Abstract
Background
Previous research estimates that the majority of athletes with sport-related concussion (SRC) will recover between 7–10 days following injury. This short, temporal window of recovery is predominately based on symptom resolution and cognitive improvement, and does not accurately reflect recent advances to the clinical assessment model.
Objective
To characterize SRC recovery at 1-week post-injury time intervals on symptom, neurocognitive, and vestibular-oculomotor outcomes, and examine gender differences on SRC recovery time.
Methods
A prospective, repeated measures design was used to examine the temporal resolution of neurocognitive, symptom, and vestibular-oculomotor impairment in 66 subjects (16.5 ± 1.9 years, range 14–23, 64% male) with SRC.
Results
Recovery time across all outcomes was between 21–28 days post SRC for most athletes. Symptoms demonstrated the greatest improvement in the first 2 weeks, while neurocognitive impairment lingered across various domains up to 28 days post SRC. Vestibular-oculomotor decrements also resolved between one to three weeks post injury. There were no gender differences in neurocognitive recovery. Males were more likely to be asymptomatic by the fourth week and reported less vestibular-oculomotor impairment than females at weeks 1 and 2.
Conclusion
When utilizing the recommended “comprehensive” approach for concussion assessment, recovery time for SRC is approximately three to four weeks, which is longer than the commonly reported 7–14 days. Sports medicine clinicians should use a variety of complementing assessment tools to capture the heterogeneity of SRC.
Keywords: sport-related concussion, recovery time, comprehensive assessment
Sport-related concussions (SRC) are purported to have a relatively short recovery time, with over 90% of injured athletes returning to play (RTP) within 7–14 days of injury 1, 2. However, there is a growing body of literature that suggests recovery may be longer for some athletes due to demographic differences (e.g., younger, females, those with concussion history) and/or to the heterogeneous nature of this injury 3–6. The current literature is also wrought with methodological inconsistencies including how symptom resolution and clinical recovery are defined 7. The majority of studies examining SRC recovery included male football players and only examined symptom and cognitive recovery outcomes e.g., 8, 9–11, limiting the generalizability of these findings. Additional research on SRC recovery trajectories across multiple domains including assessments of symptoms, neurocognitive testing, balance, and vestibular and oculomotor outcomes in more diverse samples is warranted.
Post-concussion symptom reporting, although limited by self-report, remains an important clinical marker of recovery and readiness for RTP upon successful completion of recommended RTP exertional protocols 1, 2, 12. The literature documenting the time to symptom resolution is disparate, as some studies report alleviation within 5–10 days 10, 11, 13–16, while others have documented post-concussion symptoms beyond 7–14 days 3, 4, 8, 17. These inconsistencies in symptom recovery are attributable in part to how symptom resolution was determined. Some studies 10, 11, 14, 16 defined symptom resolution (i.e., asymptomatic status) by statistically comparing symptoms between concussed athletes and their baseline or to non-concussed controls, whereas other studies defined recovery as date of medical clearance 3, 9. Symptom reporting is noted in the context of clinical examination, balance assessment, neurocognitive testing, and throughout physical exertion RTP protocols as a means of gauging overall symptom presentation but also specific to different assessments that may elicit symptoms within more specific parameters.
The subjectivity of self-reported symptoms underscores the importance of using more objective evaluations such as those afforded by neuropsychological assessment 1, 12. Some studies report that neurocognitive impairment resolves 10, 11, 14, 16, 18 within 14 days of injury, while other researchers have documented longer neurocognitive recovery trajectories lasting up to 21 days post-injury 8, 19, 20. Methodological differences such as the type of tests used (e.g., paper-and-pencil versus computer-based tests) and when the tests are administered after SRC likely account for the wide range of reported recovery times for cognitive function.
Several studies examining recovery following SRC have employed measures designed to assess the immediate (i.e., sideline) and acute (i.e., 1–3 days post-injury) effects of SRC well past these timeframes. McCrea and colleagues10, 11 used the Standard Assessment of Concussion (SAC) to assess athletes within 2 days and up to 1-week post-injury. At 1–2 days post injury, concussed athletes performed more poorly than their non-concussed counterparts, but were equivalent at 1-week post-injury. Similar findings using the SAC have been reported in other studies 10, 11, 14, 16 and researchers have concluded that the SAC lacks sensitivity in detecting cognitive impairment beyond the acute time period following SRC 7. In addition to the SAC, the Balance Error Scoring System (BESS) has also been used to measure SRC recovery 10, 11, 14, 16 and researchers have reported that balance impairment resolves within 7 days of SRC in over 90% of cases 7, 21. Similar to the SAC, researchers report that the BESS lacks sensitivity beyond the first 3 days22–24 following SRC and this measure is subject to practice and learning effects25, 26.
Clinical balance measures such as the BESS focus on postural stability, which involves the vestibulo-spinal system. Recent clinical research findings support the use of vestibular and oculomotor measures as part of a comprehensive assessment of SRC 27, 28. However, little is known about the recovery trajectory of vestibular and oculomotor outcomes, and how they compare to symptoms and neurocognitive outcomes, as they have not yet been assessed with regard to SRC recovery.
Although recent consensus papers did not list gender as a modifying factor for SRC 1, gender differences on the clinical presentation of SRC have been documented in several studies29, 30. Specifically, concussed females have demonstrated greater symptoms and lower neurocognitive performance compared to concussed males 31–33. While these data provide clinical insight to the presentation of concussion between males and females, no study to date has directly compared the recovery rates among males and females across multiple clinical domains (e.g., symptoms, neurocognitive, balance, vestibular, oculomotor) 14, 30. Comparing and documenting the multimodal recovery from SRC between genders is warranted and would further inform the clinical management of SRC for male and female athletes.
