abstract
More than 50% of the U.S. population is infected with herpes simplex virus type-I (HSV-1) and global infectious estimates are nearly 90%. HSV-1 is normally seen as a harmless virus but debilitating diseases can arise, including encephalitis and ocular diseases. HSV-1 is unique in that it can undermine host defenses and establish lifelong infection in neurons. Viral reactivation from latency may allow HSV-1 to lay siege to the brain (Herpes encephalitis). Recent advances maintain that HSV-1 proteins act to suppress and/or control the lysosome-dependent degradation pathway of macroautophagy (hereafter autophagy) and consequently, in neurons, may be coupled with the advancement of HSV-1-associated pathogenesis. Furthermore, increasing evidence suggests that HSV-1 infection may constitute a gradual risk factor for neurodegenerative disorders. The relationship between HSV-1 infection and autophagy manipulation combined with neuropathogenesis may be intimately intertwined demanding further investigation.
KEYWORDS: autophagy, herpes simplex virus, ICP34.5, immune evasion, neurodegeneration
Introduction
Herpes simplex virus type-1 (HSV-1), the prototype virus of the family Herpesviridae, has a high prevalence rate around the world, nearly 90%, while in the United States seroprevalence is around 54%.1,2 Similar to all Herpesviridae members, HSV-1 has a profound ability to infect due to a special ability to cause latent infection. HSV-1 is normally of mild consequence, but infrequently can lead to disastrous clinical outcomes. Upon primary infection of the oral or genital regions, herpes simplex retreats to nearby neurons to reside indefinitely.3 Latency may lead to recurrent lytic infections that could ultimately disseminate to the immune privileged regions of the brain, causing deadly encephalitis or an array of ocular diseases that may lead to blindness.4 Although the global impact of HSV-1 on neural functions has not been envisioned, growing evidence suggests that HSV-1 infection may represent a risk factor for Alzheimer disease (AD).5-7 As we unveil the molecular mechanisms underlying HSV-1 interaction with neurodegenerative processes, our understanding of seemingly different cellular processes may move a step further.
Herpes simplex virus Type-1 primary infection
HSV-1 gains entrance to the body through an abrasion in the skin or mucosa, where it initiates cytolytic infection in epithelial cells.4 Initial tegument proteins begin to interact with host factors and in doing so practically take over the cellular replication machinery. While viral-host interactions occur in the cytosol, the nucleocapsid attaches to the nuclear pore via the capsid portal created by UL6 portal proteins.8 Viral DNA is then injected into the nucleus. When this occurs, host sensors alert innate immune responses, which will be discussed below.
Expression of viral protein is initialized by tegument protein VP16/Vmw65, which transactivates viral α-gene transcription.4,9 Other important tegument proteins, e.g., ICP0, ICP4, and vhs/UL41 (virion-associated host shut off protein) help ensure a robust expression of viral proteins.9 vhs plays a crucial role in degrading routine and stress response mRNAs that encourage further immune consequences.10 While these proteins prevent host cellular protein synthesis, VP16 is able to complex with 2 host factors, POU2F1 (POU class 2 homeobox 1) and HCFC1 (host cell factor C1), forming a transcriptional regulatory complex that turns on the viral gene expression cascade.11 The initial group of genes promoted by VP16 complex is termed the α-genes, and each subsequent grouping β1, β2, γ1, γ2, has its own transcriptional regulator. The α-proteins ICP0, ICP4, and ICP27 play a crucial role in establishing the remaining gene cascade.12-14 During active lytic infection fever blisters or cold sores may form on the lips and areas around the mouth. The clinical name is herpes simplex labialis (HSL), also known as oral herpes. Although there is no cure, HSL is of little clinical risk and usually spontaneously goes away within 10 d.15 The displeasing blistery rash may warrant patients to seek treatment, such as acyclovir, to reduce the pain and shorten the length of illness.16 When symptoms subside, HSV-1 retreats to nearby neurons, most commonly the trigeminal ganglia, for permanent residence, and to our knowledge is never eradicated.17 Periodically, dormant HSV-1 may reactivate from latency, and virus particles are then transported along sensory neurons to the skin or other mucosa, causing recurrent HSL.18 Although HSV-1 allows itself to be silenced in neurons, reactivated virus may prevail by overcoming various host defenses19 to cause disseminated disease,18,20 which will be discussed later. An important cellular defense mechanism against HSV-1 infection is the lysosome degradation pathway of autophagy, which constitutes a quality control of intracellular entities of eukaryotic cells.21 The interplay between the herpes pathogen and host cell autophagy reflects a constant battle for control.21,22
Autophagy restriction of HSV-1 infection
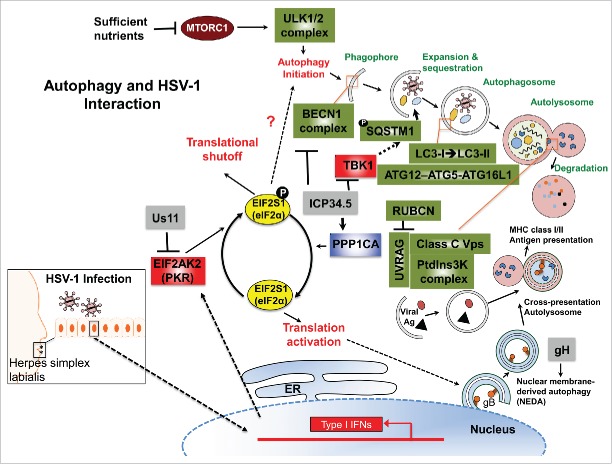
Autophagy is a fundamentally important mechanism to maintain cellular homeostasis.23 One striking aspect of autophagy in anti-microbial infection is achieved by its selective capture of foreign contents, such as bacterial or viral proteins, into phagophores, which subsequently mature into autophagosomes, allowing for their degradation, a process termed xenophagy,24 to differentiate it from the degradation of self-constituents. HSV-1 infection of cells activates the interferon (IFN) response and EIF2AK2 (eukaryotic translation initiation factor 2 α kinase 2).21 EIF2AK2 is a host-defense molecule that phosphorylates EIF2S1/eIF2α (translation initiation factor 2 subunit α) and promotes the induction of autophagy.25 Phosphorylation of EIF2S1 inhibits its function and shuts off protein synthesis in infected cells, thereby impeding viral replication (Fig. 1).26,27 As a countermeasure, the HSV-1 encoded neurovirulence factor ICP34.5 is expressed to recruit a host phosphatase, PPP1CA/PP1α (protein phosphatase 1, catalytic subunit, α isozyme), which dephosphorylates EIF2S1 and, thus, reverses EIF2AK2 effects in host-cell translational shutoff.26,27 Furthermore, ICP34.5 targets BECN1 and inhibits BECN1-mediated autophagy.28 Accordingly, mutant HSV-1 deficient in ICP34.5 elicits higher levels of autophagic response in permissive cells compared to wild-type virus, which disrupts autophagy; ICP34.5-null HSV-1 virions are sequestered within autophagosome-like membrane structures, leading to the consumption or xenophagic degradation of virions.29,30 However, it remains uncertain whether autophagy machinery contributes to HSV-1 induced autophagosome-like structures. If so, one would expect that elimination of the autophagic pathway in cells would rescue the infectivity of ICP34.5-null HSV-1, at least to a certain extent. Surprisingly, removing autophagy appears not to affect the replication of ICP34.5-null virus in cell culture,30 suggesting that xenophagy may not represent a predominant anti-HSV-1 mechanism in de novo HSV-1 infection in vitro. In contrast to this view, a recent report from Yakoub et al.31 demonstrates that inducing autophagy activity of host cells can indeed limit HSV-1 infection in vitro, and that autophagy manipulation may serve as a powerful means for the disease control of HSV-1. Despite seemingly discrepant findings, it is plausible to speculate that an alternative mechanism other than xenophagic degradation might be involved in the immune control by autophagy, especially considering the well-accepted fact that HSV-1 suppresses cellular autophagy in a multitude of ways.28,29,32,33
Figure 1.
