Abstract
Background
Genetic polymorphisms of drug-metabolizing enzymes and transporters have been extensively studied with regard to tamoxifen treatment outcomes. However, the results are inconclusive. Analysis of organ-specific metastasis may reveal the association of these pharmacogenetic factors. The aim of this study is to investigate the impact of CYP3A5, CYP2D6, ABCB1, and ABCC2 polymorphisms on the risk of all distant and organ-specific metastases in Thai patients who received tamoxifen adjuvant therapy.
Methods
Genomic DNA was extracted from blood samples of 73 patients with breast cancer who received tamoxifen adjuvant therapy. CYP3A5 (6986A>G), CYP2D6 (100C>T), ABCB1 (3435C>T), and ABCC2 (−24C>T) were genotyped using allelic discrimination real-time polymerase chain reaction assays. The impacts of prognostic clinical factors and genetic variants on disease-free survival were analyzed using the Kaplan–Meier method and Cox regression analysis.
Results
In the univariate analysis, primary tumor size >5 cm was significantly associated with increased risk of distant metastasis (P=0.004; hazard ratio [HR] =3.05; 95% confidence interval [CI], 1.44–6.47). In the multivariate analysis, tumor size >5 cm remained predictive of distant metastasis (P<0.001; HR=5.49; 95% CI, 2.30–13.10). ABCC2 −24CC were shown to be associated with increased risk of distant metastasis (P=0.040; adjusted HR=2.34; 95% CI, 1.04–5.27). The combined genotype of ABCC2 −24CC − ABCB1 3435 CT+TT was associated with increased risk of distant and bone metastasis (P=0.020; adjusted HR=2.46; 95% CI, 1.15–5.26 and P=0.040; adjusted HR=3.70; 95% CI, 1.06–12.89, respectively).
Conclusion
This study indicates that polymorphisms of ABCC2 and ABCB1 are independently associated with bone metastasis. Further prospective studies with larger sample sizes are needed to verify this finding.
Keywords: breast cancer, tamoxifen, ABCB1, ABCC2, pharmacogenetics, distant metastasis
Introduction
Tamoxifen, a selective estrogen receptor modulator, has been used as adjuvant therapy in breast cancer patients with hormone receptor-positive tumors for decades. Five years of oral tamoxifen treatment reduces the risk of cancer recurrence by ~60%. However, up to 40% of patients who receive tamoxifen experience distant or local recurrence, or die.1,2 Tamoxifen undergoes extensive metabolism by the CYP2D6 enzyme in the liver leading to the formation of more pharmacologically active metabolites, 4-hydroxyta-moxifen and endoxifen.3 CYP2D6 polymorphisms have been extensively studied for the pharmacogenetic association in breast cancer patients treated with tamoxifen. However, several lines of inconsistent evidence have been reported.4–14 In addition to CYP2D6, CYP3A5 is another enzyme involved in tamoxifen metabolism. The impact of CYP3A5 6986A>G on the treatment outcome of tamoxifen has been studied, however, the results are controversial.15–18
Tamoxifen active metabolites, 4-hydroxytamoxifen and endoxifen, have been established to bind with the ABC subfamily B member 1.19 ABCB1 is an active drug efflux transporter. It is a multiple drug resistance transporter, which may act as a barrier and limit the accessibility of active metabolites of tamoxifen to various critical target tissues. Recently, ABCB1 3435C>T has been demonstrated to be associated with increased risk of recurrence in Asian women who received tamoxifen.20 In addition to ABCB1, overexpression of ABCC2, efflux transporter, was observed in tamoxifen-resistant breast cancer cells.21 Interestingly, a polymorphism of the ABCC2 gene has been associated with 5-year tamoxifen treatment outcomes in Japanese subjects with breast cancer.8 Therefore, genetic variants of these metabolizing enzymes and drug transporters are likely to play a role to a variable degree in the clinical outcome of tamoxifen treatment. Our earlier study reported the impact of ABCB1 polymorphism on 3-year tamoxifen effectiveness in Thai populations.22 However, the impact of clinical prognostic factors and genetic variants that contributed to 5-year tamoxifen effectiveness in Thai populations has never been evaluated. In this study, genetic variants of CYP3A5 (6986A>G), CYP2D6 (100C>T), ABCB1 (3435C>T), and ABCC2 (−24C>T) in Thai breast cancer patients were investigated. The retrospective analysis of patients with primary breast cancer who developed distant metastatic disease during tamoxifen treatment was conducted. The risk of distant metastasis within 5 years was evaluated by using clinical and genetic prognostic factors in terms of organ-specific metastasis and associated patient outcomes.
Materials and methods
Patient selection criteria
Thai patients with primary breast cancer who visited Ramathibodi Hospital, Bangkok, Thailand, during the period between February 1997 and January 2008 were selected for this study. The inclusion criteria for this study were: age ≥18 years, non-pregnant women, histological confirmation of primary breast cancer with estrogen receptor (ER)+ and/or progesterone receptor (PR)+ testing, received 20 mg/day tamoxifen as an adjuvant treatment for breast cancer. In addition, all subjects were selected with regard to the consistency of pathological parameters including stage of the cancer, fundamental characteristics for the existence of metastasis and evolution of the pathology. Exclusion criteria included concurrent medications that induce or inhibit CYP2D6, CYP3A4/5, and efflux transporters. The study was approved by Ethics Committee of Ramathibodi Hospital and written informed consent was obtained from all patients. According to our criteria, the retrospective study was conducted in 73 breast cancer patients. All patients were pathologically diagnosed with invasive breast cancer without distant spread. Most patients were treated with a modified radical mastectomy. The regimens of adjuvant chemotherapy which are composed of cyclophosphamide, methotrexate, and 5-fluorouracil, adriamycin based, and adriamycin-taxane based regimens were given to nearly all patients. Thirty patients were treated with radiation. The median follow-up time of all patients was 5 (range 0.2–14.3) years.
