Abstract
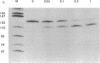
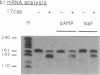
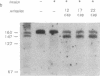
We have demonstrated that the synthesis of cDNA by avian myeloblastosis virus and Moloney murine leukemia virus reverse transcriptases can be prevented by oligonucleotides bound to the RNA template approximately 100 nucleotides remote from the 3' end of the primer. The RNA was truncated at the level of the antisense oligonucleotide-RNA duplex during the reverse transcription. The key role played by the reverse transcriptase-associated RNase H activity in the inhibition process was shown by the use of (i) inhibitors of RNase H (NaF or dAMP), (ii) Moloney murine leukemia virus reverse transcriptase devoid of RNase H activity, or (iii) alpha-analogues of oligomers that do not elicit RNase H-catalyzed RNA degradation. In all three cases the inhibitory effect was either reduced (NaF, dAMP) or totally abolished. However, an alpha-oligomer bound to the sequence immediately adjacent to the primer-binding site prevented reverse transcription. Therefore, initiation of polymerization can be blocked by means of an RNase H-independent mechanism, whereas arrest of a growing cDNA strand can be achieved only by an oligonucleotide mediating cleavage of the template RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L., Wallace D. M., Puskas R. S., Eschenfeldt W. H. Reverse transcriptase and its associated ribonuclease H: interplay of two enzyme activities controls the yield of single-stranded complementary deoxyribonucleic acid. Biochemistry. 1983 May 10;22(10):2365–2372. doi: 10.1021/bi00279a010. [DOI] [PubMed] [Google Scholar]
- Bloch E., Lavignon M., Bertrand J. R., Pognan F., Morvan F., Malvy C., Rayner B., Imbach J. L., Paoletti C. Alpha-anomeric DNA: beta-RNA hybrids as new synthetic inhibitors of Escherichia coli RNase H, Drosophila embryo RNase H and M-MLV reverse transcriptase. Gene. 1988 Dec 10;72(1-2):349–360. doi: 10.1016/0378-1119(88)90162-x. [DOI] [PubMed] [Google Scholar]
- Boiziau C., Blonski C., Thuong N. T., Shire D., Toulme J. J. Inhibition of reverse transcription by unmodified and modified antisense oligodeoxynucleotides. Nucleic Acids Symp Ser. 1991;(24):121–125. [PubMed] [Google Scholar]
- Boiziau C., Kurfurst R., Cazenave C., Roig V., Thuong N. T., Toulmé J. J. Inhibition of translation initiation by antisense oligonucleotides via an RNase-H independent mechanism. Nucleic Acids Res. 1991 Mar 11;19(5):1113–1119. doi: 10.1093/nar/19.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer L. C., Wells R. D. Mechanistic independence of avian myeloblastosis virus DNA polymerase and ribonuclease H. J Virol. 1974 Dec;14(6):1494–1502. doi: 10.1128/jvi.14.6.1494-1502.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Chevrier M., Nguyen T. T., Hélène C. Rate of degradation of [alpha]- and [beta]-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 1987 Dec 23;15(24):10507–10521. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Inhibition of DNA synthesis by cross-linking the template to platinum-thiol derivatives of complementary oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 26;17(12):4783–4798. doi: 10.1093/nar/17.12.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStefano J. J., Buiser R. G., Mallaber L. M., Myers T. W., Bambara R. A., Fay P. J. Polymerization and RNase H activities of the reverse transcriptases from avian myeloblastosis, human immunodeficiency, and Moloney murine leukemia viruses are functionally uncoupled. J Biol Chem. 1991 Apr 25;266(12):7423–7431. [PubMed] [Google Scholar]
- Furfine E. S., Reardon J. E. Reverse transcriptase.RNase H from the human immunodeficiency virus. Relationship of the DNA polymerase and RNA hydrolysis activities. J Biol Chem. 1991 Jan 5;266(1):406–412. [PubMed] [Google Scholar]
