Abstract
Aims
The purpose was to determine the exposure−response relationship of everolimus in patients with angiomyolipoma from the EXIST‐2 trial and to analyze the correlation between exposure and plasma concentrations of angiogenic biomarkers in these patients.
Methods
One hundred and eighteen patients with angiomyolipoma associated with tuberous sclerosis complex (TSC) or sporadic lymphangioleiomyomatosis (sLAM) were randomly assigned 2 : 1 to receive everolimus 10 mg (n = 79) or placebo (n = 39) once daily. Blood samples for determining everolimus concentration were collected at weeks 2, 4, 12, 24 and 48 during double‐blind treatment. Plasma samples for biomarker analysis were collected at baseline and weeks 4, 12, 24, 36, 48 and at the end of treatment. Concentrations of eight angiogenic biomarkers associated with tumour growth were determined by enzyme‐linked immunosorbent assay (ELISA).
Results
Peak and trough concentrations of everolimus in blood remained stable over time and similar to those reported in other indications. Substantial pharmacodynamic effects were observed in the everolimus, but not placebo, arm for three biomarkers: After 24 weeks of treatment, reduction of vascular endothelial growth factor D (VEGF‐D) and collagen type IV (COL‐IV) (mean fold‐changes with 95% confidence intervals [CI] were 0.36 [0.33, 0.40], and 0.54 [0.51, 0.57], respectively, P < 0.001 for both), along with increased VEGF‐A (mean fold‐change of 1.59 [1.39, 1.80], P < 0.001), were seen. Furthermore, baseline VEGF‐D and COL‐IV levels were associated with angiomyolipoma size at baseline and with angiomyolipoma response to everolimus.
Conclusions
These findings suggest that plasma angiogenic markers may provide an objective measure of patient response to everolimus.
Keywords: angiomyolipoma, biomarkers, collagen‐IV, lymphangioleiomyomatosis, tuberous sclerosis complex, VEGF‐D
What is Already Known about this Subject
Everolimus has been shown to effectively reduce renal angiomyolipoma volume on imaging in patients with tuberous sclerosis complex (TSC) or sporadic lymphangioleiomyomatosis (sLAM)
Identification of blood tests to monitor efficacy of medication in patients at increased risk from the effects of imaging assessments (such as anaesthetic side effects) might be of clinical relevance
What this Study Adds
Trough concentrations (C min) of everolimus correlated with the percentage change in angiomyolipoma volume from baseline
Everolimus modulated plasma levels of multiple angiogenesis‐related molecules, including collagen‐IV, VEGF‐A, and VEGF‐D. The measurement of plasma collagen‐IV and VEGF‐D levels may offer an alternative method for disease monitoring, thus reducing the need for imaging
Introduction
Tuberous sclerosis complex (TSC) is a genetic disorder caused by mutations in the TSC1 (encodes hamartin) or TSC2 (encodes tuberin) tumour suppressor genes that normally inhibit the mTOR complex‐1 (mTORC1), a central controller of cell growth and proliferation 1, 2. Possessing an inactivating mutation in either gene leads to a loss of a functional hamartin‐tuberin protein heterodimer, which in turn leads to constitutive mTORC1 activation, aberrant downstream signalling and growth of non‐cancerous hamartomas in the kidney (renal angiomyolipomas), brain (subependymal giant cell astrocytomas [SEGA], cortical tubers), heart (cardiac rhabdomyomas), liver, eyes and skin (angiofibromas) 1, 3, 4, 5.
Renal angiomyolipomas occur in up to 80% of patients with TSC and cause the largest proportion of deaths in adults with TSC 5, 6. Angiomyolipomas are highly vascularized tumours that appear to arise from a vascular pericyte lineage 7. As the tumours enlarge, there is an increased risk that blood vessels will develop aneurysms that subsequently rupture and haemorrhage 5, 8, 9, 10. Lymphangioleiomyomatosis (LAM), a disorder of the lung, occurs in 30% to 80% of women with TSC and increases in prevalence with increasing age 5, 11. It is characterized by infiltration of the lungs by smooth muscle‐like cells and pulmonary remodelling that results in diffuse pulmonary cysts. Following neurologic and renal disease, LAM is the third most common cause of death in patients with TSC 5. Sporadic LAM (sLAM) is a rare form of LAM that occurs in patients without TSC as a result of somatic mosaicism at the TSC2 gene locus 5, 12, 13, 14, 15. LAM and sLAM are associated with angiomyolipomas in 93% and 50% of cases, respectively 5, 16.
Loss of the TSC1 or TSC2 genes has been associated with increased production of vascular endothelial growth factor (VEGF)‐A 17. The VEGF family of growth factors are essential mediators of tumour angiogenesis 18. Elevated VEGF‐D levels in blood have been shown in patients with renal angiomyolipomas or LAM and have been correlated with angiomyolipoma size and LAM disease severity 19, 20, 21, 22. Sirolimus (also known as rapamycin), an oral mammalian target of rapamycin (mTOR) inhibitor, has been shown to inhibit production of VEGF‐A, tumour growth and angiogenesis, both in vitro in TSC1‐ or TSC2‐null fibroblasts and in vivo in a mouse model of TSC 17.
Historically, therapeutic options for patients with TSC‐related angiomyolipomas were limited to watchful waiting until the need for invasive interventions such as embolization or nephrectomy 5, 6. The efficacy and safety of the mTOR inhibitor everolimus for treating non‐life threatening angiomyolipomas was demonstrated in EXIST‐2 (NCT00790400), the first large, randomized phase 3 trial to evaluate a systemic treatment for renal angiomyolipomas (not requiring immediate surgery) in adult patients with either TSC or sLAM 20. Everolimus, at a dose of 10 mg daily, significantly reduced renal angiomyolipoma volume compared with placebo. The renal angiomyolipoma response rate (primary analysis, database cutoff 30 June 2011) was 42% for everolimus vs. 0% for placebo (P < 0.0001), where response was defined as 50% reduction of angiomyolipoma volume from baseline. During the study, the patients reported mostly mild to moderate adverse events, which were managed through everolimus dose reduction and/or interruption of therapy 20. The long term extension phase of the EXIST‐2 study (median duration of everolimus exposure of 28.9 months, database cutoff 01 May 2013) reported sustained reductions in angiomyolipoma volumes, with an overall angiomyolipoma response rate of 54% (95% confidence interval [CI], 43.9%, 63.0%) 23.
The current analyses characterized the pre‐dose trough everolimus concentration (C min) and 2 h post‐dose everolimus concentration (C 2 h), and examined the relationship between everolimus C min, the pharmacodynamic changes of various circulating proteins and treatment efficacy in the EXIST‐2 patient population. The goal of these analyses was to better discern the variability of everolimus exposure and the pharmacodynamic effect of everolimus on a broad panel of angiogenic growth factors. Patients with TSC often may require sedation or general anaesthesia for imaging to follow their renal disease. We therefore also explored whether changes in the pharmacodynamics could be used as an alternative to multiple, repeated imaging to monitor disease progression.
Methods
The details of EXIST‐2 have been previously published 20. In brief, the trial enrolled 118 patients with angiomyolipoma associated with TSC or sLAM who were randomized 2 : 1 to receive 10 mg of everolimus (n = 79) or placebo (n = 39) once daily, stratified by enzyme‐inducing anti‐epileptic drug (EIAED) use or no use. EIAEDs are strong inducers of CYP3A4 activity and included phenytoin, mephenytoin, carbamazepine, phenobarbital, pentobarbital, primidone and oxcarbazepine. The everolimus dose could be reduced to 5 mg daily or further to 5 mg every other day if necessary to manage any unacceptable toxicity. Patients were eligible for the study if they were aged ≥ 18 years, had at least one renal angiomyolipoma ≥ 3 cm in its longest diameter and had either a definite diagnosis of TSC (per consensus criteria) or sLAM (per biopsy or chest computed tomography [CT] scan). Independent ethics committees and/or local ethics review boards approved the protocol and all patients or their guardians provided written informed consent. A list of all institutional review boards is provided in Supplementary Table 1. The primary endpoint of the study was the angiomyolipoma response rate, defined as the proportion of patients with renal angiomyolipoma with a confirmed response of ≥ 50% reduction from baseline in the sum of volumes of all target lesions, in the absence of new lesions ≥ 1 cm in longest diameter, kidney volume increase of > 20% from nadir and angiomyolipoma‐related bleeding of grade ≥ 2 (CTCAE, version 3.0).
