Abstract
In the brain, mitochondrial uncoupling protein 2 (UCP2) has emerged as a stress signal associated with neuronal survival. In the retina UCP2 is expressed primarily by retinal ganglion cells. Here we investigated the functional relevance of UCP2 in the mouse retina. Increased expression of UCP2 significantly reduced apoptosis during the critical developmental period resulting in elevated numbers of retinal ganglion cells in the adult. Elevated UCP2 levels also protected against excitotoxic cell death induced by intraocular injection of either NMDA or Kainic Acid. In monolayer cultures of retinal cells, elevated UCP2 levels increased cell survival and rendered the cells independent of the survival promoting effects of the neurotrophic factors BDNF and CNTF. Taken together these data implicate UCP2 as an important regulator of retinal neuron survival both during development and in adult animals.
Keywords: reactive oxygen species, neuroprotection, excitoxicity, programmed cell death, development, retinal ganglion cell
Regulation of cell number in the nervous system involves complex interaction between the generation of neurons and neuronal death (Oppenheim, 1991; Fraser, et al, 1996; Bahr, 2000; Buss and Oppenheim, 2004; Dekkers et al., 2013). Neurons exhibit a type of cell death that has been referred to as physiological, developmental, or programmed cell death (PCD). This involves the selective loss of significant numbers of cells in a given population, generally thought to be those that have not or only incorrectly formed synaptic connections with target regions (Clarke, 1985).
Retinal Ganglion Cell (RGC) death has been studied as a model to understand natural and pathological cell death under both in vivo and in vitro conditions. (Nurcombe and Bennett, 1981; Cunningham et al, 1982; McCaffery et al, 1982; Dreher et al, 1983 ; Bonfanti et al., 1996 ; Nickells, 2004). RGCs undergo PCD during postnatal development until they reach their adult numbers. The number of RGCs in various strains of adult mice ranges from 45,000 to 76,000, down from numbers of 131,000 to 224,000 at birth (Sefton et al., 1985; Strom & Williams, 1998; Jeon et al., 1998). RGCs also undergo apoptotic cell death after axonal damage, exposure to excitotoxins as well as in diseases such as glaucoma (reviewed in Isenmann et al., 2003; Nickells 2012).
The regulation of developmental RGC death is not fully understood but is linked, in part, to appropriate functional connections to target cells. Neurotrophic factors can delay or reduce RGC death and factors derived from postsynaptic cells may play a regulatory role in the process (McCaffery et al., 1982; Spalding et al., 2004; Murphy and Clarke, 2006; Cooper et al., 2008).
Mitochondrial Uncoupling Proteins (UCP)s are inner mitochondrial membrane proteins that regulate energy metabolism, reactive oxygen species production and mitochondrial calcium levels (Castella, L et al, 2001; Andrews et al., 2005; Trenker et al., 2007; Diano and Horvath, 2012; Sluse 2012). The UCP gene family contains five members, UCP1, UCP2, UCP3, UCP4 and BMCP1 (UCP5), expressed in specific tissue patterns (Ledesma et al., 2002; Kim-Han and Dugan, 2005).
The expression of UCP2 in brain regions that regulate autonomic, metabolic and endocrine processes is an indication of its function in these processes. Modulation of UCP2 expression and function protects neonatal neurons from excitotoxicity by preventing mitochondrial dysfunction (Sullivan et al, 2003; Diano et al., 2003; Andrews et al., 2005; Conti et al., 2005; Trenker et al., 2007). Further, UCP2 prevents neuronal death occurring during epileptic seizures and is upregulated following acute brain injury, a response interpreted as a part of a protective mechanism (Bechmann et al, 2002; Mattiasson et al, 2003; Diano, et al, 2003). Thus, UCP2 acts both as a neuromodulator and neuroprotector in the central nervous system (Horvath, et al., 2003a).
We have previously shown that retinal ganglion cells in the mouse express UCP2 (Barnstable et al., 2003). Here we show that increased expression of UCP2 leads to a decrease in programmed cell death in retinal ganglion cells during the first two postnatal weeks. Increased UCP2 levels also protect ganglion cells from the effects of both neurotrophin withdrawal and excitotoxin treatment, indicating the broad spectrum of protection that this molecule can provide.
MATERIALS AND METHODS
Transgenic and Knockout mice
Transgenic animals were produced as described previously (Horvath et al., 2003b) In brief, an 80-kb human bacterial artificial chromosome (BAC) clone containing the natural genes and promoters was used to produce transgenic mice that overexpress UCP2 and UCP3. The human UCP2 is active as judged by measures of coupling and reactive oxygen species production in the transgenic animals (Horvath et al., 2003b). Male Tg32 transgenic animals and wild-type littermates were used from the sixth or higher generation backcrossed to C57BL/6J. All experimental procedures were approved by the IACUCs of Yale University and Penn State College of Medicine.
Breeding pairs of UCP2 knockout animals were generously provided by Dr. Bradford Lowell, whose laboratory generated this knockout line (Zhang et al., 2001). The knockout mice were generated by insertional mutagenesis using a neomycin gene inserted between two to seven at exon gene seven disrupting the UCP2 gene. For the generation of these mice, a c129/SVJ genomic library was used, and thus, it is anticipated that animals generated by the construct would retain genetic traces from the SV-129 strain regardless of repeated backcrossing on C57BL/6J strain (Zhang et al., 2001). For this reason heterozygous wild type littermates were used as controls for all experiments involving UCP2 KO animals.
