Abstract
Sweet taste receptors (STRs) on the tongue mediate gustatory sweet sensing, but their expression in the gut, pancreas, and adipose tissue suggests a physiological contribution to whole body nutrient sensing and metabolism. However, little is known about the function and contribution of these sugar sensors during metabolic stress induced by overnutrition and subsequent obesity. Here, we investigated the effects of high-fat/low-carbohydrate (HF/LC) diet on glucose homeostasis and energy balance in mice with global disruption of the sweet taste receptor protein T1R2. We assessed body composition, energy balance, glucose homeostasis, and tissue-specific nutrient metabolism in T1R2 knockout (T1R2-KO) mice fed a HF/LC diet for 12 wk. HF/LC diet-fed T1R2-KO mice gained a similar amount of body mass as did WT mice, but had reduced fat mass and increased lean mass relative to WT mice. T1R2-KO mice were also hyperphagic and hyperactive. Ablation of the T1R2 sugar sensor protected mice from HF/LC diet-induced hyperinsulinemia and altered substrate utilization, including increased rates of glucose oxidation and decreased liver triglyceride (TG) accumulation, despite normal intestinal fat absorption. Finally, STRs (T1r2/T1r3) were upregulated in the adipose tissue of WT mice in response to HF/LC diet, and their expression positively correlated with fat mass and glucose intolerance. The chemosensory receptor T1R2, plays an important role in glucose homeostasis during diet-induced obesity through the regulation of yet to be identified molecular mechanisms that alter energy disposal and utilization in peripheral tissues.
Keywords: sweet taste receptors, obesity, hyperinsulinemia, body composition, diabetes
obesity and its comorbidities have become a global epidemic (37). In the United States alone, about two thirds of all adults are overweight and one third are classified as obese (12). Obesity is associated with an increased risk for the development of insulin resistance and type 2 diabetes (T2D) (21, 38). Although the etiology of obesity is a complex interplay of environmental and genetic components, it is largely linked to excessive caloric intake and decreased physical activity (13). Disturbances in energy balance induce the activation of cellular biochemical sensors that attempt to restore homeostasis by the activation of metabolic, hormonal, and neuronal pathways. For instance, glucose influx and metabolism causes the accumulation of malonyl coenzyme A (CoA), which inhibits lipid oxidation in favor of triglyceride synthesis and glucose oxidation (35, 44). Nevertheless, the range and nature of nutrient-sensing pathways that regulate adaptive responses to nutrient surplus and their role in the development of obesity are not completely understood.
A series of G protein-coupled receptors (GPRs) were deorphanized, revealing that nutrients are ligands for receptor-mediated signaling pathways. Sweet taste receptors (STRs) comprise the heterodimer of T1R2/T1R3 proteins and, along with free fatty acids (GPR40, -41, -43, -119, -120) and amino acid (umami) receptors (T1R1/T1R3), sense ingested or circulating dietary nutrients to exert pleiotropic effects independently of nutrient metabolism (5, 29). For instance, STRs on enteroendocrine cells in the gut induce the secretion of glucagon-like peptide-1 (GLP-1) and other incretin hormones in response to sugars and noncaloric artificial sweeteners (NCASs) to regulate nutrient absorption (4, 20, 32, 33). In accord, we showed that STRs on β-cells sense glucose, fructose, and NCASs to regulate basal and stimulated insulin secretion (27, 28). Similar to sugars, fatty acids of various chain lengths activate GPRs on enteroendocrine cells, islets, and adipocytes to modulate gut-derived hormones (GLP-1, PYY, CCK, GIP), insulin, glucagon, and leptin secretion, respectively (50). Interestingly, T1R2/T1R3 and fatty acid receptors on adipocytes can also mediate endocrine-independent functions such as lipolysis, glucose uptake, and adipogenesis (16, 18, 34, 45, 46). The diverse tissue distribution of nutrient-sensing GPRs suggests multiple roles in delivery, processing, and storage of energy in the integration of whole body nutrient metabolism.
How these chemosensory receptors integrate signals in response to nutrient oversupply and their specific contribution to obesity and diabetes are still largely unknown. A role for fatty acid receptors in the development of metabolic diseases has been demonstrated in studies where disruption of GPR120 led to obesity in both mice and humans (18), while ablation of GPR40 induced abnormalities in glucose homeostasis in mice (22, 47). In contrast, disruption of T1R2 that sense simple sugars did not spontaneously induce glucose dysregulation (20, 28). Nevertheless, we showed that islets from hyperglycemic obese mouse models (i.e., db/db and diet-induced obese mice) have suppressed T1r2 and T1r3 expression and reduced function (27). Additionally, short-term exposure to mild hyperglycemic conditions downregulate islet STR expression, directly linking glycemic control with STR function (27). Consistent with these findings, intestinal STR expression in patients with type 2 diabetes is negatively correlated with fasting glycemia (54) and is dysregulated in response to luminal glucose during hyperglycemia (53). Taken together, these reports suggest that the chemosensory role of STRs in tissues such as the intestine and the pancreas may manifest itself only under conditions of metabolic stress, such as during the development of obesity.
To shed light on the contribution of STRs to energy homeostasis during nutrient oversupply, we investigated the effects of diet-induced obesity (DIO) on several metabolic end points in mice globally lacking the T1r2 gene of STRs (T1R2-KO). We demonstrate that after, 12 wk of a high-fat/low-carbohydrate (HF/LC) diet feeding, T1R2-KO mice have reduced adipose tissue mass and increased lean mass relative to WT mice. Ablation of T1R2 also prevented HF/LC diet-induced hyperinsulinemia and liver triglyceride (TG) accumulation, along with elevated rates of carbohydrate oxidation. STRs are upregulated in the adipose tissue of WT mice in response to HF/LC diet, and their expression positively correlates with glucose intolerance. Collectively, these data highlight a critical, unrecognized role of the STR T1R2 in the regulation of glucose homeostasis during HF/LC diet and exemplify the significance of glucose-sensing pathways independent of its metabolism.
METHODS
Animals and cell culture.
All animal experiments were approved by the Sanford Burnham Prebys Medical Discovery Institute (SBPMDI) Institutional Animal Care and Use Committee. Mice with a homozygous deletion of all seven predicted transmembrane helices of the T1r2 gene (kindly provided by Dr. Charles Zuker, Columbia University) were back-crossed on the C57Bl\6J strain for 10 generations and genotyped in-house for all experiments (55, 56). We used age-matched, sex-matched, in-house-bred non-littermate WT mice (C57Bl\6J) as controls. Cohorts of 8- to 10-wk-old male WT and T1R2-KO mice were placed on a HF/LC diet (58% fat, 26% carbohydrate, 16% protein D12330i) or control low-fat/high-carbohydrate (LF/HC; 11% fat, 73% carbohydrate, 16% protein, D12328i; Research Diets, NJ) for 12 wk. The diets were based on plant oils (fats) and starch/maltodextrin without sucrose (carbohydrates).
Body composition and energy balance.
Whole body composition (fat mass, lean mass, free fluids) was determined in conscious mice by use of a minispec NMR (LF90II, Bruker) instrument each week, beginning at week 0 of the diet. Indirect calorimetry was assessed using the Comprehensive Lab Animal Monitoring System (Columbus Instruments) at week 13. Mice were placed in individual metabolic cages for at least 24 h to acclimate to the new environment. Following the acclimation period, measurements were taken every 15 min over a 48-h period. Oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were measured to obtain estimates of energy expenditure and substrate utilization. Energy expenditure was calculated using the following equation: [3.815+1.232·(V̇co2/V̇o2)]·V̇o2 (1). The respiratory exchange ratio (RER), an index of substrate utilization, was calculated as V̇co2/V̇o2. Estimated rates of glucose and lipid oxidation were calculated using modified Ferrannini equations (11), where urinary nitrogen excretion was assumed to be equal between genotypes. Locomotor activity was measured by infrared beam breaks and converted to distance traveled. Food intake (manual) was assessed at week 10 of the diet. Mice were individually housed and allowed to acclimate for 3 days. A predetermined amount (g) of the diets (LF/HC and HF/LC) was provided to each mouse, and food intake was measured every 24 h for 5 consecutive days. The amount of food weighed was subtracted from the previous day's to determine grams of food consumed the past 24-h period. Calories per day was calculated by multiplying grams consumed by caloric content of each diet (control diet = 4.07 kcal/g; high-fat diet = 5.56 kcal/g).
