Abstract
The use of chemical flame-retardants (FR) in consumer products has steadily increased over the last 30 years. Toxicity data exist for legacy FRs such as pentabromodiphenyl ether (pentaBDE), but less is known about effects of new formulations. To address this issue, the toxicity of seven FR chemicals and formulations was assessed on the freshwater crustacean Daphnia magna. Acute 48-h nominal LC50 values for penta- and octabromodiphenyl ether (pentaBDE, octaBDE), Firemaster 550 (FM550), Firemaster BZ-54 (BZ54), bis(2-ethylhexyl) tetrabromophthalate (BEH-TEBP), triphenyl phosphate (TPhP), and nonbrominated BEH-TEBP analog bis(2-ethylhexyl) phthalate (BEHP) ranged from 0.058 mg/L (pentaBDE) to 3.96 mg/L (octaBDE). mRNA expression, 1H NMR-based metabolomic and lipidomic profiling at 1/10 LC50 revealed distinct patterns of molecular response for each exposure, suggesting pentaPBDE affects transcription and translation, octaBDE and BEH-TEBP affect glycosphingolipid biosynthesis and BZ54 affects Wnt and Hedgehog signal pathways as well as glycosaminoglycan degradation. Brominated components of FM550 (i.e., BZ54) were significantly higher in Daphnia after 48 h following 1/10 LC50 exposure. FM550 elicited significant mRNA changes at five concentrations across a range from 1/106 LC50 to 1/2 LC50. Analyses suggest FM550 impairs nutrient utilization or uptake in Daphnia.
Graphical Abstract
INTRODUCTION
The use of chemical flame-retardants (FR) in furniture became common in the United States and around the world in the 1970s. The state of California, for example, passed TB117, a mandate requiring the use of FR chemicals in all upholstered furniture. FRs such as polybrominated diphenyl ethers (PBDE) were used to meet this requirement.1,2 Recent laws amended decades-old flammability standards,3 but FR chemicals are still used in consumer products and are present in homes and the environment. Leaching of PBDEs from consumer products led to global contamination in soil,4 sewage sludge,5 costal sediments,6 the atmosphere,7 and the arctic.8 PBDEs have been detected in biota including sea turtle eggs,9 lake trout, Chinook salmon,10 humpback dolphins,11 and frogs.12 Humans can be exposed to PBDEs through dietary sources and from inadvertent ingestion of contaminated house dust particles.13,14 Toxicity data for PBDEs and other FRs exist for humans and terrestrial animals (see Dishaw et al. 2014 for a recent review15).
PBDEs can accumulate in marine copepods,16,17 affect molting and cause toxicity in aquatic invertebrates,18 and biomagnify in aquatic food chains.19 Studies on Daphnia manga show that hexaBDE affects reproduction in the µg/L range.20 Tetra- and triBDE can delay molting in adult daphnids,21 while pentaBDE changes retinoid status in zebrafish.22 PBDEs induce many adverse effects, but specific molecular mechanisms of toxicity are not fully understood.23
PBDEs are found globally and therefore have the potential to affect terrestrial and aquatic organisms. As persistent and bioaccumulative chemicals prone to long-range global transport,24,25 pentaBDE and octaBDE were banned from use in the European Union, added to the Stockholm Convention as Annex A chemicals slated for elimination, and voluntarily phased-out of use in the United States.24,26–28 As a result, levels of PBDEs are decreasing in homes, and there is a corresponding increase in a pentaBDE replacement chemical, Firemaster 550 (FM550).29
FM550 was first used as a pentaBDE replacement in 2004.2 It is a mixture of four different chemicals: two brominated components, bis (2-ethylhexyl) tetrabromophthalate (BEH-TEBP, 8%) and 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB, 30%), and two aryl phosphate ester compounds, triphenyl phosphate (TPhP, 17%) and isopropylated triaryl phosphates (ITP, 45%). ITP is a mixture of ortho-, meta-, and para-substituted isomers of mono-, di-, tri-, and tetra-ITPs.30–32 Related mixture Firemaster BZ54 consists of BEH-TEBP (30%) and EH-TBB (70%).33
FM550 is applied to polyurethane foam in furniture34 and some infant products.35 The brominated components BEH-TEBP and EH-TBB have been detected in house dust,31,36 marine biota,11 mysid shrimp,37 a bivalve, and a gastropod.38 FM550 component triphenyl phosphate (TPhP, also called TPP) is a EU high-production volume plasticizer and flame-retardant that can enter the environment by diffusive volatilization, leaching, and abrasion.39 It has been detected in sewage treatment plant influent and effluent,40 air, water, house dust, and sediment.41
In fathead minnow liver cells, EH-TBB and BEH-TEBP caused a significant increase in DNA strand breaks during exposure but not after a recovery period.42 Fathead minnow and carp hepatic subcellular fractions metabolize EH-TBB and BEH-TEBP in vitro.33 EH-TBB and BEH-TEBP affect fecundity, perhaps through an endocrine effect, in Japanese medaka.43 TPhP and monoITP exposures affect zebra fish embryo heart development through AhR-independent (TPhP, monoITP) and AhR-dependent (monoITP) pathways.32,44 96-h LC50 values for TPhP on fish species range from 0.3 to 1.2 mg/L, but can be as high as 300 mg/L; LC50 values for TPhP on Daphnia range from 1 to 1.35 mg/L.45 The present study is the first to look at mechanistic effects of FM550 to the lower-level trophic species Daphnia magna.
Aquatic toxicants are commonly divided into categories based on chemical properties and presumed mechanism of action: nonpolar hydrophobic chemicals causing Type I narcosis, polar hydrophobic chemicals causing Type II narcosis, unselective reactive chemicals, and chemicals with specific modes of action.46 Hydrophobic chemicals with a log Kow ≥ 2.7 are often considered narcotics. Toxicity attributed to narcosis is postulated to result from chemical disruption of lipid-based cellular membranes.47,48 Type I molecules are believed to move three-dimensionally through membranes, while Type II molecules interact with charged phospholipid headgroups;49 but toxicity is attributed to a shared mechanism.48 Some hydrophobic chemicals cause greater toxicity than is predicted from Kow,49 which cannot be attributed to narcosis.46
Daphnia are parthenogenetic filter feeders used to evaluate invertebrate response to environmental pollutants.50,51 Exposure of biota to FRs may occur through disposal of consumer goods containing FRs and subsequent leaching, and it is therefore important to understand the effects of FRs on environmentally relevant Daphnia manga. This work represents the first comprehensive analysis of the acute toxicity of seven emerging and legacy chemical FR formulations: PentaBDE, octaBDE, FM550, BZ54, BEH-TEBP, TPhP, and BEHP. Our goals were to determine LC50 values for each chemical and formulation and to investigate molecular mechanisms of toxicity by assessing effects on mRNA with microarrays, metabolic changes by 1H NMR-based metabolomics and lipidomic profiling. In addition, we determined if the bromine-containing FM550 components accumulate in Daphnia, and identified potential biomarkers of exposure for FM550.
MATERIALS AND METHODS
Daphnia Culture
Daphnia magna were cultured asexually in a growth chamber (Conviron) at 21 ± 1 °C with 16 h of light and 8 h of dark per day in COMBO52 media. They were fed 1 mL/L Pseudokirchneriella subcapitata (formerly Selenastrum capricornutum) from a 3.0 × 107 cells/mL stock and 1 mL/L yeast cereal-leaf and trout chow mix (YCT) three times per week following renewal of media. Media was aerated overnight to increase dissolved oxygen levels. pH was maintained at 7.4–7.8. Media chemical composition is described in Supporting Information (SI) Table S1. All chemical exposures were done in glass beakers (acute: 50 mL, for RNA extraction: 1 L, for absorption: 4 L). Animals and feed were obtained from Aquatic Research Organisms.
