Abstract
Protein deamidation has been considered a nonenzymatic process associated with protein functional decay or “aging.” Recent studies implicate protein deamidation in regulating signal transduction in fundamental biological processes, such as innate immune responses. Work investigating gammaherpesviruses and bacterial pathogens indicates that microbial pathogens deploy deamidases or enzyme-deficient homologues (pseudoenzymes) to induce deamidation of key signaling components and evade host immune responses. Here, we review studies on protein deamidation in innate immune signaling and present several imminent questions concerning the roles of protein deamidation in infection and immunity.
INTRODUCTION
Innate immunity is the first line of defense against invading pathogens. Central to host immune responses is the detection of pathogen-associated molecular patterns (PAMPs) by cellular pattern recognition receptors (PRRs) (1). Retinoic acid-induced gene I (RIG-I) is a cytosolic receptor that senses double-stranded RNA (dsRNA) originating from pathogens such as viruses (2–5). Binding to dsRNA disrupts an intramolecular interaction that keeps RIG-I in an autoinhibitory state (6, 7), triggering an overall conformational change that releases the N-terminal CARD domain (8, 9). The N-terminal CARD of RIG-I undergoes homotypic oligomerization and heterooligomerization with that of the mitochondrion antiviral signaling (MAVS) adaptor molecule (10). Oligomerized MAVS forms prion-like filaments that are capable of activating two kinase complexes, IκB kinase alpha beta gamma (IKKαβγ) and IKKε–TBK-1, which, in turn, activate NF-κB and interferon (IFN) regulatory factors (IRFs) (11–13). Along with other transcription factors, NF-κB and IRFs upregulate the expression of intrinsic antiviral molecules (e.g., Mx and viperin) and the secretion of various cytokines (e.g., interferon) that further induce the expression of a network of a few hundred antiviral genes (14). Given the potent activity of RIG-I in inducing inflammatory responses, it is not surprising that RIG-I activation is regulated by multiple mechanisms in response to viral infection. For example, noncovalent binding and covalent conjugation of the Lys63-linked polyubiquitin chain to the CARD domain are reported to activate RIG-I (15–17), whereas phosphorylation by protein kinase C and casein kinase represses and dephosphorylation promotes RIG-I-mediated signaling (18–20). These are key cellular events that have been evolved to tightly regulate RIG-I-mediated immune activation in response to viral infection.
Viruses often evolve intricate mechanisms to deflect host immune responses. While RNA viruses deploy various proteins to blunt RIG-I-mediated innate defenses by hampering key signaling components such as RIG-I and MAVS, DNA viruses can manipulate the signaling cascade to benefit their infection (21–23) (Fig. 1). Studies of RNA viruses have identified distinct viral factors that target RIG-I and MAVS. Influenza virus NS1 derails RIG-I ubiquitination by nullifying the essential TRIM25 E3 ligase (24). Notably, hepatitis C virus uses its NS3/4A protease to cleave MAVS and release it from the mitochondrial membrane (25–27), thereby halting RIG-I-dependent antiviral immune responses. A similar strategy is employed by hepatitis G virus, hepatitis A virus, enterovirus 71, and coxsackievirus to derail IFN production (28–31). DNA viruses utilize strategies that are more intricate than those utilized by RNA viruses to evade these innate immune signaling cascades. The manipulation of innate and adaptive immune responses by herpesviruses has been previously well reviewed (21). One interesting example is murine gammaherpesvirus 68 (γHV68), which requires MAVS for efficient lytic replication. γHV68 is a model herpesvirus for human Kaposi's sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV). With a combination of genetic and biochemical analyses, Dong et al. showed that the downstream IKKβ kinase is usurped to phosphorylate viral replication trans-activator (RTA) and thereby promotes viral lytic gene expression (32). Additionally, IKKβ is also coopted to phosphorylate p65 (also known as RelA), which primes p65 for proteasome-mediated degradation in conjunction with the RTA E3 ligase, thereby terminating NF-κB activation (33, 34). Thus, MAVS-dependent signaling is critical for efficient productive replication of γHV68.