Fecal lipid content and fat absorption.
Fecal and liver lipids were extracted using the method of Folch et al. (30). For the analysis of fecal lipids, mice were individually housed, feces were collected daily for 4–5 days, and food intake was measured manually. Feces were dried at 60°C overnight, and then 1 g of dried feces was incubated in 10 ml of distilled water at 4°C overnight. Feces were then homogenized by 2–3 min of vortexing; 25 ml of chloroform-methanol (2:1) was added, samples were vortexed for 30 s, incubated at room temperature for 15 min, and phase separation was induced by low-speed centrifugation (2,000 rpm for 10 min). The lower chloroform phase was then removed and transferred to a new tube, and the fecal samples were reextracted with 10 ml of chloroform-methanol (2:1) and pooled with the first chloroform extract. Samples were brought up to 25 ml with chloroform. To measure total lipid content, glass tubes were weighed, 5 ml of sample were then dried under nitrogen gas, and glass tubes were weighed again. Fecal lipid content was normalized to dried fecal weight. Fat absorption was measured by comparing fecal lipid percent and fecal output vs. food lipid percent and food intake {%lipid absorption = 1 − [(%fecal fat × fecal output g)/(%food lipid × food intake g)] × 100}. For analysis of liver lipids, 50 mg of tissue was homogenized in 1.0 ml of chloroform-methanol, and phase separation was induced with the addition of 300 μl of water, vortexed, and centrifuged at low speed (1,000 g for 20 min). The lower chloroform phase was removed, transferred to a new tube, and evaporated to dryness. The samples were then resuspended in 1.0 ml of chloroform. An aliquot of sample was then transferred to a new tube, and 30 μl of chloroform-Triton X-100 (2:1) was added, vortexed, then evaporated to dryness. The quantities of TG (Thermo Scientific, Waltham, MA) and cholesterol (Thermo Scientific) were assayed using enzymatic kits according to the manufacturer's protocol, with the exception that the corresponding standards were also treated with 30 μl of chloroform-Triton X-100 (2:1).
Plasma lipids, glycerol, and insulin.
Plasma TG (Stanbio, Boerne, TX), cholesterol (Stanbio), and glycerol (Biovision, Milpitas, CA) were measured using enzymatic kits according to the manufacturers' protocols. Plasma insulin was measured using an ultrasensitive mouse insulin ELISA (Mercodia, Sweden).
Intraperitoneal glucose tolerance test and insulin tolerance test.
The ipGTT was performed after a 16-h fast, and the ipITT on fed (ad libitum) 8- to 10-wk-old male mice after 12 wk of either control diet or HF/LC diet. Animals were injected with glucose (1g/kg body wt ip; GTT) or insulin (0.5 U/kg body wt ip; ITT). Blood glucose was sampled from the tail and analyzed with an AlphaTRAK blood glucose monitoring meter (North Chicago, IL).
Quantitative real-time PCR.
Total RNA from liver, gonadal white adipose tissue, or intestine was isolated using Invitrogen PureLink RNA kit (Carlsbad, CA) and was reverse-transcribed to cDNA (1.0 μg) using the Protoscript First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA). qPCR reactions were performed on a Bio-Rad CFX Real-Time PCR Detection system using iQ SYBR Green (Bio-Rad, Hercules, CA) using the protocol and primer sequences previously published (28).
Statistical analysis.
Results are shown as means + SE. The level of significance was set at P < 0.05. Statistical tests and significance was calculated as shown in the figure legends. ANOVA results are listed in Tables 1 and 2.
Table 1.
Two-way ANOVA results for Figs. 1–3
| ANOVA Table | F(DFn,DFd) | P Value | |
|---|---|---|---|
| Fig. 1A: fat mass | Interaction | F(36,432) = 67.58 | P < 0.0001 |
| Time | F(12,432) = 321.5 | P < 0.0001 | |
| G & D | F(3,36) = 46.18 | P < 0.0001 | |
| Subjects (matching) | F(36,432) = 41.11 | P < 0.0001 | |
| Fig. 1A: lean mass | Interaction | F(36,432) = 6.558 | P < 0.0001 |
| Time | F(12,432) = 116.8 | P < 0.0001 | |
| G & D | F(3,36) = 4.595 | P = 0.0080 | |
| Subjects (matching) | F(36,432) = 76.32 | P < 0.0001 | |
| Fig. 1B | Interaction | F(1,78) = 10.18 | P = 0.0020 |
| Diet | F(1,78) = 70.60 | P < 0.0001 | |
| Genotype | F(1,78) = 5.979 | P = 0.0167 | |
| Fig. 1C | Interaction | F(1,84) = 0.8595 | P = 0.3565 |
| Diet | F(1,84) = 0.4647 | P = 0.4973 | |
| Genotype | F(1,84) = 11.94 | P = 0.0009 | |
| Fig. 1D | Interaction | F(1,23) = 27.78 | P < 0.0001 |
| Diet | F(1,23) = 11.26 | P = 0.0027 | |
| Genotype | F(1,23) = 12.94 | P = 0.0015 | |
| Fig. 1E | Interaction | F(1,35) = 3.547 | P = 0.0680 |
| Diet | F(1,35) = 20.59 | P < 0.0001 | |
| Genotype | F(1,35) = 10.19 | P = 0.0030 | |
| Fig. 2A | Interaction | F(1,84) = 12.08 | P = 0.0008 |
| Diet | F(1,84) = 40.44 | P < 0.0001 | |
| Genotype | F(1,84) = 1.630 | P = 0.2052 | |
| Fig. 2B | Interaction | F(1,84) = 6.011 | P = 0.0163 |
| Diet | F(1,84) = 250.6 | P < 0.0001 | |
| Genotype | F(1,84) = 18.31 | P < 0.0001 | |
| Fig. 2C | Interaction | F(1,69) = 0.6137 | P = 0.4361 |
| Diet | F(1,69) = 414.6 | P < 0.0001 | |
| Genotype | F(1,69) = 54.38 | P < 0.0001 | |
| Fig. 2D | Interaction | F(1,78) = 0.1395 | P = 0.7098 |
| Diet | F(1,78) = 413.6 | P < 0.0001 | |
| Genotype | F(1,78) = 42.75 | P < 0.0001 | |
| Fig. 2E | Interaction | F(1,78) = 4.121 | P = 0.0458 |
| Diet | F(1,78) = 328.7 | P < 0.0001 | |
| Genotype | F(1,78) = 30.82 | P < 0.0001 | |
| Fig. 3A: glucose | Interaction | F(1,43) = 3.629 | P = 0.0635 |
| Diet | F(1,43) = 0.7054 | P = 0.4056 | |
| Genotype | F(1,43) = 2.806 | P = 0.1012 | |
| Fig. 3A: insulin | Interaction | F(1,43) = 1.771 | P = 0.1903 |
| Diet | F(1,43) = 8.305 | P = 0.0061 | |
| Genotype | F(1,43) = 1.930 | P = 0.1720 | |
| Fig. 3A: I/G | Interaction | F(1,43) = 3.258 | P = 0.0781 |
| Diet | F(1,43) = 8.689 | P = 0.0052 | |
| Genotype | F(1,43) = 3.830 | P = 0.0568 | |
| Fig. 3B: glucose | Interaction | F(1,43) = 0.4326 | P = 0.5142 |
| Diet | F(1,43) = 51.71 | P < 0.0001 | |
| Genotype | F(1,43) = 2.614 | P = 0.1132 | |
| Fig. 3B: insulin | Interaction | F(1,43) = 16.26 | P = 0.0002 |
| Diet | F(1,43) = 36.53 | P < 0.0001 | |
| Genotype | F(1,43) = 11.34 | P = 0.0016 | |
| Fig. 3B: I/G | Interaction | F(1,43) = 11.18 | P = 0.0017 |
| Diet | F(1,43) = 9.522 | P = 0.0035 | |
| Genotype | F(1,43) = 11.07 | P = 0.0018 | |
| Fig. 3D | Interaction | F(1,20) = 7.235 | P = 0.0141 |
| Time | F(1,20) = 56.35 | P < 0.0001 | |
| G & D | F(1,20) = 21.84 | P = 0.0001 | |
| Subjects (matching) | F(20,20) = 5.029 | P = 0.0003 | |
| Fig. 3E | Interaction | F(1,42) = 6.382 | P = 0.0154 |
| Diet | F(1,42) = 4.610 | P = 0.0376 | |
| Genotype | F(1,42) = 3.465 | P = 0.0697 | |
| Fig. 3F | Interaction | F(3,66) = 1.481 | P = 0.2277 |
| Time | F(3,66) = 102.5 | P < 0.0001 | |
| G & D | F(1,22) = 5.137 | P = 0.0336 | |
| Subjects (matching) | F(22,66) = 1.986 | P = 0.0171 | |
| Fig. 3G | Interaction | F(1,43) = 1.047 | P = 0.3118 |
| Diet | F(1,43) = 26.11 | P < 0.0001 | |
| Genotype | F(1,43) = 1.107 | P = 0.2985 |
G & D, genotype and diet; I/G, glucose/insulin.
