Abstract
Objectives To evaluate the efficacy and safety of endovascular treatment, particularly adjunctive intra-arterial mechanical thrombectomy, in patients with ischaemic stroke.
Design Systematic review and meta-analysis.
Data sources Medline, Embase, Cochrane Central Register of Controlled Trials, Web of Science, SciELO, LILACS, and clinical trial registries from inception to December 2015. Reference lists were crosschecked.
Eligibility criteria for selecting studies Randomised controlled trials in adults aged 18 or more with ischaemic stroke comparing endovascular treatment, including thrombectomy, with medical care alone, including intravenous recombinant tissue plasminogen activator (rt-PA). Trial endpoints were functional outcome (modified Rankin scale scores of ≤2) and mortality at 90 days after onset of symptoms. No language or time restrictions applied.
Results 10 randomised controlled trials (n=2925) were included. In pooled analysis endovascular treatment, including thrombectomy, was associated with a higher proportion of patients experiencing good (modified Rankin scale scores ≤2) and excellent (scores ≤1) outcomes 90 days after stroke, without differences in mortality or rates for symptomatic intracranial haemorrhage, compared with patients randomised to medical care alone, including intravenous rt-PA. Heterogeneity was high among studies. The more recent studies (seven randomised controlled trials, published or presented in 2015) proved better suited to evaluate the effect of adjunctive intra-arterial mechanical thrombectomy on its index disease owing to more accurate patient selection, intravenous rt-PA being administered at a higher rate and earlier, and the use of more efficient thrombectomy devices. In most of these studies, more than 86% of the patients were treated with stent retrievers, and rates of recanalisation were higher (>58%) than previously reported. Subgroup analysis of these seven studies yielded a risk ratio of 1.56 (95% confidence interval 1.38 to 1.75) for good functional outcomes and 0.86 (0.69 to 1.06) for mortality, without heterogeneity among the results of the studies. All trials were open label. Risk of bias was moderate across studies. The full results of two trials are yet to be published.
Conclusions Moderate to high quality evidence suggests that compared with medical care alone in a selected group of patients endovascular thrombectomy as add-on to intravenous thrombolysis performed within six to eight hours after large vessel ischaemic stroke in the anterior circulation provides beneficial functional outcomes, without increased detrimental effects.
Systematic review registration PROSPERO CRD42015019340.
Introduction
Stroke is the second leading cause of death worldwide,1 its incidence is rising in those aged less than 75 years,2 and the global burden attributable to stroke is increasing.3 Along with preventive measures, effective treatments are therefore needed to reduce the deleterious consequences of stroke.
Arterial occlusion is the cause of ischaemic stroke. Over time, deprivation of blood leads to progressive cell death. Early reversal of vascular occlusion limits the volume of damaged tissue and correlates with outcome.4 By achieving timely reperfusion, thrombolysis improves functional recovery, but only in 33% of patients.5 6 Furthermore, the recanalisation rates associated with intravenous recombinant tissue plasminogen activator (rt-PA)—approximately 46%7—are not ideal, and the use of endovascular interventions may reverse vessel occlusion more effectively and thus help further improve outcomes. Both drug and mechanical endovascular interventions have been evaluated in acute ischaemic stroke. Thrombectomy can be performed using devices that disrupt, aspirate, or retrieve clots. The procedure can be used alone or as an add-on to intravenous or intra-arterial chemical thrombolysis—that is, adjunctive intra-arterial mechanical thrombectomy.
Results for adjunctive intra-arterial mechanical thrombectomy from published randomised controlled trials are heterogeneous, and the clinical benefit of the procedure are uncertain.8 9 10 11 We conducted a systematic review with meta-analysis to compare the efficacy and safety of endovascular treatment (in particular adjunctive intra-arterial mechanical thrombectomy) with standard medical care alone (in particular intravenous rt-PA) in adults with ischaemic stroke.
Methods
Protocol and guidance
The study protocol was reported following PRIMA-P guidelines12 and was registered at PROSPERO. The methods and reporting of the systematic review followed PRISMA13 guidelines. Reporting of statistical data followed SAMPL14 guidelines.
Eligibility criteria
We included randomised controlled trials reporting on the efficacy and safety of adjunctive intra-arterial mechanical thrombectomy, independently of the device used, compared with medical care alone, including intravenous rt-PA for ischaemic stroke in adults (≥18 years). To be included, studies had to mention functional outcome and mortality at 90 days after symptom onset as trial endpoints. We did not exclude studies a priori owing to poor quality, language, or time restrictions, but we excluded observational, non-controlled, or non-randomised interventional studies. Since our primary aim was to evaluate adjunctive intra-arterial mechanical thrombectomy compared with intravenous rt-PA, we excluded randomised controlled trials in which patients were not submitted to mechanical thrombectomy in the experimental arm (for example, trials only evaluating patients submitted to other types of endovascular treatment, such as intra-arterial rt-PA and urokinase-type plasminogen activator) or in which patients were not submitted to intravenous rt-PA in the control arm.
Information sources and search strategy
Electronic identification of reports was conducted in Medline, Embase, Cochrane Central Register of Controlled Trials, Web of Science, SciELO, and LILACS. Grey literature was searched through appropriate databases (OpenGrey, Database of Abstracts of Reviews of Effects, British Library Thesis Service). We also consulted clinical trial registries (ClinicalTrials.gov, European Union Clinical Trials Register, World Health Organization International Clinical Trials Registry Platform, ISRCTN Registry, Stroke Trials Registry). The last electronic search was on 14 December 2015.
We also cross checked the references of potentially eligible randomised controlled trials.
For the search strategy we combined the terms (cerebrovascular disorder OR stroke) with (mechanical thrombolysis OR embolectomy OR thrombectomy). The Cochrane highly sensitive search strategy was used to retrieve randomised controlled trials (see supplementary file S1 for details of the search strategy).15
Study selection
Two reviewers (FBR, JBN) independently screened the titles and abstracts of retrieved reports for potential eligibility. They then screened the full text of potentially relevant trials. Disagreements were resolved by consensus or with the help of a third reviewer (DC). Interobserver bias (the percentage of agreement achieved) was calculated.16
Data collection process
Two reviewers (FBR, JBN) independently extracted data from the included randomised controlled trials using a standardised electronic form. Disagreements were resolved by consensus or with the help of a third reviewer (DC). Another reviewer (JC) double checked the extracted data. When possible we used data from intention to treat populations. When such data were not available, we used data from modified intention to treat populations, defined as participants who were included and completed the study (that is, some initially randomised who were excluded from analysis) regardless of compliance with the allocated interventions. We extracted per protocol data only when data from intention to treat or modified intention to treat populations were unavailable.
Outcomes and prioritisation
The primary efficacy outcome was the proportion of patients achieving a good functional outcome at 90 days after the onset of symptoms, defined as a modified Rankin scale17 score between 0 and 2, representing functional independence. The primary safety outcome was all cause mortality at 90 days. The secondary efficacy outcome was the proportion of patients achieving an excellent functional outcome at 90 days (modified Rankin scale score ≤1). The secondary safety outcome was the proportion of patients with symptomatic intracerebral haemorrhage as defined in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) study.18 When symptomatic intracerebral haemorrhage was not defined using SITS-MOST criteria, we accepted other definitions.
Risk of bias in individual studies
Two reviewers (FBR, JBN) independently assessed the risk of bias of individual studies using the Cochrane Collaboration risk of bias tool.15 Three additional criteria were sought: independent funding, early stopping of trial, and clinical trial registration to determine whether the trial was retrospectively or prospectively registered. If a trial was retrospectively registered, we considered the risk of bias to be high because of the risk of reporting bias.