Pharmacokinetic analysis
Pre‐dose blood samples (2 ml) for determination of everolimus C min were collected immediately prior to everolimus dosing at weeks 2, 4, 12, 24 and 48 during the double‐blind treatment period 20 to 28 h after the patient's last dose of study drug. To estimate peak everolimus concentrations (C max), blood samples were collected 2 h post‐dose (C 2 h) as a surrogate for C max reflecting that a daily dose of everolimus 10 mg is rapidly absorbed with a median t max of 1 to 2 h 24. The everolimus concentration in whole blood was determined by a validated liquid chromatography‐tandem mass spectroscopy (WuXi App Tec, Shanghai, China) method 25 as detailed previously 26. The lower limit of quantification was 0.3 ng ml−1. The method consists of protein precipitation followed by solid phase extraction. The extract was analyzed by reversed phase HPLC with C18 column (CAPCELL PAK MG, Shiseido) by gradient elution with aqueous ammonium acetate buffer and acetonitrile. The detection was made by API4000 (Applied Biosystems) with selected reaction monitoring 975.6 m/z to 908.6 m/z for everolimus and 980.8 m/z to 913.8 m/z for the internal standard of everolimus. The mobile phase consisted of gradient with water and 95% of acetonitrile with ammonium acetate. The coefficient of variation of the assay ranged from 4.7% to 7.8%.
Biomarker analysis
Plasma samples for biomarker analysis were prepared from 3 ml peripheral blood collected from all trial participants prior to everolimus dosing, at baseline and day 1 of treatment weeks 4, 12, 24, 36 and 48 and at the end of treatment. The concentrations of eight biomarkers associated with the angiogenesis and tumour growth were determined by enzyme‐linked immunosorbent assay (ELISA) for VEGF‐D and COL‐IV or by electrochemiluminescence using a multiplexed Meso Scale Discovery® (Meso Scale Diagnostics LLC, Rockville, MD, USA) platform formatted as four‐plex (placental growth factor [PLGF], VEGF‐A, basic fibroblast growth factor [bFGF] and soluble VEGF receptor 1 [sVEGFR‐1]) and two‐plex (soluble VEGF receptor 2 [sVEGFR‐2] and c‐Kit) combinations. All assays were validated prior to use according to the manufacturer's specifications. Coefficients of variation and lower limit of determination are listed in Supplementary Table 2. All assays were performed by a central laboratory.
Statistical analysis
Summary statistics were provided by planned dose (10 mg day−1) and by time window for C min and C 2 h. Because a number of patients treated with everolimus had their dose reduced from 10 mg day−1 to 5 mg day−1, an additional analysis was undertaken by actual dose received. The relationship between everolimus exposure and change from baseline in sum of volumes of target renal angiomyolipoma was investigated by a linear mixed model that included the sum of volumes at baseline, the time‐normalized C min between the previous and current imaging assessments (CT or MRI) as fixed effect and patient as random effect. A similar linear model was also fitted using log‐transformed variables.
Descriptive statistics (mean, median, percentiles and outliers) were used to describe the distribution of biomarkers at baseline. Values below the lower limit of quantitation (LLOQ) were imputed as 50% of LLOQ. Pearson correlation was used to investigate the relationship among baseline biomarker levels, and Spearman's correlation was used to determine the relationship between biomarker level and angiomyolipoma volume (sum of volumes of target angiomyolipoma) at baseline. For the correlation between the baseline biomarker levels and angiomyolipoma response rate, everolimus‐treated patients were divided into subgroups defined by ≤ or > overall median value for each biomarker at baseline. Odds ratios for response rate were obtained by logistic regression.
The effects of everolimus on biomarker levels over time were evaluated using summary statistics at each time point. Mean fold‐change and 95% CI were obtained using a linear mixed model, including terms for baseline value, treatment, time and interaction between time and treatment, P values for test of effect were calculated for each covariate in the model to assess the dynamic change of biomarkers over time. In addition, estimate at week 24 was used to report descriptively fold‐change from baseline for each biomarker, because all pharmacodynamic effects had achieved steady‐state and the number of patients with evaluable biomarker data was large enough for a meaningful analysis and interpretation of the results.
The relationship between percentage change from baseline in biomarkers and everolimus exposure was investigated by a linear mixed model, with log‐value of biomarker as dependent variable, log‐baseline value of biomarker and time‐normalized C min as fixed effect independent variables, and patient as random effect independent variable. In this analysis, the time‐normalized C min was calculated for each patient as area under the concentration curve between the current biomarker assessment and the previous one, divided by the time of this corresponding period. Percentage change in biomarker from baseline for 2‐unit C min increase was derived from the mixed model estimates as 100 × (exp(2 × C min) − 1), and 95% CI was calculated in the same way. All provided P values are nominal. Because this was a post hoc exploratory analysis, no multiplicity adjustments were performed. Therefore, statistical interpretation should be made with caution. Statistical analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient baseline demographic and disease characteristics
The baseline demographic and disease characteristics have been described previously and, in general, were well balanced between the treatment arms 20. Overall, 78% of patients had bilateral renal angiomyolipomas and 29% of patients had a large (≥ 8 cm in longest diameter) renal angiomyolipoma. Almost 40% of patients had received a previous invasive intervention, including 19% with prior nephrectomy 20.
Everolimus exposure
The mean (standard deviation [SD]) relative dose intensity (RDI) for everolimus patients (n = 79) through the double‐blind phase of the study from which the PK data were derived was 0.85 (± 0.263) and the median RDI was 1.0 (range 0.2–1.8). A total of 59.5% of patients had an RDI between 0.90 and 1.10, indicating that the doses they received were close to the planned daily dose of 10 mg.
Everolimus pharmacokinetics
Median C min and C 2 h remained relatively stable over time (Table 1). In patients who required a dose reduction to 5 mg day−1 (n ≤ 5), everolimus blood concentrations were correspondingly lower after dose reduction (Table 1). Intersubject variability of C min and C 2 h was relatively high in the total patient population listed in Table 1, due to inclusion of data from patients receiving both a 5 mg day−1 and 10 mg day−1 dose and data from patients with or without co‐administration of a CYP3A4 inducer. In patients who received an actual dose of 10 mg day−1 with or without co‐administration of a CYP3A4 inducer, the intersubject variability in C min ranged from 56.7% to 105% and in C 2 h ranged from 39.5% to 57.6%. In the small number of patients (n ≤ 9) whose daily everolimus dose had been reduced to 5 mg day−1, the intersubject variability in C min ranged from 3.6% to 57.6% and in C 2 h ranged from 14.6% to 33.6% (Table 1).
Table 1.