Table 2.
Two-way ANOVA results for Figs. 4 and 5
| ANOVA Table | F(DFn,DFd) | P Value | |
|---|---|---|---|
| Fig. 4D: Chrebp | Interaction | F(1,27) = 0.1709 | P = 0.6826 |
| Diet | F(1,27) = 12.52 | P = 0.0015 | |
| Genotype | F(1,27) = 22.56 | P < 0.0001 | |
| Fig. 4D: Acc | Interaction | F(1,27) = 0.8218 | P = 0.3727 |
| Diet | F(1,27) = 0.08480 | P = 0.7731 | |
| Genotype | F(1,27) = 11.48 | P = 0.0022 | |
| Fig. 4D: Cd36 | Interaction | F(1,28) = 0.05558 | P = 0.8153 |
| Diet | F(1,28) = 4.376 | P = 0.0456 | |
| Genotype | F(1,28) = 9.934 | P = 0.0038 | |
| Fig. 4D: Scd1 | Interaction | F(1,28) = 0.2567 | P = 0.6164 |
| Diet | F(1,28) = 0.06836 | P = 0.7957 | |
| Genotype | F(1,28) = 9.147 | P = 0.0053 | |
| Fig. 4D: Pparg | Interaction | F(1,28) = 0.4805 | P = 0.4939 |
| Diet | F(1,28) = 3.551 | P = 0.0699 | |
| Genotype | F(1,28) = 10.25 | P = 0.0034 | |
| Fig. 4D: Adipoq | Interaction | F(1,25) = 5.103 | P = 0.0329 |
| Diet | F(1,25) = 22.01 | P < 0.0001 | |
| Genotype | F(1,25) = 13.69 | P = 0.0011 | |
| Fig. 4E | Interaction | F(1,31) = 0.6082 | P = 0.4414 |
| Diet | F(1,31) = 0.8126 | P = 0.3743 | |
| Genotype | F(1,31) = 35.65 | P < 0.0001 | |
| Fig. 5A | Interaction | F(1,27) = 1.364 | P = 0.2530 |
| Diet | F(1,27) = 20.38 | P = 0.0001 | |
| Genotype | F(1,27) = 2.000 | P = 0.1687 | |
| Fig. 5B | Interaction | F(1,27) = 2.308 | P = 0.1403 |
| Diet | F(1,27) = 31.62 | P < 0.0001 | |
| Genotype | F(1,27) = 4.535 | P = 0.0425 | |
| Fig. 5C | Interaction | F(1,62) = 1.517 | P = 0.2227 |
| Diet | F(1,62) = 59.81 | P < 0.0001 | |
| Genotype | F(1,62) = 4.325 | P = 0.0417 | |
| Fig. 5D | Interaction | F(1,62) = 5.101 | P = 0.0274 |
| Diet | F(1,62) = 0.1055 | P = 0.7464 | |
| Genotype | F(1,62) = 5.085 | P = 0.0277 | |
| Fig. 5E: Cpt1 | Interaction | F(1,26) = 3.875 | P = 0.0598 |
| Diet | F(1,26) = 29.92 | P < 0.0001 | |
| Genotype | F(1,26) = 6.231 | P = 0.0192 | |
| Fig. 5E: Hmgs2 | Interaction | F(1,27) = 4.673 | P = 0.0397 |
| Diet | F(1,27) = 16.36 | P = 0.0004 | |
| Genotype | F(1,27) = 1.707 | P = 0.2024 | |
| Fig. 5E: Cd36 | Interaction | F(1,25) = 2.018 | P = 0.1678 |
| Diet | F(1,25) = 32.95 | P < 0.0001 | |
| Genotype | F(1,25) = 6.522 | P = 0.0171 | |
| Fig. 5E: Fasn | Interaction | F(1,24) = 8.292 | P = 0.0082 |
| Diet | F(1,24) = 1.373 | P = 0.2529 | |
| Genotype | F(1,24) = 6.500 | P = 0.0176 | |
| Fig. 5E: Scd1 | Interaction | F(1,26) = 4.459 | P = 0.0445 |
| Diet | F(1,26) = 5.844 | P = 0.0229 | |
| Genotype | F(1,26) = 4.382 | P = 0.0462 | |
| Fig. 5E: Hmgcr | Interaction | F(1,27) = 1.490 | P = 0.2328 |
| Diet | F(1,27) = 19.11 | P = 0.0002 | |
| Genotype | F(1,27) = 0.1238 | P = 0.7276 | |
| Fig. 5E: Hmgs1 | Interaction | F(1,21) = 160.1 | P < 0.0001 |
| Diet | F(1,21) = 339.2 | P < 0.0001 | |
| Genotype | F(1,21) = 205.0 | P < 0.0001 | |
| Fig. 5E: Pck | Interaction | F(1,23) = 1.845 | P = 0.1875 |
| Diet | F(1,23) = 0.4674 | P = 0.5010 | |
| Genotype | F(1,23) = 10.46 | P = 0.0037 |
RESULTS
T1R2-KO mice on HF/LC diet have reduced fat mass and increased lean mass.
