Abstract
Although caloric restriction (CR) could delay biologic aging in humans, it is unclear if this would occur at the cost of significant bone loss. We evaluated the effect of prolonged CR on bone metabolism and bone mineral density (BMD) in healthy younger adults. Two-hundred eighteen non-obese (BMI:25.1±1.7 kg/m2), younger (age:37.9±7.2 yr.) adults were randomly assigned to 25% CR (CR group;n=143) or ad libitum (AL group;n=75) for 2 years. Main outcomes were BMD and markers of bone turnover. Other outcomes included body composition, bone-active hormones, nutrient intake, and physical activity. Body weight (−7.5±0.4 vs. 0.1±0.5 kg), fat mass (−5.3±0.3 vs. 0.4±0.4 kg), and fat-free mass (−2.2±0.2 vs. −0.2±0.2 kg) decreased in the CR group compared with AL (all between group p<0.001). Compared with AL, the CR group had greater changes in BMD at 24 months: lumbar spine (−0.013±0.003 vs. 0.007±0.004 g/cm2; p<0.001), total hip (−0.017±0.002 vs. 0.001±0.003 g/cm2; p<0.001), and femoral neck (−0.015±0.003 vs. −0.005±0.004 g/cm2; p=0.03). Changes in bone markers were greater at 12 months for C-telopeptide (0.098±0.012 vs. 0.025±0.015 μg/L; p<0.001), tartrate-resistant acid phosphatase (0.4±0.1 vs. 0.2±0.1 U/L; p=0.004), and bone-specific alkaline phosphatase (BSAP) (−1.4±0.4 vs. −0.3±0.5 U/L; p=0.047) but not procollagen type 1 N-propeptide; at 24 months only BSAP differed between groups (−1.5±0.4 vs. 0.9±0.6 U/L; p=0.001). The CR group had larger increases in 25-hydroxyvitamin D, cortisol, and adiponectin and decreases in leptin and insulin compared with AL. However, parathyroid hormone and IGF-1 levels did not differ between groups. The CR group also had lower levels of physical activity. Multiple regression revealed that body composition, hormones, nutrients, and physical activity changes explained ~31% of the variance in BMD and bone marker changes in the CR group. Therefore, bone loss at clinically important sites of osteoporotic fractures represents a potential limitation of prolonged CR for extending lifespan. Further long-term studies are needed to determine if CR-induced bone loss in healthy adults contributes to fracture risk and if bone loss can be prevented with exercise.
Keywords: Nutrition, bone-fat interactions, bone-muscle interactions, DXA, Fracture prevention
Introduction
Caloric restriction (CR) is the most promising intervention for extending healthy lifespan of humans.(1) Data from CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) phase 1 suggested that CR is feasible and could confer ample health benefits.(2-5) However, there is considerable evidence in the literature linking weight loss with increased bone turnover and reductions in bone mineral density (BMD) in older adults.(6-12) Conversely, weight loss has not been shown to consistently increase bone turnover and decrease BMD in younger adults.(13-16) Moreover, findings of bone loss with weight loss mostly come from relatively short-term weight loss studies aiming to reduce body fat in obese adults.(8-12) There has been little or no scientific study of the possibility that CR initiated with the intent of slowing the aging process in healthy adults could occur at the cost of bone loss. In CALERIE phase 1, one year of CR in older adults (age~57 yrs.; body mass index [BMI]~27 kg/m2) increased bone turnover and decreased BMD by ~3% at the hip and spine,(17) whereas 6 months of CR in younger adults (age~37 yrs.; BMI~26 kg/m2) increased bone turnover but did not change hip BMD.(14) The long-term effects of CR (>1 year) on bone metabolism at the clinically relevant sites of osteoporotic fractures in non-obese younger adults are unknown. Furthermore, the underlying mechanisms behind any changes in BMD need to be elucidated. Such studies could yield important information as to whether CR has adverse effects on bone health and, if so, identify interventions to reduce effects of weight loss on bone.
The purpose of the present study, therefore, was to evaluate the effects of two-year CR on bone metabolism and BMD in non-obese younger adults enrolled in a 24-month, randomized controlled trial (RCT). The data reported here were obtained as secondary outcomes of CALERIE phase 2, which has the overall aim of examining the effects of sustained ~25% CR on slowing aging in non-obese humans.(18)
Methods
Overview
CALERIE phase 2 was a two-year, multicenter RCT that recruited healthy volunteers to receive an intervention designed to either reduce energy intake by ~25% (CR group) or to maintain intake on an ad libitum basis (AL group). The study was approved by the institutional review boards at Pennington Biomedical Research Center, Washington University, Tufts University, and Duke University. Details of the protocol have been reported.(19-21) Study oversight was provided by a Data and Safety Monitoring Board.
Participants
Healthy volunteers were recruited and each provided written informed consent. Men were between 20 and 50 years of age and women between 21 and 47 years of age and were required to be normal weight or slightly overweight (22.0 ≤ BMI < 28.0 kg/m2) at the screening visit. Participants underwent a comprehensive screening procedure to identify volunteers who were healthy enough to participate in a two-year RCT of CR and adhere to the rigors of the study. Details regarding the screening/recruitment process and eligibility can be found elsewhere.(19,21)
Study Design
Participants were randomized to 25% caloric restriction (CR) or ad libitum control (AL) in a 2:1 ratio. Randomization was stratified for site, sex and BMI.
The CR intervention goal was 25% reduction in energy intake prescribed based on the energy requirement to maintain baseline weight as determined by doubly-labeled water.(22) The CR intervention was driven by a mathematical model derived from CALERIE Phase 1 data that predicted the anticipated trajectory of body weight changes for each participant.(23) This trajectory predicted weight loss in the first 12 months, followed by weight maintenance. A weight loss graph showing these targeted trajectories was used as a tool to facilitate adherence.(23) Nutritional/behavioral guidance was customized to decrease the degree to which participants’ weight trajectories differed from their targets.(20) Food provision was used to educate participants on portion size and diet changes. The behavioral component included a structured curriculum and manual driven counseling in group and individual sessions. Further details about the CR intervention have been reported.(20) No specific diet composition was mandated. Rather, the CR intervention employed an algorithmic approach that combined specific nutritional and behavioral guidance so that each CR participant could maintain the prescribed level of CR. AL participants were advised to continue their current diets on an AL basis. No specific level of physical activity was recommended for either group. All participants received a multivitamin supplement (1000 IU vitamin D/d, Nature-Made Multi Complete, Mission Hills, CA) and calcium supplement (1000 mg/d, Douglas laboratories, Pittsburgh, PA).
Outcome Measures
Outcomes for this report included changes in BMD and bone turnover markers. Other outcomes included body composition, bone-active hormones, dietary/nutritional intake, physical activity, and strength. All outcomes were assessed at baseline, 12-months, and 24-months. Bone markers were also assessed at 6 months. Personnel who conducted the assessments were not aware of the group assignments.
