Abstract
Objectives
Toll-like receptors (TLRs) and complement are two components of the innate immunity. Factor B (cfB) is essential for the alternative pathway (AP) of complement activation. We have recently reported that cfB is significantly up-regulated in the kidney and may contribute to acute tubular injury in an animal model of sepsis. The current study investigates the mechanisms responsible for the cfB up-regulation and its role in sodium transporter expression in tubular cells during sepsis.
Design
Animal study.
Setting
Laboratory investigation.
Subjects
C57BL/6 J wide-type (WT), cfB−/−, and Nfkb1tm1Bal p50−/− mice.
Interventions
Human proximal tubular cells (HK-2) and mouse tubular epithelial cells (MTECs) were stimulated with TLR agonists. Bay 11-7082 was used to block NF-κB pathway. AP activation was detected by C3 zymosan deposition. Polymicrobial sepsis was created by cecal ligation and puncture (CLP). Sodium transporter gene expression was determined by qRT-PCR.
Measurements and Main Results
The agonists for TLR4 (LPS) or TLR3 (poly I:C) induced a marked increase in cfB expression in HK-2 and MTECs both at gene and protein levels. The TLR1/2 agonist, Pam3cys, induced cfB production only in HK-2 cells, not MTEC. The TLR9 ligand, CpG, failed to induce cfB production either in HK-2 cells or MTEC. LPS/poly I:C-induced cfB up-regulation was blocked by Bay 11-7082, a potent inhibitor of NF-κB signaling, and in MTECs deficient in p50 subunit of NF-κB. Media from the LPS-treated MTEC cultures contained de novo synthesized cfB and led to functional AP activation. In a CLP model, WT septic mice had down-regulated expression of sodium transporters in the kidney compared with the sham. In comparison, cfB−/− mice or mice treated with anti-cfB displayed preserved levels of Na+/K+ ATPase-α1 following sepsis.
Conclusions
1) TLR3/4 activation is sufficient to induce cfB production via NF-κB pathway and to enhance AP activation in the kidney tubular epithelial cells; 2) cfB may contribute to the down-regulation of certain sodium transporter expression during sepsis.
Keywords: complement factor B, acute kidney injury, sepsis, tubular cells, Toll-like receptors
INTRODUCTION
Sepsis is the most common cause of acute kidney injury (AKI) in critically ill patients [1]. There are estimated 700,000 cases of sepsis each year in the United States and more than 225,000 of them die [2]. The rate of acute renal failure is directly proportional to the severity of sepsis. The combination of AKI and sepsis is associated with a 70% mortality rate, as compared with a 45% mortality rate in patients with AKI alone [3]. While septic AKI is a common clinical condition, our understanding of this critical disease and the associated mechanisms is very limited. Evidence indicates that the mechanisms of sepsis-induced AKI are multi-factorial [4], including microvascular blood flow disruption leading to kidney hypoperfusion and hypoxia, and acute tubular cell necrosis and apoptosis induced by inflammation and oxidative stress. But, the mechanism by which local inflammation leads to tubular cell death is unclear. Moreover, renal tubules play an important role in the maintenance of systemic acid-base balance and blood pressure regulation [5]. Tubular epithelial cells transport sodium out of the lumen, and the efficient transport of sodium is necessary for many of the other tubular epithelial functions. Thus, understanding the tubular pathology of septic AKI has significant clinical implication.
Toll-like receptors (TLRs) and complement are two important components of host innate immunity and they interact with each other in health and disease [6]. TLRs recognize both pathogen-associated molecular components [7, 8], such as lipopolysaccharide of gram-negative bacteria and peptidoglycan of gram-positive bacteria, and endogenous danger-associated molecular patterns, such as heat shock proteins and extracellular RNA [9–11]. To date, ten TLR family members in human and thirteen in mice have been identified [12]. Previous studies indicate that TLRs, including TLR2, TLR3, TLR4 and TLR9, play a role in the pathogenesis of sepsis [13–16]. For example, in a sepsis model of AKI, TLR2 and TLR4 reportedly mediate neutrophil migration [17]. TLR9 also plays a contributory role in the development of AKI during sepsis [18, 19].
The complement system is activated by three different but convergent pathways, classical pathway (CP), lectin pathway (LP), and alternative pathway (AP). Each pathway responds to different recognition molecules and converge at C3 cleavage [20]. Activation of the complement system serves to recognize and eliminate invading organisms, but over activation may excessively amplify inflammation and result in tissue damage [21]. The AP is spontaneously activated by hydrolysis of the internal C3 thioester bond and further triggered by microbial molecules and some other proteins [22]. AP inhibition by anti-factor D blocks more than 80% of C5b-9 complex formation induced by CP or LP activation [23, 24], demonstrating the predominant role of AP in the complement activation even when initiated through the other two pathways. The AP also reportedly mediates the complement activation and is responsible for kidney injury during ischemia-reperfusion [25] and inflammatory joint disease [26]. Complement factor B (cfB) is essential for the AP activation. Genetic deletion of cfB abrogates AP activation [27].
We and others have recently shown that upon TLR activation, macrophages [28, 29] and cardiomyocytes [29] have a marked increase in de novo production of cfB. In an animal model of polymicrobial sepsis, our previous study has documented a possible role of cfB in septic AKI. Within 12–24 hours of cecal ligation and puncture, animals develop AKI. At 48 hours, there are marked histological changes that are consistent with cortical tubular necrosis. Animals lacking cfB or treated with a neutralizing anti-cfB antibody have reduced AKI, improved tubular integrity, and better survival [29]. These data demonstrate an important role of cfB in the pathogenesis of AKI during severe sepsis. However, the underlying mechanism by which local cfB is upregulated and then contributes to tubular injury in septic kidney remains unexplored.
In the current study, we tested the hypothesis that TLRs mediate cfB expression in renal tubular cells and that cfB contributes to the down-regulation of the sodium transporter expression in the kidney during polymicrobial sepsis. We found that activation of TLR3 and TLR4 induced cfB production in renal tubular cells and led to AP activation. Compared to WT mice, mice deficient in cfB had preserved expression of the sodium transporter Na+/K+−2Cl− co-transporter (NKCC2) and Na+/K+-ATPase-α1 in the kidney during polymicrobial sepsis. Mice treated with anti-cfB antibody also had a preserved effect in Na+/K+ ATPase-α1 mRNA level in sepsis.
MATERIALS AND METHODS
Reagents
Lipoteichoic acid (LTA), lipopolysaccharides (LPS), Type 1A collagenase, transferrin, 3′3′5-Triiodo-L-thyronine (T3), hydrocortisone, amphotericin B, TRIzol reagent, and Bay 11-7082 were purchased from Sigma-Aldrich (St Louis, MO). Pam3cys-Ser-(Lys)4 (Pam3cys), polyinosinic-polycytidylic acid (poly (I:C)) and ODN1826 (CpG) were from Enzo Life Science (Farmingdale, NY). RPMI 1640, 0.5% trypsin-EDTA, penicillin/streptomycin (P/S) and DMEM/F12 were from Corning (Manassas, VA). Fetal bovine serum (FBS) and insulin were from Gibco (Grand island, NY). Phosphatase inhibitor and protease inhibitor cocktail tablets were from Roche Diagnostics Corporation (Indianapolis, IN).