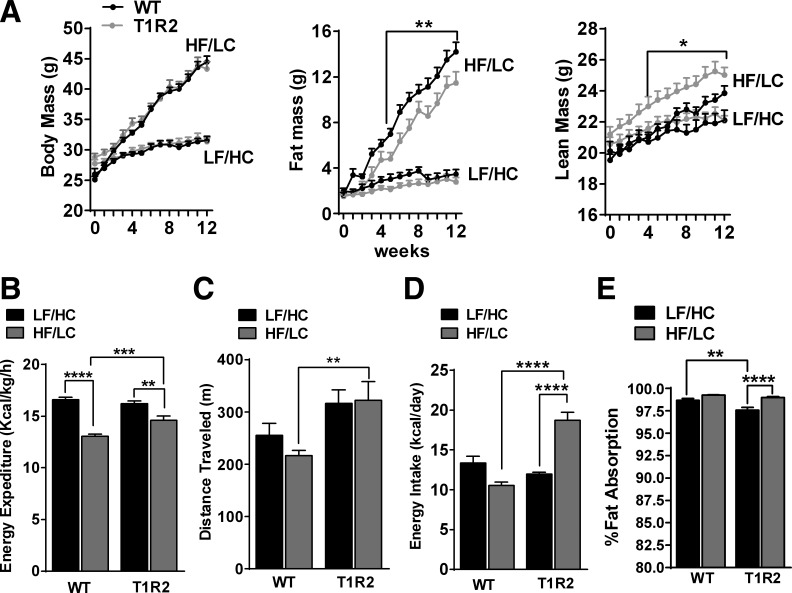
We initially characterized the metabolic effects of DIO in mice deprived of the STR T1R2, challenged by 12 wk of HF/LC diet or LF/HC diet. Although total body mass of WT and T1R2-KO mice was not different in response to HF/LC diet feeding, T1R2-KO mice had reduced fat mass and increased lean mass compared with WT mice (Fig. 1A). Next, we assessed energy balance using a Comprehensive Lab Animal Monitoring System (CLAMS). Relative to WT mice, T1R2-KO mice had increased energy expenditure in response to HF/LC diet feeding (Fig. 1B), likely due to hyperactivity (Fig. 1C), although T1R2-KO mice were more active than WT mice independently of diet. Nevertheless, T1R2-KO mice on HF/LC diet were hyperphagic (at week 10) but maintained similar food intake as WT when fed LF/HC diet (Fig. 1D). T1R2 mice on HF/LC diet had reduced fat mass despite increased energy intake, so we measured intestinal fat absorption, but no significant differences were observed between genotypes on HF/LC diet (Fig. 1E). Taken together, these data show that ablation of T1R2 attenuates HF/LC diet-induced increases in fat mass and better preserves lean mass. These effects are not due to fat malabsorption.
Fig. 1.
Effects of high-fat/low carbohydrate HF/LC) diet on body composition, energy balance and lipid absorption in sweet taste receptor 2 knockout (T1R2-KO) mice. A: body mass (left), fat mass (middle), and lean mass (right) in WT and T1R2-KO mice during 12-wk control (LF/HC) or HF/LC diet feeding (n = 12/group) measured with MRI. Two-way ANOVA with Tukey's multiple comparisons, *P < 0.05, **P < 0.01. B: dark cycle average of energy expenditure (kcal·kg−1·h−1). C: total activity (distance traveled; m) measured for 2 days in CLAMS (n = 24/group). D: average daily energy intake (kcal/day) measured manually for 7 consecutive days (n = 8/group). E: calculated percent fat absorption based on fecal lipid content and food intake (see methods; n = 12/group). Two-way ANOVA with Sidak's multiple comparisons, **P < 0.01, ***P < 0.001, ****P < 0.0001.
T1R2-KO mice preferentially utilize carbohydrates.
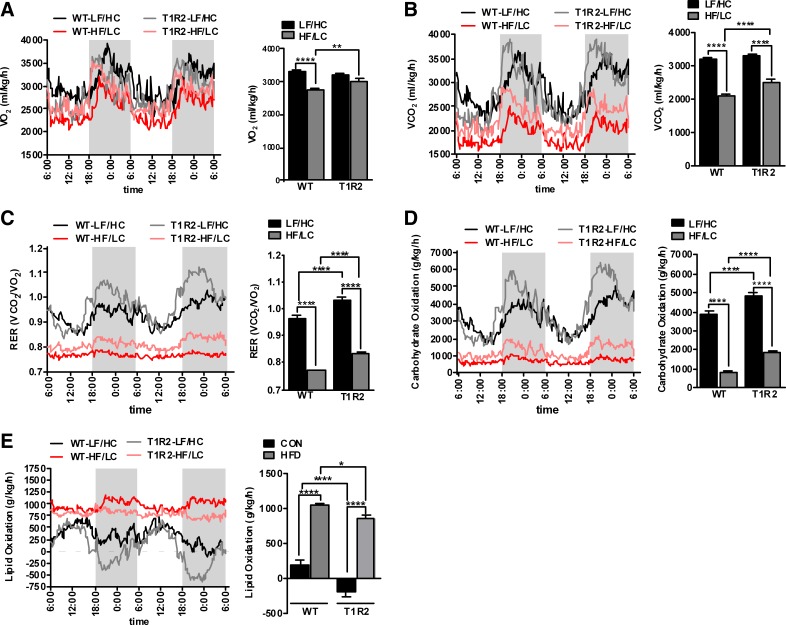
Considering that T1R2-KO mice had altered body composition and exhibited small increases in energy intake and expenditure in response to HF/LC diet, we measured respiratory gases and evaluated whole body substrate utilization. T1R2-KO mice on the HF/LC diet exhibited increased rate of V̇co2 and V̇o2 (Fig. 2, A and B) compared with WT mice on HF/LC diet. Because the genotype differences in V̇co2 were more prominent than in V̇o2, respiratory exchange ratio (RER) was also increased in T1R2-KO mice (Fig. 2C). Interestingly, RER was elevated in T1R2-KO mice independently of diet, suggesting that STRs modulate fuel preference utilization. Consistent with these data, carbohydrate oxidation (Fig. 2D) was significantly enhanced in T1R2-KO mice independently of diet, whereas the oxidation of fats (Fig. 2E) was suppressed within each dietary treatment group. Taken together, these data suggest that the STR protein T1R2 is a carbohydrate sensor important for the regulation of substrate utilization independently of diet.
Fig. 2.
Effects of HF/LC diet on expiratory gases and whole body substrate utilization in T1R2-KO mice. Traces (left) and dark cycle average (right) of oxygen consumption (V̇o2; ml·kg−1·h−1; A), carbon dioxide production (V̇co2; ml·kg−1·h−1; B), respiratory exchange ratio (RER; V̇o2/V̇co2; C), carbohydrate oxidation (g·kg−1·h−1; D), and lipid oxidation (g·kg−1·h−1; E) measured for 2 days in CLAMS in WT and T1R2-KO mice in response to 12 wk of LF/HC and HF/LC diets (n = 24/group). Two-way ANOVA with Sidak's multiple comparisons, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
T1R2-KO mice are protected against HF/LC diet-induced hyperinsulinemia.