The primary purpose of the current study was to characterize recovery at 1-week post-injury time intervals during the first month following SRC using a comprehensive concussion assessment that included symptoms, neurocognitive, and vestibular-oculomotor outcomes. We expected that recovery curves would demonstrate significant improvement from the 1-week to 2-week assessment and plateau (i.e., recovery) around the 3–4 week post-injury time points. A secondary purpose of the study was to examine the effect of gender on recovery time. We expected that females would take longer to recover than males across each domain.
Methods
Design and Participants
A prospective, repeated measures design was used for this study. A total of 66 patients met inclusion/exclusion criteria and were initially enrolled in the protocol. All participants were between 14–22 years of age and had suffered a SRC within 7 days of initial assessment. Exclusion criteria included any one or more of the following: history of special education; history of neurological or psychiatric disorders; previous moderate-to-severe traumatic brain injury (TBI: Glasgow Coma Scale <13); previous brain surgery; current use of CNS-affecting medications; history of 3 or more concussions; or previous concussion within the past 6 months. Participants with a history of migraine were eligible for participation. Only three male subjects reported a prior history of migraine and were included in the current analyses. Following enrollment, 17% (n= 11) had incomplete data, missing either the third and/or fourth week assessments. These subjects were included in analysis where data was complete. In total, 55/66 (83%) subjects completed the study for all time points. There were a total of 42 (63.6%) males and 24 (36.4%) females in the study. Table 1 provides a summary of demographic characteristics for the sample.
Table 1.
Subject characteristics at time of injury by gender with comparisons (chi square except where indicated)
| Male (n = 42) | Female (n = 24) | p | |
|---|---|---|---|
| Age (years, mean ± SD) | 16.5 ± 1.8 | 16.4 ± 2.1 | .879* |
| Disorientation, n (%) | 25 (60) | 12 (50) | .388 |
| Anterograde Amnesia, n (%) | 11 (26) | 4 (17) | .374 |
| Retrograde Amnesia, n (%) | 4 (10) | 3 (13) | .699** |
| Loss of Consciousness, n (%) | 6 (14) | 3 (13) | 1.000** |
| Signs Present, n (%) | 31 (74) | 12 (50) | .051 |
Independent samples t-test
Fisher’s exact probability test
Measures
Neurocognitive Performance
The Immediate Post-concussion Assessment and Cognitive Test (ImPACT) is a computerized neurocognitive battery comprised of three sections that include demographic/health history questionnaire, the 22-item Post-concussion Symptom Scale (PCSS- see below), and six neurocognitive test modules covering memory, attention, learning, processing speed, and reaction time. The six neurocognitive modules comprise four composite scores for verbal and visual memory (% correct), visual motor processing speed #- with higher scores= better performance), and reaction time (sec). Reliability data for the ImPACT is reported elsewhere 34.
Post-concussion Symptoms
The Post-Concussion Symptom Scale (PCSS) is a 22-item symptom report covering physical (e.g., headaches, dizziness), cognitive (e.g., mental fogginess, memory problems), sleep-related (e.g. fatigue, change in sleep patterns), and affective (e.g., increased emotionality, irritability, anxiety) symptoms commonly reported after concussive injuries. Each symptom is rated on a seven-point Likert scale ranging from 0 (not experiencing this symptom) to 6 (severe). The PCSS, which is embedded at the beginning of the ImPACT battery, is a widely used and validated tool to assess post-concussion symptoms35–37.
Dizziness and Vestibular-Oculomotor Performance
A brief interview and clinical exam were used to assess vestibular symptoms and impairment including dizziness, imbalance, and oculomotor components. The interview section contains eight questions adapted from the Dizziness Handicap Inventory (DHI) 38. This measure is comprised of items assessing general dizziness, as well as specific items inquiring about when/where dizziness occurs (i.e., dizziness when reading, dizziness in wide open spaces). The items are rated on a 7-point Likert scale (0=none to 6=severe). This modified DHI was used to calculate a total dizziness score reflected by participants’ interview responses to the eight DHI questions (Table 2). Researchers and clinicians specializing in vestibular disorders and concussion developed the clinical exam28. The vestibular-oculomotor score is comprised of participants’ responses to screening tests in a recently developed clinical tool used to screen concussion patients who might benefit from more thorough examination and subsequent referral for vestibular or oculomotor therapies28. This screening tool is described more thoroughly by Mucha and colleagues28. The vestibular-oculomotor examination consists of assessments of symptoms following the performance of smooth pursuits, horizontal and vertical saccadic eye movements, vertical and horizontal gaze stability, near point convergence (NPC), vestibular ocular reflex (VOR), and visual-motion sensitivity (VMS) (Table 3). All measures from the aforementioned screening test 28 were included in the current study with the exception of NPC.
Table 2.
Modified Dizziness Handicap Inventory
Participants responded verbally to each question and answers were recorded by the clinician. This Likert scale is identical to that used in the PCSS.
| During the past week have you experienced: | None | Mild | Moderate | Severe | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | Dizziness? (if no, mark 0 for all other questions) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 2 | Dizziness when looking up? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 3 | Dizziness when walking down aisles, hallways, etc.? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 4 | Dizziness when turning over, getting out of bed, or when lying down? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 5 | Dizziness when reading? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 6 | Dizziness during quick head movements? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 7 | Dizziness when bending over? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 8 | Dizziness in open spaces? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
Table 3.
