Abstract
The cornea requires constant epithelial renewal to maintain clarity for appropriate vision. A subset of stem cells residing at the limbus is primarily responsible for maintaining corneal epithelium homeostasis. Trauma and disease may lead to stem cell deficiency and therapeutic targeting to replenish the stemness capacity has been stalled by the lack of reliable corneal epithelial stem cell markers. Here we identified the location of Lhx2 in mice (mLhx2) cornea and conjunctival tissue using an Lhx2eGFP reporter model and in human tissues (hLHX2). Lhx2 localized to the basal cells of central cornea, the conjunctiva and the entire limbal epithelium in humans and mice. To ascribe a functional role we generated Lhx2 conditional knockout (cKO) mice and the phenotypic effects in corneas were analyzed by slit lamp microscopy, in cell‐based assays and in a model of corneal epithelium debridement. Immunodetection on corneal sections were used to visualize conjunctivalization, a sign of limbal barrier failure. Lhx2cKO mice produced reduced body hair and spontaneous epithelial defects in the cornea that included neovascularization, perforation with formation of scar tissue and opacification. Cell based assays showed that Lhx2cKO derived corneal epithelial cells have a significantly lower capacity to form colonies over time and delayed wound‐healing recovery when compared to wildtype cells. Repeated corneal epithelial wounding resulted in decreased re‐epithelialization and multiple cornea lesions in Lhx2cKO mice compared to normal recovery seen in wildtype mice. We conclude that Lhx2 is required for maintenance of the corneal epithelial cell compartment and the limbal barrier. Stem Cells 2016;34:493–503
Keywords: LIM homeobox domain 2, Cornea, Stem Cells, Wound healing, Lhx2cKO, Limbus
Significance Statement.
Over 1.2 million Americans suffer from cornea damage and over 40,000 people per year undergo corneal transplantation therapy to repair irreversible corneal injury. Corneal epithelial stem cell transplantation to stem cell deficient corneas has the potential to restore vision. In order to improve patient rehabilitation with limbal stem cell deficiency, the ability to better harness and induce the regenerative capacity of adult corneal epithelial stem cells (CESCs) using reliable genetic markers is required. The proposed research will aid in identifying, purifying and expanding stem cell populations and induce corneal epithelial cells to behave stem cell‐like necessary for stem cell maintenance.
Introduction
Transparency of the cornea is essential for vision. The integrity of the corneal epithelium, the outermost layer of the cornea, is maintained by corneal limbal epithelial stem cells localized between the edge of the cornea and the conjunctiva, in a zone known as the limbus. Trauma, disease or genetic alteration in the limbus, can result in corneal limbal stem cell deficiency that manifests clinically as recurrent epithelial defects leading to significant corneal decompensation with signs of neovascularization, conjunctivalization, opacification, and in extreme cases ulceration, cornea perforation, or blindness 1, 2, 3. Treatment of limbal stem cell deficiency requires the direct involvement and application of corneal epithelial stem cells, such as donor limbal stem cell transplantation. Presently there are no established limbal stem cell markers that enable sensitive and specific direct identification of corneal stem cells for selection and expansion for transplantation. Allogeneic limbal transplantation is at risk of rejection and requires systemic immunosuppression, as the corneal limbus is not an immune privileged tissue, unlike the rest of the cornea. Thus, further investigation of corneal epithelial stem cell biology remains a relevant and important area for advancing the treatment options for limbal stem cell deficiency.
Lim homeobox domain 2 (Lhx2) plays an important role in a variety of epithelial–mesenchymal interactions and in the regulation of various stem/progenitor cell populations identified in skin and hair, liver, blood, and the brain 4, 5, 6, 7, 8. Lhx2 has been shown to be crucial in the maintenance of stemness in murine hair follicle stem cells (HFSCs) 5, 9. The cornea is an epithelial tissue derived from neuroepithelial ectodermal origin, similar to skin. As both tissues share a common developmental origin, our hypothesis is that previously identified stem cell markers in skin may also exist in the cornea. In support of this idea, there is evidence that cofactors of LIM domains (CLIMS), which interact with LIM domains such as Lhx2, regulate maintenance of HFSCs as well as corneal homeostasis 10. Furthermore, Lhx2cKO mice driven by the K14 promoter, results in reduced hair formation from the failure to maintain HFSC quiescence and hair anchoring 11. Although the skin functions differently from the cornea, it has shown the potential to transdifferentiate into cells of a corneal phenotype 12. This apparent connection between epidermal and corneal epithelial cells suggests that Lhx2 may not only be important in maintaining stem cells of the skin, but may also play a role in corneal epithelial stem cell maintenance. We used a mouse genetics approach to identify Lhx2 by using a green fluorescent protein (GFP) reporter gene tagged to the Lhx2 promoter, known as the Lhx2eGFP model and a conditional knockout mating K14‐Cre with Lhx2 fl/fl animals, conditionally ablating Lhx2 in keratin 14 driven cells. Our findings demonstrate that Lhx2 is required for the maintenance of corneal limbal stem cells and the preservation of the ocular surface structure.
Materials and Methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) from Weill Cornell Medical College, in accordance with the US NIH Guide for the Care and Use of Laboratory Animals and guidelines of the Association for Research in Vision and Ophthalmology Statement for the use of Animals in Ophthalmic and Vision Research. Wild‐type (WT) CD1 mice were obtained from Jackson Laboratories (Bar Harbor, ME). The transgenic mice Lhx2eGFP 13 and the floxed LIM homeobox 2 (Lhx2fl/fl) mice 14 crossed with K14‐Cre mice 15 to obtain Lhx2cKO lines were obtained as a collaborative study with Dr. Elaine Fuchs (Rockefeller University, NY).
Immunofluorescence and Preparation of Corneal–Conjunctival Wholemounts and Sections
The Lhx2eGFP reporter allowed us to detect the expression of Lhx2 in corneal tissue. First, the expression of Lhx2 and GFP was detected in Lhx2eGFP corneal–conjunctival wholemount tissue. For Lhx2 detection, 20‐week‐old nonfixed mouse corneas were incubated with rabbit polyclonal LHX2Ab at 1:5,000 dilution (Gift from Dr. E. Fuchs, Rockefeller University) overnight at 4°C followed by secondary anti rabbit Cy3 (Jackson Immuno Research: 711‐165‐152, Westgrove, PA, https://www.jacksonimmuno.com/catalog/products/711-165-152). Samples were mounted in vectashield containing 4′,6‐diamidino‐2‐phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA, http://vectorlabs.com/vectashield-mounting-medium-with-dapi.html).