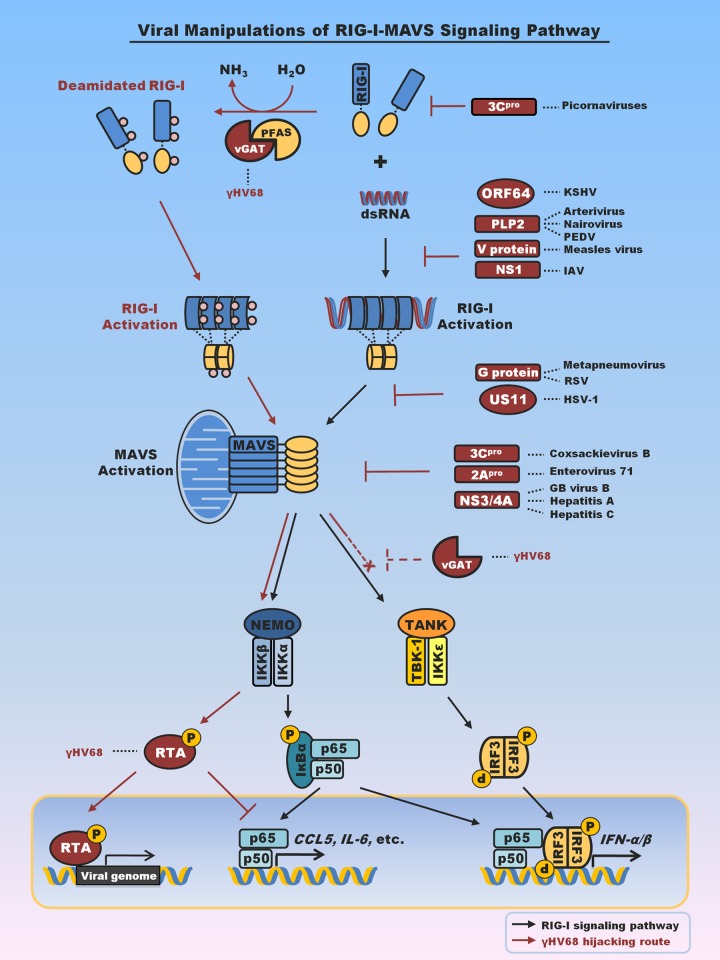
FIG 1.
Summary of viral factors that interfere with or hijack RIG-I-mediated innate immune signaling. Emphasis is placed on viral proteins that interfere with RIG-I or MAVS to evade antiviral cytokine production. Notably, viral proteins that target TBK-1 and IRF3 to block interferon production are not included here. vGAT proteins of KSHV and γHV68 recruit PFAS to deamidate and activate RIG-I. Activation of RIG-I and its downstream signaling events, specifically, those associated with IKKβ, result in p65 degradation and suppress inflammatory cytokine production (33–35). vGAT appears to blunt IRF activation by an unknown mechanism (indicated by dashed inhibition sign). PEDV, porcine epidemic diarrhea virus; IAV, influenza A virus; RSV, respiratory syncytial virus; HSV-1, herpes simplex virus 1; Hepatitis A, hepatitis A virus; Hepatitis C, hepatitis C virus; GB virus, hepatitis G virus; pro, protease; NEMO, NF-κB essential modulator; TANK, TRAF family member-associated NF-κB activator. RTA, replication and transcription activator.