Body weight, body composition, and BMD
Body weight was measured after participants fasted for ~12 hours. Fat mass (FM), fat-free mass (FFM), and BMD of the whole body and at the lumbar spine, total hip, and distal radius were measured by dual-energy x-ray absorptiometry (DXA) using Hologic 4500A, Delphi W or Discovery A scanners. Scans were analyzed at University of California San Francisco, also responsible for centralized quality control. Machine performance was monitored with baseline and longitudinal phantom measurements.
Biomarkers of bone turnover and hormones
Venous blood samples were obtained after fasting overnight and analyzed in a central laboratory. Enzyme-linked immmunosorbent assay (ELISA) was used to measure C-terminal telopeptide of type I collagen (CTX) (Nordic Bioscience, Herlev, Denmark) and tartrate-resistant acid phosphatase isoform-5b (TRAP5b) (Quidel, San Diego, CA) as markers of bone resorption and bone-specific alkaline phosphatase (BAP) (MicroVue, San Diego, CA) as marker of bone formation. Radiommunoassay was used to measure N-terminal propeptide of type I procollagen (PINP) (Orion, Espoo, Finland) as an additional marker of bone formation. ELISA was used to measure high-molecular weight adiponectin (Alpco, Salem, NH) and Insulin-like growth factor-1 (Quantikine, Minneapolis, MN). Chemiluminescent immunoassays were used to measure parathyroid hormone (PTH) (Roche Elecsys, Indianapolis, IN) and cortisol (ADVIA Centaur, Malvem, PA). Multiplex immunoassay was used to measure leptin (Millipore, Billerica, MA). Liquid chromatography-tandem mass spectrometry was used to measure 25-hydroxyvitamin D (25OHD) (Mayo Laboratories, Rochester, MN). CV's for these measurements were 3.4-8.8%.
Dietary intake, physical activity, and strength
Dietary intakes were determined using 6-day food diaries which were analyzed with Nutrition Data System for Research (Minneapolis, MN). Physical activity was determined using the Stanford 7-day Physical Activity Recall (PAR).(24) Grip strength was assessed using Jamar dynamometer (Asimow, Los Angeles, CA).
Statistical Analyses
The same statistical methodologies used in the parent RCT were applied.(18) Briefly, intention-to-treat (ITT) analyses were performed by including all available observations in the analysis. Baseline characteristics were compared using Wilcoxon-Mann-Whitney test and Fisher exact test. Repeated measures analysis of covariance(25,26) was applied with change from baseline as the dependent variable, and treatment, time, and the treatment × time interaction as independent variables; site, sex, BMI, and baseline value were included as covariates. Hypotheses of specific interest were tested by defining contrasts among the regression parameters; the predicted mean ± standard error (se) are the adjusted values from this model. Consistency between sexes was evaluated by adding and testing interaction terms in this model. Type-I error was controlled using a gatekeeping strategy.(27) The treatment × time interaction term was tested first. If significant, then following standard statistical practice, between-group differences at each time point were tested at α=.05. If not, the treatment main effect was tested next. If significant, then between-group differences at each time point were tested at α=.05. Otherwise a Bonferroni correction was applied at each time point, with the p-values adjusted by multiplying the nominal p-value by the number of tests (truncated at 1.0).(28) Linear regression was used at baseline to generate equations for predicting BMD from body weight, age, and sex. The equation was applied at the 24-month time-point, and the difference between observed and predicted BMD (i.e., the residual) interpreted as the effect of the CR intervention beyond BMD loss expected due to weight loss. Pearson's correlation was used to examine relationships in the CR group among changes in variables and changes in BMD and bone markers; stepwise multiple regression was used to identify which variables were independent contributors to changes in BMD and bone markers. All statistical tests were two-tailed; p<0.05 was considered statistically significant. Data analysis was generated using SAS version 9.2.
Results
Study population
The CONSORT diagram has been reported previously (Appendix Figure 1).(18) Briefly, 238 individuals deemed eligible, underwent baseline assessments. Ten were subsequently determined to be ineligible and 8 withdrew, so that 220 individuals were randomized. Two individuals in the CR group dropped out prior to starting the intervention resulting in an ITT cohort of 218 participants (75 in the AL group 143 in the CR group).Thirty participants (4 in the AL group, 26 in the CR group) discontinued the intervention due to personal and other reasons; however, any data provided were included in the ITT analyses.
The AL group and CR group did not differ on baseline demographic and clinical characteristics, including age, sex, ethnicity, marital status, education, weight, BMI, and BMD (Table 1).
Table 1.
Baseline Characteristics of Study Participants*
| AL N=75 | CR N=143 | P value† | |
|---|---|---|---|
| Age, y | 37.9 ± 6.9 | 38.0 ± 7.2 | 0.89 |
| Sex, no (%) | |||
| Male | 22 (29.3) | 44 (30.8) | 0.88 |
| Female | 53 (70.7) | 99 (69.2) | |
| Race, no (%) | |||
| Black or African American | 11 (14.7) | 15 (10.5) | 0.62 |
| White | 57 (76.0) | 111 (77.6) | |
| Other | 7 (9.3) | 17 (11.9) | |
| Ethnicity, no (%) | |||
| Hispanic or Latino | 4 (5.3) | 3 (2.1) | 0.27 |
| Not Hispanic or Latino | 71 (94.7) | 138 (96.5) | |
| Unknown | 0 (0.0) | 2 (1.4) | |
| Marital Status, no (%) | |||
| Married | 44 (58.7) | 86 (60.1) | 0.81 |
| Divorced | 6 (8.0) | 9 (6.3) | |
| Single | 24 (32.0) | 47 (32.9) | |
| Widowed | 0 (0.0) | 1 (0.7) | |
| Separated | 1 (1.3) | 0 ( 0.0) | |
| Education, no (%) | |||
| Elementary | 0 (0.0) | 0 ( 0.0) | 0.10 |
| 9-12th grade | 6 (8.0) | 2 (1.4) | |
| College | 46 (61.3) | 88 (61.5) | |
| Non doctoral graduate degree | 17 (22.7) | 37 (25.9) | |
| Doctoral degree | 6 (8.0) | 16 (11.2) | |
| Height, m | 168.4 ± 8.3 | 168.9 ± 8.6 | 0.91 |
| Weight, kg | 71.3 ± 8.6 | 71.8 ± 9.2 | 0.96 |
| Body mass index, kg/m2 | 25.1 ± 1.6 | 25.1 ± 1.7 | 0.90 |
| Bone mineral density, z-score | |||
| Lumbar spine | −0.5 ± 1.5 | −0.2 ± 1.0 | 0.26 |
| Total hip | −0.0 ± 0.8 | −0.0 ± 0.8 | 0.73 |
| Femoral neck | −0.2 ± 0.9 | −0.2 ± 0.9 | 0.90 |
| Oral contraceptive use in women, no (%) | |||
| Yes | 10 (18.9) | 22 (22.2) | 0.68 |
| No | 43 (81.1) | 77 (77.8) |
Values are given as mean ± SD or number (percentage) of participants unless otherwise specified.