Next, to detect corneal, limbal, and conjunctival expression of Lhx2eGFP reporter, 9‐week‐old corneas were fixed in 4% paraformaldehyde (PFA) for 40 minutes and embedded in Tissue Tek Optical Cutting Temperature compound (Sakura Finetek Japan Co., Tokyo, Japan) and snap frozen in liquid nitrogen. Cornea sections of 8 μm were mounted onto Superfrost Plus Gold slides (Fisherscientific, Waltham, MA, http://www.thermoscientific.com/content/tfs/en/product/superfrost-plus-gold-slides.html) and incubated with chicken polyclonal to GFP antibody, at 1:1,000 (Abcam: 13970, Cambridge, MA, http://www.abcam.com/gfp-antibody-chip-grade-ab290.html) followed by secondary antibody (as above) anti chicken Alexa 488 (Life Technologies: A11039, South San Francisco, CA, https://webshop.fishersci.com/insight2_uk/getProduct.do?productCode=10286672&resultSetPosition=0).
For LHX2 in human tissues, 10 μm corneal and conjunctival sections, were obtained from human corneas donated by the Eversight Eye Bank and processed for immunofluorescence as above using Cy3 as secondary antibody.
Expression of Stem Cell Markers on Corneal Tissue
The GFP and Lhx2 expression in mice harboring the Lhx2eGFP reporter was determined in the central and limbal corneal epithelium of 6‐week‐old mice. Corneal tissues from the center and limbus were isolated using a 2‐mm biopsy punch (Miltex, Inc., York, PA) (n = 4 mice). Corneal epithelia were separated from stroma by incubating the tissues at 4°C overnight in CnT50 media (CELLnTEC, Bern, Switzerland) containing 100 mg/ml of dispase (Roche Diagnostics, Basel, Switzerland) and 100 mg/ml D‐sorbitol (Sigma Aldrich, St. Louis, MO). Total RNA was extracted using the RNA Easy Mini Kit (Qiagen, Gaithersburg, MD, https://www.qiagen.com/us/shop/sample-technologies/rna/rna-preparation/rneasy-mini-kit), quantified and used in equal concentration of limbal and central corneal epithelium to generate cDNA using a reverse transcriptase polymerase chain reaction (RT‐PCR) kit according to the manufacturer's protocol (Invitrogen, Grand Island, NY, http://www.thermofisher.com/us/en/home/life-science/pcr/reverse-transcription/rt-pcr.html). cDNA was used as a template for real‐time PCR, using the Step One Plus Applied Biosystems (Thermo Fisher Scientific, Waltham, MA). For GFP detection, forward and reverse primers were diluted to 2 μM from 20 μM stock concentration in RNAse free water. The GFP probe was diluted to 1 μM from 100 μM stock concentration. GFP primer sequence Egfp‐fp: AGC AAA GAC CCC AAC GAG AA, Egfp‐rp: GGC GGC GGT CAC GAA, and GFP probe (Applied Biosystems, 6 FAM‐CGC GAT CAC ATG GTC CTG CTG G‐TAMRA). Primer pairs and TaqMan MGB probes labeled with 6‐carboxyfluorescin (FAM) at the 5′ end and nonfluorescent quencher at the 3′ end were designed with Applied Biosystems, Assay By Design mouse Lhx2 (Mm00839783_m1) and all primers normalized to GAPDH, (Mm00000015g1). The initial concentration of TaqMan MGB probes (Lhx2 and GAPDH) of the forward and reverse primers was 900 nM and the probe was 250 nM. The working concentration required dilution of the primer/probe mix to 0.6 μl in RNAse free water, Taqman PCR master mix to 6 μl in 0.4 μl RNAse free water per well. The thermocycling program was an initial cycle at 95°C for 20 seconds, followed by 45 cycles of 95°C for 3 seconds and 60°C for 30 seconds. All samples were tested in triplicate. For the detection of Lhx2, Trp63 and ABCG2 expression in WT and Lhx2cKO mice, RNA was isolated from the entire corneal epithelium (n = 10 mice), cDNA and RT‐PCR was performed as described above. The primer pairs and TaqMan MGB probes (Applied Biosystems, Grand Island, NY) used included mouse GAPDH, (Mm00000015g1), Trp63 (Mm00495788m1), ABCG2 (Mm00496364m1), and Lhx2 (Mm00839783_m1).
To study the Lhx2 expression on human samples, approval from Weill Cornell Medical College Institutional Review Board was obtained for use of human corneal specimens for research. Healthy corneas from male donors aged 57, 68, and 63 were separated into central and limbal segments using a trephine ring and the epithelium was scraped from the stroma by using a #64 Beaver blade (Becton Dickinson, Franklin Lakes, NJ). The RNA was extracted as described and cDNA was used as a template to test for the expression of LHX2 in the limbal versus central corneal epithelium. The GAPDH (Hs02758991g1) and LHX2 (Hs00180351m1) primers were obtained from Applied Biosystems, and the RT‐PCR procedure was performed as described above.
Evaluation of Cornea Phenotypes
Mice were continuously evaluated to follow up the distinct phenotype that some Lhx2cKO mice presented along this study. Mice were when significant symptoms of distress were found. Cornea images from 2‐ to 26‐week‐old mice were obtained using a slit lamp (WT and Lhx2cKO, n = 12 mice each).
Isolation of Corneal Epithelial Cells
To obtain isolated corneal epithelial cells for cell culture or RNA analysis, the eyes from WT and Lhx2cKO mice were enucleated, washed in phosphate‐buffered saline and incubated at 4°C overnight in CnT‐50 media containing dispase and D‐sorbitol as described above. The detached epithelial sheets were collected and incubated with 0.05% trypsin/0.03% EDTA (Life Technologies, Grand Island, NY, http://www.thermofisher.com/order/catalog/product/25300054) at 37°C for 10 minutes to isolate single cells using a spatula to peel off the corneal epithelium attached to the eyeball. The enzymatic reactions were stopped by adding CnT‐50 media supplemented with fetal calf serum (FCS) (Sigma, St Louis, MO, http://www.sigmaaldrich.com/catalog/product/sigma/12133c?lang=en®ion=US).
Colony Forming Assay
Isolated epithelial cells from adult WT and Lhx2cKO (6–10 weeks old) were seeded at 2,000 cells per well on Mitomycin C‐treated (4 μg/ml) mouse embryonic fibroblast 3T3 feeder layer (Cell line: CCL‐92) cultured in Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, NY, http://www.thermofisher.com/us/en/home/life-science/cell-culture/mammalian-cell-culture/classical-media/dmem.html) containing 10% FCS in triplicate on six‐well culture plates. The number of colonies generated expressed as a percentage of the total number of epithelial cells plated in a well was defined as the colony forming efficiency (CFE), determined at days 4–12. The clone growth capacity was evaluated on days 12–14 and the cultures stained with 1% crystal violet. SPSS statistics version 19 was used for statistical analysis; a Student's t test was used for comparing the CFE (%) with a p < 0.05 as statistically significant.
In Vitro Scratch Assay
To determine the direct role of Lhx2 on epithelium renewal, we evaluated the wound healing capacity of WT corneal cells and compared with cells derived from Lhx2cKO mice. Corneal cells from adult WT and Lhx2cKO (adult 6–9 weeks old) were isolated as described above and seeded at a density of 2.7 × 104 per square centimeter on tissue culture dishes coated with rat‐tail I collagen (Life Technologies, Grand Island, NY, http://www.thermofisher.com/order/catalog/product/A1048301) at a concentration of 5 μg/cm2. Cells were grown to confluence over 7 days and scratched using a P200 pipette tip creating a cell‐free area as described by Liang et al. 16. The scratched areas were photographed in the same location at 0, 8, 16, 24, and 48 hours. The cell‐free areas were measured using Image J Software (Image J 1.47v, NIH, Thornwood, MD, Bethesda) and only closely matching areas were selected for analysis. The experiments were repeated three times.
