Abstract
Experimental autoimmune encephalomyelitis (EAE) is an animal model of demyelinating autoimmune disease, such as multiple sclerosis (MS), which is characterized by central nervous system white matter lesions, microglial activation, and peripheral T cell infiltration secondary to blood-brain barrier disruption. We have previously shown that treatment with tuftsin, a tetrapeptide generated from IgG proteolysis, dramatically improves disease symptoms in EAE. Here, we report that microglial expression of Neuropilin-1 (Nrp1) is required for tuftsin-driven amelioration of EAE symptom. Nrp1 ablation in microglia blocks microglial signaling and polarization to the anti-inflammatory M2 phenotype, and ablation in either the microglia or immunosuppressive regulatory T cells (Tregs) reduces extended functional contacts between them and Treg activation, implicating a role for microglia in the activation process, and more generally, how immune surveillance is conducted in the CNS. Taken together, our findings delineate the mechanistic action of tuftsin as a candidate therapeutic against immune-mediated demyelinating lesions.
Keywords: Mice, Treg, microglia, anti-inflammatory, EAE
Introduction
Multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE) are characterized by progressive demyelination and degeneration of central nervous system (CNS) neurons (Swanborg 1995). The course of MS/EAE is strongly affected by microglia (Raivich and Banati 2004), which are capable of polarizing into pro- and anti-inflammatory subsets known as M1 and M2. Classically-activated M1 microglia release pro-inflammatory cytokines associated with increasing disease severity, while alternatively-activated, anti-inflammatory M2 microglia suppress ongoing severe EAE symptoms (Mikita et al. 2011) and promote recovery (Kigerl et al. 2009).
Regulatory T cells (Tregs) function as immunosuppressants (Kohm et al. 2002) and are critical in attenuating MS/EAE symptoms. Tregs differentiate in response to TGFβ (Glinka and Prud'homme 2008), and once activated, disrupt the function and activation of other immune cells including helper T cells, B cells (Miyara and Sakaguchi 2007), and microglia (Reynolds et al. 2007). Antigen presentation by innate immune cells is an essential component of T cell activation and functional modulation (Almolda et al. 2011). In the periphery, interaction of Tregs with dendritic cells (DC) is required for their activation (Tarbell et al. 2006). While dendritic cells are a robust population of antigen-presenting cells (APCs), they are rarely found within the CNS parenchyma in the healthy state, although they can be detected in vascular-rich areas such as the meninges and the choroid plexus, and infiltrate the CNS during EAE (McMenamin 1999). Thus, within the uninjured CNS, it is the microglia that function as MHC II-expressing APCs (Olson et al. 2001; Wlodarczyk et al. 2014). CD4+Foxp3+ Tregs were recently found to be present in the physiological CNS (Olmos-Serrano et al. 2010), and upon injury, to interact with microglia in an antigen-specific manner (Ebner et al. 2013) to dampen the inflammatory environment (Chen et al. 2014).
Initial work in our laboratory demonstrated that modulation of the microglial activation state affects EAE severity and duration (Bhasin et al. 2007). In particular, the microglial activator tuftsin was shown to decrease EAE symptom severity and dramatically improve recovery. Tuftsin is a naturally-occurring tetrapeptide (threonine-lysine-proline-arginine) which readily crosses into the CNS (Bonfoco et al. 2000) that has been shown to increase phagocytic activity in a variety of cells of monocytic origin, including macrophages and microglia, and can stimulate cell migration, chemotaxis, and respiratory burst (Siemion and Kluczyk 1999). When administered to immature bone marrow cells, tuftsin strongly promoted the development of macrophage-lineage cells (Babcock et al. 1983). Most importantly, tuftsin administration to macrophages in the presence of splenic T lymphocytes promoted the antigen presenting capabilities of cells of monocytic origin (Tzehoval et al. 1978). We also showed that tuftsin functions under activating conditions by promoting a microglial shift towards the M2 phenotype in vitro, which then promotes polarization of T cell populations towards anti-inflammatory subsets (Floriddia et al. 2012). Tuftsin, which has been reported to bind to several different receptors, specifically stimulates microglia through the receptor Nrp1 and the canonical TGFβ signaling pathway (Nissen et al. 2013). These findings suggested that microglial modulation by tuftsin through Nrp1 underlies the beneficial effects of tuftsin in EAE.
Nrp1 plays an important role in the initiation of primary immune responses in the periphery by facilitating interactions between DCs and Tregs (Tordjman et al. 2002) through homotypic binding of Nrp1 in trans (Sarris et al. 2008). Nrp1 is typically expressed by Tregs but not other T cells (Bruder et al. 2004); overexpression of FoxP3, a Treg transcription factor, in naïve T cells, causes them to express Nrp1 (Bruder et al. 2004; Sarris et al. 2008). These observations raised the possibility that Nrp1 might similarly mediate extended contacts and functional interactions between Tregs and microglia.
Many molecules are involved in the functional interaction between T cells and APCs, especially in the periphery where DCs are the primary APCs. The contact zone between these cells is often referred to as the “immunological synapse” (Friedl et al. 2005). It has been shown that immature DCs (iDCs) preferentially interact with Tregs (Sarris et al. 2008). This is not due to differential expression of adhesion or co-activating signals (Dustin et al. 1997), but to the selective Treg expression of Nrp1, e.g., blocking Nrp1 on either the DCs or Tregs eliminates the preference (Sarris et al. 2008). As microglia are the resident antigen-presenting Nrp1-expressing cells of the CNS (Olson et al. 2001), we hypothesized that Nrp1 might also mediate this type of functional interaction for Tregs and microglia.
To investigate the hypothesis that microglial modulation by tuftsin through Nrp1 underlies the beneficial effects of tuftsin in EAE, we have induced EAE in mice lacking Nrp1 expression in their macrophages and microglia. Here, we show that this selective Nrp1 ablation renders tuftsin treatment ineffective for EAE. Nrp1, which is expressed on both Tregs and DCs, homodimerizes in trans to facilitate cell-cell interactions between them. We show here that Nrp1 similarly mediates extended contacts between microglia and Tregs, which have not previously been shown to interact by direct contact, and that the interaction triggers TGFβ release. Taken together, our work identifies molecular determinants for the cellular interactions between microglia and Tregs and suggests therapeutic promise for the use of tuftsin in treating EAE/MS.
Experimental Procedures
Animals
Csf1R-cre (Deng et al. 2010), and Nrp1fl/fl (Anthonypillai et al. 2004) mice were bred in-house under pathogen-free conditions with a 12-hour light/dark cycle. Access to food and water was ad libitum. All procedures were approved by the SBU IACUC committee. For EAE experiments, 13-17 animals were used per experimental group.
Induction of EAE with MOG35-55 peptide
MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) was synthesized by the Yale University Peptide Synthesis Facility and purified using reverse-phase (C18) HPLC. EAE was induced (Arvidsson et al. 2002; Bernard et al. 1997; Bhasin et al. 2007) in female mice by subcutaneous injection on day 0 with 300μg of MOG35-55 thoroughly emulsified in complete Freund's adjuvant (CFA) containing 500μg of heat-inactivated Mycobacterium tuberculosis (Difco, Detroit, MI). A week later the mice received 300μg of MOG35-55 peptide subcutaneously in the other flank. 500ng Pertussis toxin (List Biologicals, Campbell, CA) in 200μl of PBS was injected intraperitoneally on days 0 and 2. All EAE experiments were performed using littermate controls. After immunization with MOG, mice were observed and weighed daily blindly. The severity of disease symptoms were scored on a five-point scale ranging from 0 to 5 with gradations of 0.5 for intermediate symptoms. The score is defined as follows (Hjelmstrom 1998): 0, no detectable symptoms; 1, loss of tail tone; 2, hindlimb weakness or abnormal gait; 3, complete hindlimb paralysis; 4, complete hindlimb paralysis with forelimb weakness or paralysis; 5, moribund or dead.