P-values as calculated by Wilcoxon test for continuous and ordinal values and Fisher exact test for categorical values.
CR and body weight
Significant CR was achieved in the CR group throughout the 24-month study, reaching 19.5±0.8% over the first 6 months, 10.8±0.7% over the second 6 months, and 8.3±0.8% over the remaining 12 months. Accordingly, there were significant decreases in body weight in the CR group, which were significantly different than those observed in the AL group (Table 2).
Table 2.
Effect of Caloric Restriction on Body Composition and Bone Mineral Density*
| AL N=75 | CR N=143 | P value† | |
|---|---|---|---|
| Body composition | |||
| Weight (kg) | |||
| Baseline | 71.5 ± 1.0 | 72.0 ± 0.8 | 0.93 |
| Change at 6 months | −0.8 ± 0.3 | −7.3 ± 0.2 | <0.001 |
| Change at 12 months | −0.7 ± 0.4 | −8.4 ± 0.3 | <0.001 |
| Change at 24 months | 0.1 ± 0.5 | −7.5 ± 0.4 | <0.001 |
| Fat mass (kg) | |||
| Baseline | 23.8± 0.6 | 23.5 ± 0.4 | 0.61 |
| Change at 12 months | −0.3 ± 0.3 | −6.1 ± 0.2 | <0.001 |
| Change at 24 months | 0.4 ± 0.4 | −5.3 ± 0.3 | <0.001 |
| Fat-free mass (kg) | |||
| Baseline | 47.6 ± 1.0 | 48.5 ± 0.8 | 0.48 |
| Change at 12 months | −0.3 ± 0.2 | −2.2 ± 0.1 | <0.001 |
| Change at 24 months | −0.2 ± 0.2 | −2.2 ± 0.2 | <0.001 |
| Bone mineral density | |||
| Lumbar spine (g/cm2) | |||
| Baseline | 1.043 ± 0.014 | 1.042 ± 0.010 | 0.85 |
| Change at 12 months | 0.006 ± 0.003 | −0.011 ± 0.002 | <0.001 |
| Change at 24 months | 0.007 ± 0.004 | −0.013 ± 0.003 | <0.001 |
| Total Hip (g/cm2) | |||
| Baseline | 0.982 ± 0.013 | 0.984 ± 0.009 | 0.84 |
| Change at 12 months | 0.002 ± 0.002 | −0.012 ± 0.002 | <0.001 |
| Change at 24 months | 0.001 ± 0.003 | −0.017 ± 0.002 | <0.001 |
| Femoral neck (g/cm2) | |||
| Baseline | 0.842 ± 0.013 | 0.839 ± 0.009 | 0.95 |
| Change at 12 months | 0.000 ± 0.003 | −0.011 ± 0.002 | 0.002 |
| Change at 24 months | −0.005 ± 0.004 | −0.015 ± 0.003 | 0.03 |
| Intertrochanter (g/cm2) | |||
| Baseline | 1.158 ± 0.016 | 1.167 ± 0.011 | 0.49 |
| Change at 12 months | 0.002 ± 0.003 | −0.015 ± 0.002 | <0.001 |
| Change at 24 Months | 0.001 ± 0.004 | −0.021 ± 0.003 | <0.001 |
| Trochanter (g/cm2) | |||
| Baseline | 0.742 ± 0.013 | 0.737 ± 0.008 | 0.95 |
| Change at 12 Months | 0.003 ± 0.002 | −0.006 ± 0.002 | 0.002 |
| Change at 24 Months | 0.002 ± 0.003 | −0.009 ± 0.002 | 0.002 |
| One third radius (g/cm2) | |||
| Baseline | 0.726 ± 0.009 | 0.728 ± 0.006 | 0.49 |
| Change at 12 months | 0.007 ± 0.002 | 0.011 ± 0.002 | 0.32 |
| Change at 24 months | 0.008 ± 0.002 | 0.010 ± 0.002 | 0.64 |
| Whole body (g/cm2) | |||
| Baseline | 1.162 ± 0.011 | 1.168 ± 0.008 | 0.68 |
| Change at 12 months | 0.011 ± 0.004 | 0.019 ± 0.003 | 0.07 |
| Change at 24 months | 0.011 ± 0.004 | 0.012 ± 0.003 | 0.86 |
Baseline values are the observed mean (SE); change scores are the least-squares adjusted means (SE) from the repeated measures analysis.
P values, from the Wilcoxon test for comparison of the baseline value, and the repeated measures of analyses of covariance for comparisons of change values at time points. All P values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
Body composition and BMD
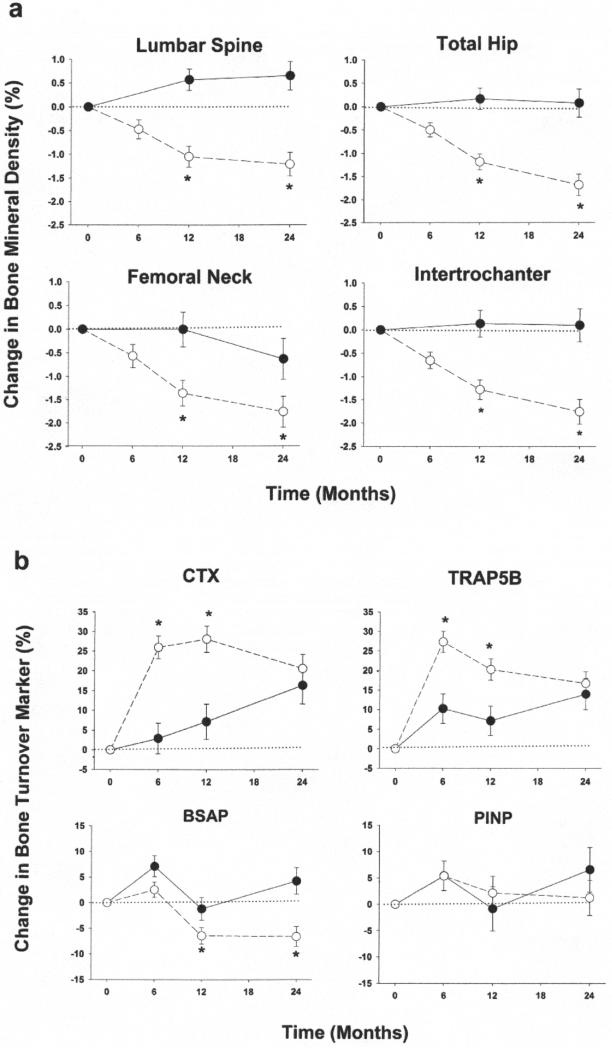
The CR group relative to AL also had significant reductions in FM and FFM at 12 months, with maintenance of lower FM and FFM at 24 months. The changes in body weight and composition in the CR group were accompanied by significant decreases in BMD relative to the AL group, at the lumbar spine, total hip, femoral neck and other regional hip sites at 12 months and 24 months (Table 2, Figure 1a).
