Abstract
Ageing causes loss of function in tissues and organs, is accompanied by a chronic inflammatory process and affects life‐ and healthspan. Calorie restriction (CR) is a non‐genetic intervention that prevents age‐associated diseases and extends longevity in most of the animal models studied so far. CR produces a pleiotropic effect and improves multiple metabolic pathways, generating benefits to the whole organism. Among the effects of CR, modulation of mitochondrial activity and a decrease in oxidative damage are two of the hallmarks. Oxidative damage is reduced by the induction of endogenous antioxidant systems and modulation of the peroxidability index in cell membranes. Mitochondrial activity changes are regulated by inhibition of IGF‐1 and Target of Rapamycin (TOR)‐dependent activities and activation of AMP‐dependent kinase (AMPK) and the sirtuin family of proteins. The activity of PGC‐1α and FoxO is regulated by these systems and is involved in mitochondria biogenesis, oxidative metabolism activity and mitochondrial turnover. The use of mimetics and the regulation of common factors have demonstrated that these molecular pathways are essential to explain the effect of CR in the organism. Finally, the anti‐inflammatory effect of CR is an interesting emerging factor to be taken into consideration. In the present revision we focus on the general effect of CR and other mimetics in longevity, focusing especially on the cardiovascular system and skeletal muscle.
Abbreviations
- AMPK
AMP‐dependent kinase
- ATG
autophagy‐related
- CR
calorie restriction
- FoxO
Forkhead box proteins
- NRF2
nuclear factor erythroid 2‐related factor 2
- PMRS
plasma membrane redox system
- TOR
Target of Rapamycin
Introduction
Ageing can be defined as a natural and progressive process occurring in all the living organisms that is characterized by the deterioration of structure and functional capacities. These alterations are multifactorial and involve diverse processes such as genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intracellular communication (Lopez‐Otin et al. 2013; Kennedy et al. 2014). Ageing is not a disease and therefore, disease‐oriented research and treatment approaches are not adequate. It has thus been proposed that the use of health‐oriented and preventive strategies is more beneficial than disease‐oriented treatments (Rattan, 2014). As a process that affects the different organs, ageing is characterized for the accumulation of multiple chronic diseases caused by the same mechanisms that drive ageing. The study of this multimorbidity and the identification of molecular markers can help us to understand the biology of ageing and to develop strategies to delay it (Burkle et al. 2015; Fabbri et al. 2015).
Calorie restriction (CR) is, to date, the most successful intervention to delay ageing progression or the development of age‐related chronic diseases. CR has been defined as the reduction of energy intake without malnutrition. During the last few years it has been demonstrated that CR extends lifespan, extending the healthspan by delaying the onset of age‐related diseases in many of the animal models studied (reviewed in Speakman & Mitchell, 2011). This effect of CR on longevity was explained in a unified theory of ageing proposed by David Sinclair (Sinclair, 2005). He considered that CR is not a simple and passive effect but an active, highly conserved stress response that increases the organism's chance of surviving adversity. Thus, CR produces a response that modifies key process in cell protection, reparation mechanisms and modulation of metabolism that permits a higher survival against adversity. This has been supported by the ‘Hormesis hypothesis of CR’ that suggests that the induction of a moderate stress causes adaptive responses of cells and organs, preventing further damage due to a stronger stress (Hipkiss, 2007; Masoro, 2007; Mattson, 2008; Rattan, 2008; Martins et al. 2011; Barbieri et al. 2013; Stankovic et al. 2013; Testa et al. 2014). As in CR, other factors such as exercise and some bioactive compounds found in fresh vegetables have been considered factors that induce hormetic responses in the organism affecting life and healthspan (Mattson, 2008).
In this review we will focus on the effect of CR and other mimetics on longevity and healthspan, focusing especially on cardiovascular system and skeletal muscle. Ageing is associated with a slow, but progressive muscle weakness, which is largely attributable to muscle wasting. CR is able to prevent muscle damage (McKiernan et al. 2011) and reverse ageing cardiomyopathy (Bales & Kraus, 2013; Yan et al. 2013).
Calorie restriction and lifespan extension
Since the first reports of Osborne et al. and Loeb and Northrop in 1917 (Loeb & Northrop, 1917; Osborne et al. 1917) and the seminal report of McCay in 1935 (McCay et al. 1935), it is accepted that the rate of ageing can be affected by the amount of food consumed and hundreds of reports have confirmed the effect of CR on longevity, affecting most of the organisms tested to date. In these studies CR shows the capacity to extend longevity, affecting both, median and maximum lifespan in different organisms from yeasts to mammals (Testa et al. 2014). These species include S. cerevisiae, C. elegans, D. melanogaster and mammals such as M. musculus and R. norvegicus as model organisms. Other reports have shown effects on several other species such as rotifers, silkworms, spiders, fishes, dogs, etc. (Le Bourg, 2010). In primates, three separated studies of the effect of CR started at the 1980s using rhesus monkeys as a model (Macaca mulatta). These animals show many affinities to humans (Roth et al. 2004). Although the experiments with these animals are still ongoing, the results obtained to date are positive and, at least, an increase in healthspan has been found in CR‐fed animals (Zainal et al. 2000; Roth et al. 2001; Mattison et al. 2012; Mercken et al. 2013; Colman et al. 2014). In humans, the most complete study on the effect of CR is the CALERIE (Comprehensive Assessment of Long‐term Effects of Reducing Intake of Energy) study, which started around seven years ago. The first reports have demonstrated similar CR‐dependent effects as in mammalian models such as improving cardiovascular factors and insulin sensitivity (Pittas et al. 2005; Redman et al. 2014)
Although it is widely considered that the reduction in caloric intake itself is the main factor responsible for life extension, other studies have demonstrated that the reduction of one component in the diet such as methionine produces similar effects (Masoro, 2006). The main problem is the variability of diets and ingredients used in CR studies (Pugh et al. 1999). The importance of the dietary composition on retardation of ageing has been recently reinforced by two studies carried out in rhesus macaques where the discrepancies between the effect of CR on longevity seem to depend on the different diets used (Cava & Fontana, 2013; Colman et al. 2014). Interestingly, a new and controlled medium used for Drosophila feeding permits control of the specific nutrient that mimics the CR effect. Although effecting minimum changes in longevity, this medium permits determination of which nutrient is more effective in the response of the organism to CR (Piper et al. 2005, 2014). Further, the use of other models of CR such as every‐other‐day feeding produces a lower intake of calories but induces changes similar to those found with classic CR (Rodriguez‐Bies et al. 2010, 2015). On the other hand, recent studies performed on the macronutrients involved in CR indicate that the protein/carbohydrate ratio also plays an important role in the response of nutrient sensors (Solon‐Biet et al. 2014). Thus, it seems that many factors influence the response of the organism to diets such as the number of calories, the quality of the source of calories and the induction of a nutritional stress by the every‐other day feeding procedure which activates evolutionarily conserved molecular mechanisms involved in the CR effect on longevity (Goodrick et al. 1982; Anson et al. 2005).
There are no detailed reports about the effect of CR on longevity in humans. The longer life expectancy of humans in comparison with other animals and the low number of persons tested makes it difficult to reach conclusions about the effect of CR on human longevity (Robert, 2013). It is not yet clear if the reported effects on longevity and healthspan found in humans are due to the decrease in the calorie intake or are the result of a high quality diet (Rizza et al. 2014). However, it seems clear that a reduction in calorie intake in humans improves healthspan (Anderson & Weindruch, 2012), and delays cardiac ageing, improving cardiovascular function, one of the main causes of death in humans (Bales & Kraus, 2013).
Mitochondrial activity and ROS production are modulated by CR
In spite of the enormous number of articles published about the mechanism involved in the effect of CR on longevity, these mechanisms have not been clarified to date, although an important role of the maintenance of a balanced activity in mitochondria is supported by a large body of evidence. Interestingly, although differences are found in some pathways in the models used in the study of ageing and CR, the downregulation of genes participating in energy production in Drosophila and rodents indicates similar physiological effects during ageing (Augustin & Partridge, 2009).
Ageing is associated with the impairment of mitochondria, with a significant increase in ROS generation and a decrease in antioxidant defences, causing accumulation of mitochondrial DNA and oxidative damage (Merry, 2002; Chistiakov et al. 2014). In fact, the two parameters that have shown strong correlation with longevity are the increase in ROS production at mitochondria and other systems and the degree of fatty acid unsaturation in membranes (Barja, 2014). It is accepted that the longevity‐promoting effects of CR can be explained, at least in part, by the modulation of mitochondrial and antioxidant activities in cells and tissues (Lopez‐Lluch et al. 2006, 2008). Furthermore, a recent review has concluded that mitochondria play a key role in the pathophysiology of ageing and in earlier stages of events leading to the ageing phenotype (Gonzalez‐Freire et al. 2015). The mitochondrial electron transport chain is considered the main source of ROS in cells. Damaged mitochondria show reduced efficiency of energy production at the same time as higher levels of ROS release. It has been suggested that all the complexes of the mitochondrial electron transport chain are involved in ROS production in the resting state of muscle (Goncalves et al. 2015). Further, it is accepted that respiratory complexes assembled in supercomplexes regulate ROS production, and that a progressive deterioration of supercomplexes in ageing cause an irreversible increase in ROS (Genova & Lenaz, 2015). Thus, the maintenance of the structure of complexes is essential to avoid accumulation of ROS sources in mitochondria.
It has been suggested that ROS are the main source of mitochondrial DNA (mtDNA) mutations, affecting both point mutations and deletions that accumulate in different tissues during ageing in mammals, including humans (Trifunovic et al. 2004). These mutations are responsible for respiratory chain age‐associated deficiencies in different tissues such as heart, skeletal muscle and brain. Thus, mice showing deficiency in mtDNA polymerase develop a mtDNA mutator phenotype, including higher levels of point mutations and deleted mtDNA that have been associated with the premature onset of ageing and ageing‐related phenotypes (Trifunovic et al. 2004).
Mitochondrial malfunction can also affect the whole physiology of cells. For example, and increase in ROS in skeletal muscle has been associated with insulin resistance in obese individuals, appearance of type 2 diabetes and muscle malfunction during normal ageing (Barbieri et al. 2013), indicating that a rise in ROS levels can lead to deterioration of the physiology of the whole cell, tissue and organ.