HIJACKING RIG-I TO EVADE CYTOKINE PRODUCTION VIA DEAMIDATION
In order to characterize the virus-host interactions that instigate MAVS-dependent signaling, a screen for open reading frames from γHV68 that activate MAVS-dependent signaling revealed that ORF75C is a potent activator of RIG-I and MAVS signaling (35). Genetic and biochemical studies demonstrated functional and direct physical interactions between ORF75c and RIG-I. ORF75 genes are highly conserved in all gammaherpesviruses. While the KSHV genome encodes ORF75 and EBV encodes BNRF1, ORF75 genes are duplicated in herpesvirus saimiri (ORF3 and ORF75) and triplicated in murine γHV68 (i.e., ORF75a, -b, and -c). Accumulating studies describe antagonism of members of the promyelocytic leukemia (PML) family as a common immune evasion function of gammaherpesvirus ORF75 proteins (36–38). These studies have been extensively reviewed elsewhere (70). Here, we discuss the deamidating activity of ORF75 proteins of gammaherpesviruses.
The gammaherpesvirus ORF75 shares homology with phosphoribosyl-formylglycinamidine synthetase (PFAS; also known as FGARAT of phosphoribosyl-formylglycinamidine [FGAM]), a cellular glutamine amidotransferase (GAT), and is thus also referred to as vGAT. PFAS catalyzes reaction 4 of the 10 steps of purine de novo synthesis. However, the vGAT proteins of γHV68 cannot complement cells deficient in PFAS (40). The carboxyl-terminal GAT domain of vGAT is sufficient to interact with RIG-I but fails to activate RIG-I. Coupled with the fact that vGAT proteins share homology with cellular GATs, this observation suggests that vGAT-induced RIG-I activation may require the enzymatic activity of GAT. Indeed, treatment of cells expressing vGAT with a GAT inhibitor specifically diminished signaling downstream of RIG-I, but not that downstream of IKKβ, indicating that GAT activity is specifically required for events upstream of IKKβ (e.g., RIG-I). Two-dimensional gel electrophoresis showed that vGAT reduced the RIG-I charge, and mass spectrometry analysis identified three site-specific deamidations within RIG-I: Q10 in the CARD domain and N245 and N445 within the helicase 1 domain. Simultaneous deamidation of all three residues led to constitutive activation of RIG-I in the absence of RNA (35), revealing a new means to activate the RIG-I receptor. Consistent with previous findings on MAVS and IKKβ (32, 33), activated RIG-I is usurped to evade antiviral cytokine production via inducing the degradation of p65, the key subunit of the transcriptionally active NF-κB dimer. Whether deamidated and activated RIG-I is required for efficient replication of γHV68 remains an open question.
vGAT proteins lack the catalytic triad essential for amidotransfer/deamidation catalysis (35), suggesting that vGAT may recruit a cellular enzyme(s) to induce the deamidation and concomitant activation of RIG-I. Enzymes involved in nucleotide biosynthesis have a propensity to form homo-oligomers in order to regulate enzymatic activity (39, 41), so it is possible that vGAT proteins recruit cellular PFAS to deamidate RIG-I. Indeed, vGATs of KSHV, EBV, and γHV68 interact with PFAS, but only vGATs of KSHV and γHV68 induce RIG-I deamidation and activation. Depletion or pharmacological inhibition of PFAS recapitulates the phenotype of fibroblasts deficient in RIG-I and MAVS, i.e., increased cytokine production in response to γHV68 infection (33–35). Furthermore, purified vGAT and PFAS deamidated RIG-I in vitro. Neither vGAT nor PFAS alone was sufficient to induce RIG-I deamidation, suggesting that vGAT activates PFAS by an intrinsic mechanism. For the first time, these studies demonstrated that a PRR is activated by a deamidase consisting of a metabolic enzyme and a viral pseudoenzyme rather than by its conventional ligand. This work also describes a new function of the cellular metabolic PFAS enzyme in deamidating asparagines and glutamines of RIG-I to regulate innate immune signaling (35), suggesting that protein deamidation could play pivotal roles in regulating innate immune signaling.