Modified Vestibular-Ocular Motor Screening description
Before beginning the screening test, participants rate headache, dizziness, nausea, and mental fogginess on a 6-point Likert scale in a similar fashion to the PCSS. After each component, participants provide a rating for each of the four symptoms. The targeted function is described in the above table for each component of the screening.
| Component | Targeted Functional Ability |
|---|---|
| Smooth Pursuits | Ability to follow a slowly moving target |
| Saccades- Horizontal Saccades-Vertical |
Ability of the eyes to move between targets without head movement in each directional plane |
| VOR-Horizontal VOR-Vertical |
Ability to stabilize vision during head movement in each directional plane |
| Visual Motion Sensitivity Test |
Ability to inhibit vestibular-induced eye movements using vision and motion sensitivity |
Procedures
The current study received Institutional Review Board Approval prior to any research activities. The researchers informed participants about the study, screened participants for inclusion/exclusion criteria, and obtained written informed consent (adult/parent) for all participants and assent from child participants when applicable. Participants completed the ImPACT and PCSS followed by the DHI and vestibular-oculomotor measures. Researchers conducted the testing individually with each participant in a private laboratory testing room. The tests required approximately 1 hour total at each test session. Participants completed a total of four test sessions at 7–10 day post-injury time intervals.
Data Analysis
Subject demographic characteristics and outcome measures (neurocognitive composite scores, total symptom scores, dizziness, and vestibular-oculomotor responses) were described within 1 week of SRC and at subsequent 1-week post-injury intervals over four weeks. Gender group differences were estimated with contingency table analysis (Chi square or fisher’s exact test) and two-sample t-test for age. A series of 2 (gender) x 4 (time- one, two, three, and four week post SRC) repeated measures ANOVAs were conducted for neurocognitive composite scores (verbal and visual memory, visual processing speed, and reaction time), total symptom score, and the dizziness and vestibular-oculomotor scores. In addition, the likelihood of becoming symptom-free at each time point was estimated using a Cox proportional hazards model with gender as a between group factor. Within group changes between time points and between group differences due to gender were determined to be significant at the p< .05 level using a Bonferroni correction for multiple comparisons. All statistical analyses were conducted by a statistician (GM), who was blind to all hypotheses.
Results
Demographics
Sixty-six subjects (mean age 16.5 ± 1.9 years, range 14–22, 64% male) post-concussion received evaluation at week-one post-concussion. Sixty and 55 subjects were subsequently evaluated at weeks 3 and 4 post-concussion, respectively. Participants sustained SRCs across a variety of sports: basketball: 4, cheerleading: 4, skiing/snowboarding: 2, field hockey: 1, football: 16, hockey: 10, lacrosse: 5, rugby: 1, soccer: 13, softball: 6, volleyball: 3, and wrestling: 2. By three weeks post-injury, six subjects were lost to follow-up, and an additional five were lost by week 4. Female and male subjects did not differ in age or in number of associated signs/symptoms at time of injury (Table 1). As a matter of clinical treatment, all subjects were given academic accommodations based on his/her level of symptom report and neurocognitive impairment, consistent with the protocol described by Sady and colleagues 39. Further, return to activity recommendations followed a commonly used graded exertion protocol40, 41. All repeated measures analyses were tested for the proportionality of the error covariance matrix. Degrees of freedom were adjusted using a Greenhouse-Geissser correction for any comparison not meeting the proportionality assumption.
Total Symptoms
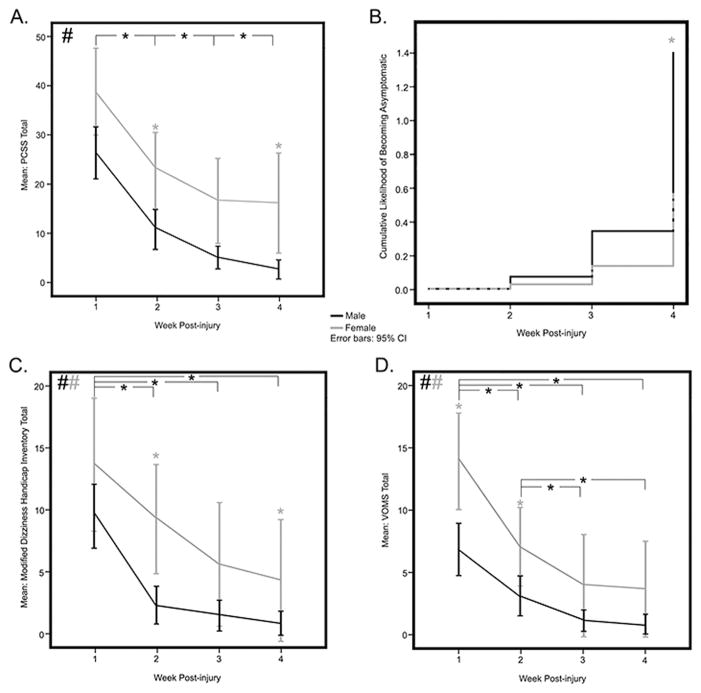
Total symptom scores demonstrated the greatest change across the time period of the study (F2,82 = 53.40, P<.001, ES (effect size) = 1.14). Thirty subjects (45.5% of the total sample, 54.4% of the sample remaining at week 4) were symptom-free by 4 weeks post injury. Gender-adjusted mean (95% CI) total symptom scores were: 1) week 1= 32.9 (27.3–38.5); 2) week 2= 17.2 (13.0–21.4); 3) week 3= 11.2 (7.6–14.7); and 4) week 4= 9.5 (5.7–13.2). Significant improvements in total symptom scores were supported between each post injury time point (p< .001). A significant between-group effect of gender was evident for the total symptom score (F1,53 = 14.03, p< .001, ES = .21). Mean total symptom scores by 4 weeks post-injury were 2.8 ± 5.8 and 16.2 ± 21.2 for males and females, respectively (Figure 1A). Males had significantly lower total symptom scores than females in weeks 2 (p= .002), 3 (p= .014), and 4 (p=.014) post-concussion. Results from a Cox proportional hazard function model demonstrated that males were more likely than females to be symptom-free within 4 weeks post injury (Hazard Ratio = 2.48, 95% CI 1.29–4.75, p< .006) (Figure 1B).
Figure 1.