It has been demonstrated that CR lowers mitochondrial membrane potential and consequently the production of ROS at the same time that it modifies the saturation/unsaturation index in mitochondrial membranes preventing oxidative damage and maintaining membrane fluidity. Recently, we have demonstrated that the production of H2O2 linked to complexes I and III is reduced by CR in both muscle (Chen et al. 2012, 2014) and liver (Chen et al. 2013). This effect was found in young animals after just 1 month of CR (Chen et al. 2012) and was maintained after 8 months of CR (Chen et al. 2014).
Another important factor involved in the accumulation of damaged mitochondria during ageing is the decline of the mitochondrial turnover by inhibition of mitophagy; the specific autophagy process that removes damaged mitochondria (Chistiakov et al. 2014). It is clear that the renovation of mitochondrial network plays a key role in healthspan increase after CR (Lopez‐Lluch et al. 2008). Further, the correct functioning of the quality control mechanism of mitochondria has been suggested as a crucial factor in counteracting the ageing process (Weber & Reichert, 2010).
Besides these strong lines of evidence, the age of the organisms at the onset of CR seems to play an important role in the regulation of mitochondrial activity. In rhesus monkey studies, ageing resulted in up‐regulation of transcripts affecting inflammation and oxidative stress and down‐regulation of genes involved in mitochondrial electron transport chain and oxidative phosphorylation (Kayo et al. 2001). These authors did not observe beneficial effects of adult‐onset CR at the transcriptional level (Kayo et al. 2001), although the same animal cohort showed several physiological beneficial effects of CR such as higher insulin sensitivity (Kemnitz et al. 1994) and decreased production of pro‐inflammatory cytokines by peripheral blood mononuclear cells (Kim et al. 1997). Interestingly, in previous studies, more of the 80% of age‐related changes in gene expression found in skeletal muscle were partially or completely suppressed by early onset of CR in mice (Lee et al. 1999). These different results probably indicate an age‐dependent effect of CR, as we have found in murine models (Rodriguez‐Bies et al. 2015; Tung et al. 2015 b).
Importance of membrane lipid composition on the CR effect
As indicated above, the decrease in the oxidative damage in organic structures is one of the main factors contributing to lifespan extension induced by CR (Sohal & Weindruch, 1996; Gredilla & Barja, 2005). The fatty acid composition of cell membranes is another important factor involved in ageing progression because it influences the lipid peroxidation rate during ageing (Hulbert, 2005). Thus, several findings indicate that the increase in lipid peroxidation during ageing (Yu, 2005), which is due to different factors such as enrichment in more oxidizable polyunsaturated fatty acids (e.g. 22:6), reduction of membrane‐linked antioxidant activities and higher production of ROS, affects the main processes in cells from plasma membrane activities to mitochondrial function.
It is still unclear whether lifespan extension induced by CR can also be explained by changes in membrane fatty acid composition conferring higher resistance to peroxidation. We have recently found that lipid composition in the diet can modulate the effect of CR on longevity (Lopez‐Dominguez et al. 2015). One month of CR produced minor changes in fatty acid composition of muscle mitochondrial phospholipids, mainly affecting phosphatidyl‐choline (Chen et al. 2012) and increasing monounsaturated fatty acid (MUFA) levels in liver mitochondria (Chen et al. 2013). These small changes in membrane lipid composition have been related to other changes such as mitochondrial proton leak, mitochondrial electron transport chain activities, ROS generation and lipid peroxidation (Chen et al. 2012, 2013) and also mitochondrial fission/fusion dynamics and apoptotic signalling (Khraiwesh et al. 2013, 2014; Lopez‐Dominguez et al. 2013).
Antioxidant activities in ageing and CR
Oxidative damage is prevented by endogenous antioxidant activities in cells and organs. A battery of enzymes are involved in the elimination of ROS such as superoxide (mitochondrial Mn‐SOD and cytosolic Cu/Zn‐SOD) and hydroperoxides (catalase and glutathione peroxidase), with the glutathione‐dependent system being the most important in cells (Lenaz et al. 1999). Although one of the most popular theories to explain the prolongevity effect of CR on different organisms is based on higher protection against the increase in oxidative stress and subsequent cell damage, the role of antioxidants in CR effect is not clear. Many lines of evidence indicate that CR reduces age‐associated accumulation of oxidized molecules (Gredilla & Barja, 2005). However, the lack of lifespan extension in antioxidant enzyme overexpression experiments casts doubt on the importance of antioxidants in the CR effect (Muller et al. 2007). Higher levels of antioxidants do not necessarily indicate a higher antioxidant protection and imbalances produced by the overexpression or higher activity of one antioxidant enzyme must be taken into consideration. Besides the important number of studies that indicate a modulation of antioxidant activities in cells and organs by CR, this effect seems to be organ dependent and studies designed to clarify how ageing and CR affect antioxidant enzymes in each individual tissue and organ must be performed (Ji et al. 1990; Tung et al. 2015 b).
Importantly, oxidoreductases such as CytB5Rase and NAD(P)H:quinone oxidoreductase 1 (NQO1) are involved in the prevention of lipid peroxidation through a coenzyme Q‐dependent system present in cell membranes and especially in plasma membrane. This system is known as the plasma membrane redox system (PMRS) and is also involved in the maintenance of the redox cycle of vitamin E (Fig. 1 Navas et al. 2005, 2007). Several years ago, we demonstrated that CR induces redox coenzyme Q‐dependent activities at the plasma membrane in old mouse and rat liver but not in young animals (De Cabo et al. 2004; Lopez‐Lluch et al. 2005). This age‐dependent effect was further confirmed in brain (Hyun et al. 2006) and in muscle (Rodriguez‐Bies et al. 2015). Therefore, the age of the organisms seems to play an important role in the relationship of CR with membrane‐linked antioxidant activities.
Figure 1. Activities of the plasma membrane redox system .
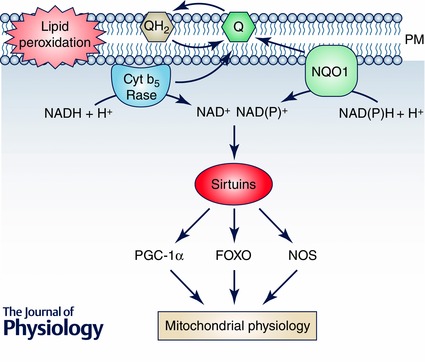
The Q‐dependent enzymatic activities at the plasma membrane (PM) not only prevent oxidative damage affecting lipid peroxidation through reduced Q or by maintaining vitamin E turnover in the membrane, but can also show other regulatory activities in the cytosol. The oxidation of NADH or NAD(P)H in the PM environment can regulate the activity of NAD+‐dependent deacetylases such as sirtuins, mainly SIRT1. These sirtuins will further regulate the activity of many other proteins involved in the control of metabolism and mitochondrial turnover such as PGC‐1α, FOXO or NOS among others. These proteins are involved in the regulation of mitochondrial biogenesis, turnover and oxidative activity. Thus, upregulation of PMRS by CR in old animals can be linked to a higher SIRT1 activity and the regulation of mitochondrial function in old animals.
The activity of the PMRS is also involved in the maintenance of mitochondrial homeostasis. In a recent work, it has been demonstrated that overexpression of NQO1 is associated with higher neuroprotection against energetic and proteotoxic stress but not against oxidative stress, indicating the importance of this system in the maintenance of bioenergetics and a secondary role in oxidative prevention (Hyun et al. 2012). Furthermore, NQO1 up‐regulates mitochondrial function in human neuroblastoma cells (Kim et al. 2013). Experiments performed in S. cerevisiae have demonstrated that the activation of NQR1, an orthologue of NQO1, is associated with the transition from fermentation to mitochondrial‐dependent oxidative metabolism (Jimenez‐Hidalgo et al. 2009). Further, Sqstm1/p62, a protein that mediates nuclear factor erythroid 2‐related factor 2 (NRF2) activation and increases NQO1, is essential in the maintenance of mitochondrial membrane activity during ageing in mice (Kwon et al. 2012), indicating the importance of this member of the coenzyme Q‐dependent oxidoreductases in maintaining mitochondrial integrity and longevity. It seems clear that the induction of the NRF2/ARE pathway during CR not only increases antioxidant protection in membranes by activating PMRS but also is essential for control of metabolic homeostasis during ageing (Ungvari et al. 2008).
Interestingly, recent studies performed in humans demonstrated that resveratrol, a compound able to induce many of the CR‐triggered pathways, up‐regulates human erythrocyte PMRS, mitigating the alterations induced by oxidation during ageing (Pandey & Rizvi, 2013). The same authors have suggested the direct relationship of this activity with the higher lifespan found in long‐living organisms (Rizvi et al. 2011). However, the mechanisms involved in the prolongevity effect of PMRS activation and its importance in ageing are not clear and the difficulties in the determination of PMRS activity in some tissues obscures its role in ageing and the effect of CR.
Taking into consideration the deleterious effect of lipid peroxidation on membrane function, can the higher activity of PMRS be related to membrane‐linked cell signalling processes? Is it related to a higher sensitivity to local factors or hormones? Some evidence indicates that activation of the PMRS system and the prevention of the oxidation of membranes can be involved in the maintenance of the cell response to external stimuli. In fact, deletion of NRF2 impairs glucose tolerance in diabetic mice (Aleksunes et al. 2010) and NQO1 has been associated with both the maintenance of the metabolic machinery in pancreatic β‐cells (Gray et al. 2011) and the prevention of its destruction induced by different stressors (Yeo et al. 2013). It has also been suggested that the regulation of this system can modulate the activity of different proteins at the plasma membrane and the nutrient‐sensors sirtuins, importantly related to many CR effects in organisms (Crane & Low, 2005) and in the control of cell survival by apoptosis (Villalba & Navas, 2000).
Molecular mechanisms involved in the effect of CR on longevity
A decrease in the uptake of nutrients produces an imbalance in the metabolic system. To return to a new equilibrium, several regulatory pathways interact. The new equilibrium responds to the relationship between growth‐associated pathways, such as the insulin–insulin growth factor‐1 (IGF‐I) receptor system and Target of Rapamycin (TOR), and regulators of a more efficient respiratory metabolism such as AMP‐dependent kinase (AMPK) and sirtuins, the NAD+‐dependent deacetylases. Interestingly, the relationship between these mechanisms is conserved during evolution from yeasts to humans. In simple terms, the decrease in calories in the diet activates systems involved in a more efficient metabolism, a higher protection against cellular damage and the activation of remodelling mechanisms, whereas less efficient metabolism and synthetic pathways are blocked. Interestingly, these mechanisms regulate each other in all the organisms studied, indicating that CR activates an evolutionarily conserved response that avoids fruitless energy expenditure and use recycled structures for the organisms’ survival (Fig. 2). In this section we provide a resumé of the main molecular mechanisms involved in the CR effect on lifespan and healthspan (more detailed information can be found in Michan, 2014; Testa et al. 2014).