Sample preparation and genotyping
Blood samples were collected (5 mL) in ethylenediaminetetraacetic acid tubes and stored at −20°C until isolation of genomic DNA for genotype analysis. All samples were isolated with phenol-chloroform method. DNA purity was assessed by using NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA).
In this study, candidate gene approach based on tamoxifen pharmacokinetics was performed. All selected variants are single-nucleotide polymorphisms (SNPs) that can affect protein function or expression. The rationale for selecting each is described as follows. As mentioned earlier, hepatic CYP2D6 plays a major role in tamoxifen biotransformation. The common reduced-activity allele in Asians is CYP2D6*10 where as CYP2D6*4, a non-functional allele, is commonly found in Caucasians.23,24 CYP2D6 100C>T, being part of both CYP2D6*4 and CYP2D6*10 haplotypes, is likely to be the causative SNP for the reduced function of CYP2D6*10.25 CYP3A5*3 (6986A>G), a non-expressor allele, has been reported to be involved in variable metabolism of CYP3A5 substrates, including tamoxifen.23 ABCB1 3435C>T is the common SNP associated with altered P-gp expression and/or function.26 Recently, it was reported that ABCB1 could transport endoxifen out of the blood–brain barrier in an animal model.19 The overexpression of ABCC2 has been found in tamoxifen-resistant breast cancer cells.21 Thus, the possibility that active metabolites could be pumped out from breast cancer cells by ABCB1 and ABCC2 has been suggested. The genotype of each candidate SNP was determined using Taqman drug metabolism genotyping assays (Thermo Fisher Scientific, Waltham, MA, USA) as follows: ABCB1 (3435 C>T) (Assay ID: C__7586657_20), ABCC2 (−24C>T) (Assay ID: C__2814642_10), CYP3A5 (6986A>G) (Assay ID: C__26201809_30) and CYP2D6 (100C>T) (Assay ID: C__11484460_40). The genotyping experiments were carried out by using real-time polymerase chain reaction Viia™ 7 instrument and performed according to manufacturer’s protocol.
Statistical analysis
The impact of clinical and genetic prognostic factors in breast cancer patients treated with 5-year tamoxifen was evaluated in terms of overall and organ-specific metastases. Disease-free survival (DFS) time was defined as the period from starting date of tamoxifen to the date of first disease recurrence (distant recurrence). Patients who had lived without disease recurrence for 5 years were defined as the non-metastasis group, whereas patients who had relapsed within 5 years were defined as the metastasis group. In this study, Fisher’s exact test was used to test the difference of clinical parameters and genotype frequency between metastasis and non-metastasis groups. Then, the difference of DFS estimated by Kaplan–Meier method across genotypes was examined by log-rank test. Cox proportional hazard analysis was used to identify significant clinical prognostic factors and to test for an independent contribution of genetic factors to the variable outcomes. Multivariate analysis was evaluated by step-wise forward Cox’s regression analysis. The result was considered to be statistically significant when bilateral P-values were <0.05. Statistical tests were performed using Stata software version 12 (StataCorp LP, College Station, TX, USA).
Results
The comparison of patient characteristics
All patients were estrogen receptor positive and were recommended to receive 5-year adjuvant tamoxifen therapy. Their median age at the time of surgery was 50 years old (range 28–76). The median follow-up time of non-metastasis was 87.6 months (range 60–171.6). The median follow-up time of bone, lung, and liver metastasis group was 48.0 months (range 2.0–172.0), 93.5 months (range 59.0–172.0), and 22.0 months (range 2.0–62.0), respectively. The number of pre- and postmenopausal patients was 47 and 26, respectively. The comparisons of clinical prognostic factors between non-metastasis and metastasis group according to first disease metastasis are shown in Table 1.
Table 1.
The comparisons of clinical characteristics between non-metastasis and metastasis group according to first disease metastasis
| Characteristics | Non-metastasis (n=39)
|
First disease metastasis (n=34)
|
|||||
|---|---|---|---|---|---|---|---|
| Bone (n=13)
|
Lung (n=13)
|
Liver (n=8)
|
|||||
| n (%) | n (%) | P-valuea | n (%) | P-valuea | n (%) | P-valuea | |
| Age at diagnosis; years | 0.110 | 1.000 | 1.000 | ||||
| ≤50 | 19 (48.7) | 10 (76.9) | 7 (53.9) | 4 (50.0) | |||
| >50 | 20 (51.3) | 3 (23.1) | 6 (46.1) | 4 (50.0) | |||
| Menstrual status | 0.099 | 0.523 | 1.000 | ||||
| Pre-menopause | 22 (56.4) | 11 (84.6) | 9 (69.2) | 5 (62.5) | |||
| Post-menopause | 17 (43.6) | 2 (15.4) | 4 (30.8) | 3 (37.5) | |||
| Tumor size; cm | 0.632 | 0.033* | 0.084 | ||||
| ≤5 | 35 (89.7) | 11 (84.6) | 8 (61.5) | 5 (62.5) | |||
| >5 | 4 (10.3) | 2 (15.4) | 5 (38.5) | 3 (37.5) | |||
| Lymph node status | 0.518 | 0.198 | 0.437 | ||||
| Positive | 21 (53.9) | 9 (69.2) | 10 (76.9) | 6 (75.0) | |||
| Negative | 18 (46.1) | 4 (30.8) | 3 (23.1) | 2 (25.0) | |||
| Tumor grading | 0.640 | 0.150 | 1.000 | ||||
| 1 | 6 (26.1) | 1 (11.1) | 0 (0.0) | 1 (16.7) | |||
| 2+3 | 17 (73.9) | 8 (88.9) | 9 (100.0) | 5 (83.3) | |||
| Unknownb | 16 (–) | 4 (–) | 4 (–) | 2 (–) | |||
| LVIc | 0.495 | 0.717 | 0.659 | ||||
| Positive | 13 (50.0) | 4 (36.4) | 4 (40.0) | 2 (33.3) | |||
| Negative | 13 (50.0) | 7 (63.6) | 6 (60.0) | 4 (66.7) | |||
| Unknownb | 13 (–) | 2 (–) | 3 (–) | 2 (–) | |||
| ER and/or PR status | n/a | n/a | n/a | ||||
| Positive | 39 (100.0) | 13 (100.0) | 13 (100.0) | 8 (100.0) | |||
| Negative | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
Notes:
Fisher’s exact test;
unknown statuses were excluded from Fisher’s exact test;
LVI (lymphovascular invasion) is considered as spreading of cancer to blood vessels and/or lymphatic system.