Data synthesis
We used random effects meta-analyses (RevMan 5.3.3 software) weighted by the inverse variance method to estimate pooled risk ratios and 95% confidence intervals. When using the Mantel-Haenszel method we considered sample size and event rates. We chose risk ratios as effect measures owing to the greater similarity of relative estimates between studies with different designs, populations, and lengths of follow-up.19 Raw data were converted to risk ratios. We assessed heterogeneity with the Cochran Q test and the I2 test.20 When statistically significant differences in risk were found (P<0.05), we also determined absolute effects and derived the additional number of participants with events per 1000 who benefitted or experienced harm from receiving the studied intervention.
To explore whether cumulative data were adequately powered to evaluate outcomes, we carried out trial sequential analyses for primary outcomes using TSA version 0.9 beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark, 2011).21 22 We calculated the required information size and computed the trial sequential monitoring boundaries using the O’Brien-Fleming approach.23 Our analysis was based on a two sided 5% risk of a type I error, 20% risk of a type II error (power of 80%), risk reduction based on pooled analysis, the weighted incidence of events in the control group, and heterogeneity. Power of the primary outcomes findings was interpreted if statistical significance was reached with either a minimum sample size or crossing a trial sequential alpha spending monitoring boundary. Trial sequential analysis evaluates whether statistically significant results of meta-analysis are reliable, taking into account information size (number of participants in the meta-analysis required to accept or reject a prespecified intervention effect). The technique is analogous to sequential monitoring boundaries in a single trial. Trial sequential analysis adjusts the threshold of statistical significance and may reduce the risk of random errors due to repetitive testing of accumulating data.
Because of inequalities in trial design, including patient populations and interventions,24 we present data separately for all outcomes a priori according to the year of publication or the presentation of the trial results (2013 and 2015). We planned further subgroup analyses for sex, trials with different risk of bias, thrombectomy devices (≥85% v <85% rate of stent retriever use), time to treatment, the administering of intravenous rt-PA, and characteristics of stroke. We carried out a sensitivity analysis by excluding data from unpublished trials.
Meta-biases
If more than 10 studies were available for each outcome, we assessed publication bias by visual inspection of asymmetry in funnel plots.15 We also carried out Egger’s and Peters’ tests.25 26
Confidence in cumulative evidence
We evaluated the quality of evidence using the grading of recommendations assessment, development and evaluation (GRADE) working group methods.27
Results
Study selection
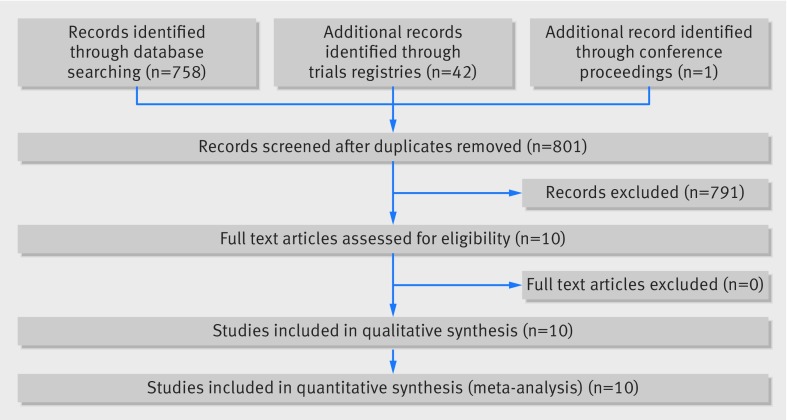
Electronic searches yielded 758 records after removal of duplicates. The interobserver agreement between screeners was good (Cohen’s κ coefficient 0.75, 95% confidence interval 0.56 to 0.93).16 Ten studies were included (fig 1); three published in 2013 (Interventional Management of Stroke (IMS) III Trial (IMS III), Intra-arterial Versus Systemic Thrombolysis for Acute Ischemic Stroke (SYNTHESIS Expansion), Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE)),28 29 30 five in 2015 (Endovascular treatment for acute ischemic stroke in the Netherlands (MR CLEAN), Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE), Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial (EXTEND-IA), Solitaire With the Intention For Thrombectomy as PRIMary Endovascular Treatment (SWIFT-PRIME), and Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours (REVASCAT)),31 32 33 34 35 and two presented in 2015 but not yet published (Assess the Penumbra System in the Treatment of Acute Stroke (THERAPY) and Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke (THRACE)).36 37 When needed, we consulted the published protocols, supplementary material, and press releases of these studies.38 39 40 41 42 43 44 45 46 47 The principal investigators of THERAPY and THRACE were unsuccessfully contacted for data. Therefore, data extraction for these trials was based solely on results presented at scientific meetings and in press releases.
Fig 1 Study flow selection
Study characteristics
All studies were multicentre, parallel, prospective randomised open blinded endpoint clinical trials (table 1). All but four (SYNTHESIS, MR CLEAN, REVASCAT, and THRACE) were international. The number of participants ranged from 70 to 656. Overall, the studies involved 2925 participants—1564 in the endovascular treatment arm and 1361 in the standard medical care (intravenous thrombolysis) arm, based on either an intention to treat population or a modified intention to treat population.
Table 1.
Details of included studies
| Trial | Source | Trial period | Country | No of centres | No of patients* | Primary outcome | Enrolment criteria | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Symptom onset (hours) | NIHSS | ||||||||
| rt-PA | AIMT | |||||||||
| IMS III28 | Broderick et al, 2013 | 2006-12 | USA, Canada, Australia, Spain, Germany, France, Netherlands | 58 | 656 | mRS ≤2 at 90 days | 18-82 | 3 | 5 | ≥10‡ |
| SYNTHESIS29 | Ciccone et al, 2013 | 2008-12 | Italy | 24 | 362 | mRS ≤1 at 90 days | 18-80 | 4.5 | 6 | ≤25 |
| MR RESCUE30 | Kidwell et al, 2013 | 2004-11 | USA, Canada | 22 | 127 | mRS scores at 90 days | 18-85 | 4.5† | 8 | 6-29 |
| MR CLEAN31 | Berkhemer et al, 2015 | 2010-14 | Netherlands | 16 | 500 | mRS scores at 90 days | ≥18 | 4.5† | 6 | ≥2 |
| ESCAPE32 | Goyal et al, 2015 | 2013-14 | Canada, USA, South Korea, Republic of Ireland, UK | 22 | 315 | Median mRS at 90 days | ≥18 | 4.5† | 12 | Unrestricted |
| EXTEND-IA33 | Campbell et al, 2015 | 2012-14 | Australia, New Zealand | 10 | 70 | Reperfusion at 24 hours and NIHSS at 3 days | ≥18 | 4.5 | 6 | Unrestricted |
| SWIFT PRIME34 | Saver et al, 2015 | 2012-15 | USA, France, Germany, Spain, Switzerland, Denmark, Austria | 39 | 196 | mRS scores at 90 days | 18-80 | 4.5 | 6 | 8-29 |
| REVASCAT35 | Jovin et al, 2015 | 2012-14 | Spain | 4 | 206 | mRS scores at 90 days | 18-85 | 4.5† | 8 | ≥6 |
| THERAPY36 | Mocco et al, 2015 | 2012-15 | USA, Germany | 36 | 108 | mRS ≤2 at 90 days | 18-85 | 4.5§ | 5¶ | ≥8 |
| THRACE37 | Bracard et al, 2015 | 2010-15 | France | 26 | 385 | mRS scores at 90 days | 18-80 | 4 | 5 | 10-25 |
rt-PA=recombinant tissue plasminogen activator; AIMT=adjuvant intra-arterial mechanical thrombolysis; NIHSS=National Institute of Health stroke scale; mRS=modified Rankin scale.
*Intention to treat population.
†If ineligible.
‡≥8 if computed tomography or magnetic resonance imaging angiographic evidence of internal carotid artery, first division of middle cerebral artery (M1), or basilar artery occlusion.
§A time limit of three hours was used for participants aged more than 80 years, with a history of stroke and diabetes, anticoagulant use, and NIHSS >25.