UCP2 expression
Total RNA was extracted from retinas and cDNA prepared as previously described (Ahmad et al., 1994). Primers used to amplify either all UCP2 or only human UCP2 are shown in Table 1. The common primers amplified a 396 bp band and the human specific primers a 1046 bp band. The PCR reactions were performed as previously described, reactions were analyzed by gel electrophoresis, and the identity of the bands was confirmed by sequencing of selected samples(Ahmad et al., 1994).
Table 1.
PCR primers used to detect human plus mouse UCP2 or human UCP2 only.
| Common fwd: 5′-CTACAAGACCATTGCACGAGAGG-3′ |
| Common rev: 5′-AGCTGCTCATAGGTGACAAACAT-3′ |
| hUCP2 fwd: 5′-CCGTGAGACCTTACAAAGC-3′ |
| hUCP2 rev: 5′-GGGAGCCTCTCGGGAAGTG-3′ |
ERG recordings and data analysis
Electroretinograms (ERGs) were obtained from eyes of adult male Tg32 transgenic and UCP2 knockout mice (average weight 30-35g and age 10-15weeks) and eyes of wild-type control mice. The ERG recordings used standard methods with little modification (Vistamehr and Tian, 2004). Briefly, after at least 1 hour dark adaptation, animals were anesthetized with a mixture of 100mg/kg ketamine + 10mg/kg xylazine. Pupils were dilated with 2.5% phenylephrine and 1% atropine (Bausch & Lomb Pharmaceuticals, Inc., Tampa, FL). 0.5% proparacaine hydrochloride (Bausch & Lomb Pharmaceuticals, Inc., Tampa, FL) was used before the contact electrodes were applied to the corneas. ERGs were evoked by 100 ms white flashes generated by light-emitting diode (LED) arrays built into a pair of miniature Ganzfield stimulators for both eyes (EPIC-3000, LKC Technologies Inc., Gaithersburg, MD). Signals were band pass filtered between 0.3 Hz to 500 Hz. For each of the intensities between 0.008 cd * s/m2 and 0.8 cd * s/m2, ERGs were averaged from five single flashes with 30s interstimulus interval. ERGs were averaged from three single flashes for the intensities between 2.5 cd * s/m2 and 25 cd * s/m2 with 60s interstimulus interval. The amplitudes ERG components a-wave, b-wave and oscillatory potentials were measured. A one-way ANOVA analysis was used to examine the amplitudes difference among these strains.
Intraocular Injections
Adult mice were anesthetized using avertin (0.03 ml/g body weight). Intraocular injections were administered to the right eye through a 28G needle attached to a 10μl Hamilton syringe inserted near the temporal region. The needle tip was positioned in the vitreous between the lens and retina and 1μl administered slowly over a period of 1–1.5 minutes. The needle was left in place for 2 minutes to minimize reflux on withdrawal. Mice received either vehicle (physiological buffered saline) alone, NMDA, or kainic acid.
Retinal Cultures
Retinal neurons were grown in vitro from PN3 wild type, transgenic and knockout pups as described previously (Han et al., 2001; Wei et al., 2002; Zhang et al., 2008). Retinas were dissected and cells dissociated mechanically by gentle passage through a fire-polished Pasteur pipette. The cells were suspended in DMEM (GIBCO) supplemented with 10% bovine serum, 40 ng/ml BDNF, 40 ng/ml CNTF and 10 μg/ml gentamycin and seeded on poly-L-lysine (50 μg/ml) coated multi-well plates. Parallel sets of cultures were treated with growth factors in each experiment. Cell numbers in the cultures were counted using the mitotracker stain and counterstained with DAPI. The average counts of each set in each group of animals were expressed as mean ± standard deviation. Analysis of the data was carried out using appropriate statistical tests using SPW program. p values < 0.05 were considered significant.
Tissue Processing and Sectioning of the retina
Animals that received intraocular injections were sacrificed 24 hours later. Eyes were collected during the first two weeks of postnatal development of all three groups of animals. Eyes were immersion fixed overnight with cold 4% paraformaldehyde in phosphate buffer (0.1M, pH 7.4). After rinsing with phosphate buffer, eyes were cryoprotected in 30% sucrose in phosphate buffer and then embedded in OCT compound. Cryostat sections of 12 μm thickness were mounted on subbed slides and used for quantification purpose.
Identification of degenerating neurons in the Ganglion Cell Layer using Cresyl Violet (CV) stain
Retinal sections were dehydrated through grades of alcohol before staining with 0.5 % aqueous cresyl violet stain at pH 3.5 - 3.8. After re-hydration and differentiation degenerating neurons could be clearly identified in the ganglion cell layer (GCL).
Immunostaining procedure using anti-caspase-3 and TUNEL
For immunostaining of caspase-3, retinal sections were blocked with PBS containing 5% normal goat serum and stained with caspase-3 antibody (R & D Systems, Inc) for 1 hour at 37°C. Fluorescein-conjugated secondary antibody was used to visualize the labeled cells. Sections were counterstained with DAPI to identify all nuclei.
A TUNEL kit (ApopTag, Emdmillipore, Billerica, MA) was used to localize apoptotic cells and was performed according to the manufacturer’s protocol.