In Vivo Corneal Epithelial Wounding and Histological Processing
To evaluate the role of Lhx2 on corneal epithelium renewal in vivo, the right eyes of WT (n = 8) and Lhx2cKO (n = 9) mice were subjected to epithelial debridement without causing injury to the underlying stroma. The left eyes were untouched and serve as controls. For this procedure, mice were anesthetized with intraperitoneal injections of a combination of ketamine (100 mg/kg) and xylazine (5 mg/kg; Phoenix Scientific, St. Joseph, MO) and 10 μl of proparacaine hydrochloride ophthalmic solution (0.5%) as a local anesthesia. A 2‐mm trephine was used to mark the area for the epithelial debridement and blunt scalpel used to gentle detach the epithelium. Animal received analgesic, and topical fluorescein solution was administered to the corneal surfaces and the epithelial defects imaged at 0, 8, 16, 32, 48, 72, 96, and 144 hours. To avoid infection of the eye, 10 μl of vigamox (moxifloxacin, Alcon, Inc., Fort Worth, TX) was applied after epithelial debridement. The epithelial debridement experiments were repeated three times, once per week during 3 weeks, allowing the corneal epithelium to heal between procedures. This challenge to the epithelium renewal was performed on the same experimental group of mice. After 144 hours from the final epithelial debridement surgery, WT and Lhx2cKO mice were euthanized, the eyes enucleated and an orbital exenteration was performed including the forniceal, palpebral conjunctiva, and cornea. The tissues were then fixed in 4% PFA for 40 minutes at room temperature and embedded in OCT as described above, cut at 8 μm sections and mounted adjacently over four slides for immunofluorescence and periodic acid staining (PAS). For the detection of conjunctival tissue, sections were processed as above and rabbit polyclonal Keratin 15 antibody (Protein Tech Group, IL:10137‐1‐AP, Rosemont, IL, http://www.ptglab.com/Products/KRT15-Antibody-10137-1-AP.htm) at 1:200 dilution was used.
Periodic Acid Staining
For the detection of goblet cells on the cornea, frozen sections from WT and Lhx2cKO corneas after the third debridement surgery were rinsed in water, incubated in 0.5% periodic acid solution, and washed in water. Slides were stained in Schiff (Electron Microscopy Sciences, PA, Hatfield, PA, http://www.sigmaaldrich.com/catalog/product/sigma/3952016?lang=en®ion=US) reagent, washed in tap water, and counterstained in Harris haemotoxylin. Slides were rinsed in water, dipped in 0.5% acid alcohol and further rinsed in water to mount in DePeX mounting medium to coverslip.
Imaging
Bright field and immunofluorescence images were captured using an observer Z1 fluorescent microscope with the AxioCam HRm digital camera and AxioVision software (Carl Zeiss, Thornwood, NY). Wholemount images were captured using a Zeiss LSM‐710 confocal microscope (Carl Zeiss, Thornwood, NY). Images were compiled in Photoshop CS5.1 and Illustrator CS5.1 (Adobe systems Inc., San Jose, CA.).
Statistics for Wound Healing Assays
All wound healing experiments were statistically analyzed only in those mice that healed by calculating the mean ± SD for each group and a Student's unpaired t test was applied by Prism (Graph Pad Software, La Jolla, CA) and graphed. Test results were reported as two‐tailed p values, where p < 0.05 were considered statistically significant.
Results
Lhx2 Is Expressed in Murine Corneal Epithelium and Preferentially Located Within the Limbus
To detect Lhx2 expression, we used a mouse genetics approach where the Lhx2 gene promoter was fused to eGFP and could be detected by direct fluorescence or immunostaining for GFP. Our studies on wholemount cornea, limbus, and conjunctival tissue demonstrated consistent localization that was diffusely distributed in central cornea, with more focal expression in the limbus, and high levels of expression in the conjunctival tissue at 20 weeks old. There was a distinct area of localization at the limbus which was not seen in adjacent sclera (Fig. 1A, 1B indicated by dashed yellow lines). GFP expression in the cornea is found in few central basal cells and in all epithelial layers of the limbus, which showed high expression (Fig. 1C, yellow line separate central cornea from limbus). The localization of human followed the same expression pattern as observed in the mouse. The central cornea expressed Lhx2 in few basal epithelial cells, whereas limbal corneal expression included basal and a few suprabasal layers. The conjunctival expression, as seen in mouse was highly pronounced in the basal layer and included few cells above (Fig. 1D). To corroborate the immunostaining, we isolated cells from both areas of the cornea and performed a RT‐PCR analysis. We found that both the expression of GFP and Lhx2 were significantly higher in the limbus as compared to central cornea (4.5 and 0.6 fold respectively) (Fig. 1E). We also investigated the relevance of the findings in mice to humans and found that this preferential expression of Lhx2 in limbal versus central cornea was even more pronounced in human cornea. Real‐time PCR demonstrated a 32‐fold higher levels of Lhx2 in human limbus versus central corneal epithelium (Fig. 1F).
Figure 1.
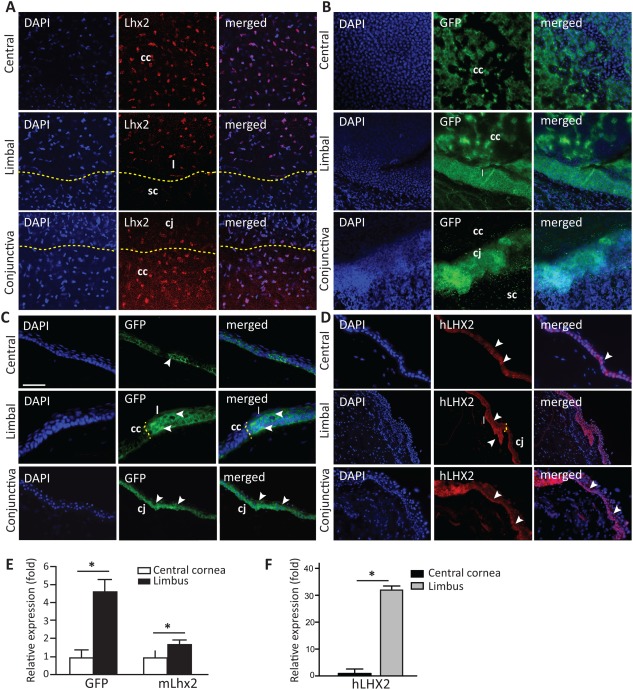
Lhx2 is expressed in the central cornea and limbus.