¶Initial protocol allowed up to eight hours, but revision limited to up to five hours (6.5% of participants were over five hours).
The main inclusion criteria were adults after stroke with time from symptom onset to intravenous thrombolysis of 3 to 4.5 hours and time from symptom onset to endovascular treatment between 5 and 12 hours. In contrast with IMS III, SYNTHESIS, EXTEND-IA, SWIFT-PRIME, THERAPY, and THRACE trials, which only included patients who were also treated with intravenous rt-PA, some trials (MR RESCUE, MR CLEAN, ESCAPE, and REVASCAT) included patients who were not eligible for intravenous thrombolysis.
All the studies focused on strokes involving the anterior circulation, although IMS III, SYNTHESIS, and THRACE also allowed strokes involving the posterior circulation. MR RESCUE, ESCAPE, EXTEND-IA, MR CLEAN, and THERAPY included strokes within the location of the internal carotid artery and/or M1 and/or M2 portions of the middle cerebral artery, whereas SWIFT-PRIME, REVASCAT, and THRACE included only strokes involving the internal carotid or M1. The Alberta Program Stroke Early Computed Tomography Score (ASPECTS) was also an enrolment criterion in IMS III, ESCAPE, SWIFT PRIME, and REVASCAT (see supplementary table S1). Radiological confirmation of large vessel occlusion was an inclusion criterion in all 2015 studies. This was not the case in trials done in 2013 (IMS III, SYNTHESIS, and MR RESCUE). Perfusion imaging depicting potentially salvageable brain tissue was only a requirement for patient inclusion in ESCAPE, EXTEND-IA, and SWIFT-PRIME.
The baseline characteristics of included patients were similar between arms across studies (table 2). Mean age ranged from 62 to 71 years, and sex distribution was approximately 1:1 in all studies. The severity of stroke ranged from 13 to 19 points (moderate, to moderate to severe stroke severity) in the National Institute of Health stroke scale.
Table 2.
Characteristics of included patients
| Trial | AIMT arm | Medical care (intravenous rt-PA) arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | No of patients* | Mean (SD) age (years) | No (%) men | Mean (SD) NIHSS | Intervention | No of patients* | Mean (SD) age (years) | No (%) men | Mean (SD) NIHSS | ||
| IMS III28 | IV rt-PA with or without IV heparin with or without thrombectomy and/or IA rt-PA | 434 | 63 (SD) 11.07 | 218 (50.2) | 20 (SD) 5.54 | IV rt-PA | 222 | 61 (SD) 10.23 | 122 (55.0) | 18 (SD) 3.69 | |
| SYNTHESIS29 | IV heparin with or without thrombectomy and/or IA rt-PA | 181 | 66 (SD) 11 | 106 (59) | 13 (SD) 5.98 | IV rt-PA | 181 | 67 (SD) 11 | 103 (57) | 13 (SD) 6.73 | |
| MR RESCUE30 | Thrombectomy with or without IA rt-PA with or without IV heparin with or without IV rt-PA | 70*/64† | 64 (SD) 12.78† | 30 (46.9)† | 17 (SD) 4.72† | With or without IV rt-PA | 57*/54† | 67 (SD) 16.48† | 27 (50)† | 17 (SD) 5.73† | |
| MR CLEAN31 | With or without IV rt-PA+thrombectomy with or without IA rt-PA or IA uPA | 233 | 65 (SD) 16.04 | 135 (57.9) | 17 (SD) 5.22 | With or without IV rt-PA | 267 | 66 (SD) 15.58 | 157 (58.8) | 18 (SD) 5.96 | |
| ESCAPE32 | Thrombectomy with or without IV rt-PA | 165 | 71 (SD) 15.71 | 79 (47.9) | 16 (SD) 5.24 | With or without IV rt-PA | 150 | 70 (SD) 15.72 | 71 (47.3) | 16 (SD) 5.99 | |
| EXTEND-IA33 | IV rt-PA with or without thrombectomy | 35 | 69 (SD) 12.3 | 17 (49) | 17 (SD) 5.41 | IV rt-PA | 35 | 70 (SD) 11.8 | 17 (49) | 14 (SD) 7.73 | |
| SWIFT PRIME34 | IV rt-PA with or without thrombectomy | 98*/98‡ | 65 (SD) 12.5† | 54 (55.1)† | 17 (SD) 5.27† | IV rt-PA | 98*/93‡ | 66 (SD) 11.3† | 45 (48.4)† | 16 (SD) 4.52† | |
| REVASCAT35 | Thrombectomy with or without IV rt-PA | 103 | 66 (SD) 11.3 | 55 (53.4) | 17 (SD) 4.51 | With or without IV rt-PA | 103 | 67 (SD) 9.5 | 54 (52.4) | 16 (SD) 5.26 | |
| THERAPY36 | IV rt-PA with or without thrombectomy | 55 | 67 (SD) 11.4 | 34 (61.8) | 17 (SD) 6.05 | IV rt-PA | 53 | 70 (SD) 10.3 | 23 (43.9) | 18 (SD) 5.38 | |
| THRACE37 | IV rt-PA with or without thrombectomy | 190 | NS | NS | NS | IV rt-PA | 195 | NS | NS | NS | |
AIMT=adjuvant intra-arterial mechanical thrombolysis; IA=intra-arterial; IV=intravenous; NIHSS=National Institute of Health stroke scale; NS=not specified; rt-PA=recombinant tissue plasminogen activator; uPA=urokinase-type plasminogen activator.
*Intention to treat population.
†Modified intention to treat population.
All studies evaluated endovascular treatment (with or without intravenous rt-PA) compared with standard medical treatment—namely, intravenous rt-PA (table 3). In the intervention arm, use of thrombolysis (intravenous rt-PA) ranged from 0% in SYNTHESIS to 100% in IMS III, EXTEND-IA, SWIFT PRIME, THERAPY, and THRACE. In SYNTHESIS, intravenous rt-PA was not administered owing to the study design (the study compared endovascular treatments, such as intra-arterial rt-PA and thrombectomy, with intravenous thrombolysis). In IMS III, the study design contemplated a planned dose reduction in intravenous rt-PA in the thrombectomy arm owing to intra-arterial rt-PA being administered concomitantly. Intravenous rt-PA was administered in the control arms (standard medical treatment) of all studies. However, in MR RESCUE, only 28.1% of patients received intravenous thrombolysis because they were considered unsuitable candidates. In the other trials, 77% to 100% of the patients in the medical care arm received intravenous rt-PA.
Table 3.
Characteristics of intervention within treatment arms
| Trial | Both arms | AIMT arm | Medical care (IV rt-PA) arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients* | No (%) IV rt-PA | No of patients* | No (%) receiving thrombectomy | No (%) receiving IV rt-PA | No (%) receiving IA rt-PA | No (%) receiving thrombectomy+IV rt-PA | No of patients* | No (%) of IV rt-PA | |||
| IMS III28 | 656 | 656 (100) | 434 | 170 (39.2) | 434 (100)† | 266 (61.3) | 170 (39.2) | 222 | 222 (100) | ||
| SYNTHESIS29 | 362 | 178 (49.2) | 181 | 56 (30.9) | 0 (0) | 109 (60.2) | 0 (0)/56 (30.9)‡ | 181 | 178 (98.3) | ||
| MR RESCUE30 | 127 | 44 (34.6) | 70 | 61 (87.1) | 28 (40.0) | 8 (11.4) | 28 (40.0) | 57 | 16 (28.1) | ||
| MR CLEAN31 | 500 | 445 (89.0) | 233 | 195 (83.7) | 203 (87.1) | 25 (10.7) | NS | 267 | 242 (90.6) | ||
| ESCAPE32 | 315 | 238 (75.6) | 165 | 151 (91.5) | 120 (72.7) | NA | 120 (72.7) | 150 | 118 (78.7) | ||
| EXTEND-IA33 | 70 | 70 (100) | 35 | 27 (77.1) | 35 (100) | NA | 27 (77.1) | 35 | 35 (100) | ||
| SWIFT PRIME34 | 191 | 191 (100) | 98§ | 87 (88.8)§ | 98 (100)§ | NA | 87 (88.8)‡ | 93‡ | 93 (100)§ | ||
| REVASCAT35 | 206 | 150 (72.8) | 103 | 98 (95.1) | 70 (68.0) | 1 (1.0) | NS | 103 | 80 (77.7) | ||
| THERAPY36 | 108 | 108 (100) | 55 | NS | 55 (100) | 0 (0.0) | NS | 53 | 53 (100) | ||
| THRACE37 | 385 | 385 (100) | 190 | NS | 190 (100) | 0 (0.0) | NS | 195 | 195 (100) | ||
AIMT=adjuvant intra-arterial mechanical thrombolysis; IV=intravenous; IA=intra-arterial; NA=not applicable; NS=not specified; rt-PA=recombinant tissue plasminogen activator.