Toxicity Assays
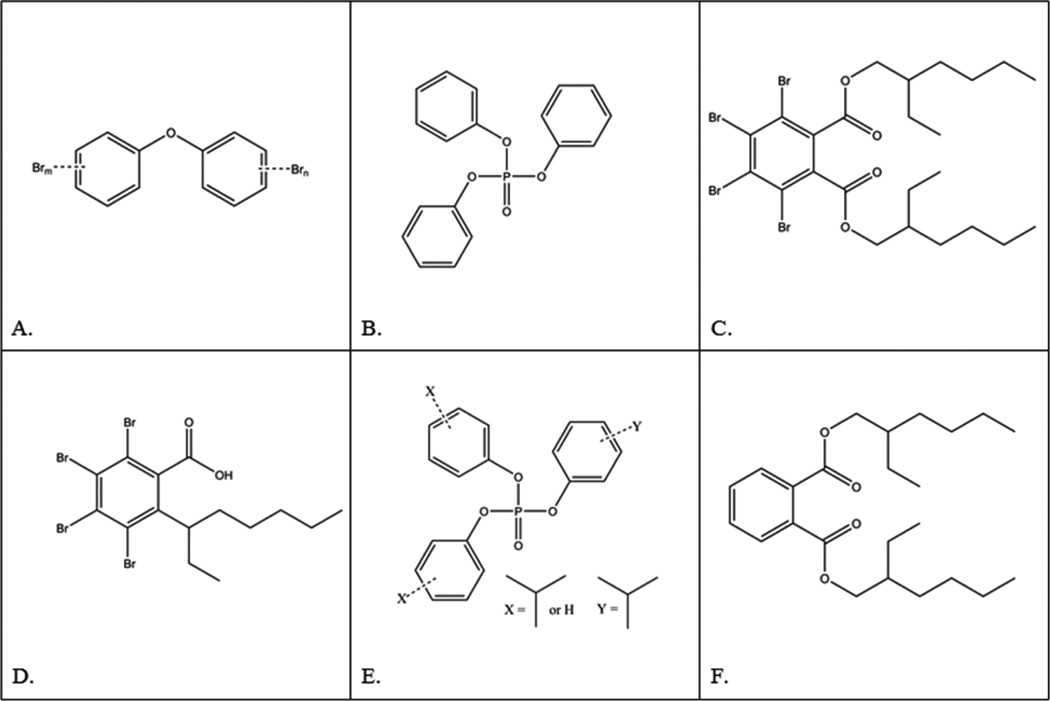
Acute toxicity assays were similar to U.S. EPA Whole Effluent Toxicity guidelines.53 Five first instar (<24 h old) daphnids were added to 35 mL COMBO media. Chemical FRs were dissolved in 0.05–0.1% dimethyl sulfoxide (DMSO) (EMD Chemicals, Inc.). Typically, four replicates of five daphnids were exposed to five different concentrations of FR or DMSO-control simultaneously. Concentrations tested can be found in SI Table S2. At least three sets of four replicates each were conducted per FR, with the exception of BZ54, for which a significant LC50 was determined after fewer exposures. Animals were fed algae after 24 h, and lethality was measured after 48 h. Acute LC50 values were determined using probit53 or Spearman–Karber method.54 Three statistical methods were used to investigate correlation between log Kow and log LC50 for each compound (SI). Briefly, log Kow values were taken from published studies and, for PBDEs, log Kow values were weighted by congener abundance (SI Table S3).32,55–58 Chemicals were manufactured by Chemtura (FM550 and BZ54), Unitex (BEH-TEBP), Aldrich (TPhP), Larodan Chemicals (pentaBDE), Bromine Compounds Ltd. (octaBDE), and Aldrich (BEHP). Chemical abbreviation nomenclature is from Bergman et. al 2012.59 Chemical structures are shown in Figure 1. All subsequent assays were done at 1/10 LC50 (1/10 of one toxic unit) or dilutions thereof.
Figure 1.
Molecular structures of chemical flame-retardants. A. Polybromodiphenyl ether. B. Triphenyl phosphate (TPhP). C. bis(2-ethylhexyl) tetrabromophthalate (BEH-TEBP). D. bis(2-ethylhexyl) tetrabromobenzoate (EH-TBB). E. Isopropylated triaryl phosphates (ITP). F. bis(2-ethylhexyl) phthalate (BEHP). B–E are components of Firemaster 550. C and D are components of Firemaster BZ-54. E contains a mixture of molecules with one, two or three isopropyl-substituted phenol rings.
Uptake of FM550
100 adult (14 day old) daphnids were exposed in 4 L COMBO media to 1/10 LC50, 1/10 000 of the LC50 (0.0486 mg/L, 0.0486 µg/L), or to a DMSO control. Exposure concentrations are based on LC50 values and are not necessarily environmentally relevant. Animals were fed algae after 24 h, removed from exposure media at 48 h and put in chemical-free media with food for 24 h to clear the alimentary canal. Animals were removed and frozen at –80 °C for analytical analyses, or, for microarray analyses, RNA was extracted immediately with methods described below. Exposures were repeated until a total of four biological replicates of ~400 daphnids were collected per concentration. Samples were analyzed for EH-TBB and BEH-TEBP concentration using gas chromatography mass spectrometry operated in negative chemical ionization mode (GC/ECNI-MS).31 Detailed methods are in the SI. A Welch two-sample t test was performed to determine significant differences in concentration of FRs between exposure groups. Samples with p-value <0.05 were considered significant. EH-TBB was not detected in laboratory blanks (<0.5 ng) but was detected in the control samples (83.6 ±22.8 ng). BEH-TEBP was not detected in laboratory blanks (1.2 ± 1.1 ng) and was above the method detection limit (MDL) in one control sample.
BEH-TEBP and EH-TBB were also measured in the YCT daphnia food. One L YCT was centrifuged in 250 mL aliquots at 3000 rpm for 20 min. The aqueous supernatant was removed and samples were dried at 80 °C for 4 h. Dried samples were then analyzed with the above analytical and computational methods.
mRNA Microarray
Effects of exposure to each chemical (pentaBDE, octaBDE, FM550, BZ54, BEH-TEBP, TPhP, or BEHP) was assayed via mRNA microarray. For each chemical, 15–20 adult (14 day old) daphnids were exposed to 1/10 LC50 or to an equal-volume DMSO control in 800 mL COMBO media. Animals were fed algae after 24 h, and after 48 h RNA was extracted in Trizol reagent (Invitrogen) with a hand-held homogenizer (Biospec Products Inc.). RNA quality was assessed with spectrophotometry and agarose gel electrophoresis. 200 ng RNA was then reverse-transcribed, amplified and hybridized onto a custom Agilent Oligonucleotide DNA microarray (AMADID #023710) with the Agilent Low-Input Quick-Amp one-color array kit and protocol (Santa Clara, CA). Four exposed and three or four control arrays were done for each condition. Arrays were scanned with a 16-bit GenePix 4000B Microarray Scanner with 5-µm resolution. Features were edited and regression analysis was performed with GenePix Pro, and the resultant data were processed as in Loguinov et al.60 Detailed statistical analysis methods are available in the SI. Array data are available at http://www.ncbi.nlm.nih.gov/geo.
Quantitative Reverse Transcription PCR
To independently verify microarray results, transcription of nine genes in ten conditions was analyzed with qPCR. Genes were chosen based on q-value, degree of differential transcription or potential mode of toxicity. One µg RNA (from independent biological replicates) was reverse transcribed and amplified as in Scanlan et al.61 Actin and GAPDH were used as housekeeping genes. Primer sequences are shown in SI Table S4. Detailed methods are available in the SI.
Gene Ontology, Pathway Enrichment and Cluster Analysis
The Daphnia magna array was annotated with a protein blast as in Antczak et al. 2013,62 which identified 4958 Daphnia pulex homologues with an expect (E) value less than or equal to 10−4. The list was matched with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database annotation (www.genome.jp/kegg) and genes were sorted into respective pathways. Of these, 1425 mapped onto 114 Daphnia pulex KEGG pathways. Pathways representing less than five genes in the array were removed, leaving 95 pathways and 1402 genes total in an unbiased sample of the original 371 KEGG pathways. Significance was calculated using a modified Fisher Exact Probability p-value.63,64
The R package hopach (implementation of Hierarchical Ordered Partitioning And Collapsing Hybrid algorithm65) from bioconductor.org was applied to data to compute ordered distance matrices using the cosangle-based distance metric65 as a measure of similarity. HOPACH is considered superior to standard hierarchical clustering because it applies nonparametric bootstrap resampling to determine the probability of “cluster membership” for each gene or chemical. Nonparametric strapping (resampling with replacement) analyses were run 1000 times to determine cluster membership probability for each gene and each main cluster. Further methodological and statistical details are in the SI.
Blast2GO66 (B2G) gene ontology enrichment analysis was performed on mRNA data to determine gene functions affected in each exposure. Microarray gene probes and EST sequences were annotated with B2G (default parameters) to create a reference gene set. Data for each chemical was compared to the reference set via Enrichment Analysis (default, two-tailed settings). Enrichment was performed with 0.01, 0.05, and 0.15 False Discovery Rates (FDR, measure of significance).
Metabolomics of FM550 and pentaBDE-exposed Daphnia
For instrument optimization, hemolymph from 80 unexposed adult (14 day old) daphnids was collected from daphnids incubated in 800 mL COMBO media with feeding after 24 h. After 48 h, hemolymph was extracted by piercing the carapace with a needle and aspirating hemolymph with a small pipet.52 For metabolomics analysis, animals were exposed to 1/10 LC50 FM550 or pentaBDE or DMSO control (with equal volume chemical), fed after 24 h and frozen on dry ice after 48 h. Seven biological replicates of 40 animals each were collected for each condition and stored at −80 °C. Hemolymph was extracted using a dual phase extraction. Detailed extraction, analysis and statistical methods are available in the SI. Briefly, a mixture of methanol, chloroform and water in the volume ratio of 4:4:2.85 was used to generate a two-phase extract.67 1H NMR spectra were acquired at 20 °C on an Agilent Inova 600 MHz NMR spectrometer with a cryogenic triple-resonance flow probe using direct-injection NMR analysis.68,69 Multivariate data analysis, principal components analysis and partial least-squares discriminant analysis were performed with SIMCA-P+ (Umetrics Inc., Umea, Sweden); univariate analysis of the binned spectra was conducted using Excel. Results were further analyzed with MetaboAnalyst 2.0 (http://www.metaboanalyst.ca)70,71 to determine overrepresentation of biological function groups and biological pathways.
Lipidomics of FM550 and pentaBDE-exposed Daphnia
Daphnids were exposed to 1/10 LC50 FM550 or pentaBDE or DMSO control, as described above for mRNA assays. After 48 h, animals were removed from culture, flash-frozen on dry ice, ground in 1.6 mL ultrapure water with a hand-held homogenizer (Biospec Products Inc.) and frozen at −80 °C. Lipids were extracted and analyzed by the Kansas State University Lipidomics Research Center. Five replicates of 10–11 animals were collected for each condition. Data were log transformed and significance was determined with a standard two-sample t test and a Wilcox rank sum test for two sample data. Additional details on extraction methods and statistical analyses are available in SI.