Lhx2 wholemount cornea and conjunctival nuclear staining (red) were detected on Lhx2eGFP corneal tissue in central, limbal, and conjunctival tissue (A). GFP expression of Lhx2eGFP in corneal wholemounts was detected in the central cornea and spanned the entire limbal region, while conjunctival tissue highly expressed Lhx2eGFP (B). In tissue cross sections, GFP‐positive cells were detected in the basal layer of the central corneal epithelium. In the peripheral cornea where the limbus is located, GFP expression was more abundant including suprabasal and basal layers. Basal cells of the conjunctiva stained positive for GFP as well (C). In human tissue, LHX2 nuclear staining in the central cornea localized to few cells in the basal layer, and included basal and suprabasal cells in the limbus. The conjunctiva distinctly expressed LHX2 in basal cells (D). Using quantitative reverse transcriptase polymerase chain reaction analysis, expression of GFP and Lhx2 was found significantly higher in murine epithelial cells originating from the limbus when compared with those from the central cornea (GFP = 4.5‐fold higher, Lhx2 = 1.6‐fold higher vs. central corneal epithelium) (E). Similarly, human LHX2 was expressed at significantly higher levels in limbal versus central corneal epithelium (32‐fold higher) (F). White arrows indicate Lhx2eGFP + and hLHX2 + cells, respectively; yellow dashed line separates the central cornea, from the limbus, conjunctiva, and sclera. Scale bar = 50 µm for images in (A–D). Data are means ± SE (human n = 3, mouse n = 4). *, p ≤ 0.05. Abbreviations: cc, central cornea; cj, conjunctiva; DAPI, * * *; GFP, green fluorescent protein; l, limbus; sc, sclera.
Lhx2cKO Mice Exhibit Abnormal Skin and Corneal Phenotypes
We next generated Lhx2cKO mice to study how the deletion of Lhx2 influences corneal epithelium homeostasis. These mice were obtained by breeding K14Cre+/− and Lhx2fl/fl and gave rise to K14Cre−/− Lhx2fl/fl or WT and K14Cre+/− Lhx2fl/fl or Lhx2cKO. When comparing postnatal day 10 (P10) littermates, it was clearly visible that the Lhx2cKO mice have reduced body hair when compared with WT (Fig. 2A). When we analyzed the cornea, all WT mice were normal (data not shown) and Lhx2cKO corneas were normal up to 6 weeks and devoid of vessels or ocular surface disorders (Fig. 2Ba). Some adult Lhx2cKO corneas exhibited a spontaneous phenotype including neovascularization (Fig. 2Bb), perforation (Fig. 2Bc), and opacification (Fig. 2Bd). These phenotypic changes became more common and severe as the mice aged (Table 1). For instance, at 6 weeks of age, 8% of mice showed neovascularization, that increased to 17% at 9 weeks, and become more prominent as with age, resulting in 83% of animal presenting this phenotype at 26 weeks. Perforated corneas sometimes occurred independently of vascularization and sometimes together (recorded at 8 weeks). Opacified corneas were noticed around 8 weeks old and increased rapidly over the weeks likely due to the healing from those mice exhibiting perforations. At 13 weeks, 25% of mice showed signs of neovascularization, while greater numbers (33%) were perforated. By 26 weeks only 16% of mice were normal in appearance while the majority were neovascularized, perforated, or opacified.
Figure 2.
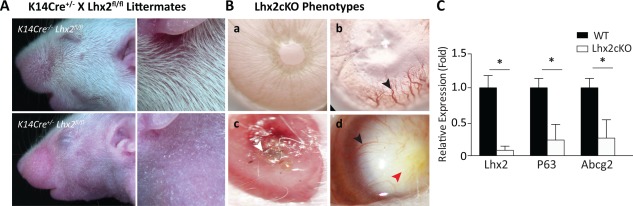
Lhx2cKO mice exhibiting spontaneous corneal phenotypes P10 littermates from K14Cre+/− and Lhx2fl/fl gave rise to K14Cre−/− Lhx2fl/fl or WT (with hair) and K14Cre+/− Lhx2fl/fl or Lhx2cKO (reduced hair) (A). Slit lamp images of adult Lhx2cKO corneas from 6 to 8 weeks old mice (B) exhibiting the phenotype of Lhx2cKO cornea as clear and devoid of vessels (a), vascularized (b), perforated (c), and opaque infiltrated with vessels (d). Black arrows point to vessels in the cornea, white arrow to the perforation and red arrow to neovessels in the opaque cornea. Quantitative reverse transcriptase polymerase chain reaction from freshly isolated WT and Lhx2‐derived corneal epithelial cells showed a significant low level of expression of genes involved in cell stemness, including Lhx2, P63, and ABCG2 (C). Data are means ± SE (n = 10). *, p ≤ 0.05. Abbreviation: WT, wild type.
Table 1.
Corneal abnormalities in Lhx2cKO mice increase with age.
| Phenotype (%) | ||||
|---|---|---|---|---|
| Age (weeks) | 1. Normal | 2. NV | Opacification | Perforation |
| 2 | 3. 100 | 4. 0 | 5. 0 | 6. 0 |
| 3 | 7. 100 | 8. 0 | 9. 0 | 10. 0 |
| 4 | 11. 100 | 12. 0 | 13. 0 | 14. 0 |
| 5 | 15. 100 | 16. 0 | 17. 0 | 18. 0 |
| 6 | 19. 83 | 20. 8 | 21. 0 | 22. 17 |
| 7 | 23. 75 | 24. 17 | 25. 0 | 26. 25 |
| 8 | 27. 75 | 28. 17 | 29. 8 | 30. 25 |
| 9 | 31. 75 | 32. 17 | 33. 17 | 34. 8 |
| 13 | 35. 60 | 36. 25 | 37. 17 | 38. 33 |
| 22 | 39. 30 | 40. 50 | 41. 25 | 42. 42 |
| 26 | 43. 16 | 44. 83 | 45. 75 | 46. 42 |
This table represents the result of slit‐lamp imaging obtained from animals followed from 2–26 weeks old. The percentage of normal corneal architecture decreased over time, with increased percentages of mice exhibiting neovascularization, opacification and perforation as age increased.
The knockdown in Lhx2 expression and previously identified corneal stem cell markers was corroborated by quantitative RT‐PCR on freshly isolated cornea epithelial cells derived from the entire cornea. The analysis revealed that P63 and ABCG2 expression were significantly reduced in Lhx2cKO as compared to WT mice. Lhx2 expression was decreased in the Lhx2cKO as expected (Fig. 2C).