Animals
All animal experiments were performed with the approval of the Subcommittee on Research Animal Care of Massachusetts General Hospital (Boston, Massachusetts). 8–12 week-old age- and gender-matched mice were used for the studies. Wild-type (WT) (C57BL/6) and NF-κB p50−/− (B6.Cg-Nfkb1tm1Bal) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). cfB−/− mice were kindly provided by Dr. Xiaobo Wu of Washington University at St. Louis. All mice were housed under temperature-controlled and pathogen-free conditions, and maintained in individual cages. The animal rooms were set at 12h light/12h dark cycles and the mice were fed with the same bacteria-free diet (Prolab Isopro RMH 3000; LabDiet, Brentwood, MO).
Human proximal tubular epithelial cell (HK-2)
HK-2 cell line, derived from normal human kidney, was purchased from American Type Culture Collection (Cat.CRL-2190, Manassas, VA) and cultured in RPMI 1640 containing 2% FBS and 1% penicillin/streptomycin. Cells were incubated in a T-75 flask at 37°C in a humidified air with 5% CO2 and allowed to reach 70–80% confluence before each passage.
Mouse tubular cell (MTEC) isolation and culture
Renal tubular epithelial cells were isolated from mice and cultured as previously described [30]. In brief, mice were euthanized with CO2 and the kidneys were isolated from WT or NF-κB p50−/− mice. Cortical tissues were minced with sterile blades after removing the capsule. After washed once with PBS, the tissue sections were mixed with 6 ml of type 1A collagenase solution (1 mg/ml in PBS) and incubated at 37°C for 30 min with vortex every 10 minutes. The cell suspension was then passed sequentially over 100-μm, 70-μm, 40-μm cell strainers (BD Falcon, Franklin Lakes, New Jersey). The filtered cells were centrifuged at 1000 rpm for 5 minutes and cultured in DMEM/F12 medium containing 2% heat-inactivated FBS, 1% penicillin/streptomycin, 5mg/L insulin, 5mg/L transferrin, 10−12 M T3, 40ng/L hydrocortisone, 2.5mg/ml amphotericin B. Culture media were changed at 48–72 h after seeding. Cell cultures were ready for experiments at 80% confluence.
qRT-PCR analysis
Total RNA was extracted from cells or tissues using TRIzol reagent. RNA concentration was determined by NanoDrop (ND-1000) and cDNA transcribed by reverse transcription reaction from 1μg of RNA. The PCR product was quantified by measuring fluorescence intensity generated by binding of SYBR Green dye (Thermo Fisher Scientific, Waltham, MA) to double-stranded DNA. All specific primers used are listed in Table 1. The transcript levels of cfB, TLRs, sodium transporters, i.e., renal outer medullary potassium channel (ROMK), Na+/K+−2Cl− co-transporter (NKCC2), epithelial sodium channel (ENaC-α), Na+/K+-ATPase -α1, and Na+/H+-exchanger (NHE3), were determined as relative gene expression normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the relative Ct method [31].
Table 1.
Primer sequence for qRT-PCR
| Gene | Primer Sequence for qRT-PCR |
|---|---|
| Mouse ROMK | Forward: 5′-TCA GAC CAA TAT AAA CTT-3′ Reverse:5′-TGG GAA AGA GTT TCT GCCG-3′ |
| Mouse NKCC2 | Forward:5′-ATC TTT GCT TTT GCC AAT GC-3′ Reverse:5′-ATG TCA TTG GTT GGA TCC AC-3′ |
| Mouse NHE3 | Forward:5′-GTA GTT GTG TGC CAG GTT CTC-3′ Reverse:5′-GGA ACA GAG GCG GAG GAG CAT-3′ |
| Mouse Na+/K+-ATPase _α1 | Forward:5′-CTC CAG CAA CAG GAC CCG GCG-3′ Reverse:5′-GAT CTC AGC GGC CCT TGC AGG-3′ |
| Mouse ENaC-α | Forward:5′-TAC GCG ACA ACA ATC CCC AAGTGG-3′ Reverse:5′-ATG GAA GAC ATC CAG AGA TTGGAG-3′ |
| Mouse TLR2 | Forward:5′-AAGGCATTAAGTCTCCGGAATTATC-3′ Reverse:5′-TCGCTTAAGTGAAGAGTCAGGTGAT-3′ |
| Mouse TLR3 | Forward:5′-CTGCACGAACCTGACAGAAC-3′ Reverse:5′-CGCAACGCAAGGATTTTATT-3′ |
| Mouse TLR4 | Forward:5′-TGACTTCATTCAAGACCAA-3′ Reverse:5′-TCCTTCCATGATAGAGGGT-3′ |
| Mouse TLR9 | Forward:5′-CTCTGAGAGACCCTGGTGTG-3′ Reverse:5′-CTCACAGGGTAGGAAGGCAG-3′ |
| Mouse GAPDH | Forward:5′-AGAACATCATCCCTGCATCC-3′ Reverse:5′-CACATTGGGGGTAGGAACAC-3′ |
Immunoblotting of medium and cellular proteins
To detect cfB protein, cell culture media were mixed with SDS sample buffer and separated in a 4–12% gradient Criterion™ XT Bis-Tris Precast gel (Bio-Rad, Hercules, CA) and transferred to PVDF membrane (Bio-Rad, Hercules, CA). The membranes were blocked in 5% nonfat dry milk in Tris buffer (Bioproduct, Boston) with 0.1% Tween-20 for 1h at room temperature. The membrane was incubated with goat anti-human/mouse complement factor B (CompTech, Tyler, Texas) antibody diluted in 5% non-fat milk at 4 °C overnight. HRP-conjugated donkey anti-goat IgG was used as secondary antibody (Sigma Aldrich, St Louis, MO). To determine phospho-IκBα and total IκBα in HK-2 cells, cells were passaged to 6-well plate and ready for treatment when reached 80% confluences. Bay 11-7082 or DMSO (final concentration of 0.2%) was added to each well containing medium RPMI 1640 with 0.05% BSA at a final concentration of 10 μM, and incubated at 37°C in a 5% CO2 incubator for 3–4 hours. LPS (100 μg/ml) or poly (I:C) (25 μg/ml) was added to the cell cultures in the presence of DMSO or Bay 11-7082. The reaction was terminated by adding cold DPBS to the cell cultures. After washing with cold DPBS 3 times, cells were lysed in NP-40 lysis buffer containing phosphatase and protease inhibitors and were detached with a cell scraper. The lysates were subjected to SDS-PAGE and immunoblotting of phospho-IκBα and total IκBα (Cell Signaling Technology, Danvers, MA). To quantify specific protein levels, protein bands were visualized in a chemiluminescence detection system (Bio-Rad ChemiDOC™ XRS+) using Luminata Western HRP substrate (Millipore, Billerica, MA). The chemilminescent density of protein bands was quantified using an NIH Image J software.
AP activation
AP activation was determined by measuring C3 deposition on zymosan as described previously with minor modifications [29]. MTECs were treated with LPS or CpG for 48h. Culture media were collected and incubated with zymosan (107 particles) in the presence of 10 mM of EGTA (to block the classical and lectin pathway), 5 mM of Mg2+ and, 10% cfB−/− serum (containing all complement factors except cfB) for 1h at 37°C. The zymosan was spun down and resuspended in 100 μl of FITC-conjugated goat anti-mouse complement C3 (Cappel Research Products, Durham, N.C.) diluted 1:100, and mean fluorescence intensity was defined using flow cytometry.