RESULTS
Flame-Retardant Toxicity in Daphnia magna
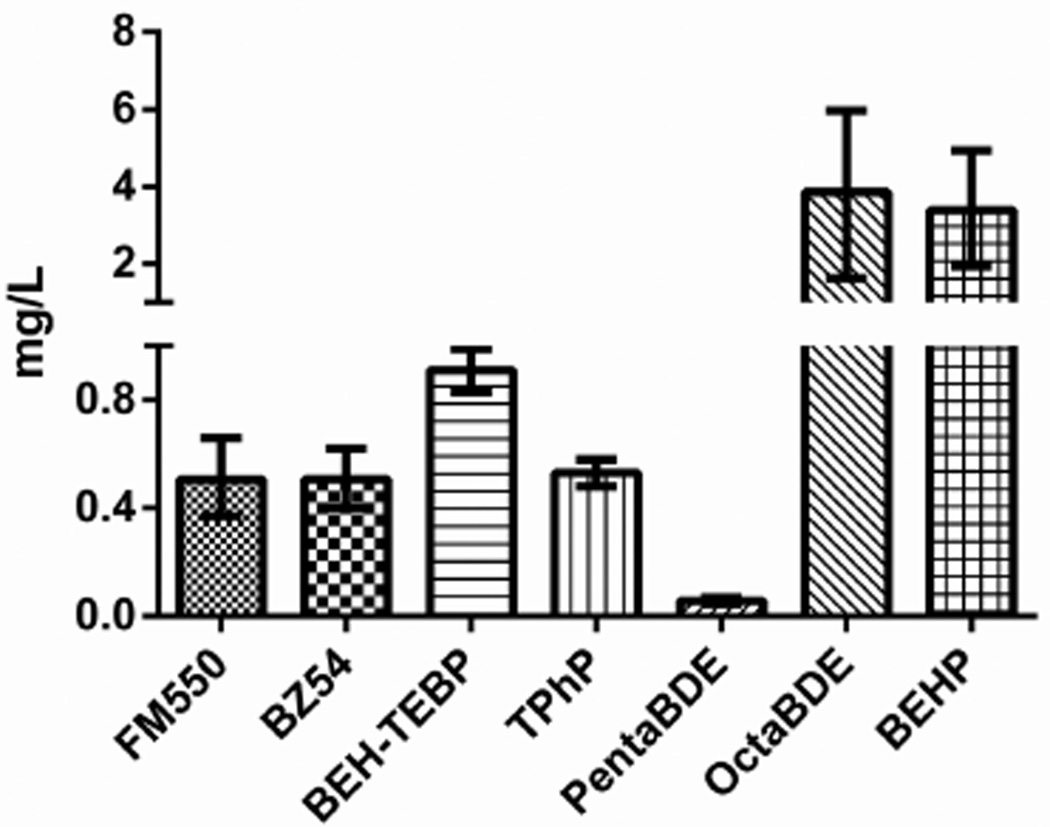
LC50 values were determined for each FR after a 48 h exposure (SI Table S5 and Figure 2) and are reported as milligrams chemical per liter COMBO media (mg/L). PentaBDE was significantly more toxic than any other FR (LC50 = 0.058 mg/L); the second most toxic chemical was 1 order of magnitude less toxic (FM550, 0.0486 mg/L). Significance was determined by binary comparison of LC50 values and corresponding confidence intervals.72 OctaBDE and BEHP were the least toxic (3.96 and 3.31 mg/L). Of note, BEHP was significantly less toxic to Daphnia than its brominated homologue BEH-TEBP. No correlation was found between the log Kow and the log LC50 for each compound (SI), although sample size precludes robust statistical confidence.
Figure 2.
Acute, 48-h LC50 values for flame-retardants on Daphnia magna. LC50 values were determined with probit or Spearman–Karber statistical programs. Error bars represent 95% confidence interval.
FM550 Detected in Daphnia magna after a 48-h Exposure to 1/10 LC50
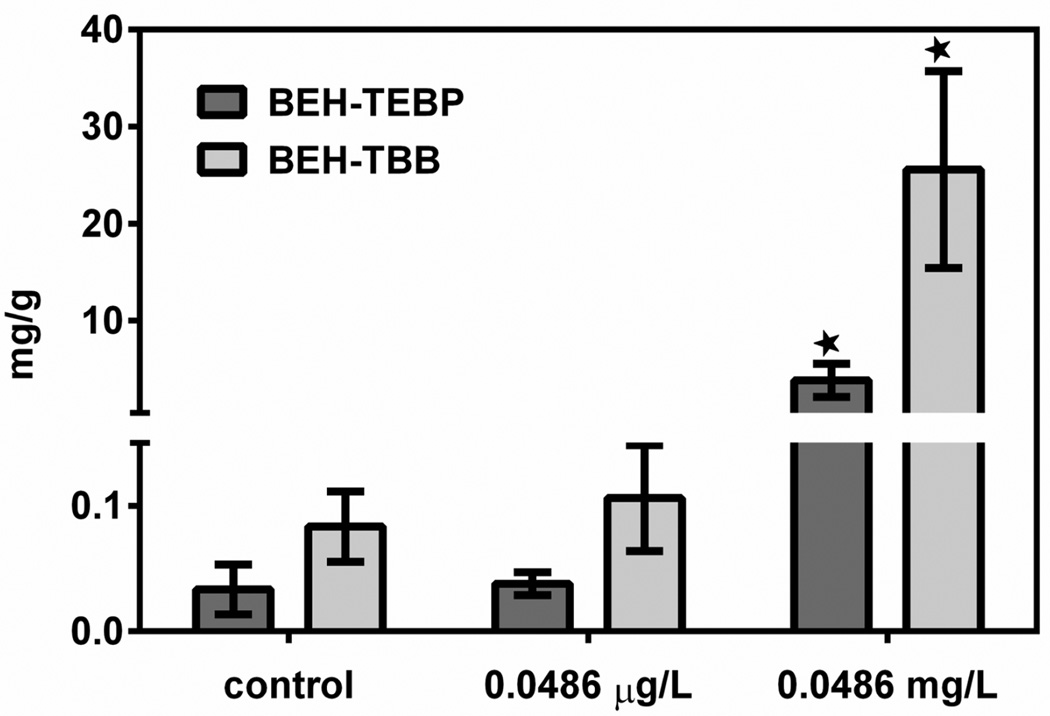
Daphnids exposed to the higher concentration (0.0486 mg/L) for 48 h showed a significant increase in both BEH-TEBP and EH-TBB as compared to control (Figure 3). Exposure at the lower concentration (0.0486 µg/L) was not significantly higher than control. Daphnid food YCT had an average of 0.625 ng/g (dry) EH-TBB and 0.629 ng/g BEH-TEBP (n = 3). Measured concentrations are reported in SI Table S6.
Figure 3.
Absorption of BEH-TEBP and EH-TBB was measured after 48-h exposure to 0.0486 µg/L (1/10 LC50) or 0.0486 mg/L FM550. Concentrations: nanograms chemical per gram daphnid, dry weight (ng/g).
Each Flame-Retardant Caused Distinct Patterns of Change in mRNA levels at 1/10 LC50
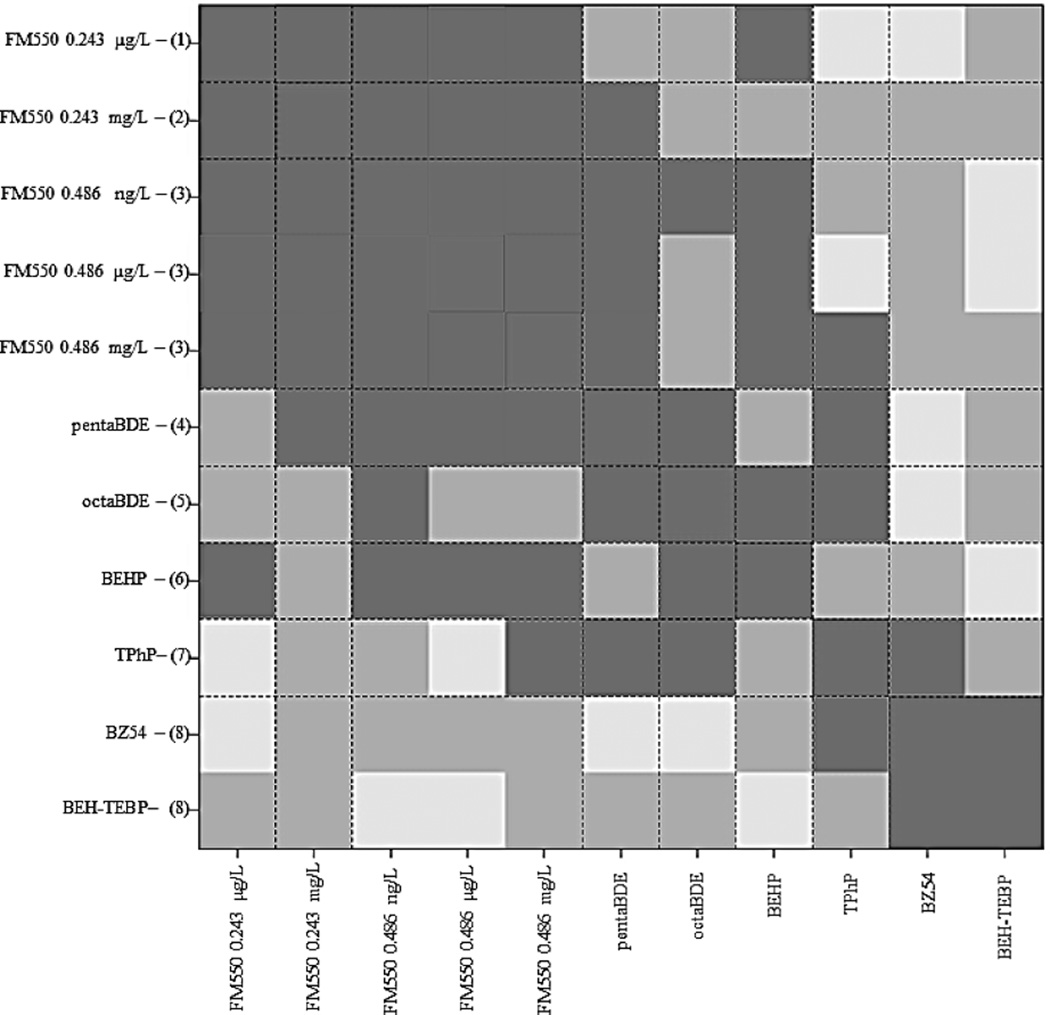
mRNA microarray, qPCR and computational analyses were used to investigate and compare biological effects of FR exposure. SI Table S7a lists the number of differentially expressed genes in each condition. qPCR independently verified microarray results for 25 out of 29 genes tested (SI Table S8). HOPACH cluster analysis of differential mRNA levels grouped BEH-TEBP and BZ54 together, while all other FRs clustered individually (Figure 4). KEGG pathway enrichment analysis found a total of 12 pathways overrepresented (Table 1), four in common to two conditions.