Figure 2. Complex interaction of proliferative and protective mechanisms in the CR prolongevity effect in organisms .
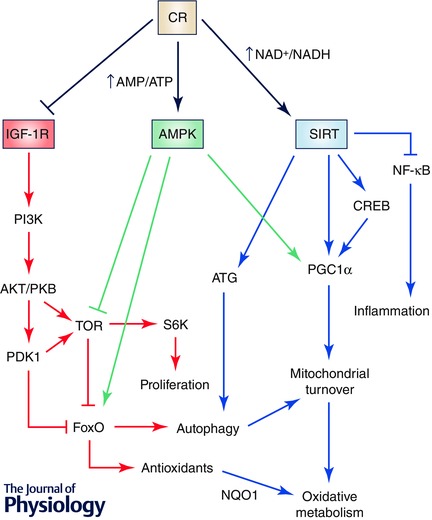
Nearly all the organisms studied to date have shown similar mechanisms in response to CR. Basically, CR induces mechanisms involved in energy efficacy and inhibits mechanisms involved in less efficient energy consumption and proliferation. Thus, CR inhibits IGF‐I‐dependent signalling which activates TOR and protein synthesis and inhibits FoxO, blocking antioxidant expression and autophagy. On the other hand, the balance between AMP and ATP and NAD+ and NADH serves as a signal to activate AMPK and sirtuins, respectively. These nutrient sensors and regulators block the signal dependent on IGF‐I and TOR at the same time as they activate mechanisms to enhance more efficient energy production through oxidative metabolism and mechanisms to enhance cell protection through antioxidants and organelle turnover through autophagy. At the same time, CR, through sirtuins, can block inflammation mediators. This regulation occurs at different levels and many mediators can produce a redundant response such as autophagy activation by blocking TOR‐dependent FoxO inhibition or by inducing the activity of autophagy‐related (ATG) proteins.
IGF‐I
Among the hypotheses to explain ageing, several findings indicate that changes in the insulin–IGF‐I receptor signalling system are involved in the modulation of ageing in both, invertebrates (Tatar et al. 2003; Kenyon, 2011) and vertebrates (Dupont & Holzenberger, 2003; Selman et al. 2008). CR reduces plasma levels of IGF‐I, insulin and glucose in rodents (Argentino et al. 2005) and also in humans (Weiss et al. 2006). Studies performed in C. elegans have shown that decrease in the activity of Daf‐2 (similar to IGF‐I receptor in these organisms) doubles lifespan (Kenyon, 2010). Activation of the IGF‐I‐dependent signalling cascade induces protein synthesis by activating TOR and ribosomal protein 6 kinase (S6K) and also inhibits the transcriptional activity of the forkhead box transcription factors (FoxO). These are important regulatory elements in the effect of CR on lifespan since the inhibition of the FoxO orthologue daf‐16 in both, C. elegans and D. melanogaster by IGF‐I‐dependent signalling decreases lifespan in these organisms (Kenyon, 2010). In humans, a genetic variation of the Fox3A gene has been found in long‐lived and healthy men showing benefits in cardiovascular disease and insulin sensitivity (Willcox et al. 2008).
Interestingly, FoxO is involved in the induction of several stress response genes (Goto & Takano, 2009), indicating that this is an important mediator of the induction of protective mechanisms in the organisms after CR. This agrees with the ‘Hormesis hypothesis’ explaining the CR effect on ageing based on the effect that a moderate stress produces by induction of protective responses in the organisms (Masoro, 2007; Rattan, 2008).
Target of Rapamycin (TOR)
TOR protein members are a conserved family of kinases that respond to stress, nutrient and growth factors. TOR stimulates cell growth when food is available. TOR inhibits autophagy and stimulates protein synthesis and cell proliferation (Wullschleger et al. 2006). The importance of TOR in longevity induced by CR was also demonstrated in invertebrates such as C. elegans and D. melanogaster. In these organisms, down‐regulation of TOR produces an increase in lifespan (Sharp, 2011). This same effect has been also found in mice (Harrison et al. 2009; Selman et al. 2009). Interestingly, a decrease in S6K activity, a protein kinase involved in protein synthesis and activated by TOR in yeast, worms, flies and mice, also extends lifespan and delays the progression of age‐related diseases (Selman et al. 2009). At the same time, the increase in FoxO‐dependent autophagy found when TOR is inhibited is essential for lifespan extension in yeast (Kamada et al. 2004) and in C. elegans (Toth et al. 2008).
AMP‐dependent protein kinase (AMPK)
AMPK is a very sensitive energy sensor in cells and organisms. AMPK is activated in response to an increase in the AMP/ATP ratio, for example, when cells are deprived of glucose, whereas its activity decreases when cells are full of energy, indicated by a lower AMP/ATP ratio (Hardie, 2011). As in the case of other regulators such as sirtuins, its effect on longevity has been observed in several organisms from yeast to mammals. It has been clearly demonstrated that an increase in AMPK activity is associated with a longer lifespan while its inhibition shortens it (Apfeld et al. 2004; Harkness et al. 2004; Tohyama & Yamaguchi, 2010). However, in mammals, the importance of this kinase is under debate since it has been reported that its activity is not affected by CR (Gonzalez et al. 2004) or is even reduced (To et al. 2007). However, other studies indicate an increase in AMPK activity in heart and skeletal muscle (Jager et al. 2007; Miller et al. 2012). These discrepancies could be due to differences in the amount of time under CR or the degree of CR which can play an important role in nutrient balance. Further, as has been indicated in antioxidant responses (Rodriguez‐Bies et al. 2015; Tung et al. 2015 b), the age of the organism can be an important factor in the importance of each component that responds to CR.
Sirtuins
One of the best known effectors of CR is the family of NAD+‐dependent protein deacetylases. They are found in all the organisms, from prokaryotes to humans, affecting the activity of many enzymes by removing acetyl residues. Some time ago it was demonstrated that the orthologue of mammalian SIRT‐1, Sir2, was able to increase lifespan in S. cerevisiae and in invertebrates such as C. elegans and D. melanogaster (Kaeberlein et al. 1999; Tissenbaum & Guarente, 2001; Wood et al. 2004). In mammals, it is clear that CR induces the expression and the activity of sirtuins in many organs and their activities are associated with many of the metabolic effects found in these organisms after CR (Imai & Guarente, 2010).
Interestingly, sirtuins seem to play a central role in the response to CR. Sirtuins act as nutrient and metabolic sensors by detecting fluctuations in the NAD+/NADH ratio. When nutrients, especially glucose, decrease, NAD+ accumulates and sirtuins are activated. Thus sirtuins have an opposite effect to TOR activation after glucose input. The increasing number of proteins regulated by deacetylation, involved in several key aspects of cell physiology from the cell cycle to metabolism and antioxidant protection, highlights the importance of sirtuins in the regulation of metabolism, insulin response, cell cycle, autophagy, etc. In fact, the role of sirtuins in the effect of CR on longevity and the increase in healthspan has been extensively reviewed (Guarente, 2011, 2013). However, sirtuin‐independent mechanisms have been also proposed to explain the increase in longevity in yeast induced by CR (Kaeberlein et al. 2004). Also, the complexity of sirtuins in mammals has promoted the idea that they can show both pro‐ and anti‐ageing capacities in mice (Kaeberlein, 2008; Li et al. 2008).
Mitochondrial modifications induced by CR
Taking into consideration the role of the factors induced by CR in metabolic regulation, their modulation can improve cellular physiology and maintain an organism's capacity for extended ageing. Several studies found that mitochondrial biogenesis is impaired during ageing, especially in high‐energy‐demanding tissues such as muscle, brain or heart (Conley et al. 2000; Short et al. 2005). CR and other interventions such as exercise or nutraceuticals such as resveratrol induce mitochondrial biogenesis in heart and skeletal muscle in humans and other organisms (Feige et al. 2008; Baur et al. 2010; Rodriguez‐Bies et al. 2015), indicating their role in the maintenance of the mitochondrial activity in these organs during ageing.
At least three different pathways have been associated with this effect on mitochondrial biogenesis in muscle: eNOS induction, PGC‐1α activation and adiponectin‐dependent activation of the SIRT1/AMPK axis. Sirtuins seem to play a key regulatory role in these pathways since SIRT1 knockout (KO) in skeletal muscle blunts the physiological changes induced by CR in this tissue (Schenk et al. 2011).
Nitric oxide synthase is induced by CR in mice and human muscle (Nisoli et al. 2005; Civitarese et al. 2007) and plays an important role in CR‐induced mitochondrial biogenesis, since in eNOS−/− mice, the effect of CR is blocked (Nisoli et al. 2005). SIRT1 has also been suggested to play an essential role, since this enzyme activates eNOS by deacetylation (Mattagajasingh et al. 2007).
PGC‐1α is the main factor involved in the regulation of mitochondrial mass and the adaptation to energy demand. PGC‐1α activity is related to many of the beneficial effects of physical activity in model organisms (Li et al. 2011). PGC‐1α also stimulates mitochondrial biogenesis, regulates mitochondrial dynamics, modulates oxidative phosphorylation and controls mitochondrial genome copy number (Handschin & Spiegelman, 2006; Gouspillou et al. 2014). AMPK and SIRT1, the two main metabolic sensors in cells, directly modulate PGC‐1α activity through phosphorylation and deacetylation (Nemoto et al. 2005; Jager et al. 2007). In fact, it has been proposed that AMPK and SIRT1 are the most important effectors that activate mitochondrial biogenesis through increasing PGC‐1α activity at the transcriptional and post‐translational level (Martin‐Montalvo & de Cabo, 2013). Recently, it has been demonstrated that both skeletal muscle‐specific AMPK deficiency and AMPKα2 KO mice impair the beneficial effects of CR on glucose tolerance due to reduced SIRT1 levels. Thus, considerable evidence indicates that the activity of AMPK and SIRT1 are essential for physiological responses induced by CR in muscle.