Mouse model of polymicrobial sepsis
The sepsis model was generated by cecal ligation and puncture (CLP) as previously described [13]. Mice were anesthetized with ketamine (120 mg/kg) and xylazine (4 mg/kg). The fur was removed and disinfected with 70% ethanol. The abdominal cavity was opened in layers in the midline with an 1.5 cm incision. The cecum was ligated at 1 cm from the tip using 5-0 silk suture, and punctured through to through in the middle with a 18-gauge needle. A small amount of faeces (around 1 mm height) was squeezed out of the puncture site gently before the cecum was returned back to the abdominal cavity. Sham-operated mice underwent laparotomy but without CLP. After surgery, 0.05 ml/g (body weight) pre-warmed normal saline was administered subcutaneously. Postoperative pain control was managed with subcutaneous injection of bupivacaine (3 mg/kg) and buprenorphine (0.1 mg/kg).
In vivo anti-cfB antibody administration
The monoclonal anti-mouse cfB (mAb1379) was administrated as previously described [29]. One mg of mAb1379 or control mouse IgG was administrated by peritoneal injection at 1h before surgery in both sham and CLP group. Kidneys were harvested 12h after surgeries, frozen in liquid nitrogen immediately and stored at −80 °C for future analysis.
Statistical analysis
Statistical analysis was performed using Prism 5 software (GraphPad software Inc., La Jolla, CA). The distribution of the continuous variables was expressed as the mean ± standard error. Data were analyzed by one-way ANOVA with Tukey or two-way ANOVA with Bonferroni post hoc tests for statistical significance. Of note, these specific comparisons were made based on a priori hypotheses rather than pure statistic consideration. The null hypothesis was rejected for P < 0.05 with the two-tailed test.
RESULTS
Activation of TLRs leads to cfB production in renal tubular cells
We treated human proximal tubular epithelial cells (HK-2) and primary cultures of mouse renal tubular epithelial cells (MTEC) with various TLR ligands and measured cfB mRNA and protein expression. As illustrated in Fig. 1A, activation of TLR1/2 by Pam3cys, TLR3 by poly (I:C) and TLR4 by LPS, but not TLR2/6 by LTA or TLR9 by CpG, induced a significant increase in cfB mRNA expression in HK-2 cells. Similarly, in MTECs, both poly (I:C) and LPS induced a significant increase in cfB mRNA expression although with different kinetics (Fig. 1B). To validate the mRNA data, we further tested the dose-response effect of the LPS and poly I:C on cfB protein expression. Consistent with the mRNA data, LPS and poly (I:C) induced a dose-dependent increase in cfB protein expression in HK-2 cells. Similarly, Pam3cys, but not LTA or CpG, induced modest cfB protein expression in HK-2 cells (Fig. 1C). Finally, consistent with mRNA data in MTECs (Fig. 1B), LPS and poly (I:C), but not CpG or Pam3cys, induced a substantial increase in cfB protein expression (Fig. 1D). Taken together, these data demonstrated that activation of TLR3 and TLR4, but not TLR9, induced cfB de novo production as well as secretion in both human and mouse proximal tubular cells, whereas activation of TLR1/2 only induced cfB production in human but not mouse tubular cells.
Fig 1. TLR ligands induce cfB production in kidney tubular cells.
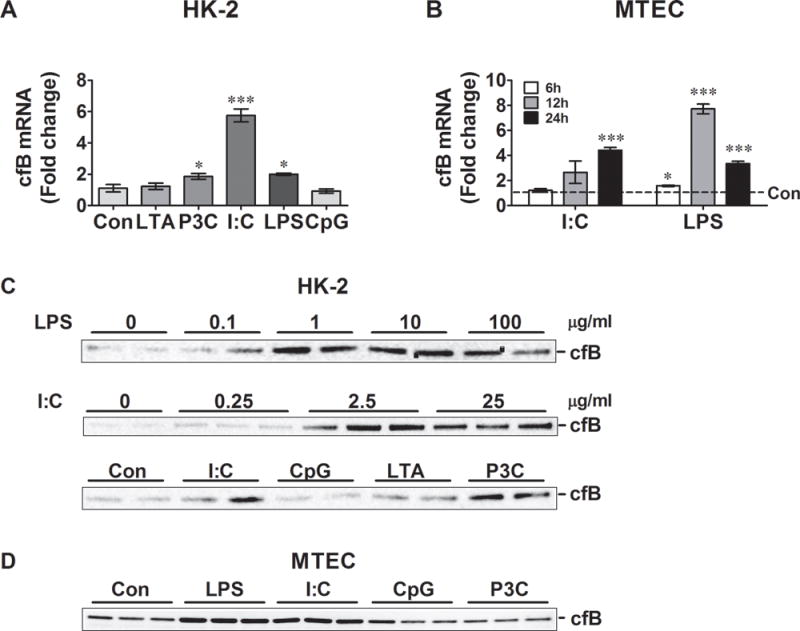
A. cfB mRNA production in HK-2 cells. HK-2 cells were stimulated with LTA (2 μg/ml), Pam3cys (1 μg/ml), poly(I:C) (25 μg/ml), LPS (100 μg/ml) or CpG (0.25 μM) for 6 hours, respectively. cfB mRNA expression was quantified by qRT-PCR. N=3–4 samples/group. *P< 0.05, ***P< 0.001 versus control. B. cfB mRNA production in MTECs. MTECs were cultures stimulated with poly(I:C) (25μg/ml), LPS (500ng/ml) for 6, 12, or 24 hours, respectively. N=3–4 samples/group. *P< 0.05, ***P< 0.001 versus control. C. cfB protein in HK-2 cell culture media. HK-2 cell cultures were stimulated with various doses of poly(I:C) or LPS. In some experiments, HK-2 cells were treated with Pam3cys (1 μg/ml), LTA (2 μg/ml), poly(I:C) (25 μg/ml), or CpG (0.25 μM) for 48 hours. At the end of the treatment, the medium cfB was detected by Western blot. D. cfB protein MTEC culture media. MTECs were stimulated with Pam3cys (1 μg/ml), poly(I:C) (25 μg/ml), LPS (500 ng/ml), or CpG (0.25 μM) for 48 hours. All the experiments were repeated at least twice. HK-2 = human proximal tubular epithelial cell line, MTEC = mouse tubular epithelial cell, LTA = lipoteichoic acid, P3C = Pam3cys-Ser-(Lys)4, I:C = poly(I:C), LPS = lipopolysaccharide, Con = control.
LPS-induced cfB expression is functionally capable of activating alternative pathway
We sought to determine whether or not the TLR-mediated cfB production could enhance AP activation. To achieve this, we collected the culture media of MTECs that were treated without or with LPS or CpG for 48h, incubated the media with cfB−/− mouse serum and zymosan to detect AP activation in the presence of Mg2+ and EGTA. AP activation was measured by C3 deposition into zymosan as determined by flow cytometry [29]. As shown in Fig. 2, the media of LPS-treated cells, which contained elevated levels of cfB (Fig. 1B, D), markedly increased C3 deposition on zymosan (891±72.8 vs. 555±7.5, MFI, P<0.01). On the hand, media from CpG-treated MTECs, which had the same levels of cfB as untreated cultures (Fig. 1D), failed to facilitate the AP activation (Fig. 2). This data suggests that the newly synthesized cfB in MTEC following TLR4 activation is functionally active by enhancing the complement AP activation.
