [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Gollnick S. O., Trounstine M. L., Yamashita L. C., Kehry M. R., Moore K. W. Isolation, characterization, and expression of cDNA clones encoding the mouse Fc receptor for IgE (Fc epsilon RII)1. J Immunol. 1990 Mar 1;144(5):1974–1982. [PubMed] [Google Scholar]
- Gordon J., Webb A. J., Guy G. R., Walker L. Triggering of B lymphocytes through CD23: epitope mapping and studies using antibody derivatives indicate an allosteric mechanism of signalling. Immunology. 1987 Apr;60(4):517–521. [PMC free article] [PubMed] [Google Scholar]
- Halberg D. F., Wager R. E., Farrell D. C., Hildreth J., 4th, Quesenberry M. S., Loeb J. A., Holland E. C., Drickamer K. Major and minor forms of the rat liver asialoglycoprotein receptor are independent galactose-binding proteins. Primary structure and glycosylation heterogeneity of minor receptor forms. J Biol Chem. 1987 Jul 15;262(20):9828–9838. [PubMed] [Google Scholar]
- Hoyle G. W., Hill R. L. Molecular cloning and sequencing of a cDNA for a carbohydrate binding receptor unique to rat Kupffer cells. J Biol Chem. 1988 Jun 5;263(16):7487–7492. [PubMed] [Google Scholar]
- Ikeda K., Sannoh T., Kawasaki N., Kawasaki T., Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987 Jun 5;262(16):7451–7454. [PubMed] [Google Scholar]
- Ikuta K., Takami M., Kim C. W., Honjo T., Miyoshi T., Tagaya Y., Kawabe T., Yodoi J. Human lymphocyte Fc receptor for IgE: sequence homology of its cloned cDNA with animal lectins. Proc Natl Acad Sci U S A. 1987 Feb;84(3):819–823. doi: 10.1073/pnas.84.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehry M. R., Yamashita L. C. Low-affinity IgE receptor (CD23) function on mouse B cells: role in IgE-dependent antigen focusing. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7556–7560. doi: 10.1073/pnas.86.19.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Leung J. O., Holland E. C., Drickamer K. Characterization of the gene encoding the major rat liver asialoglycoprotein receptor. J Biol Chem. 1985 Oct 15;260(23):12523–12527. [PubMed] [Google Scholar]
- Loeb J. A., Drickamer K. The chicken receptor for endocytosis of glycoproteins contains a cluster of N-acetylglucosamine-binding sites. J Biol Chem. 1987 Mar 5;262(7):3022–3029. [PubMed] [Google Scholar]
- Luo H. Y., Hofstetter H., Banchereau J., Delespesse G. Cross-linking of CD23 antigen by its natural ligand (IgE) or by anti-CD23 antibody prevents B lymphocyte proliferation and differentiation. J Immunol. 1991 Apr 1;146(7):2122–2129. [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Lüdin C., Hofstetter H., Sarfati M., Levy C. A., Suter U., Alaimo D., Kilchherr E., Frost H., Delespesse G. Cloning and expression of the cDNA coding for a human lymphocyte IgE receptor. EMBO J. 1987 Jan;6(1):109–114. doi: 10.1002/j.1460-2075.1987.tb04726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D. Analysis of periodic patterns in amino acid sequences: collagen. Biopolymers. 1977 Jun;16(6):1271–1297. doi: 10.1002/bip.1977.360160609. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983 Mar 15;164(4):605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M., Smillie L. B. Sequence repeats in alpha-tropomyosin. J Mol Biol. 1975 Oct 25;98(2):281–291. doi: 10.1016/s0022-2836(75)80118-5. [DOI] [PubMed] [Google Scholar]
- Nakayama E., von Hoegen I., Parnes J. R. Sequence of the Lyb-2 B-cell differentiation antigen defines a gene superfamily of receptors with inverted membrane orientation. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1352–1356. doi: 10.1073/pnas.86.4.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Evidence that the leucine zipper is a coiled coil. Science. 1989 Jan 27;243(4890):538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- Oas T. G., McIntosh L. P., O'Shea E. K., Dahlquist F. W., Kim P. S. Secondary structure of a leucine zipper determined by nuclear magnetic resonance spectroscopy. Biochemistry. 1990 Mar 27;29(12):2891–2894. doi: 10.1021/bi00464a001. [DOI] [PubMed] [Google Scholar]
- Saudek V., Pastore A., Castiglione Morelli M. A., Frank R., Gausepohl H., Gibson T., Weih F., Roesch P. Solution structure of the DNA-binding domain of the yeast transcriptional activator protein GCN4. Protein Eng. 1990 Oct;4(1):3–10. doi: 10.1093/protein/4.1.3. [DOI] [PubMed] [Google Scholar]
- Saxon A., Ke Z., Bahati L., Stevens R. H. Soluble CD23 containing B cell supernatants induce IgE from peripheral blood B-lymphocytes and costimulate with interleukin-4 in induction of IgE. J Allergy Clin Immunol. 1990 Sep;86(3 Pt 1):333–344. doi: 10.1016/s0091-6749(05)80096-x. [DOI] [PubMed] [Google Scholar]
- Suter U., Bastos R., Hofstetter H. Molecular structure of the gene and the 5'-flanking region of the human lymphocyte immunoglobulin E receptor. Nucleic Acids Res. 1987 Sep 25;15(18):7295–7308. doi: 10.1093/nar/15.18.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner A. J., Robinson S. L., Borchelt J., Wright J. R. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem. 1989 Aug 15;264(23):13923–13928. [PubMed] [Google Scholar]
- Thiel S., Reid K. B. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989 Jun 19;250(1):78–84. doi: 10.1016/0014-5793(89)80689-1. [DOI] [PubMed] [Google Scholar]
- Vercelli D., Helm B., Marsh P., Padlan E., Geha R. S., Gould H. The B-cell binding site on human immunoglobulin E. Nature. 1989 Apr 20;338(6217):649–651. doi: 10.1038/338649a0. [DOI] [PubMed] [Google Scholar]
- Von Hoegen I., Nakayama E., Parnes J. R. Identification of a human protein homologous to the mouse Lyb-2 B cell differentiation antigen and sequence of the corresponding cDNA. J Immunol. 1990 Jun 15;144(12):4870–4877. [PubMed] [Google Scholar]



