Abstract
Inflammation drives asthma and atherosclerosis. Clinical studies suggest that asthmatic patients have a high risk of atherosclerosis. Yet this hypothesis remains uncertain, given that Th2 imbalance causes asthma whereas Th1 immunity promotes atherosclerosis. In this study, chronic allergic lung inflammation (ALI) was induced in mice by ovalbumin sensitization and challenge. Acute ALI was induced in mice by ovalbumin and aluminum sensitization and ovalbumin challenge. Atherosclerosis was produced in apolipoprotein E-deficient (Apoe−/−) mice with a Western diet. When chronic ALI and atherosclerosis were produced simultaneously, ALI increased atherosclerotic lesion size, lesion inflammatory cell content, elastin fragmentation, smooth muscle cell (SMC) loss, lesion cell proliferation, and apoptosis. Production of acute ALI before atherogenesis did not affect lesion size, but increased atherosclerotic lesion CD4+ T cells, lesion SMC loss, angiogenesis, and apoptosis. Production of acute ALI after atherogenesis also did not change atherosclerotic lesion area, but increased lesion elastin fragmentation, cell proliferation, and apoptosis. In mice with chronic ALI and diet-induced atherosclerosis, daily inhalation of a mast cell inhibitor or corticosteroid significantly reduced atherosclerotic lesion T-cell and mast cell contents, SMC loss, angiogenesis, and cell proliferation and apoptosis, although these drugs did not affect lesion area, compared with those that received vehicle treatment. In conclusion, both chronic and acute ALI promote atherogenesis or aortic lesion pathology, regardless whether ALI occurred before, after, or at the same time as atherogenesis. Anti-asthmatic medication can efficiently mitigate atherosclerotic lesion pathology.
Keywords: allergic lung inflammation, chronic and acute asthma, atherosclerosis, ovalbumin
INTRODUCTION
Inflammation drives both asthma and atherosclerosis, diseases that share many pathologies. This includes the accumulation of inflammatory cells in the allergic lung airway and arterial wall, including macrophages, T cells, mast cells, eosinophils, or neutrophils.1–5 These cells release various inflammatory cytokines to induce airway narrowing and arterial wall thickening, matrix protein catabolism, cell proliferation, or apoptosis.4–7 In humans and mice, the development of allergic asthma associates with an increase of peripheral Th2 cytokines, including IL4, IL5, and IL13.8,9 In contrast, patients with atherosclerosis often have reduced plasma IL4, IL5, and IL13, but increased circulating Th1 cytokines, such as IFN-γ, IL6, and TNF-α,10,11 although patients with either asthma or atherosclerosis all have increased plasma IgE and chemokines, including monocyte chemoattractant protein-1 (MCP-1) and eotaxin, that mediate blood-borne leukocyte migration and accumulation at the site of injury.12–19 These pathological differences and similarities suggest an interaction between these two common human inflammatory diseases.
Prior studies in a survey of patients from several U.S. states demonstrated that patients with adult-onset asthma had significantly larger carotid artery intima-media thickness (IMT) than those of non-asthmatics.20 Patients with allergic disorders, including allergic rhinitis and asthma selected from random samples from Bruneck, Italy, had several folds higher risk of atherosclerosis (odds ratio [OR]: 3.8; 95% confidential interval [CI]: 1.4–10.2, P=0.007).21 A small cross-sectional evaluation of 141 men aged 17–18 years in Innsbruck, Austria, showed that participants with the same allergic disorders have several folds higher risk of developing large IMT (OR: 2.5; 95% CI: 1.1–5.5; P=0.01).21 In a much larger cohort of 70,047 men and 81,573 women from Northern California, evidence again showed asthma as a significant risk factor (OR: 1.22, 95% CI: 1.14–1.31, P<0.001) of coronary heart disease before or after adjusting for several common risk factors of atherosclerosis, including smoking, alcohol consumption, body mass index, plasma cholesterol levels, white blood cell count, hypertension, diabetes, and several others.22 Yet a large biracial cohort of 13,501 middle-aged adults between 45–64 years old with 14 years of follow up study did not reveal an association between asthma and cardiovascular disease incidence.23 It is possible that the varying risk of asthma or atherosclerosis among different races caused this insignificance.24,25
A recent study assessed the risk of allergic asthma in atherosclerosis-prone apolipoprotein E-deficient (Apoe−/−) mice sensitized with ovalbumin (OVA) together with aluminum, followed by OVA nebulization three times a week. OVA-induced airway allergic inflammation increased atherosclerotic lesion sizes in the aortic root, along with increased lesion macrophages, increased splenic Th17 and Th2 cells, and reduced splenic regulatory T cells, without affecting splenic Th1 cell population.26 Using OVA to sensitize Apoe−/− mice followed by OVA weekly nebulization to develop chronic asthma, the present study tested the influence of the concurrent development of allergic lung inflammation (ALI) on atherosclerosis. OVA and aluminum hydroxide to sensitize Apoe−/− mice followed by 3-day OVA nebulization to develop acute ALI helped assess whether pre-ALI or post-ALI affects atherosclerosis. Finally, the use of ketotifen, a mast cell inhibitor, and corticosteroid budesonide, two common anti-allergic medications, helped examine whether these anti-allergic asthma medications affect chronic ALI and atherosclerosis.
MATERIALS AND METHODS
Mouse atherosclerosis and allergic lung inflammation production
Eight to ten-week-old male Apoe−/− mice (C57BL/6) from the Jackson Laboratory (Bar Harbor, ME, USA) were used to produce atherosclerosis and acute or chronic ALI sequentially or together. To promote atherosclerosis, 8 to 10-week-old male mice from each group were fed a high-cholesterol (1.25%) Western diet (Research Diets Inc.) for 3 months. To produce acute ALI, mice were immunized intraperitoneally with 50 μg of OVA in 1 mg of Al(OH)3 in 200 μl saline on days 0, 7, and 14. On days 21, 22, and 23, mice were nebulized with 300 mg of OVA in 5 mL saline for 25 minutes per day.27,28 To produce chronic ALI, mice were immunized with 10 μg of OVA in 200 μl saline on days 0, 3, and 6, and were nebulized with 100 mg of OVA in 5 ml saline on day 13, and once per week thereafter for 3 months.28 At the endpoint of each protocol, mice were sacrificed with carbon dioxide narcosis, followed by cardiac puncture blood collection, bronchoalveolar lavage fluid (BALF) preparation, and aortic tissue harvest. Each experimental group contained 8 to 12 Apoe−/− mice at the same age. Mice were grouped randomly after purchase. All animal procedures conformed to the Guideline for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and were approved by the Harvard Medical School Standing Committee on Animals (protocols # 03759 and #04923).
The present study included four experiments with different combinations of ALI and atherosclerotic mice to test the interactions between ALI and atherosclerosis in Apoe−/− mice: (1) to produce chronic ALI and atherosclerosis at the same time; and (2) to produce acute ALI first followed by atherosclerosis. These two protocols were used to test whether the development of ALI affected atherosclerosis formation and progression; (3) to produce atherosclerosis first, and then induce acute ALI. This protocol tested whether consequent ALI exacerbated pre-established atherosclerosis and whether pre-existing atherosclerosis affected airway allergic response development; and (4) finally, 70 mg of ketitofen or 7 mg of budesonide was added into 5 mL saline for daily nebulization to treat mice with concurrent atherosclerosis and chronic ALI.
Mouse aortic arch tissue immunohistochemical analysis and lung tissue histology analysis
Serial cryostat cross-sections (6 μm) of mouse aortic arches were used for immunostaining to detect macrophages (Mac-3, 1:900, BD Biosciences, San Jose, CA, USA), T cells (CD4, 1:90, BD Biosciences; and CD8, 1:100, Chemicon International, Inc., Temecula, CA), major histocompatibility complex class II (MHC-II, 1:250, BD Biosciences), elastin (Modified Verhoeff Van Gieson Elastic Stain Kit, Sigma-Aldrich, St. Louis, MO), collagen (0.1% Sirius Red; Polysciences Inc., Warrington, PA), smooth muscle cells (SMC, 1:750, α-actin, Sigma-Aldrich), Ki67 (cell proliferation marker, 1:400, Thermo Fisher Scientific Inc., Rockford, IL), and CD31 (angiogenesis marker, 1:1500, BD Biosciences). Apoptotic cells in lesions were determined with the in situ apoptosis detection kit according to the manufacturer’s instructions (EMD Millipore, Billerica, MA). The frozen slides were also stained with toluidine blue to quantify mast cells. Collagen content, elastin fragmentation and media SMC accumulation were graded according to the grading keys that were described previously.29,30 CD31-positive microvessels, Ki67-positive cells, T cells, mast cells, and apoptotic cells were counted manually in a blinded fashion and presented as numbers per aortic arch section. Images of the relative macrophage and MHC-II contents within the aortas were captured by a Microscope VS120 Whole Slide Scanner (Olympus) and quantified by measuring the immunostaining signal-positive areas using computer-assisted image analysis software (Image-Pro Plus; Media Cybernetics, Bethesda, MD). Lung fragments were fixed in 10% formalin in saline and embedded in paraffin. Sections (4–5 μm thick) were stained with hematoxylin-eosin staining for lung histology analysis. All mouse experiments were performed, and data were analyzed in a blinded fashion by at least two observers.
Plasma and bronchoalveolar lavage fluid analysis
Plasma and BALF were collected from mice at harvest. BALF cellular typing was determined by cytospin preparation and Wright-Giemsa staining, followed by counting blindly the numbers of macrophages, lymphocytes, eosinophils, and neutrophils. Plasma and BALF IgE (BD Biosciences), MCP-1 (PeproTech, Rocky Hill, NJ), eotaxin (PeproTech), IL4 (eBioscience, San Diego, CA), IL5 (eBioscience), IL13 (PeproTech), TGF-β (eBioscience), IFN-γ (eBioscience), and TNF-α (PeproTech) were determined using ELISA kits according to the manufacturers’ instructions. Plasma total cholesterol, triglyceride, and high-density lipoprotein (HDL) levels were determined using reagents from Pointe Scientific (Canton, MI) and the level of low-density lipoproteins (LDL) was calculated as following: LDL = total cholesterol - HDL -(triglycerides/5). Investigators were blinded to the sources of samples during the assay.