*Intention to treat population.
†Approximately two thirds of standard dose.
‡IA rt-PA.
§Modified intention to treat population.
All the studies included thrombectomy as an endovascular treatment option. About two thirds of the patients randomised to the intervention arm (64.1%) underwent thrombectomy. IMS III, MR RESCUE, SYNTHESIS, and MR CLEAN allowed other endovascular interventions (intra-arterial rt-PA and urokinase-type plasminogen activator) in addition to thrombectomy; in SYNTHESIS and IMS III, only 30.9% and 39.2% of the patients were treated with adjunctive intra-arterial mechanical thrombectomy, respectively. In SYNTHESIS, the intervention arm included intra-arterial thrombolysis with rt-PA, mechanical disruption or retrieval of clots, or a combination of these approaches. In IMS III, the intervention arm included thrombectomy or endovascular delivery of rt-PA. In the other trials, 77.1% to 91.5% of the patients in the intervention arm were treated with adjunctive intra-arterial mechanical thrombectomy (data on patients in the THERAPY and THRACE trials are not yet available).
The thrombectomy devices selected among the studies varied, and some studies used more than one device (see supplementary table S2). In the 2013 studies and THERAPY early generation devices were mostly used (such as Merci retriever, Penumbra system, other aspiration systems, wire disruption), and only a small number of patients was treated with stent retrievers. Whereas in MR RESCUE and THERAPY no stent retrievers were used, in IMS III and SYNTHESIS the rate of stent retriever use was low—2.9% and 41%, respectively. However, among most 2015 studies the rate of stent retriever use (such as Solitaire FR, Solitaire 2, Trevo, Catch) was more than 86%. The time from acute stroke to endovascular treatment ranged from 225 to 355 minutes.
In the intervention arm, recanalisation rates varied between 25.0% and 88.0% according to a score of ≥2b/3 (perfusion of half or greater of the vascular distribution of the occluded artery) on the thrombolysis in cerebral infarction perfusion scale or modified thrombolysis in cerebral infarction perfusion scale (see supplementary table S3). SYNTHESIS did not report reperfusion rates. For THRACE, these data are still unavailable. Recanalisation rates greater than 58% were observed in MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME, REVASCAT, and THERAPY. With the exception of participants in the THERAPY trial, most (86.1% to 100%) of the patients in these last trials with higher recanalisation rates were treated with stent retrievers.
The follow-up period was 90 days in all the trials and all included data for our primary efficacy and safety outcomes. In IMS III, MR RESCUE, SYNTHESIS, and ESCAPE, symptomatic intracerebral haemorrhage was defined by the authors’ own criteria or according to previously defined criteria other than definition of the SITS-MOST study.18 In THRACE, the criteria used for symptomatic intracerebral haemorrhage is still unknown.
Risk of bias within studies
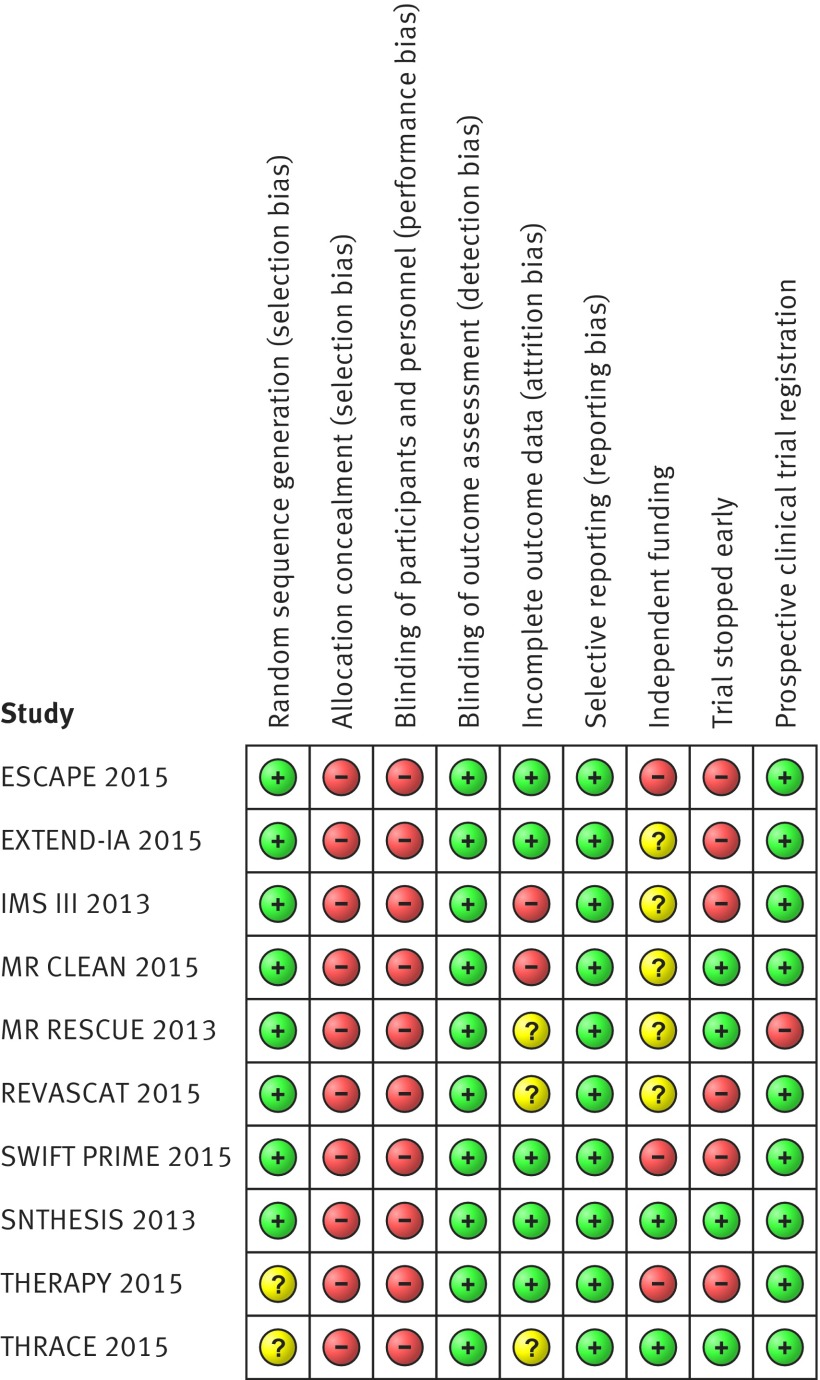
The overall risk of bias was moderate among studies (fig 2). Random sequence generation, blinding of outcome assessment, and selective reporting were considered as low risk items across studies. For THERAPY and THRACE, the bias associated with random sequence generation is not known owing to lack of information. Outcome assessment at 90 days was conducted in person in ESCAPE, EXTEND-IA, and SWIFT-PRIME, in person or by video visualisation in REVASCAT, by video visualisation in THERAPY, and by telephone in SYNTHESIS and MR CLEAN. IMS III and MR RESCUE did not report the method used for evaluation of outcome assessment—this information was absent from all available documents pertaining to both trials, including protocol and published and unpublished reports. This information is still unavailable for THRACE. Allocation concealment and blinding of participants and staff were classified as high risk owing to study design (that is, prospective randomised open blinded endpoint design). All the studies except for THRACE were at least partially funded by industry. SYNTHESIS, IMS III, and MR RESCUE were publicly funded but also had some support from industry. ESCAPE, MR CLEAN, EXTEND-IA, and REVASCAT had both mixed funding from governmental bodies and unrestricted grants from industry. SWIFT PRIME and THERAPY had only industry support.