Figure 4.
HOPACH cluster analysis of chemical profiles shows visual representation of the corresponding ordered distance matrix. Darker colored squares indicate more similarity. Clusters are numbered 1–8 on the y-axis. Three FM550 concentrations were significantly similar (cluster 3), as were BZ54 and BEH-TEBP (cluster 8).
Table 1.
KEGG Pathway Analyses of Gene Transcription Data Show That Exposure to Flame-Retardants Largely Affected Different Biological Pathwaysa
| biological pathways affected by exposure to different chemical flame-retardants | ||||||
|---|---|---|---|---|---|---|
| KEGG biological pathway | FM550 | octaBDE | pentaBDE | BZ54 | BEH-TEBP | BEHP |
| ribosome | 0.061 | 0.030 | ||||
| glycosphingolipid biosynthesis | 0.086 | 0.089 | ||||
| spliceosome | 0.066 | |||||
| pyrimidine metabolism | 0.002 | |||||
| proteasome | 0.074 | |||||
| WNT signaling pathway | 0.089 | |||||
| porphyrin and chlorophyll metabolism | 0.020 | 0.026 | ||||
| glycosaminoglycan degradation | 0.052 | |||||
| hedgehog signaling pathway | 0.052 | |||||
| arginine and proline metabolism | 0.083 | |||||
| tyrosine metabolism | 0.048 | 0.042 | ||||
| phenylalanine metabolism | 0.044 | |||||
Numbers in this graph represent the p-value of each analysis. Because annotation of the array is limited, p-values up to 0.1 were included.
FM550 Dose Response: All Concentrations Changed mRNA
Microarrays analysis was performed with RNA from daphnids exposed to five FM550 concentrations (N = four replicates of ~20 animals each). Concentrations were: 1/2 LC50 (0.243 mg/L), 1/10 LC50 (0.0486 mg/L), and three additional dilutions: 0.243 µg/L, 0.0486 µg/L, and 0.0486 ng/L. Exposure at 1/10,000 LC50 (0.0486 µg/L) resulted in the largest number of differentially expressed genes when compared to the untreated control. All concentrations caused differential mRNA levels; the no-observed transcriptional effect level73,74 was not reached (SI Table S7b). Three genes (a trichohyalin-like protein, peroxidase and an unknown protein) were differentially expressed at each concentration (Table 2a). When compared to other FRs, HOPACH clustered all concentrations together, with three concentrations significantly similar to each other (SI Figure S1). KEGG pathway enrichment found significant enhancement of pathways (p-value ≤0.1) for three concentrations (0.0486 mg/L, 0.0486 µg/L, and 0.0486 ng/L). A total of seven pathways were affected; each was unique to one concentration (SI Table S9). Analyses for linear trend75–77 found three genes significantly, negatively associated with concentration (Table 2b) and a large decrease in gene response at the highest concentrations (not shown). Clustering based on mRNA levels found 44 clusters (not shown). B2G66 gene ontology analysis with GOSSIP78 on the largest cluster indicated overexpression of functions related to oxygen binding, oxygen transporter activity and the hemoglobin complex (SI Table S10).
Table 2.
Potential Gene Transcription Biomarkers of Daphnia Exposure to Firemaster 550a
| A. genes differentially expressed in all Firemaster 550 exposures | ||||||
|---|---|---|---|---|---|---|
| gene ID | 0.243 mg/L | 0.0486 mg/L | 0.0486 µg/L | 0.243 µg/L | 0.0486 ng/L | gene function |
| DM05947 | −1.3 | −1.3 | −1.3 | −1.3 | −2 | peroxidase |
| DM12670 | −1.1 | −1.6 | −2.3 | −2 | −2.3 | unknown |
| DM09101 | −1.1 | −1.6 | −1.9 | −1.8 | −2.2 | ABC transporter |
| B. genes associated linearly with FM550 concentration | ||||||
|---|---|---|---|---|---|---|
| gene ID | trend | slope | rawp | Holm | BH | gene function |
| DM02757 | negative | −0.0129 | 6.7 × 10−05 | 0.0226 | 0.0226 | eukaryotic translation initiation factor |
| DM12108 | negative | −0.0328 | 1.9 × 10−04 | 0.0646 | 0.0324 | unknown |
| DM12547 | negative | −0.0032 | 4.0 × 10−04 | 0.1343 | 0.0450 | unknown |
RNA sequences from the microarray (Gene ID) were functionally annotated with Blast2Go. Part A shows log2 change of differential gene transcription in exposed Daphnia magna compared to control. Part B lists genes that showed a small but significant negative linear trend with increasing concentration. Holm: correction for multiple comparisons. BH (Benjamini–Hochberg procedure): controls false discovery rate.
FM550 Metabolite Profiles Are Distinguishable from Control, while PentaBDE Profiles Are Not
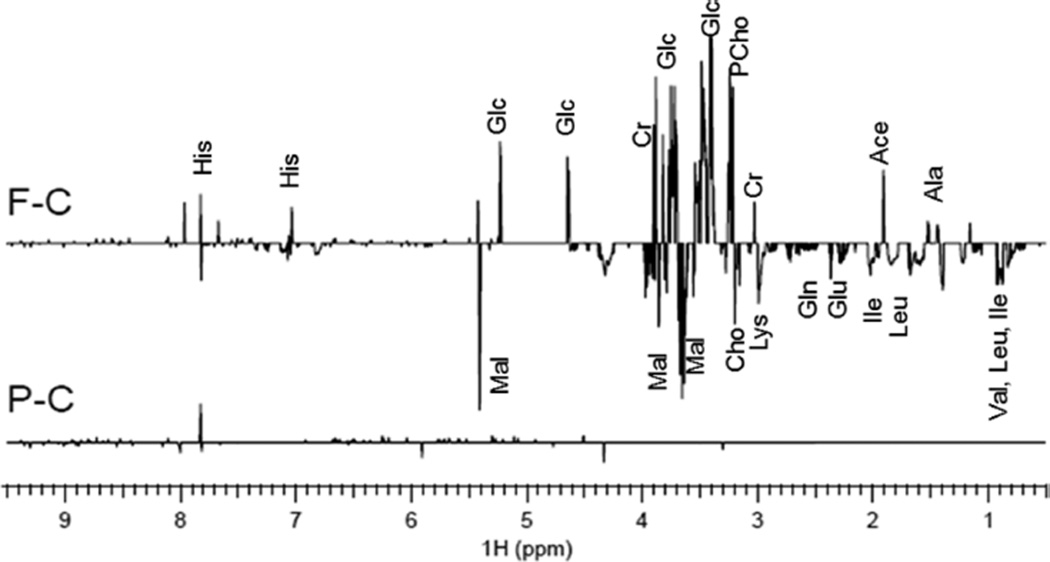
1H NMR was used to investigate changes in hemolymph metabolomic profiles between control and 1/10 LC50-exposed Daphnia (Figure 5). PLS-DA scores plots (SI Figure S2) show considerable separation between control and FM550-exposed daphnids (validated with ANOVA, p-value = 0.04). FM550 exposure changed levels of 14 small-molecule metabolites (Table 3). Pathway analysis with MetaboAnalyst showed an increase in nitrogen metabolism, arginine and proline metabolism, a decrease in valine, leucine, and isoleucine degradation and changes in aminoacyl-tRNA biosynthesis (Table 4). Enrichment analysis with MetaboAnalyst showed an increase in ammonia recycling, a decrease in valine, leucine, and isoleucine degradation, and changes in protein biosynthesis (Table 5). There was no significant difference between pentaBDE-exposed and control groups (Figure 5 and SI Figure S2).
Figure 5.
1H NMR t test filtered difference spectra showing metabolomics changes in Daphnia exposed to 1/10 LC50 FM550 (F–C) or pentaBDE (P–C) as compared to control. The t test filtered difference spectra were obtained using 7 replicates for each class. Abbreviations: acetate (Ace), alanine (Ala), choline (Cho), creatine (Cr), glucose (Glc), glutamate (Glu), glutamine (Gln), histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), malate (Mal), phosphocholine (PCho), and valine (Val). Spectra were t test filtered difference and include the p-values (less than 0.05).
Table 3.