Statistical analysis
All data are expressed as means ± SEM. This study used the non-parametric Mann-Whitney U test for unpaired data sets to examine statistical significance for all data. P < 0.05 was considered to be statistically significant.
RESULTS
OVA sensitization-induced ALI promotes atherosclerosis and lesion inflammation
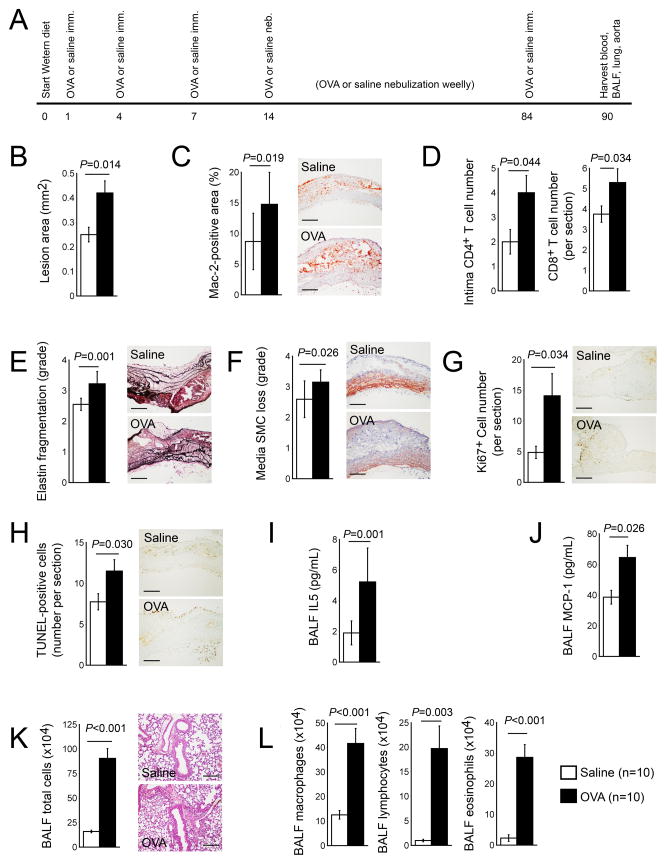
The induction of both ALI and atherosclerosis in Apoe−/− mice helped test whether asthmatic mice remained prone to atherosclerosis. Initial experiments concurrently produced OVA-induced chronic ALI and Western diet-induced atherosclerosis in Apoe−/− mice (Figure 1A). Compared with those mock (saline)-sensitized mice (control group), OVA-sensitized mice showed a significantly enlarged atherosclerotic lesion area in the aortic arches (0.42±0.049 mm2 vs. 0.25±0.03 mm2, P=0.014) and increased lesion Mac-2+ macrophage-positive area (14.76±5.22 % vs. 8.69±4.62 %, P=0.019) (Figure 1B, 1C). Furthermore, aortic arch lesion intima CD4+ T-cell content increased in OVA-induced ALI mice (P=0.044), as well as CD8+ T cells (P=0.034), elastin fragmentation (P=0.001), SMC loss (P=0.026), lesion cell proliferation (P=0.034), and apoptosis (P=0.030) (Figure 1D–1H).
Figure 1.
Chronic ALI promotes atherosclerosis in Apoe−/− mice. A. Experimental protocol. Aortic arch atherosclerotic lesion area (B), Mac-2-positive macrophage contents (C), intima CD4+ T cell and lesion CD8+ T cell numbers (D), media elastin fragmentation grade (E), media SMC loss grade (F), Ki67+ proliferating cell numbers (G), and TUNEL-positive apoptotic cell numbers (H). BALF levels of IL5 (I), MCP-1 (J), total cell numbers (K), and macrophages, lymphocytes, and eosinophils (L). Representative data in panels C, E–H, and K are shown to the right. Scale: 200 μm.
The collection of BALF and the tallying of total inflammatory cells and individual cell types verified the successful production of ALI in these mice. Although the OVA-induced ALI did not affect serum and BALF IgE levels (Table 1), likely due to the production of atherosclerosis in these mice that also increases plasma IgE levels,12 OVA-sensitized mice showed significantly higher BALF IL5 (P=0.001) and MCP-1 (P=0.026) levels than mock-sensitized mice (Figure 1I, 1J). BALF total leukocytes and individual cell types, including macrophages, lymphocytes, and eosinophils all significantly increased in lungs from OVA-sensitized mice, compared with those from mock-sensitized control mice (Figure 1K, 1L). OVA-induced chronic ALI increased plasma LDL, decreased plasma HDL, but did not affect plasma triglyceride and total cholesterol levels and inflammatory proteins, including IFN-γ, TNF-α, eotaxin, MCP-1, IL4, IL5, IL13, and TGF-β, compared with those from mock-sensitized mice (Table 1, left).
Table 1.
Production of ALI at the same time or before atherosclerosis enhances atherosclerosis progression in Apoe−/− mice.
| Variable | Chronic ALI and atherosclerosis together
|
P | Acute ALI first, then atherosclerosis
|
P | ||
|---|---|---|---|---|---|---|
| OVA (n=10) | Saline (n=10) | OVA (n=12) | Saline (n=12) | |||
| Measurements | ||||||
| Body weight (g) | 31.35±0.83 | 31.62±0.87 | 0.825 | 33.65±0.95 | 33.39±0.48 | 0.815 |
| SBP (mmHg) | 103.50±1.84 | 101.20±3.25 | 0.548 | 104.36±2.23 | 95.09±2.72 | 0.016 |
| DBP (mmHg) | 77.10±2.24 | 74.50±3.99 | 0.588 | 78.27±2.80 | 72.09±2.14 | 0.096 |
| Serum lipid proteins | ||||||
| Total cholesterol (mg/dL) | 577.36±51.69 | 407.80±60.74 | 0.064 | 528.31±22.97 | 494.43±39.63 | 0.469 |
| LDL (mg/dL) | 416.21±41.15 | 145.29±20.42 | <0.001 | 369.42±22.44 | 398.99±41.60 | 0.540 |
| HDL (mg/dL) | 110.16±13.40 | 203.44±38.54 | 0.049 | 108.25±6.60 | 69.55±8.28 | 0.002 |
| Triglyceride (mg/dL) | 254.97±32.99 | 210.77±38.72 | 0.399 | 253.20±26.78 | 129.44±38.12 | 0.015 |
| Serum inflammatory proteins | ||||||
| IgE (ng/mL) | 607.01±107.67 | 552.38±143.21 | 0.767 | 689.04±116.70 | 713.71±153.32 | 0.899 |
| IFN-γ (pg/mL) | 10.85±4.62 | 1.81±1.81 | 0.102 | 8.55±2.90 | 5.98±1.86 | 0.468 |
| TNF-α (pg/mL) | 63.42±72.89 | 30.24±33.75 | 0.690 | 29.09±38.03 | 96.73±67.24 | 0.393 |
| Eotaxin (pg/mL) | 81.86±14.87 | 283.51±50.25 | 0.066 | 490.27±56.74 | 412.51±32.47 | 0.256 |
| MCP-1 (pg/mL) | 94.64±11.27 | 203.77±115.53 | 0.517 | 386.38±29.51 | 276.21±23.05 | 0.010 |
| IL4 (pg/mL) | 76.43±21.30 | 70.21±24.75 | 0.853 | 26.69±9.28 | 32.83±16.31 | 0.747 |
| IL5 (pg/mL) | 21.99±14.84 | 1.50±1.50 | 0.251 | 38.86±3.03 | 34.67±4.88 | 0.475 |
| IL13 (pg/mL) | 7.62±0.66 | 11.81±2.48 | 0.327 | 44.21±7.98 | 68.60±28.69 | 0.428 |
| TGF-β (ng/mL) | 4.93±1.41 | 5.58±1.40 | 0.766 | 3.70±1.15 | 2.76±0.38 | 0.458 |
| BALF cell typing | ||||||
| Total Cell in BALF (x104) | 90.47±9.99 | 15.85±1.31 | <0.001 | 22.68±1.88 | 18.95±1.51 | 0.139 |
| Macrophage (x104) | 41.65±6.12 | 12.52±1.74 | <0.001 | 11.78±1.51 | 16.67±1.51 | 0.033 |
| Lymphocyte (x104) | 19.74±4.58 | 0.97±0.25 | 0.003 | 4.59±0.95 | 1/60±0.36 | 0.012 |
| Eosinophil (x104) | 28.62±4.18 | 2.27±1.09 | <0.001 | 6.20±1.06 | 0.65±0.30 | <0.001 |
| Neutrophil (x104) | 0.53±0.45 | 0.09±0.09 | 0.365 | 0.12±0.12 | 0.03±0.03 | 0.509 |
| BALF proteins | ||||||
| IgE (ng/mL) | 80.64±14.58 | 56.64±8.69 | 0.090 | 57.08±8.03 | 48.08±9.84 | 0.487 |
| IFN-γ (pg/mL) | 562.43±101.29 | 563.64±80.44 | 0.993 | 9.02±1.62 | 12.44±2.14 | 0.109 |
| TNF-α (pg/mL) | 970.93±269.78 | 848.91±134.82 | 0.700 | 86.74±10.66 | 91.00±13.22 | 0.804 |
| Eotaxin (pg/mL) | 62.29±7.96 | 67.89±6.89 | 0.303 | 23.03±1.83 | 22.00±2.48 | 0.741 |