Fig 2 Risk of bias summary
Six studies were stopped early—IMS III because of futility according to the interim analysis as per protocol, after 72.3% of the planned patients had been enrolled. The other five trials were stopped because of efficacy, after the publication of positive results in MR CLEAN: interim analyses were brought forward in ESCAPE (63.2% of the planned sample size), EXTEND-IA (70.0% of the planned sample size), and SWIFT PRIME (23.5% of the planned sample size), and enrolment was stopped because efficacy boundaries were met. In REVASCAT and THERAPY, indication of lack of equipoise led to enrolment being stopped before the efficacy boundary was reached—in REVASCAT after enrolling 25.4% of the planned sample size and in THERAPY after enrolling 15.6% of the planned sample size.
One trial (MR RESCUE) was retrospectively registered in 2006, two years after the study start. Concerning attrition bias, IMS III and MR CLEAN showed imbalances between withdrawals in the intervention and control arms. In MR RESCUE and REVASCAT, the lower number of participants between arms limited considerations about the effect of withdrawals on study results. Owing to lack of information for THRACE, attrition bias was not evaluable.
Synthesis of results
All studies, with the exception of THRACE (lacked details for modified Rankin scale score ≤1 and symptomatic intracerebral haemorrhage), reported the sought outcomes (table 1 describes the primary outcomes for each study). Results of individual studies were incorporated in forest plots (figures 3 and 4, and supplementary figures S1 and S2 and S5 to S7).
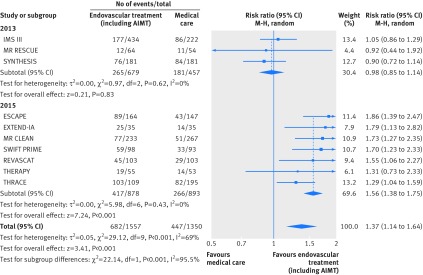
Fig 3 Forest plot for a good functional outcome (modified Rankin scale core ≤2) at 90 days, including subgroup analysis by year of study publication. AIMT=adjunctive intra-arterial mechanical thrombectomy
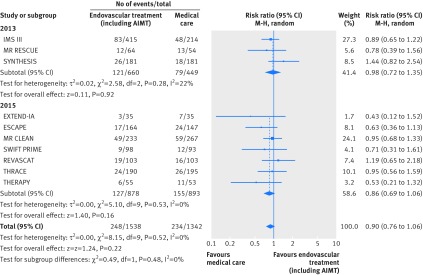
Fig 4 Forest plot for mortality at 90 days, including subgroup analysis by year of study publication. AIMT=adjunctive intra-arterial mechanical thrombectomy
Overall, 1129 out of 2907 patients (38.8%) achieved a good functional outcome at 90 days. Patients receiving endovascular treatment had a higher chance of achieving a good outcome (risk ratio 1.37, 95% confidence interval 1.14 to 1.64; fig 3), with an increase of 123 patients (95% confidence interval 46 to 212 patients) attaining a good outcome for each 1000 additional patients receiving endovascular treatment compared with medical care alone. Considerable statistical heterogeneity (I2=69%, P=0.0006) was present for overall pooled results of studies, but not for pooled results of studies published in 2013 (I2=0%; P=0.62) and in 2015 (I2=0%; P=0.43), which further support our a priori hypothesis that heterogeneity would exist between the results of trials done in 2013 and those done in 2015 owing to inequalities in study design, including patient populations and interventions. Indeed, the results for efficacy outcomes were significantly different (P<0.001) between these two subgroups of trials. No differences were found in the proportion of patients reaching modified Rankin scale scores of ≤2 (fig 3) or ≤1 (supplementary figure S1) among 2013 trials. In contrast, the pooled risk ratio for 2015 trials was 1.56 (95% confidence interval 1.38 to 1.75), representing an increase of 167 patients (95% confidence interval 113 to 223 patients) attaining a good outcome (modified Rankin scale score ≤2) for each 1000 additional patients receiving endovascular treatment compared with medical care alone. Additionally, the pooled risk ratio for 2015 trials for a modified Rankin scale score of ≤1 (supplementary figure S1) was 2.03 (95% confidence interval 1.62 to 2.53; I2=0, P=0.99), representing an increase of 131 patients (79 to 195 patients) attaining an excellent outcome for each 1000 additional patients receiving endovascular treatment compared with medical care alone. Data on outcomes for THRACE and THERAPY are not yet completely published. Sensitivity analysis excluding these studies from pooled risk ratio for 2015 trials yielded similar results: modified Rankin scale scores ≤2 (risk ratio 1.73, 95% confidence interval 1.49 to 2.01; I2=0, P=0.97) and ≤1 (2.04, 1.62 to 2.58; I2=0, P=0.99). Further sensitivity analysis excluding trials with low rates of patients treated with intravenous rt-PA in the control arm (MR RESCUE) or with low rates of adjunctive intra-arterial mechanical thrombectomy in the endovascular treatment arm (IMS III and SYNTHESIS) also yielded similar results for all efficacy outcomes; all these trials were published in 2013.
At 90 days, 482 out of 2880 participants (16.7%) died, without differences between arms in all cause mortality (risk ratio 0.90, 95% confidence interval 0.76 to 1.06; I2=0%, P=0.52; fig 4). Furthermore, the results did not differ between trials published in 2013 and 2015 (P=0.48). Sensitivity analysis excluding THRACE and THERAPY from the pooled risk ratio for 2015 trials yielded similar results (0.87, 0.68 to 1.11; I2=0%, P=0.43).
Overall, 129 out of 2526 patients (5.1%) experienced symptomatic intracerebral haemorrhage, with no significant differences between treatment groups (risk ratio 1.02, 95% confidence interval 0.72 to 1.44; I2=0%, P=0.85; supplementary figure S2). Furthermore, there were no significant differences between the results of trials published in 2013 and 2015 (P=0.86). Sensitivity analysis excluding THERAPY from the pooled risk ratio for 2015 trials yielded similar results (1.08, 0.63 to 1.83; I2= 0%, P=0.48).
Additional analysis
The number of included studies limited the evaluation of publication bias using funnel plots. Egger’s (P=0.435) and Peters’ (P=0.483) tests were not suggestive of publication bias or small studies’ effects.
For the trial sequential analysis, the proportion of patients with a favourable outcome (modified Rankin scale score ≤2) was 33%, and an increase in risk ratio of 37% was assumed based on the risk ratio of 1.37 estimated for the independency outcome. The cumulative evidence overcame the minimum information size required (1873 patients), adjusted for the obtained increase in risk ratio and heterogeneity (see supplementary figure S3). The cumulative evidence was not adequately powered for evaluation of mortality, reaching 20.1% of the required information size for a 9% risk ratio reduction of mortality (see supplementary figure S4).
Predetermined subgroup analysis for the primary efficacy outcome based on sex (see supplementary figure S5) and administered intravenous rt-PA across all patients (rt-PA versus no rt-PA; supplementary figure S6) was not significant between subgroups (P=0.61 and P=0.05, respectively).