Statistically significant at P-value <0.05.
Abbreviations: ER, estrogen receptor; n/a, not applicable; PR, progesterone receptor.
Bone metastasis was found more frequently in premenopausal patients than in postmenopausal patients, but the difference did not reach statistical significance (P-value =0.099). In addition, a number of patients with tumor size >5 cm also showed incidence of metastasis to lung and liver. A significant difference was found in the lung metastasis group, but not in the liver metastasis group (P-value =0.033 and 0.084, respectively).
Associations between genotypes and clinical outcomes
ABCB1 3435C>T, ABCC2 −24C>T, CYP3A5 6986A>G, and CYP2D6 100C>T were successfully genotyped in all patients. The genotype frequencies of these variants were compared between non-metastasis and metastasis groups according to first dominant site of metastasis as shown in Table 2. There was no significant difference between genotype frequency and first dominant site of metastasis. Regardless of any first dominant site of metastasis, Kaplan–Meier analysis indicated that patients carrying ABCC2 −24CC had significantly shorter DFS than those who carry −24CT (log-rank test, P=0.044; Figure 1). Regarding bone metastasis, patients carrying ABCC2 −24CC and ABCB1 3435CT or TT genotypes had significantly shorter DFS time than those who carry ABCB1 3435CC and ABCC2 −24CC or ABCC2 −24CT (log-rank test, P=0.040; Figure 2 and Table S1). No such variants of CYP3A5 6986A>G and CYP2D6 100C>T were found to be associated with tamoxifen treatment outcomes.
Table 2.
The comparisons of pharmacogenetic factors between non-metastasis and metastasis group according to first disease metastasis
| Polymorphisms | Non-metastasis (n=39)
|
First disease metastasis (n=34)
|
|||||
|---|---|---|---|---|---|---|---|
| Bone (n=13)
|
Lung (n=13)
|
Liver (n=8)
|
|||||
| n (%) | n (%) | P-valuea | n (%) | P-valuea | n (%) | P-valuea | |
| ABCB1 3435C>T | 0.284 | 0.841 | 0.780 | ||||
| CC | 17 (43.6) | 3 (23.1) | 5 (38.5) | 3 (37.5) | |||
| CT | 14 (35.9) | 8 (61.5) | 6 (46.1) | 4 (50.0) | |||
| TT | 8 (20.5) | 2 (15.4) | 2 (15.4) | 1 (12.5) | |||
| ABCC2 −24C>T | 0.194 | 0.194 | 0.269 | ||||
| CC | 20 (51.3) | 10 (76.9) | 10 (76.9) | 6 (75.0) | |||
| CT | 19 (48.7) | 3 (23.1) | 3 (23.1) | 2 (25.0) | |||
| CYP3A5 6986A>G | 0.311 | 0.707 | 1.000 | ||||
| AA | 24 (61.5) | 5 (38.5) | 8 (61.5) | 5 (62.5) | |||
| AG | 12 (30.8) | 6 (46.1) | 3 (23.1) | 3 (37.5) | |||
| GG | 3 (7.7) | 2 (15.4) | 2 (15.4) | 0 (0.0) | |||
| CYP2D6 100C>T | 0.610 | 0.593 | 0.474 | ||||
| CC | 12 (30.8) | 5 (38.4) | 2 (15.4) | 1 (12.5) | |||
| CT | 18 (46.1) | 4 (30.8) | 8 (61.5) | 4 (50.0) | |||
| TT | 9 (23.1) | 4 (30.8) | 3 (23.1) | 3 (37.5) | |||
Note:
Fisher’s exact test.
Figure 1.
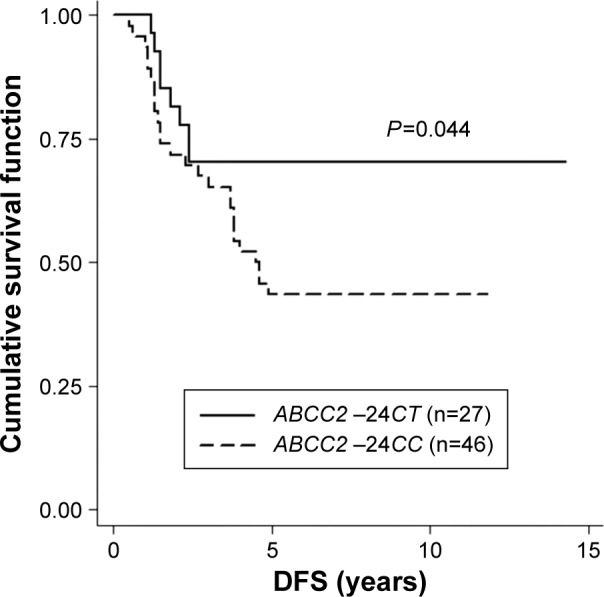
The Kaplan–Meier survival curve compares 46 patients carrying ABCC2 −24CC to 27 patients carrying ABCC2 −24CT in all groups of distant metastasis with respect to disease-free survival (DFS).