| MCP-1 (pg/mL) | 64.58±7.90 | 38.6±4.51 | 0.026 | 30.68±3.14 | 25.57±2.39 | 0.106 |
| IL4 (pg/mL) | 12.07±23.35 | 3.04±2.80 | 0.128 | 5.30±3.99 | 8.05±4.20 | 0.065 |
| IL5 (pg/mL) | 5.23±2.21 | 1.90±0.78 | 0.001 | 3.28±1.42 | 3.63±1.60 | 0.873 |
| IL13 (pg/mL) | 64.77±15.82 | 51.96±6.84 | 0.488 | 68.34±2.00 | 63.91±2.42 | 0.087 |
| TGF-β (pg/mL) | 295.78±44.21 | 178.46±37.41 | 0.059 | 148.65±25.18 | 196.33±24.36 | 0.094 |
| Atherosclerotic lesion analysis | ||||||
| Area (mm2) | 0.42±0.049 | 0.25± 0.03 | 0.014 | 0.32±0.06 | 0.26±0.04 | 0.235 |
| Macrophage (%) | 14.76± 5.22 | 8.69±4.62 | 0.019 | 10.52±3.67 | 9.53±3.15 | 0.506 |
| Mast cell (# per section) | 4.80±1.64 | 3.25±1.08 | 0.442 | 3.45±1.23 | 2.55±0.85 | 0.550 |
| CD4+ T-cell (# per section) | 6.60±1.18 | 4.25±0.67 | 0.107 | 8.54±0.95 | 4.10±0.95 | 0.004 |
| CD4+ T-cell intima | 4.00±0.71 | 2.00±0.57 | 0.044 | 4.27±0.79 | 2.30±0.53 | 0.028 |
| CD8+ T-cell (# per section) | 5.30±0.67 | 3.75±0.41 | 0.034 | 5.00±1.11 | 3.6±0.83 | 0.327 |
| MHC-II (%) | 3.58±1.97 | 3.17±2.08 | 0.678 | 3.29±1.46 | 2.82±1.33 | 0.434 |
| SMC loss (grade) | 3.15±0.44 | 2.59±0.63 | 0.026 | 3.17±0.36 | 2.48±0.47 | 0.001 |
| Collagen (grade) | 0.92±0.28 | 1.40±0.26 | 0.219 | 0.71±0.25 | 1.12±0.26 | 0.263 |
| Elastin frag. (grade) | 3.22±0.40 | 2.55±0.20 | 0.001 | 3.00±0.51 | 2.75±0.34 | 0.172 |
| Ki67+ cell (# per section) | 14.1±3.62 | 4.87±1.16 | 0.034 | 10.00±2.31 | 13.00±1.98 | 0.336 |
| Ki67+ cell media | 0.90±0.23 | 1.25±0.77 | 0.019 | 1.27±0.71 | 3.64±0.78 | 0.037 |
| Microvessel (# per section) | 7.80±1.95 | 5.87±1.20 | 0.207 | 8.73±2.25 | 3.54±0.86 | 0.026 |
| TUNEL+ cell (# per section) | 11.50±1.42 | 7.75±1.18 | 0.030 | 9.30±1.52 | 6.82±0.66 | 0.080 |
| TUNEL+ cell intima | 5.90± 0.77 | 4.87± 0.72 | 0.172 | 5.90±0.26 | 4.00±0.29 | 0.047 |
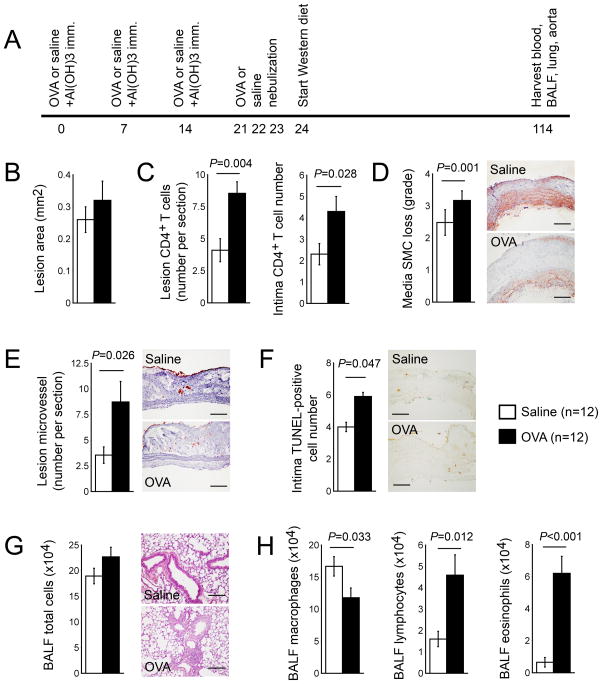
The production of acute ALI by immunizing mice with OVA together with aluminum hydroxide, followed by the production of atherosclerotic mice with a Western diet, tested whether pre-established ALI affects the consequent development of atherosclerosis (Figure 2A). In these mice, pre-established ALI did not significantly enhance aortic arch atherosclerotic lesion size, compared with that of mock-sensitized mice (Figure 2B). Compared with mock-sensitized mice, however, aortic arch atherosclerotic lesions from mice with pre-established ALI contained significantly higher total (P=0.004) and intima (P=0.028) CD4+ T cells, media SMC loss (P=0.001), lesion microvessel contents (P=0.026), and intima cell apoptosis (P=0.047) (Figure 2C–2F). Although BALF total cell numbers did not differ between mice with and without pre-established ALI, BALF macrophage content (P=0.033) decreased in OVA-sensitized mice, and OVA-immunized mice revealed significantly higher numbers of BALF lymphocytes (P=0.012) and eosinophils (P<0.001) than mock-treated control mice (Figure 2H). Further, mice with pre-established ALI revealed higher levels of both systolic blood pressure (P=0.016) and plasma levels of HDL (P=0.002), triglyceride (P=0.015), and MCP-1 (P=0.01) than those treated with saline alone (Table 1, right). Yet most tested cytokines or IgE levels in serum or BALF were not significantly different between atherosclerotic mice that developed ALI at the same time and those that were immunized with saline alone, or between atherosclerotic mice with pre-established ALI and pre-treated with saline (Table 1).
Figure 2.
Production of acute ALI promotes subsequent atherogenesis in Apoe−/− mice. A. Experimental protocol. Aortic arch atherosclerotic lesion area (B), lesion total and intima CD4+ T cell numbers (C), media SMC loss grade (D), CD31+ microvessel numbers (E), and intimal TUNEL-positive apoptotic cell numbers (F). BALF total cell numbers (G) and macrophages, lymphocytes, and eosinophils (H). Representative data in panels D–G are shown to the right. Scale: 200 μm.
Allergic lung inflammation exacerbates pre-established atherosclerosis
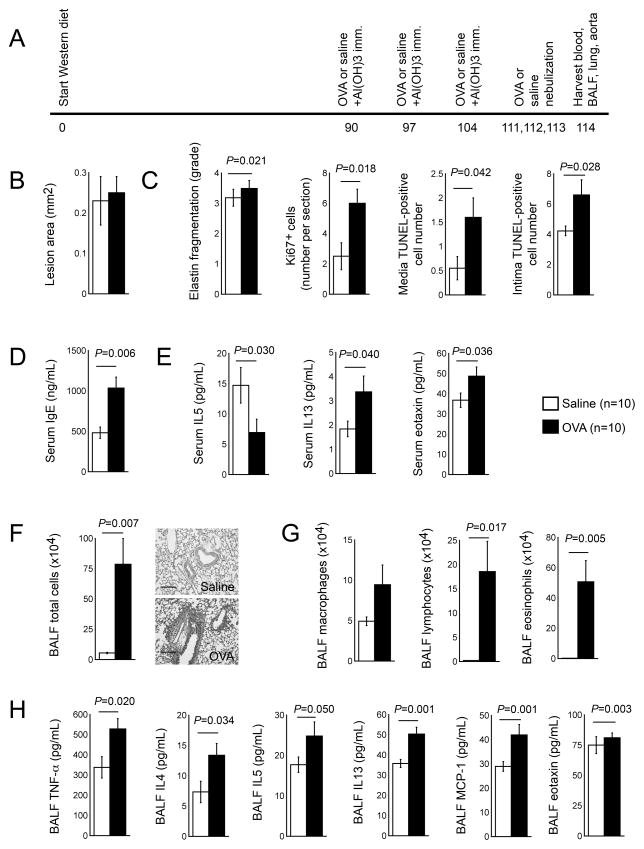
Post-ALI may affect pre-established atherosclerosis. Therefore, Apoe−/− mice were maintained on a Western diet for 3 months to develop atherosclerosis, followed by production of acute ALI (Figure 3A). Consequent acute ALI did not affect lesion size of pre-established atherosclerosis (Figure 3B), but significantly increased aortic arch media elastin fragmentation (P=0.021), lesion cell proliferation (P=0.018), and media (P=0.042) and intima (P=0.028) cell apoptosis (Figure 3C). As expected, acute ALI increased serum IgE levels (P=0.006) (Figure 3D), but reduced serum IL5 (P=0.030) and increased serum IL13 (P=0.04) and eotaxin (P=0.036) levels (Figure 3E). In BALF, acute ALI increased total cell contents (P=0.007) as well as macrophages, lymphocytes (P=0.017), and eosinophils (P=0.005) (Figure 3F, 3G). BALF levels of cytokines TNF-α (P=0.02), IL4 (P=0.034), IL5 (P=0.05), and IL13 (P=0.001), and BALF chemokines MCP-1 (P=0.001) and eotaxin (P=0.003) all increased in atherosclerotic mice that had consequent acute ALI than those challenged with saline (Figure 3H). The development of ALI alone increased aortic arch CD4+ T cells (P=0.037) and Ki67+ proliferating cells (P=0.023), but did not affect any other atherosclerosis-associated variables in the aortic arch nor serum lipid profile (Table 2, left). Of note, sequential development of ALI after atherosclerosis did not affect significantly plasma lipid profile (Table 2, right).