Subjective Symptom Measures. Representation of mean Post-Concussion Symptom Scale (PCSS) over four weeks post-injury (A), Likelihood of becoming symptom-free using a cox proportional hazard model (B), mean Dizziness symptoms reported using a modified Dizziness Handicap Inventory (DHI) over four weeks post-injury (C) and mean reported symptom provocation using a vestibular-oculomotor screening (VOMS) exam (D). Self-reported symptoms diminished significantly over the four weeks with individual week-to-week comparisons significant between weeks 1–2, 2–3, and 3–4 (A). The cox proportional hazard model (B) shows that males are significantly more likely than females to report being symptom-free by week 4 post-injury. Reported dizziness also diminished significantly over the four weeks with significant differences from weeks 1–2, 1–3, and 2–3 (C). There was also an overall significant effect of gender with females reporting more dizziness, with significant differences at weeks 2 and 4 (C). Provoked reported symptoms on the VOMS showed a similar overall diminution over the four weeks with significant differences from weeks 1–2, 1–3, 1–4 and weeks 2–3 and 2–4 (D). There was also an overall significant effect of gender with females reporting greater symptom provocation, with significant differences at weeks 1 and 2 (D). Black # depicts an overall change across the four post-injury weeks while black * depicts significant effects between specified weeks. Gray # represents an overall significant effect of gender. Gray * depicts significant gender differences at the specified time point.
Neurocognitive
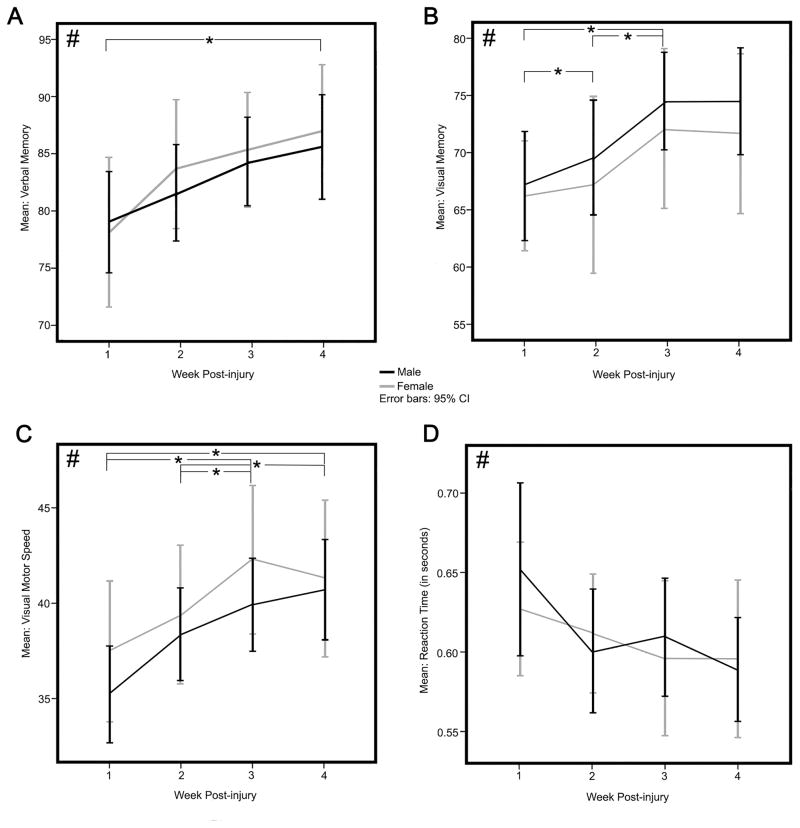
Descriptive statistics for neurocognitive and oculomotor symptom scores for all subjects across weeks 1–4 are presented in Table 4. Significant within-group improvement was seen between post-concussion weeks 1 and 4 in verbal memory scores (F2,129= 4.71, p= .007, ES = .42)(Figure 2A). Verbal memory scores did not significantly improve from week 1 until week 4 (Bonferroni p= .018). Visual memory scores improved across 4 weeks post injury (F3,143 = 5.39, p= .002, ES = .38) (Figure 2B). Significant changes were evident between weeks 1 and 3 (Bonferroni p= .041) and weeks 2 and 3 (Bonferroni p= .015), with no significant differences between weeks 3 and 4. Visual motor speed scores improved significantly (F2, 119= 10.73, p< .001, ES = .58) across 4 weeks post injury with significant changes found between weeks 1 and 3, weeks 1 and 4 (all Bonferroni p< .004), weeks 2 and 3, and weeks 2 and 4 (all Bonferroni p< .015), suggesting gradual improvement between weeks 1–3, plateauing between week 3 and 4 (Figure 2C). Reaction time demonstrated significant improvement across post-injury weeks 1–4 (F2,103=3.20 p= .046, ES = .28) with no significant individual time point differences (Figure 2D). There was no significant effect of gender across 4 weeks of follow-up on any of the neurocognitive scores.
Table 4.
Outcome measure values at 1 to 4 weeks post-concussion: Mean ± SD except where indicated
Means and standard deviations of all outcome measures at each time point. The Repeated measure ANOVA F and p values are reported for each.