Time-controlled Drug Delivery
Alzet mini-osmotic pumps (Durect, Cupertino, CA) were used for time-controlled drug delivery. 28-day pumps (rate of infusion 0.25μl/hr, 250μl total volume) were filled with PBS or 500μM tuftsin (Sigma) and incubated at 37°C overnight. The pumps were implanted subcutaneously in the back of adult female C57BL/6 mice (8-10 weeks old) under anesthesia the same day as the initial MOG immunization.
Tissue collection and processing
Mice were deeply anesthetized with intraperitoneal injection of 2.5% avertin (0.02ml/g body weight) and transcardially perfused using PBS (pH 7.4) followed by 4% paraformaldehyde (PFA) in PBS (pH 7.4). Spinal cords were isolated, post-fixed in 4% PFA, and dehydrated in 30% sucrose. After the meninges were removed, the spinal cord was cut into equal sections, embedded in optimal cutting temperature compound (Tissue Tek), frozen and stored at −80°C.
Immunofluorescence
Cells used for immunofluorescence were fixed for 30 minutes at room temperature in 4%PFA, while slides were incubated for 5 minutes in PBS to remove residual OCT. After washing with PBS, samples were blocked in serum of the host of the secondary antibody (5% serum and 0.3% BSA in PBS with 0.2% Triton-X 100), and then incubated overnight with rabbit anti-mouse Iba1 (1:500, Wako), mouse anti-mouse iNOS (1:500, BD Biosciences), mouse anti-mouse Arg-1 (1:500, BD Biosciences), rat anti-mouse CD206 (1:50, R&D Systems), rat anti-mouse CD86 (1:50, Millipore), rat anti-mouse CD11b (1:200, Serotec), and rabbit anti-mouse p-Stat6 (1:100, Cell Signaling) in 0.3% BSA in PBS with 0.2% Triton-X 100. After washing with PBS, sections were incubated with fluorescence-conjugated FITC or Cy3 goat anti-rabbit or rabbit anti-mouse secondary antibody and Streptavidin-conjugated Cy3 (to detect bound biotinylated tuftsin) for 1 hour at room temperature, washed 3 times with PBS, and mounted using Fluoromount-G with DAPI (Southern Biotech, USA). For experiments where two markers were used for staining, (e.g. Iba1/iNOS or Iba1/Arg1), yellow fluorescence is indicative of double-positive signal. DAPI was included in images as an indicator of cell density in lesion areas. The cells were imaged using a Nikon Eclipse E600 microscope or a Zeiss LSM 510 confocal microscope.
Fluoromyelin staining
Slides were rehydrated in PBS for 5min, incubated with fluoromyelin-staining solution (1:300, Invitrogen) for 20min at room temperature, washed, and mounted with Fluoromount-G (Southern Biotech). ImageJ freeware (NIH) was used to measure the demyelinated and total areas of the white matter. In brief, images were cropped to remove the grey matter regions prior to quantification. To distinguish between positive-staining white matter areas and demyelinated regions, thresholding was utilized in order to obtain a binary signal. The demyelinated area was determined by subtracting the myelinated region from the total area, and percentage calculated as shown below. Six full coronal sections were analyzed for each biological replicate.
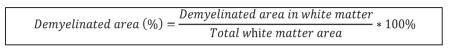 |
Flow cytometry
After undergoing EAE for 21 days as described above, animals were euthanized by saline perfusion to clear tissues of blood with the aim of reducing circulating macrophage/monocyte contamination. Spinal cords were isolated and the lumbar region was digested in papain. Following trituration, cells underwent density centrifugation in 30% Percoll to remove myelin debris, and blocked with CD16/32 Fc Block (1:50) for 30 min. These were then stained with CD11b-APC (1:100), CD86-PE (1:50), and CD206-FITC (1:50) (Biolegend) for 30 min, and washed 2x with FACS Buffer. Samples were analyzed using a BD FACSCalibur machine.
Mixed cortical cultures for primary microglia isolation
Microglia were isolated as described (Rogove and Tsirka 1998). In short, newborn (d0-d2) pups of wild-type mice were used to isolate cortical cells. The brains were removed, and cortices were freed from meninges, hippocampi and basal ganglia, and then digested in 0.25% Trypsin/EDTA (Sigma) at 37°C for 20min. To obtain a single-cell suspension, the tissue was triturated and filtered through a 40 μm cell strainer, and plated in mixed cortical medium (DMEM, 10% FBS, 40μg/ml Gentamycin). Tissue culture plates used for plating mixed cortical cultures were coated overnight at 4°C with 5μg/ml poly-D-lysine (PDL, Sigma).
The medium was changed 3 days later and microglia harvested at day 10. Briefly, lidocaine was added directly to the culture medium at a final concentration of 1mM and the culture left at room temperature for 15 minutes. The medium containing the floating microglia was collected and centrifuged at 500g for 5 min, following which the cell pellet was resuspended in microglia medium (DMEM, 1% FBS) and cells were counted on a hemocytometer. The cultures typically are >98% pure (Caito et al. 2010; Rogove and Tsirka 1998; Siao and Tsirka 2002).
Phagocytosis assay
As previously described (Rogove et al. 1999), primary microglial cultures from Nrp1 WT and KO were plated on coverslips. The following day the medium above the cells was replaced with fresh medium containing pre-stained latex beads (0.8 μm diameter, Sigma) at a concentration of 0.1 μl bead suspension/ml medium. The cells were incubated at 37°C/5% CO2 for 2 hours. After thorough washing, cells were fixed in 4% paraformaldehyde for 30 minutes at room temperature. The cells were permeabilized with PBS containing 0.1% Triton-X 100 for 15 minutes at room temperature, and identified by staining with FITC-conjugated isolectin-B4 for two hours at room temperature. After mounting on slides, microglia were imaged using a Zeiss LSM 510 confocal microscope. The rhodamine-conjugated latex beads were visualized using excitation 529 nm/emission 550 nm and appear red; the FITC-stained microglia appear green. The content of 25-31 cells of each genotype and treatment in five fields on one plane of focus was counted, with images consistently taken in the four corners and center of each coverslip. The numbers of latex beads inside the cells were averaged, and the standard deviation was calculated over the total number of cells counted. In addition images in small slice z-stacks were taken, which allowed the visualization of beads at different depths within individual cells.