Fig. 2. cfB released from LPS-treated MTECs leads to activation of the complement alternative pathway.
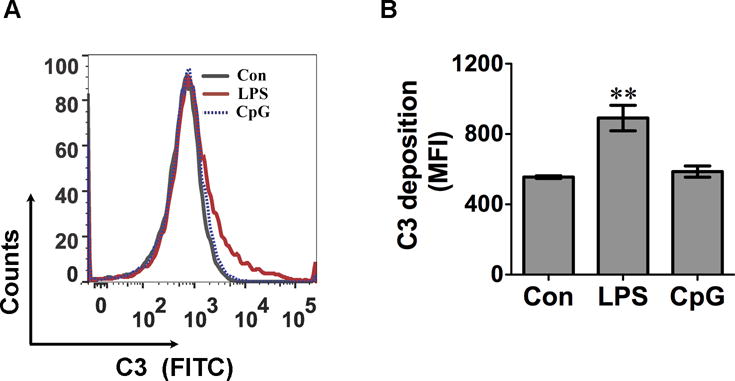
MTECs were stimulated with 500 ng/ml of LPS or 0.25 μM of CpG for 48 hours respectively. Media were collected and incubated with zymosan in the presence of 5 mM of Mg2+, 10 mM of EGTA, and 10% cfB−/− serum at 37 °C for 1 hour. C3 deposition on zymosan was detected by flow cytometry. A. Representative histogram of C3 zymosan deposition. B. Accumulated data of C3 zymosan deposition. The data are presented as mean fluorescence intensity (MFI). N = 3 in each group. **P<0.01 versus control (Con). The experiment was repeated twice. MTEC = mouse tubular epithelial cell, FITC = fluorescein isothiocyanate.
TLR ligand-induced cfB production is NF-κB dependent
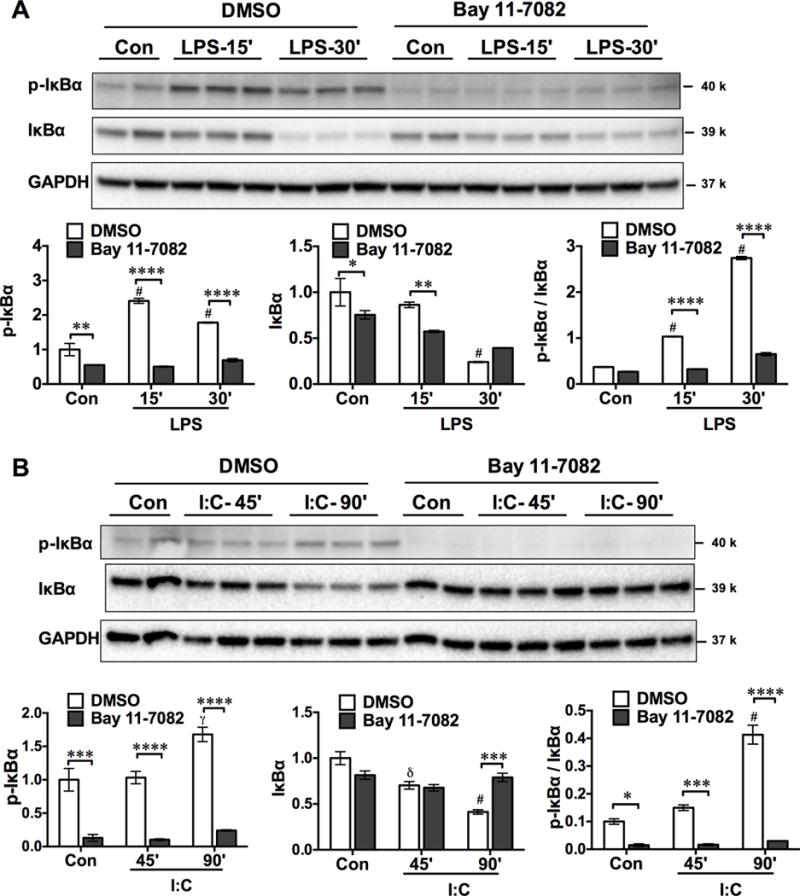
NF-κB is the major downstream effector of TLR signaling. To test the involvement of NF-κB in TLR-induced cfB expression, we treated HK-2 cells with Bay 11-7082, an inhibitor of IκB kinase (IKK), and a key enzyme that controls NF-κB signaling. Activation of IKK leads to phosphorylation as well as degradation of IκBα, which results in NF-κB nuclear translocation and transcription activation. To ensure the efficacy of Bay 11-7082 in blocking the IKK-IκBα-NF-κB signaling, we tested phosphorylation and degradation of IκBα in HK-2 cells treated with LPS or poly I:C in the absence or presence of Bay 11-7082. As illustrated in Fig. 3A–B, in the absence of Bay 11-7082 (DMSO control), treatment with LPS or poly I:C led to phosphorylation and degradation of IκBα although each followed a different kinetics. At 30 min and 90 min, respectively, LPS and poly I:C induced a marked increase in the ratio of phospho-IκBα/IκBα. Bay 11-7082 almost completely blocked the phosphorylation and degradation of IκBα, demonstrating its strong inhibitory effect on the IKK-IκBα-NF-κB signaling.
Fig. 3. Bay 11-7082 inhibits NF-κB signaling.
HK-2 cells were preincubated with DMSO (0.2%) or Bay 11-7082 (10 μM) for 3–4 h before the treatment with LPS (100 μg/ml) (A) or poly (I:C) (25 μg/ml) (B) for the indicated time. At the end of the treatments, cells were lysed and tested for phospho-IκBα, total IκBα, and GAPDH. The expression levels of p-IκBα and total IκBα were presented as fold change to the untreated control in DMSO group. The ratio of p-IκBα/IκBα was calculated to quantify the inhibitory effect of Bay 11-7082 on IKK activity. * P < 0.05, ** P<0.01, *** P <0.001, **** P<0.0001, versus DMSO. δ P <0.05, γ P <0.01, # P<0.001 versus control. n=2–3 samples. Con=control, LPS = lipopolysaccharide, I:C= poly (I:C).