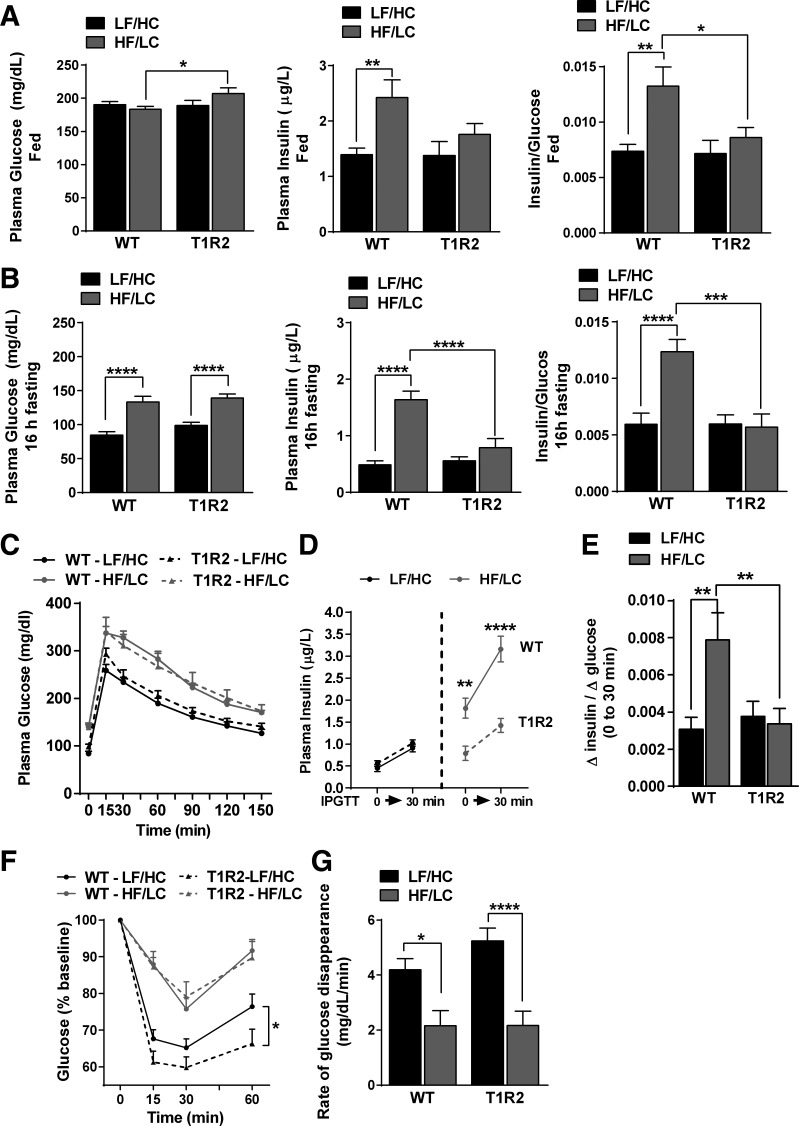
Next, we tested whether changes in body composition and substrate utilization in T1R2-KO mice were accompanied by changes in glucose homeostasis. Compared with control diet (LF/HC), HF/LC diet did not exacerbate fed (ad libitum) glucose in WT or T1R2-KO mice; however, T1R2-KO mice fed HF/LC diet had slightly higher glucose than WT mice on the same diet (Fig. 3A, left). Whereas WT mice became hyperinsulinemic (Fig. 3A, middle) following the HF/LC diet feeding, T1R2-KO mice had a normal plasma insulin and insulin/glucose ratio, an index of insulin resistance (Fig. 3A, right) (15). Because the feeding state alters the homeostatic mechanisms for glucose control (31, 51), we assessed blood glucose and plasma insulin after overnight fasting (16 h). Both WT and T1R2-KO mice fed HF/LC diet demonstrated fasting hyperglycemia; but, similar to the fed state, T1R2-KO mice maintained lower fasting insulin levels (Fig. 3B). To gain further insight, we performed ipGTT and ITT. The ipGTT bypasses the gut (and oral cavity) and assesses glucose tolerance dependent on peripheral mechanisms (i.e., pancreas, muscle, liver, adipose tissue). Both WT and T1R2-KO mice became glucose intolerant after 12 wk of HF/LC diet (Fig. 3C). Because T1R2-KO mice were resistant to HF/LC diet-induced hyperinsulinemia, we assessed plasma insulin in response to an ipGTT (t = 0 vs. t = 30 min) and calculated the insulinogenic index (ΔIt = 0–30/ΔGt = 0–30) (15). Consistent with previous observations, T1R2-KO mice on HF/LC diet responded normally to a glucose challenge and maintained glycemic control without exacerbated hyperinsulinemia compared with WT mice (Fig. 3, D and E). Although both genotypes became insulin resistant (Fig. 3F) and had reduced rates of glucose disappearance (t = 0–15 min; Fig. 3G) in response to a HF/LC diet, T1R2-KO mice on control (LF/HC) diet had improved glucose responses following an insulin challenge (Fig. 3F). Taken together, our data show that T1R2-KO mice on a HF/LC diet partially preserve glycemic control in the absence of hyperinsulinemia, suggesting the presence of mechanisms that alter glucose disposal.
Fig. 3.
Effects of HF/LC diet on glucose homeostasis in T1R2-KO mice. A: fed (ad libitum) plasma glucose (left), insulin (middle), and insulin sensitivity index (right) assessed by calculating the ratio of plasma insulin to glucose levels for each mouse in WT and T1R2-KO mice during 12-wk control (LF/HC) or HF/LC diet feeding. B: long-term fasting (16 h) plasma glucose (left), insulin (middle), and insulin sensitivity index (right) assessed by calculating the ratio of plasma insulin to glucose levels for each mouse. C: blood glucose levels during ipGTT (1.0 g/kg) as described in methods. D: plasma insulin responses (t = 0 and 30 min post) during ipGTT. E: assessment of insulinogenic index (increment in plasma insulin/increment in plasma glucose) during first 30 min of the ipGTT. F: relative blood glucose responses during ipITT (left) expressed as %change from baseline (set at 100%). G: assessment of rate of glucose disappearance (from baseline to 15 min). In all experiments, n = 12–14/group unless otherwise stated. Two-way ANOVA with Sidak's multiple comparisons, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Expression of STRs in white adipose tissue is increased during HF/LC diet and is associated with glucose intolerance.
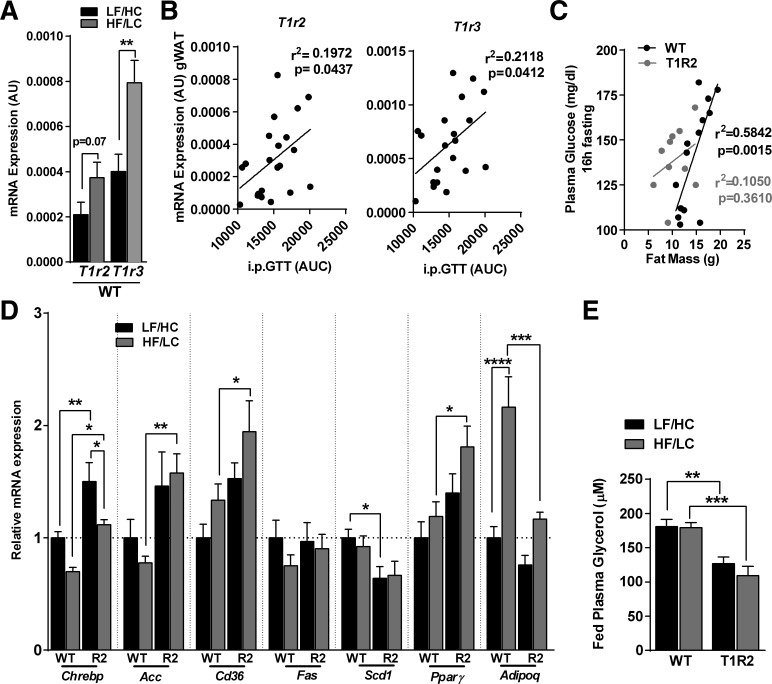
Ablation of T1R2 leads to reduced fat mass gain during HF/LC diet. Because T1R2/T1R3 receptors operate as nutrient sensors likely to regulate energy utilization and storage, we assessed the role of STRs on gonadal white adipose tissue (gWAT). Following the HF/LC diet, t1r2 was marginally upregulated, while its heterodimer, t1r3 was increased twofold in WT mice (Fig. 4A), but no differences in t1r3 expression were observed in T1R2-KO gWAT (CON: 0.00031 ± 6.0 × 10−5 vs. HF/LC diet: 0.00047 ± 1.0 × 10−5, P = 0.25, n = 6–8). These effects were specific to gWAT, since intestinal expression of STRs in WT mice remained unaltered (data not shown) while we confirmed our previous work showing that expression of STRs in β-cells was downregulated (27). Notably, the expression of STRs (t1r2 and t1r3) in gWAT was positively correlated with glucose intolerance (Fig. 4B). Adiposity is associated with hyperglycemia (2), so we evaluated whether ablation of STRs altered this relationship. As expected, fat mass was highly correlated with fasting plasma glucose in WT mice fed a HF/LC diet, but no association was observed in T1R2-KO mice fed a HF/LC diet (Fig. 4C). Because STRs may regulate adipocyte function (34, 45) we assessed the gene expression of several markers of lipid metabolism in WAT and observed that the expressions of chrebp, acc, cd36, and pparγ were increased in the gWAT of T1R2-KO mice fed a HF/LC diet compared with WT mice (Fig. 4D). Because T1R2-KO mice have reduced adiposity, plasma glycerol levels were used as an index of the lipolytic activity of gWAT. Accordingly, T1R2-KO mice displayed reduced plasma glycerol concentrations compared with WT mice independent of diet (Fig. 4E). Taken together, our data suggest that STR expression and function in adipocytes may be regulated by diet to modulate yet to be determined aspects of glucose homeostasis.