The autophagy pathway and its interaction with HSV-1. Upon HSV-1 infection, autophagy is stimulated through the activation of an IFN-inducible EIF2AK2/PKR-EIF2S1/eIF2α signaling cascade, which shuts off host protein synthesis and concomitantly turns on autophagy by hitherto unclear mechanisms (indicated by the question mark). Autophagy induction sequesters cytoplasmic contents, forming autophagosomes characterized by the LC3-I → LC3-II conversion and ATG12–ATG5-ATG16L1 supercomplex association. As lysosomes and/or endosomes fuse, many factors contribute to the formation of the autolysosome, enabling degradation of contents by hydrolytic enzymes. Digested materials can be recycled back into the cytosol for use in energy production, protein manufacturing or be delivered to antigen presentation pathways in response to infection. As such, autophagy is shown to be able to directly capture the neuroattenuated ICP34.5-mutant HSV-1 virions or viral components, delivering them for lysosomal degradation and/or for the antigen presentation of viral peptides to the MHC-I/-II pathway for adaptive immune activation. To counteract the antiviral role of EIF2AK2 and cellular autophagy, viral protein Us11 prevents EIF2AK2-mediated EIF2S1 phosphorylation. Interestingly, ICP34.5 acts to reverse phosphorylated EIF2S1 by recruiting of host phosphatase PPP1CA/PP1α. In addition, ICP34.5 restricts autophagic initiation and maturation by targeting BECN1, preventing BECN1 autophagy complex formation. ICP34.5 also engages TBK1 to inhibit TBK1-mediated antiviral signaling, and may also prevent autophagic cargo recruitment through TBK1-mediated SQSTM1 phosphorylation. Although the response of nuclear envelope-derived autophagosomes (NEDA) can be triggered by ICP34.5-associated active protein translation or independently by expression of abundant viral late proteins (e.g., gH [glycoprotein H]), ICP34.5 can restrict the NEDA maturation that engages in viral antigen presentation. The interplay between the herpes pathogen and its host cell reflects a constant battle for control. RUBCN, RUN domain and cysteine-rich domain containing, Beclin 1-interacting protein; VPS, vacuolar protein sorting.
Other than the controversial xenophagic degradation, autophagic influence is observed in both major histocompatibility complex class I (MHC-I) and class II (MHC-II) antigen presentation pathways, which aids adaptive immune activation.33,34 Infection with mutant HSV-1, which is defective in BECN1 binding and therefore in counteracting autophagy, stimulates a more robust HSV-1-specific CD4+ T cell response in vivo, as compared to its marker-rescued wild-type counterpart.35 This viral-unleashed autophagy response enables more rapid removal of virion from all tissues compared to wild-type HSV-1 infection. In addition to boosting CD4+ T cell immunity, autophagy also facilitates CD8+ T cell response.33,36 Recent studies of murine macrophage infected with HSV-1 provided a mechanistic view of how autophagy assists in the processing and loading of viral antigen gB (glycoprotein B) onto MHC-I molecules.33,36 In this case, unlike the conventional double-membrane-bound autophagosome, a novel form of autophagosome originates from coiling of the inner and outer nuclear membranes, which creates a 4-layered structure.36 A recent study by Radtke et al.,37 further showed that this nuclear envelope-derived autophagy (NEDA) response is triggered by late viral protein production. This unique autophagosome was observed to be able to ‘cross-present’ endogenous HSV-1 antigens within the MHC-I pathway, enabling responses from CD8+ T cells.33,36 In line with these findings, mutant HSV-1, unable to suppress autophagy, causes increased proliferation of CD8+ T cells responding to virally infected cells compared to the outcome with wild-type virus.38 Given the broad involvement of autophagy in antigen presentation, it may serve as a target for the host to boost adaptive immunity against HSV-1 infection.
HSV-1 inhibition of cellular autophagy
HSV-1 neurovirulence factor ICP34.5 is critical for fatal encephalitis both in mice and in human.28,40,41 As noted above, ICP34.5 directly targets BECN1 to block BECN1-mediated autophagy execution, independent of its interaction with PPP1CA (Fig. 1).28,42 Of note, a mutant HSV-1 virus that retains PPP1CA interaction but lacks BECN1 binding of ICP34.5 fails to inhibit autophagy in neurons and demonstrates impaired ability to cause lethal encephalitis in mice.28 Intriguingly, the attenuated neurovirulence of mutant HSV-1 deficient in BECN1 inhibition is fully rescued in mice genetically lacking EIF2AK2, suggesting that HSV-1-induced EIF2AK2 activation is epistatic to BECN1-mediated autophagy in vivo.28,42 In addition to PPP1CA and BECN1, TBK1 (TANK-binding kinase 1) serves as a more recently discovered target of HSV-1 ICP34.5.43 ICP34.5 interaction with TBK1 inhibits the induction of antiviral signaling exerted by TBK1, facilitating viral replication and neuroinvasion.43,44 Given that TBK1 is a necessary factor for autophagic maturation, which can phosphorylate the autophagic receptors SQSTM1/p62 (sequestosome 1) and OPTN (optineurin) to regulate cargo recruitment into phagophores for degradation,45,46 direct inhibition of TBK1 may represent an additional way that ICP34.5 suppresses autophagic function, yet this conclusion awaits further investigation. In addition to ICP34.5, tegument protein Us11 has also been implicated in the regulation of the autophagy pathway.32 Us11 is an abundant viral protein produced in the late stage of the viral life cycle, which physically interacts with EIF2AK2 to prevent EIF2AK2-mediated phosphorylation of EIF2S1 (Fig. 1).47,48 A recent study by Lussignol et al., showed that immediate-early expression of Us11 renders cells infected with ICP34.5-null HSV-1 resistant to EIF2AK2-elicited autophagy.32 Moreover, ectopic expression of Us11 fails to block autophagy in EIF2AK2-deficient cells in vitro,32 suggestive of a EIF2AK2-dependent effect of Us11 on autophagy regulation. However, the in vivo relevance of this finding and the underlying mechanism of Us11-mediated inhibition of cellular autophagy need to be further investigated. Given the conventional role of autophagy in extraneous and aggregated protein degradation, it is plausible to speculate that this late onset of autophagy inhibition allows for the more efficient production of viral progeny by keeping levels of protein degradation low. Furthermore, late inhibition of autophagy will limit antigen presentation by helping prevent the delivery of viral peptides to the immune system. More insight into the mechanisms of these autophagy inhibitors of HSV-1 can help to decipher how these events act synergistically during infection and how they contribute to viral life cycle and pathogenesis.