Small Molecule Metabolomics Changes in FM550-Exposed Daphnidsa
| metabolite | abbreviation | % change | p-value (t test) |
|---|---|---|---|
| Histidine | His | 37 | 0.0140 |
| Glucose | Glc | 24 | 0.0010 |
| Phosphocholine | PCho | 17 | 0.0020 |
| Glutamine | Gln | 11 | 0.0005 |
| Creatine | Cre | 10 | 0.0110 |
| Acetate | Ace | 7 | 0.0470 |
| Alanine | Ala | 6 | 0.0043 |
| Valine | Val | −12 | 0.0280 |
| Glutamate | Glu | −14 | 0.0007 |
| Choline | Cho | −16 | 0.0070 |
| Leucine | Leu | −16 | 0.0120 |
| Isoleucine | Ile | −17 | 0.0240 |
| Malate | Mal | −23 | 0.0030 |
| Lysine | Lys | −24 | 0.0004 |
Analysis was done with HNMR and subsequent statistical analyses. Data is from seven exposed and control biological replicates of 40 daphnids each.
Table 4.
Molecular Pathway Analysis on Metabolomic Data from FM550-Exposed Daphidsa
| pathway analysis of Firemaster 550 metabolomic data | |||||||
|---|---|---|---|---|---|---|---|
| increased metabolites | |||||||
| totalb | expectedc | hitsd | raw pe | Holm adjustf | FDRg | organismh | |
| nitrogen metabolism | 9 | 0.047 | 2 | 8.29 × 10−04 | 0.067 | 0.067 | D.r.h |
| arginine and proline metabolism | 43 | 0.225 | 2 | 0.019 | 1 | 0.699 | D.r. |
| aminoacyl-tRNA biosynthesis | 67 | 0.350 | 2 | 0.044 | 1 | 0.749 | D.r. |
| decreased metabolites | |||||||
|---|---|---|---|---|---|---|---|
| totalb | expectedc | hitsd | raw pe | Holm adjustf | FDRg | organismh | |
| valine, leucine and isoleucine biosynthesis | 13 | 0.090 | 3 | 5.62 × 10−05 | 0.004 | 0.004 | D.r. and D.m. |
| aminoacyl-tRNA biosynthesis | 67 | 0.463 | 4 | 5.28 × 10−04 | 0.041 | 0.021 | D.r. and D.m. |
| valine, leucine and isoleucine degradation | 35 | 0.242 | 3 | 0.001 | 0.093 | 0.032 | D.r. and D.m. |
Analysis was performed with MetaboAnalyst 2.0 and included D. rerio (D.r.) and D. melanogaster (D.m.) pathways with more than one hit and a raw p-value <0.05.
Total genes in biological pathway.
Number of genes expected in a random data set.
Number of hits in FM550 data.
p-Value calculated from raw data.
p-Value adjusted for multiplicity.
False discovery rate.
Organism metabolome that resulted in significant findings.
Also seen in D. melanogaster. If pathway was affected in both species, numbers represent data for the more significant species.
Table 5.
Enrichment Analysis on Metabolomic Data from FM550-Exposed Daphnids Compared to All Known Metabolites in the MetaboAnalyst Databasea
| enrichment analysis of Firemaster 550 metabolomic data | ||||||
|---|---|---|---|---|---|---|
| increased metabolites | ||||||
| totalb | expectedc | hitsd | raw pe | Holm pf | FDRg | |
| ammonia recycling | 18 | 0.153 | 2 | 0.009 | 0.709 | 0.394 |
| protein biosynthesis | 19 | 0.161 | 2 | 0.010 | 0.779 | 0.394 |
| decreased metabolites | ||||||
|---|---|---|---|---|---|---|
| totalb | expectedc | hitsd | raw pe | Holm pf | FDRg | |
| protein biosynthesis | 19 | 0.161 | 4 | 6.77 × 10−06 | 0.001 | 0.001 |
| valine, leucine and isoleucine degradation | 36 | 0.305 | 3 | 0.002 | 0.187 | 0.095 |
Biological functions with more than one hit and with raw p ≤ 0.05 are included as significant.
Total genes in biological pathway.
Number of genes expected in a random data set.
Number of hits in FM550 data.
p-Value calculated from raw data.
p-Value adjusted for multiplicity.
False discovery rate.
Changes in Lipidome Were Detected after Exposure to both FM550 and PentaBDE
To complement transcriptomic and metabolomic studies, changes in the lipidome of FM550 and pentaBDE-exposed animals were analyzed. Both pentaBDE and FM550 significantly changed the level of two fats out of 352 tested (p-value ≤0.04, Table 6). FM550 increased a phosphatidylcholine with 42 carbons and seven double bonds (PC 42:7), while pentaBDE increased lysophosphatidylcholine (LPC 20:0). Both increased phosphatidylcholine (PC 44:9).
Table 6.
Changes in Daphnia magna Lipidomea
| lipidome changes after flame-retardant exposure | ||||
|---|---|---|---|---|
| mass of lipid | formula | name | exposure | fold change |
| 552.4 | C28H58O7PN | LPC(20:0) | pentaBDE | 6.60 |
| 884.6 | C52H86O8PN | PC(44:9) | pentaBDE | 2.18 |
| 860.6 | C50H86O8PN | PC(42:7) | FM550 | 1.36 |
| 884.6 | C52H86O8PN | PC(44:9) | FM550 | 2.91 |
Endogenous lipids were measured after 48 h exposure to 1/10 LC50 pentaBDE or 1/10 LC50 FM550 or to solvent-exposed controls.
p-value ≤0.04.
LPC: lysophosphatidylcholine, PC: phosphatidylcholine.
DISCUSSION
Flame-Retardants Are Toxic to Daphnia magna
LC50 values show that FM550 and its components are highly toxic to aquatic organisms as defined by the U.S. EPA (LC50 values of 0.1–1 mg/L). In situ toxicity to Daphnia magna could cause ecologically relevant physiological disruptions and results in wide-reaching effects in freshwater ecosystems.79 PentaBDE was one to 2 orders of magnitude more toxic than any other FR; octaBDE was one of the least toxic. The phenomenon of increased toxicity with less-brominated PBDEs has been seen in other organisms,80–82 but the trend is not always true in Daphnia magna.21 BZ54, which contains BEH-TEBP and EH-TBB, was significantly more toxic than BEH-TEBP alone. TPhP, BEHP, and BZ54 LC50 values agree with those previously derived.32,83–85 Actual dissolved chemical concentrations for each FR are unknown as quantification in aqueous media is difficult and inaccurate. This work establishes for the first time nominal LC50 values for the FM550 mixture, BEH-TEBP, and octaBDE on Daphnia magna.
Molecular Effects May Be Unique for Each Chemical and Formulation
HOPACH analysis (Figure 4) showed that BEH-TEBP and BZ54 were the only exposures to cause similar effects, which is not surprising as BZ54 contains 30% BEH-TEBP and 70% BEH-TBB.33 Three of the five FM550 concentrations grouped into one cluster, indicating similar but not identical effects. Analyses with KEGG suggest distinct molecular effects by each chemical (Table 1). For example, octaBDE and BEH-TEBP increased the glycosphingolipid biosynthesis pathway, which is involved in growth factor signaling and morphogenesis in arthropods.86 Glycosphingolipid function is not comprehensively understood,87 but changes in function or abundance could affect cell proliferation, apoptosis, necrosis, senescence, and differentiation, as well as inflammation and autophagy and the stress response.88 BZ54, on the other hand, affected Wnt signal transduction (active during developmental processes89), Hedgehog signal transduction and glycosaminoglycan degradation (the latter two are largely unstudied in Daphnia). Changes to Wnt signaling could affect embryonic patterning and morphogenesis, and result in population effects. Results for pentaBDE suggest an effect on transcription and translation. In mammals, pentaBDE affect thyroid hormone homeostasis.15 Thyroid hormone acts as a translation factor that promotes differentiation and maturation of various tissues in vertebrates.90 In Daphnia, thyroid hormone has been shown to cause an increased growth rate.90 If pentaBDE causes increased growth in Daphnia, natural daphnid populations could be affected (reduced food availability, overcrowding, etc.). It is important to note that the microarray does not represent the entire Daphnia transcriptome and Daphnia gene-functions are not well characterized, so additional mechanisms may be involved.
Hydrophobic chemicals with a log Kow ≥ 2.7 are often considered “narcotic.” If chemicals are more toxic than predicted by Kow, then effects cannot be attributed to narcosis alone.46,49 To investigate narcosis, we compared the log Kow and log LC50 of each chemical,91 and did not find a relationship between the two variables (SI). This work is limited because we were not able to measure actual aquatic FR concentrations. Previous work by Vandenbrouck et al. in Daphnia on two presumed narcotics (pyrene and fluoranthene) did not find clear separation between gene transcription profiles.92 However, in the present work, clear differences were seen at the mRNA, metabolomic, and lipidomic levels. Taken together, these results suggest that the traditional definitions of Type I and Type II narcosis48,49 may not sufficiently encompass the biological effects of hydrophobic chemicals.
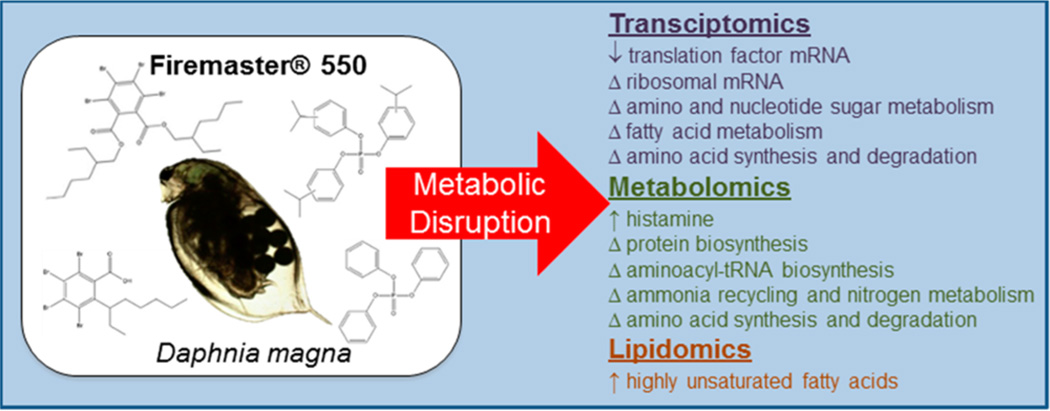
FM550 May Cause Nutritional Dysregulation in Daphnia magna
Transcriptomic, metabolomic, and lipidomic data indicate general xenobiotic response and effects related to nutritional status in Daphnia after exposure to FM550 (Figure 6). Specific metabolites with the largest changes (Table 3) are histidine, glucose, and lysine. Histaminergic signaling in the Daphnia central nervous system controls phototactic behavior (response to UV light) and is moderated by food abundance and quality.93 Changes to the system can effect Daphnia populations and ecosystems.93
Figure 6.