Fig. 4.
Effects of HF/LC diet on adipose tissue function in T1R2-KO mice. A: STR gene expression (T1r2 and T1r3) in gonadal white adipose tissue (gWAT) of WT mice fed control (LF/HC) diet or HF/LC diet measured by quantitative real-time RT-PCR. Arbitrary units (AU) shown normalized to GAPDH (n = 12 mice). Student's t-test, **P < 0.01. B: correlation of T1r2 and T1r3 expression in gWAT with ipGTT expressed as area under curve (AUC). Linear regression analysis (n = 20–21 mice). C: correlation of fasting (16 h) plasma glucose with fat mass in WT and T1R2-KO mice fed HF/LC diet (n = 10–14 mice). D: gWAT expression of selected genes involved in lipid metabolism in WT and T1R2-KO (R2) mice fed LF/HC or HF/LC diet measured by quantitative real-time RT-PCR and normalized to GAPDH. Data are expressed as relative expression to WT mice on LF/HC diet (set at value 1; n = 8/group). E: fed (ad libitum) plasma glycerol as index of lipolysis in WT and T1R2-KO mice fed LF/HC or HF/LC diet (n = 9 mice/group). Two-way ANOVA with Sidak's multiple comparisons, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
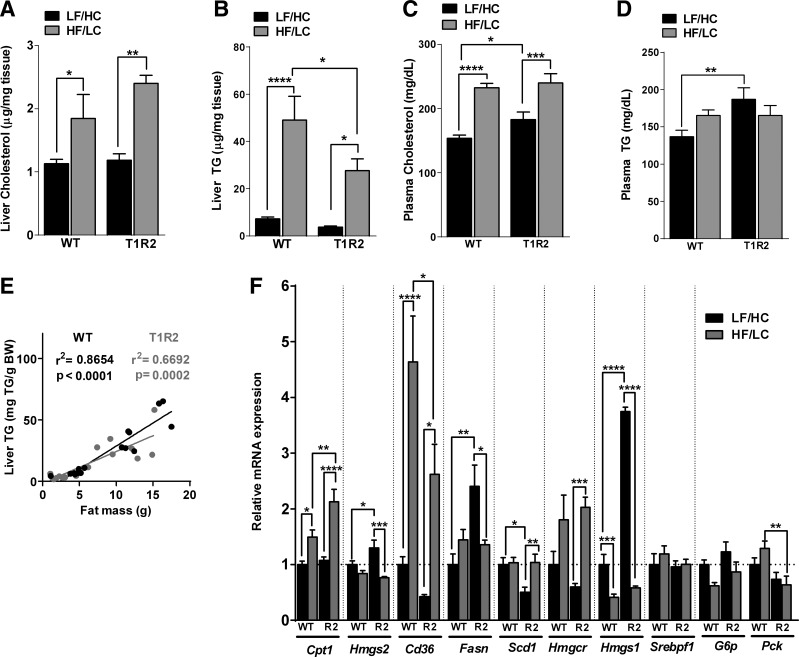
T1R2-KO mice are protected against HF/LC diet-induced hepatic steatosis.
We showed that T1R2-KO mice have reduced fat mass and reduced fatty acid oxidation in response to 12-wk of HF/LC diet feeding, suggesting that tissues other than adipose may also have altered lipid accumulation. As expected, HF/LC diet increased hepatic cholesterol and triglyceride (TG) content in both genotypes (Fig. 5, A and B). Nevertheless, T1R2-KO mice had reduced liver TG accumulation compared with WT mice in response to the HF/LC diet (Fig. 5B). As expected, HF/LC diet led to hypercholesterolemia irrespective of genotype (Fig. 5C), but unexpectedly plasma cholesterol and TGs were slightly elevated in T1R2-KO mice on the control diet compared with WT (Fig. 5D). Fat mass positively correlates with liver TG content (17). Because T1R2-KO fed a HF/LC diet have reduced fat mass, we assessed whether its relationship with liver TG is altered, but no differences between WT and T1R2-KO mice were observed (Fig. 5E). To shed light on the potential mechanisms to account for the differences in hepatic TG content between WT and T1R2-KO mice, we measured the expression of genes involved in lipid metabolism in the liver. HF/LC diet feeding upregulated genes involved in fatty acid transport (i.e., cd36), and oxidation (i.e., cpt1) as well as cholesterol synthesis (i.e., hmgcr) in both WT and T1R2-KO livers (Fig. 5F). We also observed a T1R2-KO-specific upregulation of genes involved in lipogenesis (i.e., fasn, hmgs1), suggesting indirect effects of STRs in the liver on the regulation of lipid metabolism independent of diet. Taken together, our data suggest STRs may regulate hepatic TG accumulation by regulating their uptake and metabolism.
Fig. 5.
Effects of HF/LC diet on liver function in T1R2-KO mice. Liver cholesterol content (A), liver triglyceride (TG; B) content, plasma cholesterol concentration (C), and plasma TG concentration (D) in WT and T1R2-KO mice fed control (LF/HC) or HF/LC diet (n = 8 mice/group). E: correlation of liver TG content and fat mass in WT and T1R2 mice on LF/HC and HF/LC diets. Linear regression analysis (n = 16 mice/group). F: liver expression of selected genes involved in lipid metabolism in WT and T1R2-KO (R2) mice fed LF/HC or HF/LC diet measured by quantitative real-time RT-PCR and normalized to L32. Data are expressed as relative expression to WT mice on LF/HC diet (set at value 1) (n = 8/group). Two-way ANOVA with Sidak's multiple comparisons, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
DISCUSSION
Dietary nutrient metabolism is essential not only for providing the necessary energy for cellular functions, but also in the generation of metabolic intermediates, which regulate substrate preference and utilization (39, 43). These nutrient sensory pathways regulate autonomous cellular effects dependent on the quality and quantity of nutrient supply while integrating signals from hormonal or neuronal sources (10). Recent evidence suggests that nutrient chemosensation can also be mediated via cell surface GPRs independently of nutrient metabolism. For example, glucose and other simple sugars can be sensed by the obligate GPR heterodimer T1R2/T1R3 to regulate incretin and insulin secretion in endocrine cells from the gut (20, 24, 25, 33) and the pancreas (27, 28, 36) as well as lipid metabolism in adipocytes (14, 45). We hypothesized that the T1R2/T1R3 is an essential chemosensory receptor that regulates nutrient metabolism during conditions of metabolic stress. Here, we show that genetic deletion of the T1r2 gene alters glucose homeostasis and energy metabolism in response to 12 wk of DIO. Specifically, T1R2-KO mice fed a HF/LC diet demonstrate altered body composition, energy balance, and fuel utilization. These effects are accompanied by improved glucose homeostasis and adipocyte function as well as decreased liver lipid accumulation.