Besides epithelial infection, presenting as herpes simplex labialis or genital herpes, HSV-1 can further infect and reside latent within neurons. Autophagic mechanisms to prevent viral spread and replication may have more profound effects in neuronal infection as opposed to epithelial infection.49 Before discussing the neurovirulent control of HSV-1 infection by autophagy, it is important to note some specific features of autophagy in neurons, especially since the most debilitating HSV-1 clinical manifestations (e.g., encephalitis) arise through neural infection.
Neuronal autophagy
It is important to reiterate that neurons are specialized for cellular communication, being composed of cell body, axon, dendrites and synapses. Long processes that relay signals from individual synapses to neuron cell bodies drastically increase the jurisdiction of autophagy.50 Presumably, autophagy may be more robust in neurons due to the increased size and the fact that neurons are terminally differentiated. However, basal autophagy levels in healthy neurons have been observed to be lower than other bodily tissues, as manifested by decreased numbers of autophagosomes.51,52 This may be due to neurons' rapacious attitude toward nutrients, limiting the need to induce autophagic recycling mechanisms. Despite a lower quantity of autophagosomes, higher efficiency and faster turnover of autophagosomes has been observed in neuronal cells than non-neural counterparts, which may explain the need for less autophagosomes but tighter regulation.53,54 Interestingly, the main regulatory factor in non-neural cells, the mechanistic target of rapamycin (serine/threonine kinase) complex 1 (MTORC1), seems to play a less dominant role in neurons.55 Rapamycin, a potent stimulator of autophagy by inhibiting MTORC1 activity, fails to upregulate autophagy in primary neural cells.55 Moreover, Mizushima et al., showed that although mice exhibit reduced activity of MTORC1 after a 48-h fast, accumulation of GFP-LC3 or GFP-ATG5 puncta is not observed in hypothalamic neurons.51 Thus, even the inhibition of MTORC1 in neurons may not actually be sufficient to allow an autophagic response. There must be other MTORC1-independent signaling mechanisms to induce autophagy. One such pathway through Ca2+-CAPN/calpain-GNAS/Gsα and cAMP–RAPGEF3/Epac-PLCE1/PLCϵ (phospholipase C epsilon 1)-IP3 (inositol 1,4,5-triphosphate) signaling has been revealed to contribute to upregulation of autophagy in neurons.56
Although the dynamics of autophagy initiation are unclear, other differences exist involving the trafficking of autophagosomes throughout the cell body to nerve terminals and synapses.50,57,58 During axonal damage, autophagosomes accumulate near damage sites and actually assist in axon remodeling.59 Autophagic recycling is observed at the far reaches of dendritic terminals, where synapses are constantly involved in the re-uptake of cleft proteins.60 Therefore, it is crucial to maintain the bidirectional transportation through long axons, and highly effective regulation of autophagosomes may be a result of this requirement. In developing new synaptic connections, increased levels of autophagy correlate with increased synaptic size. This is exemplified by Atg1 overexpression in Drosophila, which induces higher levels of autophagy, and concomitantly, displays increased synaptic size.61,62 Reduction or mutating of autophagy proteins leads to developmental defects of the synapse.63 Moreover, knockdown of crucial autophagy proteins in mice, ATG5 and ATG7, causes debilitating neurodegeneration.64-66 It seems certain that proper basal autophagy maintains the neuronal equilibrium and prevents neurodegeneration.67 In this context, HSV-1 inhibition of autophagy in neurons may contribute to the malfunctions of the nervous system as described below.
HSV-1-infected neurons and autophagy
HSV-1 latency has mostly been found in trigeminal ganglia but also in geniculate and vestibular ganglia.68,69 Latency contributes greatly to HSV-1 pathogenesis, because it can appear one day and reappear decades later, and manifest in advanced clinical diseases. In addition to causing mucosal lesions, reactivated HSV-1 in neurons can relocate to ultimately reach the central nervous system (CNS), where acute encephalitis may occur; or under certain circumstances a latent infection may be established in CNS neurons, as shown in HSV-1 latently infected mice and humans.70-77 The type I-IFN response to HSV-1 infection in neurons is not as robust as that in epithelial cells, presumably to prevent IFN-associated cell toxicity in nonrenewable neurons.49,78,79 While neural infection continues, an increasing amount of HSV-1 latency-associated transcripts accumulate, which limits lytic gene infection and maintains latency.80-82 Notably, low levels of lytic genes are constantly being transcribed yet viral progeny are not produced.83-86 Although the neural transcription of HSV-1 ICP34.5 is not profound, HSV-1 ICP34.5-deficient viruses are more neuroattenuated than wild-type counterparts,87 suggesting that ICP34.5-mediated autophagy inhibition and/or other functionalities may be involved in the neuropathogenesis of HSV-1. However, functional importance regarding the specific role of autophagy in the context of HSV-1 latency is still lacking.
In the neuron, autophagy plays a larger role interacting with replicating or re-activated viruses than in epithelial cells.28,88 Neonatal infection of HSV-1 is particularly predisposed to disseminated disease or encephalitis.89 Reactivated HSV-1 is thought to cause encephalitis by retrograde transport of virus through nerve axons to the CNS.20 Upon viral reactivation, expression of ICP34.5 is necessary for fatal encephalitis in mice and humans.28,40,41 In fact, wild-type ICP34.5 in comparison to ICP34.5-mutant or -knockout HSV-1 strains causes nearly a 107-fold increase in neurovirulence.90 How does ICP34.5 cause neurovirulence? As previously mentioned, ICP34.5 recruitment of PPP1CA causes a reverse of host translational shutoff, and direct BECN1 binding manifests as inhibition of autophagy.28 In fact, mutant ICP34.5 HSV-1 deficient in BECN1-binding only, but capable of PPP1CA-binding is highly neuroattenuated in vivo,28 indicating that neuropathogenesis may stem from the disruption of autophagy through ICP34.5 binding to BECN1. In support of this view, Yordy et al. 49 showed that nearly 50% of mice develop advanced clinical symptoms (e.g., neuropathogenesis) upon infection with wild-type HSV-1 (rescued strain), whereas no advanced symptoms result upon infection with an ICP34.5-mutant HSV-1 strain defective in autophagy inhibition.49 By contrast, no difference is detected between the wild-type and mutant strain in viremia and viral clearance in the genital mucosa, suggesting a minimal role of autophagy in controlling HSV-1 disease in epithelial cells in vivo.49 Interestingly, unlike in adults, autophagy is not stimulated by type-I IFN response in newborns, nor is autophagy inhibition by ICP34.5 required for the progression of HSV-1 encephalitis.91 In fact, activated autophagy is associated with increased apoptosis in the HSV-infected newborn brain, which may even aggravate neonatal HSV encephalitis,89 highlighting an age-dependent difference in autophagy functionality in HSV-associated neuropathogenesis. Taken together, ICP34.5 inhibition of BECN1-mediated autophagy is, at least in part, the neurovirulent mechanism that has important implications in HSV-1 disease progression from infected neurons.