Figure 2.
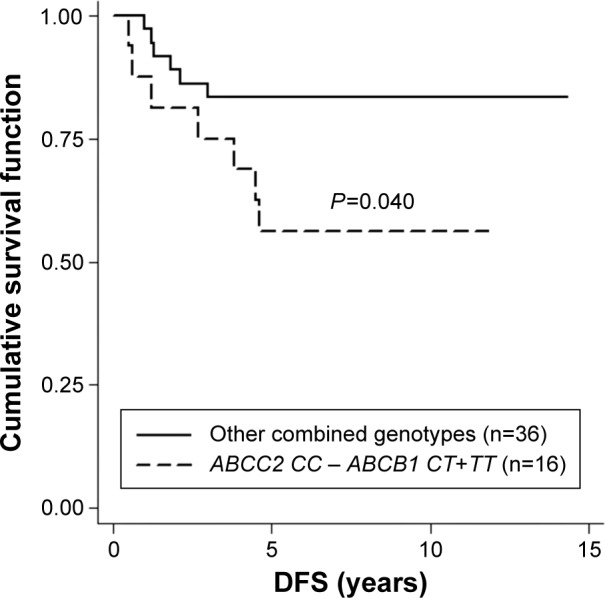
The Kaplan–Meier survival curve compares 16 patients carrying ABCB1 3435CT or TT and ABCC2−24CC to 36 patients carrying other combined genotypes of ABCC2 and ABCB1 in bone metastasis with respect to disease-free survival (DFS).
Correlation of prognostic clinical factors and pharmacogenetic factors with the risk of distant metastasis
In the univariate Cox proportional hazard analysis for distant metastasis-free survival, only tumor size >5 cm was considered to be a significant associated factor (P=0.004; hazard ratio, 3.05; 95% confidence interval, 1.44–6.47, Table 3). In the multivariate analysis, tumor size >5 cm remained predictive of distant metastasis after adjustment for age, menstrual status, lymph node status, and pharmacogenetic factors (P<0.001; adjusted hazard ratio, 5.49; 95% confidence interval, 2.30–13.10). Moreover, the ABCC2 genotype (CT vs CC) was shown to be associated with increased risk of distant metastasis in 5-year tamoxifen treatment after adjustment for age, menstrual status, tumor size, and lymph node status (P=0.040; adjusted hazard ratio, 2.34; 95% confidence interval, 1.04–5.27 for ABCC2 CC). Nevertheless, the combined genotype of ABCB1 and ABCC2 (other combined genotypes vs ABCC2 CC − ABCB1 CT+TT) was shown to be associated with increased risk of distant metastasis after adjustment for age, menstrual status, tumor size, and lymph node status (P=0.020; adjusted hazard ratio, 2.46; 95% confidence interval, 1.15–5.26 for ABCC2 CC − ABCB1 CT+TT).
Table 3.
Univariate and multivariate disease-free survival Cox analyses in all breast cancer patients with distant metastasis
| Covariate | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Clinical factors | ||||||
| Age (≤50 vs >50 years) | 0.68 | 0.34–1.36 | 0.283 | 0.60 | 0.27–1.31 | 0.202 |
| Menstrual status (post- vs pre-menopause) | 1.77 | 0.82–3.80 | 0.140 | 2.02 | 0.91–4.48 | 0.081 |
| Tumor size (≤5 vs >5 cm) | 3.05 | 1.44–6.47 | 0.004* | 5.49 | 2.30–13.10 | <0.001* |
| Lymph node status (negative vs positive) | 1.84 | 0.86–3.95 | 0.116 | 1.65 | 0.76–3.59 | 0.204 |
| Pharmacogenetic factors | ||||||
| ABCB1 3435C>T (CC vs CT+TT) | 1.25 | 0.61–2.57 | 0.540 | n/a | n/a | n/a |
| ABCC2 −24C>T (CT vs CC) | 2.19 | 0.99–4.85 | 0.052 | 2.34 | 1.04–5.27 | 0.040* |
| CYP3A5 6986A>G (AA+AG vs GG) | 1.72 | 0.60–4.89 | 0.307 | n/a | n/a | n/a |
| CYP2D6 100C>T (CC+CT vs TT) | 1.22 | 0.58–2.55 | 0.596 | n/a | n/a | n/a |
| Combined genotypes of ABCB1 and ABCC2 | ||||||
| Other combined genotypes vs ABCC2 CC − ABCB1 CT+TT | 1.76 | 0.89–3.46 | 0.100 | 2.46 | 1.15–5.26 | 0.020a,* |
Notes:
Statistically significant at P-value <0.05;
P-value was calculated from the covariates which were all clinical factors and the combined genotypes of ABCB1 and ABCC2. Abbreviations: CI, confidence interval; HR, hazard ratio; n/a, not applicable.
Regarding bone metastasis, none of the prognostic clinical factors were shown to be associated with an increased risk of recurrence in both univariate and multivariate analysis (Table 4). Unexpectedly, the combined genotype of ABCC2 −24CC and ABCB1 3435CT or TT was significantly correlated with increased bone metastasis risk after adjustment for age, menstrual status, tumor size, and lymph node status (P=0.040; adjusted hazard ratio, 3.70; 95% confidence interval, 1.06–12.89 for ABCC2 CC − ABCB1 CT+TT, Table 4).
Table 4.