Figure 3.
Production of acute ALI enhances pre-established atherosclerosis in Apoe−/− mice. A. Experimental protocol. B. Aortic arch atherosclerotic lesion area. C. Lesion media elastin fragmentation grade, Ki67+ proliferating cell numbers, and TUNEL-positive apoptotic cell numbers. D. Serum IgE levels. E. Serum IL5, IL13, and eotaxin levels. F. BALF total cell numbers. G. BALF macrophages, lymphocytes, and eosinophils. H. BALF TNF-α, IL4, IL5, IL13, MCP-1, and eotaxin levels. Representative data in panel F are shown to the right. Scale: 200 μm.
Table 2.
Production of ALI enhances pre-established atherosclerosis in Apoe−/− mice.
| Variable | Acute ALI only
|
P | Atherosclerosis first, then acute ALI
|
P | ||
|---|---|---|---|---|---|---|
| OVA (n=8) | Saline (n=8) | OVA (n=10) | Saline (n=10) | |||
| Measurements | ||||||
| Body weight (g) | 30.45±1.15 | 30.84±1.29 | 0.825 | 30.44±0.51 | 33.04±0.82 | 0.022 |
| SBP (mmHg) | 106.25±4.08 | 103.00±2.26 | 0.501 | 96.40±2.07 | 101.11±3.08 | 0.241 |
| DBP (mmHg) | 75.25±3.13 | 78.00±2.97 | 0.534 | 72.80±1.91 | 77.00±2.54 | 0.222 |
| Serum lipid proteins | ||||||
| Total cholesterol (mg/dL) | 415.72±37.23 | 422.30±22.02 | 0.882 | 417.55±34.24 | 448.04±23.98 | 0.476 |
| LDL (mg/dL) | 120.73±33.44 | 161.31±23.39 | 0.339 | 313.66±35.50 | 339.56±21.13 | 0.540 |
| HDL (mg/dL) | 243.23±17.05 | 215.81±17.24 | 0.277 | 76.91±10.71 | 54.48±8.47 | 0.119 |
| Triglyceride (mg/dL) | 258.78±28.05 | 225.92±21.35 | 0.368 | 162.59±30.84 | 270.00±50.73 | 0.093 |
| Serum inflammatory proteins | ||||||
| IgE (ng/mL) | 269.45±60.71 | 57.03±13.55 | 0.010 | 1034.64±136.00 | 482.16±70.98 | 0.006 |
| IFN-γ (pg/mL) | 7.74±3.42 | 5.51±2.56 | 0.557 | 71.21±9.49 | 68.72±18.01 | 0.905 |
| TNF-α (pg/mL) | 165.71±81.10 | 57.93±45.14 | 0.134 | 23.74±3.83 | 19.68±0.56 | 0.340 |
| Eotaxin (pg/mL) | 19.42±2.24 | 18.45±2.28 | 0.767 | 48.71±4.50 | 36.78±3.56 | 0.036 |
| MCP-1 (pg/mL) | 131.94±31.35 | 179.30±21.18 | 0.242 | 94.88±8.05 | 86.70±16.19 | 0.660 |
| IL4 (pg/mL) | 67.29±9.08 | 134.67±76.69 | 0.411 | 39.20±8.20 | 24.50±7.10 | 0.097 |
| IL5 (pg/mL) | 13.09±2.91 | 7.65±2.40 | 0.101 | 6.92±2.21 | 14.71±2.93 | 0.030 |
| IL13 (pg/mL) | 7.11±0.88 | 6.15±1.39 | 0.575 | 3.37±0.65 | 1.84±0.32 | 0.040 |
| TGF-β (ng/mL) | 6.94±0.92 | 5.05±1.34 | 0.133 | 2.69±0.48 | 3.19±0.36 | 0.417 |
| BALF cell typing | ||||||
| Total Cell in BALF (x104) | 4.66±0.45 | 4.18±0.45 | 0.462 | 78.64±21.08 | 5.37±0.53 | 0.007 |
| Macrophage (x104) | 0.72±0.23 | 3.72±0.42 | <0.001 | 9.44±2.44 | 4.92±0.54 | 0.102 |
| Lymphocyte (x104) | 1.63±0.27 | 0.35±0.10 | 0.002 | 18.54±6.27 | 0.22±0.05 | 0.017 |
| Eosinophil (x104) | 2.27±0.24 | 0.12±0.05 | <0.001 | 50.66±14.04 | 0.24±0.07 | 0.005 |
| Neutrophil (x104) | 0.00±0.00 | 0.00±0.00 | 0.361 | 0.00±0.00 | 0.00±0.00 | – |
| BALF proteins | ||||||
| IgE (ng/mL) | 128.99±13.94 | 88.68±13.34 | 0.028 | 106.19±12.40 | 104.96±8.79 | 0.937 |
| IFN-γ (pg/mL) | 12.86±2.31 | 10.25±1.74 | 0.385 | 11.05±1.65 | 11.00±1.67 | 0.982 |
| TNF-α (pg/mL) | 365.51±59.86 | 158.94±41.10 | 0.013 | 528.13±51.82 | 337.89±53.04 | 0.020 |
| Eotaxin (pg/mL) | 36.04±5.48 | 23.58±1.19 | 0.029 | 75.77±7.73 | 80.82±3.92 | 0.003 |
| MCP-1 (pg/mL) | 30.09±3.64 | 23.14±0.99 | 0.050 | 41.95±4.34 | 28.92±2.11 | 0.001 |
| IL4 (pg/mL) | 5.98±1.94 | 7.88±1.79 | 0.241 | 13.38±1.93 | 7.33±1.77 | 0.034 |
| IL5 (pg/mL) | 21.92±2.65 | 19.09±2.72 | 0.234 | 24.76±3.54 | 17.70±1.90 | 0.050 |
| IL13 (pg/mL) | 34.66±2.51 | 27.67±0.68 | 0.028 | 50.33±3.41 | 35.75±2.17 | 0.001 |
| TGF-β (pg/mL) | 372.15±57.07 | 283.71±53.02 | 0.067 | 362.19±33.28 | 278.17±35.79 | 0.052 |
| Atherosclerotic lesion analysis | ||||||
| Area (mm2) | 0.14±0.02 | 0.12±0.03 | 0.508 | 0.25±0.04 | 0.23±0.06 | 0.740 |
| Macrophage (%) | 5.85±4.20 | 5.86±3.50 | 0.997 | 13.83±6.52 | 13.04±3.99 | 0.752 |
| Mast cell (# per section) | 1.12±0.29 | 1.43±0.40 | 0.571 | 3.80±0.81 | 3.78±1.13 | 0.987 |
| CD4+ T-cell (# per section) | 4.25±0.72 | 2.62±0.37 | 0.037 | 5.30±0.75 | 4.89±0.77 | 0.706 |
| CD8+ T-cell (# per section) | 2.88±0.64 | 3.00±0.92 | 0.913 | 5.00±0.82 | 3.78±0.70 | 0.272 |
| MHC-II (%) | 2.38±0.00 | 2.35±1.42 | 0.950 | 4.08±3.26 | 4.00±2.99 | 0.958 |
| SMC loss (grade) | 1.56±0.38 | 1.67±0.46 | 0.607 | 2.86±0.58 | 2.85±0.52 | 0.986 |
| Collagen (grade) | 0.64±0.22 | 0.67±0.21 | 0.925 | 2.03±0.35 | 2.10±0.37 | 0.892 |
| Elastin frag. (grade) | 1.84±0.45 | 1.99±0.45 | 0.517 | 3.49±0.26 | 3.18±0.28 | 0.021 |
| Ki67+ cell (# per section) | 3.12±0.44 | 1.50±0.46 | 0.023 | 6.00±0.93 | 2.50±0.89 | 0.018 |
| Microvessel (# per section) | 0.87±0.29 | 0.87±0.29 | 0.500 | 5.90±0.95 | 5.00±1.75 | 0.329 |
| TUNEL+ cell (# per section) | 1.87±0.40 | 2.62±0.56 | 0.149 | 10.50±1.46 | 7.89±0.71 | 0.066 |
| TUNEL+ cell intima | 0.87±0.29 | 1.25±0.31 | 0.199 | 6.60±1.07 | 4.22±0.32 | 0.028 |
| TUNEL+ cell media | 0.37±0.26 | 0.25±0.16 | 0.347 | 1.60±0.4 | 0.55±0.24 | 0.042 |
| TUNEL+ cell adventitia | 0.62±0.18 | 1.12±0.29 | 0.088 | 2.30±0.60 | 3.11±0.65 | 0.187 |
Pre-induced atherosclerosis aggravates OVA-sensitized mouse allergic responses
Three independent experiments tested whether airway allergic responses that occurred before, at the same time, or after atherosclerosis affected atherosclerotic lesion formation or lesion pathology. Apoe−/− mice develop atherosclerosis under a normal chow diet, but an atherogenic diet promotes atherogenesis in these mice. The production of ALI in mice with pre-established atherosclerosis by feeding mice an atherogenic diet tested whether consequent airway allergic responses affected atherosclerosis formation. The same experiment also tested whether pre-established atherosclerosis affected ALI. The comparison of OVA-sensitized mice with and without pre-established atherosclerosis revealed significantly increased plasma levels of IgE (269.45±60.71 ng/ml vs. 1034.64±136 ng/ml, P<0.001), IFN-γ (P<0.001), and eotaxin (P=0.001), but lower levels of IL4 (P=0.036), IL13 (P=0.007), and TGF-β (P=0.002) in mice with atherosclerosis. Mice with atherosclerosis and consequent ALI also had higher plasma LDL, but lower HDL and triglyceride levels than those that only had ALI (Table 3, left).