Measurement of cytokine levels
To measure TGFβ levels, the Ready-Set-GO TGFβ cytokine ELISA kit from eBioscience was utilized. Briefly, 96-well plates were coated overnight with the appropriate dilution of capturing antibody in coating buffer (0.2M sodium phosphate buffer pH 6.5). The plate was washed three times with PBS-T (PBS, 0.05% Tween-20) and blocked with Assay Diluent for 1 hour at RT. To activate latent TGFβ1, samples were treated with 20 μl of 1N HCl per 100μl of sample for 10 minutes, and then neutralized with 1N NaOH. After washing as before, 100μl of sample or cytokines standard prepared in assay diluent were added followed by 2 hour incubation at RT. After 5 washes with PBS-T, 100μl of assay diluent containing biotin-conjugated detection antibody and Avidin-HRP reagent at the appropriate dilutions were added and incubated for 1 hour at RT. Following 7 washes with PBS-T, 100μl of Substrate Solution was added to each well. After 30 min incubation in the dark, 50μl of Stop Solution (2N H2SO4) were added and absorbance at 450nm was read within 30 min on a SpectraMax microplate reader using the Softmax Pro software. Final readings were calculated with a dilution factor of 1.4 to account for acid activation/neutralization.
Primary T cell culture
Primary splenic CD4+ T cells were isolated using a CD4+ mouse T cell negative-isolation kit (Invitrogen). Briefly, a mixture of monoclonal antibodies against unwanted cells (B cells, NK cells, monocytes, dendritic cells, CD8+ T cells, erythrocytes and granulocytes) was added to spleen cells. T cells were isolated by removing the antibody-labeled cells using mouse-depletion Dynabeads and a Dynal MPC.
Tregs were generated using the Millipore FlowCellect Treg differentiation kit. The CD4+ helper T cells were cultured in a CD3-coated plate and treated with two stages of T cell activator solutions as per kit instructions. On day 4 of the seven-day procedure, Tregs for Nrp1-KO groups were treated with retroviral-Cre containing media isolated from Phoenix-Eco cells for 3 days to allow for incorporation of the Cre gene and excision of the Nrpfl/fl sequence to generate a Treg-specific Nrp1 knockout.
Generation of retroviral Cre
Retrovirus containing a Cre expression construct (HR-MMPCreGFP) was generated as described (Silver and Livingston 2001). Phoenix cells were transfected with a plasmid containing Cre and GFP sequences using Lipofectamine in serum-free Opti-MEM media. After 12 hours, the media was changed to DMEM with 10% FBS and pen/strep. After 24 hours, virus-containing media were collected and used freshly.
RT-PCR
RNA was isolated from microglia and T cells using RNA-Bee (Tel-Test), by the manufacturer’s protocol. cDNA was generated by reverse-transcribing one microgram of RNA using the High Capacity Reverse Transcription cDNA kit (Applied Biosystems) on a Veriti Thermocycler. Nrp1 and GAPDH transcripts were amplified using Taq DNA polymerase with Standard Taq buffer (New England Biosystems). Primer sequences are as follows: Nrp1 forward, 5′-GGGCAGAGACTGCAAGTATGA-3′; Nrp1 reverse, 5′-AGAAATGGCCCTGAAGACAC-3′; GAPDH forward, 5′-GCACAGTCAAGGCCGAGAAT-3′; GAPDH reverse, 5′-GCCTTCTCCATGGTGGTGGA-3′.
Statistics
For multiple comparisons within a group, statistical analysis was performed using one-way ANOVA followed by a Bonferroni-Dunn test. For comparisons between groups, a two-tailed t-test was used, as indicated by the figure legends. For all figures, p<0.05 was considered significant and is marked by *; p<0.01 and p<0.001 are marked by ** and *** respectively. All results are represented as averages with error bars indicating the standard error of the mean. In all experiments, n refers to the number of biological replicates used for each condition.
Results
Elimination of microglial/macrophage Nrp1 expression prevents tuftsin-mediated reduction of disease score and weight loss in EAE
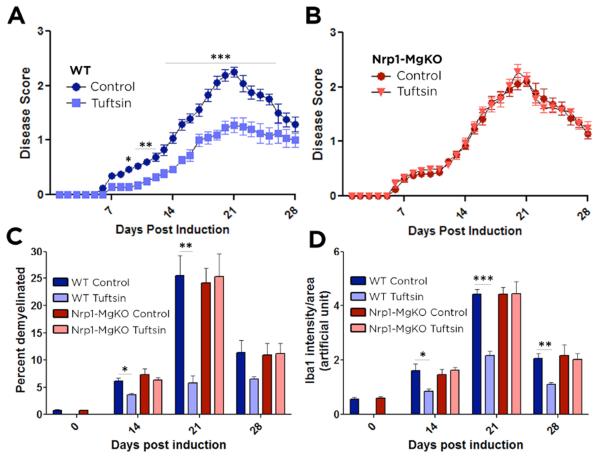
To evaluate the role of Nrp1 in mediating tuftsin’s beneficial effects, we assessed the clinical course of MOG-induced EAE in mice lacking macrophage and microglial Nrp1 expression. The mice were generated by crossing Csf1R-iCre mice (Deng et al. 2010) to Nrp1fl/fl mice (Anthonypillai et al. 2004) in the C57Bl/6 background; Csf1R-cre/ Nrp1fl/fl F2 progeny hereafter are denoted as Nrp1-MgKO mice. Nrp1fl/fl littermates lacking the Csf1R-iCre transgene were used for controls (“WT”).
Tuftsin was delivered for 28 days starting at day 0 via implantation of an osmotic pump. Parallel delivery of the diluent, saline, as a control had no effect on EAE disease score in WT or Nrp1-MgKO mice (Fig. S1); thus the changes observed following tuftsin treatment can be attributed to its pharmacological effects. Clinical scores were recorded blinded to genotype. Induction of EAE in control mice elicited clinical symptoms at day 6 that gradually increased in severity through day 21 and then subsided (Fig. 1A). In contrast, tuftsin treatment significantly attenuated the symptoms from day 9 onwards. Nrp1-MgKO mice exhibited EAE disease symptoms identical in severity to WT mice (Fig. 1A, B), but for these mice, tuftsin administration was without effect (Fig. 1B). To quantify the role of Nrp1 in mediating tuftsin’s effects, peak and cumulative scores were compared. The peak score for WT mice (2.3±0.1) signifies that their most severe symptom was partial hindlimb paralysis (Fig. S2). In contrast, WT mice treated with tuftsin had a peak score of 1.2±0.1, corresponding to a limp tail. Nrp1-MgKO mice attained a WT-like peak score (2.2±0.1) that was not improved with tuftsin (2.3±0.1). Cumulative scores were similar: WT control, 30.0±1.7; tuftsin-treated WT, 18.5±2.3; Nrp1-MgKO, 28.3±2.1; and tuftsin-treated Nrp1-MgKO, 28.4±1.6 (Fig. S2). Taken together, tuftsin significantly reduces EAE severity via Nrp1 signaling in macrophages and microglia.
Figure 1. Tuftsin infusion has no effect on EAE disease symptoms in Nrp1-MgKO mice.
EAE was induced by injection of MOG35-55 in CFA and pertussis toxin. Osmotic pumps filled with 500μM tuftsin in PBS were implanted subcutaneously on day 0 after MOG immunization. Comparisons between WT control (untreated) and tuftsin-infused mice (A) and Nrp1-MgKO control (untreated) and tuftsin-infused mice (B). Data are mean ± SEM. n= 13-17, *, p<0.05; **, p<0.01, ***, p<0.001. (C) Frozen spinal cord sections were isolated from control and tuftsin-infused WT and KO mice at 0, 14, 21, and 28 days post induction of EAE. Demyelination was quantified by fluoromyelin staining and demyelinated areas were measured using ImageJ and quantified. (D) Quantification of Iba1 signal intensity staining was utilized to detect microglia in control and tuftsin-infused WT and Nrp-MgKO mice at 0, 14, 21, and 28 days post induction of EAE.