Everolimus C min and C 2 h at weeks 2, 4, 12, 24 and 48 of the study irrespective of the actual dose received by the patients or by the actual dose received by the patients (10 mg day‐1 or 5 mg day‐1) for all evaluable everolimus‐treated patients during double‐blind period
| Week 2 | Week 4 | Week 12 | Week 24 | Week 48 | |
|---|---|---|---|---|---|
| All patients, irrespective of the dose received | |||||
| C min (ng ml‐1 ) | |||||
| n | 43 | 44 | 49 | 46 | 15 |
| Mean ± SD | 7.63 ± 4.32 | 7.72 ± 4.35 | 8.79 ± 6.75 | 9.37 ± 8.83 | 11.49 ± 12.01 |
| CV% | 56.7% | 56.4% | 76.8% | 94.2% | 104.5% |
| Median (range) | 6.6(0.6–19.6) | 7.0(1.4–22.2) | 7.5(1.0–32.6) | 6.7(0.0–52.8) | 6.9 (2.5–50.0) |
| C 2 h (ng ml‐1 ) | |||||
| n | 55 | 49 | 56 | 50 | 14 |
| Mean ± SD | 33.38 ± 15.66 | 30.89 ± 14.96 | 34.48 ± 15.10 | 39.27 ± 22.25 | 33.20 ± 18.45 |
| CV% | 46.9% | 48.4% | 43.8% | 56.7% | 55.6% |
| Median (range) | 31.5(4.9–75.8) | 28.2(4.3–75.9) | 34.4(10.5–77.9) | 38.9(5.4–98.6) | 29.0(10.0–71.2) |
| Patients who received 10 mg day ‐1 dose | |||||
|---|---|---|---|---|---|
| C min (ng ml‐1 ) | |||||
| n | 43 | 41 | 42 | 39 | 11 |
| Mean ± SD | 7.63 ± 4.32 | 7.85 ± 4.49 | 9.40 ± 7.03 | 10.13 ± 9.34 | 13.10 ± 13.69 |
| CV% | 56.7% | 57.2% | 74.8% | 92.2% | 105% |
| Median (range) | 6.6(0.6–19.6) | 7.2(1.4–22.2) | 7.6(1.0–32.6) | 6.9(0.0–52.8) | 7.8(2.5–50.0) |
| C 2 h (ng ml‐1 ) | |||||
| n | 55 | 46 | 45 | 39 | 10 |
| Mean ± SD | 33.38 ± 15.66 | 31.44 ± 15.28 | 37.39 ± 14.76 | 44.18 ± 22.55 | 36.54 ± 21.03 |
| CV% | 46.9% | 48.6% | 39.5% | 51.0% | 57.6% |
| Median (range) | 31.5(4.9–75.8) | 29.1(4.3–75.9) | 36.2(10.5–77.9) | 41.5(5.4–98.6) | 35.0(10.0–71.2) |
| Patients who received 5 mg day ‐1 dose | |||||
|---|---|---|---|---|---|
| C min (ng ml‐1 ) | |||||
| n | 0 | 3 | 5 | 5 | 3 |
| Mean ± SD | 6.04 ± 0.22 | 4.89 ± 2.82 | 4.57 ± 1.92 | 5.30 ± 0.93 | |
| CV% | 3.6% | 57.6% | 41.9% | 17.6% | |
| Median (range) | 6.0(5.8–6.3) | 3.9(2.1–8.0) | 5.0(2.5–6.8) | 5.7 (4.2–6.0) | |
| C 2 h (ng ml‐1 ) | |||||
| n | 0 | 3 | 9 | 9 | 4 |
| Mean ± SD | 22.47 ± 3.29 | 19.40 ± 6.26 | 19.93 ± 6.69 | 24.88 ± 4.34 | |
| CV% | 14.6% | 32.3% | 33.6% | 17.5% | |
| Median (range) | 21.6(19.7–26.1) | 19.7(11.4–30.9) | 19.6(12.6–35.7) | 24.0(20.8–30.7) | |
C min, predose trough concentration; C 2 h, 2 h post‐dose concentration; CV, coefficient of variation; SD, standard deviation.
At randomization, 13 patients in the everolimus arm and seven patients in the placebo arm were using an EIAED. Irrespective of the actual administered dose, C min and C 2 h values were lower by approximately one‐third to one‐half in patients using an EIAED at randomization compared with patients who were not (Table 2). In patients using EIAEDs at randomization, the intersubject variability in C min ranged from 39.0% to 59.2% and in C 2 h ranged from 44.5% to 66.4%. In patients not using an EIAED at randomization, the intersubject variability in C min ranged from 52.4% to 108.2% and in C 2 h ranged from 42.4% to 53.4% (Table 2). Analyses using linear mixed models based on the actual dose received also demonstrated lower mean C min and C 2 h values in patients using EIAEDs at randomization compared with the respective exposure indices in patients who were not using EIAEDs at randomization. For patients receiving an actual dose of 10 mg day−1, the geometric mean (90% CI) for C min was 3.79 (2.85, 5.03) ng ml−1 in patients using EIAEDs (n = 11) compared with 8.21 (7.19, 9.37) ng ml−1 for patients not using EIAEDs (n = 49), and for C 2 h was 19.74 (16.23, 24.02) ng ml−1 in patients using EIAEDs (n = 11) compared with 35.04 (32.01, 38.34) ng ml−1 for patients not using EIAEDs (n = 51). The difference in the number of patients between C min and C 2 h for EIAED non‐users was due to the number of evaluable samples available. Data for the EIAED users receiving 5 mg daily dose were not interpretable due to the small sample size (n = 0 and n = 1 for C min and C 2 h, respectively).
Table 2.
Everolimus C min and C 2 h irrespective of the actual dose administered to the patient at weeks 2, 4, 12, 24 and 48 of the study period, stratified by enzyme‐inducing antiepileptic drug (EIAED) use or non‐use at randomization (double‐blind period)
| Week 2 | Week 4 | Week 12 | Week 24 | Week 48 | |
|---|---|---|---|---|---|
| EIAED use at randomization | |||||
| C min (ng ml‐1 ) | |||||
| n | 8 | 8 | 10 | 9 | 3 |
| Mean ± SD | 4.36 ± 1.70 | 4.77 ± 2.78 | 4.30 ± 1.96 | 5.10 ± 3.02 | 8.26 ± 3.94 |
| CV% | 39.0% | 58.2% | 45.5% | 59.2% | 47.7% |
| Median (range) | 4.8(0.6–6.0) | 4.6(1.4–10.3) | 3.8(2.3–8.8) | 4.3(1.8–10.2) | 7.8 (4.6–12.4) |
| C 2 h (ng ml‐1 ) | |||||
| n | 10 | 9 | 10 | 10 | 2 |
| Mean ± SD | 21.38 ± 11.03 | 18.98 ± 8.82 | 26.92 ± 11.97 | 25.94 ± 14.37 | 18.78 ± 12.47 |
| CV% | 51.6% | 46.5% | 44.5% | 55.4% | 66.4% |
| Median (range) | 21.3(4.9–41.7) | 22.7(4.3–29.3) | 24.1(10.5–47.0) | 20.3(5.4–45.3) | 18.8(10.0–27.6) |
| EIAED non‐use at randomization | |||||
|---|---|---|---|---|---|
| C min (ng ml‐1 ) | |||||
| n | 35 | 36 | 39 | 37 | 12 |
| Mean ± SD | 8.38 ± 4.41 | 8.38 ± 4.39 | 9.94 ± 7.07 | 10.41 ± 9.47 | 12.30 ± 13.31 |
| CV% | 52.6% | 52.4% | 71.1% | 91.0% | 108.2% |
| Median (range) | 7.3(2.7–19.6) | 7.4(2.2–22.2) | 8.0(1.0–32.6) | 6.8(0.0–52.8) | 6.7(2.5–50.0) |
| C 2 h (ng ml‐1 ) | |||||
| n | 45 | 40 | 46 | 40 | 12 |
| Mean ± SD | 36.04 ± 15.37 | 33.57 ± 14.82 | 36.13 ± 15.32 | 42.60 ± 22.75 | 35.61 ± 18.55 |
| CV% | 42.6% | 44.1% | 42.4% | 53.4% | 52.1% |
| Median (range) | 34.8(5.0–75.8) | 30.7(10.6–75.9) | 34.9(11.4–77.9) | 40.4(11.5–98.6) | 30.6(10.3–71.2) |
C min, predose trough concentration; C 2 h, 2 h post‐dose concentration; CV, coefficient of variation; EIAED, enzyme‐inducing anti‐epileptic drug; SD, standard deviation.