Figure 1.
Mean percent changes ± SEM in bone mineral density (panel a) and biochemical markers of bone turnover (panel b) in non-obese younger adults randomized to ad libitum group (●) or caloric restriction (○). P value significantly different (<0.05) from ad libitum value. Abbreviations: CTX = C-terminal telopeptide of type I collagen; BAP = bone specific alkaline phosphatase; TRAP5b = tartrate-resistant acid phosphatase isoform 5b; PINP = intact N-terminal propeptide of type I
The treatment effects on BMD did not differ between males and females (sex × treatment interaction p>0.05). There were no treatment effects on BMD at the distal radius or whole body.
Bone turnover and hormones
Compared with the AL group, serum CTX and TRAP5B concentrations significantly increased as early as 6 months and remained elevated at 12 months in the CR group (Table 3, Figure 1b). Conversely, serum BAP and PINP concentrations did not change at 6 months; serum levels of BAP significantly decreased at 12 months and remained suppressed at 24 months in the CR group compared with the AL group. Serum 25OHD concentrations significantly increased in the CR group compared with AL at 12 months and 24 months, unaccompanied by changes in PTH. The changes in 25OHD concentrations in the CR group were inversely correlated with the changes in body weight (r=−0.21, p=0.02) and FM at 12 months (r=−0.22, p=0.01) although not at 24 months (both p>0.05). Serum cortisol levels slightly but significantly increased at 12 months but not at 24 months in the CR group compared with AL as previously reported.(29) Serum levels of adiponectin significantly increased and leptin and insulin significantly decreased at 12 months and 24 months in the CR group compared with AL. Serum IGF-1 did not significantly change as previously reported.(29)
Table 3.
Effect of Caloric Restriction on Bone Markers and Hormones*
| AL N=75 | CR N=143 | P value† | |
|---|---|---|---|
| Biochemical markers of bone turnover | |||
| CTX (μg/L) | |||
| Baseline | 0.358 ± 0.015 | 0.344 ± 0.013 | 0.26 |
| Change at 6 months | 0.010 ± 0.013 | 0.090 ± 0.010 | <0.001 |
| Change at 12 months | 0.025 ± 0.015 | 0.098 ± 0.012 | <0.001 |
| Change at 24 months | 0.057 ± 0.016 | 0.071 ± 0.013 | 0.47 |
| TRAP5B (U/L) | |||
| Baseline | 2.4 ± 0.1 | 2.1 ± 0.1 | 0.02 |
| Change at 6 months | 0.2 ± 0.1 | 0.6 ± 0.1 | <0.001 |
| Change at 12 months | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.004 |
| Change at 24 months | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.58 |
| BSAP (U/L) | |||
| Baseline | 22.9 ± 0.9 | 21.7 ± 0.6 | 0.25 |
| Change at 6 months | 1.6 ± 0.5 | 0.6 ± 0.3 | 0.06 |
| Change at 12 months | −0.3 ± 0.5 | −1.4 ± 0.4 | 0.047 |
| Change at 24 months | 0.9 ± 0.6 | −1.5 ± 0.4 | 0.001 |
| P1NP (μg/L) | |||
| Baseline | 43.3 ± 1.9 | 39.5 ± 1.3 | 0.08 |
| Change at 6 months | 2.0 ± 1.5 | 2.2 ± 1.1 | 1.00 |
| Change at 12 months | −0.3 ± 1.7 | 0.9 ± 1.3 | 1.00 |
| Change at 24 months | 2.7 ± 1.8 | 0.5 ± 1.4 | 0.93 |
| Bone-active hormones | |||
| 25-hyroxyvitamin D (ng/mL) | |||
| Baseline | 29.3 ± 0.9 | 29.6 ± 0.6 | 0.74 |
| Change at 12 months | 0.3 ± 0.8 | 2.9 ± 0.5 | 0.004 |
| Change at 24 months | −0.3 ± 0.9 | 1.9 ± 0.7 | 0.047 |
| Parathyroid hormone (pg/mL) | |||
| Baseline | 39.0 ± 1.4 | 41.5 ± 1.0 | 0.12 |
| Change at 12 months | 0.0 ± 1.2 | −2.0 ± 0.9 | 0.34 |
| Change at 24 months | 0.4 ± 1.3 | −0.4 ± 1.0 | 1.00 |
| Adiponectin (ng/mL) | |||
| Baseline | 4856 ± 376 | 4807 ± 240 | 0.78 |
| Change at 12 months | −435 ± 187 | 875 ± 142 | <0.001 |
| change at 24 months | −1011 ± 195 | 314 ± 152 | <0.001 |
| Leptin (pg/mL) | |||
| Baseline | 17717 ± 1650 | 16950 ± 1150 | 0.73 |
| Change at 12 months | −2812 ± 749 | −11041 ± 583 | <0.001 |
| Change at 24 months | −954 ± 966 | −9660 ± 749 | <0.001 |
| Insulin (uIU/mL) | |||
| Baseline | 5.8 ± 0.3 | 5.4 ± 0.2 | 0.27 |
| Change at 12 months | −0.1 ± 0.2 | −1.6 ±0.2 | <0.001 |
| Change at 24 months | 0.1 ± 0.2 | −1.7 ± 0.2 | <0.001 |
Abbreviations: CTX = C-telopeptide of type I collagen; BSAP = bone specific alkaline phosphatase; TRAP5b = tartrate-resistant acid phosphatase; PINP = intact N-terminal propeptide of type I procollagen
Baseline values are the observed mean (SE); change scores are the least-squares adjusted means (SE) from the repeated measures analysis.
P values, from the Wilcoxon test for comparison of the baseline value, and the repeated measures of analyses of covariance for comparisons of change values at time points. All P values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
Energy intake, physical activity, and strength
Food records showed that the CR group restricted their energy intake while the AL group maintained their intake (Table 4). Only fat intake significantly decreased among the macronutrients while micronutrients did not change or increased in the CR group compared with AL. PAR indicated that total self-reported physical activity significantly decreased in the CR group compared with AL. Changes in grip strength did not differ significantly.
Table 4.