Lhx2cKO‐Derived Epithelial Cells Showed Reduced Colony Forming Efficiency
We assessed the proliferative capacity, population doublings, and colony forming efficiency (CFE) of WT versus Lhx2cKO corneal epithelial cells (CECs) in culture. Cells were isolated from the entire cornea and evaluated in a time dependent manner for CFE, and at day 12, the cultures were stained with crystal violet to visualize the colonies. In general, Lhx2cKO‐derived cells showed reduced cell proliferation and CFE efficiency when compared with WT cells (Fig. 3A). Initially at day 5, Lhx2cKO CECs showed increased CFE (calculated by the number of colonies divided by the total number of plated cells on a 3T3 feeder layer) by 37% (CFE WT = 1 ± 0.05% vs. Lhx2cKO = 1.37 ± 0.06%, p = 0.0027) compared with the WT cells. The trend changed after day 7 and WT cells were 30.5% higher than Lhx2cKO cells (CFE WT = 2.3 ± 0.2% vs. Lhx2cKO = 1.6 ± 0.1%, p = 0.006) and at day 9, CFE were 50% higher (CFE WT = 4.1 ± 0.30% vs. Lhx2cKO = 2.1 ± 0.2%, p = 0.001) (Fig. 3B). Lhx2cKO corneal epithelial cells were able to form colonies in culture, though greater numbers of colonies were predominantly seen in WT cultures and rarely identified in the Lhx2 deficient cells.
Figure 3.
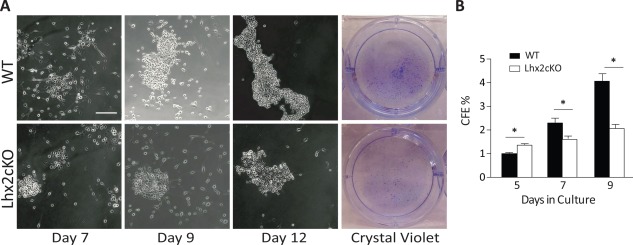
Lhx2cKO‐derived corneal epithelial cells had a reduced capacity to form colonies in vitro freshly isolated WT and Lhx2cKO corneal epithelial cells were seeded in six‐well plates and allowed to form colonies as indicated in Material and Methods section. Phase contrast and crystal violet staining showed that Lhx2cKO‐derived cells have a reduced capacity to form colonies when compared with WT cells (A). Initially Lhx2cKO‐derived cells formed more colonies at day 5 in culture, after day 9, they only reached 50% of those formed by WT‐derived cells. Quantification of the colony forming efficiency on crystal violet staining showed a significant decrease in Lhx2cKO compared with WT mice (B). Scale bar = 200 µM. Data are means ± SE (n = 3). *, p ≤ 0.05. Abbreviations: CFE, colony forming efficiency; WT, wild type.
Lhx2cKO Corneal Epithelial Cells Showed Delayed Wound Healing In Vitro
Tissue injury is known to activate stem cells to proliferate and form new tissue in various adult organs 17, 18, 19, 20. In culture, we mimicked the corneal wound healing procedure by scratching confluent cultures of the WT and Lhx2cKO murine CECs and monitored the closure of the wound. Images were taken at 0, 8, 16, 24, and 48 hours at the same locations in the cultures using phase‐contrast microscopy. The cell‐free area of the scratch in WT and Lhx2cKO cells was measured at time 0 and selected for direct comparisons only if they had approximately the same wound area between replicas to monitor healing over time. We found a significant decrease in Lhx2cKO wound closure when compared with WT (Fig. 4A). Quantification of the cell‐free areas showed that Lhx2cKO cells delay wound healing as early as 8 hours (2.2 ± 1.16) when compared with the WT (7.5 ± 1.46, p = 0.0092). These differences remained significant overtime and at 24 hours WT cells completely close the scratch while Lhx2cKO‐derived cells presented only 39.7 % closure. Lhx2cKO corneal cells fully recovered at 48 hours after wounding (Fig. 4B).
Figure 4.
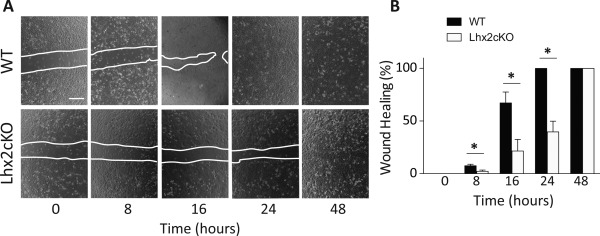
Lhx2cKO derived corneal epithelial cells showed delayed wound healing.
Corneal epithelial cells were isolated from WT and Lhx2cKO mice and cultured as indicated in Material and Methods. Cells were seeded in culture plates and when confluent a scratch was made through the cell monolayer and the wound closure followed via time‐lapse microscopy (A). Scratches in WT cells closed in less than 24 hrs, while closure of scratches made in Lhx2cKO cultures were significantly delayed, healing by 48hrs (B). Data are means SE ± (n = 3). *P ≤ 0.05. Abbreviation: WT, wild type.
Lhx2cKO Mice Showed Impaired Corneal Epithelial Wound Healing
Corneal injury is repaired from proliferating corneal epithelial cells, originating from the limbus. Re‐epithelialization can be visualized during this process by fluorescein that stains the cornea devoid of epithelium. To determine the role of Lhx2 in maintaining the stemness capacity of the limbus, we challenged the corneal epithelium with multiple epithelial debridements to establish if the re‐epithelialization of the injured cornea proceeds in a similar fashion in Lhx2cKO and WT mice. The mice (WT n = 8, Lhx2cKO n = 9) were subjected to epithelial debridement repeatedly once a week for 3 weeks. We used the right eyes for debridement and the contralateral eye as a control. We found that Lhx2cKO mice have impaired capacity to heal the injured corneal epithelium. Slit lamp examination demonstrated that WT corneas routinely healed by 32 hours after a third debridement, whereas Lhx2cKO corneas had a marked delay in re‐epithelialization with only 30% of these corneas healed at 144 hours (Fig. 5A). Experimental data from epithelial debridement 1, 2, and 3 from WT (left) and Lhx2cKO (right) are collectively represented in Figure 5B. We registered the epithelial wound healing response found in the cornea of both WT and Lhx2cKO after these series of debridements. We observed no phenotypic alteration in the cornea of WT mice in any of the experiments. WT mice showed faster epithelial wound healing in the second and third debridement when compared with the initial first debridement (Fig. 5B). However, in the corneas of Lhx2cKO mice, we found that some mice healed within the normal time frame after the first debridement and some took longer to heal. After the second and third debridement, we observed an increase in the number of mice that showed epithelial defects or did not heal (Fig. 5B). The epithelial defects usually started with cornea neovascularization (white squares) that lead to perforation (red squares), keratinization, opacification, and loss of vision.
Figure 5.
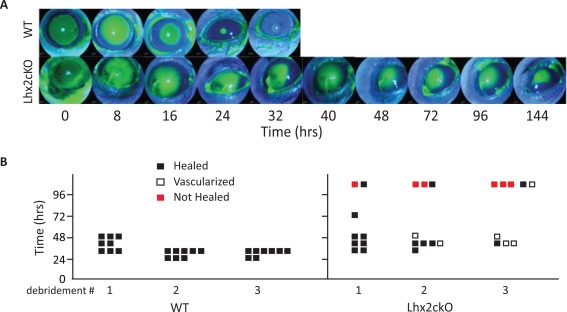
Corneal epithelial wound healing is impaired in Lhx2cKO mice.