Another classical hallmark of MS and EAE is weight loss, which parallels the severity of motor symptoms (Emerson et al. 2009). As scores represent a subjective value determined by the observer, recording weight as an indicator of disease severity provides a measure that avoids relying on interpretation of behavior. As shown in Fig. S2, control mice steadily lost weight until day 21, after which weight gain occurred during the recovery phase in parallel to their course of disease (Fig. 1A). However, mice treated with tuftsin did not lose weight and in fact gained weight throughout the EAE disease course, despite the mild loss of tail tone. Conversely, Nrp1-MgKO mice in both the control and tuftsin-treated groups lost weight similarly to that seen for the WT control mice (Fig. S2). Thus, loss of macrophage and microglial Nrp1 disrupts tuftsin’s prevention of weight loss during EAE.
Persistent demyelination in mice lacking expression of Nrp1 in macrophages and microglia regardless of tuftsin administration
Demyelination is a well-defined characteristic of MS and EAE. Fluoromyelin was used to visualize myelin in the spinal cord white matter. Demyelination was evident in WT mice at EAE day 14 (6.2±0.5%), reached a peak at day 21 (25.5±3.7%), and was improved but incompletely resolved at day 28 (Fig. 1C, S3). Tuftsin infusion significantly reduced the demyelination at days 14 (3.6±0.2%) and 21 (5.8±1.3%). WT-like demyelination was observed in the Nrp1-MgKO mice but tuftsin treatment conferred no protection (Fig. 1C, S3). The groups with substantial demyelination exhibited remyelination by day 28, although not to the level of protection provided by tuftsin treatment for the WT mice. There was no significant change in demyelination as a result of tuftsin treatment in either group at day 7 (Fig. S4). Thus, Nrp1 expression by microglia is required for tuftsin to exert its beneficial effects on three physical and pathological hallmarks of MS - paralysis, weight loss, and demyelination.
Suppression of microglial activation by tuftsin requires Nrp1 signaling
During EAE, the infiltrating T cell attack on myelin results in the accumulation of cellular debris in injured areas. Activated microglia then arrive to clear the debris via phagocytosis (Benveniste 1997). As the timing and extent of microglial activation contribute significantly to the ultimate outcome of EAE (Bhasin et al. 2007), we immunostained coronal spinal cord sections for Iba1, which is expressed by resting microglia/ macrophages and becomes upregulated upon activation.
Iba1 staining of brain sections from WT and KO mice on day 0 revealed the presence of resting microglia/macrophages, as defined by their ramified morphology and long, thin processes (asterisks, Fig. S5). By day 14, WT control and both Nrp1-MgKO control and tuftsin-treated mice exhibited clustering of microglia with enlarged cell bodies and retracted cell processes in the white matter, presumably in demyelinating lesions, which was not observed in the WT tuftsin-treated mice. As the disease progressed to the peak at day 21, microglia with amoeboid morphology characteristic of full activation (arrowheads) as well as extensive microglial/macrophage infiltration were observed in WT mice and in Nrp1-MgKO mice with or without tuftsin treatment, whereas the WT tuftsin-treated mice displayed reduced microglial/macrophage infiltration and the cells that were observed had a ramified, resting morphology (Fig. S5). At day 28, although the microglia in all groups were returning to their baseline status, those in the WT tuftsin-treated mice remained significantly less activated morphologically. There was no significant change in microglial activation between the groups at day 7 (Fig. S4). Quantification of the Iba1 signal yielded a similar outcome (Fig. 1D). These results indicate that tuftsin treatment of WT mice, but not Nrp1-MgKO mice, attenuates microglial activation during EAE.
Tuftsin infusion shifts microglia to an M2-like state in an Nrp1-dependent manner
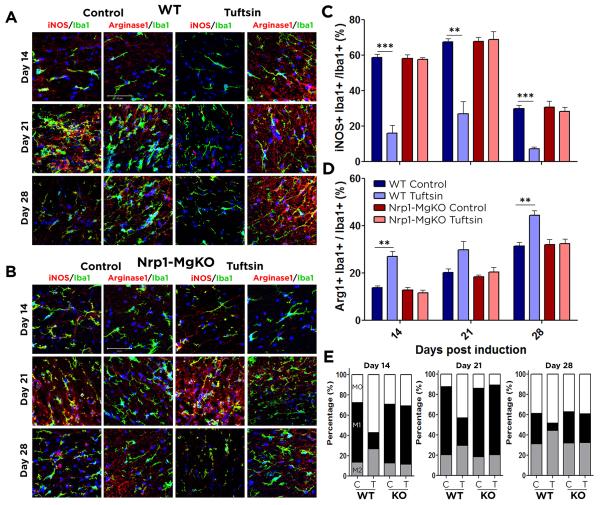
Microglia can play both a detrimental and beneficial role in MS/EAE due to their ability to polarize along a spectrum between pro-inflammatory, M1-like or anti-inflammatory, M2-like subsets. M1-like microglia dominate in the CNS in the early stages of EAE but are then replaced by M2-like microglia during recovery (Kigerl et al. 2009). As the balance between the M1 and M2 populations strongly affects the MS/EAE disease course (Mikita et al. 2011), we sought to determine tuftsin’s effect on M2 polarization.
To delineate the microglial activation state, Iba1+ cells expressing low levels of both the M1 marker iNOS and the M2 marker arginase-1 (Arg1) were defined as M0 resting microglia. Cells expressing elevated levels of iNOS or Arg1 were considered to be M1 or M2 activated microglia, respectively. At day 7 post EAE induction, Arg1 and iNOS were difficult to detect in any group (Fig. S4). At day 14 of EAE in WT control mice, 72.6% of the microglia were activated (Fig. 2). Consistent with prior studies, M1 microglia predominated, representing 81% of the activated microglia (58.8±1.7% iNOS+ cells vs 13.7±0.9% Arg1+ M2 cells). This predominance continued through day 21, when 87.8% of the microglia were activated and 77% of these were in the M1 state (67.5±1.8% vs 20.3±1.5% Arg1+ M2 cells). In contrast, only 43.0% of the microglia were activated in WT tuftsin-treated mice at day 14, and of those, only a minority (37%) were M1 (15.9±4.6% iNOS+ cells vs 27.0±2.1% Arg1+ M2 cells). Of critical importance, despite the decreased total number of activated microglia with tuftsin treatment (43% vs 73% in control mice), the absolute number of M2 microglia was increased (27% vs 14%). This trend continued through day 21. Similar outcomes were observed during the recovery phase at day 28, by which time the WT control mice had converted to M1-M2 equivalence, but there was a 5:1 M2:M1 dominance in the tuftsin-treated mice. Consistent with the prior findings in this study, the tuftsin-driven M2 predominance was abolished in the Nrp1-MgKO mice (Figure 2B-E). These observations were successfully confirmed using additional M1 (CD86) and M2 (CD206) markers at day 21, by immunostaining and flow cytometry (Fig. S6).