Table 3.
Production of atherosclerosis deteriorates ALI in Apoe−/− mice.
| Variable | OVA
|
P | Saline
|
P | ||
|---|---|---|---|---|---|---|
| Acute ALI only (n=8) | Atherosclerosis first, then acute ALI (n=10) | Saline only (n=8) | Atherosclerosis first then saline (n=10) | |||
| Measurements | ||||||
| Body weight (g) | 30.45±1.15 | 30.44±0.51 | 0.994 | 30.84±1.29 | 33.04±0.82 | 0.262 |
| SBP (mmHg) | 106.25±4.08 | 96.40±2.07 | 0.056 | 103.00±2.26 | 101.11±3.08 | 0.985 |
| DBP (mmHg) | 75.25±3.13 | 72.80±1.91 | 0.516 | 78.00±2.97 | 77.00±2.54 | 0.610 |
| Serum lipid proteins | ||||||
| Total cholesterol (mg/dL) | 415.72±37.27 | 417.55±34.24 | 0.972 | 422.30±22.02 | 448.04±23.98 | 0.441 |
| LDL (mg/dL) | 120.73±33.44 | 313.66±35.50 | 0.001 | 161.31±23.39 | 339.56±21.13 | <0.001 |
| HDL (mg/dL) | 243.23±17.05 | 76.91±10.71 | <0.001 | 215.81±17.24 | 54.48±8.47 | <0.001 |
| Triglyceride (mg/dL) | 258.78±28.05 | 162.59±30.84 | 0.035 | 225.92±21.35 | 270.00±50.73 | 0.441 |
| Serum inflammatory proteins | ||||||
| IgE (ng/mL) | 269.45±60.71 | 1034.64±136.00 | <0.001 | 57.03±13.55 | 482.16±70.98 | <0.001 |
| IFN-γ (pg/mL) | 7.74±3.42 | 71.21±9.49 | <0.001 | 5.51±2.56 | 68.72±18.01 | 0.009 |
| TNF-α (pg/mL) | 165.71±81.10 | 23.74±3.83 | 0.124 | 57.93±45.23 | 19.68±0.56 | 0.863 |
| Eotaxin (pg/mL) | 19.42±2.24 | 48.71±4.50 | 0.001 | 18.45±2.28 | 36.78±3.56 | 0.002 |
| MCP-1 (pg/mL) | 131.94±31.35 | 94.88±8.05 | 0.298 | 179.30±21.18 | 86.70±16.19 | 0.005 |
| IL4 (pg/mL) | 67.29±9.08 | 39.20±8.20 | 0.036 | 134.67±76.69 | 24.50±7.10 | 0.195 |
| IL5 (pg/mL) | 13.09±2.91 | 6.92±2.21 | 0.136 | 7.65±2.40 | 14.71±2.93 | 0.090 |
| IL13 (pg/mL) | 7.11±0.88 | 3.37±0.65 | 0.007 | 6.15±1.39 | 1.84±0.32 | 0.021 |
| TGF-β (ng/mL) | 6.94±0.92 | 2.69±0.48 | 0.002 | 5.05±1.34 | 3.19±0.36 | 0.216 |
| BALF cell typing | ||||||
| Total Cell in BALF (x104) | 4.66±0.45 | 78.64±21.08 | 0.006 | 15.85±1.31 | 5.37±0.53 | <0.001 |
| Macrophage (x104) | 0.72±0.23 | 9.44±2.44 | 0.006 | 12.52±1.74 | 4.92±0.54 | 0.002 |
| Lymphocyte (x104) | 1.63±0.27 | 18.54±6.27 | 0.025 | 0.97±0.25 | 0.22±0.05 | 0.016 |
| Eosinophil (x104) | 2.27±0.24 | 50.66±14.04 | 0.007 | 2.27±1.09 | 0.24±0.07 | 0.094 |
| Neutrophil (x104) | 0.00±0.00 | 0.00±0.00 | – | 0.09±0.09 | 0.00±0.00 | 0.343 |
| BALF proteins | ||||||
| IgE (ng/mL) | 128.99±13.94 | 106.19±12.40 | 0.351 | 88.68±13.34 | 104.96±8.79 | 0.648 |
| IFN-γ (pg/mL) | 12.86±2.31 | 11.05±1.65 | 0.535 | 10.25±1.74 | 11.00±1.67 | 0.762 |
| TNF-α (pg/mL) | 365.51±59.86 | 528.13±51.82 | 0.028 | 158.94±41.10 | 337.89±53.04 | 0.018 |
| Eotaxin (pg/mL) | 36.04±5.48 | 75.77±7.73 | 0.006 | 23.58±1.19 | 80.82±3.92 | 0.036 |
| MCP-1 (pg/mL) | 30.09±3.64 | 41.95±4.34 | 0.002 | 23.14±0.99 | 28.92±2.11 | 0.030 |
| IL4 (pg/mL) | 5.98±1.94 | 13.38±1.93 | 0.016 | 7.88±1.79 | 7.33±1.77 | 0.829 |
| IL5 (pg/mL) | 21.92±2.65 | 24.76±3.54 | 0.531 | 19.09±2.72 | 17.70±1.90 | 0.681 |
| IL13 (pg/mL) | 34.66±2.51 | 50.33±3.41 | 0.002 | 27.67±0.68 | 35.75±2.17 | 0.006 |
| TGF-β (pg/mL) | 372.15±57.07 | 362.19±33.28 | 0.883 | 283.71±53.02 | 278.17±35.79 | 0.648 |
| Atherosclerotic lesion analysis | ||||||
| Area (mm2) | 0.14±0.02 | 0.25±0.04 | 0.021 | 0.12±0.03 | 0.23±0.06 | 0.050 |
| Macrophage (%) | 5.85±4.20 | 13.83±6.52 | 0.006 | 5.86±3.50 | 13.04±3.99 | 0.002 |
| Mast cell (# per section) | 1.12±0.29 | 3.80±0.81 | 0.010 | 1.43±0.40 | 3.78±1.13 | 0.040 |
| CD4+ T-cell (# per section) | 4.25±0.72 | 5.30±0.75 | 0.328 | 2.62±0.37 | 4.89±0.77 | 0.022 |
| CD8+ T-cell (# per section) | 2.88±0.64 | 5.00±0.82 | 0.029 | 3.00 ±0.92 | 3.78±0.70 | 0.257 |
| MHC-II (%) | 2.38±0.00 | 4.08±3.26 | 0.070 | 2.35±1.42 | 4.00±2.99 | 0.090 |
| SMC loss (grade) | 1.56±0.38 | 2.86±0.58 | <0.001 | 1.67±0.46 | 2.85±0.52 | <0.001 |
| Collagen (grade) | 0.64±0.22 | 2.03±0.35 | 0.004 | 0.67±0.21 | 2.10±0.37 | 0.002 |
| Elastin frag. (grade) | 1.84±0.45 | 3.49±0.26 | <0.001 | 1.99±0.45 | 3.18±0.28 | <0.001 |
| Ki67+ cell (# per section) | 3.12±0.44 | 6.00±0.93 | 0.016 | 1.50±0.46 | 2.50±0.89 | 0.364 |
| Microvessel (# per section) | 0.87±0.29 | 5.90±0.95 | 0.001 | 0.87±0.29 | 5.00±1.75 | 0.047 |
| TUNEL+ cell (# per section) | 1.87±0.40 | 10.50±1.46 | 0.001 | 2.62±0.56 | 7.89±0.71 | <0.001 |
BALF proteins TNF-α (P=0.028), eotaxin (P=0.006), MCP-1 (P=0.002), IL4 (P=0.016), and IL13 (P=0.002) increased in mice with atherosclerosis than in mice without pre-established atherosclerosis (Table 3, left). As expected, aortic arches revealed enlarged lesion area (P=0.021) and increased lesion macrophage content (P=0.006), mast cell numbers (P=0.010), CD8+ T cell content (P=0.029), SMC loss (P<0.001), collagen degradation (P=0.004), elastin fragmentation (P<0.001), lesion cell proliferation (P=0.016), lesion microvessel contents (P=0.001), and cell apoptosis (P=0.001), compared with those in mice with ALI alone (Table 3, left).
In saline-treated mice without ALI, pre-established atherosclerosis increased serum IgE (57.03±13.55 ng/ml vs. 482.16±70.98 ng/ml, P<0.001), IFN-γ (P=0.009), eotaxin (P=0.002), and LDL (P<0.001), and decreased HDL (P<0.001) levels, but also decreased serum MCP-1 (P=0.005) and IL13 (P=0.021) levels. Aortic arches from mice with pre-established atherosclerosis had significantly larger aortic lesion areas and lesion contents of macrophages (P=0.002), mast cells (P=0.040), CD4+ T cells (P=0.022), lesion SMC loss (P<0.001), collagen degradation (P=0.002), elastin fragmentation (P<0.001), microvessel proliferation (P=0.047), and apoptotic cells (P<0.001) (Table 3, right). BALF IgE did not show a significant difference in this study, but TNF-α (P=0.018), eotaxin (P=0.036), MCP-1 (P=0.030), and IL13 (P=0.006) levels increased in mice with pre-established atherosclerosis (Table 3, right). These observations suggest that atherosclerosis helps promote BALF inflammation independent of ALI.