Effect of Caloric Restriction on Nutrient Intake, Physical Activity, and Strength*
| AL N=75 | CR N=143 | P value† | |
|---|---|---|---|
| Nutrient intake‡ | |||
| Energy (kcal/d) | |||
| Baseline | 2045.3 ± 55.50 | 2126.3 ± 46.7 | 0.46 |
| Change at 12 months | −83.0 ± 38.6 | −279.5 ± 29,3 | <0.001 |
| Change at 24 months | −121.1 ± 42.7 | −216.3 ± 33.1 | 0.07 |
| Protein (g/d) | |||
| Baseline | 86.6 ± 2.8 | 86.5 ± 1.9 | 0.97 |
| Change at 12 months | 0.13 ± 2.1 | −2.4 ± 1.6 | 0.6 |
| Change at 24 months | −4.9 ± 2.4 | −2.6 ± 1.9 | 0.85 |
| Fat (g/d) | |||
| Baseline | 80.8 ± 2.6 | 81.3 ± 2.1 | 0.63 |
| Change at 12 months | −3.2 ± 2.1 | −20.2 ± 1.7 | <.001 |
| Change at 24 months | −4.4 ± 2.3 | −15.3 ± 1.8 | <0.001 |
| Carbohydrates (g/d) | |||
| Baseline | 234.9 ± 7.1 | 254.3 ± 6.3 | 0.09 |
| Change at 12 months | −12.0 ± 5.8 | −9.8 ± 4.5 | 1.00 |
| Change at 24 months | −15.1 ± 6.1 | −9.5 ± 4.7 | 0.90 |
| Calcium (mg/d) | |||
| Baseline | 917.6 ± 40.7 | 911.2 ± 25.0 | 0.82 |
| Change at 12 months | −24.8 ± 30.1 | 48.3 ± 22.8 | 0.09 |
| Change at 24 months | −28.6 ± 32.1 | −4.6 ± 24.6 | 1.00 |
| Vitamin D (μg/d) | |||
| Baseline | 5.1 ± 0.4 | 4.7 ± 0.2 | 0.73 |
| Change at 12 months | 0.1 ± 0.4 | 0.5 ± 0.3 | 0.79 |
| Change at 24 months | 0.3 ± 0.4 | −0.1 ± 0.3 | 0.81 |
| Vitamin A, (IU/d) | |||
| Baseline | 6951.9 ± 478.5 | 7718.4 ± 384.7 | 0.23 |
| Change at 12 months | 901.9 ± 822.9 | 3415.9 ± 618.5 | 0.01 |
| Change at 24 months | 1446.1 ± 827.5 | 3174.4 ± 631.1 | 0.09 |
| Vitamin K (μg/d) | |||
| Baseline | 127.6 ± 12.8 | 130.8 ± 6.2 | 0.31 |
| Change at 12 months | 22.6 ± 12.2 | 32.8 ± 9.2 | 0.49 |
| Change at 24 months | −0.7 ± 15.8 | 55.2 ± 12.0 | 0.01 |
| Magnesium (mg) | |||
| Baseline | 299.3 ± 11.8 | 311.5 ± 7.6 | 0.27 |
| Change at 12 months | 1.3 ± 9.3 | 39.2 ± 7.1 | 0.001 |
| Change at 24 months | −4.4 ± 10.2 | 43.6 ± 7.9 | <0.001 |
| Phosphorus (mg) | |||
| Baseline | 1344.1 ± 43.9 | 1349.6 ± 28.1 | 0.99 |
| Change at 12 months | −27.5 ± 32.3 | −29.7 ± 24.5 | 0.28 |
| Change at 24 months | −67.0 ± 35.2 | 7.4 ± 27.0 | 0.16 |
| Physical activity and strength | |||
| Total physical activity (kcal/d)§ | |||
| Baseline | 1532.1 ± 31.4 | 1572.1 ± 23.2 | 0.38 |
| Change at 12 months | −7.9 ± 20.4 | −102.4 ± 15.5 | <0.001 |
| Change at 24 months | −16.5 ± 23.1 | −83.8 ± 17.9 | 0.02 |
| Grip strength (kg) | |||
| Baseline | 33.8 ± 1.2 | 33.8 ± 0.9 | 0.92 |
| Change at 12 months | 1.8 ± 0.9 | 0.6 ± 0.7 | 0.54 |
| Change at 24 months | 0.8 ± 0.6 | 0.8 ± 0.5 | 1.00 |
Baseline values are the observed mean (SE); change scores are the least-squares adjusted means (SE) from the repeated measures analysis.
P values, from the Wilcoxon test for comparison of the baseline value, and the repeated measures of analyses of covariance for comparisons of change values at time points. All P values reflect Bonferroni corrections, truncated at 1.0, as appropriate (see text).
Does not include multivitamin and calcium supplements as provided in the study.
Self-reported using the Standard 7-day Physical Activity Recall(24)
Variables associated with BMD and bone marker changes in the CR group
Results of bivariate correlation are provided in Appendix Table 1 while results of multiple regression analyses are provided in Table 5. Stepwise multiple regression revealed that change in FFM was the only factor associated with change in total hip BMD at 24 months in the CR group. Different combinations of changes in body composition, bone-active hormones, nutrition intake, and physical activity explained 10%-31% of the variance in changes in spine BMD and bone markers at 12 months and 24 months in the CR group.
Table 5.