Univariate and multivariate disease-free survival Cox analyses in breast cancer patients with bone metastasis
| Covariate | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Clinical factors | ||||||
| Age (≤50 vs >50 years) | 0.33 | 0.09–1.19 | 0.092 | 0.30 | 0.07–1.21 | 0.091 |
| Menstrual status (post- vs pre-menopause) | 3.59 | 0.79–16.22 | 0.096 | 4.00 | 0.86–18.50 | 0.076 |
| Tumor size (≤5 vs >5 cm) | 1.48 | 0.33–6.69 | 0.610 | 3.74 | 0.66–21.08 | 0.134 |
| Lymph node status (negative vs positive) | 1.66 | 0.51–5.39 | 0.398 | 1.31 | 0.39–4.36 | 0.658 |
| Pharmacogenetic factors | ||||||
| ABCB1 3435C>T (CC vs CT+TT) | 2.23 | 0.61–8.13 | 0.221 | n/a | n/a | n/a |
| ABCC2 −24C>T (CT vs CC) | 2.69 | 0.74–9.78 | 0.133 | 3.16 | 0.84–11.89 | 0.088 |
| CYP3A5 6986A>G (AA+AG vs GG) | 2.20 | 0.48–9.98 | 0.304 | n/a | n/a | n/a |
| CYP2D6 100C>T (CC+CT vs TT) | 1.51 | 0.47–4.92 | 0.489 | n/a | n/a | n/a |
| Combined genotypes of ABCB1 and ABCC2 | ||||||
| Other combined genotypes vs ABCC2 CC − ABCB1 CT+TT | 2.95 | 0.99–8.80 | 0.051 | 3.70 | 1.06–12.89 | 0.040a,* |
Notes:
Statistically significant at P-value <0.05;
P-value was calculated from the covariates which were all clinical factors and the combined genotypes of ABCB1 and ABCC2. Abbreviations: CI, confidence interval; HR, hazard ratio; n/a, not applicable.
Regarding lung and liver metastasis, tumor size was the only prognostic factor that significantly contributed to shorter DFS in both univariate and multivariate analyses (data not shown).
Discussion
In developing countries, the majority of breast cancer occurs among premenopausal women with a mean age of 50.27 Five-year treatment with tamoxifen is the main endocrine therapy for premenopausal women with ER+ breast cancer. In postmenopausal women only, aromatase inhibitor (AI) treatment offers more benefits than tamoxifen by greatly reducing estrogen concentrations, hence avoiding stimulation of ER+ breast cancer cells.28 However, postmenopausal patients with intolerance to AIs could switch to tamoxifen after at least 2 years of treatment with AIs.29 The variability in tamoxifen response among ER+ individuals is consistently observed. It is mainly caused by the differences in various clinical prognostic factors and characteristics of the tumor. Since genomic knowledge is becoming increasingly relevant, prognosis and breast cancer treatment could be partly explained by pharmacogenomics.
CYP2D6 plays a major role in the conversion of tamoxifen to endoxifen, which is the most potent anti-estrogen metabolite.30 CYP2D6*10/*10, a common reduced-activity allele in Asians including Thais, was demonstrated to be associated with significantly lower endoxifen level compared to CYP2D6*1/*1 at steady-state of tamoxifen adjuvant therapy.8,31,32 Consequently, Asian women carrying CYP2D6*10/*10 genotype had significantly shorter DFS than those who carry CYP2D6*1/*1 genotype treated with the equivalent dose of tamoxifen.6,7,10,31 Furthermore, CYP2D6*10/*10 was independently correlated to unfavorable outcomes in tamoxifen treatment, significant in the multivariate analyses adjusted for clinical prognostic factors.7,31 The pharmacogenetic association of CYP2D6 and tamoxifen is mostly observed in the retrospective study, however, two prospective clinical trials recently showed that CYP2D6 genotypes were not able to predict clinical benefit of tamoxifen treatment.11,12 In addition, they found that CYP2D6 alleles predicting reduced and deficient enzyme activities were correlated with increased side effects, such as hot flushes, rather than the variation of tamoxifen effectiveness. A high level of tamoxifen still poses clinically significant adverse effects, such as thromboembolic events and endometrial cancer. However, a low endoxifen level might unchangingly exert an anti-estrogen effect, as indicated by Ki-67 proliferative index that was observed in patients who received low-dose tamoxifen treatment.33 There is only one piece of evidence that supports the association of CYP3A5*3/*3 representing an inactive form of the enzyme and favorable response to tamoxifen.18 However, homozygous CYP3A5*3 has never been shown to be associated with endoxifen level, or even to increase the risk of recurrence in women prescribed tamoxifen.22,23 In our study, we did not find any associations of CYP2D6*10 (100C>T) and CYP3A5*3 (6986A>G) with an increased risk of 5-year recurrence in Thai patients with early breast cancer receiving adjuvant tamoxifen.
Apart from metabolizing enzymes, drug transporter polymorphisms have been addressed in an issue of tamoxifen-outcome variation. Japanese women carrying wild-type allele ABCC2 68231A>G were associated with an increased risk of recurrence during adjuvant tamoxifen therapy.6 Homozygous CC genotype of ABCB1 3435C>T was recently shown to correlate with shorter DFS in Asian breast cancer patients treated with tamoxifen.20 Noticeably, both studies demonstrated the significance of drug transporter genes with wild-type allele when analyses were performed in combination with impaired or absent functional variants of CYP2D6. The consistent results may reflect the important role of drug transporters in limiting intracellular drug concentration caused by defective hepatic CYP2D6. However, we could not determine the combined genotype of drug metabolizing enzyme (CYP3A5, CYP2D6) and drug transporter (ABCC2, ABCB1) polymorphisms affecting either DFS or recurrence risk even in subgroup analysis.