Quantification of Neurons
Cell counts of degenerating neurons were analyzed from PN1 to PN14 in wild type, UCP2 transgenic and UCP2 knockout mice. The average diameter of a ganglion cell is approximately 10-12 μm during development in mice. To avoid sampling the same cells in GCL and to reduce the probability of counting same degenerating cells more than once, every alternate section was used for counting purposes (O’Leary et al, 1986). Serial sections of retina from each eyeball were collected and the mid-peripheral sections were used for quantification purpose. The complete length of retinal section was divided into equal segments and quantification was done in repeated sample units all along the length of retina. The total number of degenerating neurons counted in different samples per retina was summed and represented at each postnatal day studied. We analyzed the retinas of 3 pups at every developmental stage in all three groups of mice. Retinas from adult mice after intraocular injections of NMDA and kainate were also sectioned and quantified using the same procedure.
Statistical Analysis
The mean number of degenerating neurons of the retina was estimated based on previously described methods (Pienado–Ramon et al, 1996). Student’s t-test was used to compare the mean number of degenerating neurons between groups.
RESULTS
UCP2 expression affects the mouse ERG
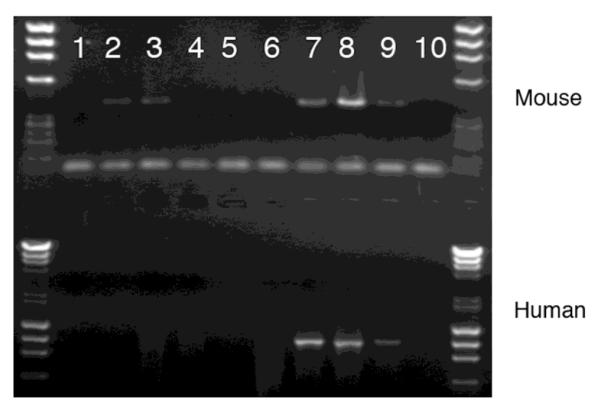
The superficial appearance of the eyes of UCP2 overexpressing transgenic and UCP2 knockout mice was indistinguishable from their wild type controls. Previous studies have documented the normal expression of the UCP2 transgene in many CNS regions using RT-PCR, in situ hybridization and western blots (Diano et al., 2003). To confirm that the UCP2 transgene was expressed in the eye, we used RT-PCR with primers that distinguished human and mouse UCP2 (Figure 1). The results were as expected where wild type animals showed only mouse UCP2, transgenic animals showed bands for both human and mouse UCP2 and the knockouts showed no bands.
Figure 1.
RT-PCR detection of UCP2 expression in mouse retinas. cDNAs were amplified using primers specific for mouse (upper gel) or for human (lower gel) UCP2. Wild type and transgenic mice contain mouse UCP2 RNA but only the transgenic retinas contain human UCP2 RNA. Lanes 1 and 10, negative controls; lanes 2 and 3, wild type mice; lanes 4-6, UCP2 knockout mice; lanes 7-9, UCP2 transgenic mice.
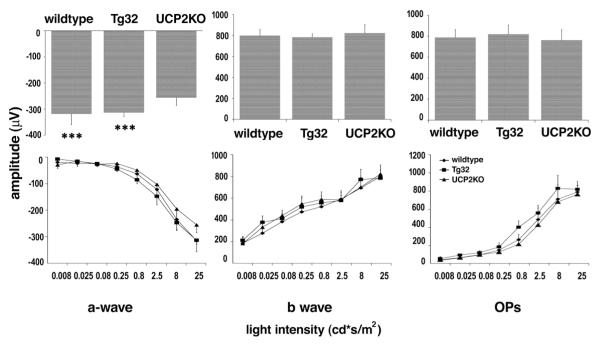
The retinas of the two genetically altered mouse strains were fully functional as shown by the relatively normal ERG profiles obtained (Figure 2). We measured the a-wave which is indicative of photoreceptor function; the b-wave which is indicative of ON bipolar cell function and reflects both photoreceptor and synaptic function in the outer plexiform layer of the retina (Stockton & Slaughter, 1989; Tian & Slaughter, 1995) and the Oscillatory Potential (OP), which reflects interactions among bipolar, amacrine, and ganglion cells, and is a measure of inner retinal function (Wachtmeister, 1998).
Figure 2.
Electroretinograms of wild type, UCP2 transgenic and UCP2 knockout mice. Average amplitudes of ERG a-wave (left), b-wave (middle), and OPs (right) recorded from wild type, Tg32 and UCP2KO mice evoked by 25 cd * s/m2 flash light stimulus. The variation of response with light intensity is shown in the lower traces.
Fig 2 shows the average amplitudes of the ERG a-wave (left), b-wave (middle), and OPs (right) recorded from wild type, Tg32 and UCP2KO mice evoked by 25 cd * s/m2 flash light stimulus. UCP2KO mice achieved a smaller a-wave compared to wild type (19.04% less) and Tg32 (18.53% less). A one-way ANOVA analysis showed that these differences were statistically significant (p<0.001). In the b-wave and oscillatory potential measurements the amplitudes showed no significant difference among the three strains. The amplitudes of all three components varied with the intensity of light stimulus as expected.
