Abstract
Diabetes mellitus is a major health concern of the developing and developed nations across the globe. This devastating disease accounts for the 5% deaths around the world annually. The current treatment methods do not address the underlying causes of the disease and have severe limitations. Stem cells are unique cells with the potential to differentiate into any type of specialized cells. This feature of both adult and embryonic stem cells was explored in great detail by the scientists around the world and are successful in producing insulin secreting cells. The different type of stem cells (induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs) and adult stem cells) proves to be potent in treating diabetes with certain limitations. This article precisely reviews the resources and progress made in the field of stem cell research for diabetic treatment.
Keywords: Stem cells, Diabetes, β-Cells, Induced pluripotency
1. Introduction
Diabetes mellitus (DM) is most devastating, chronic, common non-communicable disease (NCD) and has become a serious problem globally. The number of the diabetic population around the globe is continuously increasing with a current estimation of 371 million cases in 2012 and it is expected to reach 552 million by 2030 (IDF diabetes atlas, 2012). It is also estimated that 5% of all deaths in the world are caused by diabetes and the number is rapidly increasing. Among two general types of diabetes, type 1 diabetes (TIDM) is characterized by immune complex mediated attack on insulin producing β cells of the pancreas (Atkinson and Eisenbarth, 2001). Type 2 diabetes (T2DM) arises due to either insufficient insulin synthesis or the body’s inability to respond secreted insulin and leading to glucose build-up in the blood (DeFronzo, 1997). The impairment in glucose control leads to both micro and macro vascular complications that often result in the other clinical conditions associated with diabetes.
The chemical methods of treatment for diabetes do not address the causes of the disease and have side effects. Thus, there is an obvious search for the suitable alternative treatment methods. The current cellular based therapeutic method for the treatment of diabetes is focused on the transplantation of either the pancreas or islet-cells to reconstitute the insulin-secreting functional β cells. However, this technique is hampered by a shortage of donor organs. All these issues paved the way to explore the research possibilities of generating pancreatic β cells from stem cells. The unique regenerative properties of stem cells could be a vital tool which can be exploited in the treatment of diabetes. Developing a renewable source of islets with stem cells would circumvent the current supply/demand issues in islet transplantation and provide patients with a long-term source of insulin-producing β-cells. Thus, stem cell investigation has become the centre of attraction for diabetic treatment (Mccall et al., 2009). This article reviews the progress of stem cell research made in the field of diabetic treatment and practical hurdles associated with it.
2. Stem cells and their therapeutic potential
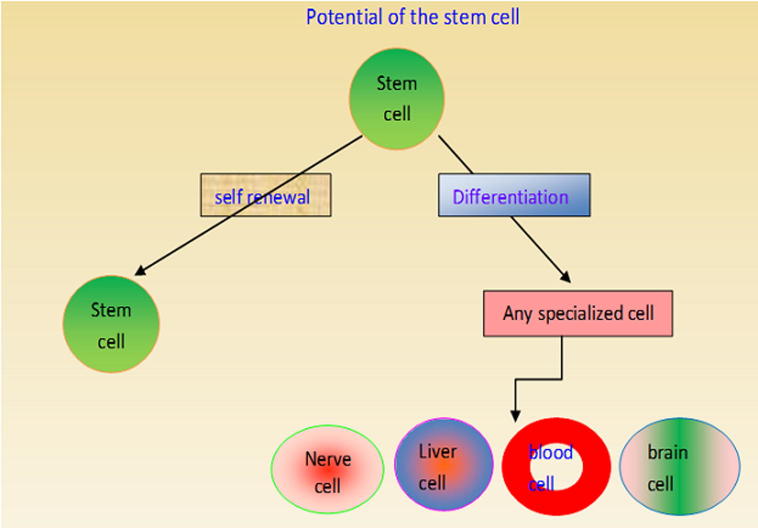
The stem cells are more gifted and are responsible for the formation of different types of cells during the early embryonic life and the later growth of the organism. Stem cells possess an exceptional quality to replenish itself and to produce any specialized cell types under appropriate microenvironment. A rapidly dividing stem cell produces two new cells, each having two choices depending upon the requirement of the organism. Thus, a newly produced cell either may remain as a stem cell or it may undergo further differentiation to become a more specialized cell with specific function. The stem cells have the potential to become any type of specialized cell such as a myocyte, blood cell, hepatocyte and brain cell (Fig. 1). The stem cells can rapidly divide in some organs such as bone marrow and gut to repair and replace the damaged tissues. However, in some other organs such as in the heart and pancreas, the stem cells remain as a resident cell and undergo division only under specific requirement.