Schematic showing proposed toxic effects of chemical flame-retardant formulation FM550 on Daphnia magna. Omic techniques picked up changes related to nutritional status.
Glucose is used as fuel as well as conjugate for Phase II metabolism, to increase xenobiotic excretion.94 Daphnia have genes that encode enzymes for gluconeogenesis and for breakdown of glycogen, to maintain the minimal glucose levels for survival.95 Increased glucose levels, detected by metabolomics, could be caused by reduced Phase II metabolism, or by glycogen breakdown. However, changes to the related enzymes and pathways were not detected with microarray analysis.
Enrichment analysis of 1H NMR metabolomic data (Table 5) shows changes in ammonia recycling, protein biosynthesis, and amino acid degradation. Daphnia has two main forms of nitrogen: amino acids (AAs) and ammonia (NH3).96 Essential AAs are highly correlated to changes in nitrogen status and occur during fasting and growth.97 During starvation, NH3 excretion increases; NH3 is primarily sourced from amino acids and secondarily from nucleic acids.98 Ammonia cycling is related to protein catabolism in Daphnia99 and can increase along with the rate of ammonia excretion when Cladocern are starving.100,101
Daphnia growth is primarily associated with AA abundance.102 Pathway analysis (Table 4) detected effects on valine, leucine, and arginine metabolism and changes in aminoacyl-tRNA. Valine, leucine, proline, and arginine are three of the most common Daphnia AAs.97 Proline is an antioxidant in Daphnia.94 Aminoacyl-tRNA is used by the ribosome to make peptide chains, and aminoacyl-tRNA synthase activity is correlated with somatic growth in Daphnia.102 Together, the results indicate a clear effect on metabolites mediating growth.
KEGG analysis complements the metabolomics data. While the KEGG findings were not the same for each of the five FM550 concentrations tested, all results indicate changes to nutritional status and growth (ribosome, fatty acid metabolism, valine, leucine and isoleucine degradation, amino sugar, and nucleotide sugar metabolism) or xenobiotic metabolism (peroxisome and glutathione metabolism). Glycosphingolipid is involved in growth and in the stress response.88 Transcripomic data also show possible effects on oxygen transport (SI Table S10). One differentially expressed gene common to all FM550 exposure concentrations (translation initiation factor) decreased as the concentration of FM550 increased, again indicating effects on protein synthesis and growth. Lipidomic analysis showed an increase in one highly unsaturated fatty acid (HUFA) (42:7, Phosphatidly choline). Growth in Daphnia is secondarily associated with HUFA availability.102 HUFA are important for cell membrane support and are conserved during starvation.94
Three genes differentially expressed in all five concentrations may be useful biomarkers of exposure to FM550. Validation of biomarkers would require further studies to identify and verify gene products in Daphnia and in other organisms.
The brominated compounds in FM550 and BZ54, BEH-TBPH, and EH-TBB, are detected in marine mammals,11 accumulate in fathead minnows,42 and have potential to accumulate in other aquatic organisms. We found the compounds in D. magna after 48 h (Figure 3), suggesting the possibility for trophic transfer. Low levels of the compounds were also detected in YCT daphnid food (BEH-TBB: 0.625 ± 0.198 ng/g; BEH-TEBP: 0.629 ± 0.018 ng/g), which is made from yeast, fermented trout chow and cereal leaves. YCT contamination is the probable source of detected FRs in control animals (SI Table S6) and illuminates the broader problem of global FR contamination.
Supplementary Material
Acknowledgments
This work was supported by NSF CBET-1066358 to C.D.V., NIEHS R01ES016099 to H.M.S., and NERC Grant NE/1028246/2 to F.F. The lipid analyses performed at Kansas Lipidomics Research Center Analytical Laboratory were supported by NSF EPS 0236913, MCB 0455318, and DBI 0521587. The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. TOC graphic image was taken at the U.C. Berkeley College of Natural Resources Biological Imaging Facility.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Detailed methods on differential mRNA determination, qPCR, FM550 dose–response analysis, and metabolomic and lipidomic analyses; Table S1, chemical components of COMBO media; Table S2, raw acute toxicity data; Table S3, PentaBDE and OctaBDE congener profiles and weight; Table S4, primer sequences used in qPCR; Table S5, acute, 48-h LC50 values of flame-retardants; Table S6, concentration data from absorption studies; Table S7a, numbers of differentially expressed genes in each 1/10 LC50 FR exposure; Table S7b, numbers of differentially expressed genes in FM550 dose–response; Table S8, qPCR results; Table S9, KEGG pathway analysis on FM550 dose–response data; Table S10, gene ontology enrichment analysis on FM550 dose–response data; Figure S1, HOPACH clustering based on chemical profiles of FM550 dose–response data; and Figure S2, PLS-DA plots of FM550 and pentaBDE metabolomic data. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.5b00977.
The authors declare no competing financial interest.
REFERENCES
- 1.Shaw SD, Blum A, Weber R, Kannan K, Rich D, Lucas D, Koshland CP, Dobraca D, Hanson S, Birnbaum LS. Halogenated flame retardants: do the fire safety benefits justify the risks? Rev. Environ. Health. 2010;25(4):261–305. doi: 10.1515/reveh.2010.25.4.261. [DOI] [PubMed] [Google Scholar]
- 2.Blum A. The fire retardant dilemma. Science (New York NY) 2007;318(5848):194–195. doi: 10.1126/science.318.5848.194b. [DOI] [PubMed] [Google Scholar]
- 3.Bureau of Electronic and Appliance Repair, Home Furnishings; and Thermal Insulation, editor. State of California Department of Consumer Affairs, Technical Bulletin 117-2013. CA: 2013. [Google Scholar]
- 4.Gao S, Hong J, Yu Z, Wang J, Yang G, Sheng G, Fu J. Polybrominated diphenyl ethers in surface soils from e-waste recycling areas and industrial areas in South China: Concentration levels, congener profile, and inventory. Environ. Toxicol. Chem./SETAC. 2011;30(12):2688–2696. doi: 10.1002/etc.668. [DOI] [PubMed] [Google Scholar]
- 5.Daso AP, Fatoki OS, Odendaal JP, Olujimi OO. Occurrence of selected polybrominated diphenyl ethers and 2,2′,4,4′,5,5′-hexabromobiphenyl (BB-153) in sewage sludge and effluent samples of a wastewater-treatment plant in Cape Town, South Africa. Arch. Environ. Contam. Toxicol. 2011;62(3):391–402. doi: 10.1007/s00244-011-9720-9. [DOI] [PubMed] [Google Scholar]
- 6.Kimbrough KL, Johnson WE, Lauenstein GG, Christensen JD, Apeti DA. In: An Assessment of Polybrominated Diphenyl Ethers (PBDEs) in Sediments and Bivalves of the U.S. Costal Zone. Memorandum NT, editor. Silver Spring, MD: 2009. p. 87. Vol. NOS NCCOS. [Google Scholar]
- 7.Birgul A, Katsoyiannis A, Gioia R, Crosse J, Earnshaw M, Ratola N, Jones KC, Sweetman AJ. Atmospheric polybrominated diphenyl ethers (PBDEs) in the United Kingdom. Environ. Pollut. 2012;169:105–111. doi: 10.1016/j.envpol.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Xiao H, Hung H, Wania F, Lao R, Sabljic E, Sverko E, Lei YD, Fellin P, Barresi E. Field evaluation of a flow-through sampler for measuring pesticides and brominated flame retardants in the arctic atmosphere. Environ. Sci. Technol. 2012;46(14):7669–7676. doi: 10.1021/es301481w. [DOI] [PubMed] [Google Scholar]
- 9.Alava JJ, Keller JM, Wyneken J, Crowder L, Scott G, Kucklick JR. Geographical variation of persistent organic pollutants in eggs of threatened loggerhead sea turtles (Caretta caretta) from southeastern United States. Environ. Toxicol. Chem./SETAC. 2011;30(7):1677–1688. doi: 10.1002/etc.553. [DOI] [PubMed] [Google Scholar]
- 10.Kuo YM, Sepulveda MS, Hua I, Ochoa-Acuna HG, Sutton TM. Bioaccumulation and biomagnification of polybrominated diphenyl ethers in a food web of Lake Michigan. Ecotoxicology (London) 2010;19(4):623–634. doi: 10.1007/s10646-009-0431-1. [DOI] [PubMed] [Google Scholar]
- 11.Lam JC, Lau RK, Murphy MB, Lam PK. Temporal trends of hexabromocyclododecanes (HBCDs) and polyrominated diphenyl ethers (PBDEs) and detection of two novel flame retardants in marine mammals from Hong Kong, South China. Environ. Sci. Technol. 2009;43(18):6944–6949. doi: 10.1021/es901408t. [DOI] [PubMed] [Google Scholar]
- 12.Liu PY, Du GD, Zhao YX, Mu YS, Zhang AQ, Qin ZF, Zhang XY, Yan SS, Li Y, Wei RG, Qin XF, Yang YJ. Bioaccumulation, maternal transfer and elimination of polybrominated diphenyl ethers in wild frogs. Chemosphere. 2011;84(7):972–978. doi: 10.1016/j.chemosphere.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ. Sci. Technol. 2008;42(21):8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 14.Huwe JK, West M. Polybrominated diphenyl ethers in U.S. meat and poultry from two statistically designed surveys showing trends and levels from 2002 to 2008. J. Agric. Food Chem. 2011;59(10):5428–5434. doi: 10.1021/jf2003915. [DOI] [PubMed] [Google Scholar]