Subgroup analysis according to stent retriever use reached significance (P=0.04; supplementary figure S7), favouring high (≥85%) stent retriever use (risk ratio 1.69, 95% confidence interval 1.42 to 2.01) over low to no use (1.18, 0.88 to 1.58).
Subgroup analysis according to risk of bias, characteristics of stroke, and time to treatment were not done because of the similar risk of bias across studies and because of lack of robust data for strokes involving the posterior circulation and time to endovascular treatment.
Discussion
In this systematic review we found that there is moderate to high quality evidence suggesting that the addition of endovascular treatment, in particular thrombectomy with stent retriever, to best medical care including intravenous rt-PA, improves the probability of a patient being functionally independent at 90 days after ischaemic stroke, without increased mortality or symptomatic intracerebral haemorrhage.
These conclusions are based on 10 randomised controlled trials enrolling 2925 patients with ischaemic stroke. Although pooled analysis of these trials yielded statistically significant and clinically relevant effects, statistically significant heterogeneity was found among the results of the studies. This heterogeneity was driven by differences in methodological and clinical features between studies. There were disparities in inclusion criteria and in the interventions considered in both the standard medical treatment and the endovascular treatment arms; in particular, the proportion of patients who underwent intravenous thrombolysis and adjunctive intra-arterial mechanical thrombectomy, as well as the type of devices used for thrombectomy. These differences led us to look at the results of the 10 included randomised controlled trials separately, using two distinct subgroups: trials published in 2013, including IMS III, SYNTHESIS, and MR RESCUE, and trials published in 2015, including MR CLEAN, ESCAPE, EXTEND-IA, SWIFT-PRIME, REVASCAT, THERAPY, and THRACE.
All the studies focused on strokes involving the anterior circulation, but IMS II, SYNTHESIS, and THRACE also included strokes in the posterior circulation as part of their inclusion criteria. THRACE also included strokes involving the proximal artery (internal carotid or M1). The former were the only types of strokes included in REVASCAT and SWIFT-PRIME. ESCAPE, MR CLEAN, REVASCAT, SWIFT-PRIME, and THERAPY also included strokes of the M2 portion of the middle cerebral artery.
Imaging evidence of large vessel occlusion (the index problem amenable by thrombectomy) was not required for enrolment in IMS III and SYNTHESIS but was an obligatory criterion in MR RESCUE and in all 2015 studies. In four studies it was also needed to document potentially salvageable brain tissue: perfusion imaging showing evidence of penumbra was required in three of the 2015 studies (ESCAPE, EXTEND-IA, and SWIFT PRIME), and REVASCAT only included patients with a high ASPECTS score—that is, with imaging features suggestive of less extensive brain damage. Although MR RESCUE evaluated the existence of penumbra, this was not a criterion for enrolment.
Most patients in the medical care arm (>77%) were treated with intravenous rt-PA (the only exception being MR RESCUE) as well as in the endovascular treatment arm (>68% of the patients), except in SYNTHESIS and MR RESCUE (where the rate of administered intravenous thrombolysis was low). In IMS III the dose of intravenous rt-PA was reduced because of the study design and safety issues.
Although all studies evaluated patients subjected to thrombectomy in the endovascular arm, the rate of patients who underwent adjunctive intra-arterial mechanical thrombectomy varied between studies. In 2015 trials this rate was high (>77%). However, less than 40% of the patients were treated with thrombectomy in IMS III and SYNTHESIS but were given intra-arterial rt-PA, a strategy that has proved to be of little benefit because of an increase in complications.48 Also important, the use of stent retrievers was more prominent in the most recent trials, which were the studies with the highest rates for reperfusion. Solitaire FR, the most commonly used stent retriever, is a newer generation device that has been shown to contribute to higher recanalisation rates and reduced deployment times compared with previous devices.49 The use of outdated first generation devices may have led to the suboptimal revascularisation rates observed in IMS III and MR RESCUE (41% and 25%, respectively) and, at least in IMS III, may have contributed to substandard groin puncture to reperfusion times.50
The focus on large vessel occlusion scenarios, the selection of patients with less extensive brain tissue damage, the use of two simultaneous endovascular reperfusion techniques (intravenous rt-PA and thrombectomy), and the use of more efficient devices are probably pivotal factors that help to explain the difference between the statistically significant and clinically relevant results observed among the 2015 randomised controlled trials, but not among the 2013 trials. Unsurprisingly, some previous systematic reviews and meta-analyses focusing mainly on 2013 publications9 10 12 failed to detect differences between treatments. However, our results are supported by the quantitative analysis of current meta-analytical studies that include more recent published randomised controlled trials.51 52 53
Considering the pathophysiology of ischaemic stroke and the knowledge acquired from IMS III54 and SYNTHESIS,55 as well as from previous trials of rt-PA,6 it can be concluded that faster, more efficient recanalisation is of paramount importance to reduce the infarction of penumbral brain tissue and thus contribute to improved clinical outcomes. As such, the prompt administering of intravenous rt-PA as well as timely intravascular intervention achieved in 2015 studies may have contributed to less brain tissue damage.
Because of the reasons discussed previously and the rate and dosage of intravenous rt-PA used in both study arms, the trials published or presented in 2015 are more suited to test the true effect of endovascular thrombectomy on its index disease. We therefore consider that pooled results from these studies evaluate more accurately the benefit of endovascular treatment in general and adjunctive thrombectomy after intravenous rt-PA in particular, for ischaemic stroke caused by large vessel occlusion. Based on these results, we conclude that patients undergoing adjunctive intra-arterial mechanical thrombectomy are twice as likely to be without disability and 1.5 times as likely to be functionally independent 90 days after an ischaemic stroke caused by occlusion of anterior large vessels.
Weaknesses of this study
Despite using data from multicentre randomised controlled trials, the information was not powered enough to evaluate the safety of endovascular treatment, including adjunctive intra-arterial mechanical thrombectomy. Furthermore, observational studies may be more adequate than randomised controlled trials to evaluate safety, as these may include patients who are usually excluded from randomised controlled trials, and follow-up is often longer. Lastly, the magnitude of effects may have been exaggerated by stricter patient selection and by a higher level of study site selection and interventionist proficiency compared with the real world.
The prospective randomised open blinded endpoint design of all studies has greater similarities with everyday clinical practice and is more cost effective than double blinded randomised controlled trial designs.56 None the less, such studies eliminate a placebo effect—a phenomenon not discarded in blind sham controlled trials—and are more likely to lead to researcher and patient biases56 and to patient drop-out after randomisation.
In stroke trials it is customary to provide outcomes at 90 days.57 However, spontaneous neurological recovery may take longer to attain its maximal potential,57 so longer follow-ups could have contributed to a better understanding of the evolution of functional endpoints through time.
Another limitation was the overall moderate risk of bias—all the trials used the prospective randomised open blinded endpoint design, some were mostly funded by industry, six were stopped early, and one was registered retrospectively. Nevertheless, previous reports noted that industry sponsored studies can accurately report outcomes58 and that efficacy treatment effects in truncated trials may not be substantially larger than for completed trials.59 Finally, data from the THRACE and THERAPY trials have not yet been officially published. These data was extracted from scientific conferences and press releases. Therefore it is possible that the available information is not definitive.
Implications for clinical practice
Recommending endovascular treatment, particularly adjunctive intra-arterial mechanical thrombectomy with stent retrievers, as standard of care in ischaemic stroke caused by occlusion of large vessels in the anterior circulation requires a restructuring of comprehensive stroke centres and of interventional neuroradiologists’ training to enhance the available resources.