Figure 1.
Self renewal and differentiation potential of the stem cells.
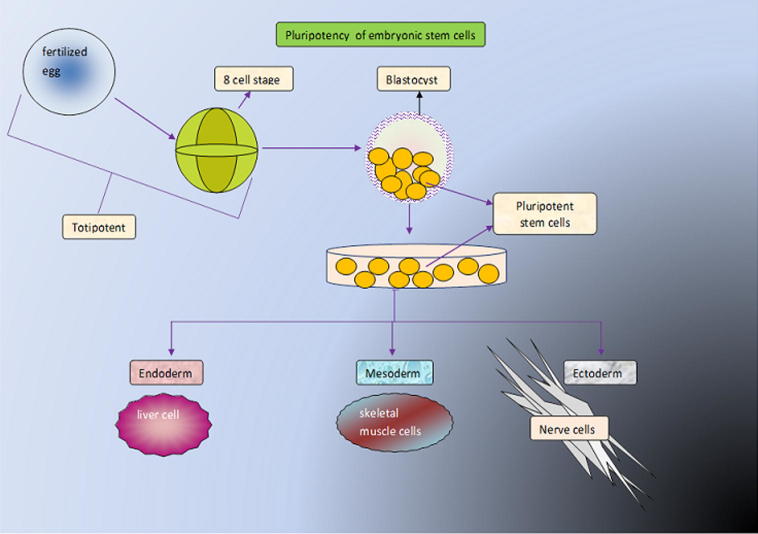
The stem cells can be classified in many ways based on the origin, potential, source and method of derivation etc. In general, based on the source, stem cells can be broadly classified into two types as embryonic stem cells (ESCs) and adult stem cells. The ESCs are obtained from the inner cell mass of a 4–5-day old embryo (blastocyst). Nearly, three decades ago, the researchers isolated the ESCs from the mouse embryos (Evans and Kaufman, 1981, Martin, 1981). Later, Thomson et al. (1998) were successful enough to isolate and continuously culture the ESCs of a human blastocyst (Thomson et al., 1998). The ESC are capable of forming the cells of all three germ layers and they are termed as pluripotent stem cells (Fig. 2). The adult stem cells are very rare stem cells found in almost all major organs and are referred as multipotent cells due to their limited ability to differentiate.
Figure 2.
Pluripotent stem cell forms the cells of all there germ layers.
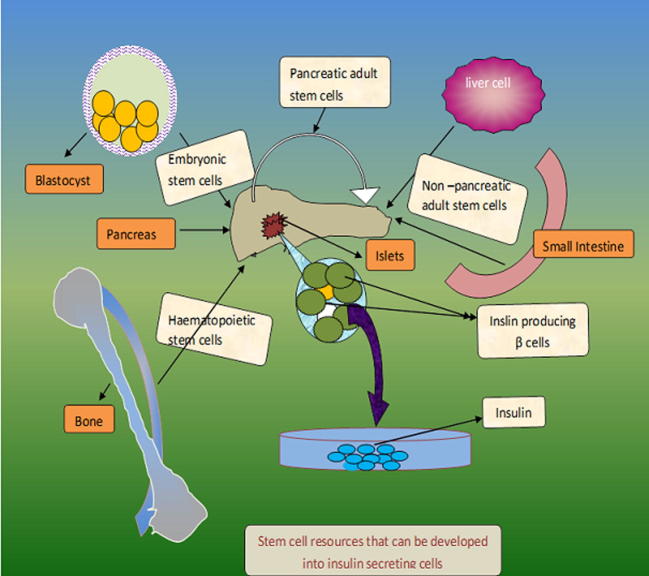
Scientists around the globe are making a considerable effort to make use of different type stem cells for the treatment of various medical conditions (Liu et al., 2013). Although as yet there have been no approved treatments using ESCs, adult stem cell use has become fairly common in medical practice. Bone marrow transplantation employs hematopoietic stem cells (HSCs) taken from donor marrow to successfully treat leukaemia and other haematological malignancies. This success has led researchers to explore other uses, including the treatment of stroke (Haas et al., 2005), blindness (Adler, 2008) and even myocardial infarctions (Meyer et al., 2007). The stem cell based therapy for diabetes, aims to replace the diseased or lost cells of the pancreas by using pluripotent or multipotent stem cells. The research to date has explored the potential of different type of stem cells (induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs) and adult stem cells) by various approaches to generate surrogate β cells or to restore β-cell function (Fig. 3).