HSV-1-associated autophagy dysfunction: A risk factor for neurodegeneration?
There has been mounting evidence suggesting the involvement of HSV-1 in the pathogenesis of neurodegneration.92-94 While patients rarely exhibit signs of encephalitis, many postmortem studies find a high prevalence of HSV-1 in the brain.95-98 Strikingly, Alzheimer disease brains contain a high localization of HSV-1 DNA within amyloid plaques, 72% in AD patients, whereas only 24% of the DNA associates with plaques in age-matched non-AD patients, who accumulate plaques at a much lower rate.7 Furthermore, animal models and acute HSV-1 encephaltitis patients show that the virus targets brain regions overlapping with AD: frontal and temporal cortices and the hippocampus.99-102 Although HSV-1 preferentially lies dormant in neurons, the detection of HSV-1 DNA in the cerebrospinal fluid suggests that asymptomatic replication of HSV-1 may occur in the CNS under certain cirucumstances.103,104 Given the intense interplay between HSV-1 and host cells, it is likely that recurrent HSV-1 reactivation may cumulatively arouse neuronal dysfunction, as demonstrated recently by Martin et al.,105 which shows that HSV-1 reactivation from latency induces neuroinflammation and the appearance of early neurodegenerative markers including MAPT/Tau phosphorylation. In line with this, independent analyses of large cohorts of subjects revealed a significantly increased risk for AD in patients with anti-HSV-1 IgM, a marker of viral primary infection or reactivation.106,107 Another study by Mancuso et al.108 revealed that elevated HSV-1-antibody titers are significantly more frequent in patients with mild AD than in healthy controls, and that they are positively correlated with cortical gray matter, suggesting a protective role of HSV-1-specific antibody in the early stages of AD. Despite these advancements, the mechanisms underlying AD association with HSV-1 infection remain incompletely explored.
As noted above, HSV-1 has developed strategies to sabotage autophagy activation, degradation, and antigen presentation. Recurrent HSV-1 replication in CNS and its jeopardizing of the autophagy machinery may act, in a step-by-step fashion, to hasten disease progression of neurodegeneration. On the one hand, as immune systems decline in advancing age, the chance of HSV-1 penetration into the brain from an earlier latent infection might increase, causing gradual local damage through recurrent reactivation. On the other hand, aging declines autophagy function, which may be hastened via HSV-1 replication, especially through neurovirulence factor ICP34.5. The reduction of autophagy not only assists the viral lifecycle but also lessens the degradation of both viral and host protein aggregates.109 Aggregation of β-amyloid protein fragments accumulate to ultimately cause synapse destruction, a hallmark of Alzheimer disease.110,111 Neural cell death may also occur in these circumstances. Although autophagy serves as a direct response to the accumulation of β-amyloid aggregates, reduced expression of BECN1 found in prodromal Alzheimer disease patients indicates autophagy falters before severe disease.112 Furthermore, Becn1-heterozygous mice exhibit aggravation of Alzheimer pathology by increased and rapid accumulation of β-amyloid plaques.112 All these studies point to the direction of necessity of autophagy in clearing the accumulation of pathogenic β-amyloid aggregates.113,114 In line with this, the reversal of neurodegeneration is observed in PC12 cells upon stimulation of autophagy through rapamycin or the novel small molecule AR-12 (small molecule OSU-03012; likely to be independent of MTOR).115 Another hallmark of Alzheimer disease is the accumulation of autophagic vesicles filled with incompletely digested proteins in axons, suggesting abnormalities in axonal transport or a consequence of jammed vesicles and/or not enough space to transport.116 Notably, neuronal communication is dependent upon the robustness of synaptic size and response, which is positively correlated with autophagy. In this specific context, autophagy's role in synaptic viability may also serve the underlying cause in promoting the development of neurodegenerative disorders. Hence, autophagy rehabilitation may hold the key to prevent neurodegeneration from early on. Interestingly, It is unclear whether autophagy abnormalities are a direct cause or consequence of these specific diseases, but recent evidence is mounting that points to its crucial role.117 It further stands to reason if HSV-1 infection has such a profound effect on influencing autophagic processes that the three, HSV-1 infection, autophagy, and neuropathogenesis, are uniquely intertwined.118
Conclusion
HSV-1 plays an integral role in debilitating autophagy in epithelial cells, local neurons, and in the central nervous system. Autophagy abnormalities hold the key in the disruption of neuron homeostasis, and likely contribute to the development of neurodegeneration. Despite many unanswered questions about HSV-1 in neurons, HSV-1 infection along with the age-related decline of autophagy may explain the consequences behind a gradual disease progression spanning decades.118 Many studies point toward HSV-1 being a prime candidate as a neural gene vector.119-121 Delivery of autophagy-promoting factors using recombinant HSV-1 may be a step forward in order to decipher the exact mechanism of pathogenesis and prevent or slow the decline of neurodegenerative diseases. Nevertheless, although the correlation of HSV-1 and AD remains controversial, autophagy may be an interesting therapeutic facet between the two.
Abbreviations
- AD
Alzheimer disease
- BECN1
Beclin 1, autophagy related
- CNS
central nervous system
- EIF2AK2/PKR
eukaryotic translation initiation factor 2 α kinase 2
- EIF2S1
eukaryotic translation initiation factor 2 subunit α
- ER
endoplasmic reticulum
- HSV-1
herpes simplex virus type-1
- ICP34.5
infected cell protein 34.5
- IFN
interferon
- MHC-I
major histocompatibility complex class I
- MHC-II
major histocompatibility complex class II
- MTORC1
mechanistic target of rapamycin (serine/threonine kinase) complex 1
- PNS
peripheral nervous system
- PPP1CA
protein phosphatase 1, catalytic subunit, α isozyme
- TBK1
TANK-binding kinase 1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was partly supported by the Margaret E. Early Medical Research Trust, American Cancer Society (RSG-11–121–01-CCG), and National Institutes of Health grants R01 CA140964 to C. Liang.