Indeed, the association of the combined genotype, ABCC2 −24C>T and ABCB1 3435C>T, with tamoxifen treatment outcomes was unexpectedly discovered. The combination of ABCC2 −24CC and ABCB1 3435CT+TT, along with tumor size, was displayed to be associated with increased risk of 5-year distant metastasis in Thai patients with ER+ breast cancer receiving adjuvant tamoxifen. In general, tumor size and lymph node status are the fundamental predictors of prognosis in solid cancers. However, most of our patients with bone metastasis had small size primary tumors and less lymph node involvement, hence, their recurrence risk could not be estimated by clinical prognostic factors. Nevertheless, pharmacogenetic factors served as predictive markers for them due to the combined genotype of drug transporters which exclusively expressed the significant association. Homozygous wild-type ABCC2 −24C>T and ABCB1 3435C>T were associated with increased efflux protein expression, which may result in the reduction of active tamoxifen metabolites’ bioavailability in patients, at both intracellular and plasma levels, finally, leading to treatment failure.34–36 As mentioned above, homozygous wild-type ABCC2 −24C>T and ABCB1 3435C>T need to be combined with reduced or non-functional variants of CYP2D6 predicted phenotype to increase risk of recurrence. Because of our limitations, not all of the variants for CYP2D6 phenotype prediction were determined, thus, we could not reveal any such association. However, our results are partly consistent with the hypothesis regarding the significant association of ABCC2 −24CC with short DFS. Interestingly, some reports gave us a clue that the effects of ABCB1 3435C>T may be exerted differently in an organ-specific manner. In Asian studies, ABCB1 3435TT genotype was revealed to be associated with high risk of death from osteosarcoma,37 while breast cancer patients carrying the same TT genotype had a favorable prognosis.38 Standard chemotherapy regimens were prescribed in both studies. Thus, it may be postulated that ABCB1 3435TT genotype “prefers” to facilitate drug therapy against breast tumors in contrast to bone tumors. Nevertheless, it could be speculated that there is an organ-specific association in a case of drug resistant epilepsy where ABCC2 −24CC genotype was significantly correlated with responders.39 In contrast, it was found that ABCC2 −24CC genotype was associated with tamoxifen non-responders in breast cancer patients in this study and a previous one.20 The organ-specific effects of ABCB1 and ABCC2 polymorphisms might be underestimated and need to be proved.
According to multivariate analysis, ABCC2 −24CC and ABCB1 3435CT+TT were significantly associated with bone metastasis adjusted for age, menstrual status, tumor size, and lymph node status. However, both markers need to be verified in a prospective study with a large sample size. Tamoxifen monotherapy should be the inclusion criteria because adjuvant chemotherapy followed by tamoxifen was associated with bone loss in premenopausal patients, and thus could confound the study outcome.40 In addition, all of the predicted CYP2D6 phenotypes and other significant prognostic factors must be completely determined to confirm validation of our results.
Conclusion
This finding presented the prognostic value of pharmacogenetic factors in adjuvant tamoxifen therapy where conventional factors were weakly predictive of breast cancer prognosis. The combined genotype of ABCC2 −24C>T and ABCB1 3435C>T was indicated for the increased risk of specific disease recurrence such as bone metastasis. Novel prognostic factors are needed to improve the clinical outcomes for all breast cancer patients treated with tamoxifen. The incorporation of pharmacogenetic tests into routine clinical practice is also necessary for the development of a health care system in Thailand.
Supplementary material
Table S1.
A combination of the ABCC2 and ABCB1 genotypes and bone-metastasis-free survival profile of individual patients
| Patient number | Combined genotypes of ABCC2 −24C>T and ABCB1 3435C>T | DFS time (years) |
|---|---|---|
| 1 | −24CT +3435TT | 7.8 |
| 2 | −24CC +3435CC | 5.7 |
| 3 | −24CC +3435TT | 9.8 |
| 4 | −24CT +3435CC | 6.1 |
| 5 | −24CT +3435CT | 8.6 |
| 6 | −24CC +3435CC | 10.2 |
| 7 | −24CC +3435CC | 6.8 |
| 8 | −24CT +3435TT | 8.3 |
| 9 | −24CT +3435CT | 7.9 |
| 10 | −24CT +3435CC | 8.9 |
| 11 | −24CC +3435CT | 5.6 |
| 12 | −24CC +3435CC | 7.3 |
| 13 | −24CC +3435CC | 5.2 |
| 14 | −24CC +3435CC | 10.1 |
| 15 | −24CT +3435TT | 5.0 |
| 16 | −24CC +3435CT | 8.9 |
| 17 | −24CT +3435TT | 8.9 |
| 18 | −24CT +3435CT | 10.5 |
| 19 | −24CT +3435CC | 14.3 |
| 20 | −24CT +3435CT | 7.3 |
| 21 | −24CC +3435CC | 8.4 |
| 22 | −24CT +3435CC | 5.1 |
| 23 | −24CT +3435CT | 5.0 |
| 24 | −24CC +3435CT | 5.0 |
| 25 | −24CT +3435TT | 8.1 |
| 26 | −24CT +3435CC | 10.3 |
| 27 | −24CT +3435CC | 7.3 |
| 28 | −24CC +3435CC | 5.6 |
| 29 | −24CC +3435CT | 6.2 |
| 30 | −24CC +3435CT | 11.4 |
| 31 | −24CC +3435CT | 5.8 |
| 32 | −24CT +3435TT | 5.5 |
| 33 | −24CC +3435CT | 5.6 |
| 34 | −24CT +3435CT | 5.5 |
| 35 | −24CC +3435CC | 7.5 |
| 36 | −24CT +3435CT | 5.2 |
| 37 | −24CC +3435CC | 5.4 |
| 38 | −24CT +3435CC | 5.5 |
| 39 | −24CC +3435TT | 11.8 |
| 40 | −24CC +3435CT | 4.6 |
| 41 | −24CC +3435TT | 4.5 |
| 42 | −24CC +3435CT | 3.8 |
| 43 | −24CC +3435CC | 3.0 |
| 44 | −24CC +3435TT | 2.7 |
| 45 | −24CC +3435CT | 1.2 |
| 46 | −24CC +3435CT | 0.6 |
| 47 | −24CT +3435CT | 1.2 |
| 48 | −24CC +3435CT | 0.5 |
| 49 | −24CT +3435CT | 1.8 |
| 50 | −24CC +3435CC | 1.3 |
| 51 | −24CC +3435CC | 1.0 |
| 52 | −24CT +3435CT | 2.1 |
Abbreviation: DFS, disease-free survival.