Figure 3.
Different types of stem cell resources with a potential to be developed into insulin secreting cells.
3. Adult stem cells and diabetes
3.1. Pancreatic stem cells
The technological advancements over a decade enabled the scientific community to derive stem cells from various types of tissue sources including bone marrow, umbilical cord blood, adipose tissue, skin, periosteum, dental pulp etc. The pancreas is an organ of first choice to be looking for the potential stem cells. Animal studies have shown that the availability of small amounts of pancreatic tissue would restore the maximum pancreatic β-cell mass (Bonner-Weir et al., 1993). This is due to the replication of differentiated β-cells of pancreatic ducts and dedifferentiation of these cells to pluripotent cells that in turn produce more β-cells. Further investigation revealed that this population of ductal cells could, in vitro, be cultivated and directed to form insulin producing clusters (Bonner-Weir et al., 2000, Gao et al., 2003). Seaberg et al. (2004) developed a clonal population of adult pancreatic precursor cells from ductal cells that can produce both C-peptide and insulin. Studies have shown that the islets of both rodent and human contains multipotent stem cells (Eberhardt et al., 2006, Zulewski et al., 2001). Earlier, many researchers raised their speculation about the existence of pancreatic adult stem, despite the progress and promise of pancreatic stem cells (Dor et al., 2004). Later, Xu et al. (2008) have provided a strong evidence for the existence of multipotent progenitor cells in the pancreatic ducts of mice that can give rise to new β-cells (Xu et al., 2008). These studies and other reports conclusively prove two facts, first the existence of pancreatic stem cell and second the β-cells can be formed from non-β-cells. However, further research needs to focus on finding and activating pancreatic stem cells in diabetic patients to promote β-cell formation. More research strategies are required to develop suitable methods to address the issue of isolation and ex vivo expansion of these stem cells for transplantation (Michael et al., 2010).
3.2. Haemopoietic progenitor cells
The adult stem cells of the haemopoietic system, like HSCs and mesenchymal stem cells (MSCs) are having the ability to transdifferentiate into a number of other cell lineages, including the liver, brain, lung and even gastrointestinal tract cells (Brazelton et al., 2000, Jiang et al., 2002, Krause et al., 2001, Lagasse et al., 2000). This multipotent differentiation of haemapoietic progenitors was explored in greater detail by various groups of researchers to replenish the β-cell population in T1DM.
The mouse bone marrow cells were differentiated into functionally competent β-cells in an in vivo experiment (Ianus et al., 2003). Similar experiments with mice showed that bone marrow cells can be targeted to the pancreas and that hyperglycaemia can be reversed (Hess et al., 2003). Studies carried out with autologous HSCs showed improvement in both type 1 (Couri et al., 2009) and type 2 diabetes mellitus (Estrada et al., 2008). These trials provide promising results for the use of autologous HSCs in the treatment of diabetes.
3.3. Other adult stem cells
The liver and small intestine are extensively studied as potential sources of pancreatic β-cells. These organs have an edge over others as they are derived from endoderm along with the pancreas. Different research groups around the nations have successfully transdifferentiated rodent hepatic cells into insulin-producing cells via multiple genetic approaches (Ber et al., 2003, Kim et al., 2007, Sapir et al., 2005, Yang et al., 2002). Regardless of the method used, amelioration of hyperglycaemia was achieved by these cells in the mouse models. This created hope among the researchers to search for extra-pancreatic sources of insulin (Zalzman et al., 2003).