| Week 1 n = 66 |
Week 2 n = 66 |
Week 3 n = 60 |
Week 4 n = 55 |
Within-group effect significance* (n = 55) | |
|---|---|---|---|---|---|
| Verbal Memory | 78.7±14.6 | 82.2±13.4 | 84.7±11.6 | 86.0±13.0 |
F2,129 = 4.71 p= .007 |
| Visual Memory | 66.8±13.9 | 68.7±16.9 | 73.7±13.9 | 73.5±14.1 |
F3,143 = 5.39 p= .002 |
| Visual Motor Speed | 36±8.4 | 38.7±8.0 | 40.7±7.9 | 40.9±8.0 |
F2,119=10.73 p< .001 |
| Reaction Time | .64±.15 | .60±.11 | .60±.11 | .59±.10 |
F2,103=3.20 p= .046 |
| Post-Concussion Symptom Score | 30.9±19.3 | 15.1±16.1 | 8.9±13.2 | 7.4±14.6 |
F2,87=53.40 p< .001 |
| Symptom free n (%)† | 1(1.5) | 10 (15.2) | 22(36.7) | 30(45.5) | N/A |
| Dizziness Interview score | 9.5±8.2 n = 61 |
4.6±6.3 n = 62 |
2.1±5.5 n = 56 |
1.8±5.4 n = 54 |
F2,82=29.97 p< .001 n = 48 |
| Vestibular-Oculomotor symptom score | 11.0±9.9 n = 61 |
5.0±8.1 n = 63 |
3.0±7.4 n = 58 |
2.1±6.9 n = 54 |
F2,73=29.26 p< .001 n =50 |
Repeated measures ANOVA;
Impact Symptom Score = 0
Figure 2.
Neurocognitive Measures from the Immediate Post-Concussion and Cognitive Test (ImPACT). Representation of the mean performances across the four clinical composite scores including Verbal Memory (A), Visual Memory (B), Visual Motor Speed (C) and Reaction Time (D). Verbal Memory scores improve across the four post-injury weeks incrementally with significant week-to-week differences apparent between weeks 1 and 4 only (A). Visual memory scores also improve across the four post-injury weeks with significant week-to-week differences from weeks 1–2, 1–3, and 2–3 (B). Visual Motor Speed improves across the four post-injury weeks with significant week-to-week differences apparent between weeks 1–3, 1–4 and weeks 2–4 (C). Reaction Time improves significantly over the four weeks post-injury, but no single week-to-week comparisons are significant (D). Black # depicts an overall change across the four post-injury weeks while black * depicts significant effects between specified weeks.
Vestibular-Oculomotor
Mean modified dizziness scores decreased significantly post-injury (F2,73 = 29.26, p< .001, ES = .96), with significant post-hoc changes observed between weeks 1 and 2, weeks 1 and 3, weeks 1 and 4 (all p< .001 with Bonferroni correction), and weeks 2 and 4 (p < .011) (Figure 1C). A between-group effect of gender was observed (F1,48 = 8.07, p= .007, ES = .56) with males exhibiting lower overall mean dizziness scores by week 4. Post-hoc individual time point comparisons demonstrated significantly lower mean dizziness scores in males (2.06, 95% CI 0–4.48) than in females (8.89, 95% CI 5.66–12.12) at week 2 post-injury (Figure 1C).
Vestibular-oculomotor symptom scores decreased by week 4 post injury (F2,97= 35.91, p< .001 , ES = .98) with significant declines from week 1 to weeks 2 through 4 (p< .001), and from week 2 through weeks 3 and 4 (p< .002) after Bonferroni correction for multiple comparisons (Figure 1D). A between-group effect due to gender was observed in vestibular-oculomotor symptom scores (F1,60 =11.59, p= .001, ES = .75) with males displaying lower overall mean scores compared with females by 4 weeks. Post-hoc comparisons showed that males had lower mean vestibular-oculomotor symptom scores at weeks 1 (males 14.47, 95% CI 9.41-19.54; females 27.0, 95% CI 20.17-33.87) and 2 (males 4.8, 95% CI .82–8.78; females 16.1, 95% CI 10.81–21.55). Differences at weeks 3 and 4 were not significant.
Discussion
The current study reexamines SRC recovery trajectories in a mixed gender cohort of adolescent and young adult athletes using multiple outcome recovery measures. Recovery outcomes for most athletes were between 21–28 days, which is longer than the purported timeframe of 7–14 days 11, 14. Specifically, symptoms improved from week 1 to 2, but slowed thereafter. Gender does not play a role in initial symptom severity, but from week 2 and onward males showed a sharper reduction in symptoms than females. Neurocognitive recovery is highly heterogeneous with different cognitive domains recovering at different rates, taking up to 28 days to show recovery across all domains. There were no gender differences in neurocognitive recovery. Showing steadier rates of recovery, both dizziness and vestibular-oculomotor function improved between weeks 1 through 3, plateauing thereafter. There was a clear gender difference on these measures, with males recovering more quickly than females. Overall, the results indicate a heterogeneous pattern of recovery across domains, underscoring the importance of multiple measures and assessments across time to ensure recovery.
Self-reported symptoms resolved in a linear recovery pattern, improving each week from 1–4 weeks post injury. Gender played a significant role in symptom reporting in general and also across dizziness and vestibular-oculomotor measures. For both males and females, symptoms decreased significantly from week 1 to week 2 with marginal means from each gender not showing significant differences thereafter. However, males reported significantly lower symptom totals relative to females from weeks 2–4. Males were also significantly more likely than females to be asymptomatic by the fourth week. Despite the associated pitfalls, self-reported symptoms remain an important factor in monitoring and managing recovery from SRC. The results of the current study indicate steady symptom reduction in the first 3 weeks post-injury, suggesting that nearly half (45.5%) of the athletes were recovered at this time-point. By 4 weeks post-injury, 56% were symptom-free, with an even larger percentage (67%) being minimally symptomatic (i.e., total symptom score ≤4). The current findings are considerably more conservative than much of the previously reported findings 10, 14, 16, 18, 42 where a higher percentage of athletes were deemed recovered based primarily on symptom reports. We chose a symptom total of 0 to mean full resolution, whereas the aforementioned studies used a group-based statistical approach (either regression or comparison to a control group). The approach taken in the current study is statistical, but instead of using regression or comparison to a control group, we chose to look at the probability of being symptom-free, and as such have a more rigid definition of recovery (i.e., PCSS total = 0), which better accounts for the trajectory of an individual patient within the group.