Relationship between everolimus concentration and response to treatment
Fitting a linear mixed model for the absolute change from baseline in angiomyolipoma lesion volume resulted in a slope of −8.12 cm3 per 1‐unit log C min increase (95% CI −18.80, 2.55, P = 0.135), which was not statistically significant. An interaction between angiomyolipoma lesion volume at baseline and the absolute volume reduction was noted, suggesting that the exposure−response relationship may have varied in patients with different angiomyolipoma lesion volumes at baseline. To assess this, the data were further analyzed by categorizing the angiomyolipoma size at baseline (<100 cm3, 100–200 cm3 and >200 cm3). No significant correlation was found between C min and the absolute change of angiomyolipoma lesion volume from baseline in any of the subgroups. An analysis that fitted a linear mixed model for the percentage change from baseline in angiomyolipoma lesion volume did indicate a statistically significant 10.37% reduction in tumour size from baseline (95% CI −15.96%, −4.40%, P = 0.001) for a two‐fold increase in C min. Thus, data suggest percent change, rather than absolute change, from baseline in angiomyolipoma lesion volume was correlated with everolimus C min concentration.
Baseline biomarker analysis
VEGF‐A, VEGF‐D, placental growth factor (PLGF), collagen type IV (COL‐IV), and basic fibroblast growth factor (bFGF), soluble VEGF receptor 1 and 2 (sVEGFR1 and 2) and c‐Kit/mast/stem cell growth factor receptor/CD117, all related to angiogenesis and cell growth, were selected for the exploratory biomarker analysis of this disease with highly vasculature lesions. The plasma concentrations of these eight biomarkers were determined in triplicate in samples collected at baseline and in up to five on‐treatment time points. Evaluable results were obtained from 83% to 94% of the trial population (biomarker population), except those for bFGF. The number of samples with evaluable bFGF data was too small (<25% of the trial population) for a meaningful analysis and therefore the data were excluded from reported results.
The everolimus‐naïve baseline biomarker concentrations and their distribution in their respective biomarker populations are shown in Figure 1. All biomarkers appeared to follow a normal distribution. Interestingly, significant, strong correlations were observed between baseline levels of three pairs of biomarkers: VEGF‐D and COL‐IV (r = 0.71, P < 0.001), VEGF‐A and sVEGFR1 (r = 0.57, P < 0.001), and sVEGFR1 and PLGF (r = 0.47, P < 0.001) (Table 3), suggesting that the levels of biomarkers in the circulation may be regulated in a coordinated fashion. To our knowledge, this is the first time these correlations have been reported in a large number of TSC patients with angiomyolipomas.
Figure 1.
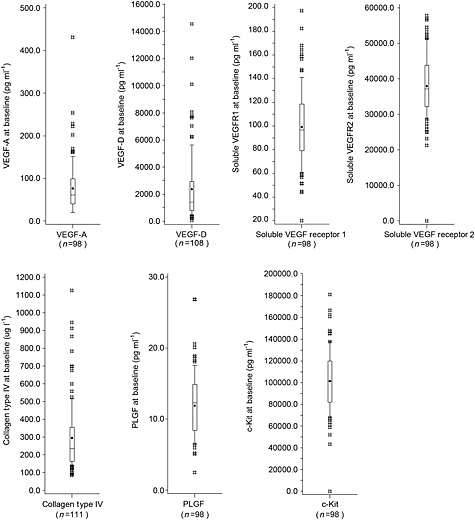
Boxplots of biomarker distribution at baseline from pooled everolimus and placebo patients. Medians are displayed as a line and means are displayed as dots. Boxes are drawn from the 25th to 75th percentiles, and whiskers extend to the 10th to 90th percentiles. Values that lay outside the 10th to 90th percentiles are indicated by a hash symbol (#). PLGF, placental growth factor; VEGF‐A, vascular endothelial growth factor A; VEGF‐D, vascular endothelial growth factor D
Table 3.
Pearson correlation coefficients were calculated to determine the relationship among angiogenic biomarkers at baseline in the trial population (everolimus plus placebo). Strong correlations were noted between baseline levels of VEGF‐D and Col IV, VEGF‐A and sVEGFR1, and sVEGFR1 and PLGF
| Biomarker | VEGF‐A | sVEGFR1 | PLGF | VEGF‐D | COL‐IV | sVEGR2 | c‐Kit |
|---|---|---|---|---|---|---|---|
| VEGF‐A | – | 0.568 P < 0.001 | 0.294 P = 0.003 | −0.009 P = 0.931 | −0.038 P = 0.708 | 0.049 P = 0.632 | 0.038 P = 0.712 |
| sVEGFR1 | – | 0.474 P < 0.001 | 0.184 P = 0.074 | 0.204 P = 0.044 | 0.103 P = 0.315 | 0.150 P = 0.141 | |
| PLGF | – | 0.166 P = 0.108 | 0.317 P = 0.002 | 0.149 P = 0.142 | 0.121 P = 0.237 | ||
| VEGF‐D | – | 0.711 P < 0.001 | 0.275 P = 0.007 | −0.098 P = 0.343 | |||
| Col IV | – | 0.205 P = 0.043 | −0.075 P = 0.465 | ||||
| sVEGFR2 | – | 0.222 P = 0.028 | |||||
| c‐Kit | – |
COL‐IV, collagen type IV; PLGF, placental growth factor; VEGF‐A, vascular endothelial growth factor A; VEGF‐D, vascular endothelial growth factor D; sVEGFR1, soluble VEGF receptor 1; sVEGFR2, soluble VEGF receptor 2.
Biomarker response to everolimus
The levels of all biomarkers were very similar between everolimus and placebo arms at baseline (Table 4). After everolimus treatment, the levels of four biomarkers were modulated by everolimus. Significant decreases of the VEGF‐D and COL‐IV levels from baseline were seen in the everolimus arm, with mean fold‐changes at week 24 of 0.36 (95% CI 0.33, 0.40, P < 0.001) and 0.54 (95% CI 0.51, 0.57, P < 0.001), respectively, compared with their baseline levels. Minimal change was seen for either biomarker in the placebo arm. A moderate decrease in sVEGFR2 level was also seen at week 24, compared with its baseline level (mean fold‐change of 0.81, 95% CI 0.72, 0.93, P = 0.002) in the everolimus arm, whereas no such decrease was observed in the placebo arm. In contrast, VEGF‐A level was increased with a mean fold‐change of 1.59 (95% CI 1.39, 1.80, P < 0.001), compared with baseline level at week 24, but there was no increase noticed in the placebo arm. These changes were noticeable as early as week 4, the first on‐treatment time point of evaluation, reached steady‐state by week 12, and were sustained until the end of the study after 48 weeks of treatment (Figure 2, Table 4). Based on a linear mixed model, VEGF‐D and COL‐IV levels between weeks 4 and 48 were significantly reduced with everolimus compared with placebo (P < 0.001 for treatment by time effect), and the changes in VEGF‐A and sVEGFR‐2 levels over time also reached statistical significance, compared with placebo (P = 0.032 and P = 0.015 for treatment by time effect, respectively) (Table 5). These observations demonstrated that the biomarker changes were due to pharmacodynamic effects of everolimus. Mean levels of sVEGFR‐1, c‐Kit and PLGF remained about the same over time compared with their baseline levels in either treatment arm (Figure 2, Table 4).
Table 4.