We assessed the renewal capacity of the corneal epithelium in WT and Lhx2cKO mice by inducing consecutive epithelial injuries and visualizing the area of the debridement by fluorescein staining. The mice were subjected to cornea epithelial debridement three times, allowing a week to recover between each procedure (see Materials and Methods). In general, WT mice healed their epithelium consistently at 32hrs post‐injury, whilst a significant subset of Lhx2cKO mice demonstrated markedly delayed wound closure. After a third epithelial debridement, WT mice healed their epithelium at 32hrs while most Lhx2cKO had persistent epithelial defects at 144 hrs post wounding (typical example of this marked delay in healing shown) (A). A detailed graphical representation of all epithelial debridements showed that Lhx2cKO mice (far right panel) have a reduced healing capacity after repeated debridement and presented corneal complications, such as corneal neovascularization and perforation that were not observed in the WT (far left panel) (B). Abbreviation: WT, wild type.
Using data from only those mice that did heal in <96 hours, a mild but not significant delay in wound healing was shown in the transgenic Lhx2cKO mice after the first debridement (Supporting Information Fig. S1). After the second epithelial debridement, there was a statistically significant delay in wound healing seen in Lhx2cKO (mean = 3.64 ± 5.08%, p = 0.0067) at the early time points of 8 hours compared with WT mice (mean = 18.48 ± 10.29%). These differences extended to 16 hours between Lhx2cKO (mean = 42.7 ± 21.08%, p = 0.0399) versus WT control (mean = 61.9 ± 12.16%). After 16 hours, however, no significant difference was seen in the rate of wound healing of both mice. After the final debridement, the wound healing rate for Lhx2cKO (mean = 39.7 ± 16.6%, p = 0.0176) was also significantly lower at early time points up to 16 hours when compared with WT (mean = 61.97 ± 10.10%). However, no significant difference was seen at later time points (Supporting Information Fig. S1).
Lhx2 Contributes to Maintenance of the Limbal Barrier
In the normal eye, the limbus separates the avascular cornea from the conjunctiva. The limbus is rich in stem cells and highly vascularized and by mechanisms that are not totally understood, it prevents blood vessels penetrating into the cornea. We observed that after epithelial debridement, some corneas in our Lhx2cKO population showed signs of neovascularization. Therefore, we investigated whether WT and Lhx2cKO corneas showed histological signs of neovascularization and conjunctivalization by looking for specific markers, such as presence of goblet cells (normally only present in the conjunctiva) using PAS and keratin 15 (K15), a marker of conjunctival and limbal epithelial tissue in mice 21. Nondebrided and after the third debridement corneas from WT and Lhx2cKO mice were sectioned and stained, including the cornea, bulbar, forniceal, and palpebral conjunctiva. Higher power images are shown (large white boxes) for immunofluorescence and PAS (Fig. 6).
Figure 6.
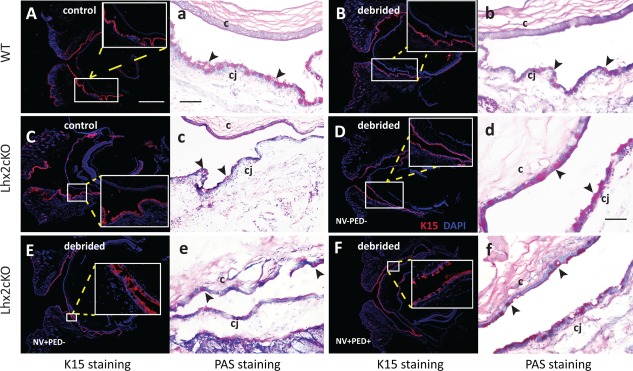
Lhx2 contributes to maintenance of the limbal barrier.
Detection of debridement‐induced conjunctivalization of the cornea in Lhx2cKO mice was visible in eye sections stained with anti Keratin 15 (K15) antibody (red) and Periodic Acid Schiff (PAS) staining which is specific for goblet cells. In normal (A,a) and debrided WT corneas (B, b) no changes in morphology were observed, and K15 and goblet cells were detected only in the conjunctiva. In non‐debrided Lhx2cKO cornea a similar pattern is observed with no alteration in the cornea (C,c), but upon epithelial debridement, goblet cells and K15 positive staining were present in both the conjunctiva and the cornea (D,d). Furthermore, some Lhx2cKO mice developed neovascularization (NV) (E, e) or neovascularization plus persistent epithelial defects (PED) (F, f). In the immunofluorescence images, selected areas (small box) are enlarged for better visualization of K15. Black arrows indicate goblet cells. c = cornea; cj = conjunctiva. Scale bars: A–F = 1000μm; a,b,c,e,f = 200μm; and d = 100μm.
In the absence of epithelium injury, we found normal distribution of K15‐ and PAS‐positive cells only in the conjunctival epithelium in both WT (Fig. 6Aa) and Lhx2cKO (Fig. 6Bb) mice. After consecutive corneal epithelial injury induced by debridement, WT mice showed normal K15 and PAS restricted to the conjunctiva (Fig. 6Cc). However, Lhx2cKO mice revealed PAS‐positive cells and K15 expression in the corneal epithelium consistent with conjuctivalization of the cornea in all the mice that underwent the procedure, regardless of external clinical appearance (Fig. 6D–6F). This includes eyes that clinically did not exhibit any signs of corneal neovascularization or persistent epithelial defects (no NV, no PED) (Fig. 6Dd), eyes with signs of corneal neovascularization but no persistent epithelial defects (NV, no PED) (Fig. 6Ee), as well as eyes with both corneal neovascularization and persistent epithelial (NV and PED) defects (Fig. 6Ff). Thus conjunctivalization of Lhx2cKO corneas indicates loss of a limbal barrier, therefore, ascribing a new role for Lhx2 in maintaining the cornea free from epithelium derived from the conjunctiva.
Discussion
The corneal epithelium is constantly renewed via corneal stem cells that are classically believed to reside at the limbus 3, 22, 23. The location of Lhx2 and the fate of corneal epithelial cells in K14 driven Lhx2cKO mice in the cornea have not been described previously. In our study, Lhx2 is highly expressed at the limbus, although it was also identified in the basal layer of the conjunctiva and central cornea. However, the strong localization at the limbus may suggest that Lhx2 is mobilized to the center cornea during normal epithelial renewal, growth or upon injury induced re‐epithelialization.
Reports from Majo et al. have shown that stem cells may not be exclusive to the limbal region of mice and that populations of central corneal cells also possess regenerative capacity in the context of wound healing 24. Previous studies have shown that limbal ablation did not induce corneal opacity and that limbal grafts did not migrate centripetally to the central cornea for up to 4 months. These data suggest that the limbus may not exclusively participate in normal corneal homeostasis and that there may be stem cells within the central cornea 24, 25. Amitai‐Lange et al. used multicolor lineage tracing of limbal and corneal K14‐positive cells in R26R‐Confetti/K14Cre ERT mice and showed that limbal epithelial cells underwent slow centripetal migration of 4–5 months until reaching the corneal center 26. Although the limbus greatly contributed to corneal renewal, the progenitor cells had longer turnover, while limbal clones contributed to renewing itself to remain in a cluster at the limbus, creating radial streaks migrating to the center for normal homesostasis to occur. Upon mild injury, the central corneal cells greatly contributed to the repair 26.