Figure 2. Tuftsin promotion of the M2 phenotype is abolished in Nrp1-MgKO mice.
Pro-inflammatory (M1) and anti-inflammatory (M2) microglia were identified by co-localization of iNOS/Iba1 and Arg1/Iba1, respectively, in the spinal cords of control (C) and tuftsin-infused (T) WT EAE mice in (A) and control and tuftsin-infused EAE Nrp1-MgKO mice in (B). iNOS+/Iba1+ (C) and Arg1+/Iba1+ (D) cells were enumerated. Percentages of each population after treatment are indicated in (E). C: Control, T: Tuftsin. Nuclei are stained with DAPI (blue). Data are represented as mean ± SEM. n=3, **, p<0.01, ***, p<0.001. Scale bar: 50μm.
These results indicate that tuftsin signaling through Nrp1 on macrophages/microglia polarizes them towards the protective M2 phenotype early in the disease, promoting anti-inflammatory responses that attenuate clinical symptoms.
Tuftsin promotes microglial phagocytosis in an Nrp1-dependent manner
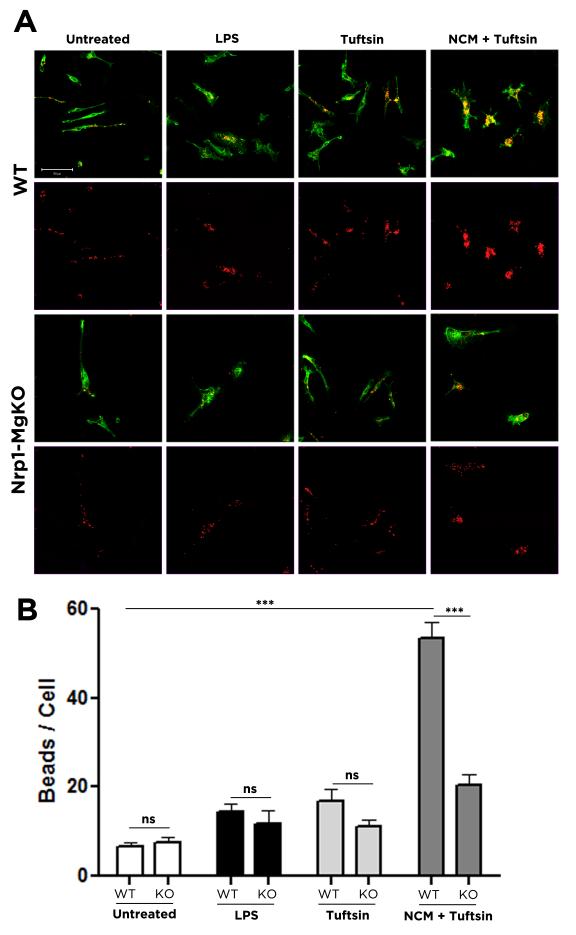
A critical role of microglia in degenerative neurological disorders is to phagocytose cell debris to allow for functional recovery (Neumann et al. 2009); disruption of the phagocytosis during EAE exacerbates demyelination (Matta et al. 2007). As M2 microglia are more phagocytic than their M1 counterparts (Durafourt et al. 2012), we predicted that the suppression of M2 polarization in tuftsin-treated Nrp1-MgKO animals would decrease phagocytic clearance and prevent recovery.
Microglia isolated from WT and Nrp1-MgKO pups were cultured to achieve a fully resting state before being polarized to the M1 state by LPS, to the M2 state using neuronal conditioned media (NCM) in the presence of tuftsin (Wu et al. 2012), or were treated with tuftsin alone or left untreated as controls. These conditions were intended to represent the state of microglia in animals during the degeneration (M1) and recovery (M2) stages of EAE, with untreated cells serving as representatives of M0 resting microglia. After exposure to fluorescently-tagged latex beads for two hours, the number of phagocytosed particles was quantified (Fig. 3A). To bypass the potential issue of bead clustering, we analyzed the cells post ingestion through quantification of the intracellular number of beads on the z-axis (Fig. S7). While M1 microglia were significantly more phagocytic than M0 resting ones, they were still much less phagocytic than M2-polarized cells. However, loss of Nrp1 expression resulted in the loss of this increased phagocytic capability in response to tuftsin under M2 activating conditions. In the inflammatory M1 state, as well as the resting state or in response to tuftsin alone, there was no difference in the phagocytic capacity of Nrp1 WT or KO microglia (Fig. 3B).
Figure 3. Nrp1 expression on microglia is necessary for increased phagocytic activity of microglia in the presence of tuftsin.
Microglia isolated from Nrp1-MgKO or WT pups were untreated, treated with 100ng/ml LPS for 4 hours, or treated with 100ug/ml tuftsin in the presence or absence of neuronal conditioned medium (NCM) for 10 hours, and then incubated with 0.1ul/ml suspension of red fluorescent beads (0.8 um diameter, Sigma) (A). The number of beads in 25-31 cells per condition was quantified (B). ***, p<0.001; ns, not significant.
Nrp1 promotes extended contacts and functional interactions between microglia and Tregs
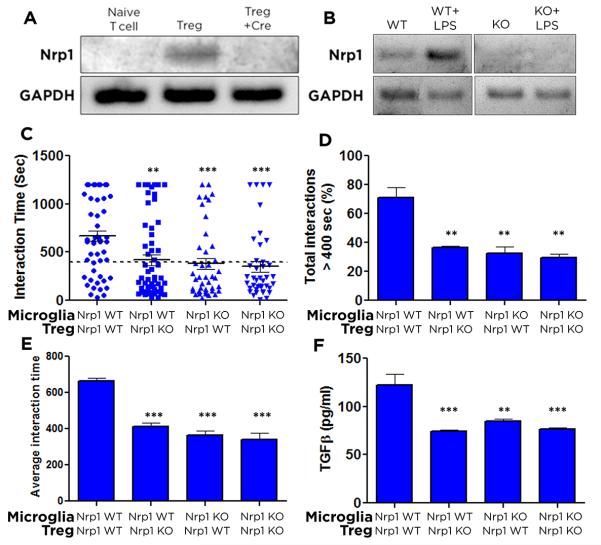
Although interactions between DCs and Treg have been observed in real-time (Sarris et al. 2008), this has not been investigated for microglia. Using time-lapse video microscopy, we examined contact times between microglia and Treg in the presence or absence of Nrp1. Microglia were isolated from Nrp1-MgKO and WT littermates. To ablate Nrp1 from Tregs, T cells from Nrp1fl/fl mice were polarized to the Treg subset, during which time they were also transfected with a retroviral-Cre expressing construct (HR-MMPCreGFP) (Silver and Livingston 2001). The resulting cells are referred to as Nrp1-TregKO, while cells not exposed to retrovirus functioned as the WT control. To confirm loss of Nrp1 expression, RT-PCR was performed to probe Nrp1 transcripts in naïve, Treg-polarized, and Treg-polarized in the presence of retroviral Cre cells (Fig. 4A). Microglia lacking Nrp1 expression were isolated from Nrp1-MgKO mice (Fig. 4B) and used in the resting state.
Figure 4. Loss of Nrp1 expression on microglia and/or Tregs reduces long contacts and TGFβ release.