PROTEIN DEAMIDATION
Protein deamidation was initially reported more than half a century ago (42). Early work focused on the nonenzymatic deamidation of asparaginyl and, to a lesser extent, glutaminyl residues of proteins in vivo and in vitro. Analyzing a large set of proteins, Robinson and Robinson showed that the rate of asparaginyl deamidation was determined by the primary sequence, secondary and tertiary structures of the protein, and cellular environment (such as pH) (43). The ubiquitous distribution of asparaginyl/glutaminyl residues and frequent deamidation thereof in proteins, coupled with the finding that surrounding sequences determine the rate of protein deamidation, prompted the postulation that nonenzymatic protein deamidation serves as an internal clock to time the biological events of a particular protein (44). Thus, nonenzymatic deamidation may be a built-in clock to time protein functional decay or “aging.”
(i) Enzymes (deamidases) that catalyze protein deamidation.
Emerging studies indicate that microbes deploy protein deamidation to manipulate key signaling components to promote their infection. Microbial enzymes constitute the founding members of the protein deamidase family and offer an opportunity to answer fundamental questions concerning protein deamidation. Work on the PFAS-vGAT protein-deamidating complex has described a new regulatory function of the metabolic PFAS enzyme, and likely other glutamine amidotransferases, in innate immune signal transduction. This work raises a number of imminent questions that are fundamental to enzymology and innate immune signaling. First, how does the amidotransferase activity of PFAS in purine synthesis correlate with its protein-deamidating activity in virus-infected cells? PFAS is known to catalyze amidotransfer from free glutamine to synthesize phosphoribosyl-formylglycinamidine (FGAM) in purine synthesis (45), whereas the PFAS-vGAT complex is capable of deamidating asparaginyl and glutaminyl residues of RIG-I (35). Cellular GAT enzymes catalyze the biosynthesis of nucleotides, amino acids, glycoprotein, and NAD (46). The fact that PFAS can participate in both purine synthesis and RIG-I activation suggests that nucleotide metabolism is linked to innate immune signaling. Indeed, inhibitors targeting dihydroorotate dehydrogenase, a key enzyme of the de novo pyrimidine synthesis pathway, reduced viral replication (47–49). Later, it was shown that this antiviral activity relies on an elevated cellular immune response (50). The molecular link between reduced pyramidine biosynthesis and increased antiviral gene expression remains unknown. Presumably, the nucleotide pool within cells infected by a virus in vivo is small, so activating nucleotide biosynthesis is imperative for efficient viral transcription and genome replication. An elegant example is how cellular SAMHD1 nucleotide hydrolase restricts human immunodeficiency virus (HIV) via depleting the nucleotide pool available for HIV replication (51). If vGAT activates PFAS for purine biosynthesis and RIG-I-dependent evasion of antiviral cytokine production, a proper division between the two enzymatic activities (i.e., protein deamidation and purine synthesis) is critical for viral lytic replication. In support of this notion, murine γHV68 robustly upregulates the protein expression of PFAS, partly by increasing its mRNA level (35). Future work concerning PFAS regulation in virus-infected cells will likely further elucidate the possible cross talk between innate immune signaling and nucleotide biosynthesis.
Second, how does RIG-I access the active site of the PFAS deamidase domain? Cellular glutamine amidotransferases consist of a GAT domain and a metabolite synthetase domain connected by an ammonia channel. Structural analysis of the Salmonella enterica serovar Typhimurium homolog of PFAS revealed a relatively buried catalytic triad for deamidation facing the internal ammonia channel (39). If human PFAS is structurally similar to Salmonella PFAS, binding to vGAT perhaps triggers a conformational change that exposes the catalytic triad to accommodate asparaginyl and glutaminyl residues of RIG-I. The GAT domain is loosely connected to the FGAM synthetase domain (39), which likely provides flexibility and enables substrate accessibility. Such a conformational change may uncouple deamidation from ammonia channeling to the catalytic center of the FGAM synthetase, thereby facilitating the release of free ammonia. Interestingly, mutations blocking the ammonia channel between the GAT domain and the neighboring synthetase domain of imidazole-glycerolphosphate synthase increased the deamidating activity of the glutaminase domain by more than 3 orders of magnitude (52), suggesting that ammonia release is a mechanism to significantly elevate glutamine hydrolysis. vGAT may deploy a similar mechanism of uncoupling the ammonia channel from the enzymatic domain to deamidate RIG-I, although the detailed mechanism of vGAT activation of PFAS requires further investigation.