Among the other members of the sirtuin family, SIRT3 is located within mitochondria, suggesting its immediate regulation of mitochondrial activity. In fact, SIRT3 increases in skeletal muscle after CR and decreases on feeding a high‐fat diet, indicating that regulation depends on the calorie intake (Palacios et al. 2009). This sirtuin has been linked to the induction of the catabolism of lipids (Kendrick et al. 2011). Among other important roles of SIRT3 in mitochondria, we can highlight the regulation of succinate‐dehydrogenase activity by deacetylation (Kendrick et al. 2011), the modulation of the activity of Mn‐SOD (Qiu et al. 2010) or the autophagy regulation by deacetylation of FoxO3 in CR‐fed animals (Kume et al. 2010). Interestingly, SIRT3 KO mice show PGC‐1α down‐regulation and low levels of AMPK phosphorylation after CR, indicating a key role for this mitochondrial sirtuin in energy regulation in muscle (Palacios et al. 2009). On the other hand, PGC‐1α exerts a positive feedback, stimulating SIRT3 expression, in muscle and liver (Kong et al. 2010).
Interestingly, the role of SIRT3 in acetate metabolism has been also related to ageing (Shimazu et al. 2010). Acetate plays an important role in cell metabolism, being an important product of ethanol and fatty acid metabolism, especially during fasting or starvation (Seufert et al. 1974). Acetate can be converted into acetyl‐CoA by the activity of acetyl‐CoA synthase enzymes in cytosol (AceCS1) or mitochondria (AceCS2). These enzymes are activated by deacetylation by both SIRT1 in cytosol and SIRT3 in mitochondria (Shimazu et al. 2010). An important role for SIRT3 in acetate metabolism has been suggested, since both, SIRT3 KO and AceCS2 KO mice show overlapping phenotypes. However, to date, no clear data about the role of acetate in the ageing progress has been shown although its AceCS‐mediated synthesis in yeast has been associated with higher longevity (Falcon et al. 2010).
Another interesting factor involved in mitochondrial activity regulation after CR is the hormetic response linked to mitochondrial activity, called mitohormesis. Apart of the above‐indicated factors that regulate mitochondrial biogenesis, a role for ROS production in mitohormesis has been proposed (Ristow & Zarse, 2010; Ristow & Schmeisser, 2011, 2014; Ristow, 2014). It has been proposed that higher ROS production after CR induces the antioxidant response, increases pathways and molecules involved in removing cell damage and modulates mitochondrial activity (Ristow, 2014). Interestingly, it has been demonstrated that ROS are involved in the modulation of glucose metabolism and the increase in oxidative metabolism and stress resistance in CR and also in exercise (Barbieri et al. 2013). However, the relationship of ROS with mitohormesis and the regulation of enzymatic regulators such as AMPK, sirtuins or FoxO is still controversial, although an AMPK‐dependent increase in SIRT3 activity has been associated with the activation of protection against oxidative stress (Brandauer et al. 2015) and the ROS‐dependent activation of AMPK has recently been associated with higher longevity in C. elegans (Hwang et al. 2014) and with the increase in autophagy in HUVEC cells (Li et al. 2015). Therefore, it seems that mild oxidative stress could be associated with the response to CR. Although the mechanism involved in the ROS‐induced increase in mitochondrial metabolism is not clear, it seems that AMPK‐dependent modulation of FoxO or PGC‐1α could be involved. But more importantly, these findings indicate that the abuse of exogenous antioxidants during ageing may reduce lifespan rather than increasing it. It is more important to induce endogenous antioxidant systems that add antioxidants to compromised organisms lacking enzymes able to use them.
Further research is needed to clarify the complex network of interactions and protein modifications involved in metabolism regulation induced by CR and related to a higher energetic efficiency. However, it is clear that regulation of sirtuins activities are, together with AMPK activity and PGC‐1α‐dependent pathways, at the centre of the response to CR (Fig. 3).
Figure 3. Role of sirtuins in the control of mitochondrial physiology .
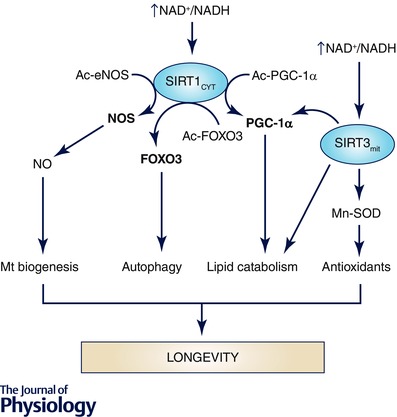
The increase in the NAD+/NADH ratio produced by CR in cells may induce the activity of the cytosolic SIRT1 and the mitochondrial SIRT3 forms. Both have been involved in the induction of many processes involved in the modulation of mitochondrial physiology such as induction of mitochondrial biogenesis by activating NOS and PGC‐1α, which, coupled with the activation of autophagy by deacetylation of FOXO3, promotes the renovation of mitochondria, eliminating damaged and unbalanced mitochondria at the same time as undamaged and active mitochondria increase. At the same time, lipid catabolism is promoted and the activity of mitochondrial antioxidants increases through the activity of SIRT3. Thus, sirtuins seem to be at the centre of mitochondrial modulation during CR.
CR mimetics
During the past few years, a group of molecularly unrelated compounds have emerged as CR mimetics, able to produce, at least partially, similar effects in different organisms. In general, all these compounds have a common denominator, the activation of the above‐described molecular pathways involved in the response to CR such as AMPK and sirtuins (Fig. 2).
Among these compounds, polyphenols have been extensively studied. These compounds are characterized by the presence of one or more hydroxyl groups bound to an unsaturated aromatic hydrocarbon ring such as benzene and are produced by plants in response to stress or fungal infections (Bravo, 1998). Among these compounds, resveratrol has emerged as the compound that best mimics the CR effect on several organisms (Testa et al. 2014). Among the demonstrated effects of resveratrol we can highlight: a decrease in insulin secretion and insulin level, an increase in insulin sensitivity, a decrease in fat mass, an increase in mitochondrial biogenesis and oxidative phosphorylation, an increase in the NAD+/NADH ratio and the subsequent activation of sirtuins, an increase in AMPK activity and increase in mitophagy and autophagy (Testa et al. 2014). Interestingly, a recent study of humans demonstrates that treatment with resveratrol produces similar effects to CR in obese people. Thirty days of resveratrol treatment activated AMPK, and increased SIRT1 and PGC‐1α, improving muscle mitochondrial respiration based on fatty acids similarly to the effect found with CR (Timmers et al. 2011).
Another interesting compound is rapamycin (sirolimus), an inhibitor of TOR that induces significant lifespan extension in several organisms (Selman et al. 2009; Sharp, 2011). This compound has been shown to increase lifespan in yeast, nematodes, flies and human cells by decreasing protein synthesis, through inhibition of S6K, and by inducing autophagy through the inhibition of TOR and the subsequent activation of FoxO. However, in a recent study performed in mice, rapamycin has shown a selective effect on female mice and different features in comparison with CR (Miller et al. 2014). Thus, its use as mimetic of CR needs more study, at least, in mammals.
In 1998, Lane and colleagues proposed that feeding animals with glycolysis blockers such as 2‐deoxy‐d‐glucose (2DG) could mimic the physiological effects of CR (Lane et al. 1998). This compound increases C. elegans lifespan (Schulz et al. 2007) and produces several CR‐related effects in rats such as a decrease in insulin levels (Ingram & Roth, 2011), an increase in glucocorticoids (Wan et al. 2004) and an increase in lifespan (Wan et al. 2003). As in the other cases, it seems that 2DG can also activate AMPK and SIRT1 (Wang et al. 2011; Yang et al. 2011). However, the main problem with this compound is that it can be cardiotoxic and increases the incidence of tumours in adrenal medulla in long‐term treatments (Minor et al. 2010).
Another CR mimetic of interest is metformin, a biguanine drug, clinically used in the treatment of type‐2 diabetes that has increased the lifespan of C. elegans and D. melanogaster, probably by activating AMPK (Slack et al. 2012). Experiments performed in mice indicate that low doses of metformin produce similar effects to those found with CR, such as higher insulin sensitivity, reduction of low‐density lipoproteins and cholesterol levels in plasma, an increase in antioxidant protection and reduction of chronic inflammation. As in the above‐indicated cases, the effect of metformin seems to be through activation of AMPK (Martin‐Montalvo et al. 2013). Furthermore, the effect of metformin has recently been associated with the activation of SIRT1‐mediated autophagy in an AMPK‐independent pathway (Song et al. 2015). This is therefore a promising compound, since, in in vitro experiments with human fibroblasts, metformin has shown the capacity to restore the mitochondrial dysfunction found in aged cells (Alcocer‐Gomez et al. 2015).
All these compounds have shown, at least in part, similar effects to CR on cells, tissues and organs and all of them have produced mitochondrial regulation by increasing turnover and activating oxidative metabolism through activation of the AMPK/SIRT1 axis and inhibition of TOR. It seems clear then that the regulation of mitochondrial metabolism by these nutrient sensors is at the centre of the effect of CR and its mimetics on healthspan and longevity.
CR and inflammation
Inflammation is also an important factor in ageing. Proinflammatory factors such as TNF‐α, IFN‐γ, IL‐1β, IL‐18 and others increase systemically during ageing (Chung et al. 2002; Salvioli et al. 2006; Goto, 2008). It has been shown that the increase in oxidative stress during ageing can be involved in the incidence of age‐related diseases and the induction of a chronic inflammatory process (Solana et al. 2006; Candore et al. 2010). Evidence indicates that sterile inflammation, a chronic low‐level inflammation, is caused by either stress or environmental conditions and increases during ageing (Feldman et al. 2015). Accumulation of damage during ageing increases the production of danger‐associated molecular patterns (DAMPs) that are recognized by immune receptors and activates the inflammasome (Feldman et al. 2015). In fact, chronic inflammatory status has been associated with many chronic diseases of ageing (Pawelec et al. 2014) and ‘inflammaging’ has been coined as a new concept for chronic inflammation associated with ageing (Franceschi & Campisi, 2014).