Biomarker levels at baseline and over time for patients receiving everolimus or placebo
| Baseline | Week 4 | Week 12 | Week 24 | Week 36 | Week 48 | ||
|---|---|---|---|---|---|---|---|
| VEGF‐A (pg ml −1 ) | |||||||
| Everolimus | Mean ± SD | 79.2 ± 69.8(n = 63) | 117.9 ± 137.3(n = 66) | 120.4 ± 85.9(n = 66) | 111.3 ± 76.5(n = 63) | 94.7 ± 54.5(n = 32) | 144.7 ± 89.8(n = 18) |
| Mean fold‐change (95% CI) | – | 1.51(1.33, 1.71)(n = 56) | 1.68(1.48, 1.91)(n = 56) | 1.59(1.39, 1.80)(n = 53) | 1.31(1.10, 1.56)(n = 26) | 1.79(1.45, 2.22)(n = 16) | |
| Placebo | Mean ± SD | 71.9 ± 49.7(n = 35) | 86.4 ± 73.8(n = 36) | 64.8 ± 45.8(n = 39) | 67.2 ± 42.3(n = 36) | 72.1 ± 45.3(n = 22) | 77.0 ± 55.1(n = 10) |
| Mean fold change (95% CI) | – | 1.10(0.92, 1.31)(n = 28) | 0.97(0.82, 1.16)(n = 29) | 1.04(0.88, 1.24)(n = 29) | 1.12(0.90, 1.38)(n = 18) | 0.90(0.66, 1.21)(n = 8) | |
| VEGF‐D (pg ml −1 ) | |||||||
|---|---|---|---|---|---|---|---|
| Everolimus | Mean ± SD | 2379.7 ± 2716.1(n = 73) | 1363.2 ± 1614.5(n = 74) | 795.6 ± 714.5(n = 72) | 762.8 ± 679.0(n = 72) | 707.2 ± 645.8(n = 60) | 674.5 ± 602.3(n = 35) |
| Mean fold‐change (95% CI) | – | 0.58(0.53, 0.64)(n = 69) | 0.38(0.35, 0.42)(n = 68) | 0.36(0.33, 0.40)(n = 67) | 0.34(0.31, 0.38)(n = 56) | 0.34(0.30, 0.38)(n = 33) | |
| Placebo | Mean ± SD | 2397.0 ± 2184.9(n = 35) | 2499.5 ± 2230.5(n = 37) | 2493.0 ± 2230.9(n = 38) | 2719.9 ± 2525.8(n = 35) | 2816.7 ± 2720.2(n = 32) | 2823.5 ± 2923.8(n = 19) |
| Mean fold‐change (95% CI) | – | 1.07(0.93, 1.22)(n = 33) | 1.05(0.92, 1.20)(n = 34) | 1.08(0.94, 1.23)(n = 32) | 1.09(0.95, 1.25)(n = 29) | 1.12(0.95, 1.32)(n = 16) | |
| sVEGFR1 (pg ml −1 ) | |||||||
|---|---|---|---|---|---|---|---|
| Everolimus | Mean ± SD | 98.8 ± 35.8(n = 63) | 106.6 ± 35.6(n = 66) | 107.0 ± 39.3(n = 66) | 101.1 ± 33.1(n = 63) | 101.1 ± 23.5(n = 32) | 108.6 ± 42.9(n = 18) |
| Mean fold‐change (95% CI) | – | 1.08(1.00, 1.16)(n = 59) | 1.05(0.98, 1.13)(n = 59) | 1.02(0.94 1.09)(n = 55) | 0.98(0.89, 1.08)(n = 28) | 0.98(0.87, 1.12)(n = 16) | |
| Placebo | Mean ± SD | 100.3 ± 27.1(n = 35) | 97.8 ± 32.3(n = 36) | 94.2 ± 28.3(n = 39) | 96.8 ± 23.0(n = 36) | 101.0 ± 23.6(n = 22) | 94.2 ± 34.5(n = 10) |
| Mean fold‐change (95% CI) | – | 0.93(0.85, 1.03)(n = 32) | 0.97(0.88, 1.07)(n = 35) | 0.98(0.89, 1.08)(n = 33) | 1.02(0.91, 1.15)(n = 19) | 1.03(0.87, 1.22)(n = 9) | |
| sVEGFR2 (pg ml −1 ) | |||||||
|---|---|---|---|---|---|---|---|
| Everolimus | Mean ± SD | 37918.2 ± 8015.3(n = 63) | 31437.6 ± 7296.3(n = 65) | 29633.1 ± 6440.4(n = 66) | 30779.7 ± 6038.1(n = 64) | 32472.6 ± 6637.1(n = 32) | 29808.1 ± 6997.9(n = 18) |
| Mean fold‐change (95% CI) | – | 0.83(0.73, 0.95)(n = 58) | 0.79(0.69, 0.89)(n = 59) | 0.81(0.72, 0.93)(n = 56) | 0.85(0.71, 1.02)(n = 28) | 0.79(0.62, 1.00)(n = 16) | |
| Placebo | Mean ± SD | 38219.5 ± 10697.6(n = 35) | 35383.8 ± 11169.0(n = 36) | 36729.3 ± 9633.7(n = 39) | 38213.1 ± 8503.9(n = 36) | 40973.8 ± 8023.8(n = 22) | 41663.6 ± 9975.1(n = 10) |
| Mean fold‐change (95% CI) | – | 0.70(0.59, 0.83)(n = 32) | 1.01(0.86, 1.19)(n = 35) | 1.01(0.86, 1.20)(n = 33) | 1.06(0.85, 1.32)(n = 19) | 1.07(0.78, 1.48)(n = 9) | |
| COL‐IV (μg l −1 ) | |||||||
|---|---|---|---|---|---|---|---|
| Everolimus | Mean ± SD | 285.1 ± 189.4(n = 75) | 165.2 ± 60.7(n = 74) | 144.4 ± 46.2(n = 75) | 137.9 ± 43.5(n = 73) | 137.5 ± 43.6(n = 63) | 137.8 ± 34.4(n = 36) |
| Mean fold‐change (95% CI) | – | 0.64(0.60, 0.67)(n = 71) | 0.56(0.53, 0.60)(n = 72) | 0.54(0.51, 0.57)(n = 70) | 0.53(0.50, 0.56)(n = 61) | 0.52(0.49, 0.52)(n = 34) | |
| Placebo | Mean ± SD | 320.9 ± 204.7(n = 36) | 299.3 ± 190.3(n = 38) | 315.4 ± 198.6(n = 39) | 329.4 ± 209.9(n = 36) | 326.4 ± 207.4(n = 33) | 327.2 ± 257.2(n = 20) |
| Mean fold‐change (95% CI) | – | 0.97(0.90, 1.05)(n = 35) | 1.01(0.93, 1.09)(n = 36) | 1.04(0.96, 1.13)(n = 34) | 1.03(0.96, 1.12)(n = 31) | 1.05(0.96, 1.14)(n = 18) | |
| c‐Kit (pg ml ‐1 ) | |||||||
|---|---|---|---|---|---|---|---|
| Everolimus | Mean ± SD | 100027.8 ± 27442.2(n = 63) | 105171.3 ± 28493.6(n = 65) | 100331.2 ± 25554.5(n = 66) | 100340.7 ± 26737.2(n = 64) | 101462.2 ± 25297.2(n = 32) | 100332.3 ± 21889.4(n = 18) |
| Mean fold‐change (95% CI) | – | 1.04(0.90, 1.20)(n = 58) | 1.00(0.87, 1.15)(n = 59) | 0.99(0.86, 1.15)(n = 56) | 1.02(0.83, 1.24)(n = 28) | 0.99(0.76, 1.29)(n = 16) | |
| Placebo | Mean ± SD | 103951.6 ± 30678.4(n = 35) | 93409.0 ± 32289.6(n = 36) | 96593.5 ± 27125.8(n = 39) | 99908.5 ± 22516.5(n = 36) | 106747.4 ± 22122.6(n = 22) | 91277.6 ± 14335.0(n = 10) |
| Mean fold‐change (95% CI) | – | 0.68(0.56, 0.82)(n = 32) | 1.03(0.86, 1.23)(n = 35) | 1.02(0.85, 1.23)(n = 33) | 1.07(0.84, 1.37)(n = 19) | 0.98(0.69, 1.39)(n = 9) | |
| PLGF (pg ml ‐1 ) | |||||||
|---|---|---|---|---|---|---|---|
| Everolimus | Mean ± SD | 11.8 ± 5.2(n = 63) | 11.1 ± 4.8(n = 66) | 12.5 ± 4.7(n = 66) | 12.1 ± 4.8(n = 63) | 12.8 ± 5.1(n = 32) | 13.9 ± 5.5(n = 18) |
| Mean fold‐change (95% CI) | – | 0.90(0.81, 0.99)(n = 55) | 1.05(0.95, 1.16)(n = 56) | 1.04(0.94 1.16)(n = 52) | 0.98(0.86, 1.12)(n = 28) | 1.03(0.87, 1.23)(n = 16) | |
| Placebo | Mean ± SD | 12.2 ± 4.0(n = 35) | 11.6 ± 4.3(n = 36) | 12.0 ± 3.9(n = 39) | 13.2 ± 4.2(n = 36) | 12.3 ± 3.7(n = 22) | 12.4 ± 3.4(n = 10) |
| Mean fold‐change (95% CI) | – | 0.92(0.81, 1.05)(n = 32) | 1.04(0.91, 1.18)(n = 34) | 1.07(0.94, 1.22)(n = 33) | 1.05(0.89, 1.23)(n = 19) | 1.05(0.83, 1.33)(n = 9) | |
COL‐IV, collagen type IV; PLGF, placental growth factor; VEGF‐A, vascular endothelial growth factor A; VEGF‐D, vascular endothelial growth factor D; sVEGFR1, soluble VEGF receptor 1; sVEGFR2, soluble VEGF receptor 2.