Acknowledgments
This research project was financially supported by the joint grant between the Faculty of Science and the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, and Thailand Research Fund (IR5780011). Additional support for this work has been provided by the Ramathibodi Cancer Center, Ramathibodi Hospital, Bangkok, Thailand.
Footnotes
Author contributions
All authors contributed toward data analysis, interpretation of the results, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 3.Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer. 2009;9(8):576–586. doi: 10.1038/nrc2683. [DOI] [PubMed] [Google Scholar]
- 4.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302(13):1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saladores P, Murdter T, Eccles D, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J. 2015;15(1):84–94. doi: 10.1038/tpj.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Sun Y, Yao L, et al. Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol. 2008;19(8):1423–1429. doi: 10.1093/annonc/mdn155. [DOI] [PubMed] [Google Scholar]
- 7.Kiyotani K, Mushiroda T, Sasa M, et al. Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci. 2008;99(5):995–999. doi: 10.1111/j.1349-7006.2008.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiyotani K, Mushiroda T, Imamura CK, et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28(8):1287–1293. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirachainan E, Jaruhathai S, Trachu N, et al. CYP2D6 polymorphisms influence the efficacy of adjuvant tamoxifen in Thai breast cancer patients. Pharmgenomics Pers Med. 2012;5:149–153. doi: 10.2147/PGPM.S32160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukasem C, Sirachainan E, Chamnanphon M, et al. Impact of CYP2D6 polymorphisms on tamoxifen responses of women with breast cancer: a microarray-based study in Thailand. Asian Pac J Cancer Prev. 2012;13(9):4549–4553. doi: 10.7314/apjcp.2012.13.9.4549. [DOI] [PubMed] [Google Scholar]
- 11.Rae JM, Drury S, Hayes DF, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(6):452–460. doi: 10.1093/jnci/djs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regan MM, Leyland-Jones B, Bouzyk M, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1–98 trial. J Natl Cancer Inst. 2012;104(6):441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HS, Choi JY, Lee MJ, et al. Association between genetic polymorphisms of CYP2D6 and outcomes in breast cancer patients with tamoxifen treatment. J Korean Med Sci. 2011;26(8):1007–1013. doi: 10.3346/jkms.2011.26.8.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Province MA, Goetz MP, Brauch H, et al. CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations. Clin Pharmacol Ther. 2014;95(2):216–227. doi: 10.1038/clpt.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 16.Tucker AN, Tkaczuk KA, Lewis LM, et al. Polymorphisms in cytochrome P4503A5 (CYP3A5) may be associated with race and tumor characteristics, but not metabolism and side effects of tamoxifen in breast cancer patients. Cancer Lett. 2005;217(1):61–72. doi: 10.1016/j.canlet.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Murdter TE, Schroth W, Bacchus-Gerybadze L, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89(5):708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 18.Wegman P, Elingarami S, Carstensen J, et al. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007;9(1):R7. doi: 10.1186/bcr1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iusuf D, Teunissen SF, Wagenaar E, et al. P-glycoprotein (ABCB1) transports the primary active tamoxifen metabolites endoxifen and 4-hydroxytamoxifen and restricts their brain penetration. J Pharmacol Exp Ther. 2011;337(3):710–717. doi: 10.1124/jpet.110.178301. [DOI] [PubMed] [Google Scholar]
- 20.Teh LK, Mohamed NI, Salleh MZ, et al. The risk of recurrence in breast cancer patients treated with tamoxifen: polymorphisms of CYP2D6 and ABCB1. AAPS J. 2012;14(1):52–59. doi: 10.1208/s12248-011-9313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi HK, Yang JW, Roh SH, Han CY, Kang KW. Induction of multidrug resistance associated protein 2 in tamoxifen-resistant breast cancer cells. Endocr Relat Cancer. 2007;14(2):293–303. doi: 10.1677/ERC-06-0016. [DOI] [PubMed] [Google Scholar]