The progressive muscle weakness linked to ageing has been associated with a diminished function of satellite cells. The activity of satellite cells can be affected by systemic inflammatory factors such as TNF‐α that negatively affect muscle‐regenerating capacity (Degens, 2010). In a recent study, it has been demonstrated that the chronic‐inflammation‐related acute phase C‐reactive protein (CRP) negatively affects skeletal muscle mass in elderly women and serum from these elderly women reduces myoblasts growth (Wahlin‐Larsson et al. 2014).
It is likely that the maintenance of mitochondria biogenesis by CR can increase the resistance of muscle against inflammation. The decrease in PGC‐1α protein during ageing has been associated with, among other factors, inflammatory markers in white adipose and liver tissues (Sczelecki et al. 2014). Furthermore, exercise prevents the age‐associated increase in systemic IL‐6 and TNF‐α in a PGC1‐α‐dependent mechanism in mice (Olesen et al. 2013). CR also decreases inflammation and insulin resistance in an age‐associated inflammation rat model (Horrillo et al. 2011) and shows anti‐inflammatory activity by modulating GSH redox status, NF‐κB, SIRT1, PPARs, and FoxOs (Chung et al. 2011). And, recently we have also found that resveratrol is able to decrease both the production of proinflammatory cytokines and the induction of inflammasome in aged mice liver (Tung et al. 2015 a).
Effect of CR on age‐associated diseases in humans
Two of the main age‐associated diseases in humans are type 2 diabetes and cardiovascular disease (CVD). In both cases, models of CR, dietary interventions or exercise have shown important improvements in the onset and development of these diseases. The onset of insulin resistance and type 2 diabetes, probably due to the impairment of fatty acid oxidation by mitochondrial dysfunction, is a hallmark of ageing (Civitarese et al. 2006; Rolo & Palmeira, 2006). Interestingly, PGC‐1α expression and mitochondrial biogenesis stimulated by AMPK and SIRT1 in muscle has been associated with the reduction of insulin resistance (Reznick & Shulman, 2006; Gerhart‐Hines et al. 2007). Thus, the use of CR or CR mimickers such as resveratrol or metformin can help to prevent the onset and the progression of these diseases.
CVD is one of the main problems in humans associated to ageing by accumulation of factors that impair cardiovascular function such as hypercholesterolaemia, high glucose or endothelial dysfunction. A 20% CR for 2—6 years in humans has been associated with lower levels of many of CVD markers and diabetes such as body weight, blood pressure, and blood cholesterol and glucose (Everitt & Le Couteur, 2007). But, as CR in humans is a complex and difficult therapy, exercise can be used as a mimetic. In fact, physical activity has shown positive effects by increasing lifespan without CVD (Franco et al. 2005) and lowering mortality risk in active subjects (Landi et al. 2004). Most of the mechanisms that maintain cardiac activity such as autophagy, proteasome‐mediated turnover, apoptosis and control of quality of mitochondria are affected during ageing and are improved by both CR and exercise (Quarles et al. 2015). The control of oxidative damage in plasma cholesterol, especially in LDL is another factor to be considered. We have recently demonstrated that increased physical activity is associated with lower oxidative damage in plasma of elderly people, probably through the increase in coenzyme Q in plasma lipoproteins, whereas sedentarism reduces coenzyme Q levels, which is associated with an increase in lipid peroxidation in plasma (Del Pozo‐Cruz et al. 2014 a,b). Similar results were obtained in mice forced to perform exercise (Rodriguez‐Bies et al. 2015). These studies indicate that exercise and CR exerts a general effect and improves CVD not only by modulating heart and endothelial physiology but also reducing the oxidation of lipids in plasma and thus the production of the atherosclerotic plaque.
Concluding remarks
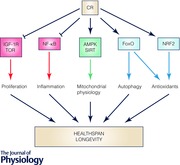
The broad effect of CR on healthspan and longevity occurs through multiple mechanisms that involve most of the metabolic pathways in tissues and organs (Fig. 4). The major effectors are sirtuin deacetylases, AMPK and PGC‐1α. CR improves aerobic metabolism by increasing efficient mitochondrial metabolism, lowering endogenous ROS production at the same time as it increases the amount and activity of endogenous antioxidant enzymes. These molecular and physiological effects have also been found with some nutraceuticals and compounds that act as CR mimetics such as resveratrol, rapamycin or metformin. CR also affects the lipid composition of membranes by lowering oxidative damage. Further, the study of the mechanisms involved in the prevention of chronic inflammation induced by CR, probably through similar mechanisms to those found in mitochondrial regulation, is increasing and offers new opportunities to understand how CR prevents endogenous damage in the organism.
Figure 4. Resumé of CR effects on cells .
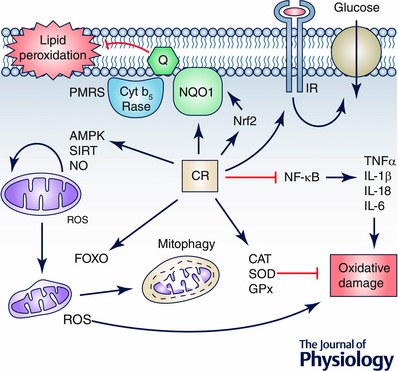
CR induces many pathways that are involved in changes in mitochondrial physiology by activating AMPK‐ and SIRT‐dependent pathways and inducing mitochondrial turnover by balancing mitochondrial biogenesis with induction of mitophagy of damaged mitochondria. This regulation reduces the production of ROS by damaged mitochondria, thus reducing oxidative damage. This damage is also reduced by the induction of cell antioxidant activities such as CAT, SOD and GPx and by preventing the activation of proinflammatory processes by blocking the NFκB pathway. On the other hand, the role of plasma membrane redox system regulation in the CR effect on cells remains to be clarified but it seems that in aged cells and tissues the induction of this system by activation of Nrf2 not only prevents lipid peroxidation in membranes but can also regulate the response to external factors such as local factors or insulin. We cannot discard a putative role of this system in the regulation of the insulin response preventing insulin‐resistance in aged cells.
Additional information
Competing interests
The authors declare no conflicts of interest in writing this manuscript.
Funding
The research group is funded by the Andalusian Government grant BIO177 (FEDER funds of European Commission). Research has been funded by the Spanish Ministry of Economy and Competitiveness grant DEP2012‐39985. The authors are also members of the CIBERER, Instituto Carlos III, of the Spanish Ministry of Health.
Biography
Guillermo López‐Lluch (left) and Plácido Navas (right) work at the Andalusian Centre for Development Biology (www.CABD.es), University of Pablo de Olavide in Seville, Spain. Their major research interest is the study of the regulation of bioenergetics pathways, mainly during ageing, and inherited mitochondrial disorders. Both approaches are based on the translational analysis of the coenzyme Q biosynthesis pathway in cell and mouse models. The biochemistry and molecular analysis of coenzyme Q in patients’ fibroblasts are used for diagnosis of the mitochondrial disorder and the molecular mechanisms of the defect are studied in transgenic mouse models and dietary interventions in ageing mice.

References
- Alcocer‐Gomez E, Garrido‐Maraver J, Bullon P, Marin‐Aguilar F, Cotan D, Carrion AM, Alvarez‐Suarez JM, Giampieri F, Sanchez‐Alcazar JA, Battino M & Cordero MD (2015). Metformin and caloric restriction induce an AMPK‐dependent restoration of mitochondrial dysfunction in fibroblasts from fibromyalgia patients. Biochim Biophys Acta 1852, 1257–1267. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Reisman SA, Yeager RL, Goedken MJ & Klaassen CD (2010). Nuclear factor erythroid 2‐related factor 2 deletion impairs glucose tolerance and exacerbates hyperglycemia in type 1 diabetic mice. J Pharmacol Exp Ther 333, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM & Weindruch R (2012). The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol 24, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Jones B & de Cabod R (2005). The diet restriction paradigm: a brief review of the effects of every‐other‐day feeding. Age (Dordr) 27, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, O'Connor G, McDonagh T, DiStefano PS & Curtis R (2004). The AMP‐activated protein kinase AAK‐2 links energy levels and insulin‐like signals to lifespan in C. elegans. Genes Dev 18, 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argentino DP, Dominici FP, Al‐Regaiey K, Bonkowski MS, Bartke A & Turyn D (2005). Effects of long‐term caloric restriction on early steps of the insulin‐signaling system in mouse skeletal muscle. J Gerontol A Biol Sci Med Sci 60, 28–34. [DOI] [PubMed] [Google Scholar]
- Augustin H & Partridge L (2009). Invertebrate models of age‐related muscle degeneration. Biochim Biophys Acta 1790, 1084–1094. [DOI] [PubMed] [Google Scholar]
- Bales CW & Kraus WE (2013). Caloric restriction: implications for human cardiometabolic health. J Cardiopulm Rehabil Prev 33, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri E, Sestili P, Vallorani L, Guescini M, Calcabrini C, Gioacchini AM, Annibalini G, Lucertini F, Piccoli G & Stocchi V (2013). Mitohormesis in muscle cells: a morphological, molecular, and proteomic approach. Muscles Ligaments Tendons J 3, 254–266. [PMC free article] [PubMed] [Google Scholar]
- Barja G (2014). The mitochondrial free radical theory of aging. Prog Mol Biol Transl Sci 127, 1–27. [DOI] [PubMed] [Google Scholar]
- Baur JA, Chen D, Chini EN, Chua K, Cohen HY, de Cabo R, Deng C, Dimmeler S, Gius D, Guarente LP, Helfand SL, Imai S, Itoh H, Kadowaki T, Koya D, Leeuwenburgh C, McBurney M, Nabeshima Y, Neri C, Oberdoerffer P, Pestell RG, Rogina B, Sadoshima J, Sartorelli V, Serrano M, Sinclair DA, Steegborn C, Tatar M, Tissenbaum HA, Tong Q, Tsubota K, Vaquero A & Verdin E (2010). Dietary restriction: standing up for sirtuins. Science 329, 1012–1013; author reply 1013–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandauer J, Andersen MA, Kellezi H, Risis S, Frosig C, Vienberg SG & Treebak JT (2015). AMP‐activated protein kinase controls exercise training‐ and AICAR‐induced increases in SIRT3 and MnSOD. Front Physiol 6, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo L (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56, 317–333. [DOI] [PubMed] [Google Scholar]
- Burkle A, Moreno‐Villanueva M, Bernhard J, Blasco M, Zondag G, Hoeijmakers JH, Toussaint O, Grubeck‐Loebenstein B, Mocchegiani E, Collino S, Gonos ES, Sikora E, Gradinaru D, Dolle M, Salmon M, Kristensen P, Griffiths HR, Libert C, Grune T, Breusing N, Simm A, Franceschi C, Capri M, Talbot D, Caiafa P, Friguet B, Slagboom PE, Hervonen A, Hurme M & Aspinall R (2015). MARK‐AGE biomarkers of ageing. Mech Ageing Dev 151, 2–12. [DOI] [PubMed] [Google Scholar]
- Candore G, Caruso C, Jirillo E, Magrone T & Vasto S (2010). Low grade inflammation as a common pathogenetic denominator in age‐related diseases: novel drug targets for anti‐ageing strategies and successful ageing achievement. Curr Pharm Des 16, 584–596. [DOI] [PubMed] [Google Scholar]