We previously showed that T1R2 regulates insulin secretion and that obesity and diabetes alter its function on β-cells (27, 28). To address the role of T1R2 signaling during the development of DIO, we used a diet high in saturated fat (58%) that was formulated predominantly with coconut oil. We used this diet specifically because coconut oil induces moderate metabolic dysfunction compared with the commonly used lard-based HF diet (19, 52) but at the same time causes significant hyperinsulinemia (19). Similar to WT, T1R2-KO mice became obese after 12 wk of HF/LC diet, but distinct differences in body composition were observed; T1R2-KO mice had increased lean mass as well as reduced fat mass, which confirmed a recent report that used a HF/HC Western diet [41% fat, 43% carbohydrates with high sucrose (45)] to induce obesity. Evidently, differences in energy balance may account for these observations, as T1R2-KO mice on a HF/LC diet were consuming more calories than WT mice and had slightly increased energy expenditure. In contrast, WT mice on HF/LC diet became obese despite not being hyperphagic, consistent with previous observations using coconut oil instead of lard as a source for saturated fat (19). It is important to note that energy balance was assessed at the end of the HF/LC diet intervention, a time point at which the rate of weight gain and energy intake is often reduced (52).
Notably, T1R2-KO mice preferentially oxidized carbohydrates on control (LF/HC) diet compared with WT mice. As expected, HF/LC diet feeding favored fat oxidation in both genotypes, yet T1R2 mice relied less on fat as a substrate and accordingly had significantly higher RER. In contrast, the RER was not different in T1R2-KO mice fed a Western diet (45), suggesting that these effects may be diet dependent (i.e., amount and type of fat, sugar content). Similarly to our observations, ablation of the short-chain free fatty acid chemoreceptor GPR43 also increased lean mass and RER in response to the HF/LC diet (3) (40% fat, 43% carbohydrates, 17% protein), suggesting that nutrient chemoreceptors, including but not limited to T1R2/T1R3 and GPR43, could regulate whole body substrate utilization. The combination of increased lean mass and enhanced glucose oxidation may increase the capacity for glucose turnover, thus altering glycemic control. No genotype effect was observed in fed (ad libitum) or overnight (16 h) fasting glucose levels. Unlike the WT mice that developed hyperinsulinemia during HF/LC diet feeding (19), the T1R2-KO mice maintained glycemia without exacerbated insulinemia, suggestive of improved insulin sensitivity and/or enhanced basal glucose disposal. Nevertheless, the T1R2-KO mice became glucose intolerant and insulin resistant (i.e., rate of glucose disappearance from 0–15 min of the ITT) in response to a HF/LC diet but maintained a normal insulinogenic index (ΔIt = 0–30/ΔGt = 0–30) during an ipGTT. GPR43-deficient mice also became glucose intolerant during a GTT but had reduced plasma insulin responses during the HF/LC diet (3). These data demonstrate that different classes of chemoreceptors (for sugars vs. fatty acids) can affect glucose homeostasis via distinct mechanisms. The maintenance of glycemia in the absence of hyperinsulinemia suggests that T1R2-mediated chemosensory pathways may control yet to be determined mechanisms that regulate glucose disposal and oxidation in peripheral tissues such as muscle. Considering that T1R2-KO mice also have increased lean mass, this mechanism could shunt excess energy for oxidation.
Fat mass gain partially accounts for the development of hyperglycemia in HF/LC diet-fed WT mice, but ablation of T1R2 ameliorates these effects. HF/LC diet feeding induced the upregulation of the T1r3 gene and, to a much lesser degree, the T1r2 gene in the gWAT of WT mice, with their expression being positively correlated to glucose intolerance. It is not clear from existing data whether common mechanisms regulate T1r2 and T1r3 expression (27), but it is noteworthy that no upregulation of the T1r3 gene was noted in T1R2-KO mice in response to HF/LC diet, suggesting that intact T1R2/T1R3 signaling in gWAT may be required for their upregulation. We did not assess whether these mild alterations in expression affect T1R2/T1R3 protein levels, due to unreliable commercial antibodies. Our data suggest a possible role of both T1R2 and T1R3 in gWAT biology (45) and the development of glucose intolerance, which will require further investigation. Ablation of the T1R3 subunit of the T1R2/T1R3 heterodimer is often used to address the role of STRs in nongustatory physiology and metabolism (24, 33, 45). However, in addition to abolished sweet taste, T1R3-KO mice have ablated umami taste (i.e., amino acid chemosensation) (8, 56). We intentionally used T1R2-KO mice to avoid this confounding factor, yet we do not discard the possibility that part of our observations may be mediated by T1R3, which may operate as a low-affinity autonomous homodimer (30, 56). The proportional higher expression of the T1r3 transcript, compared with the T1r2, in gWAT is consistent with this possibility.
Reduced adiposity and smaller adipocyte size (45) in T1R2-KO mice may be secondary to reduced insulin levels in these mice, but they also suggest the involvement of STRs in the regulation of lipid turnover. For instance, we found increased expression of the fatty acid transporter Cd36 and Pparγ that regulate lipid uptake and oxidation, respectively, while no significant HF/LC diet effects on lipogenic genes (i.e., Fas, Scd1) were noted. Consistent with increased glucose flux, Chrebp and Acc were also upregulated. T1R2/T1R3 ligands have been shown to suppress lipolysis, although these effects were independent of their signaling (46). We showed that, independently of dietary manipulations, whole body lipid oxidation and plasma glycerol was reduced in T1R2-KO mice, suggesting suppressed lipolytic activity. Nevertheless, this observation may be an indirect manifestation of reduced adiposity due to smaller adipocyte size (45). In agreement, adiponectin expression was also reduced in WAT from T1R2-KO mice. We did not assess free fatty acids, but a Western diet had no effects on fed or fasted free fatty acids in T1R2-KO mice (45). Instead, plasma TGs were slightly elevated in T1R2-KO mice on control diet, consistent with findings showing reduced whole body fat oxidation.
T1R2-KO mice are hyperphagic in response to the HF/LC diet and have seemingly normal fat absorption (<6% fecal lipid content) (6, 40), suggesting that the absence of adipose tissue expansion in response to absorbed lipids may be due to their accumulation in peripheral tissues, although we cannot exclude the involvement of altered energy-harvesting pathways of gut microbiota (49). Accumulation of excess hepatic lipids often accompanies DIO (23), but to our surprise, T1R2-KO mice had reduced liver TG content. Hepatic steatosis is strongly associated with and parallels the development of insulin resistance (26), but paradoxically T1R2-KO mice on HF/LC diet are protected despite reduced insulin sensitivity (ipITT). Beyond insulin resistance, hyperinsulinemia can be an independent factor for the development of fatty liver (42). T1R2-KO mice do not develop HF/LC diet-induced hyperinsulinemia, which may indirectly account for the protective liver phenotype. Hyperinsulinemia can activate LXR and SREBP-1c to result in increased lipogenesis (7). We observed a diet-genotype interaction on the expression of SREBP targets (fasn, scd1, hmgcr, hmgs1), which could account for the elevated plasma cholesterol and TGs observed in T1R2-KO mice under control (LF/HC) diet, although we do exclude the possibility of altered peripheral lipoprotein metabolism in these mice (9). Moreover, it was recently shown that hyperinsulinemia can upregulate the fatty acid transporter cd36, leading to steatosis (48). As expected, HF/LC diet induced a prominent upregulation of cd36 expression, but it was less pronounced in T1R2-KO livers, further supporting the association between preservation of insulinemia and protection from liver steatosis. Elevated plasma glucose concentrations can promote hepatic TG uptake and synthesis and impair β-oxidation (41). Fed plasma glucose was marginally elevated in T1R2-KO mice in response to HF/LC diet, but expression of cpt1, a transporter of fatty acids into the mitochondria, was upregulated, suggesting enhanced β-oxidation in the liver. This is in contrast to the slightly suppressed rate of whole body lipid oxidation in T1R2-KO mice during HF/LC diet. Nevertheless, T1R2-KO mice have increased lean mass and energy expenditure, which could enhance the absolute capacity for lipid oxidation, preventing ectopic lipid accumulation. In agreement, muscle TG content was not different between T1R2-KO and WT mice on HF/LC diet (data not shown). Taken together, T1R2-KO mice are partially protected against HF/LC diet-induced liver steatosis via indirect mechanisms such as the prevention of hyperinsulinemia. This may reduce fatty acid uptake and enhance β-oxidation in the liver, while the increased lean mass can serve as a surplus sink.