T cells isolated from the spleens of Nrp1fl/fl mice were left as naïve T cells, polarized to Tregs, or polarized to Tregs in the presence of Cre retrovirus (A); microglia isolated from Nrp1-MgKO or WT pups were examined for Nrp1 expression in the presence or absence of LPS by PCR (B). Microglia were plated in chamber slides and allowed to return to a resting state. The next day, WT or Nrp1-TregKO were added and visualized using time-lapse video microscopy. Images were taken every 10 seconds for 20min and the duration of contacts were determined by the number of frames that Treg and microglia remained in steady contact. The interaction time for each individual contact is shown in (C). The percentage of interactions longer than 400sec (D), and average contact length within each biological replicate (E) were quantified. Significance is relative to WT microglia/WT Treg. After 4 days of co-culture, media were isolated and an ELISA assay used to quantify TGFβ cytokine levels (F), Data are represented as mean ± SEM. n=3-9, **, p<0.01; ***, p<0.001.
The microglial-Treg interactions were monitored for 20 minutes (Supplemental Video 1). WT microglia and Tregs had longer average interaction times than when one or both of the cell types lacked Nrp1 expression (Fig. 4A,C). Transient contacts are indicative of T cells scanning for antigen, while longer interactions are required for rearrangement of proximal signaling molecules and activation (Dustin et al. 1997). Based on previous reports, we set a threshold of 400 seconds as the reference duration (Sarris et al. 2008); longer contacts were considered long/stable; shorter interactions were considered short. Long contacts were significantly reduced when homotypic Nrp1 signaling was disrupted (Fig. 4C-E), similar to the prior study of iDCs and Treg in the periphery (Sarris et al. 2008).
TGFβ is an anti-inflammatory cytokine integral to Treg development and function (Huber et al. 2004). Binding of TGFβ directly to Nrp1 on Tregs promotes their immunosuppressive capabilities, either through activation of TGFβ from its latent form (Glinka and Prud'homme 2008), or potentially by interacting with both Nrp1 and its co-receptor TβR1 and signaling via the canonical TGFβ pathway (Nissen et al. 2013). Activated Tregs become a ‘mobile’ source of TGFβ, as they are recruited to areas of inflammation and release it there (Chen and Wahl 2003). Through cell-cell contacts, Tregs deliver inhibitory signals to target cells (Chen et al. 2001). Long, functional contacts resulting in TCR stimulation promote Treg activation and TGFβ release (Sarris et al. 2008). Since microglia can interact with Tregs (Ebner et al. 2013), we assessed TGFβ levels as a measure of Treg activation by microglia.
Following the real-time video experiments, we kept the microglia and Tregs in co-culture for 4 additional days. Media were collected and an ELISA assay performed for TGFβ. WT Tregs, when exposed to WT microglia, released significantly more TGFβ than when homotypic Nrp1 interactions were disrupted through loss of Nrp1 expression for either or both cell-types (Fig. 4F). These data indicate that Treg activation and release of TGFβ are mediated through Treg interactions with microglial APCs through Nrp1.
Discussion
Tuftsin’s broad activities on phagocytic cells, especially microglia and macrophages, make the peptide a candidate for immunotherapy as evidenced by the chemical synthesis of tuftsin and its analogs for a variety of clinical studies (Fridkin and Najjar 1989). Here, we investigated the mechanism through which tuftsin promotes recovery during EAE; Nrp1 ablation in microglia/macrophages abolished tuftsin’s beneficial effects, indicating that microglial stimulation by tuftsin is requisite for the amelioration of EAE. Further, we have generated insights into the interactions between peripheral and CNS immune cells that normally do not interact outside of injury or disease.
Specific ablation of genes from macrophages/microglia alone with no off-target deletion is complex. The Csf1rR promoter drives Cre expression in ~98% of yolk-sac derived microglia in the hindbrain during development and in 80% of adult microglia (Fantin et al. 2013). Cre expression has also been reported in ~50% of bone marrow granulocytes and splenic T cells (Deng et al. 2010), and in ~1-2% of neurons (Del Pino et al. 2013). At this time, there is no superior microglial-specific Cre line available for use (Abram et al. 2014). All Cre models have inherent limitations with respect to lack of complete efficiency of deletion, or varying amounts of deletion in non-target cells; this particular Csf1R-iCre driver line has been used widely and deemed scientifically acceptable as a research tool (Arno et al. 2014; Perdiguero et al. 2014).
Nrp1 ablation from all T cell populations in vivo results in a worsened EAE disease course characterized by a preferential Th17 lineage commitment and decreased Treg functionality (Solomon et al. 2011). These results contrast with ours, as macrophage/microglial-specific Nrp1-MgKO mice exhibit an EAE disease course comparable to WT controls. There are several potential explanations. First, we previously showed that tuftsin promotes Nrp1 upregulation on microglia that otherwise have only very low levels of Nrp1 (Wu et al. 2012), implying that loss of Nrp1 expression should not have great consequence for the endogenous state under baseline conditions. Further, the Tregs in these mice still express Nrp1, allowing them to bind and activate TGFβ (Glinka and Prud'homme 2008). The disruption of antigen presentation preference (Fig. 4) could potentially be circumvented, as during EAE DCs and iDCs from the periphery are recruited into the CNS (Sagar et al. 2012) and are capable of interacting with T cells on a Nrp1-mediated basis (Sarris et al. 2008). Thus, while infiltrating Tregs from the periphery would not be able to interact well with Nrp1-MgKO microglia, they would still make contacts with Nrp1-expressing, antigen-presenting iDCs in a Treg-preferential manner. Endogenously, Nrp1 on microglial cells has been shown to be upregulated following neuronal injury (Majed et al. 2006). So while microglial/macrophage-specific Nrp1 knockout mice respond differently to tuftsin treatment, they would still undergo a disease course similar to WT mice. However, as macrophage/microglial-specific Nrp1-KO animals fail to show any benefits from tuftsin treatment, we can conclude that tuftsin acts primarily through macrophages/microglia.
Microglial polarization along the pro- to anti-inflammatory continuum (Mikita et al. 2011) as well as their temporal activation during EAE (Bhasin et al. 2007) is essential in determining whether they will function in a beneficial or harmful manner. As evidenced here, tuftsin not only seems to delay the activation of microglia, but also gives them an early, lasting prevalence of M2-polarization. The early enhancement of this polarization (at day 14 vs day 21, Fig. 2) suggests why the disease course in tuftsin-treated WT mice is characterized by a “soft peak” that is strongly reduced compared to control mice. Regardless, mice in all treatment groups show a steady increase in M2 microglia that only becomes predominant in control and Nrp1-MgKO mice during the recovery phase. M1 microglia have been reported to promote T cell differentiation toward Th1 and Th17 fates (Becher et al. 2006), which induce neurodegeneration (Centonze et al. 2009) and impair remyelination and recovery (Li et al. 2005). M2 macrophages are associated with increased Th2 and Treg populations (Frisancho-Kiss et al. 2009), and M2 microglia drive oligodendrocyte differentiation during CNS remyelination (Miron et al. 2013). However, polarization towards to M2-subset is complex, as cells can fall into several subdivisions known as M2a, M2b, or M2c phenotypes. From our results here and previous work in our lab, we speculate that animals treated with tuftsin have their microglia activated in an M2c-like manner. Firstly, we show above that high levels of Arg1 are expressed in the microglia from WT animals exposed to tuftsin, which is consistent with an M2a or M2c phenotype, but excludes M2b (Gerber and Mosser 2001; Mills et al. 2000). Further, tuftsin-stimulated primary microglia under activating conditions produce large amounts of IL-10, which is consistent with the M2c phenotype (Gerber and Mosser 2001; Wu et al. 2012). Most interestingly, M2c macrophages appear to need a two-hit activation process, with an initial non-specific activating signal that does not induce polarization on its own, but when combined with a second stimulus reprograms macrophages to produce IL-10. This is identical to what we saw with the combination of tuftsin and NCM in our previous work (Edwards et al. 2006; Wu et al. 2012). Taken in whole, we conclude that tuftsin functions by promoting an overall anti-inflammatory environment in the CNS during EAE that is critically important for attenuating disease pathology.