Anti-allergic mast cell inhibitor and glucocorticoid in treatment of atherosclerosis
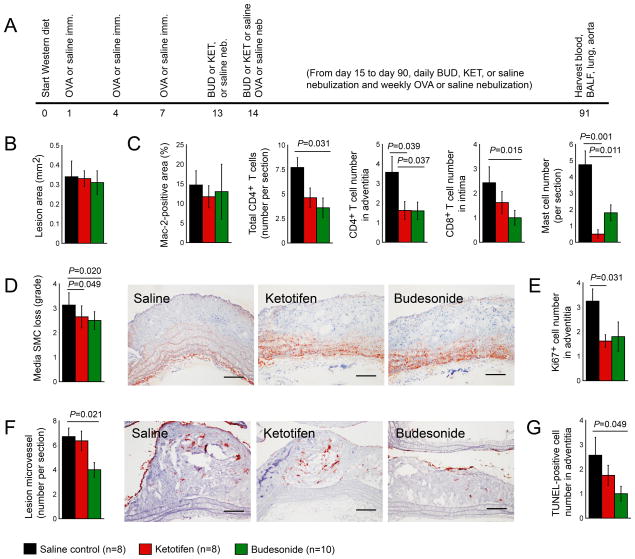
The treatment of patients with asthma and other allergic reactions often involves the use of ketotifen, a mast cell inhibitor, and budesonide, a corticosteroid.31–34 This engendered the hypothesis that these anti-allergic asthma drugs may improve both ALI and atherosclerosis in mice with concurrent ALI and atherosclerosis (Figure 1 and Table 1, left). Therefore, the production of atherosclerosis and chronic ALI preceded the daily nebulization of either ketotifen or budesonide (Figure 4A). Although nebulization of these anti-asthmatic drugs did not change atherosclerotic arch lesion area or lesion macrophage contents (Figure 4B, 4C), ketotifen or budesonide significantly reduced aortic arch lesion CD4+ and CD8+ T cells, mast cells, media SMC loss, lesion cell proliferation, angiogenesis, and apoptosis (Figure 4C–4G, Table 4). As in humans, mice with chronic ALI and atherosclerosis had increased plasma total cholesterol, LDL, and triglycerides. Corticosteroid treatment increases plasma LDL levels in humans.35,36 The present study also found that daily inhalation of budesonide increased plasma LDL and total cholesterol (Table 4). A recent randomized control study demonstrated a role of ketotifen in reducing plasma cholesterol and LDL and increased plasma HDL.37 Results did not, however, show a significant effect of this mast cell stabilizer in plasma lipid profiles in these mice with concurrent ALI and atherosclerosis (Table 4). Neither budesonide nor ketotifen affected plasma IgE and most tested cytokine and chemokine levels. These anti-asthmatic drugs also did not affect BALF cell content, IgE, or most tested BALF cytokines and chemokines, although both drugs increased BALF IL13 levels (Table 4).
Figure 4.
Anti-allergic ketotifen and budesonide reduce atherosclerotic lesion pathologies in Apoe−/− mice. A. Experimental protocol. Aortic arch atherosclerotic lesion area (B), lesion Mac-2-positive macrophage numbers, total and adventitia CD4+ T cells, total CD8+ T cells, and mast cells (C), media SMC loss grade (D), Ki67+ proliferating cells (E), lesion CD31+ microvessel numbers (F), and TUNEL-positive apoptotic cell numbers (G). Representative data in panels D and F are shown to the right. Scale: 200 μm.
Table 4.
Effects of corticosteroid (budesonide) and mast cell inhibitor (ketotifen) in Apoe−/− mice with concurrent productions of chronic ALI and atherosclerosis.
| Variable | Chronic ALI and atherosclerosis at the same time
|
P Saline vs. BUD |
P Saline vs. KET |
||
|---|---|---|---|---|---|
| BUD (n=10) | KET (n=8) | Saline (n=8) | |||
| Measurements | |||||
| Body weight (g) | 25.11±0.40 | 26.13±0.87 | 28.59±1.05 | 0.013 | 0.093 |
| SBP (mmHg) | 105.90±3.82 | 104.38±3.37 | 95.88±3.42 | 0.068 | 0.099 |
| DBP (mmHg) | 84.00±3.58 | 80.13±2.78 | 74.13±2.93 | 0.049 | 0.160 |
| Serum lipid proteins | |||||
| Total cholesterol (mg/dL) | 501.97±54.01 | 387.71±13.97 | 370.54±25.97 | 0.047 | 0.572 |
| LDL (mg/dL) | 359.83±39.75 | 257.40±26.00 | 205.28±25.01 | 0.005 | 0.171 |
| HDL (mg/dL) | 102.29±16.01 | 100.47±18.92 | 139.97±15.57 | 0.111 | 0.130 |
| Triglyceride (mg/dL) | 199.28±47.24 | 149.22±29.25 | 126.46±19.78 | 0.113 | 0.935 |
| Serum inflammatory proteins | |||||
| IgE (ng/mL) | 326.91±44.24 | 400.12±89.41 | 436.67±95.48 | 0.325 | 0.784 |
| IFN-γ (pg/mL) | 5.56±3.63 | 2.94±3.43 | 43.10±48.63 | 0.466 | 0.437 |
| TNF-α (pg/mL) | 43.61±31.25 | 60.53±46.42 | 340.56±198.86 | 0.091 | 0.104 |
| Eotaxin (pg/mL) | 52.40±4.07 | 61.02±6.97 | 37.58±6.60 | 0.088 | 0.030 |
| MCP-1 (pg/mL) | 197.17±86.72 | 120.48±17.10 | 115.01±19.27 | 0.421 | 0.835 |
| IL4 (pg/mL) | 55.06±14.87 | 71.54±12.84 | 92.38±42.85 | 0.435 | 0.653 |
| IL5 (pg/mL) | 6.50±1.87 | 4.58±1.14 | 7.71±2.10 | 0.682 | 0.109 |
| IL13 (pg/mL) | 8.98±2.60 | 11.68±3.05 | 8.70±2.80 | 0.943 | 0.484 |
| TGF-β (ng/mL) | 1.18±0.46 | 0.66±0.30 | 0.96±0.48 | 0.745 | 0.610 |
| BALF cell typing | |||||
| Total Cell in BALF (x104) | 5.16±0.48 | 8.36±0.88 | 6.75±1.40 | 0.312 | 0.359 |
| Macrophage (x104) | 2.74±0.47 | 1.86±0.38 | 2.71±0.78 | 0.979 | 0.351 |
| Lymphocyte (x104) | 1.83±0.38 | 3.02±0.82 | 2.10±0.61 | 0.708 | 0.384 |
| Eosinophil (x104) | 0.59±0.11 | 2.35±0.55 | 1.91±0.57 | 0.055 | 0.580 |
| Neutrophil (x104) | 0.01±0.01 | 0.08±0.05 | 0.03 ±0.03 | 0.584 | 0.472 |
| BALF proteins | |||||
| IgE (ng/mL) | 54.74±4.39 | 53.78±9.30 | 59.18±10.56 | 0.708 | 0.707 |
| IFN-γ (pg/mL) | 467.28±25.59 | 551.86±60.84 | 581.20±63.44 | 0.064 | 0.744 |
| TNF-α (pg/mL) | 827.00±105.25 | 692.75±78.35 | 581.91±90.61 | 0.054 | 0.372 |
| Eotaxin (pg/mL) | 41.78±3.45 | 44.68±3.55 | 37.38±1.87 | 0.304 | 0.051 |
| MCP-1 (pg/mL) | 30.56±5.29 | 34.77±4.54 | 32.00±4.10 | 0.838 | 0.658 |
| IL4 (pg/mL) | 2.57±0.63 | 1.81±0.68 | 1.84±0.51 | 0.420 | 0.977 |
| IL5 (pg/mL) | 0.66±0.37 | 0.39±0.24 | 0.48±0.26 | 0.723 | 0.806 |
| IL13 (pg/mL) | 56.66±7.35 | 53.36±5.90 | 39.01±5.58 | 0.043 | 0.050 |
| TGF-β (pg/mL) | 217.52±32.89 | 162.75±46.39 | 227.88±37.02 | 0.841 | 0.297 |
| Atherosclerotic lesion analysis | |||||
| Area (mm2) | 0.31±0.06 | 0.33±0.04 | 0.34±0.08 | 0.765 | 0.932 |
| Macrophage (%) | 13.01±7.00 | 11.71±2.82 | 14.71±3.64 | 0.529 | 0.104 |
| Mast cell (# per section) | 1.80±0.49 | 0.50±0.27 | 4.75±0.84 | 0.011 | 0.001 |
| CD4+ T-cell (# per section) | 3.60±1.08 | 4.62±1.44 | 7.71±1.22 | 0.031 | 0.136 |
| CD4+ T-cell Adventitia | 1.60±0.45 | 1.62±0.46 | 3.57±0.81 | 0.037 | 0.039 |
| CD8+ T-cell (# per section) | 3.40±0.56 | 5.37±1.49 | 5.14±0.88 | 0.070 | 0.449 |
| CD8+ T-cell Intima | 1.00±0.30 | 1.62±0.45 | 2.43±0.65 | 0.015 | 0.341 |
| MHC-II (% ) | 3.07±1.93 | 3.22±1.54 | 4.55±4.60 | 0.444 | 0.489 |
| SMC loss (grade) | 2.50±0.36 | 2.65±0.51 | 3.13±0.52 | 0.020 | 0.049 |
| Collagen (grade) | 1.21±0.17 | 1.19±0.04 | 1.07±0.32 | 0.705 | 0.724 |
| Elastin frag. (grade) | 3.31±0.32 | 3.11±0.53 | 3.01±0.54 | 0.312 | 0.730 |
| Ki67+ cell (# per section) | 11.40±1.41 | 9.75±2.68 | 12.12±1.73 | 0.749 | 0.471 |
| Ki67+ cell adventitia | 1.80±0.66 | 1.62±0.26 | 3.25±0.59 | 0.122 | 0.031 |
| Microvessel (# per section) | 4.00±0.63 | 6.37±0.82 | 6.71±0.75 | 0.021 | 0.387 |
| TUNEL+ cell (# per section)7.30±1.32 | 7.00±1.10 | 7.86±1.42 | 0.786 | 0.656 | |
| TUNEL+ cell adventitia | 1.00±0.30 | 1.75±0.41 | 2.57±0.73 | 0.049 | 0.188 |
DISCUSSION
The present study revealed a possible interaction between allergic asthma and atherosclerosis. Patients with asthma may have a higher risk of developing atherosclerosis than those without asthma,20–22 and patients with atherosclerosis may experience a higher risk of allergic reactions than those without atherosclerosis. Data showed that mice with chronic or acute ALI had larger atherosclerotic lesion size or more lesion pathological complications than those of mock-challenged mice, regardless of the production of atherosclerosis occurring before, after, or at the same time as atherogenesis (Table 1 and Table 2, right). In contrast, in mice with acute ALI, pre-established atherosclerosis increased serum and BALF IgE, BALF inflammatory cell accumulation, and ALI-relevant Th2 cytokines (Table 3, left). Yet the development of acute ALI, either before or after the development of atherosclerosis (Table 1, right, and Table 2, right), did not affect atherosclerotic lesion size, although acute ALI did affect atherosclerotic lesion pathologic complications.