References
- [1].Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis 2014; 209:325-33; PMID:24136792; http://dx.doi.org/ 10.1093/infdis/jit458 [DOI] [PubMed] [Google Scholar]
- [2].Xu F, Schillinger JA, Sternberg MR, Johnson RE, Lee FK, Nahmias AJ, Markowitz LE. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J Infect Dis 2002; 185:1019-24; PMID:11930310; http://dx.doi.org/ 10.1086/340041 [DOI] [PubMed] [Google Scholar]
- [3].Wilson AC, Mohr I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol 2012; 20:604-11; PMID:22963857; http://dx.doi.org/ 10.1016/j.tim.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis 1998; 26:541-53; quiz 54-5; PMID:9524821; http://dx.doi.org/ 10.1086/514600 [DOI] [PubMed] [Google Scholar]
- [5].Carbone I, Lazzarotto T, Ianni M, Porcellini E, Forti P, Masliah E, Gabrielli L, Licastro F. Herpes virus in Alzheimer disease: relation to progression of the disease. Neurobiol Aging 2014; 35:122-9; PMID:23916950; http://dx.doi.org/ 10.1016/j.neurobiolaging.2013.06.024 [DOI] [PubMed] [Google Scholar]
- [6].Harris SA, Harris EA. Herpes Simplex Virus Type 1 and Other Pathogens are Key Causative Factors in Sporadic Alzheimer Disease. J Alzheimers Dis 2015; 48:319-53; PMID:26401998; http://dx.doi.org/ 10.3233/JAD-142853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer disease amyloid plaques. J Pathol 2009; 217:131-8; PMID:18973185; http://dx.doi.org/ 10.1002/path.2449 [DOI] [PubMed] [Google Scholar]
- [8].Newcomb WW, Juhas RM, Thomsen DR, Homa FL, Burch AD, Weller SK, Brown JC. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J Virol 2001; 75:10923-32; PMID:11602732; http://dx.doi.org/ 10.1128/JVI.75.22.10923-10932.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Loret S, Guay G, Lippe R. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J Virol 2008; 82:8605-18; PMID:18596102; http://dx.doi.org/ 10.1128/JVI.00904-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Esclatine A, Taddeo B, Roizman B. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc Natl Acad Sci U S A 2004; 101:18165-70; PMID:15596716; http://dx.doi.org/ 10.1073/pnas.0408272102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wysocka J, Herr W. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem Sci 2003; 28:294-304; PMID:12826401; http://dx.doi.org/ 10.1016/S0968-0004(03)00088-4 [DOI] [PubMed] [Google Scholar]
- [12].Everett RD. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 2000; 22:761-70; PMID:10918307; http://dx.doi.org/ 10.1002/1521-1878(200008)22:8%3c761::AID-BIES10%3e3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- [13].Kalamvoki M, Roizman B. Interwoven roles of cyclin D3 and cdk4 recruited by ICP0 and ICP4 in the expression of herpes simplex virus genes. J Virol 2010; 84:9709-17; PMID:20660182; http://dx.doi.org/ 10.1128/JVI.01050-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sinani D, Cordes E, Workman A, Thunuguntia P, Jones C. Stress-induced cellular transcription factors expressed in trigeminal ganglionic neurons stimulate the herpes simplex virus 1 ICP0 promoter. J Virol 2013; 87:13042-7; PMID:24027338; http://dx.doi.org/ 10.1128/JVI.02476-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Opstelten W, Neven AK, Eekhof J. Treatment and prevention of herpes labialis. Can Fam Physician 2008; 54:1683-7; PMID:19074705 [PMC free article] [PubMed] [Google Scholar]
- [16].Raborn GW, Grace MG. Recurrent herpes simplex labialis: selected therapeutic options. J Can Dent Assoc 2003; 69:498-503; PMID:12954137 [PubMed] [Google Scholar]
- [17].Preston CM, Efstathiou S. Molecular basis of HSV latency and reactivation. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al., eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge, 2007. [Google Scholar]
- [18].Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol 2007; 57:737-63; quiz 64-6; PMID:17939933; http://dx.doi.org/ 10.1016/j.jaad.2007.06.027 [DOI] [PubMed] [Google Scholar]
- [19].Roizman B, Whitley RJ. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol 2013; 67:355-74; PMID:24024635; http://dx.doi.org/ 10.1146/annurev-micro-092412-155654 [DOI] [PubMed] [Google Scholar]
- [20].Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res 2006; 71:141-8; PMID:16675036; http://dx.doi.org/ 10.1016/j.antiviral.2006.04.002 [DOI] [PubMed] [Google Scholar]
- [21].Orvedahl A, Levine B. Autophagy in Mammalian antiviral immunity. Curr Top Microbiol Immunol 2009; 335:267-85; PMID:19802570 [DOI] [PubMed] [Google Scholar]
- [22].Shoji-Kawata S, Levine B. Autophagy, antiviral immunity, and viral countermeasures. Biochim Biophys Acta 2009; 1793:1478-84; PMID:19264100; http://dx.doi.org/ 10.1016/j.bbamcr.2009.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Awan MU, Deng Y. Role of autophagy and its significance in cellular homeostasis. Appl Microbiol Biotechnol 2014; 98:5319-28; PMID:24743981; http://dx.doi.org/ 10.1007/s00253-014-5721-8 [DOI] [PubMed] [Google Scholar]
- [24].Klionsky DJ, Cuervo AM, Dunn WA Jr., Levine B, van der Klei I, Seglen PO. How shall I eat thee? Autophagy 2007; 3:413-6; PMID:17568180; http://dx.doi.org/ 10.4161/auto.4377 [DOI] [PubMed] [Google Scholar]
- [25].Talloczy Z, Jiang W, Virgin HWt, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A 2002; 99:190-5; PMID:11756670; http://dx.doi.org/ 10.1073/pnas.012485299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A 1997; 94:843-8; PMID:9023344; http://dx.doi.org/ 10.1073/pnas.94.3.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chou J, Chen JJ, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 α and premature shutoff of protein synthesis after infection with gamma 134.5- mutants of herpes simplex virus 1. Proc Natl Acad Sci U S A 1995; 92:10516-20; PMID:7479831; http://dx.doi.org/ 10.1073/pnas.92.23.10516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 2007; 1:23-35; PMID:18005679; http://dx.doi.org/ 10.1016/j.chom.2006.12.001 [DOI] [PubMed] [Google Scholar]
- [29].Talloczy Z, Virgin HWt, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2006; 2:24-9; PMID:16874088; http://dx.doi.org/ 10.4161/auto.2176 [DOI] [PubMed] [Google Scholar]