Factors Associated with Changes in BMD and Bone Turnover Markers in the Caloric Restriction Group*
| Final model of variables affecting Δ spine BMD | ||
|---|---|---|
| at 12 months | ||
| β | p-value | |
| Δ cortisol | 0.248 | 0.004 |
| Δ 25OHD | 0.243 | 0.01 |
| Δ total energy intake | 0.206 | 0.02 |
| Δ FM | 0.210 | 0.02 |
| Δ FFM | −0.160 | 0.08 |
| at 24 months | ||
|---|---|---|
| β | p-value | |
| Δ total fat intake | 0.282 | 0.01 |
| Δ 25OHD | 0.218 | 0.02 |
| Δ total CHO intake | −0.193 | 0.07 |
| Final model of variables affecting Δ hip BMD | ||
|---|---|---|
| at 12 months | ||
| β | p-value | |
| Δ Cortisol | 0.393 | <.001 |
| Δ IGF-1 | −0.384 | <.001 |
| Δ adiponectin | −0.319 | <.001 |
| Δ leptin | −0.252 | 0.002 |
| Δ FFM | 0.179 | 0.02 |
| Δ total protein intake | 0.122 | 0.12 |
| at 24 months | ||
|---|---|---|
| β | p-value | |
| Δ FFM | 0.226 | 0.02 |
| Δ cortisol | 0.171 | 0.06 |
| Δ leptin | −0.143 | 0.11 |
| Final model of variables affecting Δ CTX | ||
|---|---|---|
| at 12 months | ||
| β | p-value | |
| Δ FFM | −0.266 | 0.003 |
| Δ IGF-1 | 0.248 | 0.005 |
| Δ Cortisol | −0.176 | 0.04 |
| Δ PA activity | −0.135 | 0.12 |
| at 24 months | ||
|---|---|---|
| β | p-value | |
| Δ FFM | −0.220 | 0.01 |
| Δ cortisol | −0.231 | 0.01 |
| Δ leptin | −0.240 | 0.01 |
| Δ total CHO intake | −0.236 | 0.01 |
| Final model of variables affecting Δ TRAP5b | ||
|---|---|---|
| at 12 months | ||
| β | p-value | |
| Δ FFM | −0.107 | 0.02 |
| Δ cortisol | −0.145 | 0.10 |
| Δ vitamin A intake | 0.165 | 0.10 |
| at 24 months | ||
|---|---|---|
| β | p-value | |
| Δ cortisol | −0.338 | <.001 |
| Δ leptin | −0.29 | <.001 |
| Δ FFM | −0.162 | 0.06 |
| Δ insulin | −0,145 | 0.09 |
| Final model of variables affecting Δ BAP | ||
|---|---|---|
| at 12 months | ||
| β | p-value | |
| Δ cortisol | −0.330 | 0.001 |
| Δ IGF-1 | 0.266 | 0.002 |
| Δ total energy intake | −0.125 | 0.14 |
| at 24 months | ||
|---|---|---|
| β | p-value | |
| Δ Cortisol | −0.263 | 0.004 |
| Δ IGF1 | 0.193 | 0.03 |
| Δ leptin | −0.158 | 0.08 |
| Δ PTH | −0.149 | 0.09 |
| Δ total energy intake | −0.134 | 0.14 |
| Final model of variables affecting Δ PINP | ||
|---|---|---|
| at 12 months | ||
| β | p-value | |
| Δ cortisol | −0.367 | <.001 |
| Δ IGF-1 | 0.279 | 0.001 |
| Δ total CHO intake | −0.275 | 0.001 |
| Δ weight | −0.260 | 0.002 |
| Δ 25OHD | −0.203 | 0.02 |
| at 24 months | ||
|---|---|---|
| β | p-value | |
| Δ total protein | −0.254 | 0.01 |
| Δ Cortisol | −0.250 | 0.01 |
| Δ IGF1 | 0.190 | 0.03 |
| Δ weight | −0.161 | 0.07 |
| Δ leptin | −0.140 | 0.12 |
multiple r=0.43; p<0.001
multiple r=0.31; p=0.01
multiple r=0.56; p<0.001
multiple r=0.31; p=0.01
multiple r=0.39; p=0.001
multiple r=0.51; p<.001
multiple r=0.26; p=.01
multiple r=0.55; p <.001
multiple r=0.41; p<.001
multiple r=0.55; p <.001
multiple r=0.56; p<.001
multiple r=0.52; p<.001
Abbreviations: Δ = change, BMD = bone mineral density, 25OHD = 25-hydroxyvitamin D, FM = fat mass, FFM = fat-free mass, CHO = carbohydrate, IGF-1 = insulin growth factor-1, CTX = C-telopeptide of type I collagen; BSAP = bone specific alkaline phosphatase; TRAP5b = Tartrate-resistant acid phosphatase; PINP = intact N-terminal propeptide of type I procollagen; PA = physical activity
Using stepwise multiple regression analyses
Predicting BMD changes from body weight changes
To determine whether changes in BMD were expected based on changes in body weight, we compared the BMD measured by DXA with the BMD derived from a prediction equation at baseline. There were no significant differences between the actual results and the values derived from the regression model in either group (AL: 1.047±0.122 vs. 1.042±0.021 g/cm2 and 0.984±0.116 vs. 0.983±0.043 g/cm2; CR:1.029±0.120 vs. 1.024± 0.021 g/cm2 and 0.969±0.111 vs. 0.970±0.045 g/cm2, for 24-month lumbar spine and total hip BMD, respectively) (Appendix Figure 2).
Discussion
CR could enhance healthy lifespan but it also causes weight loss, which has been associated with a reduction of bone mass in obese older adults.(6-12) The effects of long-term CR on bone health in non-obese younger adults are unknown. In this two-year RCT, prolonged CR-induced weight loss significantly decreased BMD at the clinically important sites of the lumbar spine, total hip, and femoral neck due to an increase in bone resorption and a decrease in bone formation.
The decreases in spine and hip BMD in our participants, however, were predicted from their decrease in body weight. Weight loss studies in obese older adults also found that bone loss was proportional to the amount of weight loss.(6-12) The ~2% decrease in spine and hip BMD in our non-obese younger adults is within the range reported in studies of obese older adults who lost a similar amount (~10%) of body weight.(6-12) Therefore, the effect of weight loss on BMD in non-obese younger adults is similar to that in obese older adults. Conversely, our data are in contrast to studies showing no detrimental bone effects with weight loss in obese younger adults.(13,15,16) The reason for this discrepancy could be that non-obese younger adults have less FFM than obese younger adults to protect against bone loss.(30)
BMD decreased at the central sites of the spine and hip but not at the peripheral site of the wrist or whole body. A possible explanation is that the spine and hip contain more trabecular bone, which has a greater rate of turnover than does cortical bone; sites where trabecular bone predominates are more sensitive to factors that alter bone turnover.(31) Another possible explanation is that the spine and hip are weight-bearing skeleton whereas the wrist is not, which makes these sites more responsive to the unloading effect of weight loss.(17) Although whole body BMD is highly reproducible, it is not sensitive in estimating BMD changes as it averages findings from many areas of the body. Given that spine and hip BMD are used to predict fracture risk and are common sites of osteoporotic fractures,(32) these site-specific effects of CR could have clinical implications.
The transient but marked increases (~30%) in CTX andTRAP5B in the CR participants indicate that bone resorption was stimulated in response to CR-induced weight loss. In fact, the peak increase in bone resorption markers coincided with the peak weight loss (6-12 months). Interestingly, although bone turnover is a tightly coupled process,(33) bone formation markers did not increase following the increase in bone resorption markers, supporting the lower BMD observed at 12 and 24 months. On the contrary, BAP decreased (~8%) whereas PINP did not change during the latter phase when bone resorption markers were reverting towards baseline coincident with weight maintenance. Our results support data from studies in obese older adults, which found that weight loss was associated with disproportionate increase in bone resorption.(8,17,34) However, our results also suggest that in non-obese younger adults undergoing CR, an absolute decrease in bone formation additionally contributes to bone loss.