When mice developed atherosclerosis, they also showed elevation of plasma LDL and reduction of plasma HDL (Table 3, right). In contrast, development of ALI alone did not affect these plasma lipoproteins (Table 2, left), although patients with asthma often have higher plasma LDL and lower plasma HDL than those from control patients.38,39 Interestingly, data revealed that ALI significantly increased plasma LDL and decreased plasma HDL without affecting plasma total cholesterol levels in mice that developed concurrently atherosclerosis (Table 1, left). These observations remain consistent to what was reported in humans. Asthma did not affect human plasma total cholesterol levels in men or women.22 Yet, ALI did not affect plasma LDL levels when ALI was produced before (Table 1, right) or after (Table 2, right) the development of atherosclerosis. Further, when ALI was produced before atherosclerosis, ALI increased plasma HDL levels (Table 1, right). Therefore, the role of ALI in regulating plasma lipid profiles in the setting of atherosclerosis may involve more complicated mechanisms, a hypothesis that merits further investigation.
Anti-asthmatic prescriptions commonly include corticosteroids.33,34 Yet evidence suggests corticosteroids produce hyperlipidemia, hypertension, and dyslipidemia, which in turn may increase adverse cardiovascular events.40,41 Conflicting case-control studies suggest an insignificant effect of corticosteroid on plasma lipid profiles.42 Here, the 25-minute daily inhalation of 7 mg of budesonide in 5 mL of saline, according to a previously described dose,43 significantly improved atherosclerotic lesion pathology, although such a medication did not affect lesion size (Figure 4, and Table 4). This dose of budesonide reduced serum and BALF IgE and BALF lymphocytes and eosinophils, but none of these changes reached statistical significance (Table 4B). Higher doses of budesonide44 or a different drug administration route may exert a more robust inhibition of ALI and the reduction of atherosclerotic lesion area.
Recent studies from obese and diabetic patients showed that the oral administration of the mast cell inhibitor ketotifen (2 mg/day) for 12 weeks reduced plasma LDL and triglyceride levels and increased plasma HDL levels.37 In mice with diet-induced atherosclerosis, mast cell inhibition with intraperitoneal administration of cromolyn also attenuated atherosclerosis.45,46 This study revealed that the inhalation of ketotifen (14 mg/mL), a common anti-allergy medication,31,32 reduced aortic arch lesion pathology, but did not significantly reduce atherosclerotic lesion size (Table 4 and Figure 4B). Different from humans, ketotifen inhalation, instead of oral administration did not affect significantly plasma lipid profiles. Different ketotifen formulations showed the efficacy in changing serum lipid profile,37,46 and different doses can affect plasma lipid profile differently.37 The low dose used in this study, in addition to the drug formulation difference, likely caused insignificant differences in reducing atherosclerotic lesion size by ketotifen. Therefore, a higher dose or different drug formulations may achieve a better efficacy.
Prior studies from experimental atherosclerosis and OVA-challenge-induced ALI demonstrated that mice with ALI had significantly enlarged atherosclerotic lesions in the aortic root than those challenged with saline alone, and these ALI mice had elevated splenic Th2 and Th17 cells without affecting Th1 cells.26 Our study showed that OVA-induced ALI before, after, or at the same time as atherosclerosis increased serum or BALF levels of Th2 cytokines IL4, IL5, or IL13 (Table 1 and Table 2, right).47 Yet data did not reveal significant differences in serum Th1 cytokines IFN-γ and TNF-α with or without ALI from all three combinations of productions of ALI and atherosclerosis (Table 1 and Table 2, right). Of note, Th2 imbalance promotes asthma,8,9 whereas Th1 responses dominate atherogenesis. The production of asthma and the increase of Th2 responses should reduce atherosclerosis. A detailed analysis of both Th1 and Th2 cytokines from prior study26 and this current study did not support this hypothesis.
An increase of plasma or BALF IgE levels commonly signifies ALI production in mice48 (Table 2, left). The development of diet-induced atherosclerosis also increases serum IgE12 (Table 3, right). IgE plays detrimental roles in atherosclerosis.12 Mice that developed concurrent ALI and atherosclerosis or mice that developed ALI followed by atherosclerosis had comparable plasma IgE levels (Table 1), although serum IgE levels doubled in mice with pre-established atherosclerosis followed by ALI production, compared with mice with atherosclerosis alone (Table 2, right). In our model of chronic ALI, OVA immunization did not include Al(OH)3 (Figure 1A). Omission of Al(OH)3 in such mice is known to reduce greatly the IgE production.49 Therefore, the production of atherosclerosis may contribute mainly to the serum IgE in these chronic ALI mice (Table 1, left). Insignificant plasma and BALF IgE levels, but significantly enlarged atherosclerotic lesion size in mice with concurrent production of chronic ALI and atherosclerosis compared with mice with atherosclerosis alone, suggest a negligible role of IgE in contributing to atherosclerosis in these mice. This study, however, does not exclude the possibility of local IgE or Th1 cytokine functions at the milieu of atherosclerotic lesions.
This study reported the contribution of ALI to atherosclerosis, but did not explore further the cellular and molecular mechanisms behind these observations. ALI is known to induce protease expression, including neutrophil elastase, matrix metalloproteinases, mast cell proteases, and cysteinyl cathepsins.50,51 Increased lesion inflammation, matrix degradation and media SMC loss, cell proliferation, apoptosis, and neovascularization after ALI production suggest the participation of these proteases in ALI-induced atherosclerosis. For example, ALI induces the expression of tissue elastic cathepsin S (CatS)52–54 and cathepsin K (CatK).55 While lysosomal CatS mediates antigen presentation and T-cell activation,56,57 extracellular CatS mediates extracellular matrix protein degradation, thereby promoting neovascularization, arterial wall destruction, and atherosclerosis.58,59 CatK is also a potent elastase and collagenase60 that plays essential roles in vascular remodeling, neovascularization, and atherosclerosis.61–63 ALI-induced expression and activity of these cathepsins are probably only one of the mechanisms by which ALI exacerbates atherosclerosis. Pharmacological inactivation of these proteases may mitigate ALI-enhanced atherosclerosis.
Acknowledgments
The authors thank Ms. Eugenia Shvartz for her technical assistance and Ms. Chelsea Swallom for her editorial assistance. All authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors. This study is supported by the National Natural Science Foundation of China (81300176, CY) and the National Institutes of Health grants HL60942, HL81090, HL123568 (GPS), HL48743, and HL080472 (PL). All authors declare no conflict of interest.