- [30].Alexander DE, Ward SL, Mizushima N, Levine B, Leib DA. An analysis of the role of autophagy in replication of herpes simplex virus in cell culture. J Virol 2007; 81(22):12128-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yakoub AM, Shukla D. Herpes Simplex Virus-1 Fine-Tunes Host's Autophagic Response to Infection: A Comprehensive Analysis in Productive Infection Models. PLoS One 2015; 10:e0124646; PMID:25894397; http://dx.doi.org/ 10.1371/journal.pone.0124646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lussignol M, Queval C, Bernet-Camard MF, Cotte-Laffitte J, Beau I, Codogno P, Esclatine A. The herpes simplex virus 1 Us11 protein inhibits autophagy through its interaction with the protein kinase PKR. J Virol 2013; 87:859-71; PMID:23115300; http://dx.doi.org/ 10.1128/JVI.01158-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, Alexander D, Leib D, Norbury C, Lippé R, et al.. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol 2009; 10:480-7; PMID:19305394; http://dx.doi.org/ 10.1038/ni.1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, et al.. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A 2005; 102:7922-7; PMID:15894616; http://dx.doi.org/ 10.1073/pnas.0501190102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Leib DA, Alexander DE, Cox D, Yin J, Ferguson TA. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J Virol 2009; 83:12164-71; PMID:19759141; http://dx.doi.org/ 10.1128/JVI.01676-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].English L, Chemali M, Desjardins M. Nuclear membrane-derived autophagy, a novel process that participates in the presentation of endogenous viral antigens during HSV-1 infection. Autophagy 2009; 5:1026-9; PMID:19556870; http://dx.doi.org/ 10.4161/auto.5.7.9163 [DOI] [PubMed] [Google Scholar]
- [37].Radtke K, English L, Rondeau C, Leib D, Lippe R, Desjardins M. Inhibition of the host translation shutoff response by herpes simplex virus 1 triggers nuclear envelope-derived autophagy. J Virol 2013; 87:3990-7; PMID:23365427; http://dx.doi.org/ 10.1128/JVI.02974-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Broberg EK, Peltoniemi J, Nygardas M, Vahlberg T, Roytta M, Hukkanen V. Spread and replication of and immune response to gamma134.5-negative herpes simplex virus type 1 vectors in BALB/c mice. J Virol 2004; 78:13139-52; PMID:15542666; http://dx.doi.org/ 10.1128/JVI.78.23.13139-13152.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yakoub AM, Shukla D. Autophagy stimulation abrogates herpes simplex virus-1 infection. Sci Rep 2015; 5:9730; PMID:25856282; http://dx.doi.org/ 10.1038/srep09730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 1990; 250:1262-6; PMID:2173860; http://dx.doi.org/ 10.1126/science.2173860 [DOI] [PubMed] [Google Scholar]
- [41].Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, Hadley D, Patterson J, Brown SM, Rampling R. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther 2004; 11:1648-58; PMID:15334111; http://dx.doi.org/ 10.1038/sj.gt.3302289 [DOI] [PubMed] [Google Scholar]
- [42].Orvedahl A, Levine B. Autophagy and viral neurovirulence. Cell Microbiol 2008; 10:1747-56; PMID:18503639; http://dx.doi.org/ 10.1111/j.1462-5822.2008.01175.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Verpooten D, Ma Y, Hou S, Yan Z, He B. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J Biol Chem 2009; 284:1097-105; PMID:19010780; http://dx.doi.org/ 10.1074/jbc.M805905200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ma Y, Jin H, Valyi-Nagy T, Cao Y, Yan Z, He B. Inhibition of TANK binding kinase 1 by herpes simplex virus 1 facilitates productive infection. J Virol 2012; 86:2188-96; PMID:22171259; http://dx.doi.org/ 10.1128/JVI.05376-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weidberg H, Elazar Z. TBK1 mediates crosstalk between the innate immune response and autophagy. Sci Signal 2011; 4:pe39; PMID:21868362; http://dx.doi.org/ 10.1126/scisignal.2002355 [DOI] [PubMed] [Google Scholar]
- [46].Pilli M, Arko-Mensah J, Ponpuak M, Roberts E, Master S, Mandell MA, Dupont N, Ornatowski W, Jiang S, Bradfute SB, et al.. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 2012; 37:223-34; PMID:22921120; http://dx.doi.org/ 10.1016/j.immuni.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cassady KA, Gross M, Roizman B. The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J Virol 1998; 72:8620-6; PMID:9765401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Poppers J, Mulvey M, Khoo D, Mohr I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J Virol 2000; 74:11215-21; PMID:11070019; http://dx.doi.org/ 10.1128/JVI.74.23.11215-11221.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yordy B, Iijima N, Huttner A, Leib D, Iwasaki A. A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe 2012; 12:334-45; PMID:22980330; http://dx.doi.org/ 10.1016/j.chom.2012.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wong YC, Holzbaur EL. Autophagosome dynamics in neurodegeneration at a glance. J Cell Sci 2015; 128:1259-67; PMID:25829512; http://dx.doi.org/ 10.1242/jcs.161216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 2004; 15:1101-11; PMID:14699058; http://dx.doi.org/ 10.1091/mbc.E03-09-0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 2005; 64:113-22; PMID:15751225; http://dx.doi.org/ 10.1093/jnen/64.2.113 [DOI] [PubMed] [Google Scholar]
- [53].Nixon RA. Endosome function and dysfunction in Alzheimer disease and other neurodegenerative diseases. Neurobiol Aging 2005; 26:373-82; PMID:15639316; http://dx.doi.org/ 10.1016/j.neurobiolaging.2004.09.018 [DOI] [PubMed] [Google Scholar]
- [54].Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer disease. J Neurosci 2008; 28:6926-37; PMID:18596167; http://dx.doi.org/ 10.1523/JNEUROSCI.0800-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mitra S, Tsvetkov AS, Finkbeiner S. Protein turnover and inclusion body formation. Autophagy 2009; 5:1037-8; PMID:19838079; http://dx.doi.org/ 10.4161/auto.5.7.9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 2009; 16:46-56; PMID:18636076; http://dx.doi.org/ 10.1038/cdd.2008.110 [DOI] [PubMed] [Google Scholar]
- [57].Fu MM, Nirschl JJ, Holzbaur EL. LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Dev Cell 2014; 29:577-90; PMID:24914561; http://dx.doi.org/ 10.1016/j.devcel.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yue Z, Friedman L, Komatsu M, Tanaka K. The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochim Biophys Acta 2009; 1793:1496-507; PMID:19339210; http://dx.doi.org/ 10.1016/j.bbamcr.2009.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci 2006; 26:8057-68; PMID:16885219; http://dx.doi.org/ 10.1523/JNEUROSCI.2261-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sumpter R Jr., Levine B. Selective autophagy and viruses. Autophagy 2011; 7:260-5; PMID:21150267; http://dx.doi.org/ 10.4161/auto.7.3.14281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J Cell Biol 2009; 187:71-9; PMID:19786572; http://dx.doi.org/ 10.1083/jcb.200907109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wairkar YP, Toda H, Mochizuki H, Furukubo-Tokunaga K, Tomoda T, Diantonio A. Unc-51 controls active zone density and protein composition by downregulating ERK signaling. J Neurosci 2009; 29:517-28; PMID:19144852; http://dx.doi.org/ 10.1523/JNEUROSCI.3848-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron 2000; 26:313-29; PMID:10839352; http://dx.doi.org/ 10.1016/S0896-6273(00)81166-6 [DOI] [PubMed] [Google Scholar]