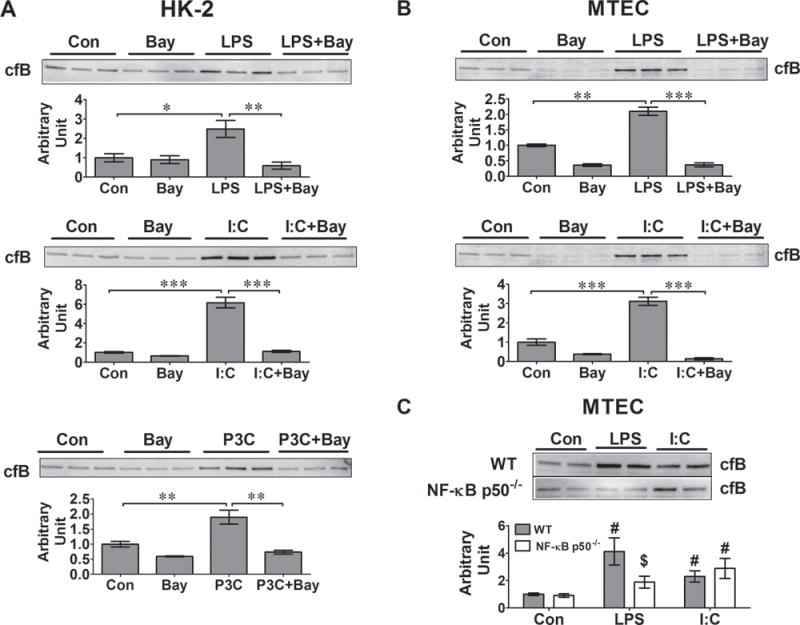
We found that treatment of HK-2 cells and MTEC with Bay 11-7082 completely blocked the TLR ligand (LPS and poly I:C)-induced cfB production in both HK-2 cells and MTECs (Fig. 4A–B). These data suggest that LPS- and poly I:C-induced cfB production in both human and mouse renal tubular cells is NF-κB dependent. To further confirm the role of NF-κB in the TLR ligand-induced cfB expression in MTECs, we isolated MTECs from both WT and NF-κB p50−/− mice, and treated the cells with different TLR ligands. Forty-eight hours after the treatment, LPS induced robust cfB production in WT but not in NF-κB p50−/− MTECs. In comparison, poly (I:C) markedly increased cfB production in both WT and NF-κB p50−/− MTECs (Fig. 4C). These data suggest that LPS- but not poly (I:C)-induced cfB production in MTECs is dependent on the NF-κB p50 subunit.
Fig. 4. TLR-induced cfB synthesis is NF-κB-dependent in kidney tubular cells.
NF-κB inhibitor, Bay 11-7082 (10 μM), was added 30 min prior to the TLR ligand treatments in HK-2 cell or METCs. LPS (100 μg/ml in HK-2 cells, and 500 ng/ml in MTECs), poly (I:C) (25 μg/ml) or Pam3cys (1 μg/ml). Twenty-four hours later, the culture media were collected and cfB was detected by Western blot. A. Effect of Bay 11-7082 on cfB production in HK-2 cells. *P<0.05, ** P<0.01, *** P<0.001. N=3 in each group. B. Effect of Bay 11-7082 on cfB production in MTECs. ** P<0.01, *** P<0.001. N=3 in each group. C. Effect of NF-κB p50 deficiency on LPS- or poly (I:C)-induced cfB production. MTECs were isolated from WT or NF-κB p50−/− mice and stimulated with 25 μg/ml of poly (I:C) or 500 ng/ml of LPS for 48 hours, respectively. Medium cfB was detected by Western blot. The cfB protein bands were quantified using NIH Image J. Con, control. #P<0.01 versus corresponding control, $P<0.05 versus WT-LPS. N = 9 in each group. HK-2 = human proximal tubular epithelial cell line, MTEC = mouse tubular epithelial cell, I:C = poly (I:C), P3C = Pam3cys-Ser-(Lys)4, Bay = Bay 11-7082.
Activation of TLRs leads to cytokine production in renal tubular cells
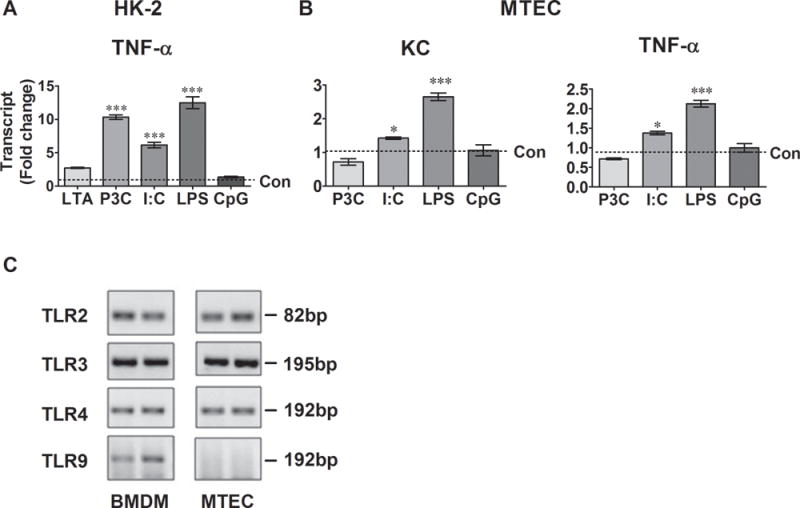
We treated HK-2 cell and MTEC with various TLR ligands and measured cytokine gene expression. As illustrated in Fig. 5A, activation of TLR1/2 by Pam3cys, TLR3 by poly (I:C) and TLR4 by LPS, but not TLR2/6 by LTA or TLR9 by CpG, induced a significant increase in TNF-α gene expression in HK-2 cells compared to the control. Similarly, in MTECs, both poly (I:C) and LPS induced a significant increase in KC and TNF-α gene expression (Fig. 5B). To determine why CpG failed to induce cfB and cytokine production in MTEC, we detected mRNA expression of TLRs in MTECs and compared with mouse bone marrow derived macrophages (BMDM). As shown in Fig 5C, MTECs expressed TLR2, TLR3, and TLR4 mRNA but not TLR9. On the other hand, BMDM expressed all four TLRs.
Fig. 5. TLR ligands stimulate cytokine production in kidney tubular epithelial cells.
A. Cytokine production n HK-2 cells. Cells were stimulated with 2 μg/ml of LTA, 1 μg/ml of Pam3cys, 25 μg/ml of poly (I:C), 100 μg/ml of LPS, or 0.25 μM of CpG for 6 hours. N=3 samples/group. ***P<0.001 versus the control (Con). B. Cytokine production n MTECs. Cells were stimulated with 1 μg/ml of Pam3cys, 25 μg/ml of poly(I:C), 500 ng/ml of LPS, or 0.25 μM of CpG for 6 hours. N=3 samples/group. *P<0.05, *** P<0.001 versus the control. C. Representative picture of TLR transcript products following qRT-PCR. RNA was extracted from MTECs and BMDM. cDNAs were transcribed and amplified with the primers specific for the indicated TLRs. The PCR products were electrophoresed and visualized in a 1.5% agarose gel containing ethidium bromide. The pictures represent the duplicate samples and the experiments were repeated twice. HK-2 = human proximal tubular epithelial cell line, METC = mouse tubular epithelial cell, BMDM = bone marrow-derived macrophage, LTA = lipoteichoic acid, P3C = Pam3cys-Ser-(Lys)4, I:C = poly (I:C), LPS = lipopolysaccharide, Con = control.
Expression of tubular sodium transporters during sepsis
Down-regulation of tubular sodium transporters is strongly associated with tubular dysfunction during inflammation [32]. Our previous study has shown that cfB deficiency leads to reduced acute kidney injury with attenuated tubular cell necrosis in sepsis [29]. To test the possible mechanism by which cfB impacts kidney injury during sepsis, we tested the gene expression levels of several sodium transporters in the kidney after sham or CLP surgeries. As illustrated in Fig. 6A, there was a 33% reduction in Na+-K+-2Cl− co-transporter (NKCC2), a 20% reduction in Na+/K+ ATPase-α1, and a 85% reduction in the renal outer medullary potassium channel (ROMK) at 12 h after CLP procedure in WT mice. There was no change in the expression of the other two transporters ENaC-α and NHE3 in the kidney following sepsis. In comparison, in the cfB-deficient mice, the expression of NKCC2 and Na+/K+ ATPase-α1 was largely preserved in sepsis and maintained at the same level as the sham mice (Fig. 6A). However, cfB deficiency had no impact on the sepsis-induced marked reduction in the ROMK tubular expression.