In summary, STRs, whose expression and function can be modulated by ambient glucose (27), regulate endocrine secretion in the gut and the pancreas as well as energy storage in the adipocyte (16, 18, 27, 28, 34, 45, 46, 50). Taken together, these data suggest that STRs play a role in regulating energy homeostasis. We used whole body deletion of the T1R2 STR to address the significance of glucose chemosensation during metabolic stress induced by a HF/LC diet. Our findings further established the role of STRs in the regulation of adipose tissue biology, although the precise molecular mechanisms are still under investigation. We also found a novel unexpected role of T1R2 in the regulation of energy disposal and utilization during which T1R2-mediated chemosensation alters fuel oxidation in peripheral tissues. These latter observations may account for the protection from liver steatosis, expansion of adipocyte mass, and hyperinsulinemia that are typical manifestations of DIO. Nevertheless, the primary tissue(s) implicated in the regulation of energy utilization by STRs and the corresponding cellular events remain unknown, partly because global deletion of the T1r2 gene limits specific conclusions during data interpretation. Tissue-specific ablation of STRs will be necessary to decipher the pleiotropic functions of sugar chemosensors in the regulation of metabolism and the development of metabolic disorders.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-097757 and by institutional funds to R. E. P and G. A. K.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.R.S., T.H., E.K.A., and G.A.K. performed experiments; K.R.S., J.E.A., R.E.P., and G.A.K. analyzed data; K.R.S. and G.A.K. prepared figures; K.R.S., T.H., E.K.A., J.L.S., J.E.A., R.E.P., and G.A.K. approved final version of manuscript; J.L.S., J.E.A., R.E.P., and G.A.K. interpreted results of experiments; J.L.S., R.E.P., and G.A.K. edited and revised manuscript; G.A.K. conception and design of research; G.A.K. drafted manuscript.
ACKNOWLEDGMENTS
We thank Charles Zuker (Columbia University) for mutant mice; Emily King and Jennifer Ayala (Cardiometabolic Phenotyping Core, SBPMDI).
REFERENCES
- 1.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56: 1025–1033, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Azuma K, Heilbronn LK, Albu JB, Smith SR, Ravussin E, Kelley DE, Look AARG. Adipose tissue distribution in relation to insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 293: E435–E442, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjursell M, Admyre T, Goransson M, Marley AE, Smith DM, Oscarsson J, Bohlooly YM. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am J Physiol Endocrinol Metab 300: E211–E220, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Brown RJ, Rother KI. Non-nutritive sweeteners and their role in the gastrointestinal tract. J Clin Endocrinol Metab 97: 2597–2605, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvo SS, Egan JM. The endocrinology of taste receptors. Nat Rev Endocrinol 11: 213–227, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey MC, Small DM, Bliss CM. Lipid digestion and absorption. Annu Rev Physiol 45: 651–677, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci USA 101: 11245–11250, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem 277: 3801–3804, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature 517: 302–310, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism 37: 287–301, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Friedman JM. A war on obesity, not the obese. Science 299: 856–858, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Glendinning JI, Gillman J, Zamer H, Margolskee RF, Sclafani A. The role of T1r3 and Trpm5 in carbohydrate-induced obesity in mice. Physiol Behav 107: 50–58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, Fagot-Campagna A, Pettitt DJ, Bennett PH, Knowler WC. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 151: 190–198, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hasan AU, Ohmori K, Konishi K, Igarashi J, Hashimoto T, Kamitori K, Yamaguchi F, Tsukamoto I, Uyama T, Ishihara Y, Noma T, Tokuda M, Kohno M. Eicosapentaenoic acid upregulates VEGF-A through both GPR120 and PPARgamma mediated pathways in 3T3-L1 adipocytes. Mol Cell Endocrinol 406: 10–18, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Hui ST, Parks BW, Org E, Norheim F, Che N, Pan C, Castellani LW, Charugundla S, Dirks DL, Psychogios N, Neuhaus I, Gerszten RE, Kirchgessner T, Gargalovic PS, Lusis AJ. The genetic architecture of NAFLD among inbred strains of mice. Elife 4: e05607, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin MR, Korner A, Kiess W, Pigeyre M, Caiazzo R, Van Hul W, Van Gaal L, Horber F, Balkau B, Levy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu TA, Wolowczuk I, Tsujimoto G, Froguel P. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483: 350–354, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism 45: 1539–1546, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104: 15069–15074, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 57: 2432–2437, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med 164: 2169–2175, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann NY Acad Sci 1170: 91–94, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 90: 822S–825S, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 135: 122–130, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Kyriazis GA, Smith KR, Tyrberg B, Hussain T, Pratley RE. Sweet taste receptors regulate basal insulin secretion and contribute to compensatory insulin hypersecretion during the development of diabetes in male mice. Endocrinology 155: 2112–2121, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 109: E524–E532, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laffitte A, Neiers F, Briand L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care 17: 379–385, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X. T1R receptors mediate mammalian sweet and umami taste. Am J Clin Nutr 90: 733S–737S, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab 14: 9–19, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379–392, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masubuchi Y, Nakagawa Y, Ma J, Sasaki T, Kitamura T, Yamamoto Y, Kurose H, Kojima I, Shibata H. A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLos One 8: e54500, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest 60: 265–270, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLos One 4: e5106, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 766–781, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen NT, Nguyen XM, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes Surg 21: 351–355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obici S, Wang J, Chowdury R, Feng Z, Siddhanta U, Morgan K, Rossetti L. Identification of a biochemical link between energy intake and energy expenditure. J Clin Invest 109: 1599–1605, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petit V, Arnould L, Martin P, Monnot MC, Pineau T, Besnard P, Niot I. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res 48: 278–287, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 290: G852–G858, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Rhee EJ, Lee WY, Cho YK, Kim BI, Sung KC. Hyperinsulinemia and the development of nonalcoholic Fatty liver disease in nondiabetic adults. Am J Med 124: 69–76, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Rossetti L. Perspective: hexosamines and nutrient sensing. Endocrinology 141: 1922–1925, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Saha AK, Kurowski TG, Ruderman NB. A malonyl-CoA fuel-sensing mechanism in muscle: effects of insulin, glucose, and denervation. Am J Physiol Endocrinol Metab 269: E283–E289, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Simon BR, Learman BS, Parlee SD, Scheller EL, Mori H, Cawthorn WP, Ning X, Krishnan V, Ma YL, Tyrberg B, MacDougald OA. Sweet taste receptor deficient mice have decreased adiposity and increased bone mass. PLos One 9: e86454, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon BR, Parlee SD, Learman BS, Mori H, Scheller EL, Cawthorn WP, Ning X, Gallagher K, Tyrberg B, Assadi-Porter FM, Evans CR, MacDougald OA. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem 288: 32475–32489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab 1: 245–258, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Steneberg P, Sykaras AG, Backlund F, Straseviciene J, Soderstrom I, Edlund H. Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes hepatosteatosis and hepatic insulin resistance. J Biol Chem 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Boren J, Oresic M, Backhed F. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res 51: 1101–1112, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinolo MA, Hirabara SM, Curi R. G-protein-coupled receptors as fat sensors. Curr Opin Clin Nutr Metab Care 15: 112–116, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Wahren J, Ekberg K. Splanchnic regulation of glucose production. Annu Rev Nutr 27: 329–345, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, Suppl 3: S215–S219, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, Horowitz M, Rayner CK. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 58: 337–346, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. [DOI] [PubMed] [Google Scholar]