The baseline characteristics of the included population mean that the pooled clinical benefit attributable to adjunctive intra-arterial mechanical thrombectomy may only be applicable to patients younger than 85 years with strokes involving the large vessels of the anterior circulation where brain damage is not widespread and if the intervention is performed within six to eight hours after an acute stroke. Adding thrombectomy to standard intravenous rt-PA opens the conventional treatment window from 4.5 hours to at least six hours in these scenarios. Still, the decision to use adjunctive thrombectomy should be taken shortly after patients start to receive intravenous rt-PA.
Implications for research
Future studies should evaluate the optimal timeframe for adjunctive intra-arterial mechanical thrombectomy; its benefit in patients with contraindications to thrombolysis, strokes involving the posterior circulation, and older populations; and its safety profile. Also, longer follow-ups could help provide a better understanding of the cost effectiveness and impact on budgets of implementing adjunctive intra-arterial mechanical thrombectomy. Finally, cost effectiveness analyses should be pursued to ascertain the value of endovascular thrombectomy before the widespread implementation and restructuring of comprehensive stroke centres.
Conclusion
In contrast with some previous publications8 9 11 and the results obtained in initial trials, this systematic review and meta-analysis found that endovascular treatment, in particular thrombectomy as an add-on to intravenous rt-PA, provides beneficial functional outcomes after ischaemic stroke secondary to occlusion of anterior large vessels, without increased detrimental effects compared with medical care alone. Our results and recommendations are in accordance with other recently published systematic reviews in this specialty.51 52 53 In addition to the studies already included in those recent systematic reviews, we included data from two unpublished studies and performed a cumulative meta-analytical measurement—trial sequential analysis—that reinforces our findings and recommendations. Also, our qualitative analysis allows for an indepth view of the clinical and methodological disparities between the published trials. This, in turn, helps to explain the shift in evidence regarding endovascular thrombectomy in acute stroke as well as to explore the clinical contexts where this invasive approach seems to be more beneficial. We think that the critical discussion on how the obtained results translate into clinical practice is of particular interest to both neurologists and neuroradiologists when deciding the best treatment option for individual patients with acute stroke.
What is already known on this topic
Intravenous thrombolysis is the standard treatment for acute ischaemic stroke, but the rates for recanalisation are not ideal
The use of concomitant endovascular reperfusion techniques, such as adjunctive intra-arterial mechanical thrombectomy, may help to improve clinical outcomes further
What this study adds
This systematic review and meta-analysis of 10 randomised controlled trials provide moderate to high quality evidence suggesting that, in carefully selected patients endovascular treatment, particularly adjunctive intra-arterial mechanical thrombectomy, provided within six to eight hours after ischaemic stroke involving large vessels in the anterior circulation, leads to improved functional outcomes at 90 days without increased mortality or symptomatic intracerebral haemorrhage
This evidence supports the need to restructure current neurointerventional resources and to change clinical practice
Web extra.
Web extra supplied by authors
Appendix: additional information
Contributors: JJF and JC are the guarantors. All authors except for JMF contributed to the drafting of the manuscript, the development of the selection criteria, the risk of bias assessment strategy, and data extraction criteria. FBR developed the search strategy. FBR and JBN conducted the report screening, study inclusion, data extraction, and result interpretation and discussion. DC performed the statistical analysis and intrepted and discussed the results. JMF, JJF, and JC provided expertise on stroke. JJF and JC also provided expertise on methods. All authors read, provided feedback, and approved the final manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study received no funding.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that: no author has support for the submitted work; JJF has speaker and consultant relationships with GlaxoSmithKline, Novartis, TEVA, Lundbeck, Solvay, Abbott, Bial, Merck-Serono, Grunenthal, and Merck Sharp and Dohme that might have an interest in the submitted work in the previous three years; their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and FBR, JBN, DC, and JC have no non-financial interests that may be relevant to the submitted work. JMF received in the past three years speaker fees from Boehringer Ingelheim and consultancy fees from Boehringer Ingelheim, Lundbeck, and Daichi Sankyo.
Ethical approval: Not required.
Data sharing: No additional data available.
Transparency: The lead authors (JC and JJF) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. 10.1016/S0140-6736(12)61728-0 pmid:23245604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 2012;79:1781-7. 10.1212/WNL.0b013e318270401d pmid:23054237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thrift AG, Cadilhac DA, Thayabaranathan T, et al. Global stroke statistics. Int J Stroke 2014;9:6-18. 10.1111/ijs.12245 pmid:24350870. [DOI] [PubMed] [Google Scholar]
- 4.Ueda T, Sakaki S, Kumon Y, Ohta S. Multivariable analysis of predictive factors related to outcome at 6 months after intra-arterial thrombolysis for acute ischemic stroke. Stroke 1999;30:2360-5. 10.1161/01.STR.30.11.2360 pmid:10548671. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2014;7:CD000213.pmid:25072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emberson J, Lees KR, Lyden P, et al. Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929-35. 10.1016/S0140-6736(14)60584-5 pmid:25106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967-73. 10.1161/01.STR.0000258112.14918.24 pmid:17272772. [DOI] [PubMed] [Google Scholar]
- 8.Lin C, Li N, Wang K, et al. Efficacy and safety of endovascular treatment versus intravenous thrombolysis for acute ischemic stroke: a meta-analysis of randomized controlled trials. PLoS One 2013;8:e77849 10.1371/journal.pone.0077849 pmid:24204995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh B, Parsaik AK, Prokop LJ, Mittal MK. Endovascular therapy for acute ischemic stroke: a systematic review and meta-analysis. Mayo Clin Proc 2013;88:1056-65. 10.1016/j.mayocp.2013.07.015 pmid:24079677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fargen KM, Neal D, Fiorella DJ, Turk AS, Froehler M, Mocco J. A meta-analysis of prospective randomized controlled trials evaluating endovascular therapies for acute ischemic stroke. J Neurointerv Surg 2015;7:84-9. 10.1136/neurintsurg-2014-011543 pmid:25432979. [DOI] [PubMed] [Google Scholar]
- 11.Osanai T, Pasupuleti V, Deshpande A, et al. Acute endovascular reperfusion therapy in ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2015;10:e0122806 10.1371/journal.pone.0122806 pmid:25915905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamseer L, Moher D, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647 10.1136/bmj.g7647 pmid:25555855. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94 10.7326/0003-4819-151-4-200908180-00136 pmid:19622512. [DOI] [PubMed] [Google Scholar]
- 14.Lang TA, Altman DG. Basic Statistical Reporting for Articles Published in Biomedical Journals: The “Statistical Analyses and Methods in the Published Literature” or The SAMPL Guidelines”. Science Editors’ Handbook, European Association of Science Editors. 2013. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions.Wiley Online Library, 2008 10.1002/9780470712184. [DOI] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. 10.2307/2529310 pmid:843571. [DOI] [PubMed] [Google Scholar]
- 17.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. 10.1161/01.STR.19.5.604 pmid:3363593. [DOI] [PubMed] [Google Scholar]
- 18.Wahlgren N, Ahmed N, Dávalos A, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007;369:275-82. 10.1016/S0140-6736(07)60149-4 pmid:17258667. [DOI] [PubMed] [Google Scholar]