- Cava E & Fontana L (2013). Will calorie restriction work in humans? Aging (Albany NY) 5, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E & Team CP (2007). Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4, e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, Ravussin E & Smith SR (2006). Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 4, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R & Anderson RM (2014). Caloric restriction reduces age‐related and all‐cause mortality in rhesus monkeys. Nat Commun 5, 3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA & Esselman PC (2000). Oxidative capacity and ageing in human muscle. J Physiol 526, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane FL & Low H (2005). Plasma membrane redox and control of sirtuin. Age (Dordr) 27, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hagopian K, Bibus D, Villalba JM, Lopez‐Lluch G, Navas P, Kim K, McDonald RB & Ramsey JJ (2013). The influence of dietary lipid composition on liver mitochondria from mice following 1 month of calorie restriction. Biosci Rep 33, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hagopian K, Bibus D, Villalba JM, Lopez‐Lluch G, Navas P, Kim K & Ramsey JJ (2014). The influence of dietary lipid composition on skeletal muscle mitochondria from mice following eight months of calorie restriction. Physiol Res 63, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hagopian K, McDonald RB, Bibus D, Lopez‐Lluch G, Villalba JM, Navas P & Ramsey JJ (2012). The influence of dietary lipid composition on skeletal muscle mitochondria from mice following 1 month of calorie restriction. J Gerontol A Biol Sci Med Sci 67, 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN & Bobryshev YV (2014). Mitochondrial aging and age‐related dysfunction of mitochondria. Biomed Res Int 2014, 238463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim KW, Choi JS & Yu BP (2002). Molecular inflammation hypothesis of aging based on the anti‐aging mechanism of calorie restriction. Microsc Res Tech 59, 264–272. [DOI] [PubMed] [Google Scholar]
- Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, Jung JH & Yu BP (2011). Molecular inflammation as an underlying mechanism of the aging process and age‐related diseases. J Dent Res 90, 830–840. [DOI] [PubMed] [Google Scholar]
- De Cabo R, Cabello R, Rios M, Lopez‐Lluch G, Ingram DK, Lane MA & Navas P (2004). Calorie restriction attenuates age‐related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol 39, 297–304. [DOI] [PubMed] [Google Scholar]
- Degens H (2010). The role of systemic inflammation in age‐related muscle weakness and wasting. Scand J Med Sci Sports 20, 28–38. [DOI] [PubMed] [Google Scholar]
- Del Pozo‐Cruz J, Rodriguez‐Bies E, Ballesteros‐Simarro M, Navas‐Enamorado I, Tung BT, Navas P & Lopez‐Lluch G (2014. a). Physical activity affects plasma coenzyme Q10 levels differently in young and old humans. Biogerontology 15, 199–211. [DOI] [PubMed] [Google Scholar]
- Del Pozo‐Cruz J, Rodriguez‐Bies E, Navas‐Enamorado I, Del Pozo‐Cruz B, Navas P & Lopez‐Lluch G (2014. b). Relationship between functional capacity and body mass index with plasma coenzyme Q10 and oxidative damage in community‐dwelling elderly‐people. Exp Gerontol 52, 46–54. [DOI] [PubMed] [Google Scholar]
- Dupont J & Holzenberger M (2003). IGF type 1 receptor: a cell cycle progression factor that regulates aging. Cell Cycle 2, 270–272. [PubMed] [Google Scholar]
- Everitt AV & Le Couteur DG (2007). Life extension by calorie restriction in humans. Ann N Y Acad Sci 1114, 428–433. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Zoli M, Gonzalez‐Freire M, Salive ME, Studenski SA & Ferrucci L (2015). Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc 16, 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon AA, Chen S, Wood MS & Aris JP (2010). Acetyl‐coenzyme A synthetase 2 is a nuclear protein required for replicative longevity in Saccharomyces cerevisiae . Mol Cell Biochem 333, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Lagouge M & Auwerx J (2008). Dietary manipulation of mouse metabolism. Curr Protoc Mol Biol 66, 10.5.1–10.5.11. [DOI] [PubMed] [Google Scholar]
- Feldman N, Rotter‐Maskowitz A & Okun E (2015). DAMPs as mediators of sterile inflammation in aging‐related pathologies. Ageing Res Rev 24, 29–39. [DOI] [PubMed] [Google Scholar]
- Franceschi C & Campisi J (2014). Chronic inflammation (inflammaging) and its potential contribution to age‐associated diseases. J Gerontol A Biol Sci Med Sci 69 (Suppl. 1), S4‐9. [DOI] [PubMed] [Google Scholar]
- Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J & Nusselder W (2005). Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med 165, 2355–2360. [DOI] [PubMed] [Google Scholar]
- Genova ML & Lenaz G (2015). The interplay between respiratory supercomplexes and ROS in aging. Antioxid Redox Signal 23, 208–238. [DOI] [PubMed] [Google Scholar]
- Gerhart‐Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z & Puigserver P (2007). Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC‐1alpha. EMBO J 26, 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves RL, Quinlan CL, Perevoshchikova IV, Hey‐Mogensen M & Brand MD (2015). Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J Biol Chem 290, 209–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R & Saupe KW (2004). Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab 287, E1032–E1037. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Freire M, Rd Cabo, Bernier M, Sollott SJ, Fabbri E, Navas P & Ferrucci L (2015). Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci 70, 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR & Cider NL (1982). Effects of intermittent feeding upon growth and life span in rats. Gerontology 28, 233–241. [DOI] [PubMed] [Google Scholar]
- Goto M (2008). Inflammaging (inflammation + aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci Trends 2, 218–230. [PubMed] [Google Scholar]
- Goto T & Takano M (2009). Transcriptional role of FOXO1 in drug resistance through antioxidant defense systems. Adv Exp Med Biol 665, 171–179. [DOI] [PubMed] [Google Scholar]
- Gouspillou G, Sgarioto N, Norris B, Barbat‐Artigas S, Aubertin‐Leheudre M, Morais JA, Burelle Y, Taivassalo T & Hepple RT (2014). The relationship between muscle fiber type‐specific PGC‐1alpha content and mitochondrial content varies between rodent models and humans. PLoS One 9, e103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JP, Eisen T, Cline GW, Smith PJ & Heart E (2011). Plasma membrane electron transport in pancreatic beta‐cells is mediated in part by NQO1. Am J Physiol Endocrinol Metab 301, E113–E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredilla R & Barja G (2005). Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology 146, 3713–3717. [DOI] [PubMed] [Google Scholar]
- Guarente L (2011). Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol 76, 81–90. [DOI] [PubMed] [Google Scholar]
- Guarente L (2013). Calorie restriction and sirtuins revisited. Genes Dev 27, 2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C & Spiegelman BM (2006). Peroxisome proliferator‐activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27, 728–735. [DOI] [PubMed] [Google Scholar]
- Hardie DG (2011). Sensing of energy and nutrients by AMP‐activated protein kinase. Am J Clin Nutr 93, 891S‐896S. [DOI] [PubMed] [Google Scholar]
- Harkness TA, Shea KA, Legrand C, Brahmania M & Davies GF (2004). A functional analysis reveals dependence on the anaphase‐promoting complex for prolonged life span in yeast. Genetics 168, 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E & Miller RA (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss AR (2007). Dietary restriction, glycolysis, hormesis and ageing. Biogerontology 8, 221–224. [DOI] [PubMed] [Google Scholar]
- Horrillo D, Sierra J, Arribas C, Garcia‐San Frutos M, Carrascosa JM, Lauzurica N, Fernandez‐Agullo T & Ros M (2011). Age‐associated development of inflammation in Wistar rats: Effects of caloric restriction. Arch Physiol Biochem 117, 140–150. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ (2005). On the importance of fatty acid composition of membranes for aging. J Theor Biol 234, 277–288. [DOI] [PubMed] [Google Scholar]
- Hwang AB, Ryu EA, Artan M, Chang HW, Kabir MH, Nam HJ, Lee D, Yang JS, Kim S, Mair WB, Lee C, Lee SS & Lee SJ (2014). Feedback regulation via AMPK and HIF‐1 mediates ROS‐dependent longevity in Caenorhabditis elegans . Proc Natl Acad Sci USA 111, E4458‐4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP & de Cabo R (2006). Calorie restriction up‐regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA 103, 19908–19912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun DH, Kim J, Moon C, Lim CJ, de Cabo R & Mattson MP (2012). The plasma membrane redox enzyme NQO1 sustains cellular energetics and protects human neuroblastoma cells against metabolic and proteotoxic stress. Age (Dordr) 34, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S & Guarente L (2010). Ten years of NAD‐dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci 31, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK & Roth GS (2011). Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Exp Gerontol 46, 148–154. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St‐Pierre J & Spiegelman BM (2007). AMP‐activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC‐1alpha. Proc Natl Acad Sci USA 104, 12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL, Dillon D & Wu E (1990). Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am J Physio l Regul Integr Comp Physiol 258, R918–R923. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Hidalgo M, Santos‐Ocana C, Padilla S, Villalba JM, Lopez‐Lluch G, Martin‐Montalvo A, Minor RK, Sinclair DA, de Cabo R & Navas P (2009). NQR1 controls lifespan by regulating the promotion of respiratory metabolism in yeast. Aging Cell 8, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M (2008). The ongoing saga of sirtuins and aging. Cell Metab 8, 4–5. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S & Kennedy BK (2004). Sir2‐independent life span extension by calorie restriction in yeast. PLoS Biol 2, E296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M & Guarente L (1999). The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13, 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Sekito T & Ohsumi Y (2004). Autophagy in yeast: a TOR‐mediated response to nutrient starvation. Curr Top Microbiol Immunol 279, 73–84. [DOI] [PubMed] [Google Scholar]
- Kayo T, Allison DB, Weindruch R & Prolla TA (2001). Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc Natl Acad Sci USA 98, 5093–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST & Bergman RN (1994). Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol Endocrinol Metab 266, E540–E547. [DOI] [PubMed] [Google Scholar]
- Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, Watson PA, Birdsey N, Bao J, Gius D, Sack MN, Jing E, Kahn CR, Friedman JE & Jonscher KR (2011). Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J 433, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss‐Coray T & Sierra F (2014). Geroscience: linking aging to chronic disease. Cell 159, 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C (2011). The first long‐lived mutants: discovery of the insulin/IGF‐1 pathway for ageing. Philos Trans R Soc Lond B Biol Sci 366, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ (2010). The genetics of ageing. Nature 464, 504–512. [DOI] [PubMed] [Google Scholar]
- Khraiwesh H, Lopez‐Dominguez JA, Fernandez del Rio L, Gutierrez‐Casado E, Lopez‐Lluch G, Navas P, de Cabo R, Ramsey JJ, Buron MI, Villalba JM & Gonzalez‐Reyes JA (2014). Mitochondrial ultrastructure and markers of dynamics in hepatocytes from aged, calorie restricted mice fed with different dietary fats. Exp Gerontol 56, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiwesh H, Lopez‐Dominguez JA, Lopez‐Lluch G, Navas P, de Cabo R, Ramsey JJ, Villalba JM & Gonzalez‐Reyes JA (2013). Alterations of ultrastructural and fission/fusion markers in hepatocyte mitochondria from mice following calorie restriction with different dietary fats. J Gerontol A Biol Sci Med Sci 68, 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim SK, Kim HK, Mattson MP & Hyun DH (2013). Mitochondrial function in human neuroblastoma cells is up‐regulated and protected by NQO1, a plasma membrane redox enzyme. PLoS One 8, e69030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Aiken JM, Havighurst T, Hollander J, Ripple MO & Weindruch R (1997). Adult‐onset energy restriction of rhesus monkeys attenuates oxidative stress‐induced cytokine expression by peripheral blood mononuclear cells. J Nutr 127, 2293–2301. [DOI] [PubMed] [Google Scholar]
- Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F & Chang Y (2010). Sirtuin 3, a new target of PGC‐1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5, e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Uzu T, Horiike K, Chin‐Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A & Koya D (2010). Calorie restriction enhances cell adaptation to hypoxia through Sirt1‐dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J, Han E, Bui CB, Shin W, Lee J, Lee S, Choi YB, Lee AH, Lee KH, Park C, Obin MS, Park SK, Seo YJ, Oh GT, Lee HW & Shin J (2012). Assurance of mitochondrial integrity and mammalian longevity by the p62‐Keap1‐Nrf2‐Nqo1 cascade. EMBO Rep 13, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Cesari M, Onder G, Lattanzio F, Gravina EM & Bernabei R (2004). Physical activity and mortality in frail, community‐living elderly patients. J Gerontol A Biol Sci Med Sci 59, 833–837. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK & Roth GS (1998). 2‐Deoxy‐D‐glucose feeding in rats mimics physiologic effects of calorie restriction. Journal of Anti‐aging Medicine 1, 327–337. [Google Scholar]
- Le Bourg E (2010). Predicting whether dietary restriction would increase longevity in species not tested so far. Ageing Res Rev 9, 289–297. [DOI] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R & Prolla TA (1999). Gene expression profile of aging and its retardation by caloric restriction. Science 285, 1390–1393. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Bovina C, Formiggini G & Parenti Castelli G (1999). Mitochondria, oxidative stress, and antioxidant defences. Acta Biochim Pol 46, 1–21. [PubMed] [Google Scholar]
- Li GH, Lin XL, Zhang H, Li S, He XL, Zhang K, Peng J, Tang YL, Zeng JF, Zhao Y, Ma XF, Lei JJ, Wang R, Wei DH, Jiang ZS & Wang Z (2015). Ox‐Lp(a) transiently induces HUVEC autophagy via an ROS‐dependent PAPR‐1‐LKB1‐AMPK‐mTOR pathway. Atherosclerosis 243, 223–235. [DOI] [PubMed] [Google Scholar]
- Li L, Muhlfeld C, Niemann B, Pan R, Li R, Hilfiker‐Kleiner D, Chen Y & Rohrbach S (2011). Mitochondrial biogenesis and PGC‐1alpha deacetylation by chronic treadmill exercise: differential response in cardiac and skeletal muscle. Basic Res Cardiol 106, 1221–1234. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW & Longo VD (2008). SirT1 inhibition reduces IGF‐I/IRS‐2/Ras/ERK1/2 signaling and protects neurons. Cell Metab 8, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J & Northrop JH (1917). What determines the duration of life in Metazoa? Proc Natl Acad Sci USA 3, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Dominguez JA, Khraiwesh H, Gonzalez‐Reyes JA, Lopez‐Lluch G, Navas P, Ramsey JJ, de Cabo R, Buron MI & Villalba JM (2013). Dietary fat modifies mitochondrial and plasma membrane apoptotic signaling in skeletal muscle of calorie‐restricted mice. Age (Dordr) 35, 2027–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Dominguez JA, Ramsey JJ, Tran D, Imai DM, Koehne A, Laing ST, Griffey SM, Kim K, Taylor SL, Hagopian K, Villalba JM, Lopez‐Lluch G, Navas P & McDonald RB (2015). The influence of dietary fat source on life span in calorie restricted mice. J Gerontol A Biol Sci Med Sci 70, 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P & de Cabo R (2006). Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 103, 1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Lluch G, Irusta PM, Navas P & de Cabo R (2008). Mitochondrial biogenesis and healthy aging. Exp Gerontol 43, 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Lluch G, Rios M, Lane MA, Navas P & de Cabo R (2005). Mouse liver plasma membrane redox system activity is altered by aging and modulated by calorie restriction. Age (Dordr) 27, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Otin C, Blasco MA, Partridge L, Serrano M & Kroemer G (2013). The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowell MF & Maynard LA (1935). The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutrition 10, 63–79. [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM & Weindruch R (2011). Caloric restriction delays aging‐induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol 46, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Montalvo A & de Cabo R (2013). Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid Redox Signal 19, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye‐Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M & de Cabo R (2013). Metformin improves healthspan and lifespan in mice. Nat Commun 4, 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I, Galluzzi L & Kroemer G (2011). Hormesis, cell death and aging. Aging (Albany NY) 3, 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ (2006). Caloric restriction and aging: controversial issues. J Gerontol A Biol Sci Med Sci 61, 14–19. [DOI] [PubMed] [Google Scholar]
- Masoro EJ (2007). Role of hormesis in life extension by caloric restriction. Dose Response 5, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K & Irani K (2007). SIRT1 promotes endothelium‐dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104, 14855–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK & de Cabo R (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP (2008). Dietary factors, hormesis and health. Ageing Res Rev 7, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Majounie E, Ding J, Guo R, Kim J, Bernier M, Mattison J, Cookson MR, Gorospe M, de Cabo R & Abdelmohsen K (2013). Age‐associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany NY) 5, 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry BJ (2002). Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol 34, 1340–1354. [DOI] [PubMed] [Google Scholar]
- Michan S (2014). Calorie restriction and NAD+/sirtuin counteract the hallmarks of aging. Front Biosci (Landmark Ed) 19, 1300–1319. [DOI] [PubMed] [Google Scholar]
- Miller BF, Robinson MM, Bruss MD, Hellerstein M & Hamilton KL (2012). A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell 11, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L & Strong R (2014). Rapamycin‐mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13, 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Smith DL Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, Roth GS, Lane M, Allison DB, de Cabo R, Ingram DK & Mattison JA (2010). Chronic ingestion of 2‐deoxy‐D‐glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol 243, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A & Van Remmen H (2007). Trends in oxidative aging theories. Free Radic Biol Med 43, 477–503. [DOI] [PubMed] [Google Scholar]
- Navas P, Villalba JM & de Cabo R (2007). The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion 7 (Suppl.), S34‐40. [DOI] [PubMed] [Google Scholar]
- Navas P, Villalba JM & Lenaz G (2005). Coenzyme Q‐dependent functions of plasma membrane in the aging process. Age (Dordr) 27, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM & Finkel T (2005). SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC‐1α. J Biol Chem 280, 16456–16460. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S & Carruba MO (2005). Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317. [DOI] [PubMed] [Google Scholar]
- Olesen J, Ringholm S, Nielsen MM, Brandt CT, Pedersen JT, Halling JF, Goodyear LJ & Pilegaard H (2013). Role of PGC‐1alpha in exercise training‐ and resveratrol‐induced prevention of age‐associated inflammation. Exp Gerontol 48, 1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne TB, Mendel LB & Ferry EL (1917). The effect of retardation of growth upon the breeding period and duration of life of rats. Science 45, 294–295. [DOI] [PubMed] [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ & Tong Q (2009). Diet and exercise signals regulate SIRT3 and activate AMPK and PGC‐1alpha in skeletal muscle. Aging (Albany NY) 1, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KB & Rizvi SI (2013). Resveratrol up‐regulates the erythrocyte plasma membrane redox system and mitigates oxidation‐induced alterations in erythrocytes during aging in humans. Rejuvenation Res 16, 232–240. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Goldeck D & Derhovanessian E (2014). Inflammation, ageing and chronic disease. Curr Opin Immunol 29, 23–28. [DOI] [PubMed] [Google Scholar]
- Piper MD, Blanc E, Leitao‐Goncalves R, Yang M, He X, Linford NJ, Hoddinott MP, Hopfen C, Soultoukis GA, Niemeyer C, Kerr F, Pletcher SD, Ribeiro C & Partridge L (2014). A holidic medium for Drosophila melanogaster . Nat Methods 11, 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Mair W & Partridge L (2005). Counting the calories: the role of specific nutrients in extension of life span by food restriction. J Gerontol A Biol Sci Med Sci 60, 549–555. [DOI] [PubMed] [Google Scholar]
- Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, Stark PC, Greenberg AS & Roberts SB (2005). A low‐glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care 28, 2939–2941. [DOI] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG & Weindruch R (1999). Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging 20, 157–165. [DOI] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E & Chen D (2010). Calorie restriction reduces oxidative stress by SIRT3‐mediated SOD2 activation. Cell Metab 12, 662–667. [DOI] [PubMed] [Google Scholar]