Fig. 6. Sodium transporter gene expression in the kidney in sham and septic mice.
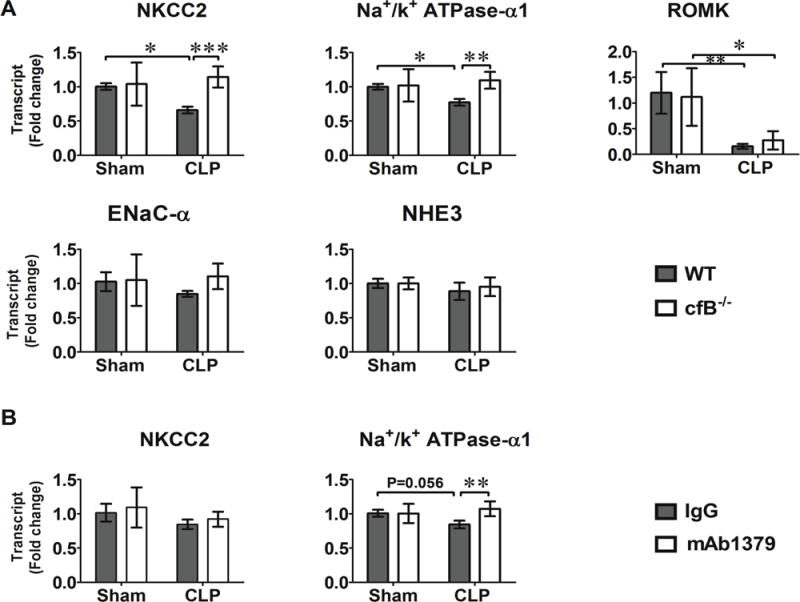
A. Impact of cfB deficiency on the sodium transporter gene expression. WT and cfB−/− mice were subjected to sham or CLP procedures. Mice were euthanized and kidneys harvested 12 hours later. NKCC2, Na+/K+ ATPase-α1, ROMK, ENaC-α, and NHE3 gene expression were determined by qRT-PCR. *P<0.05, **P<0.01, ***P<0.001. N=4 in Sham group, N=6–7 in CLP group. B. Effect of anti-cfB antibody on sodium transport gene expression. One mg of mouse control IgG or anti-cfB antibody mAb1379 was administered i.p.1 hour before sham or CLP surgery. The kidneys were harvested 12 hours later and RNA extracted. NKCC2 and Na+/K+ ATPase-α1 gene expression were determined by qRT-PCR. N=7 in sham or CLP group. **P<0.01. NKCC2 = Na+-K+-2Cl− cotransporter, ROMK = renal outer medullary potassium channel, NHE3 = Na+/H+-exchanger, ENaC-α = epithelial sodium channel.
Anti-cfB mAb1379 preserves Na+/K+ ATPase-α1 expression during sepsis
mAb1379 is an inhibitory mAb specific for mouse cfB [33]. The efficacy of mAb1379 administered one hour prior to CLP procedure is evident as serum from mAb1379-, but not control IgG-treated, mice completely inhibits AP activation in an in vitro zymosan-based AP assay [29]. When given 1 h before and again at 12 h after surgery, mAb-1379-treated mice have reduced acute kidney injury [29]. To assess the contribution of cfB/AP activation to sodium transporter down-regulation in sepsis, mice were treated with anti-cfB antibody (mAb1379) or control IgG 1 h before sham or CLP procedures. Kidneys were harvested at 12 h after surgeries and NKCC2, Na+/K+-ATPase-α1 gene expression were detected. As shown in Fig. 6B, treatment with anti-cfB mAb1379 preserved the expression of Na+/K+ ATPase-α1 mRNA level, but had no impact on the NKCC2 expression within the same time period.
DISCUSSION
We have made several findings in the current study. We show that activation of TLR3 or TLR4, but not TLR9, leads to marked cfB production in both human and mouse kidney tubular cells. The effect of TLR activation appears mediated via NF-κB pathway as Bay 11-7082, a potent IKK inhibitor, or genetic deficiency of the NF-κB p50 subunit eliminates the TLR-induced effects. We demonstrate that the enhanced cfB expression in and secretion from the tubular cells is biologically activity since the de novo synthesized cfB in the media significantly enhances the complement AP activation. Finally, we demonstrate that during severe polymicrobial sepsis, several tubular sodium transporters including NKCC2, Na+/K+ ATPase-α1, and ROMK were significantly down-regulated. Systemic deficiency of cfB, which is known to attenuate systemic inflammation, reduce acute kidney injury, and improve overall survival [29], protects against the loss of NKCC2 and Na+/K+ ATPase-α1expression. Administration of anti-cfB antibody, which attenuates kidney injury in sepsis [29], also preserves Na+/K+ ATPase-α1. Collectively, these data suggest that upon the activation of TLR3/4, kidney tubular cells are capable of producing significant amount of cfB locally and that cfB and AP activation may contribute to the observed down-regulation of sodium transporters on tubular epithelial cells during bacterial sepsis.
Our studies demonstrate that activation of TLR3 and TLR4, but not TLR9, results in cfB synthesis in both HK-2 cells and MTECs and lead to AP activation. Activation of TLR2 only induces cfB in HK-2 cell but not in MTECs. The lack of cfB response to TLR9 agonist is consistent with the absent cytokine production in the same cell type and probably due to the lack of TLR9 expression in MTECs. qRT-PCR data indicate that MTECs express the transcripts for TLR2, TLR3 and TLR4 but not for TLR9. These data are consistent with a previous report that MTECs possess TLR1, TLR2, TLR3, TLR4 and TLR6, but not TLR5 or TLR9 [34]. These data not only demonstrate the capability of renal tubular cells to produce cfB, but also exemplify the interaction of the two innate immune components – activation of TLRs promotes the complement AP activation. This is potentially significant as previous studies have shown that locally produced complement proteins may play an important role in kidney injury [35–38]. One such example is that the kidney-derived local complement amplifies inflammation following ischemia-reperfusion injury and kidney transplant rejection [39–41]. In animal models of polymicrobial sepsis, we reported that cfB expression in the kidney is markedly increased and primarily located in the tubular cells of the cortex and some in the medulla [29]. Renal tubular epithelial cells are a source of complement production and highly sensitive to injury [42]. Systemic deletion of cfB results in reduced tubular cell necrosis during sepsis [29]. Supporting these in vivo observations, the current study extends these findings and demonstrates the critical role of TLR3 and TLR4 in promoting the de novo synthesis of cfB in renal tubular epithelial cells and their role in the AP activation in the kidney cells.