When observing the release of TGFβ in cocultures of Treg and microglia, we observed slightly elevated cytokine levels when WT Tregs were exposed to Nrp1-MgKO microglia. It is possible that this is due to TGFβ acting on the Tregs themselves via binding to Nrp1, as these Tregs are still capable of responding to TGFβ via Nrp1. While the reduction of cytokine release from the 120 pg/ml range to the 75 pg/ml range may seem small, other successful MS drugs have shown a similar change in TGFβ levels. For example, FTY720, also known as Fingolimod, is an FDA-approved drug used to treat MS. Administration of FTY720 to Treg cells increases TGFβ release from the 50 pg/ml to the 110 pg/ml range (Liu et al. 2012). Removal of Treg cells (Kim et al. 2007) or interference with their recruitment or retention (Bystry et al. 2001) rapidly induces an autoimmune response even in unprimed hosts. This suggests that self-reactive T cells are constitutively present but held in check by circulating Tregs. Here, we present evidence that Nrp1 is critically important for Treg function, as it mediates the long contacts between microglia and Tregs that activate the Tregs and promote TGFβ release. These findings have implications for processes ranging from maintenance of tolerance to autoimmunity, particularly in the CNS.
Short contacts between T cells and APCs are characteristic of antigen scanning by T cells. However, long interactions seem to occur only in the presence of cognate MHC-peptide complexes, which are required for the stabilization and enhancement of immunological synapse formation (Grakoui et al. 1999). Nonetheless, Tregs have also been reported to make three times as many long interactions with iDCs than do naïve T cells in the absence of exogenous antigen (Sarris et al. 2008), possibly due to the bias of the TCR repertoire of Tregs towards endogenous antigens (Hsieh et al. 2004). This could potentially explain why we are able to observe long contacts between microglia and Treg in the absence of antigen-specific stimulation.
The Treg-microglial interaction may play a key role in many CNS disorders. Microglia have not been thought to typically interact with Tregs in the healthy state, as T cells are viewed as being normally excluded from the “immune-privileged” CNS due to the blood brain barrier (Fletcher et al. 2010). It was recently reported, however, that TCRαβ+CD4+Foxp3+ Tregs are present in the healthy CNS where they constitute 15% of the cerebral CD4+ T-cells (Xie et al. 2014), hypothetically to restrain the immune response in physiological conditions. Upon injury, though, once other T cell types infiltrate, Tregs may function to oppose neurodegenerative conditions, e.g. during intracerebral hemorrhage (Yang et al. 2014); adoptive transfer of Tregs is beneficial in optic nerve injury (Kipnis et al. 2004) and Parkinson’s disease (Appel et al. 2010). Most importantly, Tregs are critical in the EAE recovery process. Infusion of Tregs attenuates EAE symptoms (Kohm et al. 2002) and their depletion increases EAE susceptibility (Reddy et al. 2004). Mice that do not express Nrp1 in their T cell populations exhibit severe EAE and have reduced numbers of Tregs, which implies that Nrp1 is necessary for Treg differentiation and function in vivo (Solomon et al. 2011).
Our results above support the idea of Nrp1 having a dual role; first as a mediator of long interactions between microglia and Treg, and also as a ligand for tuftsin which promotes the microglial anti-inflammatory shift. We believe that these two roles work hand-in-hand to produce the overall beneficial effects we see with tuftsin infusion during EAE. Microglia preferentially interact with Treg on a Nrp1-mediated basis, which preferentially activates these immunosuppressive cells. While normally during EAE the overall environment would become more inflammatory due to demyelination and cell death, tuftsin promotes an M2 shift in microglia through Nrp1 which further supports an environment in which Treg and Th2 responses can predominate.
Taken together, our work provides evidence for direct interactions between immunocompetent microglia and Tregs in the CNS and defines Nrp1 as an essential mediator of this cell-cell communication. Our data also indicate that the effects of tuftsin on Nrp1 signaling may constitute a potential therapeutic opportunity for EAE/MS, in particular with the development of non-peptide, small molecule tuftsin mimetics.
Supplementary Material
Main Points.
Tuftsin attenuates EAE symptoms and demyelination, and promotes an anti-inflammatory shift in microglia
Deletion of microglial Nrp1 completely abolishes tuftsin’s beneficial effects
Nrp1 mediates long, functional contacts between microglia & Treg
Acknowledgments
We thank Drs. Alex Kolodkin (Johns Hopkins Univ) and Dr. Jeff Pollard (Albert Einstein College of Medicine) for sharing the Nrp1fl/fl and Csf1R-cre mice, respectively; we also thank Dr. Mike Frohman and members of the Tsirka lab for their helpful discussions. This work was supported by NIH IRACDA K12GM102778 (JN), NMSS PP1815 and NIH R01NS42168 (SET).
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
JN designed and performed the experiments, analyzed the data and wrote drafts of the manuscript. SET designed experiments, analyzed the data and wrote drafts of the manuscript.