Many other stem cell resources have been explored for the production of insulin secreting β cells and different degrees of success have been achieved with them. The resources include the stem cells of the small intestine (Suzuki et al., 2003, Yoshida et al., 2002), salivary glands (Okumura et al., 2003) and adipose tissue (Timper et al., 2006). However, many experimental results and the embryological proximity of the liver to pancreas suggest that this organ could be an ideal non-pancreatic stem cell source of β-cells that can be utilized the cure of diabetes. In the years to come, the hepatic production of insulin has the potential to become a viable source for β-cell replacement. This is possible not before addressing the practical hurdles associated with these cell lines, culture conditions, complete differentiation, and islet structure formation etc. (Michael et al., 2010).
4. ESCs and diabetes
The pluripotent nature of the ESCs has been hailed for long by the researchers and these cells are explored for their use in a number of medical conditions, including diabetes (Trounson, 2013). ESCs are viewed as an excellent resource for the generation of insulin secreting islet cells through the established developmental and differentiation pathways. Theoretically it is possible, despite the difficulties, that ESCs could be directed to differentiate into pancreatic islet cells and these cells could then be implanted in patients with diabetes, thus the β-cell deficit could be circumvented.
Over a decade ago, pancreatic islet cells were produced from mouse ESCs. The researchers developed insulin-secreting clones from a genetically engineered and drug-selected mouse ESC line. These cells were transplanted into diabetic mice and resulted in the amelioration of hyperglycaemia for few months (Soria et al., 2000). A number of other groups have also utilized both mouse (Blyszczuk et al., 2004, Hori et al., 2002, Kahan et al., 2003, Lumelsky et al., 2001, Leon-Quinto et al., 2004) and human (Assady et al., 2001, Segev et al., 2004) ESCs for their studies and have reported the different degree of success in producing islets. All these efforts have come across different issues that include final cell homogeneity (Lumelsky et al., 2001), immaturity of the differentiated cells (Segev et al., 2004), low numbers of insulin-producing cells (Hori et al., 2005) and a poor insulin response when the cells were exposed to glucose (Assady et al., 2001, Miyazaki et al., 2004). To add this, few groups of researchers also argued that these cells were not actually insulin producing cells as they could not produce C-peptide and intracellular insulin once the cells were cultured in insulin-free medium (Hansson et al., 2004, Rajagopal et al., 2003, Sipione et al., 2004). Despite the ray of hope, it was certainly proving difficult to create reliable insulin-producing β-cell phenotype from ESCs. All these issues collectively forced the researchers to rethink their differentiation strategies and Kubo et al. (2004) developed a recipe to convert mouse ESCs into definitive endoderm (Kubo et al., 2004). This protocol was redefined by D’Amour et al. to produce a near 100% pure definitive endodermal cell population (D’Amour et al., 2005). The same group had demonstrated the production of pancreatic endocrine hormone- producing cells that contained both insulin and C-peptide by a five stage in vitro differentiation process (D’Amour et al., 2006). Through these inventions, these researchers were able to produce insulin from these cells in the range of human islets; but could not establish the insulin response to glucose. Later, this response was achieved by Kroon et al.(2008) as the hyperglycaemic responsiveness is a crucial characteristic that is needed for any potential cellular diabetic therapy. This group transplanted their unique differentiated cells that resemble 6–9-week-old embryo into the immunodeficient mice and showed that the insulin release was glucose-dependent. This allowed the cells to both recover mice from STZ (streptozotocin)-induced diabetes as well as to prevent it (Kroon et al., 2008). These discoveries may lead the way for ESCs to become a strong candidate for cellular replacement therapy in T1DM in near future.