ABBREVIATIONS
- ALI
allergic lung inflammation
- Apoe
apolipoprotein E
- SMC
smooth muscle cell
- IMT
intima-media thickness
- OR
odds ratio
- CI
confidential interval
- OVA
ovalbumin
- MHC II
major histocompatibility complex class II
- BALF
bronchoalveolar lavage fluid
- MCP-1
monocyte chemoattractant protein-1
- LDL
low-density lipoproteins
- HDL
high-density lipoprotein
- cathepsin S
CatS
- cathepsin K
CatK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McFadden ER, Jr, Gilbert IA. Asthma. N Engl J Med. 1992;327:1928–37. doi: 10.1056/NEJM199212313272708. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 3.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 4.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 6.Fahy JV, Corry DB, Boushey HA. Airway inflammation and remodeling in asthma. Curr Opin Pulm Med. 2000;6:15–20. doi: 10.1097/00063198-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 7.O’Byrne PM. Airway inflammation and asthma. Aliment Pharmacol Ther. 1996;10(Suppl 2):18–24. doi: 10.1046/j.1365-2036.1996.22164016.x. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–5. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosnjak B, Stelzmueller B, Erb KJ, et al. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res. 2011;12:114. doi: 10.1186/1465-9921-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frostegard J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 11.Ait-Oufella H, Taleb S, Mallat Z, et al. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–79. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Cheng X, Xiang MX, et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe−/− mice. J Clin Invest. 2011;121:3564–77. doi: 10.1172/JCI46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dal Negro RW, Guerriero M, Micheletto C, et al. Changes in total IgE plasma concentration measured at the third month during anti-IgE treatment predict future exacerbation rates in difficult-to-treat atopic asthma: a pilot study. J Asthma. 2011;48:437–41. doi: 10.3109/02770903.2011.578316. [DOI] [PubMed] [Google Scholar]
- 14.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 15.Oettgen HC, Geha RS. IgE in asthma and atopy: cellular and molecular connections. J Clin Invest. 1999;104:829–35. doi: 10.1172/JCI8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohman MK, Wright AP, Wickenheiser KJ, et al. Monocyte chemoattractant protein-1 deficiency protects against visceral fat-induced atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1151–8. doi: 10.1161/ATVBAHA.110.205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emanuele E, Falcone C, D’Angelo A, et al. Association of plasma eotaxin levels with the presence and extent of angiographic coronary artery disease. Atherosclerosis. 2006;186:140–5. doi: 10.1016/j.atherosclerosis.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Lilly CM, Woodruff PG, Camargo CA, Jr, et al. Elevated plasma eotaxin levels in patients with acute asthma. J Allergy Clin Immunol. 1999;104:786–90. doi: 10.1016/s0091-6749(99)70288-5. [DOI] [PubMed] [Google Scholar]
- 19.Tateno H, Nakamura H, Minematsu N, et al. Eotaxin and monocyte chemoattractant protein-1 in chronic eosinophilic pneumonia. Eur Respir J. 2001;17:962–8. doi: 10.1183/09031936.01.17509620. [DOI] [PubMed] [Google Scholar]
- 20.Onufrak S, Abramson J, Vaccarino V. Adult-onset asthma is associated with increased carotid atherosclerosis among women in the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2007;195:129–37. doi: 10.1016/j.atherosclerosis.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoflach M, Kiechl S, Mayr A, et al. Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY studies. Arch Intern Med. 2005;165:2521–6. doi: 10.1001/archinte.165.21.2521. [DOI] [PubMed] [Google Scholar]
- 22.Iribarren C, Tolstykh IV, Eisner MD. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol. 2004;33:743–8. doi: 10.1093/ije/dyh081. [DOI] [PubMed] [Google Scholar]
- 23.Schanen JG, Iribarren C, Shahar E, et al. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax. 2005;60:633–8. doi: 10.1136/thx.2004.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahran HS, Bailey C. Factors associated with asthma prevalence among racial and ethnic groups--United States, 2009–2010 behavioral risk factor surveillance system. J Asthma. 2013;50:583–9. doi: 10.3109/02770903.2013.794238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budoff MJ, Yang TP, Shavelle RM, et al. Ethnic differences in coronary atherosclerosis. J Am Coll Cardiol. 2002;39:408–12. doi: 10.1016/s0735-1097(01)01748-x. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Gao S, Xu W, et al. Allergic asthma accelerates atherosclerosis dependent on Th2 and Th17 in apolipoprotein E deficient mice. J Mol Cell Cardiol. 2014;72:20–7. doi: 10.1016/j.yjmcc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Huang X, Wolters PJ, et al. Cutting edge: Deficiency of macrophage migration inhibitory factor impairs murine airway allergic responses. J Immunol. 2006;177:5779–84. doi: 10.4049/jimmunol.177.9.5779. [DOI] [PubMed] [Google Scholar]
- 28.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–9. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Sukhova GK, Yang M, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–68. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deckert V, Kretz B, Habbout A, et al. Development of abdominal aortic aneurysm is decreased in mice with plasma phospholipid transfer protein deficiency. Am J Pathol. 2013;183:975–86. doi: 10.1016/j.ajpath.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Kabra SK, Pandey RM, Singh R, et al. Ketotifen for asthma in children aged 5 to 15 years: a randomized placebo-controlled trial. Ann Allergy Asthma Immunol. 2000;85:46–52. doi: 10.1016/S1081-1206(10)62433-7. [DOI] [PubMed] [Google Scholar]
- 32.Molkhous P, Dupont C. Ketotifen treatment of atopic dermatitis and other food allergy diseases. Allergy. 1989;44(Suppl 9):117–23. [PubMed] [Google Scholar]
- 33.Volovitz B. Inhaled budesonide in the management of acute worsenings and exacerbations of asthma: a review of the evidence. Respir Med. 2007;101:85–695. doi: 10.1016/j.rmed.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880–5. doi: 10.1067/mjd.2002.120464. [DOI] [PubMed] [Google Scholar]
- 35.Ettinger WH, Klinefelter HF, Kwiterovitch PO. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis. 1987;63:167–72. doi: 10.1016/0021-9150(87)90117-1. [DOI] [PubMed] [Google Scholar]
- 36.Ross IL, Marais AD. The influence of glucocorticoids on lipid and lipoprotein metabolism and atherosclerosis. S Afr Med J. 2014;104:671–4. doi: 10.7196/samj.7979. [DOI] [PubMed] [Google Scholar]
- 37.El-Haggar SM, Farrag WF, Kotkata FA. Effect of ketotifen in obese patients with type 2 diabetes mellitus. J Diabetes Complications. 2015;29:427–32. doi: 10.1016/j.jdiacomp.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Vinding RK, Stokholm J, Chawes BL, et al. Blood lipid levels associate with childhood asthma, airway obstruction, bronchial hyperresponsiveness, and aeroallergen sensitization. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 39.Yiallouros PK, Savva SC, Kolokotroni O, et al. Asthma: the role of low high-density-lipoprotein cholesterol in childhood and adolescence. Int Arch Allergy Immunol. 2014;165:91–9. doi: 10.1159/000368405. [DOI] [PubMed] [Google Scholar]
- 40.Ng MK, Celermajer DS. Glucocorticoid treatment and cardiovascular disease. Heart. 2004;90:829–30. doi: 10.1136/hrt.2003.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varas-Lorenzo C, Rodriguez LA, Maguire A, et al. Use of oral corticosteroids and the risk of acute myocardial infarction. Atherosclerosis. 2007;192:376–83. doi: 10.1016/j.atherosclerosis.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Picado C, Deulofeu R, Lleonart R, et al. Lipid and protein metabolism in asthma. Effects of diet and corticosteroid therapy. Allergy. 1999;54:569–75. doi: 10.1034/j.1398-9995.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 43.Wattenberg LW, Wiedmann TS, Estensen RD, et al. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997;57:5489–92. [PubMed] [Google Scholar]
- 44.Olsen PC, Kitoko JZ, Ferreira TP, et al. Glucocorticoids decrease Treg cell numbers in lungs of allergic mice. Eur J Pharmacol. 2015;747:52–8. doi: 10.1016/j.ejphar.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–24. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Sjoberg S, Tia V, et al. Pharmaceutical stabilization of mast cells attenuates experimental atherogenesis in low-density lipoprotein receptor-deficient mice. Atherosclerosis. 2013;229:304–9. doi: 10.1016/j.atherosclerosis.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romagnani S. T-cell subsets (Th1 versus Th2) Ann Allergy Asthma Immunol. 2000;85:9–18. doi: 10.1016/S1081-1206(10)62426-X. quiz 18, 21. [DOI] [PubMed] [Google Scholar]
- 48.Mayr SI, Zuberi RI, Zhang M, et al. IgE-dependent mast cell activation potentiates airway responses in murine asthma models. J Immunol. 2002;169:2061–8. doi: 10.4049/jimmunol.169.4.2061. [DOI] [PubMed] [Google Scholar]
- 49.Mayr SI, Zuberi RI, Liu FT. Role of immunoglobulin E and mast cells in murine models of asthma. Braz J Med Biol Res. 2003;36:821–7. doi: 10.1590/s0100-879x2003000700001. [DOI] [PubMed] [Google Scholar]
- 50.Greene CM, McElvaney NG. Proteases and antiproteases in chronic neutrophilic lung disease - relevance to drug discovery. Br J Pharmacol. 2009;158:1048–58. doi: 10.1111/j.1476-5381.2009.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh SW, Pae CI, Lee DK, Jones F, Chiang GK, Kim HO, et al. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002;168:1992–2000. doi: 10.4049/jimmunol.168.4.1992. [DOI] [PubMed] [Google Scholar]
- 52.Fajardo I, Svensson L, Bucht A, Pejler G. Increased levels of hypoxia-sensitive proteins in allergic airway inflammation. Am J Respir Crit Care Med. 2004;170:477–84. doi: 10.1164/rccm.200402-178OC. [DOI] [PubMed] [Google Scholar]
- 53.Daheshia M, Tian N, Connolly T, Drawid A, Wu Q, Bienvenu JG, et al. Molecular characterization of antigen-induced lung inflammation in a murine model of asthma. Ann N Y Acad Sci. 2002;975:148–59. doi: 10.1111/j.1749-6632.2002.tb05948.x. [DOI] [PubMed] [Google Scholar]
- 54.Deschamps K, Cromlish W, Weicker S, Lamontagne S, Huszar SL, Gauthier JY, et al. Genetic and pharmacological evaluation of cathepsin s in a mouse model of asthma. Am J Respir Cell Mol Biol. 2011;45:81–7. doi: 10.1165/rcmb.2009-0392OC. [DOI] [PubMed] [Google Scholar]
- 55.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Poloczek A, et al. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) in chronic hypoxia- and antigen-mediated pulmonary vascular remodeling. Respir Res. 2013;14:1. doi: 10.1186/1465-9921-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Driessen C, Bryant RA, Lennon-Dumenil AM, Villadangos JA, Bryant PW, Shi GP, et al. Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J Cell Biol. 1999;147:775–90. doi: 10.1083/jcb.147.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 58.Shi GP, Sukhova GK, Kuzuya M, Ye Q, Du J, Zhang Y, et al. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res. 2003;92:493–500. doi: 10.1161/01.RES.0000060485.20318.96. [DOI] [PubMed] [Google Scholar]
- 59.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chapman HA, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annu Rev Physiol. 1997;59:63–88. doi: 10.1146/annurev.physiol.59.1.63. [DOI] [PubMed] [Google Scholar]
- 61.Hu L, Cheng XW, Song H, Inoue A, Jiang H, Li X, et al. Cathepsin K activity controls injury-related vascular repair in mice. Hypertension. 2014;63:607–15. doi: 10.1161/HYPERTENSIONAHA.113.02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang H, Cheng XW, Shi GP, Hu L, Inoue A, Yamamura Y, et al. Cathepsin K-mediated Notch1 activation contributes to neovascularization in response to hypoxia. Nat Commun. 2014;5:3838. doi: 10.1038/ncomms4838. [DOI] [PubMed] [Google Scholar]
- 63.Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]