- Quarles EK, Dai DF, Tocchi A, Basisty N, Gitari L & Rabinovitch PS (2015). Quality control systems in cardiac aging. Ageing Res Rev 23, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SI (2008). Hormesis in aging. Ageing Res Rev 7, 63–78. [DOI] [PubMed] [Google Scholar]
- Rattan SI (2014). Aging is not a disease: implications for intervention. Aging Dis 5, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, Fontana L, Wong WW, Roberts SB, Ravussin E & Group CS (2014). Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr 99, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM & Shulman GI (2006). The role of AMP‐activated protein kinase in mitochondrial biogenesis. J Physiol 574, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M (2014). Unraveling the truth about antioxidants: mitohormesis explains ROS‐induced health benefits. Nat Med 20, 709–711. [DOI] [PubMed] [Google Scholar]
- Ristow M & Schmeisser K (2014). Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 12, 288–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M & Schmeisser S (2011). Extending life span by increasing oxidative stress. Free Radic Biol Med 51, 327–336. [DOI] [PubMed] [Google Scholar]
- Ristow M & Zarse K (2010). How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45, 410–418. [DOI] [PubMed] [Google Scholar]
- Rizvi SI, Kumar D, Chakravarti S & Singh P (2011). Erythrocyte plasma membrane redox system may determine maximum life span. Med Hypotheses 76, 547–549. [DOI] [PubMed] [Google Scholar]
- Rizza W, Veronese N & Fontana L (2014). What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev 13, 38–45. [DOI] [PubMed] [Google Scholar]
- Robert L (2013). Calorie‐restriction and longevity. End of a dream, at least for primates? European Geriatric Medicine 4, 129–132. [Google Scholar]
- Rodriguez‐Bies E, Navas P & Lopez‐Lluch G (2015). Age‐dependent effect of every‐other‐day feeding and aerobic exercise in ubiquinone levels and related antioxidant activities in mice muscle. J Gerontol A Biol Sci Med Sci 70, 33–43. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Bies E, Santa‐Cruz Calvo S, Fontan‐Lozano A, Pena Amaro J, Berral de la Rosa FJ, Carrion AM, Navas P & Lopez‐Lluch G (2010). Muscle physiology changes induced by every other day feeding and endurance exercise in mice: effects on physical performance. PLoS One 5, e13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolo AP & Palmeira CM (2006). Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol 212, 167–178. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lesnikov V, Lesnikov M, Ingram DK & Lane MA (2001). Dietary caloric restriction prevents the age‐related decline in plasma melatonin levels of rhesus monkeys. J Clin Endocrinol Metab 86, 3292–3295. [DOI] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA & Ingram DK (2004). Aging in rhesus monkeys: relevance to human health interventions. Science 305, 1423–1426. [DOI] [PubMed] [Google Scholar]
- Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E & Franceschi C (2006). Inflamm‐aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des 12, 3161–3171. [DOI] [PubMed] [Google Scholar]
- Sczelecki S, Besse‐Patin A, Abboud A, Kleiner S, Laznik‐Bogoslavski D, Wrann CD, Ruas JL, Haibe‐Kains B & Estall JL (2014). Loss of Pgc‐1α expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. Am J Physiol Endocrinol Metab 306, E157–E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, McCurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K & Olefsky JM (2011). Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest 121, 4281–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M & Ristow M (2007). Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6, 280–293. [DOI] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al‐Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L & Withers DJ (2008). Evidence for lifespan extension and delayed age‐related biomarkers in insulin receptor substrate 1 null mice. FASEB J 22, 807–818. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al‐Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D & Withers DJ (2009). Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert CD, Graf M, Janson G, Kuhn A & Soling HD (1974). Formation of free acetate by isolated perfused livers from normal, starved and diabetic rats. Biochem Biophys Res Commun 57, 901–909. [DOI] [PubMed] [Google Scholar]
- Sharp ZD (2011). Aging and TOR: interwoven in the fabric of life. Cell Mol Life Sci 68, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Huang JY, Ho LT & Verdin E (2010). Acetate metabolism and aging: An emerging connection. Mech Ageing Dev 131, 511–516. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen‐Schimke J, Raghavakaimal S & Nair KS (2005). Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102, 5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA (2005). Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126, 987–1002. [DOI] [PubMed] [Google Scholar]
- Slack C, Foley A & Partridge L (2012). Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila . PLoS One 7, e47699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS & Weindruch R (1996). Oxidative stress, caloric restriction, and aging. Science 273, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana R, Pawelec G & Tarazona R (2006). Aging and innate immunity. Immunity 24, 491–494. [DOI] [PubMed] [Google Scholar]
- Solon‐Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG & Simpson SJ (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum‐fed mice. Cell Metab 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, Lee HC & Lee BW (2015). Metformin alleviates hepatosteatosis by restoring SIRT1‐mediated autophagy induction via an AMP‐activated protein kinase‐independent pathway. Autophagy 11, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR & Mitchell SE (2011). Caloric restriction. Mol Aspects Med 32, 159–221. [DOI] [PubMed] [Google Scholar]
- Stankovic M, Mladenovic D, Ninkovic M, Vucevic D, Tomasevic T & Radosavljevic T (2013). Effects of caloric restriction on oxidative stress parameters. Gen Physiol Biophys 32, 277–283. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A & Antebi A (2003). The endocrine regulation of aging by insulin‐like signals. Science 299, 1346–1351. [DOI] [PubMed] [Google Scholar]
- Testa G, Biasi F, Poli G & Chiarpotto E (2014). Calorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevity. Curr Pharm Des 20, 2950–2977. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen‐Kornips E, Hesselink MK, Kunz I, Schrauwen‐Hinderling VB, Blaak EE, Auwerx J & Schrauwen P (2011). Calorie restriction‐like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA & Guarente L (2001). Increased dosage of a sir‐2 gene extends lifespan in Caenorhabditis elegans . Nature 410, 227–230. [DOI] [PubMed] [Google Scholar]
- To K, Yamaza H, Komatsu T, Hayashida T, Hayashi H, Toyama H, Chiba T, Higami Y & Shimokawa I (2007). Down‐regulation of AMP‐activated protein kinase by calorie restriction in rat liver. Exp Gerontol 42, 1063–1071. [DOI] [PubMed] [Google Scholar]
- Tohyama D & Yamaguchi A (2010). A critical role of SNF1A/dAMPKalpha (Drosophila AMP‐activated protein kinase alpha) in muscle on longevity and stress resistance in Drosophila melanogaster . Biochem Biophys Res Commun 394, 112–118. [DOI] [PubMed] [Google Scholar]
- Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs‐Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M & Vellai T (2008). Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans . Autophagy 4, 330–338. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT & Larsson NG (2004). Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423. [DOI] [PubMed] [Google Scholar]
- Tung BT, Rodriguez‐Bies E, Talero E, Gamero‐Estevez E, Motilva V, Navas P & Lopez‐Lluch G (2015. a). Anti‐inflammatory effect of resveratrol in old mice liver. Exp Gerontol 64, 1–7. [DOI] [PubMed] [Google Scholar]
- Tung BT, Rodriguez‐Bies E, Thanh HN, Le‐Thi‐Thu H, Navas P, Sanchez VM & Lopez‐Lluch G (2015. b). Organ and tissue‐dependent effect of resveratrol and exercise on antioxidant defenses of old mice. Aging Clin Exp Res 27, 775–783. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado‐Fernandez C, Csiszar A & de Cabo R (2008). Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba JM & Navas P (2000). Plasma membrane redox system in the control of stress‐induced apoptosis. Antioxid Redox Signal 2, 213–230. [DOI] [PubMed] [Google Scholar]
- Wahlin‐Larsson B, Carnac G & Kadi F (2014). The influence of systemic inflammation on skeletal muscle in physically active elderly women. Age (Dordr) 36, 9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S & Mattson MP (2003). Intermittent fasting and dietary supplementation with 2‐deoxy‐d‐glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J 17, 1133–1134. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S & Mattson MP (2004). Dietary supplementation with 2‐deoxy‐d‐glucose improves cardiovascular and neuroendocrine stress adaptation in rats. Am J Physiol Heart Circ Physiol 287, H1186‐1193. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liang B, Shirwany NA & Zou MH (2011). 2‐Deoxy‐d‐glucose treatment of endothelial cells induces autophagy by reactive oxygen species‐mediated activation of the AMP‐activated protein kinase. PLoS One 6, e17234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber TA & Reichert AS (2010). Impaired quality control of mitochondria: aging from a new perspective. Exp Gerontol 45, 503–511. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger‐May K, Schechtman KB, Klein S, Holloszy JO & Washington University School of Medicine CG (2006). Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B & Curb JD (2008). FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA 105, 13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M & Sinclair D (2004). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R & Hall MN (2006). TOR signaling in growth and metabolism. Cell 124, 471–484. [DOI] [PubMed] [Google Scholar]
- Yan L, Gao S, Ho D, Park M, Ge H, Wang C, Tian Y, Lai L, De Lorenzo MS, Vatner DE & Vatner SF (2013). Calorie restriction can reverse, as well as prevent, aging cardiomyopathy. Age (Dordr) 35, 2177–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang NC, Song TY, Chen MY & Hu ML (2011). Effects of 2‐deoxyglucose and dehydroepiandrosterone on intracellular NAD+ level, SIRT1 activity and replicative lifespan of human Hs68 cells. Biogerontology 12, 527–536. [DOI] [PubMed] [Google Scholar]
- Yeo SH, Noh JR, Kim YH, Gang GT, Kim SW, Kim KS, Hwang JH, Shong M & Lee CH (2013). Increased vulnerability to beta‐cell destruction and diabetes in mice lacking NAD(P)H:quinone oxidoreductase 1. Toxicol Lett 219, 35–41. [DOI] [PubMed] [Google Scholar]
- Yu BP (2005). Membrane alteration as a basis of aging and the protective effects of calorie restriction. Mech Ageing Dev 126, 1003–1010. [DOI] [PubMed] [Google Scholar]
- Zainal TA, Oberley TD, Allison DB, Szweda LI & Weindruch R (2000). Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB J 14, 1825–1836. [DOI] [PubMed] [Google Scholar]