5. Induced pluripotent stem cells and diabetes
The production of pluripotent stem cells from non pluripotent resource is referred as induced pluripotency. Somatic cells can be reprogrammed to produce pluripotent stem cell under specific conditions and such cell is known as induced pluripotent stem cell (iPSC). Induced pluripotency is achieved by directed expression of specific transcription factors (Yamanaka, 2008). The induced pluripotent stem cells (iPSCs) exhibit high telomerase activity similar to that of ESCs and possess hypomethylated gene promoters (Maherali and Hochedlinger, 2008, Takahashi et al., 2007). These iPSCs are preferred choices of cell based therapy for diabetes management as they can be patient specific and eliminate the possibility of likelihood rejection. The fibroblast cells are induced to produce iPSCs and these cells are later converted to pancreatic beta like cells by a three-stage differentiation process. The transplantation of fibroblast derived beta like cells in diabetic mouse model was effective in controlling hyperglycaemia for long term (Alipio et al., 2010). Zhang and colleagues have shown that the human ESCs and iPSCs were differentiated into mature pancreatic cells that were capable of secreting both insulin and C-peptide (Zhang et al., 2009). The lentiviral transfection method is utilized to reprogramme the adult pancreatic beta-cells into iPSCs. The produced cells were capable of differentiating into cells of all three germ layers (Maehr et al., 2009, Stadtfeld et al., 2008). The fibroblastic cells of type 1 diabetic patient were induced to produce pluripotent stem cells by the lentiviral transfection method and the produced cells are identified as insulin secreting cells (Maehr et al., 2009). The above mentioned research innovations and recent progress in the field of induced pluripotency would allow the usage patient-specific iPSCs for cell based therapies in diabetes. However, the safe usage of iPSCs for diabetes management must be ensured as these cells exhibit irregular behaviour and significant variations in reprogramming (Lister et al., 2011).
6. Hurdles in the progress
Despite the valiant strides made in the field of stem cell biology over a decade, the usage of stem cells in diabetes treatment is still in its primitive stage. The effective and full-fledged usage of stem cells relies upon how well the associated issues and hurdles are resolved. The stem cell based cure of diabetes will become reality only when all the difficulties are properly identified and effectively addressed. The progress of stem cell research has been hampered by both technological as well as ethical issues. The predominant concerns associated with the development of a potential source of stem cells for the treatment of diabetes have been listed in Table 1.
Table 1.
Primary concerns associated with stem cells in the treatment of diabetes.
| Forefront issues associated with the stem cell usage for diabetic treatment | |
|---|---|
| 1 | Safe administration and potential risk of teratoma formation. |
| 2 | Transplantation complications and acclimatization in the tissue microenvironment. |
| 3 | Side effects and risk factors associated with immunosuppression. |
| 4 | Scale up potential of stem cells. |
| 5 | Ethical issues concerned with the use of ESCs. |
6.1. Safety aspects
The ability to form teratomas and the potential risk of malignancy are the key features associated with the usage of ESCs (Leon-Quinto et al., 2004). Thus, there should be a rigorous testing and screening for potential side effects before using it in clinical trials and for treatment in human population.
6.2. Transplantation issues
The autoimmune rejection is a major issue during transplantation and that requires a stable and appropriate immunosuppression regime. Current transplantation protocols need to be stabilized with the standard testing module. Stem cell transplantation requires a number of studies to discuss the issues associated with the transplanted cell survival, stability and durability in the new microenvironment with appropriate vascular and neural support.
6.3. Scale up issues
Once the appropriate developmental procedures are optimized then comes the scale up issues. The quantity of cells needs to meet the demands for further research including clinical trials. Thus it requires efficient techniques to maximize the yield by adjusting the culture requirements. To maintain the balance between the demand and usage, the scale-up potential of stem cells needs further exploration to provide an excess of transplanted cellular reserves.
6.4. Ethical issues
Due to the nature of its origin, the ESCs are the hot target for the ethicists. Generally, ESCs are usually derived from unused and/or unfertilized embryos at in vitro fertilization clinics. These ESCs need to be procured from the donor on the basis of informed consent before using them for any clinical study. However, in most of the cases the cells from the embryo are obtained by destroying the embryo and that rises the question about the origin of life and ethical rights to destroy the embryo. The answer will lead into the never ending debate and it is always advisable to follow the set of rules that are laid by the governing bodies around the globe based on the sentiments and beliefs of people from that particular geographical location. The adult stem cells are preferred over their embryonic counterpart as there is no much controversy about the usage of adult stem cells. However it is always safer to adopt the policy of transparency regarding the derivation and usage of stem cells whatever may be the source of their origin. The recent technological advancements in the field of induced pluripotent stem cell research allow the usage of persons own stem cells for different purposes (Holm, 2008, Yu et al., 2007). In such cases, adult cells are reprogrammed to the pluripotent state and subsequently differentiated into functional β-cells. This technique may eventually solve the issues associated with the embryonic stem cells but adds further safety burden probably to be solved in the near future.