Although the precise mechanisms for bone loss during CR are unknown, several contributory factors have been hypothesized.(30,35,36) Our data showed that CR-induced weight loss was accompanied by changes in body composition (FM, FFM) that could contribute to bone loss.(30) Other potential contributors to the higher bone loss were greater increases in cortisol and adiponectin and decreases in leptin and insulin observed in the CR group.(35,36) Regarding cortisol, serum cortisol increased at 12 months while BMD decreased at 12 and 24 months comparing the CR and AL groups, consistent with known negative effects of cortisol on bone. However, in models confined to the CR group investigating factors that contributed to changes in BMD and bone turnover markers in the setting of CR, we found that cortisol changes over 12 months were positively associated with BMD changes and negatively associated with bone turnover markers in adjusted (Table 5) and unadjusted (Appendix Table 1) models. We are not certain of the reasons for this unexpected finding. We cannot exclude that subjects who had lesser increases in cortisol at 12 months had greater increases in cortisol at 6 months (not measured in this study), consistent with an adaptive CR response.(37) The most rapid weight loss in CR participants occurred during the first 6 months. Cortisol levels at 12 months might not accurately reflect cumulative exposure to cortisol over the first year. Thus, future studies might include measurements of serum cortisol at earlier time points to determine if CR-induced changes in cortisol precede and predict the CR-induced bone loss. Although increases in 25OHD were greater in the CR group due to the weight loss,(38) changes in PTH or IGF1 did not differ between the CR and AL groups. Another contributing factor is the lower level of physical activity in the CR group although we did not observe group differences in strength. Analyses exploring the factors associated with bone loss suggested that the key factor correlated with changes in hip BMD were changes in FFM. For changes in spine BMD and bone markers, there was no single main predictor but a combination of predictors in the final models accounting for up to 31% of the variance, consistent with the multifactorial nature of the bone loss. Conversely, given that a large proportion of the variance was unexplained, factors not measured could underlie the bone loss with CR. One such factor is sclerostin, a protein secreted by osteocytes in states of unloading (e.g. weight loss) to inhibit Wnt/b-catenin signaling and bone formation.(39,40) A recent study reported increase in sclerostin with weight loss that was prevented by exercise.(41) It is important to note that physical activity decreased in CR participants since PA has been shown to be osteoprotective during CR;(34) this reduction in PA could have also contributed to bone loss. Supporting this premise, CR was associated with FFM loss, a change that can be prevented/attenuated by increased physical activity.(42) Given the coupling between muscle and bone during weight loss,(43) factors other than sclerostin that mediate muscle-bone interactions could also mediate bone loss during CR(44) and deserve investigation.
Typically, spine and hip BMD increase until early adulthood and remain stable until age ~50.(45) Thus, the decreases in spine and hip BMD we observed, although small, are unexpected for adults in this age range (21-50 yrs.). However, we also found that the changes in BMD were consistent with the changes in body weight. Overall, the predicted increase in fracture risk associated with BMD loss with CR appears small. At the femoral neck, the average BMD loss was −0.015 g/cm2 (−0.16 T-score units). Using the WHO Fracture Risk Assessment Tool, (46) this degree of BMD loss in a 50-year-old woman without risk factors corresponds to <0.5% increase in 10-year risk of fracture. In addition, there was no increased fracture risk in Look AHEAD, a trial testing the effects of a weight loss intervention in overweight and obese adults with type 2 diabetes although the intervention group lost an average of 8.6% of body weight.(47) Of course, longer term studies are needed to fully assess the clinical importance of these findings. Follow-up studies in older adults suggest that BMD loss continues after weight reduction(48) and/or may not fully recover with weight regain.(49)
The strengths of our study include the RCT design, unique 2-year CR duration in non-obese participants, and comprehensive assessments of bone metabolism. Although we used DXA to monitor bone loss because it is the standard for measuring BMD (≥65% of the variance in bone strength), a limitation is that DXA assesses bone quantity but not bone quality, an additional predictor of fracture risk.(50) Besides bone markers, we did not measure bone quality (e.g. bone microarchitecture). It is possible that despite BMD loss, CR preserved bone quality as suggested by low BMD but good bone quality noted in persons practicing long-term CR.(51) Another limitation is that changes in BMD after weight loss might be exaggerated because of technical limitations of DXA.(52) However, this would primarily affect interpretation of studies examining bone changes after extreme weight loss not after moderate weight loss as in the current study.(36) Moreover, our results are consistent with RCTs in older adults showing bone loss with weight loss.(6-12) Importantly, changes in BMD in the participants were corroborated by changes in bone markers.(53) Despite extensive behavioral counseling, participants did not achieve the protocol goal of 25% CR. As discussed,(18) this probably occurred because the target weight loss trajectory based on our pilot studies(23) underestimated the weight loss needed to sustain 25% CR. It may also reflect the inability of most individuals to maintain greater degrees of CR over long periods. Nonetheless, significant CR and weight loss (both ~12%) were achieved throughout the 24-month study, which suggest that even lesser degree of CR has negative effects on bone. Another limitation is that we did not include a standard exercise regime, because the overall goal of CALERIE phase 2 was to determine if CR in humans results in the same beneficial effects as observed in animals subjected to similar levels of CR.(19) In animals, both CR and exercise improve average survival, but only CR extends maximal life span.(54) Finally, our study involved a highly motivated population and very intensive behavioral intervention, thus limiting broader inferences of our results.
In conclusion, our results demonstrate that 2-years sustained CR in non-obese younger adults increases bone resorption while decreasing bone formation (i.e. uncoupling of bone turnover) and causes a modest but significant decline in BMD, expected on the basis of the weight lost, at clinically important sites of osteoporotic fractures. Further long-term studies are needed to determine if such bone loss increases fracture risk and whether therapeutic interventions can prevent bone loss that occurs with CR-induced weight loss.
Supplementary Material
Acknowledgement
Funding/Support: This study was supported by the National Institute on Aging (NIA) and National Institute of Diabetes and Kidney Diseases, National Institute of Health (U01AG022132, U01AG020478, U01AG020487, U01AG020480, and U24AG047121)
Role of the Funder/Sponsor: Consistent with an NIH cooperative agreement, the study was designed and managed by a Steering Committee consisting of the Principal Investigators of the three clinical sites and the Data Coordinating Center, and a representative from the NIA. Decisions were made by majority vote and all members, including the NIH, had one vote.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Author Contributions: Dr Villareal had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Villareal, Fontana, Redman, Das, Smith, Saltzman, Bales, Rochon, Schwartz
Acquisition, analysis, or interpretation of data: Villareal, Fontana, Redman, Das, Smith, Saltzman, Rochon, Bales, Lewis, Schwartz
Drafting of the manuscript: Villareal, Schwartz.
Critical revision of the manuscript for important intellectual content: Villareal, Fontana, Das, Redman, Smith, Saltzman, Bales, Rochon, Pieper, Huang, Lewis, Schwartz
Statistical analysis: Rochon, Pieper, Huang.
Administrative, technical, or material support: Villareal, Fontana, Das, Redman, Smith, Saltzman, Bales, Rochon, Pieper, Huang, Lewis, Schwartz.