- 15.Dishaw LV, L JM, Roberts SC, Stapleton HM. Exposures, mechanisms, and impacts of endocrine-active flame retardants. Curr. Opin. Pharmacol. 2014;19:125–133. doi: 10.1016/j.coph.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnusson K, Tiselius P. The importance of uptake from food for the bioaccumulation of PCB and PBDE in the marine planktonic copepod Acartia clausi. Aquat. Toxicol. (Amsterdam) 2010;98(4):374–380. doi: 10.1016/j.aquatox.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Magnusson K, Magnusson M, Ostberg P, Granberg M, Tiselius P. Bioaccumulation of 14C-PCB 101 and 14C-PBDE 99 in the marine planktonic copepod Calanus f inmarchicus under different food regimes. Mar. Environ. Res. 2007;63(1):67–81. doi: 10.1016/j.marenvres.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Gismondi E, Thome JP. Effects of two PBDE congeners on the moulting enzymes of the freshwater amphipod Gammarus pulex. Environ. Pollut. 2014;191:119–125. doi: 10.1016/j.envpol.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Losada S, Roach A, Roosens L, Santos FJ, Galceran MT, Vetter W, Neels H, Covaci A. Biomagnification of anthropogenic and naturally-produced organobrominated compounds in a marine food web from Sydney Harbour, Australia. Environ. Int. 2009;35(8):1142–1149. doi: 10.1016/j.envint.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Nakari T, Huhtala S. Comparison of toxicity of congener-153 of PCB, PBB, and PBDE to Daphnia magna. Ecotoxicol. Environ. Safety. 2008;71(2):514–518. doi: 10.1016/j.ecoenv.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Davies R, Zou E. Polybrominated diphenyl ethers disrupt molting in neonatal Daphnia magna. Ecotoxicology. 2012;21(5):1371–1380. doi: 10.1007/s10646-012-0891-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Hu C, Huang C, Wang Q, Wang X, Yang L, Zhou B. Alterations in retinoid status after long-term exposure to PBDEs in zebrafish (Danio rerio) Aquat. Toxicol. 2012;120:11–18. doi: 10.1016/j.aquatox.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin. Med. Res. 2003;1(4):281–290. doi: 10.3121/cmr.1.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockholm Convention on persistent organic pollutants (POPs), editor. Stockholm Convention, Decision POPRC-1/3: Pentabromodiphenyl ether. 2011. [Google Scholar]
- 25.Wania F, Dugani CB. Assessing the long-range transport potential of polybrominated diphenyl ethers: a comparison of four multimedia models. Environ. Toxicol. Chem. 2003;22(6):1252–1261. [PubMed] [Google Scholar]
- 26.The European Parliment, Directive 2002/95/EC of the European Parliment and of the Council of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electrical equipment. 2003.
- 27.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol. 2012;46(24):13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The European Parliment Directive 2003/11/EC of the European Parliment and of the Council of 6 February 2003 amending for the 24th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether, octabromodiphenyl ether), 2003.
- 29.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012;46(24):13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klosterhaus S, Konstantinov A, Davis E, Klein J, Stapleton H. Characterization of the Brominated Chemicals in a PentaBDE Replacement Mixture and their Detection in Biosolids. Ottawa, Ontario, Canada: 2009. [Google Scholar]
- 31.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 2008;42(18):6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 32.McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: Potential role of the aryl hydrocarbon receptor. Toxicol. Sci.: Off. J. Soc. Toxicol. 2013;133(1):144–156. doi: 10.1093/toxsci/kft020. [DOI] [PubMed] [Google Scholar]
- 33.Bearr JS, Mitchelmore CL, Roberts SC, Stapleton HM. Species specific differences in the in vitro metabolism of the flame retardant mixture, FiremasteR(R)) BZ-54. Aquat. Toxicol. (Amsterdam) 2012;124-125C:41–47. doi: 10.1016/j.aquatox.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 2009;43(19):7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 2011;45(12):5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014;116:54–60. doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verslycke TA, Vethaak AD, Arijs K, Janssen CR. Flame retardants, surfactants and organotins in sediment and mysid shrimp of the Scheldt estuary (The Netherlands) Environ. Pollut. (Barking) 2005;136(1):19–31. doi: 10.1016/j.envpol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 38.La Guardia MJ, Hale RC, Harvey E, Mainor TM, Ciparis S. In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the bivalve (Corbicula fluminea) and gastropod (Elimia proxima) Environ. Sci. Technol. 2012;46(11):5798–5805. doi: 10.1021/es3004238. [DOI] [PubMed] [Google Scholar]
- 39.Lin K. Joint acute toxicity of tributyl phosphate and triphenyl phosphate to Daphnia magna. Environ. Chem. Lett. 2009;7(4):309–312. [Google Scholar]
- 40.Marklund A, Andersson B, Haglund P. Organophosphorus flame retardants and plasticizers in Swedish sewage treatment plants. Environ. Sci. Technol. 2005;39(19):7423–7429. doi: 10.1021/es051013l. [DOI] [PubMed] [Google Scholar]
- 41.van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–11153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 42.Bearr JS, Stapleton HM, Mitchelmore CL. Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster 550 and Firemaster BZ-54. Environ. Toxicol. Chem. 2010;29(3):722–729. doi: 10.1002/etc.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders DM, Podaima M, Codling G, Giesy JP, Wiseman S. A mixture of the novel brominated flame retardants TBPH and TBB affects fecundity and transcript profiles of the HPGL-axis in Japanese medaka. Aquat. Toxicol. (Amsterdam) 2015;158:14–21. doi: 10.1016/j.aquatox.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Gerlach CV, Das SR, Volz DC, Bisson WH, Kolluri SK, Tanguay RL. Mono-substituted isopropylated triaryl phosphate, a major component of Firemaster 550, is an AHR agonist that exhibits AHR-independent cardiotoxicity in zebrafish. Aquat. Toxicol. (Amsterdam) 2014;154:71–79. doi: 10.1016/j.aquatox.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.United States Environmental Protection Agency. Furniture Flame Retardancy Partnership Environmental Profiles of Chemical Flame-Retardant Alternatives for Low-Desnisty Polyuretane Foam. Vol. 2. Cincinnati, OH: 2005. [Google Scholar]
- 46.Verhaar HJ, Solbe J, Speksnijder J, van Leeuwen CJ, Hermens JL. Classifying environmental pollutants: Part 3. External validation of the classification system. Chemosphere. 2000;40(8):875–883. doi: 10.1016/s0045-6535(99)00317-3. [DOI] [PubMed] [Google Scholar]
- 47.Sanderson H, Thomsen M. Ecotoxicological quantitative structure-activity relationships for pharmaceuticals. Bull. Environ. Contam. Toxicol. 2007;79(3):331–335. doi: 10.1007/s00128-007-9249-9. [DOI] [PubMed] [Google Scholar]
- 48.Veith GD, Broderius SJ. Rules for distinguishing toxicants that cause type I and type II narcosis syndromes. Environ. Health Perspect. 1990;87:207–211. doi: 10.1289/ehp.9087207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts DW, Costello JF. Mechanisms of action for general and polar narcosis: A difference in dimension. QSAR Comb. Sci. 2003;22(2):226–233. [Google Scholar]
- 50.Watanabe H, Kobayashi K, Kato Y, Oda S, Abe R, Tatarazako N, Iguchi T. Transcriptome profiling in crustaceans as a tool for ecotoxicogenomics: Daphnia magna DNA microarray. Cell Biol. Toxicol. 2008;24(6):641–647. doi: 10.1007/s10565-008-9108-4. [DOI] [PubMed] [Google Scholar]
- 51.Seda J, Petrusek A, Sehnal F. Daphnia as a model organism in limnology and aquatic biology: some aspects of its reproduction and development PREFACE. J. Limnol. 2011;70(2):336–336. [Google Scholar]
- 52.Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia. 1998;377:147–159. [Google Scholar]
- 53.Agency, U. S. E. P. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. 5th. Washington DC: U.S. Environmental Protection Agency, Office of Water; 2002. [Google Scholar]
- 54.Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977;11(7):714–719. [Google Scholar]
- 55.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Di(2-ethylhexyl)phthalate (DEHP) Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2002. [PubMed] [Google Scholar]
- 56.Reemtsma T, Quintana JB, Rodil R, Garcia-Lopez M, Rodriguez I. Organophosphorus flame retardants and plasticizers in water and air I. Occurrence and fate. Trac-Trends Anal. Chem. 2008;27(9):727–737. [Google Scholar]
- 57.La AGMJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ. Sci. Technol. 2006;40(20):6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- 58.Sjodin A. Occupational and Dietary Exposure to Organohalogen Substances, with Special Emphasis on Polybrominated Diphenyl Ethers. Stockholm: Stockholm University; 2000. [Google Scholar]