- [64].Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al.. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441:885-9; PMID:16625204; http://dx.doi.org/ 10.1038/nature04724 [DOI] [PubMed] [Google Scholar]
- [65].Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al.. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441:880-4; PMID:16625205; http://dx.doi.org/ 10.1038/nature04723 [DOI] [PubMed] [Google Scholar]
- [66].Komatsu M, Wang QJ, Holstein GR, Friedrich VL Jr., Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A 2007; 104:14489-94; PMID:17726112; http://dx.doi.org/ 10.1073/pnas.0701311104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 2015; 16:345-57; PMID:25991442; http://dx.doi.org/ 10.1038/nrn3961 [DOI] [PubMed] [Google Scholar]
- [68].Furuta Y, Takasu T, Sato KC, Fukuda S, Inuyama Y, Nagashima K. Latent herpes simplex virus type 1 in human geniculate ganglia. Acta Neuropathol 1992; 84:39-44; PMID:1323906; http://dx.doi.org/ 10.1007/BF00427213 [DOI] [PubMed] [Google Scholar]
- [69].Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, Brandt T. Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol 2001; 11:408-13; PMID:11556685; http://dx.doi.org/ 10.1111/j.1750-3639.2001.tb00408.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lewandowski G, Zimmerman MN, Denk LL, Porter DD, Prince GA. Herpes simplex type 1 infects and establishes latency in the brain and trigeminal ganglia during primary infection of the lip in cotton rats and mice. Arch Virol 2002; 147:167-79; PMID:11855629; http://dx.doi.org/ 10.1007/s705-002-8309-9 [DOI] [PubMed] [Google Scholar]
- [71].Kastrukoff L, Hamada T, Schumacher U, Long C, Doherty PC, Koprowski H. Central nervous system infection and immune response in mice inoculated into the lip with herpes simplex virus type 1. J Neuroimmunol 1982; 2:295-305; PMID:6282930; http://dx.doi.org/ 10.1016/0165-5728(82)90062-5 [DOI] [PubMed] [Google Scholar]
- [72].Dobson CB, Wozniak MA, Itzhaki RF. Do infectious agents play a role in dementia? Trends Microbiol 2003; 11:312-7; PMID:12875814; http://dx.doi.org/ 10.1016/S0966-842X(03)00146-X [DOI] [PubMed] [Google Scholar]
- [73].Efstathiou S, Minson AC, Field HJ, Anderson JR, Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J Virol 1986; 57:446-55; PMID:3003377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rock DL, Fraser NW. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature 1983; 302:523-5; PMID:6300686; http://dx.doi.org/ 10.1038/302523a0 [DOI] [PubMed] [Google Scholar]
- [75].Gordon L, McQuaid S, Cosby SL. Detection of herpes simplex virus (types 1 and 2) and human herpesvirus 6 DNA in human brain tissue by polymerase chain reaction. Clin Diagn Virol 1996; 6:33-40; PMID:15566888; http://dx.doi.org/ 10.1016/0928-0197(95)00203-0 [DOI] [PubMed] [Google Scholar]
- [76].Sequiera LW, Jennings LC, Carrasco LH, Lord MA, Curry A, Sutton RN. Detection of herpes-simplex viral genome in brain tissue. Lancet 1979; 2:609-12; PMID:90272; http://dx.doi.org/ 10.1016/S0140-6736(79)91667-2 [DOI] [PubMed] [Google Scholar]
- [77].Cabrera CV, Wohlenberg C, Openshaw H, Rey-Mendez M, Puga A, Notkins AL. Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature 1980; 288:288-90; PMID:6253827; http://dx.doi.org/ 10.1038/288288a0 [DOI] [PubMed] [Google Scholar]
- [78].Griffin DE. Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol Res 2010; 47:123-33; PMID:20087684; http://dx.doi.org/ 10.1007/s12026-009-8143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Levine B. Apoptosis in viral infections of neurons: a protective or pathologic host response? Curr Top Microbiol Immunol 2002; 265:95-118; PMID:12014197 [DOI] [PubMed] [Google Scholar]
- [80].Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 2008; 454:780-3; PMID:18596690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tang S, Bertke AS, Patel A, Wang K, Cohen JI, Krause PR. An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor. Proc Natl Acad Sci U S A 2008; 105:10931-6; PMID:18678906; http://dx.doi.org/ 10.1073/pnas.0801845105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Thompson RL, Sawtell NM. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J Virol 2001; 75:6660-75; PMID:11413333; http://dx.doi.org/ 10.1128/JVI.75.14.6660-6675.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ma JZ, Russell TA, Spelman T, Carbone FR, Tscharke DC. Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response. PLoS Pathog 2014; 10:e1004237; PMID:25058429; http://dx.doi.org/ 10.1371/journal.ppat.1004237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chen SH, Lee LY, Garber DA, Schaffer PA, Knipe DM, Coen DM. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J Virol 2002; 76:4764-72; PMID:11967293; http://dx.doi.org/ 10.1128/JVI.76.10.4764-4772.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A 2002; 99:978-83; PMID:11773630; http://dx.doi.org/ 10.1073/pnas.022301899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Giordani NV, Neumann DM, Kwiatkowski DL, Bhattacharjee PS, McAnany PK, Hill JM, Bloom DC. During herpes simplex virus type 1 infection of rabbits, the ability to express the latency-associated transcript increases latent-phase transcription of lytic genes. J Virol 2008; 82:6056-60; PMID:18400860; http://dx.doi.org/ 10.1128/JVI.02661-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bolovan CA, Sawtell NM, Thompson RL. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J Virol 1994; 68:48-55; PMID:8254758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yordy B, Iwasaki A. Cell type-dependent requirement of autophagy in HSV-1 antiviral defense. Autophagy 2013; 9:236-8; PMID:23095715; http://dx.doi.org/ 10.4161/auto.22506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Frenkel LM, Gruber WC, Rathore M, Bradley JS, Diaz PS, Kumar M, et al.. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics 2001; 108:223-9; PMID:11483781; http://dx.doi.org/ 10.1542/peds.108.2.223 [DOI] [PubMed] [Google Scholar]
- [90].Thompson RL, Wagner EK, Stevens JG. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology 1983; 131:180-92; PMID:6316650; http://dx.doi.org/ 10.1016/0042-6822(83)90544-5 [DOI] [PubMed] [Google Scholar]
- [91].Wilcox DR, Wadhwani NR, Longnecker R, Muller WJ. Differential reliance on autophagy for protection from HSV encephalitis between newborns and adults. PLoS Pathog 2015; 11:e1004580; PMID:25569138; http://dx.doi.org/ 10.1371/journal.ppat.1004580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Itzhaki RF. Herpes simplex virus type 1 and Alzheimer disease: increasing evidence for a major role of the virus. Front Aging Neurosci 2014; 6:202; PMID:25157230; http://dx.doi.org/ 10.3389/fnagi.2014.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wozniak MA, Itzhaki RF. Antiviral agents in Alzheimer disease: hope for the future? Ther Adv Neurol Disord 2010; 3:141-52; PMID:21179606; http://dx.doi.org/ 10.1177/1756285610370069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Piacentini R, De Chiara G, Li Puma DD, Ripoli C, Marcocci ME, Garaci E, Palamara AT, Grassi C. HSV-1 and Alzheimer disease: more than a hypothesis. Front Pharmacol 2014; 5:97; PMID:24847267; http://dx.doi.org/ 10.3389/fphar.2014.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hemling N, Roytta M, Rinne J, Pollanen P, Broberg E, Tapio V, Vahlberg T, Hukkanen V. Herpesviruses in brains in Alzheimer and Parkinson diseases. Ann Neurol 2003; 54:267-71; PMID:12891684; http://dx.doi.org/ 10.1002/ana.10662 [DOI] [PubMed] [Google Scholar]