Figure 2.
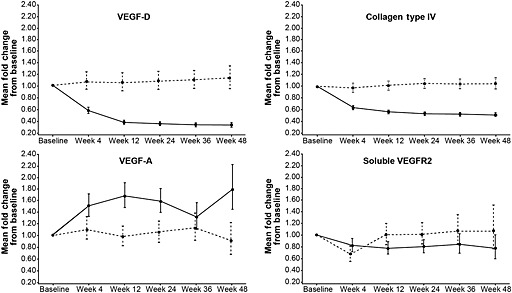
Longitudinal plots of mean fold‐change in levels of vascular endothelial growth factor D (VEGF‐D), collagen type IV (COL‐IV), vascular endothelial growth factor A (VEGF‐A) and soluble VEGF receptor 2 (VEGFR2) over time. ( ) everolimus, (
) everolimus, ( ) placebo
) placebo
Table 5.
Test of effect for covariates in the linear mixed model of fold‐change from baseline
| Biomarker | P value for test of effect | |||
|---|---|---|---|---|
| Baseline | Time | Treatment | Time × treatment | |
| VEGF‐A | <0.001 | 0.941 | <0.001 | 0.032 |
| VEGF‐D | <0.001 | <0.001 | <0.001 | <0.001 |
| sVEGFR1 | <0.001 | 0.997 | 0.484 | 0.218 |
| sVEGFR2 | <0.001 | 0.059 | 0.027 | 0.015 |
| COL‐IV | <0.001 | 0.011 | <0.001 | <0.001 |
| PLGF | <0.001 | 0.014 | 0.713 | 0.966 |
| c‐Kit | <0.001 | 0.089 | 0.414 | 0.022 |
COL‐IV, collagen type IV; PLGF, placental growth factor; VEGF‐A, vascular endothelial growth factor A; VEGF‐D, vascular endothelial growth factor D; sVEGFR1, soluble VEGF receptor 1; sVEGFR2, soluble VEGF receptor 2.
Association between everolimus Cmin and pharmacodynamics
Moderate but statistically significant associations were observed between higher everolimus C min exposure and a greater magnitude of down regulation of both VEGF‐D and COL‐IV (P < 0.001 and P = 0.003, respectively; Table 6). There was a statistically significant association between the magnitude of sVEGFR1 level increases and higher everolimus C min (P = 0.019). However, no statistically significant association between C min and the VEGF‐A level was seen (P = 0.101; Table 6).
Table 6.
Relationship between percentage change from baseline in biomarkers and time‐normalized C min (only everolimus patients)
| Biomarker | Patients, n | Samples used in the model, n | Percentage change per 2‐unit C min increase, Estimate (95% CI) | P value |
|---|---|---|---|---|
| VEGF‐A | 46 | 149 | 3.205 (−0.622, 7.180) | P = 0.101 |
| VEGF‐D | 57 | 228 | −6.429 (−9.275, −3.493) | P < 0.001 |
| sVEGFR1 | 46 | 156 | 2.203 (0.368, 4.072) | P = 0.019 |
| sVEGFR2 | 46 | 155 | −0.460 (−1.567, 0.659) | P = 0.416 |
| COL‐IV | 59 | 242 | −1.853 (−3.048, −0.644) | P = 0.003 |
| PLGF | 44 | 149 | 1.908 (−1.066, 4.972) | P = 0.208 |
| c‐Kit | 46 | 155 | 0.290 (−0.731, 1.321) | P = 0.576 |
COL‐IV, collagen type IV; PLGF, placental growth factor; VEGF‐A, vascular endothelial growth factor A; VEGF‐D, vascular endothelial growth factor D; sVEGFR1, soluble VEGF receptor 1; sVEGFR2, soluble VEGF receptor 2.
Association of baseline biomarker level with angiomyolipoma response
To explore the utility of these two biomarkers for predicting efficacy to everolimus treatment, we investigated the associations between baseline biomarker levels and both the angiomyolipoma response rate (percent of patients with ≥ 50% volume reduction from baseline) and the best response of the lesions at any assessment point in everolimus‐treated patients. When the patients were divided into high vs. low baseline biomarker level subgroups by the median levels of VEGF‐D and COL‐IV, the response rate was significantly higher in patients with low COL‐IV levels than in those with high COL‐IV levels (60.5% vs. 32.4%, respectively, odds ratio 3.19 95% CI 1.24, 8.23, P = 0.016). The same trend was seen between patients with low and high VEGF‐D levels, although without reaching statistical significance with median as the cut‐point for the subgroups (56.4% vs. 35.3%, respectively, odds ratio 2.37, 95% CI, 0.92, 6.11, P = 0.074). When baseline levels of COL‐IV and VEGF‐D were plotted against the best response to treatment (best percentage change in angiomyolipoma volume), no strong relationship was apparent between the angiomyolipoma volume reduction in response to treatment and the levels of either biomarker (Figure 3). These results suggest that baseline COL‐IV level may serve as a predictive biomarker of everolimus response at a population level, but baseline concentration of both COL‐IV and VEGF‐D biomarkers are probably unable to predict the treatment effect on individual angiomyolipoma with sufficient clinical utility.
Figure 3.
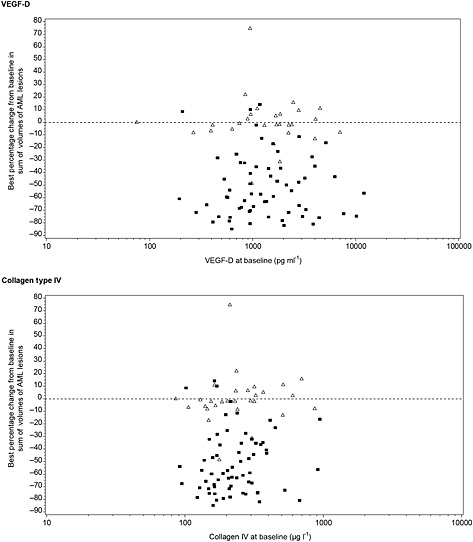
Scatter plots showing relationship between VEGF‐D and collagen type IV (COL‐IV) levels at baseline with best percentage change from baseline in the sum of volumes of target renal angiomyolipoma lesions (according to central radiology review). ( ) everolimus, (
) everolimus, ( ) placebo
) placebo
Discussion
The EXIST‐2 study was the first large randomized study to detail the effect of everolimus on angiomyolipoma growth in adult patients with TSC or sLAM 20. The present analysis of everolimus exposure and association with pharmacodynamic biomarkers will contribute to a deeper understanding of everolimus activity in this patient population. Furthermore, the findings provide new information about biomarkers that could potentially help to monitor angiomyolipoma lesion burden or predict the benefit of treatment. It is important to note that the randomized, placebo‐controlled trial design enabled both an examination of exposure indices and pharmacodynamic changes over time as well as of everolimus pharmacodynamic effects with respect to tumour and the surrogate biomarker concentrations examined.