- 19.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 2002;21:1575-600. 10.1002/sim.1188 pmid:12111921. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. 10.1002/sim.1186 pmid:12111919. [DOI] [PubMed] [Google Scholar]
- 21.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61:763-9. 10.1016/j.jclinepi.2007.10.007 pmid:18411040. [DOI] [PubMed] [Google Scholar]
- 22.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive--Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287-98. 10.1093/ije/dyn188 pmid:18824466. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549-56. [PubMed] [Google Scholar]
- 24.Fiorella DJ, Fargen KM, Mocco J, et al. Thrombectomy for acute ischemic stroke: an evidence-based treatment. J Neurointerv Surg 2015;7:314-5. 10.1136/neurintsurg-2015-011707 pmid:25735851. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629 pmid:9310563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676-80. 10.1001/jama.295.6.676 pmid:16467236. [DOI] [PubMed] [Google Scholar]
- 27.Atkins D, Best D, Briss PA, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490 10.1136/bmj.328.7454.1490 pmid:15205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broderick JP, Palesch YY, Demchuk AM, et al. Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368:893-903. 10.1056/NEJMoa1214300 pmid:23390923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciccone A, Valvassori L, Nichelatti M, et al. SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013;368:904-13. 10.1056/NEJMoa1213701 pmid:23387822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidwell CS, Jahan R, Gornbein J, et al. MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368:914-23. 10.1056/NEJMoa1212793 pmid:23394476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkhemer OA, Fransen PS, Beumer D, et al. MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11-20. 10.1056/NEJMoa1411587 pmid:25517348. [DOI] [PubMed] [Google Scholar]
- 32.Goyal M, Demchuk AM, Menon BK, et al. ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. 10.1056/NEJMoa1414905 pmid:25671798. [DOI] [PubMed] [Google Scholar]
- 33.Campbell BC, Mitchell PJ, Kleinig TJ, et al. EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-18. 10.1056/NEJMoa1414792 pmid:25671797. [DOI] [PubMed] [Google Scholar]
- 34.Saver JL, Goyal M, Bonafe A, et al. SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-95. 10.1056/NEJMoa1415061 pmid:25882376. [DOI] [PubMed] [Google Scholar]
- 35.Jovin TG, Chamorro A, Cobo E, et al. REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-306. 10.1056/NEJMoa1503780 pmid:25882510. [DOI] [PubMed] [Google Scholar]
- 36.Mocco J, Zaidat O, Von Kummer R, et al. Results of the THERAPY trial: A prospective, randomized trial to define the role of mechanical thrombectomy as adjunctive treatment to iv rtpa in acute ischemic stroke. Int J Stroke 2015;10:10.pmid:26306674.26306674 [Google Scholar]
- 37.Bracard S, Guillemin F, Ducrocq X. THRACE study: Intermediate analysis results. Int J Stroke 2015;10:31. [Google Scholar]
- 38.Khatri P, Hill MD, Palesch YY, et al. Interventional Management of Stroke III Investigators. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke 2008;3:130-7. 10.1111/j.1747-4949.2008.00151.x pmid:18706007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciccone A, Valvassori L, Nichelatti M. SYNTHESIS Expansion investigators. SYNTHESIS expansion: design of a nonprofit, pragmatic, randomized, controlled trial on the best fast-track endovascular treatment vs. standard intravenous alteplase for acute ischemic stroke. Int J Stroke 2011;6:259-65. 10.1111/j.1747-4949.2011.00587.x pmid:21557814. [DOI] [PubMed] [Google Scholar]
- 40.Kidwell CS, Jahan R, Alger JR, et al. MR RESCUE Investigators. Design and rationale of the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) Trial. Int J Stroke 2014;9:110-6. 10.1111/j.1747-4949.2012.00894.x pmid:22974139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fransen PS, Beumer D, Berkhemer OA, et al. MR CLEAN Investigators. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials 2014;15:343 10.1186/1745-6215-15-343 pmid:25179366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demchuk AM, Goyal M, Menon BK, et al. ESCAPE Trial Investigators. Endovascular treatment for Small Core and Anterior circulation Proximal occlusion with Emphasis on minimizing CT to recanalization times (ESCAPE) trial: methodology. Int J Stroke 2015;10:429-38. 10.1111/ijs.12424 pmid:25546514. [DOI] [PubMed] [Google Scholar]
- 43.Campbell BC, Mitchell PJ, Yan B, et al. EXTEND-IA investigators. A multicenter, randomized, controlled study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial therapy (EXTEND-IA). Int J Stroke 2014;9:126-32. 10.1111/ijs.12206 pmid:24207098. [DOI] [PubMed] [Google Scholar]
- 44.Saver JL, Goyal M, Bonafe A, et al. SWIFT PRIME Investigators. Solitaire™ with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) trial: protocol for a randomized, controlled, multicenter study comparing the Solitaire revascularization device with IV tPA with IV tPA alone in acute ischemic stroke. Int J Stroke 2015;10:439-48. 10.1111/ijs.12459 pmid:25777831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molina CA, Chamorro A, Rovira À, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke 2015;10:619-26. 10.1111/ijs.12157 pmid:24206399. [DOI] [PubMed] [Google Scholar]
- 46.Mocco J, Khatri P, Zaidat O. Assess the Penumbra System in the Treatment of Acute Stroke (THERAPY). 2011. https://clinicaltrials.gov/ct2/show/study/NCT01429350.
- 47.Bracard S. Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke (THRACE). 2010. https://clinicaltrials.gov/ct2/show/study/NCT01062698?term=thrace&rank=1.
- 48.Mullen MT, Pisapia JM, Tilwa S, Messé SR, Stein SC. Systematic review of outcome after ischemic stroke due to anterior circulation occlusion treated with intravenous, intra-arterial, or combined intravenous+intra-arterial thrombolysis. Stroke 2012;43:2350-5. 10.1161/STROKEAHA.111.639211 pmid:22811451. [DOI] [PubMed] [Google Scholar]
- 49.Saver JL, Jahan R, Levy EI, et al. SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012;380:1241-9. 10.1016/S0140-6736(12)61384-1 pmid:22932715. [DOI] [PubMed] [Google Scholar]
- 50.Goyal M, Almekhlafi MA, Fan L, et al. Evaluation of interval times from onset to reperfusion in patients undergoing endovascular therapy in the Interventional Management of Stroke III trial. Circulation 2014;130:265-72. 10.1161/CIRCULATIONAHA.113.007826 pmid:24815501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CJ, Ding D, Starke RM, et al. Endovascular vs medical management of acute ischemic stroke. Neurology 2015;85:1980-90;85:1980-90. 10.1212/WNL.0000000000002176 pmid:26537058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yarbrough CK, Ong CJ, Beyer AB, Lipsey K, Derdeyn CP. Endovascular Thrombectomy for Anterior Circulation Stroke: Systematic Review and Meta-Analysis. Stroke 2015;46:3177-83. 10.1161/STROKEAHA.115.009847 pmid:26396032. [DOI] [PubMed] [Google Scholar]
- 53.Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular Thrombectomy for Acute Ischemic Stroke: A Meta-analysis. JAMA 2015;314:1832-43. 10.1001/jama.2015.13767 pmid:26529161. [DOI] [PubMed] [Google Scholar]
- 54.Khatri P, Yeatts SD, Mazighi M, et al. IMS III Trialists. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol 2014;13:567-74. 10.1016/S1474-4422(14)70066-3 pmid:24784550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciccone A, Investigators SE. SYNTHESIS Expansion Investigators. Complexity of the endovascular intervention and clinical outcomes in acute ischaemic stroke. Lancet Neurol 2014;13:865 10.1016/S1474-4422(14)70174-7 pmid:25142453. [DOI] [PubMed] [Google Scholar]
- 56.Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press 1992;1:113-9. 10.3109/08037059209077502 pmid:1366259. [DOI] [PubMed] [Google Scholar]
- 57.Duncan PW, Jorgensen HS, Wade DT. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke 2000;31:1429-38. 10.1161/01.STR.31.6.1429 pmid:10835468. [DOI] [PubMed] [Google Scholar]
- 58.Naci H, Dias S, Ades AE. Industry sponsorship bias in research findings: a network meta-analysis of LDL cholesterol reduction in randomised trials of statins. BMJ 2014;349:g5741 10.1136/bmj.g5741 pmid:25281681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korn EL, Freidlin B, Mooney M. Stopping or reporting early for positive results in randomized clinical trials: the National Cancer Institute Cooperative Group experience from 1990 to 2005. J Clin Oncol 2009;27:1712-21. 10.1200/JCO.2008.19.5339 pmid:19237631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix: additional information