Study supervision: Villareal, Fontana, Das, Redman, Smith, Saltzman
Disclosures
The authors have nothing to disclose.
Reference list
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Racette SB, Weiss EP, Villareal DT, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heilbronn LK, de JL, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz AV, Johnson KC, Kahn SE, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res. 2012;27:619–627. doi: 10.1002/jbmr.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20:455–463. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141–147. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 10.Ricci TA, Heymsfield SB, Pierson RN, Jr., Stahl T, Chowdhury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73:347–352. doi: 10.1093/ajcn/73.2.347. [DOI] [PubMed] [Google Scholar]
- 11.Avenell A, Richmond PR, Lean ME, Reid DM. Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr. 1994;48:561–566. [PubMed] [Google Scholar]
- 12.Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med. 1993;95:131–140. doi: 10.1016/0002-9343(93)90253-l. [DOI] [PubMed] [Google Scholar]
- 13.Riedt CS, Schlussel Y, von TN, et al. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am J Clin Nutr. 2007;85:972–980. doi: 10.1093/ajcn/85.4.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168:1859–1866. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapses SA, Von Thun NL, Heymsfield SB, et al. Bone turnover and density in obese premenopausal women during moderate weight loss and calcium supplementation. J Bone Miner Res. 2001;16:1329–1336. doi: 10.1359/jbmr.2001.16.7.1329. [DOI] [PubMed] [Google Scholar]
- 16.Uusi-Rasi K, Rauhio A, Kannus P, et al. Three-month weight reduction does not compromise bone strength in obese premenopausal women. Bone. 2010;46:1286–1293. doi: 10.1016/j.bone.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Villareal DT, Fontana L, Weiss EP, et al. Bone Mineral Density Response to Caloric Restriction-Induced Weight Loss or Exercise-Induced Weight Loss: A Randomized Controlled Trial. Arch Intern Med. 2006;166:2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 18.Ravussin, Redman LM, Rochon J, et al. A two-year randomized controlled trial of human caloric restrcition: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–1104. doi: 10.1093/gerona/glv057. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rochon J, Bales CW, Ravussin E, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011;66:97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rickman AD, Williamson DA, Martin CK, et al. The CALERIE Study: design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials. 2011;32:874–881. doi: 10.1016/j.cct.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart TM, Bhapkar M, Das S, et al. Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Phase 2 (CALERIE Phase 2) screening and recruitment: methods and results. Contemp Clin Trials. 2013;34:10–20. doi: 10.1016/j.cct.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redman LM, Kraus WE, Bhapkar M, et al. Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr. 2014;99:71–78. doi: 10.3945/ajcn.113.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieper C, Redman L, Racette S, et al. Development of adherence metrics for caloric restriction interventions. Clin Trials. 2011;8:155–164. doi: 10.1177/1740774511398369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 25.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. 2nd ed. Oxford University Press; New York: 2002. [Google Scholar]
- 26.Jennrich RI, Schluchter MD. Unbalanced repeated-measures models with structured covariance matrices. Biometrics. 1986;42:805–820. [PubMed] [Google Scholar]
- 27.Dmitrienko A, Millen BA, Brechenmacher T, Paux G. Development of gatekeeping strategies in confirmatory clinical trials. Biom J. 2011;53:875–893. doi: 10.1002/bimj.201100036. [DOI] [PubMed] [Google Scholar]
- 28.Wright S. Adjusted p-values for simultaneous inference. Biometrics. 1992;48:1005–1013. [Google Scholar]
- 29.Fontana L, Villareal DT, Das SK, et al. Effect of 2-year caloric restriction on circulating levels of IGF-1, IGF-binding proteins, and cortisol in non-obese men and women: a randomized clinical trial. Aging Cell. doi: 10.1111/acel.12400. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 31.Bonnick SL. Appication and Interpretation. Humana Press; New York, NY: 2010. Skeletal Anatomy in Densitometry. In: Bonnick SL, editor. Bone Densitometry in Clinical Practice. pp. 35–78. [Google Scholar]
- 32.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J Cell Sci. 2011;124:991–998. doi: 10.1242/jcs.063032. [DOI] [PubMed] [Google Scholar]
- 34.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid IR, Cornish J, Baldock PA. Nutrition-related peptides and bone homeostasis. J Bone Miner Res. 2006;21:495–500. doi: 10.1359/jbmr.051105. [DOI] [PubMed] [Google Scholar]
- 36.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masoro EJ. The role of hormesis in life extension by dietary restriction. Interdiscip Top Gerontol. 2007;35:1–17. doi: 10.1159/000096552. [DOI] [PubMed] [Google Scholar]
- 38.Mason C, Xiao L, Imayama I, et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am J Clin Nutr. 2011;94:95–103. doi: 10.3945/ajcn.111.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland MK, Geoghegan JC, Yu C, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27:1215–1221. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 43.Armamento-Villareal R, Aguirre L, Napoli N, et al. Changes in thigh muscle volume predict bone mineral density response to lifestyle therapy in frail, obese older adults. Osteoporos Int. 2014;25:551–558. doi: 10.1007/s00198-013-2450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonewald LF, Kiel DP, Clemens TL, et al. Forum on bone and skeletal muscle interactions: Summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2013;28:1857–1865. doi: 10.1002/jbmr.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Looker AC, Borrud LG, Hughes JP, et al. Lumbar Spine and Proximal Femur Bone Mineral Density, Bone Mineral Content, and Bone Area: United States, 2005-2008. National Center for Health Statistics, Vital Health Stat (251); 2012. [PubMed] [Google Scholar]
- 46.Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44:734–743. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 47.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waters DL, Vawter R, Qualls C, Chode S, Armamento-Villareal R, Villareal DT. Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J Nutr Health Aging. 2013;17:3–7. doi: 10.1007/s12603-012-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villalon KL, Gozansky WS, Van Pelt RE, et al. A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity (Silver Spring) 2011;19:2345–2350. doi: 10.1038/oby.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 51.Villareal DT, Kotyk JJ, Armamento-Villareal RC, et al. Reduced bone mineral density is not associated with significantly reduced bone quality in men and women practicing long-term calorie restriction with adequate nutrition. Aging Cell. 2011;10:96–102. doi: 10.1111/j.1474-9726.2010.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tothill P, Hannan WJ. Comparisons between Hologic QDR 1000W, QDR 4500A, and Lunar Expert dual-energy X-ray absorptiometry scanners used for measuring total body bone and soft tissue. Ann N Y Acad Sci. 2000;904:63–71. doi: 10.1111/j.1749-6632.2000.tb06422.x. [DOI] [PubMed] [Google Scholar]
- 53.Civitelli R, Armamento-Villareal R, Napoli N. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int. 2009;20:843–851. doi: 10.1007/s00198-009-0838-9. [DOI] [PubMed] [Google Scholar]
- 54.Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol (1985) 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.