The mean C min and C 2 h of everolimus measured in this trial were consistent with those observed in other everolimus trials in early or advanced solid tumours (including breast cancer, colorectal cancer, gastric cancer, lung cancer, prostate cancer, kidney cancer, melanoma and sarcoma) receiving a similar dose of the drug 20, 27, 28, 29. The mean C min for patients not using EIAEDs at randomization who received the actual planned dose of 10 mg once daily was within the range of trough concentrations observed in those previous everolimus studies with the same dose. The wide interindividual variability observed for the C min and C 2 h values listed in Tables 1, 2 was possibly due to the inclusion of data from patients irrespective of actual administered dose and/or with or without coadministration of cytochrome P450 (CYP3A4) enzyme inducers. We anticipated the significant effect of EIAEDs in decreasing everolimus C min and C 2 h in those patients with EIAED use at randomization. EIAEDs induce the enzymatic activity of CYP3A4, thereby increasing the metabolism of everolimus when co‐administered because everolimus is extensively metabolized by CYP3A4 enzymes and is a substrate of P‐glycoprotein 30. In the EXIST‐2 trial, patients were stratified by EIAED use at randomization to avoid potential effects of EIAEDs on efficacy and safety outcomes. In addition, a linear model analysis suggested that relative changes, and not absolute changes, from baseline in angiomyolipoma lesion volume were associated with everolimus C min, probably due to a large inter‐subject difference in baseline angiomyolipoma lesion volume.
This analysis was the first to examine multiple angiogenesis‐related biomarkers in TSC patients with angiomyolipomas and to investigate the association of everolimus treatment with circulating biomarker levels. Circulating levels of VEGF‐A, VEGF‐D, COL‐IV and sVEGFR2 have previously been found to be increased in patients with angiomyolipomas associated with TSC, reflecting the vascular pericytic origins of angiomyolipoma lesions 7. In EXIST‐2, the observed decrease in VEGF‐D, COL‐IV and sVEGFR2 levels and concomitant increase in VEGF‐A levels from baseline in the patients treated with everolimus supports the hypothesis that everolimus may, at least partially, act through an anti‐angiogenic mechanism in these patients. Because EXIST‐2 was a placebo‐controlled trial, the present analysis was able to demonstrate that the dynamic changes of the biomarkers were directly associated with the administration of everolimus, as biomarker levels did not change markedly in the placebo arm. The moderate decrease in sVEGFR2 level and lack of everolimus effect on s‐VEGFR1, c‐Kit and PLGF levels have been observed in every everolimus trial in which these biomarkers were assessed, including trials for renal cell carcinoma and subependymal giant cell astrocytomas associated with TSC 31, 32. The observed trend of decreased sVEGFR2 accompanying increased VEGF‐A levels may suggest that a negative feedback loop exists between the sVEGFR2 receptor and its ligand, VEGF‐A. Interestingly, in a previous study, no significant change in VEGF‐A levels was observed in patients with advanced renal cell carcinoma treated with everolimus, even though sVEGFR2 levels decreased over the same period 31. In human ovarian cancer cell lines and in mouse tumour xenograft models treated with everolimus, the level of VEGF‐A had actually decreased 33. This suggests that the marked increase in VEGF‐A levels during everolimus therapy in patients with angiomyolipoma might be unique to this disease and needs further investigation.
Down regulation of circulating VEGF‐D by mTOR inhibition has previously been reported in sirolimus‐treated patients with TSC and/or LAM 21, 34, 35, 36. In a small (28 patients) single arm study, oral sirolimus decreased VEGF‐D levels by 70% at 12 months in patients with renal angiomyolipomas associated with TSC and/or LAM 21. In a placebo‐controlled trial of 89 women with LAM (MILES trial), sirolimus decreased VEGF‐D levels by more than 50% after 12 months, compared with no change in the placebo group 35. All of these findings are very similar to the 64% reduction of VEGF‐D level at 6 months observed in this study, suggesting that this pharmacodynamic effect is likely a class effect of mTOR inhibitors. The correlation observed in our study between a decrease in VEGF‐D and an increase in everolimus exposure provides further evidence that VEGF‐D down regulation is related to the effect of mTOR inhibition by everolimus.
We previously demonstrated a significant correlation between the baseline levels of both VEGF‐D and COL‐IV with the baseline angiomyolipoma volume in the trial population and, moreover, in everolimus‐treated patients, a statistically significant correlation between VEGF‐D pharmacodynamic change and response to treatment (the angiomyolipoma volume decrease) at one time point (baseline vs. week 24), suggesting that these biomarkers are of potential clinical utility in monitoring disease burden and everolimus treatment effect over time. This is of particular importance for children or disabled patients requiring general anaesthesia when performing MRI scans. Of particular interest in this study, low baseline COL‐IV levels (i.e. below median) might derive a larger everolimus benefit, as measured by a higher angiomyolipoma response rate. COL‐IV has been shown to be produced in excess by angiomyolipomas 7. These two biomarkers, therefore, could potentially help to predict the response to everolimus before treatment is initiated and then help to monitor the response to everolimus longitudinally.
In conclusion, these data provide additional important information about exposure to everolimus and pharmacodynamics of everolimus treatment in patients with renal angiomyolipomas associated with TSC or sLAM. A statistically significant relationship was found between the percentage change from baseline in angiomyolipoma lesion volume and C min. Everolimus decreased VEGF‐D, COL‐IV, and sVEGFR2 levels and increased VEGF‐A levels, suggesting that mTOR modulates the angiogenesis pathway in TSC. The lack of correlation between baseline plasma VEGF‐D and COL‐IV levels with response to treatment supported that all patients, regardless of their baseline biomarker levels, should receive everolimus treatment. The potential of using these biomarkers to monitor objectively and conveniently the response to everolimus over time in TSC patients with angiomyolipoma warrants further investigation.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that writing and editorial support have been funded by Novartis. WC, SU, TB, KS and DC were employees of Novartis in the previous 3 years. KB, JCK, BAZ, MF and JJB have received honoraria and/or research grants or served as a consultant for Novartis in the previous 3 years. KB has also served as consultant for Bristol‐Myers Squibb, Effimune, Hexal, Pfizer, and Veloxis, and has received research grants for clinical studies, speaker fees, honoraria, travel expenses and payment for development of educational presentations from Astellas, Aicuris, BmT GmbH, Bristol‐Myers Squibb, Chiesi, Fresenius, Hexal, Otsuka, Pfizer, Roche, Siemens and Veloxis.
Author contributions
JJB, DC, WC and JCK participated in the study design and discussions. DC and WC oversaw data collection. JJB, KS, TB, DC, WC, SU and JCK participated in the data analysis, interpretation and discussions. KB, JCK and JJB performed the literature review. KB, BAZ, MF, JCK and JJB recruited and enrolled patients. All authors reviewed and edited drafts and approved the final draft of the manuscript for submission.
We thank our patients for their participation and contribution to the trial and the investigators, study nurses and clinical research associates from the individual trial centres who provided ongoing support. We thank Wei He for statistical support and Traci Stuve, MA, Robert Schoen, Pharm D and Alison Comer, PhD of ApotheCom for editorial assistance, which was funded by Novartis Pharmaceuticals Corporation.
Supporting information
Table S1 List of institutional review boards
Table S2 Lower limit of determination and coefficient of variation for biomarker assessments
Supporting info item
Supporting info item
Budde, K. , Zonnenberg, B. A. , Frost, M. , Cheung, W. , Urva, S. , Brechenmacher, T. , Stein, K. , Chen, D. , Kingswood, J. C. , and Bissler, J. J. (2016) Pharmacokinetics and pharmacodynamics of everolimus in patients with renal angiomyolipoma and tuberous sclerosis complex or lymphangioleiomyomatosis. Br J Clin Pharmacol, 81: 958–970. doi: 10.1111/bcp.12834.