As Lhx2 is highly expressed at the limbus, we generated K14 driven Lhx2 conditional knockout mice to determine its role in corneal epithelial homeostasis and renewal upon injury. K14 has previously been localized to the full thickness of the limbal epithelium, occasionally restricted to the basal limbal epithelial layer, the central cornea and the conjunctiva in humans and mice 21, 25, 27, therefore, we expected a conditional deletion of Lhx2 to appropriately affect these tissues. The Lhx2cKO mice displayed various phenotypes in the unperturbed state. A subset of these animals were phenotypically normal; however, other groups demonstrated variable evidence of stem cell failure including neovascularization, persistent epithelial defect, or corneal melt with perforation. The dominant negative (DN)‐Clims transgenic, a cofactor of the LIM domain, also driven by the K14 promoter portrayed a striking phenotype resulting in hair loss, similar to our Lhx2cKO model. Clims have shown to be prominently expressed in the corneal epithelium, including the stem cell rich limbal epithelium, as seen in our Lhx2eGFP murine model. Abnormal corneal homeostasis of the model K14‐DN‐Clim and Lhx2cKO exhibit many of the same phenotypic characteristics including neovascularization, opacification in which 50% of the K14‐DN‐CLIMS were affected by 1 month old (significantly earlier than we observed in Lhx2cKO mice), and by 6 months nearly all mice were affected as seen in our Lhx2cKO model. Histologically, the DN‐Clims cornea in older mice was hyperplastic, cornified, and the stroma was vascularized as we observed in the Lhx2cKO (data not shown).
The abnormal phenotypes seen in our transgenic mice model were similar to those described by either adding high concentrations of topical benzalkonium chloride frequently to the corneal surface 28 or alkali‐burn models 29, 30, both resulting in limbal stem cell deficiency and eventually corneal neovascularization within a similar time frame of 4–12 weeks. Corneal neovascularization has been associated with a number of human corneal ailments such as chemical injury, Steven Johnson's syndrome and aniridia, and patients with these ailments exhibit limbal stem cell deficiency and goblet cells on the corneal surface. The presence of goblet cells in the corneal surface epithelium is an indication that the conjunctival tissue has migrated across the limbus that normally separates the avascular cornea from the highly vascularized conjunctiva.
In our observations of Lhx2cKO mice, we also noticed that there was a time‐dependent phenotype change in those mice having cornea alterations, whereby a subset of mice appearing normal at 5 weeks of age developed corneal neovascularization at 6 weeks, perforation at 7 weeks and opacity at 8 weeks. In mice, above 13 weeks old, corneal anomalies were more frequent, suggesting that as the cornea is exposed to environmental stress seen during the lifetime of these mice, the lack of Lhx2 affected the natural epithelial cell turnover. Other genetic models such as the Pax6 KO, representing a model of aniridia, exhibit similar phenotypes as seen in Lhx2cKO mice, including the in‐growth of blood vessels from the limbal region onto the corneal surface, the appearance of goblet cells and corneal opacity that contributed to visual loss later in life.
To further understand the role of Lhx2 in the corneal epithelium, we studied the capacity of Lhx2cKO corneal epithelial cells to behave as stem cells. Lhx2cKO‐derived cells have reduced colony‐forming efficiency as well as reduced wound healing in culture suggesting that Lhx2 can directly affect the renewal of the dividing population that includes stem cells and transient amplifying cells, both required for normal corneal epithelium homeostasis and fundamental for repair after injury. We further extended our studies by analyzing the role of Lhx2 in vivo in corneas subjected to epithelial injury. As seen in isolated cells in vitro, mice lacking Lhx2 showed an impaired capacity to renew the injured epithelium when the tissue was challenged by multiple debridements, an effect that was not observed in WT mice that renewed their corneal epithelium normally. In WT mice, the epithelium healed within 32–48 hours. In subsequent second and third debridement's performed on the same mice, this decreased to 24–32 hours, most likely due to a higher proportion of transient amplifying cells already present at the time of second and third debridement's. Before the first debridement, corneal regeneration only needed to keep pace with normal corneal homeostasis. Upon corneal wounding in the WT at the first debridement, an elevated rate of regeneration was required to repair the epithelial defect, thus accelerating the mechanisms that increase the numbers of transient amplifying cells and priming the cornea for quicker healing during subsequent debridement. This trend of reduced healing times in second and third consecutive debridement did not occur in the transgenic Lhx2cKO mice. Statistical analysis, including the healing rates of only those animals that recovered less than 96 hours in our studies, showed significantly reduced healing in Lhx2cKO especially in the early time points before 24 hours in the second and third debridement. This suggests that the ability to maintain the epithelial renewal over time in Lhx2cKO mice is lost with the depletion of subset of progenitors cells over time when injury is induced and repeated, contributing to the lack of wound healing in these mice.
The presence of Lhx2 is not only required for proper cornea homeostasis and epithelium renewal, our studies demonstrate that Lhx2 is fundamental to keep the cornea avascular. Our histological analysis showed that the barrier that the limbus provides to keep a clear cornea free of blood vessels from the rich vascularized conjunctiva is lost when the Lhx2cKO corneas were challenged by debridement. Normal murine and human conjunctiva tissue expresses Lhx2; however, when the gene is knocked out in mice, we see new blood vessels and goblet cells over the corneal surface, suggesting that Lhx2 could indeed regulate the barrier between the conjunctiva and the cornea. The presence of conjunctival elements such as goblet cells, K15‐positive staining cells and blood vessels in the central cornea in the Lhx2cKO and not in the WT mice indicate that in addition to contributing to the stemness of the limbus, Lhx2 is also required for the proper separation of these two tissues. In these mice, where the corneal epithelium appeared to heal normally on examination, possible abnormal mechanisms were contributing in part to the overall re‐epithelialization process and the presence of neovascularization that increased over repeated debridement are early signs of vision impairment and corneal disease. The absence of goblet cells in the cornea of debrided eyes in WT mice and nondebrided transgenic Lhx2cKO mice further emphasizes the importance of Lhx2 in corneal homeostasis, since these cells are associated to conjunctivalization and neovascularization 31, 32. However, it must be noted that although no PAS‐positive cells were observed in non‐debrided Lhx2cKO mice, they nevertheless may occur spontaneously in these mice. This occurs especially in eyes of older transgenic Lhx2cKO mice that harbor signs of corneal neovascularization. Although these mice were not part of the debridement experiments, they may have accumulated greater amounts of microtrauma throughout their lives that led to a limbal stem cell deficiency phenotype. 33, 34, 35.
Conclusions
Lhx2 was abundantly expressed in the limbus, the stem cell rich region of the cornea. Conditional deletion of Lhx2 in the corneal epithelium produced spontaneous corneal anomalies that resulted in impaired vision. In vitro, corneal epithelial cells from Lhx2cKO mice have reduced capacity of forming colonies and wound closure. In vivo, Lhx2 deficiency resulted in loss of corneal epithelial renewal and the limbal barrier. Lhx2 could be responsible in part for maintaining or activating quiescent cells in the corneal epithelium and its loss in the limbus resembles features of limbal stem cell deficiency. Our data further enhance the need to identify novel genes that regulate stem and progenitor cell activation and proliferation for corneal stem cell treatments.