With regard to neurocognitive recovery in the current study, there was variability across cognitive domains. Athletes’ recovery on visual memory, visual motor speed, and reaction time composites demonstrated significant linear recovery from week 1 through week 3, but plateaued thereafter. In contrast, verbal memory recovery did not demonstrate significant improvement until week 4. The improvements in verbal memory performance from weeks 1 to 2 and 2 to 3 were incremental and not significant, demonstrating a slower, more gradual recovery trajectory. The discrepancies in cognitive recovery reflect a domain-specific pattern of cognitive recovery, supporting the idea that any neurocognitive assessment for SRC must assess more than a single domain. Overall, in this sample athletes required four weeks to demonstrate significant neurocognitive recovery as measured using ImPACT. There were no significant between-gender differences on neurocognitive measures. The current results are consistent with some previous research that reported similar neurocognitive findings8, 19, 20. These studies share several common features to the current study, including mixed gender samples (with exception of the Lau et al. study, which used a male-only sample) and an average participant age of 16 years. The current findings are in contrast to studies showing shorter recovery times, which included older samples of primarily college athletes in mostly male 14 or exclusively male samples 10, 16. Further, these previous studies used either sideline assessment measures (e.g., SAC, BESS) along with paper-pencil based neuropsychological or computerized neurocognitive tests that may have limited utility beyond 10 days post-injury 43. By contrast, the studies- including the current work- that demonstrated longer recovery trajectories following SRC all employed computerized testing (i.e., ImPACT). The longer recovery times for cognitive outcomes supported in the current and previous studies may reflect the more sensitive nature of computerized neurocognitive tests to detect the subtle effects of this injury. Although some researchers and clinicians are critical of computerized neurocognitive tests44–46, there is growing empirical evidence supporting the sensitivity of such tests47–49. As such, selecting measures that sensitively and reliably measure the effects of SRC on cognition is essential to clinical and research aspects of recovery.
Dizziness and vestibular-oculomotor symptoms demonstrated similar recovery trajectories as total symptoms. Specifically, dizziness demonstrated steady, significant decreases between weeks 1–2, 2–3, and 3–4; with females reporting higher dizziness than males at each time point. While related, balance is a different construct 28 with different physiological underpinnings,50, 51 and therefore comparisons between the two are not likely meaningful. This study is the first within the SRC literature to measure the recovery trajectory of dizziness as a separate construct, rather than as one of many symptoms. Again, gender played a significant role, with females reporting greater symptom provocation than males at each time point. This finding is consistent with the gender differences in symptom reporting described above and elsewhere in the literature 29, 31, 52–54. In contrast to total concussion symptom reports, which plateaued around 3 weeks post-injury, both dizziness and reported symptoms following the vestibular-oculomotor exam were significantly different from week 3 to week 4. This finding suggests that there is added value in assessing dizziness and vestibular-oculomotor symptoms at each clinical evaluation.
Limitations
In the current study we assessed multiple outcomes to describe recovery following SRC in a sample that includes males and females representing different sports. However, the current findings are not without limitations. Symptoms and vestibular-oculomotor outcomes were assessed using self-report data, which are limited by response bias. In addition, we assumed that athletes were honest and accurate in their responses. We used asymptomatic status to indicate recovery for symptoms, as we did not have access to baseline data. However, many healthy athletes report some symptoms at baseline. As such, having access to baseline symptoms can provide valuable information when assessing recovery. Further, the sample size of the current study is small and therefore warrants further work in larger samples. As a consequence of the smaller sample size, the age range is still relatively narrow. A similar study with a larger sample and age range should be conducted. Field and colleagues 55 and Covassin and colleagues30 are the only two studies to compare high school versus collegiate athletes. Both of these studies focused on clinical presentation rather than recovery, meaning that there are still several unanswered questions about the role of age/development on recovery. It is also worth noting that even while clinical recommendations are made, patient compliance cannot be known with certainty. It may well be the case that patients in the current sample did not or could not follow the clinical recommendations, thereby delaying recovery. Further, the researchers in this study (with the exception of the statistician) were not blind to the hypotheses. While this is common in clinical research, it is important to note and acknowledge the role this might play in interpretation and, therefore, treatment ramifications. Finally, selection bias toward patients with longer recoveries is a limitation to this study, as it is in all concussion research.
Conclusion
The results of the current study reveal two important points in measuring recovery from SRC. First, athletes in the current sample demonstrated a more protracted recovery curve than has been reported in the literature. Specifically, our results indicate that recovery for most athletes approximates 3–4 weeks rather than the prevailing timeframe of 7–14 days. Our results reinforce the importance of a comprehensive assessment of SRC that includes symptoms, neurocognitive testing, and vestibular-oculomotor outcomes, as each component may have a different recovery trajectory that might be missed by focusing on only one or two assessments. Such an approach will provide clinicians with valuable information about an athlete’s recovery and how the injury might be managed or treated 56. The disparate recovery rate of symptoms, neurocognition, and equilibrium detailed in the current study provides more evidence that concussions are not simple injuries with singular recovery trajectories, but instead reflect an amalgamation of symptoms and dysfunctions that recovery differentially, not unitarily.
Footnotes
Disclosure of funding: This research was supported in part by grants to the University of Pittsburgh from the National Institute on Deafness and Other Communication Disorders (1K01DC012332-01A1) to Dr. Anthony Kontos, and through a research contract between the University of Pittsburgh and ElMindA, Ltd (Israel) with Dr. Anthony Kontos. Dr. Michael Collins is a co-developer and board member of ImPACT Applications.