References
- 1. Huang J, Manning BD. The TSC1‐TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008; 412: 179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Budde K, Gaedeke J. Tuberous sclerosis complex‐associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis 2012; 59: 276–83. [DOI] [PubMed] [Google Scholar]
- 3. Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006; 355: 1345–56. [DOI] [PubMed] [Google Scholar]
- 4. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet 2008; 372: 657–68. [DOI] [PubMed] [Google Scholar]
- 5. Franz DN, Bissler JJ, McCormack FX. Tuberous sclerosis complex: neurological, renal and pulmonary manifestations. Neuropediatrics 2010; 41: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dixon BP, Hulbert JC, Bissler JJ. Tuberous sclerosis complex renal disease. Nephron Exp Nephrol 2011; 118: e15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siroky BJ, Yin H, Dixon BP, Reichert RJ, Hellmann AR, Ramkumar T, Tsuchihashi Z, Bunni M, Dillon J, Bell PD, Sampson JR, Bissler JJ. Evidence for pericyte origin of TSC‐associated renal angiomyolipomas and implications for angiotensin receptor inhibition therapy. Am J Physiol Renal Physiol 2014; 307: F560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiol 2002; 225: 78–82. [DOI] [PubMed] [Google Scholar]
- 9. Eble JN. Angiomyolipoma of kidney. Semin Diagn Pathol 1998; 15: 21–40. [PubMed] [Google Scholar]
- 10. Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int 2004; 66: 924–34. [DOI] [PubMed] [Google Scholar]
- 11. Cudzilo CJ, Szczesniak RD, Brody AS, Rattan MS, Krueger DA, Bissler JJ, Franz DN, McCormack FX, Young LR. Lymphangioleiomyomatosis screening in women with tuberous sclerosis. Chest 2013; 144: 578–85. [DOI] [PubMed] [Google Scholar]
- 12. McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133: 507–16. [DOI] [PubMed] [Google Scholar]
- 13. Franz DN, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, Sethuraman G, Colby TV, Kwiatkowski DJ, McCormack FX. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med 2001; 164: 661–8. [DOI] [PubMed] [Google Scholar]
- 14. Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75: 591–4. [DOI] [PubMed] [Google Scholar]
- 15. Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A 2000; 97: 6085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeoh ZW, Navaratnam V, Bhatt R, McCafferty I, Hubbard RB, Johnson SR. Natural history of angiomyolipoma in lymphangioleiomyomatosis: implications for screening and surveillance. Orphanet J Rare Dis 2014; 9: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El‐Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res 2003; 63: 5173–7. [PubMed] [Google Scholar]
- 18. Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002; 20: 4368–80. [DOI] [PubMed] [Google Scholar]
- 19. Seyama K, Kumasaka T, Souma S, Sato T, Kurihara M, Mitani K, Tominaga S, Fukuchi Y. Vascular endothelial growth factor‐D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol 2006; 4: 143–52. [DOI] [PubMed] [Google Scholar]
- 20. Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ, Whittemore VH, Chen D, Sahmoud T, Shah G, Lincy J, Lebwohl D, Budde K. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST‐2): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2013; 381: 817–24. [DOI] [PubMed] [Google Scholar]
- 21. Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ Jr, Miles D, Cutler D, Krueger D, Uppot RN, Rabenou R, Camposano S, Paolini J, Fennessy F, Lee N, Woodrum C, Manola J, Garber J, Thiele EA. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF‐D levels decrease. PLoS One 2011; 6: e23379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu KF, Zhang P, Tian X, Ma A, Li X, Zhou J, Zeng N, Gui YS, Guo Z, Feng R, Zhang W, Sun W, Cai B. The role of vascular endothelial growth factor‐D in diagnosis of lymphangioleiomyomatosis (LAM). Respir Med 2013; 107: 263–8. [DOI] [PubMed] [Google Scholar]
- 23. Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Souter M, Nonomura N, Brakemeier S, de Vries PJ, Berkowitz N, Segal S, Anak O, Peyrard S, Budde K. Everolimus for renal angiomyolipoma associated with tuberous sclerosis complex (TSC): EXIST‐2 ~ 3‐year follow‐up. Poster presented at 29th Annual Congress of the European Association of Urology; April 11–15, 2014; Stockholm, Sweden.
- 24. Kirchner GI, Meier‐Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet 2004; 43: 83–95. [DOI] [PubMed] [Google Scholar]
- 25. US Department of Health and Human Services . Guidance for Industry. Bioanalytical Method Validation. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. May 2001. Accessed October 30, 2015.
- 26. Peveling‐Oberhag J, Zeuzem S, Yong WP, Kunz T, Paquet T, Bouillaud E, Urva S, Anak O, Sellami D, Kobalava Z. Effects of hepatic impairment on the pharmacokinetics of everolimus: a single‐dose, open‐label, parallel‐group study. Clin.Ther 2013; 35: 215–25. [DOI] [PubMed] [Google Scholar]
- 27. Xu B, Wu Y, Shen L, Ye D, Jappe A, Cherfi A, Wang H, Yuan R. Two dose‐level confirmatory study of the pharmacokinetics and tolerability of everolimus in Chinese patients with advanced solid tumors. J Hematol Oncol 2011; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Donnell A, Faivre S, Burris HA III, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM, Brock C, Jones S, Raymond E, Judson I. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 2008; 26: 1588–95. [DOI] [PubMed] [Google Scholar]
- 29. Okamoto I, Doi T, Ohtsu A, Miyazaki M, Tsuya A, Kurei K, Kobayashi K, Nakagawa K. Phase I clinical and pharmacokinetic study of RAD001 (everolimus) administered daily to Japanese patients with advanced solid tumors. Jpn J Clin Oncol 2010; 40: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacobsen W, Serkova N, Hausen B, Morris RE, Benet LZ, Christians U. Comparison of the in vitro metabolism of the macrolide immunosuppressants sirolimus and RAD. Transplant Proc 2001; 33: 514–5. [DOI] [PubMed] [Google Scholar]
- 31. Oudard S, Escudier BJ, Thompson JA, Grunwald V, Masini C, Bracarda S, Paneerselvam A, Gogov S, Chen D, Motzer RJ. Relationship between biomarkers and everolimus efficacy in the phase III RECORD‐1 trial of patients with metastatic renal cell carcinoma (mRCC). Proceedings of the American Society of Clinical Oncology Annual Meeting. J Clin Oncol 2013; 31 (suppl 6) : abstract 352. [Google Scholar]
- 32. Franz DN, Kingswood C, Jozwiak S, Budde K, Belousova E, Sparagana S, Zonnenberg B, Frost M, Shah G, Lebrec J, Ford J, Kalfoglou C, Chen D, Bissler JJ. Effect of everolimus on angiogenic biomarkers in patients with tuberous sclerosis complex (TSC): results from EXIST‐1 and EXIST‐2. Poster presented at: 48th Annual Meeting of the American Society of Clinical Oncology; June 1–5, 2012; Chicago, Illinois.
- 33. van der Bilt AR, Terwisscha van Scheltinga AG, Timmer‐Bosscha H, Schröder CP, Pot L, Kosterink JG, van der Zee AG, Lub‐de Hooge MN, de Jong S, de Vries EG, Reyners AK. Measurement of tumor VEGF‐a levels with 89Zr‐bevacizumab PET as an early biomarker for the antiangiogenic effect of everolimus treatment in an ovarian cancer xenograft model. Clin Cancer Res 2012; 18: 6306–14. [DOI] [PubMed] [Google Scholar]
- 34. Malinowska IA, Lee N, Kumar V, Thiele EA, Franz DN, Ashwal S, Sagalowsky A, Dimario FJ Jr, Cutler D, Krueger D, Camposano S, Paolini J, Dabora SL. Similar trends in serum VEGF‐D levels and kidney angiomyolipoma responses with longer duration sirolimus treatment in adults with tuberous sclerosis. PLoS One 2013; 8: e56199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP 3rd, Goldberg HJ, Young LR, Kinder BW, Downey GP, Sullivan EJ, Colby TV, McKay RT, Cohen MM, Korbee L, Taveira‐DaSilva AM, Lee HS, Krischer JP. Trapnell BC, National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 2011; 364: 1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Young LR, Lee HS, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP 3rd, Goldberg HJ, Downey GP, Swigris JJ, Am Taveira‐DaSilva, Krischer JP, Trapnell BC, McCormack FX, MILES Trial Group . Serum VEGF‐D concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med 2013; 1: 445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of institutional review boards
Table S2 Lower limit of determination and coefficient of variation for biomarker assessments
Supporting info item
Supporting info item