- 59.Bergman A, Ryden A, Law RJ, de Boer J, Covaci A, Alaee M, Birnbaum L, Petreas M, Rose M, Sakai S, Van den Eede N, van der Veen I. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ. Int. 2012;49:57–82. doi: 10.1016/j.envint.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loguinov AV, Mian IS, Vulpe CD. Exploratory differential gene expression analysis in microarray experiments with no or limited replication. Genome biology. 2004;5(3):R18. doi: 10.1186/gb-2004-5-3-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scanlan LD, Reed RB, Loguinov AV, Antczak P, Tagmount A, Aloni S, Nowinski DT, Luong P, Tran C, Karunaratne N, Pham D, Lin XX, Falciani F, Higgins CP, Ranville JF, Vulpe CD, Gilbert B. Silver nanowire exposure results in internalization and toxicity to Daphnia magna. ACS Nano. 2013;7(12):10681–10694. doi: 10.1021/nn4034103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antczak P, Jo HJ, Woo S, Scanlan L, Poynton H, Loguinov A, Chan S, Falciani F, Vulpe C. Molecular toxicity identification evaluation (mTIE) approach predicts chemical exposure in Daphnia magna. Environ. Sci. Technol. 2013;47(20):11747–11756. doi: 10.1021/es402819c. [DOI] [PubMed] [Google Scholar]
- 63.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 64.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Laan MJ, Pollard KS. A new algorithm for hybrid hierarchical clustering with visualization and the bootstrap. J. Stat. Plan. Inf. 2003;117(2):275–303. [Google Scholar]
- 66.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics (Oxford) 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 67.Viant MR. Revealing the metabolome of animal tissues using 1H nuclear magnetic resonance spectroscopy. Methods Mol. Biol. (Clifton) 2007;358:229–246. doi: 10.1007/978-1-59745-244-1_13. [DOI] [PubMed] [Google Scholar]
- 68.Teng Q, Ekman DR, Huang W, Collette TW. Push-through direct injection NMR: an optimized automation method applied to metabolomics. Analyst. 2012;137(9):2226–2232. doi: 10.1039/c2an16251b. [DOI] [PubMed] [Google Scholar]
- 69.Bax A, Davis DG. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J. Magn. Reson. 1985;65(2):355–360. [Google Scholar]
- 70.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37(Web Server issue):W652–W660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012;40(Web Server issue):W127–W133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wheeler MW, Park RM, Bailer AJ. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem./SETAC. 2006;25(5):1441–1444. doi: 10.1897/05-320r.1. [DOI] [PubMed] [Google Scholar]
- 73.Lobenhofer EK, Cui X, Bennett L, Cable PL, Merrick BA, Churchill GA, Afshari CA. Exploration of low-dose estrogen effects: identification of no observed transcriptional effect level (NOTEL) Toxicol. Pathol. 2004;32(4):482–492. doi: 10.1080/01926230490483324. [DOI] [PubMed] [Google Scholar]
- 74.Zarbl H, Gallo MA, Glick J, Yeung KY, Vouros P. The vanishing zero revisited: Thresholds in the age of genomics. Chem.-Biol. Interact. 2010;184(1–2):273–278. doi: 10.1016/j.cbi.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smyth GK. Limma: Linear Models for Microarray Data. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 76.R Core Development Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 77.Pollard KS, Dudoit S, van der Laan MJ. Multiple Testing Procedures: R Multtest Package and Applications to Genomics. In: Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 251–272. [Google Scholar]
- 78.Bluthgen N, Brand K, Cajavec B, Swat M, Herzel H, Beule D. Biological profiling of gene groups utilizing Gene Ontology. Genome informatics. International Conference on Genome Informatics. 2005;16(1):106–115. [PubMed] [Google Scholar]
- 79.Poynton HC, Taylor NS, Hicks J, Colson K, Chan S, Clark C, Scanlan L, Loguinov AV, Vulpe C, Viant MR. Metabolomics of microliter hemolymph samples enables an improved understanding of the combined metabolic and transcriptional responses of Daphnia magna to cadmium. Environ. Sci. Technol. 2011;45(8):3710–3717. doi: 10.1021/es1037222. [DOI] [PubMed] [Google Scholar]
- 80.Mhadhbi L, Fumega J, Beiras R. Toxicological effects of three polybromodiphenyl ethers (BDE-47, BDE-99 and BDE-154) on growth of marine algae Isochrysis galbana. Water Air Soil Pollut. 2012;223(7):4007–4016. [Google Scholar]
- 81.Pellacani C, Buschini A, Galati S, Mussi F, Franzoni S, Costa LG. Evaluation of DNA damage induced by 2 polybrominated diphenyl ether flame retardants (BDE-47 and BDE-209) in SK-N-MC Cells. Int. J. Toxicol. 2012;31(4):372–379. doi: 10.1177/1091581812449663. [DOI] [PubMed] [Google Scholar]
- 82.Usenko CY, Robinson EM, Usenko S, Brooks BW, Bruce ED. PBDE developmental effects on embryonic Zebrafish. Environ. Toxicol. Chem. 2011;30(8):1865–1872. doi: 10.1002/etc.570. [DOI] [PubMed] [Google Scholar]
- 83.Waaijers S, Bleyenberg TE, Dits A, Schoorl M, Schutt J, Kools SA, de Voogt P, Admiraal W, Parsons JR, Kraak M. Daphnid life cycle responses to new generation flame retardants. Environ. Sci. Technol. 2013;47(23):13798–13803. doi: 10.1021/es4031529. [DOI] [PubMed] [Google Scholar]
- 84.Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW. A summary of the acute toxicity of 14 phthalate-esters to representative aquatic organisms. Environ. Toxicol. Chem. 1995;14(9):1569–1574. [Google Scholar]
- 85.Chemtura USA Corporation. Material Safety Data Sheet 00896: Firemaster® 550. Middlebury, Conneticut: 2006. p. 8. [Google Scholar]
- 86.Tiemeyer M, Selleck S, Esko J. Arthropoda. In: Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi C, Hart G, Etzler M, editors. Essentials of Glycobiology. 2nd. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 87.Kojima H, Tohsato Y, Kabayama K, Itonori S, Ito M. Biochemical studies on sphingolipids of Artemia f ranciscana: complex neutral glycosphingolipids. Glycoconjugate J. 2013;30(3):257–268. doi: 10.1007/s10719-012-9436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 2002;277(29):25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 89.Janssen R, Le Gouar M, Pechmann M, Poulin F, Bolognesi R, Schwager EE, Hopfen C, Colbourne JK, Budd GE, Brown SJ, Prpic NM, Kosiol C, Vervoort M, Damen WG, Balavoine G, McGregor AP. Conservation, loss, and redeployment of Wnt ligands in protostomes: implications for understanding the evolution of segment formation. BMC Evol. Biol. 2010;10:374. doi: 10.1186/1471-2148-10-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kashian DR, Dodson SI. Effects of vertebrate hormones on development and sex determination in Daphnia magna. Environ. Toxicol. Chem. 2004;23(5):1282–1288. doi: 10.1897/03-372. [DOI] [PubMed] [Google Scholar]
- 91.Cristale J, Garcia Vazquez A, Barata C, Lacorte S. Priority and emerging flame retardants in rivers: occurrence in water and sediment Daphnia magna toxicity and risk assessment. Environ. Int. 2013;59:232–243. doi: 10.1016/j.envint.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 92.Vandenbrouck T, Jones OA, Dom N, Griffin JL, De Coen W. Mixtures of similarly acting compounds in Daphnia magna: from gene to metabolite and beyond. Environ. Int. 2010;36(3):254–268. doi: 10.1016/j.envint.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 93.McCoole MD, Baer KN, Christie AE. Histaminergic signaling in the central nervous system of Daphnia and a role for it in the control of phototactic behavior. J. Exp. Biol. 2011;214(Pt 10):1773–1782. doi: 10.1242/jeb.054486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smirnov NN. Physiology of the Cladocera. London: Academic Press; 2014. [Google Scholar]
- 95.Campos B, Garcia-Reyero N, Rivetti C, Escalon L, Habib T, Tauler R, Tsakovski S, Pina B, Barata C. Identification of metabolic pathways in Daphnia magna explaining hormetic effects of selective serotonin reuptake inhibitors and 4-nonylphenol using transcriptomic and phenotypic responses. Environ. Sci. Technol. 2013;47(16):9434–9443. doi: 10.1021/es4012299. [DOI] [PubMed] [Google Scholar]
- 96.Gardner WS, Miller WH., Iii Intracellular composition and net release rates of free amino acids in Daphnia magna. Can. J. Fish. Aquat. Sci. 1981;38(2):157–162. [Google Scholar]
- 97.Ventura M, Catalan J. Variability in amino acid composition of alpine crustacean zooplankton and its relationship with nitrogen-15 fractionation. J. Plankton Res. 2010;32(11):1583–1597. [Google Scholar]
- 98.Vonk HJ. Metabolism and Growth. In: Waterman T, editor. The Physiology of Crustacea. Vol. 1. New York: Elsevier Science; 1960. [Google Scholar]
- 99.Scavia D, Gardner WS. Kinetics of nitrogen and phosphorous release in varying food supplies by Daphnia magna. Hydrobiologia. 1982;96(2):105–111. [Google Scholar]
- 100.Hessen DO. Carbon, nitrogen and phosphorous statud in Daphnia at varying food conditions. J. Plankton Res. 1990;12(6):1239–1249. [Google Scholar]
- 101.D’Abramo L, Conklin D, Akiyama D. Nutrition I. W. G. o. C. Crustacean Nutrition. Vol. 6. Baton Rouge, FL: World Aquaculture Society; 1997. [Google Scholar]
- 102.Yebra L, Hernández-León S. Aminoacyl-tRNA synthetases activity as a growth index in zooplankton. J. Plankton Res. 2004;26(3):351–356. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.