Our data demonstrate the NF-κB inhibitor can block TLR ligand-induced cfB upregulation in renal tubular cells. This observation suggests that NF-κB family plays an essential role in the transcriptional activation of cfB gene expression. The NF-κB is the downstream molecule of TLR signaling pathway and controls a large numbers of target genes involved in inflammation [43]. TLR3 signaling is mainly through Trif pathway and induces the activation of two transcription factors: NF-κB and IRF-3 [44]. The immediate early gene induction by poly (I:C) was through TLR3, NF-κB, and IRF signaling pathways. The Bay compound completely blocks IKK activity and poly(I:C)-induced cfB production, suggesting that NF-κB is the key pathway for poly(I:C)-induced cfB synthesis. Using MTECs from NF-κB p50−/− mice, we found that NF-κB p50 plays an essential role in cfB synthesis in response to LPS, but the synthesis of cfB following poly (I:C) is independent of p50. This may not be surprising since there are five members of NF-κB family in mammalian cells: Rel A, Rel B, C-Rel, p50/p105 and p52/p100. p50 is derived from the larger precursor p105 [45] and the deletion of p50 may not be sufficient to block the poly (I:C)-induced NF-κB signaling and thus cfB production. In comparison, the Bay agent completely inhibits the IκBα phosphorylation and blocks NF-κB activation as demonstrated previously [46] and again in the current study. It is worth noting that NF-κB activation is also critical in LPS- or DNA-induced cfB gene induction in macrophages [28, 47] and that both p50 and p65 contribute to TNF-α-induced cfB expression in macrophages [48].
The current data also demonstrate the ability of tubular epithelial cells to produce cytokines in response to activation of TLR1/2, TLR3 or TLR4. These TLRs are activated during bacterial or viral infection and known to play a role in the development polymicrobial sepsis [13–15]. It is possible that in response to pathogen invasion and through TLR-dependent mechanisms, renal tubular cells produce pro-inflammatory cytokines. However, whether or not these locally produced cytokines and chemokines play a role in renal inflammation and injury to tubular cells remains unclear.
The kidney is an important organ that maintains the homeostasis in the body. Renal tubules play a particularly critical role in sodium reabsorption and are prone to various insults such as hypoperfusion and ischemic injury, bacterial infection, and septic shock. We [29] and others [49] have previously shown acute tubular necrosis during polymicrobial sepsis as evidenced by vacuolization and dysfunction of tubular cells. In comparison, animals lacking cfB display improved tubular integrity and less severe AKI [29]. To further understand the molecular basis for these observations, the current study tested the role of cfB in the expression levels of renal tubular sodium transporters in sham and septic mice. We found that there was a significant decrease in NKCC2, Na+/K+ ATPase-α1 and ROMK in WT mice subjected to polymicrobial sepsis, while others such as ENaC-α or NHE3 levels remained unchanged. However, while down-regulation of tubular sodium transporters is strongly associated with tubular dysfunction during endotoxin-induced inflammation [32], it remains to be determined whether or not the down-regulation of these sodium transporters is responsible for polymicrobial sepsis-induced AKI or sufficient to cause any tubular dysfunction. Each of these sodium transporters is responsible for the sodium reabsorption and has distinct expression patterns at the different segment of the kidney. NKCC2 mediates 50% of all sodium transport at the apical membrane in the thick ascending limb of the loop of Henle [50]. The Na+/K+ ATPase is a trans-membrane protein and transports Na+ out and K + into the tubular cells [51]. ROMK is an ATP-dependent potassium channel and is responsible for K+ secretion and control of NaCl reabsorption [52]. ENaC-α is the epithelial sodium channel located in the distal tubule and mediates sodium entry through the apical membrane of renal epithelial cells. NHE3 is expressed at the apical membrane of the proximal tubule and the thick ascending limb, and mediates sodium entry. The finding that septic cfB−/− mice had preserved expression of NKCC2 and Na+/K+ ATPase-α1 as compared with the septic WT mice suggests that cfB may play a role, directly or indirectly, in the down-regulation of these sodium transporters during polymicrobial sepsis. However, how cfB regulates the sodium transporter expression is unclear and remains to be investigated.
In this study, we used proximal tubular cell (HK-2) for its human relevance and thus focused a major portion of the in vitro studies in the cell line. However, in order to delineate the signaling mechanism, we also established mouse tubular epithelial cell (MTEC) primary cultures in the lab so we could take advantage of genetically modified models such as NF-κB p50−/− and cfB−/− for the in vivo study. Both human and mouse tubular cells act similarly in their response to TLR3 and TLR4 agonist, but not TLR9 agonist, to produce cfB. However, different from human tubular cells, mouse tubular cells failed to respond to TLR1/2 agonist. The reason for the difference is unlear.
In summary, our recent study has established the important contribution of complement factor B to the pathogenesis of sepsis-induced acute kidney injury [29]. However, the mechanisms underlying the cfB-mediated septic kidney injury has not been explored. The current investigation extends the study and demonstrates that activation of TLR3 and TLR4, both known for their important role in bacterial sepsis, leads to significant de novo production of complement factor B via a NF-κB-dependent mechanism in renal tubular cells. Moreover, in a mouse model of polymicrobial sepsis, we show a significant down-regulation of several tubular sodium transporters important for maintaining sodium and potassium hemostasis. Using genetically modified mouse models and specific antibody, we demonstrate that cfB plays an important role in the down-regulation of these sodium transporters during polymicrobial sepsis. Thus, the current study provides a mechanistic insight into how factor B is up-regulated in the kidney and may contribute to renal tubular injury during bacterial sepsis. Understanding the molecular mechanisms responsible for acute kidney injury during severe sepsis may have potential implication in the pathogenesis and treatment of this critical disease.
Acknowledgments
Disclosure: This work was supported in part by the National Institutes of Health (NIH, Bethesda, Maryland) grant GM097259 (WC), DK076690 (JMT) and a mentored research award from International Anesthesia Research Society (San Francisco, California) (LZ).
Copyright form disclosures: Dr. Chao received support for article research from the National Institutes of Health (NIH). His institution received grant support from the NIH. Dr. Zou received support for article research from the International Anesthesia Research Society. Her institution received grant support from the International Anesthesia Research Society. Dr. Thurman received support for article research from the NIH and consulted for Baxter Pharmaceuticals and Alexion Pharmaceuticals, Inc. Dr. Thurman and his institution received royalties from Alexion Pharmaceuticals, Inc. His institution received grant support from the NIH.