7. Conclusions
Over a decade of research clearly suggests that insulin producing cells may be derived from stem cells. However there is a need for further development of methods for differentiation and selection of completely functional β cells. The regeneration of β cells is possible by controlling the regulation of various factors. Exploration of these β cell regulation pathways would be logical strides towards the curative efforts in diabetes treatment. Despite the achievements and progress with the stem cells, the key issues like safety concerns, teretoma formation, transplantation issues and autoimmune response, and ethical dilemmas of ESCs limit their further exploration in clinical trials. Similarly, the issues associated with the scale up production, hamper the exploration of adult stem cells and iPSCs to be used as a choice of therapeutic resources. The scientific effort of a past decade enabled us to produce insulin secreting cells and the future years may come up with the solutions to use stem cells as a therapeutic agent to cure diabetes.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adler R. Curing blindness with stem cells: hope, reality, and challenges. Adv. Exp. Med. Biol. 2008;613:3–20. doi: 10.1007/978-0-387-74904-4_1. [DOI] [PubMed] [Google Scholar]
- Alipio Z., Liao W., Roemer E.J., Waner M., Fink L.M., Ward D.C., Ma Y. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic β-like cells. Proc. Natl. Acad. Sci. USA. 2010;107:13426–13431. doi: 10.1073/pnas.1007884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assady S., Maor G., Amit M., Itskovitz-Eldor J., Skorecki K.L., Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- Atkinson M.A., Eisenbarth G.S. Type I diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- Ber I., Shternhall K., Perl S., Ohanuna Z., Goldberg I., Barshack I., Benvenisti-Zarum L., Meivar-Levy I., Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J. Biol. Chem. 2003;278:31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- Blyszczuk P., Asbrand C., Rozzo A., Kania G., St-Onge L., Rupnik M., Wobus A.M. Embryonic stem cells differentiate into insulin-producing cells without selection of nestin-expressing cells. Int. J. Dev. Biol. 2004;48:1095–1104. doi: 10.1387/ijdb.041904pb. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Baxter L.A., Schuppin G.T., Smith F.E. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42:1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Taneja M., Weir G.C., Tatarkiewicz K., Song K.H., Sharma A., O’Neil J.J. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton T.R., Rossi F.M., Keshet G.I., Blau H.M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Couri C.E., Oliveira M.C., Stracieri A.B., Moraes D.A., Pieroni F., Barros G.M., Madeira M.I., Malmegrim K.C., Foss-Freitas M.C., Simoes B.P., Martinez E.Z., Foss M.C., Burt R.K., Voltarelli J.C. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- D’Amour K.A., Agulnick A.D., Eliazer S., Kelly O.G., Kroon E., Baetge E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D’Amour K.A., Bang A.G., Eliazer S., Kelly O.G., Agulnick A.D., Smart N.G., Moorman M.A., Kroon E., Carpenter M.K., Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- DeFronzo R.A. Pathogenesis of Type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;5:178–269. [Google Scholar]
- Dor Y., Brown J., Martinez O.I., Melton D.A. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Eberhardt M., Salmon P., von Mach M.A., Hengstler J.G., Brulport M., Linscheid P., Seboek D., Oberholzer J., Barbero A., Martin I., Müller B., Trono D., Zulewski H. Multipotential nestin and Isl-1 positive mesenchymal stem cells isolated from human pancreatic islets. Biochem. Biophys. Res. Commun. 2006;345:1167–1176. doi: 10.1016/j.bbrc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Estrada E.J., Valacchi F., Nicora E., Brieva S., Esteve C., Echevarria L., Froud T., Bernetti K., Cayetano S.M., Velazquez O., Alejandro R., Ricordi C. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 2008;17:1295–1304. doi: 10.3727/096368908787648119. [DOI] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Gao R., Ustinov J., Pulkkinen M.A., Lundin K., Korsgren O., Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- Haas S., Weidner N., Winkler J. Adult stem cell therapy in stroke. Curr. Opin. Neurol. 2005;18:59–64. doi: 10.1097/00019052-200502000-00012. [DOI] [PubMed] [Google Scholar]