References
- Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci. 2011;16:1157–71. doi: 10.2741/3781. [DOI] [PubMed] [Google Scholar]
- Anthonypillai C, Sanderson RN, Gibbs JE, Thomas SA. The distribution of the HIV protease inhibitor, ritonavir, to the brain, cerebrospinal fluid, and choroid plexuses of the guinea pig. J Pharmacol Exp Ther. 2004;308:912–20. doi: 10.1124/jpet.103.060210. [DOI] [PubMed] [Google Scholar]
- Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson's disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 2010;31:7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arno B, Grassivaro F, Rossi C, Bergamaschi A, Castiglioni V, Furlan R, Greter M, Favaro R, Comi G, Becher B. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun. 2014;5:5611. doi: 10.1038/ncomms6611. others. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Babcock GF, Amoscato AA, Nishioka K. Effect of tuftsin on the migration, chemotaxis, and differentiation of macrophages and granulocytes. Ann N Y Acad Sci. 1983;419:64–74. doi: 10.1111/j.1749-6632.1983.tb37092.x. [DOI] [PubMed] [Google Scholar]
- Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84:532–43. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med (Berl) 1997;75:165–73. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Bernard CC, Johns TG, Slavin A, Ichikawa M, Ewing C, Liu J, Bettadapura J. Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis. J Mol Med. 1997;75:77–88. doi: 10.1007/s001090050092. [DOI] [PubMed] [Google Scholar]
- Bhasin M, Wu M, Tsirka SE. Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis. BMC Immunol. 2007;8:10. doi: 10.1186/1471-2172-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfoco E, Chen W, Paul R, DA C, Cooper N. b1 integrin antagonism on adherent, differentiated human neuroblastoma cells triggers an apoptotic signaling pathway. Neuroscience. 2000;101:1145–1152. doi: 10.1016/s0306-4522(00)00429-2. [DOI] [PubMed] [Google Scholar]
- Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–30. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010;24:3145–59. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D'Amelio M, Cavallucci V, Martorana A. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29:3442–52. doi: 10.1523/JNEUROSCI.5804-08.2009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Tian H, Sicurello P, Frank M, Orenstein JM, Wahl SM. Requirement for transforming growth factor beta1 in controlling T cell apoptosis. J Exp Med. 2001;194:439–53. doi: 10.1084/jem.194.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Wahl SM. TGF-beta: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003;14:85–9. doi: 10.1016/s1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang L, Zhang IY, Liang J, Wang H, Ouyang M, Wu S, da Fonseca AC, Weng L, Yamamoto Y. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 2014;74:7285–97. doi: 10.1158/0008-5472.CAN-14-1240. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M, Ciceri G, Gabaldon MV, Moratal D, Dierssen M. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–68. doi: 10.1016/j.neuron.2013.07.010. others. [DOI] [PubMed] [Google Scholar]
- Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol. 2010;176:952–67. doi: 10.2353/ajpath.2010.090622. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–27. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:3909–13. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F, Brandt C, Thiele P, Richter D, Schliesser U, Siffrin V, Schueler J, Stubbe T, Ellinghaus A, Meisel C. Microglial activation milieu controls regulatory T cell responses. J Immunol. 2013;191:5594–602. doi: 10.4049/jimmunol.1203331. others. [DOI] [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson MR, Gallagher RJ, Marquis JG, LeVine SM. Enhancing the ability of experimental autoimmune encephalomyelitis to serve as a more rigorous model of multiple sclerosis through refinement of the experimental design. Comp Med. 2009;59:112–28. [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Plein A, Denti L, Fruttiger M, Pollard JW, Ruhrberg C. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood. 2013;121:2352–62. doi: 10.1182/blood-2012-05-424713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floriddia EM, Rathore KI, Tedeschi A, Quadrato G, Wuttke A, Lueckmann JM, Kigerl KA, Popovich PG, Di Giovanni S. p53 Regulates the neuronal intrinsic and extrinsic responses affecting the recovery of motor function following spinal cord injury. J Neurosci. 2012;32:13956–70. doi: 10.1523/JNEUROSCI.1925-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkin M, Najjar VA. Tuftsin: its chemistry, biology, and clinical potential. Crit Rev Biochem Mol Biol. 1989;24:1–40. doi: 10.3109/10409238909082550. [DOI] [PubMed] [Google Scholar]
- Friedl P, den Boer AT, Gunzer M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5:532–45. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
- Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather D. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23:649–57. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 2001;166:6861–8. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- Glinka Y, Prud'homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–10. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–4483. [PubMed] [Google Scholar]
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- Kigerl K, Gensel J, Ankeny D, Alexander J, Donnelly D, Popovich P. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Avidan H, Caspi RR, Schwartz M. Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: A dialogue with microglia. PNAS. 2004;101:14663–14669. doi: 10.1073/pnas.0404842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–6. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2005;158:58–66. doi: 10.1016/j.jneuroim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang J, Xiao H, Wang X, Li Y, Gong Y, Huang Y. The sphingosine-1-phosphate receptor agonist FTY720 and its phosphorylated form affect the function of CD4+CD25+ T cells in vitro. Int J Mol Med. 2012;30:211–9. doi: 10.3892/ijmm.2012.973. [DOI] [PubMed] [Google Scholar]
- Majed H, Chandran S, Niclou S, Nicholas R, Wilkins A, Wing M, Rhodes K, Spillantini M, Compston A. A novel role for Sema3A in neuroprotection from injury mediated by activated microglia. J Neurosci. 2006;26:1730–8. doi: 10.1523/JNEUROSCI.0702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. others. [DOI] [PubMed] [Google Scholar]
- McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–62. [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of Multiple Sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. others. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–8. doi: 10.1038/nn.3469. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–95. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen JC, Selwood DL, Tsirka SE. Tuftsin signals through its receptor neuropilin-1 via the transforming growth factor beta pathway. J Neurochem. 2013;127:394–402. doi: 10.1111/jnc.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–38. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Girvin AM, Miller SD. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J Virol. 2001;75:9780–9. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2014 doi: 10.1038/nature13989. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev. 2004;46:261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, Sobel RA, Wucherpfennig KW, Kuchroo VK. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2004;101:15434–9. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson's disease. J Leukoc Biol. 2007;82:1083–94. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Rogove A, Siao C, Keyt B, Strickland S, Tsirka S. Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system. J Cell Sci. 1999;112:4007–4016. doi: 10.1242/jcs.112.22.4007. [DOI] [PubMed] [Google Scholar]
- Rogove AD, Tsirka SE. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr Biol. 1998;8:19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, Jain P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J Neuroinflamm. 2012;9:245. doi: 10.1186/1742-2094-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris M, Andersen K, Randow F, Mayr L, Betz A. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci. 2002;22:3352–8. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemion IZ, Kluczyk A. Tuftsin: on the 30-year anniversary of Victor Najjar's discovery. Peptides. 1999;20:645–74. doi: 10.1016/s0196-9781(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Silver D, Livingston D. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol Cell. 2001;8:233–43. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- Solomon BD, Mueller C, Chae WJ, Alabanza LM, Bynoe MS. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108:2040–5. doi: 10.1073/pnas.1008721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanborg RH. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin Immunol Immunopathol. 1995;77:4–13. doi: 10.1016/0090-1229(95)90130-2. [DOI] [PubMed] [Google Scholar]
- Tarbell KV, Yamazaki S, Steinman RM. The interactions of dendritic cells with antigen-specific, regulatory T cells that suppress autoimmunity. Semin Immunol. 2006;18:93–102. doi: 10.1016/j.smim.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, Roméo P. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002;3:477–482. doi: 10.1038/ni789. [DOI] [PubMed] [Google Scholar]
- Tzehoval E, Segal S, Stabinsky Y, Fridkin M, Spirer Z, Feldman M. Tuftsin (an Ig-associated tetrapeptide) triggers the immunogenic function of macrophages: implications for activation of programmed cells. Proc Natl Acad Sci U S A. 1978;75:3400–4. doi: 10.1073/pnas.75.7.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk A, Lobner M, Cedile O, Owens T. Comparison of microglia and infiltrating CD11c(+) cells as antigen presenting cells for T cell proliferation and cytokine response. J Neuroinflammation. 2014;11:57. doi: 10.1186/1742-2094-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Nissen JC, Chen EI, Tsirka SE. Tuftsin promotes an anti-inflammatory switch and attenuates symptoms in experimental autoimmune encephalomyelitis. PLoS One. 2012;7:e34933. doi: 10.1371/journal.pone.0034933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Choudhury GR, Winters A, Yang SH, Jin K. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur J Immunol. 2014 doi: 10.1002/eji.201444823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Yu A, Liu Y, Shen H, Lin C, Lin L, Wang S, Yuan B. Regulatory T cells inhibit microglia activation and protect against inflammatory injury in intracerebral hemorrhage. Int Immunopharmacol. 2014;22:522–5. doi: 10.1016/j.intimp.2014.06.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.