Third, it is unclear whether PFAS, and other glutamine amidotransferases, can deamidate proteins in the absence of gammaherpesvirus vGAT proteins. RIG-I deamidation was not observed in cells infected with Sendai virus or γHV68 deficient in vGAT. If PFAS and other GATs can deamidate proteins in mammalian cells, it would be interesting to quantify how viral infection impacts the spectrum of deamidated proteins. This would be best assessed by a proteome-wide deamidation analysis. The herpesvirus proteome offers an excellent platform to systematically analyze protein deamidation. vGATs of KSHV, EBV, and γHV68 display similar interactions with PFAS, but only EBV vGAT failed to deamidate RIG-I, implying that vGATs and PFAS may have other functions shared by all three gammaherpesviruses such as nucleotide metabolism and evasion of intrinsic antiviral immunity. Although the genomes of herpes simplex viruses (HSV) contain no homolog of vGAT, HSV-1 infection induced a robust reduction in RIG-I's charge, indicative of deamidation. This suggests that herpes simplex viruses may have evolved a different mechanism for inducing RIG-I deamidation. Future studies may reveal a new example of protein deamidation in innate immune signaling.
In contrast to viral pseudoenzymes, several bacterial proteins appear to possess intrinsic deamidase activity toward multiple signaling molecules. Cytotoxic necrotizing factors (CNFs) produced by uropathogenic (CNF1) or enteropathogenic (CNF2) Escherichia coli deamidate and constitutively activate small G proteins (53–55). Similarly to chemotaxis D (CheD) deamidase, CNFs form a common α/β/β sandwich that contains the catalytic dyad in a shallow cavity at the top of the protein (56, 57). The members of the Cif family of effectors secreted by Burkholderia pseudomallei and enteropathogenic E. coli and the OspI effector secreted by Shigella flexneri can deamidate ubiquitin/Nedd8 and the UBC13 E2 enzyme, respectively (58, 59). Deamidation of these signaling components by bacterial effectors is essential for evading cellular immune responses and the pathogenesis of these microbes. These studies define the structure and function of protein deamidases in pathogen infection.
(ii) Protein targets of enzyme-catalyzed deamidation.
Protein deamidation catalyzed by enzymes is generally rapid and tightly regulated. To date, we have understood the functional consequences of deamidation of a small subset of proteins in prokaryotes and mammalian cells (Table 1). Initial studies showed that key cellular signaling molecules are deamidated by pathogenic microbes to facilitate their invasion, which underpins their pathogenesis (68). Small G proteins such as RhoA and Cdc42 require a key glutamine residue for GTP hydrolysis. Deamidation of the conserved glutamine by pathogenic E. coli locks these G proteins in a GTP-bound state, resulting in the constitutive activation of G proteins and stress fiber formation (53, 60, 62). Interestingly, deamidation is also crucial for regulating signal transduction in bacterial chemotaxis. In nonenteric bacteria, the chemotaxis C (CheC) phosphatase and methyl-accepting chemotaxis proteins (known as MCPs) are deamidated by the CheD polypeptide and are required for directional chemotaxis (57). Mammalian ubiquitins Nedd8 and UBC13 have recently been shown to be deamidated by effectors secreted by enteric pathogenic E. coli and Shigella, respectively (58, 59). Deamidation of these cellular signaling molecules inactivates the ubiquitin proteasome system that is critical for signal transduction downstream of tumor necrosis factor alpha (TNF-α), an important component of the host immune defense system. In response to DNA damage, anti-apoptotic Bcl-xL is targeted for degradation via deamidation (64). Recently, the deamidation of 4E-BP2 was shown to promote its association with mammalian target of rapamycin (mTOR) and modulate neuronal excitatory synaptic transmission (67). It was postulated that the deamidation of Bcl-xL and 4E-BP2 is a nonenzymatic process and results from an increase in cellular pH. Deamidation of other key proteins is implicated in regulating some fundamental processes (e.g., cell-matrix interaction) and underpins medically important diseases (e.g., Alzheimer's disease) (69).