- Gagnor C., Bertrand J. R., Thenet S., Lemaître M., Morvan F., Rayner B., Malvy C., Lebleu B., Imbach J. L., Paoletti C. alpha-DNA. VI: Comparative study of alpha- and beta-anomeric oligodeoxyribonucleotides in hybridization to mRNA and in cell free translation inhibition. Nucleic Acids Res. 1987 Dec 23;15(24):10419–10436. doi: 10.1093/nar/15.24.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnor C., Rayner B., Leonetti J. P., Imbach J. L., Lebleu B. Alpha-DNA.IX: Parallel annealing of alpha-anomeric oligodeoxyribonucleotides to natural mRNA is required for interference in RNase H mediated hydrolysis and reverse transcription. Nucleic Acids Res. 1989 Jul 11;17(13):5107–5114. doi: 10.1093/nar/17.13.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild J., Agrawal S., Civeira M. P., Sarin P. S., Sun D., Zamecnik P. C. Inhibition of human immunodeficiency virus replication by antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5507–5511. doi: 10.1073/pnas.85.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Lavignon M., Bertrand J. R., Rayner B., Imbach J. L., Malvy C., Paoletti C. Inhibition of Moloney murine leukemia virus reverse transcriptase by alpha-anomeric oligonucleotides. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1184–1190. doi: 10.1016/0006-291x(89)91367-3. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Zhang G. R., Sun D. K., Ikeuchi T., Sarin P. S. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6553–6556. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau N., Boiziau C., Verspieren P., Shire D., Toulmé J. J. Blockage of AMV reverse transcriptase by antisense oligodeoxynucleotides. FEBS Lett. 1990 Nov 12;274(1-2):53–56. doi: 10.1016/0014-5793(90)81327-k. [DOI] [PubMed] [Google Scholar]
- Majumdar C., Stein C. A., Cohen J. S., Broder S., Wilson S. H. Stepwise mechanism of HIV reverse transcriptase: primer function of phosphorothioate oligodeoxynucleotide. Biochemistry. 1989 Feb 7;28(3):1340–1346. doi: 10.1021/bi00429a060. [DOI] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Zon G., Shinozuka K., Robert-Guroff M., Shimada T., Stein C. A., Mitsuya H., Wong-Staal F., Cohen J. S., Broder S. Regulation of viral expression of human immunodeficiency virus in vitro by an antisense phosphorothioate oligodeoxynucleotide against rev (art/trs) in chronically infected cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4244–4248. doi: 10.1073/pnas.86.11.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Thenet S., Bertrand J. R., Paoletti J., Malvy C., Paoletti C. alpha-DNA II. Synthesis of unnatural alpha-anomeric oligodeoxyribonucleotides containing the four usual bases and study of their substrate activities for nucleases. Nucleic Acids Res. 1987 Apr 24;15(8):3421–3437. doi: 10.1093/nar/15.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama F., Kikuchi R., Crouch R. J., Uchida T. Intrinsic properties of reverse transcriptase in reverse transcription. Associated RNase H is essentially regarded as an endonuclease. J Biol Chem. 1989 Nov 5;264(31):18808–18817. [PubMed] [Google Scholar]
- Stevenson M., Iversen P. L. Inhibition of human immunodeficiency virus type 1-mediated cytopathic effects by poly(L-lysine)-conjugated synthetic antisense oligodeoxyribonucleotides. J Gen Virol. 1989 Oct;70(Pt 10):2673–2682. doi: 10.1099/0022-1317-70-10-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. K., Zhang J., Li Z. Y., Tarpley W. G., Downey K. M., So A. G. Functional characterization of RNA-dependent DNA polymerase and RNase H activities of a recombinant HIV reverse transcriptase. Biochemistry. 1991 Mar 12;30(10):2651–2655. doi: 10.1021/bi00224a013. [DOI] [PubMed] [Google Scholar]
- Tanese N., Goff S. P. Domain structure of the Moloney murine leukemia virus reverse transcriptase: mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1777–1781. doi: 10.1073/pnas.85.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspieren P., Cornelissen A. W., Thuong N. T., Hélène C., Toulmé J. J. An acridine-linked oligodeoxynucleotide targeted to the common 5' end of trypanosome mRNAs kills cultured parasites. Gene. 1987;61(3):307–315. doi: 10.1016/0378-1119(87)90194-6. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Rossi J. J., Murakawa G. J., Spallone P. A., Stephens D. A., Kaplan B. E., Eritja R., Wallace R. B., Cantin E. M. Inhibition of human immunodeficiency virus by using an oligonucleoside methylphosphonate targeted to the tat-3 gene. J Virol. 1988 Oct;62(10):3914–3917. doi: 10.1128/jvi.62.10.3914-3917.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]