References
- 1.McCrory P, Meeuwisse W, Aubry M, et al. Consensus statement on Concussion in Sport-The 4th International Conference on Concussion in Sport held in Zurich, November 2012. J Sci Med Sport. 2013 doi: 10.1016/j.jsams.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250–2257. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau BC, Collins MW, Lovell MR. Cutoff scores in neurocognitive testing and symptom clusters that predict protracted recovery from concussions in high school athletes. Neurosurgery. 2012;70:371–379. doi: 10.1227/NEU.0b013e31823150f0. discussion 379. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg MA, Meehan WP, 3rd, Mannix R. Duration and course of post-concussive symptoms. Pediatrics. 2014;133:999–1006. doi: 10.1542/peds.2014-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meehan WP, 3rd, Zhang J, Mannix R, Whalen MJ. Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery. 2012;71:885–891. doi: 10.1227/NEU.0b013e318265a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covassin T, Bay E. Are there gender differences in cognitive function, chronic stress, and neurobehavioral symptoms after mild-to-moderate traumatic brain injury? J Neurosci Nurs. 2012;44:124–133. doi: 10.1097/JNN.0b013e318252737d. [DOI] [PubMed] [Google Scholar]
- 7.Broglio SP, Puetz TW. The effect of sport concussion on neurocognitive function, self-report symptoms and postural control : a meta-analysis. Sports Med. 2008;38:53–67. doi: 10.2165/00007256-200838010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Lau B, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin J Sport Med. 2009;19:216–221. doi: 10.1097/JSM.0b013e31819d6edb. [DOI] [PubMed] [Google Scholar]
- 9.Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39:2311–2318. doi: 10.1177/0363546511410655. [DOI] [PubMed] [Google Scholar]
- 10.McCrea M, Barr WB, Guskiewicz K, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11:58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 11.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. Jama. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 12.McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on Concussion in Sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med. 2009;19:185–200. doi: 10.1097/JSM.0b013e3181a501db. [DOI] [PubMed] [Google Scholar]
- 13.Fazio VC, Lovell MR, Pardini JE, Collins MW. The relation between post concussion symptoms and neurocognitive performance in concussed athletes. NeuroRehabilitation. 2007;22:207–216. [PubMed] [Google Scholar]
- 14.McCrea M, Guskiewicz K, Randolph C, et al. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J Int Neuropsychol Soc. 2013;19:22–33. doi: 10.1017/S1355617712000872. [DOI] [PubMed] [Google Scholar]
- 15.Makdissi M, Cantu RC, Johnston KM, McCrory P, Meeuwisse WH. The difficult concussion patient: what is the best approach to investigation and management of persistent (>10 days) postconcussive symptoms? Br J Sports Med. 2013;47:308–313. doi: 10.1136/bjsports-2013-092255. [DOI] [PubMed] [Google Scholar]
- 16.Prichep LS, McCrea M, Barr W, Powell M, Chabot RJ. Time course of clinical and electrophysiological recovery after sport-related concussion. J Head Trauma Rehabil. 2013;28:266–273. doi: 10.1097/HTR.0b013e318247b54e. [DOI] [PubMed] [Google Scholar]
- 17.Meehan WP, 3rd, Mannix RC, Stracciolini A, Elbin RJ, Collins MW. Symptom severity predicts prolonged recovery after sport-related concussion, but age and amnesia do not. J Pediatr. 2013;163:721–725. doi: 10.1016/j.jpeds.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins MW, Grindel SH, Lovell MR, et al. Relationship between concussion and neuropsychological performance in college football players. Jama. 1999;282:964–970. doi: 10.1001/jama.282.10.964. [DOI] [PubMed] [Google Scholar]
- 19.Covassin T, Elbin RJ, Nakayama Y. Tracking neurocognitive performance following concussion in high school athletes. Phys Sportsmed. 2010;38:87–93. doi: 10.3810/psm.2010.12.1830. [DOI] [PubMed] [Google Scholar]
- 20.Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. Brain Inj. 2006;20:245–252. doi: 10.1080/02699050500487910. [DOI] [PubMed] [Google Scholar]
- 21.Quatman-Yates C, Hugentobler J, Ammon R, Mwase N, Kurowski B, Myer GD. The utility of the balance error scoring system for mild brain injury assessments in children and adolescents. Phys Sportsmed. 2014;42:32–38. doi: 10.3810/psm.2014.09.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King LA, Horak FB, Mancini M, et al. Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch Phys Med Rehabil. 2014;95:353–359. doi: 10.1016/j.apmr.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray N, Salvatore A, Powell D, Reed-Jones R. Reliability and Validity Evidence of Multiple Balance Assessments in Athletes With a Concussion. J Athl Train. 2014 doi: 10.4085/1062-6050-49.3.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnoff JT, Peterson VJ, Hollman JH, Smith J. Intrarater and interrater reliability of the Balance Error Scoring System (BESS) PM R. 2009;1:50–54. doi: 10.1016/j.pmrj.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Mulligan IJ, Boland MA, McIlhenny CV. The balance error scoring system learned response among young adults. Sports Health. 2013;5:22–26. doi: 10.1177/1941738112467755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burk JM, Munkasy BA, Joyner AB, Buckley TA. Balance error scoring system performance changes after a competitive athletic season. Clin J Sport Med. 2013;23:312–317. doi: 10.1097/JSM.0b013e318285633f. [DOI] [PubMed] [Google Scholar]
- 27.Murray NG, Ambati VN, Contreras MM, Salvatore AP, Reed-Jones RJ. Assessment of oculomotor control and balance post-concussion: a preliminary study for a novel approach to concussion management. Brain Inj. 2014;28:496–503. doi: 10.3109/02699052.2014.887144. [DOI] [PubMed] [Google Scholar]