- Hansson M., Tonning A., Frandsen U., Petri A., Rajagopal J., Englund M.C., Heller R.S., Hakansson J., Fleckner J., Skold H.N., Melton D., Semb H., Serup P. Artifactual insulin release from differentiated embryonic stem cells. Diabetes. 2004;53:2603–2609. doi: 10.2337/diabetes.53.10.2603. [DOI] [PubMed] [Google Scholar]
- Hess D., Li L., Martin M., Sakano S., Hill D., Strutt B., Thyssen S., Gray D.A., Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat. Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- Holm S. Time to reconsider stem cell ethics: the importance of induced pluripotent cells. J. Med. Ethics. 2008;34:63–64. doi: 10.1136/jme.2007.023903. [DOI] [PubMed] [Google Scholar]
- Hori Y., Rulifson I.C., Tsai B.C., Heit J.J., Cahoy J.D., Kim S.K. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2002;99:16105–16110. doi: 10.1073/pnas.252618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y., Gu X., Xie X., Kim S.K. Differentiation of insulin-producing cells from human neural progenitor cells. PLoS Med. 2005;2:e103. doi: 10.1371/journal.pmed.0020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianus A., Holz G.G., Theise N.D., Hussain M.A. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J. Clin. Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDF Diabetes Atlas, 5th edition update, Brussels, International Diabetes Federation, 2012. (www.idf.org/diabetesatlas/news/fifth-edition-release). Accessed January 2013.
- Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R., Reyes M., Lenvik T., Lund T., Blackstad M., Du J., Aldrich S., Lisberg A., Low W.C., Largaespada D.A., Verfaillie C.M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kahan B.W., Jacobson L.M., Hullett D.A., Ochoada J.M., Oberley T.D., Lang K.M., Odorico J.S. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation. Diabetes. 2003;52:2016–2024. doi: 10.2337/diabetes.52.8.2016. [DOI] [PubMed] [Google Scholar]
- Kim S., Shin J.S., Kim H.J., Fisher R.C., Lee M.J., Kim C.W. Streptozotocin-induced diabetes can be reversed by hepatic oval cell activation through hepatic transdifferentiation and pancreatic islet regeneration. Lab. Invest. 2007;87:702–712. doi: 10.1038/labinvest.3700561. [DOI] [PubMed] [Google Scholar]
- Krause D.S., Theise N.D., Collector M.I., Henegariu O., Hwang S., Gardner R., Neutzel S., Sharkis S.J. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S., Young H., Richardson M., Smart N.G., Cunningham J., Agulnick A.D., D’Amour K.A., Carpenter M.K., Baetge E.E. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Kubo A., Shinozaki K., Shannon J.M., Kouskoff V., Kennedy M., Woo S., Fehling H.J., Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I.L., Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Leon-Quinto T., Jones J., Skoudy A., Burcin M., Soria B. In vitro directed differentiation of mouse embryonic stem cells into insulin-producing cells. Diabetologia. 2004;47:1442–1451. doi: 10.1007/s00125-004-1458-8. [DOI] [PubMed] [Google Scholar]
- Lister R., Pelizzola M., Kida Y.S., Hawkins R.D., Nery J., Hon G., Antosiewicz-Bourget J., O’Malley R., Castanon R., Klugman S., Downes M., Yu R., Stewart R., Ren B., Thomson J.A., Evans R.M., Ecker J.R. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang Y., Li Y., Pei X. Research status and prospect of stem cells in the treatment of diabetes mellitus. Sci. China Life Sci. 2013;56:306–312. doi: 10.1007/s11427-013-4469-1. [DOI] [PubMed] [Google Scholar]
- Lumelsky N., Blondel O., Laeng P., Velasco I., Ravin R., McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- Maehr R., Chen S., Snitow M., Ludwig T., Yagasaki L., Goland R., Leibel R.L., Melton D.A. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N., Hochedlinger K. Induced pluripotency of mouse and human somatic cells. Cold Spring Harbor Symposia Quant. Biol. 2008;73:157–162. doi: 10.1101/sqb.2008.73.017. [DOI] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccall M.D., Toso C., Emmanuel E., Baetge E.E., Shapiro A.M. Are stem cells a cure for diabetes? Clin. Sci. (Lond) 2009;118:87–97. doi: 10.1042/CS20090072. [DOI] [PubMed] [Google Scholar]