- 22.Sensorn I, Sirachainan E, Chamnanphon M, et al. Association of CYP3A4/5, ABCB1 and ABCC2 polymorphisms and clinical outcomes of Thai breast cancer patients treated with tamoxifen. Pharmgenomics Pers Med. 2013;6:93–98. doi: 10.2147/PGPM.S44006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim JS, Chen XA, Singh O, et al. Impact of CYP2D6, CYP3A5, CYP2C9 and CYP2C19 polymorphisms on tamoxifen pharmacokinetics in Asian breast cancer patients. Br J Clin Pharmacol. 2011;71(5):737–750. doi: 10.1111/j.1365-2125.2011.03905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brauch H, Schroth W, Goetz MP, et al. Tamoxifen use in postmenopausal breast cancer: CYP2D6 matters. J Clin Oncol. 2013;31(2):176–180. doi: 10.1200/JCO.2012.44.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pratt VM, Zehnbauer B, Wilson JA, et al. Characterization of 107 genomic DNA reference materials for CYP2D6, CYP2C19, CYP2C9, VKORC1, and UGT1A1: a GeT-RM and Association for Molecular Pathology collaborative project. J Mol Diagn. 2010;12(6):835–846. doi: 10.2353/jmoldx.2010.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodges LM, Markova SM, Chinn LW, et al. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein) Pharmacogenet Genomics. 2011;21(3):152–161. doi: 10.1097/FPC.0b013e3283385a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghiasvand R, Adami HO, Harirchi I, Akrami R, Zendehdel K. Higher incidence of premenopausal breast cancer in less developed countries; myth or truth? BMC Cancer. 2014;14:343. doi: 10.1186/1471-2407-14-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Dowsett M, Forbes JF, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 29.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55(5):471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 31.Lim HS, Ju Lee H, Seok Lee K, et al. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007;25(25):3837–3845. doi: 10.1200/JCO.2007.11.4850. [DOI] [PubMed] [Google Scholar]
- 32.Areepium N, Panomvana D, Rungwanonchai P, Sathaporn S, Voravud N. Effects of CYP2D6 and UGT2B7 polymorphisms on pharmacokinetics of tamoxifen in Thai breast cancer patients. Breast Cancer (Dove Med Press) 2013;5:73–78. doi: 10.2147/BCTT.S47172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Sousa JA, Facina G, da Silva BB, Gebrim LH. Effects of low-dose tamoxifen on breast cancer biomarkers Ki-67, estrogen and progesterone receptors. Int Semin Surg Oncol. 2006;3:29. doi: 10.1186/1477-7800-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laechelt S, Turrini E, Ruehmkorf A, et al. Impact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and function. Pharmacogenomics J. 2011;11(1):25–34. doi: 10.1038/tpj.2010.20. [DOI] [PubMed] [Google Scholar]
- 35.Haenisch S, Zimmermann U, Dazert E, et al. Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenomics J. 2007;7(1):56–65. doi: 10.1038/sj.tpj.6500403. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiaohui S, Aiguo L, Xiaolin G, et al. Effect of ABCB1 polymorphism on the clinical outcome of osteosarcoma patients after receiving chemotherapy. Pak J Med Sci. 2014;30(4):886–890. doi: 10.12669/pjms.304.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HJ, Im SA, Keam B, et al. ABCB1 polymorphism as prognostic factor in breast cancer patients treated with docetaxel and doxorubicin neoadjuvant chemotherapy. Cancer Sci. 2015;106(1):86–93. doi: 10.1111/cas.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu J, Zhou BT, Yin JY, et al. ABCC2 polymorphisms and haplotype are associated with drug resistance in Chinese epileptic patients. CNS Neurosci Ther. 2012;18(8):647–651. doi: 10.1111/j.1755-5949.2012.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006;24(4):675–680. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
A combination of the ABCC2 and ABCB1 genotypes and bone-metastasis-free survival profile of individual patients
| Patient number | Combined genotypes of ABCC2 −24C>T and ABCB1 3435C>T | DFS time (years) |
|---|---|---|
| 1 | −24CT +3435TT | 7.8 |
| 2 | −24CC +3435CC | 5.7 |
| 3 | −24CC +3435TT | 9.8 |
| 4 | −24CT +3435CC | 6.1 |
| 5 | −24CT +3435CT | 8.6 |
| 6 | −24CC +3435CC | 10.2 |
| 7 | −24CC +3435CC | 6.8 |
| 8 | −24CT +3435TT | 8.3 |
| 9 | −24CT +3435CT | 7.9 |
| 10 | −24CT +3435CC | 8.9 |
| 11 | −24CC +3435CT | 5.6 |
| 12 | −24CC +3435CC | 7.3 |
| 13 | −24CC +3435CC | 5.2 |
| 14 | −24CC +3435CC | 10.1 |
| 15 | −24CT +3435TT | 5.0 |
| 16 | −24CC +3435CT | 8.9 |
| 17 | −24CT +3435TT | 8.9 |
| 18 | −24CT +3435CT | 10.5 |
| 19 | −24CT +3435CC | 14.3 |
| 20 | −24CT +3435CT | 7.3 |
| 21 | −24CC +3435CC | 8.4 |
| 22 | −24CT +3435CC | 5.1 |
| 23 | −24CT +3435CT | 5.0 |
| 24 | −24CC +3435CT | 5.0 |
| 25 | −24CT +3435TT | 8.1 |
| 26 | −24CT +3435CC | 10.3 |
| 27 | −24CT +3435CC | 7.3 |
| 28 | −24CC +3435CC | 5.6 |
| 29 | −24CC +3435CT | 6.2 |
| 30 | −24CC +3435CT | 11.4 |
| 31 | −24CC +3435CT | 5.8 |
| 32 | −24CT +3435TT | 5.5 |
| 33 | −24CC +3435CT | 5.6 |
| 34 | −24CT +3435CT | 5.5 |
| 35 | −24CC +3435CC | 7.5 |
| 36 | −24CT +3435CT | 5.2 |
| 37 | −24CC +3435CC | 5.4 |
| 38 | −24CT +3435CC | 5.5 |
| 39 | −24CC +3435TT | 11.8 |
| 40 | −24CC +3435CT | 4.6 |
| 41 | −24CC +3435TT | 4.5 |
| 42 | −24CC +3435CT | 3.8 |
| 43 | −24CC +3435CC | 3.0 |
| 44 | −24CC +3435TT | 2.7 |
| 45 | −24CC +3435CT | 1.2 |
| 46 | −24CC +3435CT | 0.6 |
| 47 | −24CT +3435CT | 1.2 |
| 48 | −24CC +3435CT | 0.5 |
| 49 | −24CT +3435CT | 1.8 |
| 50 | −24CC +3435CC | 1.3 |
| 51 | −24CC +3435CC | 1.0 |
| 52 | −24CT +3435CT | 2.1 |
Abbreviation: DFS, disease-free survival.