Ganglion cell death is modulated by UCP2
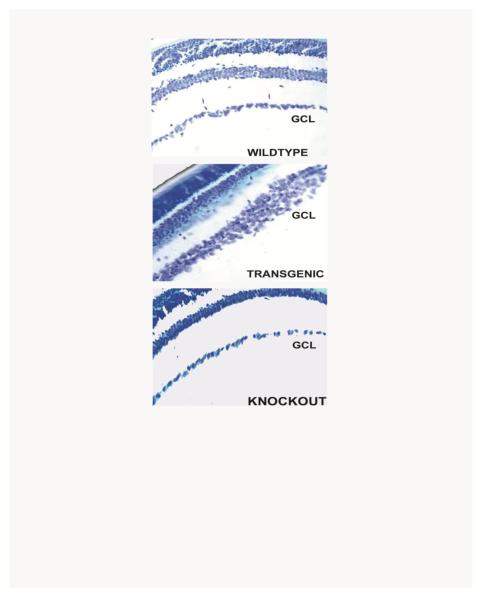
Examining sections of adult mice of the different genotypes revealed differences in the ganglion cell layers (Figure 3). When we used multiple sections from the eyes of three adult mice of each genotype to measure the numbers of neurons in the GCL of wild type, UCP2 transgenic and UCP2 knockout adult mice, we found that UCP2 transgenic mice had 48% more cell bodies in the GCL and knockout mice had 20% fewer cells than wild type controls. The ganglion cell layer of transgenic animals was frequently several cell bodies thick, a phenomenon seen only in immature retinas of normal mice. At random regions along the ganglion cell layer, even thicker clumps of ganglion cells were seen. The results suggest that increased expression of UCP2 can regulate the numbers of neurons in the GCL of adult mice.
Figure 3.
Ganglion cell layers of adult mice. Sections of adult retina from wild type, UCP2 transgenic, and UCP2 knockout mice. The only difference detectable at this level of resolution is an increased thickness of the ganglion cell layer in the UCP2 transgenic animals. GCL = Ganglion cell layer.
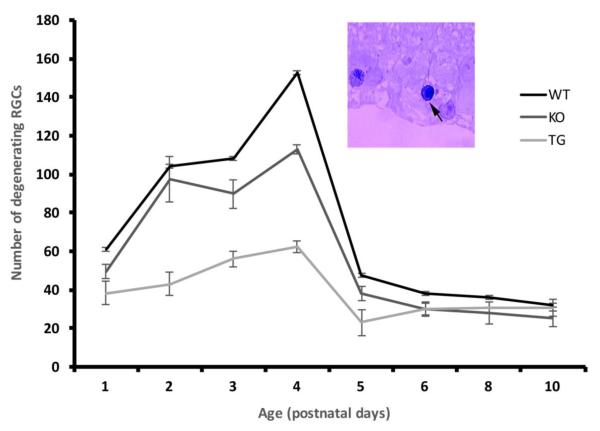
In the mouse, as in other species, there is an initial overproduction of ganglion cells that is reduced to the adult number by programmed cell death. To determine whether the larger number of cells found in the ganglion cell layer of UCP2 overexpressing mice was due to an increase in initial production or a decrease in cell death we counted degenerating cells at different stages of development using Cresyl Violet stained sections. The darkly stained neurons which showed typical degenerating features like shrunken nucleus, fragmented chromatin and irregular but intact cell membrane were considered dead cells and were distributed throughout the GCL. Viable cells on the other hand were faintly stained, generally larger in size, and showed clear cytoplasm and nucleus (Figure 4 inset).
Figure 4.
Time course of degeneration in the retinal ganglion cell layer. Dying cells were counted in the ganglion cell layer of three mice at each age. All mice showed a peak of degeneration at PN4, though the numbers at this and at all other ages were decreased in UCP2 transgenic animals. Inset: Example of degenerating ganglion cell in the GCL of a retinal section indicated by the arrow. A larger ganglion cell with fragmented chromatin enclosed within the nuclear membrane is indicated at the extreme left.
The late embryonic stages showed few degenerating neurons and hence were not quantified. Cell death was studied every 24 hrs from PN1 to PN8 which includes the known wave of maximum cell death in the GCL of the retina that occurs during the first postnatal week (Figure 4). The numbers of degenerating cells were counted in an identical number of segments from the eyes of three mice of each genotype. The average number of degenerating cells at PN1 was 61.2±12.3 in wild type, 49.6±3.5 in UCP2 knockout and 38.4±6.0 in UCP2 overexpressing transgenic mice. At this age the difference between wild type and transgenic was significant (p<0.045) but between wild type and knockout was not. The numbers of dying cells in the GCL increased to 152.9±6.4 in wild type, 113.1±2.3 in knockout and 62.4±3.2 in transgenic mice by PN4. At this age both transgenic and knockout differed significantly from wild type (p<0.001). We also quantified the degenerating neuronal counts until the end of second postnatal week but only a low level of degeneration was found after the first postnatal week and this low number was not statistically different among the three genotypes. Thus, there was a significant reduction in the number of cells which are degenerating in the UCP2 transgenic mice when compared to wild type and knockout mice through the major period of developmental cell death.
We confirmed the results from the Cresyl Violet stained sections using both TUNEL labeling and immunolabeling for activated caspase 3 (data not shown). In each case the number of degenerating cells in the GCL was fewer in transgenic mice compared with knockout and wild type mice.