- [96].Mori I, Kimura Y, Naiki H, Matsubara R, Takeuchi T, Yokochi T, Nishiyama Y. Reactivation of HSV-1 in the brain of patients with familial Alzheimer disease. J Med Virol 2004; 73:605-11; PMID:15221907; http://dx.doi.org/ 10.1002/jmv.20133 [DOI] [PubMed] [Google Scholar]
- [97].Jamieson GA, Maitland NJ, Wilcock GK, Craske J, Itzhaki RF. Latent herpes simplex virus type 1 in normal and Alzheimer disease brains. J Med Virol 1991; 33:224-7; PMID:1649907; http://dx.doi.org/ 10.1002/jmv.1890330403 [DOI] [PubMed] [Google Scholar]
- [98].Jamieson GA, Maitland NJ, Wilcock GK, Yates CM, Itzhaki RF. Herpes simplex virus type 1 DNA is present in specific regions of brain from aged people with and without senile dementia of the Alzheimer type. J Pathol 1992; 167:365-8; PMID:1328575; http://dx.doi.org/ 10.1002/path.1711670403 [DOI] [PubMed] [Google Scholar]
- [99].Sokolov AA, Reincke M. Herpes simplex encephalitis affecting the entire limbic system. Mayo Clin Proc 2012; 87:e69; PMID:22959003; http://dx.doi.org/ 10.1016/j.mayocp.2012.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ando Y, Kitayama H, Kawaguchi Y, Koyanagi Y. Primary target cells of herpes simplex virus type 1 in the hippocampus. Microbes Infect 2008; 10:1514-23; PMID:18852062; http://dx.doi.org/ 10.1016/j.micinf.2008.09.005 [DOI] [PubMed] [Google Scholar]
- [101].Taylor SW, Lee DH, Jackson AC. Herpes simplex encephalitis presenting with exclusively frontal lobe involvement. J Neurovirol 2007; 13:477-81; PMID:17994434; http://dx.doi.org/ 10.1080/13550280701491131 [DOI] [PubMed] [Google Scholar]
- [102].Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer disease. Lancet 1997; 349:241-4; PMID:9014911; http://dx.doi.org/ 10.1016/S0140-6736(96)10149-5 [DOI] [PubMed] [Google Scholar]
- [103].Plentz A, Jilg W, Kochanowski B, Ibach B, Knoll A. Detection of herpesvirus DNA in cerebrospinal fluid and correlation with clinical symptoms. Infection 2008; 36:158-62; PMID:18379728; http://dx.doi.org/ 10.1007/s15010-007-6354-y [DOI] [PubMed] [Google Scholar]
- [104].Bearer EL. HSV, axonal transport and Alzheimer disease: in vitro and in vivo evidence for causal relationships. Future Virol 2012; 7:885-99; PMID:23335944; http://dx.doi.org/ 10.2217/fvl.12.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Martin C, Aguila B, Araya P, Vio K, Valdivia S, Zambrano A, Concha MI, Otth C. Inflammatory and neurodegeneration markers during asymptomatic HSV-1 reactivation. J Alzheimers Dis 2014; 39:849-59; PMID:24296813 [DOI] [PubMed] [Google Scholar]
- [106].Feart C, Helmer C, Fleury H, Bejot Y, Ritchie K, Amouyel P, Schraen-Maschke S, Buée L, Lambert JC, Letenneur L, et al.. Association between IgM anti-herpes simplex virus and plasma amyloid-β levels. PLoS One 2011; 6:e29480; PMID:22216291; http://dx.doi.org/ 10.1371/journal.pone.0029480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Letenneur L, Peres K, Fleury H, Garrigue I, Barberger-Gateau P, Helmer C, Orgogozo JM, Gauthier S, Dartigues JF. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer disease: a population-based cohort study. PLoS One 2008; 3:e3637; PMID:18982063; http://dx.doi.org/ 10.1371/journal.pone.0003637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mancuso R, Baglio F, Cabinio M, Calabrese E, Hernis A, Nemni R, Clerici M. Titers of herpes simplex virus type 1 antibodies positively correlate with grey matter volumes in Alzheimer disease. J Alzheimers Dis 2014; 38:741-5; PMID:24072067 [DOI] [PubMed] [Google Scholar]
- [109].Itzhaki RF, Wozniak MA. Herpes simplex virus type 1 in Alzheimer disease: the enemy within. J Alzheimers Dis 2008; 13:393-405; PMID:18487848 [DOI] [PubMed] [Google Scholar]
- [110].Palop JJ, Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer disease: from synapses toward neural networks. Nat Neurosci 2010; 13:812-8; PMID:20581818; http://dx.doi.org/ 10.1038/nn.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer disease. Cold Spring Harb Perspect Biol 2012; 4(5):a005777; PMID:22491782; http://dx.doi.org/ 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, et al.. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J Clin Invest 2008; 118:2190-9; PMID:18497889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy 2005; 1:11-22; PMID:16874045; http://dx.doi.org/ 10.4161/auto.1.1.1513 [DOI] [PubMed] [Google Scholar]
- [114].Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O'Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet 2005; 37:771-6; PMID:15980862; http://dx.doi.org/ 10.1038/ng1591 [DOI] [PubMed] [Google Scholar]
- [115].Yang Y, Chen S, Zhang J, Li C, Sun Y, Zhang L, Zheng X. Stimulation of autophagy prevents amyloid-β peptide-induced neuritic degeneration in PC12 cells. J Alzheimers Dis 2014; 40:929-39; PMID:24531159 [DOI] [PubMed] [Google Scholar]
- [116].Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer-like axonal dystrophy. J Neurosci 2011; 31:7817-30; PMID:21613495; http://dx.doi.org/ 10.1523/JNEUROSCI.6412-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lee JA. Neuronal autophagy: a housekeeper or a fighter in neuronal cell survival? Exp Neurobiol 2012; 21:1-8; PMID:22438673; http://dx.doi.org/ 10.5607/en.2012.21.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Itzhaki RF, Cosby SL, Wozniak MA. Herpes simplex virus type 1 and Alzheimer disease: the autophagy connection. J Neurovirol 2008; 14:1-4; PMID:18300070; http://dx.doi.org/ 10.1080/13550280701802543 [DOI] [PubMed] [Google Scholar]
- [119].Goins WF, Huang S, Cohen JB, Glorioso JC. Engineering HSV-1 vectors for gene therapy. Methods Mol Biol 2014; 1144:63-79; PMID:24671677; http://dx.doi.org/ 10.1007/978-1-4939-0428-0_5 [DOI] [PubMed] [Google Scholar]
- [120].Liu R, Varghese S, Rabkin SD. Oncolytic herpes simplex virus vector therapy of breast cancer in C3(1)/SV40 T-antigen transgenic mice. Cancer Res 2005; 65:1532-40; PMID:15735042; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-3353 [DOI] [PubMed] [Google Scholar]
- [121].Melendez ME, Fraefel C, Epstein AL. Herpes simplex virus type 1 (HSV-1)-derived amplicon vectors. Methods Mol Biol 2014; 1144:81-98; PMID:24671678; http://dx.doi.org/ 10.1007/978-1-4939-0428-0_6 [DOI] [PubMed] [Google Scholar]