TABLE 1.
Emerging discoveries of mammalian protein deamidations
| Functional consequence | Deamidated cellular target(s) | Deamidase/other mechanism(s) | Deamidase-encoding species | Reference |
|---|---|---|---|---|
| G protein signaling pathways | ||||
| Activation of G protein signaling | Rho GTPases | Cytotoxic necrotizing factors (CNFs) | Escherichia coli (EPEC) | 60 |
| • RhoA | ||||
| • Rac | ||||
| • Cdc42 | Yersinia pseudotuberculosis | 53 | ||
| Heterotrimeric G proteins (Gαi, Gαq/11, and Gα12/13) | Pasteurella multocida toxin (PMT) | Pasteurella multocida | 61 | |
| Rho GTPases | VopC | Vibrio parahaemolyticus | 62 | |
| • Rac | ||||
| • Cdc42 | ||||
| Translation/cell cycle progression/apoptosis | ||||
| Inhibition of translation | Eukaryotic initiation factor 4A (eIF4A) | Burkholderia lethal factor 1 (BLF1) | Burkholderia pseudomallei | 63 |
| Critical switch (checkpoint) of apoptosis induced by DNA damage | B-cell lymphoma—extra large (Bcl-XL) | pH change | NAa | 64 |
| Inhibition of cell cycle progression | Ubiquitin | Cycle-inhibiting factors (Cifs) | Escherichia coli | 58 |
| Yersinia pseudotuberculosis | ||||
| NEDD8 | Burkholderia pseudomallei | |||
| Photorhabdus asymbiotica | ||||
| Photorhabdus luminescens | ||||
| Innate immunity and inflammatory responses | ||||
| Inactivation of ubiquitin system and its related pathways, e.g., NF-κB signaling pathway | Ubiquitin | Cycle-inhibiting factors (Cifs) | Escherichia coli | 58 |
| Yersinia pseudotuberculosis | ||||
| Burkholderia pseudomallei | ||||
| Photorhabdus asymbiotica | ||||
| Photorhabdus luminescens | ||||
| Inhibition of UBC13/TRAF6-dependent inflammatory responses | Ubiquitin-conjugating enzyme 13 (UBC13) | OspI | Shigella flexneri | 59 |
| Activation of RIG-I signaling to evade inflammatory signaling | Retinoic acid-inducible gene 1 (RIG-I) | vGAT (ORF75/ORF75c) + phosphoribosylformylglycinamidine synthase (PFAS) | Murine herpesvirus 68 (ORF75c) | 35 |
| Human herpesvirus 8 (ORF75) | ||||
| Homo sapiens (PFAS) | ||||
| Recognition by gut-derived T cells to promote intestinal inflammation | Gliadin (wheat), etc. | Tissue transglutaminase (TG2) | Homo sapiens | 65 |
| Others | ||||
| N-end rule pathway of protein degradation | Model substrate of N-end rule pathway | N-terminal glutamine amidase (NTQA) | Mus musculus | 66 |
| Alteration of the kinetics of excitatory synaptic transmission | Eukaryotic initiation factor 4E-binding protein 2 (4E-BP2) | pH change | NA | 67 |
NA, not applicable.