UCP2 promotes retinal cell survival in culture
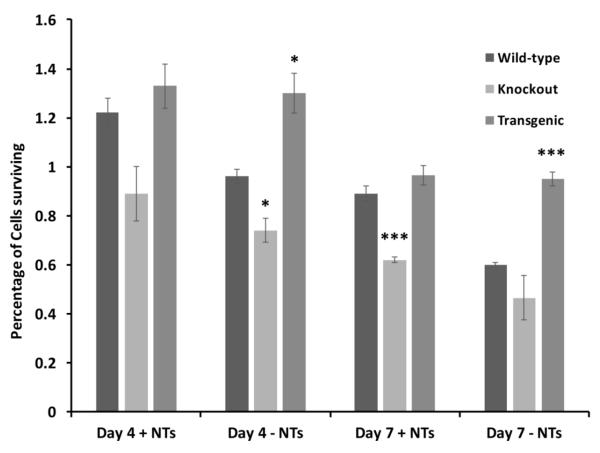
To investigate whether the results obtained under in vivo conditions also applied to in vitro studies, we cultured retinal cells of wild type, transgenic and knockout mice. Cultures were maintained for 8 DIV and neuronal counts were obtained from sets of cultures with or without an added cocktail of neurotrophic factors. Our in vitro results correspond to in vivo counts showing greater neuronal survival in transgenic mice when compared to wild type and knockout control mice. At the qualitative level, we observed greater number of cell bodies in the neuronal clusters and more neuronal clusters in the transgenic mice in comparison with the wild type mice at both 4 DIV and 7 DIV in the retinal cultures. This result did not hold for cultures from knockout mice where there was a considerable cell loss in the retinal cultures when compared to wild type cultures on all the days studied in vitro (Figure 5). Quantitative analysis indicated that at day 4 without added neurotrophic factors, cultures from transgenic mice had a significantly greater nimber of cells (p<0.05) and from knockout mice a significantly lower number of cells (p<0.05). At day 7, cultures from knockout animals had significantly lower numbers of cells (p<0.001) than wild type even in the presence on neurotrophic factors, and in the absence of neurotrophic factors, cultures from the transgenic animals had significantly higher numbers of cells (p<0.001). The addition of neurotrophic factors to the retinal cultures from transgenic mice did not alter the neuronal numbers significantly at either time point whereas neuronal survival was enhanced with growth factors in the cultures from both wild type and knockout mice.
Figure 5.
Survival of retinal cells in cultures derived from wild type, knockout (and UCP2 transgenic PN1 mice. Three sets of cultures were established from each of the different genotypes and the surviving cells counted after 4 or 7 days of culture. Cell survival in the presence (+NTs) and absence (-NTs) of BDNF (40 ng/ml) plus CNTF (40 ng/ml) were measured separately. * = p<0.05, *** = p<0.001, all comparisons with wild type.
This study demonstrates that more neurons were present in retinal primary cultures grown in vitro and their survival is independent of growth factors in UCP2 transgenic mice. There were fewer neurons in wild type and least number of neurons in UCP2 knockout cultured retina. Thus, our in vitro study correlates well with our earlier in vivo results in all three groups of mice studied.
UCP2 protects retinal cells against NMDA and KA excitotoxicity
The survival of cells in the GCL of UCP2 wild type, transgenic and knockout adult animals was studied when exposed to the excitotoxins NMDA and KA. 40 nmoles or 160 nmoles of NMDA and 5 nmole or 10 nmole of KA were injected intraocularly and the survival of neurons in GCL was examined 24 hr later.
40 nmoles NMDA had no significant effect on the neurons in the GCL. Animals that were injected with 160 nmoles of NMDA showed extensive death of cells in the GCL after 24 hours (Table 2). Wild type control mice had 64% neurons remaining in the GCL after NMDA injections, knockout mice had 62% neurons and transgenic mice had 83% neurons remaining when compared to their respective control injected retinas (p<0.0001). Injections of KA demonstrated that UCP2 overexpression was less efficient at protecting cells from this stronger excitotoxic agent (Table 1). The percentage of surviving GCL neurons in the three groups of mice was about 22% in wild type mice, 23% in knockout mice and 32% in transgenic mice, which was still highly significant when compared to wild type (P<0.0001). The results indicated that transgenic mice showed significantly more surviving neurons after exposure to NMDA than knockout and wild type control animals, suggesting that UCP2 provides strong protection against excitotoxic cell death caused by NMDA injections. UCP2 is less effective at providing protection against KA excitotoxicity, though significantly more cells survived than in the wild type or knockout animals.
Table 2.
Survival of neurons in the ganglion cell layer of mouse eyes injected with NMDA or Kainic acid.
| NMDA (160 nmoles) |
KA (10 nmoles) |
|
|---|---|---|
| Wild type | 61.66±2.1 | 21.19±1.4 |
| Transgenic | 103.66±3.6*** | 39.96±1.8*** |
| Knockout | 60.12±1.8 | 22.30±1.7 |
Numbers represent cells counted per 1000 μm length of retina.
= p<0.0001. In all experiments n=6.
DISCUSSION
Three principal findings emerge from this work. First, we found that increasing mitochondrial UCP2 activity by addition of a human UCP2 gene prevented the normal loss of neurons in the GCL during development of the retina. Second, increased UCP2 activity promoted survival of cultured retinal neurons and made them less dependent on neurotrophic support. Third, we found that UCP2 transgenic mice showed significantly less cell death in the ganglion cell layer when exposed to the excitotoxin NMDA and a similar, though less pronounced, effect when exposed to kainic acid. Together these findings suggest that UCP2 plays an important role in both physiological and pathological death of retinal ganglion cells, and probably other neurons.