- 28.Mucha A, Collins MW, Elbin RJ, et al. A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42:2479–2486. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covassin T, Elbin RJ. The female athlete: the role of gender in the assessment and management of sport-related concussion. Clin Sports Med. 2011;30:125–131. x. doi: 10.1016/j.csm.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40:1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- 31.Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT. Sex differences in outcome following sports-related concussion. J Neurosurg. 2005;102:856–863. doi: 10.3171/jns.2005.102.5.0856. [DOI] [PubMed] [Google Scholar]
- 32.Colvin AC, Mullen J, Lovell MR, West RV, Collins MW, Groh M. The role of concussion history and gender in recovery from soccer-related concussion. Am J Sports Med. 2009;37:1699–1704. doi: 10.1177/0363546509332497. [DOI] [PubMed] [Google Scholar]
- 33.Covassin T, Elbin RJ, Crutcher B, Burkhart S. The management of sport-related concussion: considerations for male and female athletes. Transl Stroke Res. 2013;4:420–424. doi: 10.1007/s12975-012-0228-z. [DOI] [PubMed] [Google Scholar]
- 34.Iverson GL, Lovell MR, Collins MW. Interpreting change on ImPACT following sport concussion. Clin Neuropsychol. 2003;17:460–467. doi: 10.1076/clin.17.4.460.27934. [DOI] [PubMed] [Google Scholar]
- 35.Barlow M, Schlabach D, Peiffer J, Cook C. Differences in change scores and the predictive validity of three commonly used measures following concussion in the middle school and high school aged population. Int J Sports Phys Ther. 2011;6:150–157. [PMC free article] [PubMed] [Google Scholar]
- 36.Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40:2375–2384. doi: 10.1177/0363546512455400. [DOI] [PubMed] [Google Scholar]
- 37.Stump JE, Lovell MR, Collins MW, Moritz K, Fu FH. The Post Concussion Symptom Scale (PCSS): a factor analysis. British Journal of Sports Medicine. 2004;38:661–662. [Google Scholar]
- 38.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Archives of otolaryngology--head & neck surgery. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 39.Sady MD, Vaughan CG, Gioia GA. School and the concussed youth: recommendations for concussion education and management. Phys Med Rehabil Clin N Am. 2011;22:701–719. ix. doi: 10.1016/j.pmr.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker JG, Freitas MS, Leddy JJ, Kozlowski KF, Willer BS. Return to full functioning after graded exercise assessment and progressive exercise treatment of postconcussion syndrome. Rehabil Res Pract. 2012;2012:705309. doi: 10.1155/2012/705309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of Concussion and Post-concussion Syndrome. Sports Health. 2012;4:147–154. doi: 10.1177/1941738111433673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuckerman SL, Lee YM, Odom MJ, Solomon GS, Forbes JA, Sills AK. Recovery from sports-related concussion: Days to return to neurocognitive baseline in adolescents versus young adults. Surg Neurol Int. 2012;3:130. doi: 10.4103/2152-7806.102945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coldren RL, Russell ML, Parish RV, Dretsch M, Kelly MP. The ANAM lacks utility as a diagnostic or screening tool for concussion more than 10 days following injury. Mil Med. 2012;177:179–183. doi: 10.7205/milmed-d-11-00278. [DOI] [PubMed] [Google Scholar]
- 44.Resch J, Driscoll A, McCaffrey N, et al. ImPact test-retest reliability: reliably unreliable? J Athl Train. 2013;48:506–511. doi: 10.4085/1062-6050-48.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Resch JE, Macciocchi S, Ferrara MS. Preliminary evidence of equivalence of alternate forms of the ImPACT. Clin Neuropsychol. 2013;27:1265–1280. doi: 10.1080/13854046.2013.845247. [DOI] [PubMed] [Google Scholar]
- 46.Bruce J, Echemendia R, Meeuwisse W, Comper P, Sisco A. 1 year test-retest reliability of ImPACT in professional ice hockey players. Clin Neuropsychol. 2014;28:14–25. doi: 10.1080/13854046.2013.866272. [DOI] [PubMed] [Google Scholar]
- 47.Schatz P, Ferris CS. One-month test-retest reliability of the ImPACT test battery. Arch Clin Neuropsychol. 2013;28:499–504. doi: 10.1093/arclin/act034. [DOI] [PubMed] [Google Scholar]
- 48.Schatz P, Sandel N. Sensitivity and specificity of the online version of ImPACT in high school and collegiate athletes. Am J Sports Med. 2013;41:321–326. doi: 10.1177/0363546512466038. [DOI] [PubMed] [Google Scholar]
- 49.Elbin RJ, Schatz P, Covassin T. One-year test-retest reliability of the online version of ImPACT in high school athletes. Am J Sports Med. 2011;39:2319–2324. doi: 10.1177/0363546511417173. [DOI] [PubMed] [Google Scholar]
- 50.Balaban CD, Jacob RG. Background and history of the interface between anxiety and vertigo. J Anxiety Disord. 2001;15:27–51. doi: 10.1016/s0887-6185(00)00041-4. [DOI] [PubMed] [Google Scholar]
- 51.Balaban CD, Thayer JF. Neurological bases for balance-anxiety links. J Anxiety Disord. 2001;15:53–79. doi: 10.1016/s0887-6185(00)00042-6. [DOI] [PubMed] [Google Scholar]
- 52.Asplund CA, McKeag DB, Olsen CH. Sport-related concussion: factors associated with prolonged return to play. Clin J Sport Med. 2004;14:339–343. doi: 10.1097/00042752-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Delaney JS, Lacroix VJ, Leclerc S, Johnston KM. Concussions among university football and soccer players. Clin J Sport Med. 2002;12:331–338. doi: 10.1097/00042752-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Dick RW. Is there a gender difference in concussion incidence and outcomes? Br J Sports Med. 2009;43(Suppl 1):i46–50. doi: 10.1136/bjsm.2009.058172. [DOI] [PubMed] [Google Scholar]
- 55.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142:546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds E, Collins MW. In-office management of sport-related concussion. Prog Neurol Surg. 2014;28:128–138. doi: 10.1159/000358770. [DOI] [PubMed] [Google Scholar]