Author Contributions
R.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, administrative support; R.‐i.C.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; J.Y.: conception and design, collection and assembly of data, final approval of manuscript; P.W.: conception and design, collection and assembly of data, data analysis and interpretation, final approval of manuscript; A.L.: animal Husbandry, conception and design, final approval of manuscript; V.G.: data analysis and interpretation, manuscript writing, final approval of manuscript; E.F.: conception and design, final approval of manuscript; M.I.R.: conception and design, financial support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, administrative support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Supporting information
Supporting Information Figure S1
Figure 1 Legend
Acknowledgments
We thank Nicole Stokes and Maria Nikolova (Howard Hughes Medical Institute, Laboratory of Mammalian Cell Biology Development, The Rockefeller University) for technical support and advice concerning the Lhx2cKO mice. This work was supported by New York State Stem Cell Science and Tri‐Institutional Stem Cell Initiative (to M.I.R and E.F), Natural Sciences Foundation of China Grant 81200656 (to P.W), NIH Grant R21EY025062 (to M.I.R), and Research to Prevent Blindness Grant EY001792.
References
- 1. Burman S, Sangwan V. Cultivated limbal stem cell transplantation for ocular surface reconstruction. Clin Ophthalmol 2008;2:489–502. [PMC free article] [PubMed] [Google Scholar]
- 2. Prabhasawat P, Ekpo P, Uiprasertkul M et al. Efficacy of cultivated corneal epithelial stem cells for ocular surface reconstruction. Clin Ophthalmol 2012;6:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun TT, Tseng SC, Lavker RM. Location of corneal epithelial stem cells. Nature 2010;463:E10–E11; discussion E11. [DOI] [PubMed] [Google Scholar]
- 4. Porter FD, Drago J, Xu Y et al. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development 1997;124:2935–2944. [DOI] [PubMed] [Google Scholar]
- 5. Tornqvist G, Sandberg A, Hagglund AC et al. Cyclic expression of lhx2 regulates hair formation. PLoS Genet 2010;6:e1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinto do OP, Richter K, Carlsson L. Hematopoietic progenitor/stem cells immortalized by Lhx2 generate functional hematopoietic cells in vivo. Blood 2002;99:3939–3946. [DOI] [PubMed] [Google Scholar]
- 7. Dahl L, Richter K, Hagglund AC et al. Lhx2 expression promotes self‐renewal of a distinct multipotential hematopoietic progenitor cell in embryonic stem cell‐derived embryoid bodies. PloS One 2008;3:e2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mardaryev AN, Meier N, Poterlowicz K et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development 2011;138:4843–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science 2006;312:1946–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X, Mannik J, Kudryavtseva E et al. Co‐factors of LIM domains (Clims/Ldb/Nli) regulate corneal homeostasis and maintenance of hair follicle stem cells. Dev Biol 2007;312:484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folgueras AR, Guo X, Pasolli HA et al. Architectural niche organization by LHX2 is linked to hair follicle stem cell function. Cell Stem Cell 2013;13:314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyer‐Blazejewska EA, Call MK, Yamanaka O et al. From hair to cornea: Toward the therapeutic use of hair follicle‐derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 2011;29:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heintz N. Gene expression nervous system atlas (GENSAT). Nat Neurosci 2004;7:483. [DOI] [PubMed] [Google Scholar]
- 14. Mangale VS, Hirokawa KE, Satyaki PR et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science 2008;319:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vasioukhin V, Degenstein L, Wise B et al. The magical touch: Genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA 1999;96:8551–8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang CC, Park AY, Guan JL. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Prot 2007;2:329–333. [DOI] [PubMed] [Google Scholar]
- 17. Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat 2003;203:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 2004;432:324–331. [DOI] [PubMed] [Google Scholar]
- 19. Urbanek K, Torella D, Sheikh F et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA 2005;102:8692–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wan M, Li C, Zhen G et al. Injury‐activated transforming growth factor beta controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells 2012;30:2498–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshida S, Shimmura S, Kawakita T et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci 2006;47:4780–4786. [DOI] [PubMed] [Google Scholar]
- 22. Dua HS, Azuara‐Blanco A. Limbal stem cells of the corneal epithelium. Survey Ophthalmol 2000;44:415–425. [DOI] [PubMed] [Google Scholar]
- 23. Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: Looking at some old problems from a new angle. Exp Eye Res 2004;78:433–446. [DOI] [PubMed] [Google Scholar]
- 24. Majo F, Rochat A, Nicolas M et al. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 2008;456:250–254. [DOI] [PubMed] [Google Scholar]
- 25. Vauclair S, Majo F, Durham AD et al. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell 2007;13:242–253. [DOI] [PubMed] [Google Scholar]
- 26. Amitai‐Lange A, Altshuler A, Bubley J et al. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 2015;33:230–239. [DOI] [PubMed] [Google Scholar]
- 27. Tanifuji‐Terai N, Terai K, Hayashi Y et al. Expression of keratin 12 and maturation of corneal epithelium during development and postnatal growth. Invest Ophthalmol Vis Sci 2006;47:545–551. [DOI] [PubMed] [Google Scholar]
- 28. Lin Z, He H, Zhou T et al. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Invest Ophthalmol Vis Sci 2013;54:6314–6325. [DOI] [PubMed] [Google Scholar]
- 29. Giacomini C, Ferrari G, Bignami F et al. Alkali burn versus suture‐induced corneal neovascularization in C57BL/6 mice: An overview of two common animal models of corneal neovascularization. Exp Eye Res 2014;121:1–4. [DOI] [PubMed] [Google Scholar]
- 30. Saika S, Miyamoto T, Yamanaka O et al. Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor‐kappaB, in treatment of corneal alkali burns in mice. Am J Pathol 2005;166:1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joussen AM, Poulaki V, Mitsiades N et al. VEGF‐dependent conjunctivalization of the corneal surface. Invest Ophthalmol Vis Sci 2003;44:117–123. [DOI] [PubMed] [Google Scholar]
- 32. Swamynathan SK. Ocular surface development and gene expression. J Ophthalmol 2013;2013:103947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lauderdale JD, Wilensky JS, Oliver ER et al. 3′ deletions cause aniridia by preventing PAX6 gene expression. Proc Natl Acad Sci USA 2000;97:13755–13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanson IM, Seawright A, Hardman K et al. PAX6 mutations in aniridia. Hum Mol Genet 1993;2:915–920. [DOI] [PubMed] [Google Scholar]
- 35. Chen YT, Chen FY, Vijmasi T et al. Pax6 downregulation mediates abnormal lineage commitment of the ocular surface epithelium in aqueous‐deficient dry eye disease. PloS One 2013;8:e77286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1
Figure 1 Legend