Both UCP2 transgenic and UCP2 knockout mice displayed normal b-waves and oscillating potentials in their ERGs. This suggests that manipulating UCP2 expression had no significant effect on the synaptic circuitry of the retina. On the other hand, the knockout animals did show a reduced a-wave. We have not detected any gross abnormality in the photoreceptor layer in these animals and the defect may either be a more subtle change in cell physiology or some abnormality in another cell type such as the RPE layer that in turn affects the visual cycle.
Mitochondrial UCP2 regulates neuronal cell death
UCP2 is upregulated following brain injury and this is thought to be part of its neuroprotective function (Bechmann et al., 2002; Liu et al., 2009). UCP2 is also known to prevent neuronal loss in an epilepsy model in mice, a Parkinson’s model in monkeys and following global ischemia (Horvath et al., 2003b; Diano et al., 2003; Deierburg et al., 2008). These studies reveal a role for UCP2 in regulating cell death under conditions of disease or injury. In the present studies we addressed the importance of UCP2 in natural form of cell death that occurs during development. Our results show that UCP2 overexpressing mice showed fewer degenerating neurons in the GCL during the first postnatal week when maximum cell death is seen in the retina at the time of development (Sefton et al., 1985). The significantly lower numbers of dying cells seen throughout the first postnatal week (Fig 4) can explain the higher numbers of cells seen in the GCL of adult mice. We found no abnormalities in the ERG signals from the inner retina and so presume that the connectivity patterns of these additional cells are normal. It will be of interest to study how the axonal arbors of the ganglion cells adapt to the additional numbers in the ganglion cell target regions. Interestingly we found a significant decrease in cell death at PN4 in the UCP2 knockout mice as compared with wild type (although it was still significantly greater than the transgenic value). Even with this decreased cell death the knockout retinas have fewer cells in the GCL in the adult. At present we do not know whether this strain has altered cell generation or cell death occurring over a broader developmental period, either of which could account for the adult result.
It is known that one of the important determining factors for cell death during development is the deprivation of neurotrophic factors (Spalding et al., 2004) that leads to cell death through the bax and caspase-3 dependent, but not the p53 dependent, pathway (Nickells, 2004). Our in vitro study revealed that cultured neurons from UCP2 transgenic mice showed more and healthier neurons both in the presence and absence of growth factors. In contrast, cultures from knockout and wild type control mice had severe neuronal loss when maintained in absence of growth factors. We have recently shown that the neurotrophic factor LIF increases the pool of UCP2 mRNA in astrocytes and this is then rapidly translated into protein following exposure to oxidative stress (Lapp et al., 2014). Our results are compatible with a model where increasing transcription of the UCP2 gene is an important component of neurotrophic factor function.
Role of UCP2 in retinas exposed to NMDA and Kainate
Although there are reports of UCP2 regulating substrate availability and calcium movements in mitochondria, the strongest evidence supports a role in regulating the production and effects of reactive oxygen species (ROS). ROS generation is an obligatory product of oxidative phosphorylation through the electron transport chain and activation of UCP2 can reduce ROS production by producing small decreases in the mitochondrial membrane potential (Arsenijevic et al., 2000; Jezek et al., 2004; Brand and Esteves, 2005; Mailloux and Harper, 2011; Toda and Diano, 2014). In addition, recent studies have shown in hypothalamus that UCP2 levels and activity are promoted by ROS and that a major function of UCP2 is the scavenging of these free radicals (Andrews et al., 2008).
Excitotoxic agents can cause production of superoxide radicals, probably related to calcium-stimulated production of NO interacting with oxygen radicals from mitochondria (Schulz et al., 1997). NMDA receptors are known to be abundant in RGCs and these cells are clearly sensitive to its toxic effects (Lipton, 2001; Seki and Lipton, 2008). Other studies, however suggest that kainate receptors also play an important role in the death of RGCs under a variety of damaging conditions (Otori et al., 1998). We found that significantly more neurons survived after injections of NMDA in the UCP2 transgenic mice. A similar, though less dramatic, effect was found following the more severe insult of kainate injection. This is similar to previous studies on adult rats where it was found that systemic L-kynurenine administration gave protection against NMDA-induced, and to a lesser extent against kainate-induced, degeneration of RGCs (Wu et al. 2002). Increased UCP2 activity would reduce the production and availability of these oxygen radicals.
In summary, enhanced mitochondrial uncoupling by overexpression of UCP2 decreases programmed cell death in retinal ganglion cells, reduces neurotrophin dependency of cultured retinal neurons and protects retinal cells against the toxic effects of glutamate agonists. This suggests that regulation of reactive oxygen production is a key feature regulating retinal cell survival or death, and that drugs that regulate UCP2 level or activity may play an important role in protecting neurons from a variety of insults.
Acknowledgements
This worked was supported by grants from the NIH (TLH, CJB) and from the Macula Vision Research Foundation.