Footnotes
This work is attributed to the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20(6):588–595. doi: 10.1097/MCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marik PE. Surviving sepsis: going beyond the guidelines. Ann Intensive Care. 2011;1(1):17. doi: 10.1186/2110-5820-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 4.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura M, Shirai A, Yamazaki O, Satoh N, Suzuki M, Horita S, Yamada H, Seki G. Roles of renal proximal tubule transport in acid/base balance and blood pressure regulation. BioMed research international. 2014;2014:504808. doi: 10.1155/2014/504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296(1):H1–H12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, Chao W. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem. 2011;286(36):31308–31319. doi: 10.1074/jbc.M111.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Feng Y, Zou L, Wang L, Chen HH, Cai JY, Xu JM, Sosnovik DE, Chao W. Role of Extracellular RNA and TLR3-Trif Signaling in Myocardial Ischemia-Reperfusion Injury. Journal of the American Heart Association. 2014;3(1):e000683. doi: 10.1161/JAHA.113.000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Y, Chen H, Cai J, Zou L, Yan D, Xu G, Li D, Chao W. Cardiac RNA induces inflammatory responses in cardiomyocytes and immune cells via Toll-like receptor 7 signaling. J Biol Chem. 2015 Sep 11; doi: 10.1074/jbc.M115.661835. pii: jbc M115.661835 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim KH, Staudt LM. Toll-like receptor signaling. Cold Spring Harb Perspect Biol. 2013;5(1):a011247. doi: 10.1101/cshperspect.a011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou L, Feng Y, Chen YJ, Si R, Shen S, Zhou Q, Ichinose F, Scherrer-Crosbie M, Chao W. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2010;38(5):1335–1342. doi: 10.1097/CCM.0b013e3181d99e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. The Journal of experimental medicine. 2008;205(11):2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009;106(7):2348–2352. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205(6):1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castoldi A, Braga TT, Correa-Costa M, Aguiar CF, Bassi EJ, Correa-Silva R, Elias RM, Salvador F, Moraes-Vieira PM, Cenedeze MA, et al. TLR2, TLR4 and the MYD88 signaling pathway are crucial for neutrophil migration in acute kidney injury induced by sepsis. PLoS One. 2012;7(5):e37584. doi: 10.1371/journal.pone.0037584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Li Y, Hu Z, Su J, Huo Y, Tan B, Wang X, Liu Y. Small interfering RNA targeting Toll-like receptor 9 protects mice against polymicrobial septic acute kidney injury. Nephron Experimental nephrology. 2012;122(1–2):51–61. doi: 10.1159/000346953. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. American journal of physiology Renal physiology. 2008;294(5):F1050–1058. doi: 10.1152/ajprenal.00461.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343(1):227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajishengallis G. Complement and periodontitis. Biochem Pharmacol. 2010;80(12):1992–2001. doi: 10.1016/j.bcp.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harboe M, Mollnes TE. The alternative complement pathway revisited. J Cell Mol Med. 2008;12(4):1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clinical and experimental immunology. 2004;138(3):439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harboe M, Garred P, Karlstrom E, Lindstad JK, Stahl GL, Mollnes TE. The down-stream effects of mannan-induced lectin complement pathway activation depend quantitatively on alternative pathway amplification. Molecular immunology. 2009;47(2–3):373–380. doi: 10.1016/j.molimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Amura CR, Renner B, Lyubchenko T, Faubel S, Simonian PL, Thurman JM. Complement activation and toll-like receptor-2 signaling contribute to cytokine production after renal ischemia/reperfusion. Mol Immunol. 2012;52(3–4):249–257. doi: 10.1016/j.molimm.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176(3):1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin DD, Colten HR. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci U S A. 1997;94(16):8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaczorowski DJ, Afrazi A, Scott MJ, Kwak JH, Gill R, Edmonds RD, Liu Y, Fan J, Billiar TR. Pivotal advance: The pattern recognition receptor ligands lipopolysaccharide and polyinosine-polycytidylic acid stimulate factor B synthesis by the macrophage through distinct but overlapping mechanisms. J Leukoc Biol. 2010;88(4):609–618. doi: 10.1189/jlb.0809588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou L, Feng Y, Li Y, Zhang M, Chen C, Cai J, Gong Y, Wang L, Thurman JM, Wu X, et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J Immunol. 2013;191(11):5625–5635. doi: 10.4049/jimmunol.1301903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renner B, Coleman K, Goldberg R, Amura C, Holland-Neidermyer A, Pierce K, Orth HN, Molina H, Ferreira VP, Cortes C, et al. The complement inhibitors Crry and factor H are critical for preventing autologous complement activation on renal tubular epithelial cells. J Immunol. 185(5):3086–3094. doi: 10.4049/jimmunol.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. BioTechniques. 2005;39(1):75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol. 2007;18(4):1072–1083. doi: 10.1681/ASN.2006050454. [DOI] [PubMed] [Google Scholar]
- 33.Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42(1):87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol. 2002;169(4):2026–2033. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- 35.Thurman JM, Lucia MS, Ljubanovic D, Holers VM. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 2005;67(2):524–530. doi: 10.1111/j.1523-1755.2005.67109.x. [DOI] [PubMed] [Google Scholar]
- 36.Lenderink AM, Liegel K, Ljubanovic D, Coleman KE, Gilkeson GS, Holers VM, Thurman JM. The alternative pathway of complement is activated in the glomeruli and tubulointerstitium of mice with adriamycin nephropathy. Am J Physiol Renal Physiol. 2007;293(2):F555–564. doi: 10.1152/ajprenal.00403.2006. [DOI] [PubMed] [Google Scholar]
- 37.Zhou W, Marsh JE, Sacks SH. Intrarenal synthesis of complement. Kidney Int. 2001;59(4):1227–1235. doi: 10.1046/j.1523-1755.2001.0590041227.x. [DOI] [PubMed] [Google Scholar]
- 38.Daha MR, van Kooten C. Is there a role for locally produced complement in renal disease? Nephrol Dial Transplant. 2000;15(10):1506–1509. doi: 10.1093/ndt/15.10.1506. [DOI] [PubMed] [Google Scholar]
- 39.Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20(2):217–226. doi: 10.1096/fj.05-4747com. [DOI] [PubMed] [Google Scholar]
- 40.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8(6):582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 41.Thurman JM, Royer PA, Ljubanovic D, Dursun B, Lenderink AM, Edelstein CL, Holers VM. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol. 2006;17(3):707–715. doi: 10.1681/ASN.2005070698. [DOI] [PubMed] [Google Scholar]
- 42.Bartels K, Grenz A, Eltzschig HK. Sphingosine-1-phosphate receptor signaling during acute kidney injury: the tissue is the issue. Kidney Int. 2014;85(4):733–735. doi: 10.1038/ki.2013.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasschaert J, Ladrière L, Urbain M, Dogusan Z, Katabua B, Sato S, Akira S, Gysemans C, Mathieu C, Eizirik DL. Toll-like receptor 3 and STAT-1 contribute to double-stranded RNA+ interferon-gamma-induced apoptosis in primary pancreatic beta-cells. J Biol Chem. 2005;280(40):33984–33991. doi: 10.1074/jbc.M502213200. [DOI] [PubMed] [Google Scholar]
- 45.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 46.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272(34):21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 47.Kaczorowski DJ, Scott MJ, Pibris JP, Afrazi A, Nakao A, Edmonds RD, Kim S, Kwak JH, Liu Y, Fan J, et al. Mammalian DNA is an endogenous danger signal that stimulates local synthesis and release of complement factor B. Mol Med. 2012;18:851–860. doi: 10.2119/molmed.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Krein PM, Muruve DA, Winston BW. Complement factor B gene regulation: synergistic effects of TNF-alpha and IFN-gamma in macrophages. J Immunol. 2002;169(5):2627–2635. doi: 10.4049/jimmunol.169.5.2627. [DOI] [PubMed] [Google Scholar]
- 49.Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. J Am Soc Nephrol. 2007;18(6):1807–1815. doi: 10.1681/ASN.2006121402. [DOI] [PubMed] [Google Scholar]
- 50.Ares GR, Caceres PS, Ortiz PA. Molecular regulation of NKCC2 in the thick ascending limb. American journal of physiology Renal physiology. 2011;301(6):F1143–1159. doi: 10.1152/ajprenal.00396.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohtomo Y, Ono S, Sahlgren B, Aperia A. Maturation of rat renal tubular response to alpha-adrenergic agonists and neuropeptide Y: a study on the regulation of Na+,K+-ATPase. Pediatr Res. 1996;39(3):534–538. doi: 10.1203/00006450-199603000-00024. [DOI] [PubMed] [Google Scholar]
- 52.Schulte U, Hahn H, Konrad M, Jeck N, Derst C, Wild K, Weidemann S, Ruppersberg JP, Fakler B, Ludwig J. pH gating of ROMK (K(ir)1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc Natl Acad Sci U S A. 1999;96(26):15298–15303. doi: 10.1073/pnas.96.26.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]