- Meyer G.P., Wollert K.C., Drexler H. The role of stem cells in the post-MI patient. Curr. Heart Failure Rep. 2007;4:198–203. doi: 10.1007/s11897-007-0013-6. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Yamato E., Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030–1037. doi: 10.2337/diabetes.53.4.1030. [DOI] [PubMed] [Google Scholar]
- Okumura K., Nakamura K., Hisatomi Y., Nagano K., Tanaka Y., Terada K., Sugiyama T., Umeyama K., Matsumoto K., Yamamoto T., Endo F. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104–113. doi: 10.1053/jhep.2003.50259. [DOI] [PubMed] [Google Scholar]
- Rajagopal J., Anderson W.J., Kume S., Martinez O.I., Melton D.A. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. doi: 10.1126/science.1077838. [DOI] [PubMed] [Google Scholar]
- Sapir T., Shternhall K., Meivar-Levy I., Blumenfeld T., Cohen H., Skutelsky E., Eventov-Friedman S., Barshack I., Goldberg I., Pri-Chen S., Ben-Dor L., Polak-Charcon S., Karasik A., Shimon I., Mor E., Ferber S. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc. Natl. Acad. Sci. USA. 2005;102:7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg R.M., Smukler S.R., Kieffer T.J., Enikolopov G., Asghar Z., Wheeler M.B., Korbutt G., van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- Segev H., Fishman B., Ziskind A., Shulman M., Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem cells. 2004;22:265–274. doi: 10.1634/stemcells.22-3-265. [DOI] [PubMed] [Google Scholar]
- Sipione S., Eshpeter A., Lyon J.G., Korbutt G.S., Bleackley R.C. Insulin expressing cells from differentiated embryonic stem cells are not β cells. Diabetologia. 2004;47:499–508. doi: 10.1007/s00125-004-1349-z. [DOI] [PubMed] [Google Scholar]
- Soria B., Roche E., Berna G., Leon-Quinto T., Reig J.A., Martin F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M., Brennand K., Hochedlinger K. Reprogramming of pancreatic β cells into induced pluripotent stemcells. Curr. Biol. 2008;18:890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Nakauchi H., Taniguchi H. Glucagon-like peptide 1 (1–37) converts intestinal epithelial cells into insulin-producing cells. Proc. Natl. Acad. Sci. USA. 2003;100:5034–5039. doi: 10.1073/pnas.0936260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stemcells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Timper K., Seboek D., Eberhardt M., Linscheid P., Christ-Crain M., Keller U., Muller B., Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem. Biophys. Res. Commun. 2006;341:1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- Trounson A. A rapidly evolving revolution in stem cell biology and medicine. Reprod. Biomed. 2013 doi: 10.1016/j.rbmo.2013.07.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Xu X., D’Hoker J., Stanǵe G., Bonńe S., De Leu N., Xiao X., Van de Casteele M., Mellitzer G., Ling Z., Pipeleers D., Bouwens L., Scharfmann R., Gradwohl G., Heimberg H. β Cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. Induction of pluripotent stemcells from mouse fibroblasts by four transcription factors. Cell Prolif. 2008;41:51–56. doi: 10.1111/j.1365-2184.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li S., Hatch H., Ahrens K., Cornelius J.G., Petersen B.E., Peck A.B. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc. Natl. Acad. Sci. USA. 2002;99:8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kajimoto Y., Yasuda T., Watada H., Fujitani Y., Kosaka H., Gotow T., Miyatsuka T., Umayahara Y., Yamasaki Y., Hori M. PDX-1 induces differentiation of intestinal epithelioid IEC-6 into insulin-producing cells. Diabetes. 2002;51:2505–2513. doi: 10.2337/diabetes.51.8.2505. [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I.I., Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zalzman M., Gupta S., Giri R.K., Berkovich I., Sappal B.S., Karnieli O., Zern M.A., Fleischer N., Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc. Natl. Acad. Sci. USA. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Jiang W., Liu M., Sui X., Yin X., Chen S., Shi Y., Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- Zulewski H., Abraham E.J., Gerlach M.J., Daniel P.B., Moritz W., Muller B., Vallejo M., Thomas M.K., Habener J.F. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]