REFERENCES
- Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GM, Zufall F, Barnstable CJ. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994;12:155–165. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Horvath B, Barnstable CJ, Elsworth J, Yang L, Beal MF, Roth RH, Matthews RT, Horvath TL. Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson's disease. J Neurosci. 2005;25:184–191. doi: 10.1523/JNEUROSCI.4269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschöp MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008:454846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Bähr M. Live or let die - retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23:483–490. doi: 10.1016/s0166-2236(00)01637-4. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Li M, Reddy R, Horvath TL. Mitochondrial uncoupling proteins: regulators of retinal cell death. Adv Exp Med Biol. 2003;533:269–275. doi: 10.1007/978-1-4615-0067-4_33. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Diano S, Warden CH, Bartfai T, Nitsch R, Horvath TL. Brain mitochondrial uncoupling protein 2 (UCP2): a protective stress signal in neuronal injury. Biochem. Pharmacol. 2002;64:363–367. doi: 10.1016/s0006-2952(02)01166-8. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou JC, Maffei L, Rabacchi SA. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. Journal of Neuroscience. 1996;16:4186–4194. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Buss RR, Oppenheim RW. Role of programmed cell death in normal neuronal development and function. Anat Sci Int. 2004;79:191–197. doi: 10.1111/j.1447-073x.2004.00088.x. [DOI] [PubMed] [Google Scholar]
- Castella L, Rigoulet M, Penicaud L. Mitochondrial ROS metabolism; modulation by uncoupling proteins. IUBMB Life. 2001;52:181–188. doi: 10.1080/15216540152845984. [DOI] [PubMed] [Google Scholar]
- Clarke PGH. Neuronal death in the development of the vertebrate nervous system. Int. Rev. Neurobiol. 1985;34:133–214. doi: 10.1016/s0074-7742(08)60098-7. [DOI] [PubMed] [Google Scholar]
- Conti B, Sugama S, Lucero J, Winsky-Sommerer R, Wirz SA, Maher P, Andrews Z, Barr AM, Morale MC, Paneda C, Pemberton J, Gaidarova S, Behrens MM, Beal F, Sanna PP, Horvath T, Bartfai T. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- Cooper NG, Laabich A, Fan W, Wang X. The relationship between neurotrophic factors and CaMKII in the death and survival of retinal ganglion cells. Prog Brain Res. 2008;173:521–540. doi: 10.1016/S0079-6123(08)01136-9. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Mohler IM, Giordano DL. Naturally occurring neuron death in the ganglion cell layer of the neonatal rat: morphology and evidence for regional correspondence with neuron death in superior colliculus. Dev. Brain Res. 1982;2:203–215. doi: 10.1016/0165-3806(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Dekkers MP, Nikoletopoulou V, Barde YA. Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity. J Cell Biol. 2013;203:385–393. doi: 10.1083/jcb.201306136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deierborg T, Wieloch T, Diano S, Warden CH, Horvath TL, Mattiasson G. Overexpression of UCP2 protects thalamic neurons following global ischemia in the mouse. J Cereb Blood Flow Metab. 2008;28:1186–1195. doi: 10.1038/jcbfm.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med. 2012;18:52–58. doi: 10.1016/j.molmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: A mechanism for preconditioning. Endocrinology. 2003;144:5014–5021. doi: 10.1210/en.2003-0667. [DOI] [PubMed] [Google Scholar]
- Dreher B, Potts RA, Bennett MR. Evidence that the early postnatal reduction in the number of rat retinal ganglion cells is due to a wave of ganglion cell death. Neurosci. Lett. 1983;36:255–260. doi: 10.1016/0304-3940(83)90009-5. [DOI] [PubMed] [Google Scholar]
- Fraser A, McCarthy N, Evan GI. Biochemistry of cell death. Curr. Opin. Neurobiol. 1996;6:71–80. doi: 10.1016/s0959-4388(96)80011-0. [DOI] [PubMed] [Google Scholar]
- Han MH, Kawasaki A, Wei JY, Barnstable CJ. Miniature postsynaptic currents depend on Ca2+ released from internal stores via PLC/IP3 pathway. Neuroreport. 2001;12:2203–2207. doi: 10.1097/00001756-200107200-00032. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, Barnstable CJ. Mitochondrial uncoupling protein 2 in the central nervous system: neuromodulator and neuroprotector. Biochemical Pharmacology. 2003a;65:1917–1921. doi: 10.1016/s0006-2952(03)00143-6. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, Miyamoto S, Barry S, Gatti S, Alberati D, Livak F, Lombardi A, Moreno M, Goglia F, Mor G, Hamilton J, Kachinskas D, Horwitz B, Warden CH. Uncoupling proteins-2 and 3 influence obesity and inflammation in transgenic mice. Int J Obes Relat Metab Disord. 2003b;27:433–442. doi: 10.1038/sj.ijo.0802257. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Kretz A, Cellerino A. Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res. 2003;22:483–543. doi: 10.1016/s1350-9462(03)00027-2. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek P, Zácková M, Růzicka M, Skobisová E, Jabůrek M. Mitochondrial uncoupling proteins--facts and fantasies. Physiol Res. 2004;53(Suppl 1):S199–211. [PubMed] [Google Scholar]
- Kim-Han JS, Dugan LL. Mitochondrial uncoupling proteins in the central nervous system. Antioxid Redox Signal. 2005;7:1173–1181. doi: 10.1089/ars.2005.7.1173. [DOI] [PubMed] [Google Scholar]
- Lapp DW, Zhang SS, Barnstable CJ. Stat3 mediates LIF-induced protection of astrocytes against toxic ROS by upregulating the UPC2 mRNA pool. Glia. 2014;62:159–170. doi: 10.1002/glia.22594. [DOI] [PubMed] [Google Scholar]
- Ledesma A1, de Lacoba MG, Rial E. The mitochondrial uncoupling proteins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-reviews3015. REVIEWS3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Retinal ganglion cells, glaucoma and neuroprotection. Prog Brain Res. 2001;131:712–718. [PubMed] [Google Scholar]
- Liu Y, Chen L, Xu X, Vicaut E, Sercombe R. Both ischemic preconditioning and ghrelin administration protect hippocampus from ischemia/reperfusion and upregulate uncoupling protein-2. BMC Physiol. 2009;9:17. doi: 10.1186/1472-6793-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- McCaffery CA, Bennett MR, Dreher B. The survival of neonatal rat retinal ganglion cells in vitro is enhanced in the presence of appropriate parts of the brain. Exp Brain Res. 1982;48:377–386. doi: 10.1007/BF00238614. [DOI] [PubMed] [Google Scholar]
- Murphy JA, Clarke DB. Target-derived neurotrophins may influence the survival of adult retinal ganglion cells when local neurotrophic support is disrupted: Implications for glaucoma. Med Hypotheses. 2006;67:1208–1212. doi: 10.1016/j.mehy.2006.04.049. [DOI] [PubMed] [Google Scholar]
- Nickells RW. The molecular biology of retinal ganglion cell death: caveats and controversies. Brain Res Bull. 2004;62:439–446. doi: 10.1016/j.brainresbull.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Nickells RW. The Cell and Molecular Biology of Glaucoma: Mechanisms of Retinal Ganglion Cell Death. IOVS. 2012;53:2476–2681. doi: 10.1167/iovs.12-9483h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe V, Bennett MR. Embryonic chick retinal ganglion cells identified “in vitro”. Their survival is dependent on a factor from the optic tectum. Exp Brain Res. 1981;44:249–258. doi: 10.1007/BF00236562. [DOI] [PubMed] [Google Scholar]
- O’Leary DDM, Fawcett JW, Cowan WM. Topographic targeting errors in the retinocollicular projection and their elimination by selective ganglion cell death. J. Neurosci. 1986;6:3692–3705. doi: 10.1523/JNEUROSCI.06-12-03692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu.Rev. Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Otori Y, Wei J-Y, Barnstable CJ. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 1998;39:972–981. [PubMed] [Google Scholar]
- Pienado–Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of Nt-4, Nt-3, and Brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- Schulz JB, Matthews RT, Klockgether T, Dichgans J, Beal MF. The role of mitochondrial dysfunction and neuronal nitric oxide in animal models of neurodegenerative diseases. Mol Cell Biochem. 1997;174:193–197. [PubMed] [Google Scholar]
- Sefton AJ, Horsburgh GM, Lam K. The development of the optic nerve in rodents. Aust N Z J Ophthalmol. 1985;13:135–145. doi: 10.1111/j.1442-9071.1985.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog Brain Res. 2008;173:495–510. doi: 10.1016/S0079-6123(08)01134-5. [DOI] [PubMed] [Google Scholar]
- Sluse FE. Uncoupling proteins: molecular, functional, regulatory, physiological and pathological aspects. Adv Exp Med Biol. 2012;942:137–156. doi: 10.1007/978-94-007-2869-1_6. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Rush RA, Harvey AR. Target-derived and locally derived neurotrophins support retinal ganglion cell survival in the neonatal rat retina. J Neurobiol. 2004;60:319–27. doi: 10.1002/neu.20028. [DOI] [PubMed] [Google Scholar]
- Stockton RA, Slaughter MM. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J Gen Physiol. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom RC, Williams RW. Cell production and cell death in the generation of variation in neuron number. J. Neurosci. 1998;18:9948–9953. doi: 10.1523/JNEUROSCI.18-23-09948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Dubé C, Dorenbos K, Steward O, Baram TZ. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Slaughter MM. Correlation of dynamic responses in the ON bipolar neuron and the b-wave of the electroretinogram. Vision Res. 1995;35:1359–1364. doi: 10.1016/0042-6989(95)98715-l. [DOI] [PubMed] [Google Scholar]
- Toda C, Diano S. Mitochondrial UCP2 in the central regulation of metabolism. Best Pract Res Clin Endocrinol Metab. 2014;28:757–764. doi: 10.1016/j.beem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistamehr S, Tian N. Light deprivation suppresses the light response of inner retina in both young and adult mouse. Vis Neurosci. 2004;21:23–37. doi: 10.1017/s0952523804041033. [DOI] [PubMed] [Google Scholar]
- Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998;17:485–521. doi: 10.1016/s1350-9462(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Wei JY, Jin X, Cohen ED, Daw NW, Barnstable CJ. cGMP-induced presynaptic depression and postsynaptic facilitation at glutamatergic synapses in visual cortex. Brain Res. 2002;927:42–54. doi: 10.1016/s0006-8993(01)03323-6. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Lee SC, Scharfman HE, Schwarcz R. L-4-chlorokynurenine attenuates kainate-induced seizures and lesions in the rat. Exp Neurol. 2002;177:222–232. doi: 10.1006/exnr.2002.7971. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li H, Liu MG, Kawasaki A, Fu XY, Barnstable CJ, Shao-Min Zhang S. STAT3 activation protects retinal ganglion cell layer neurons in response to stress. Exp Eye Res. 2008;86:991–997. doi: 10.1016/j.exer.2008.03.020. [DOI] [PubMed] [Google Scholar]