CONCLUSION AND PERSPECTIVES
Recent studies of enterobacterial effectors and gammaherpesviral pseudoenzymes implicate a new function of protein deamidation in regulating innate immune signal transduction. This is likely just the tip of iceberg concerning the general regulatory roles of protein deamidation in fundamental biological processes. That metabolic glutamine amidotransferases, such as the vGAT-PFAS complex, are capable of deamidating proteins is interesting in that these enzymes may provide a physical and functional link to other biological processes. However, these studies have generated more questions than answers in regard to fundamental principles concerning protein deamidation. For example, how are these cellular GATs regulated and delegated between metabolism and signal transduction in response to infection? What sequence and structural elements of GATs (e.g., PFAS) enable their dual functionality or substrate promiscuity in deamidating free glutamine and glutaminyl/asparaginyl residues? Is there sequence specificity of target deamidated proteins? If yes, is the specificity defined by the primary, secondary, or tertiary structure of the deamidated protein? Last, and importantly, is protein deamidation involved in other fundamental biological processes that are intrinsically linked to nucleotide biosynthesis, such as DNA damage and repair? These microbial studies have unveiled a new function of the simplest posttranslational modification of proteins, deamidation, in immune regulation and will certainly instruct us more in the years to come.
ACKNOWLEDGMENTS
Work in the Feng laboratory is supported by grants from NIDCR (DE021445) and NCI (CA134241).
We apologize to those whose work is not cited here due to limited space.
REFERENCES
- 1.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 4.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt A, Schwerd T, Hamm W, Hellmuth JC, Cui S, Wenzel M, Hoffmann FS, Michallet MC, Besch R, Hopfner KP, Endres S, Rothenfusser S. 2009. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc Natl Acad Sci U S A 106:12067–12072. doi: 10.1073/pnas.0900971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito T, Hirai R, Loo YM, Owen D, Johnson CL, Sinha SC, Akira S, Fujita T, Gale M Jr. 2007. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci U S A 104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R, Gale M Jr, Inagaki F, Fujita T. 2008. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell 29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D, Cusack S. 2011. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. 2011. Structural insights into RNA recognition by RIG-I. Cell 147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu B, Peisley A, Tetrault D, Li Z, Egelman EH, Magor KE, Walz T, Penczek PA, Hur S. 2014. Molecular imprinting as a signal-activation mechanism of the viral RNA sensor RIG-I. Mol Cell 55:511–523. doi: 10.1016/j.molcel.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 13.Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. 2011. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panne D, Maniatis T, Harrison SC. 2007. An atomic model of the interferon-beta enhanceosome. Cell 129:1111–1123. doi: 10.1016/j.cell.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. 2007. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 16.Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. 2010. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshiumi H, Miyashita M, Inoue N, Okabe M, Matsumoto M, Seya T. 2010. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe 8:496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Maharaj NP, Wies E, Stoll A, Gack MU. 2012. Conventional protein kinase C-alpha (PKC-alpha) and PKC-beta negatively regulate RIG-I antiviral signal transduction. J Virol 86:1358–1371. doi: 10.1128/JVI.06543-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Z, Ren H, Liu Y, Teeling JL, Gu J. 2011. Phosphorylation of RIG-I by casein kinase II inhibits its antiviral response. J Virol 85:1036–1047. doi: 10.1128/JVI.01734-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wies E, Wang MK, Maharaj NP, Chen K, Zhou S, Finberg RW, Gack MU. 2013. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity 38:437–449. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng P, Moses A, Fruh K. 2013. Evasion of adaptive and innate immune response mechanisms by gamma-herpesviruses. Curr Opin Virol 3:285–295. doi: 10.1016/j.coviro.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sparrer KM, Gack MU. 2015. Intracellular detection of viral nucleic acids. Curr Opin Microbiol 26:1–9. doi: 10.1016/j.mib.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo YM, Gale M Jr. 2011. Immune signaling by RIG-I-like receptors. Immunity 34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. 2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. 2005. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci U S A 102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 27.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M Jr, Lemon SM. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A 102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Benureau Y, Rijnbrand R, Yi J, Wang T, Warter L, Lanford RE, Weinman SA, Lemon SM, Martin A, Li K. 2007. GB virus B disrupts RIG-I signaling by NS3/4A-mediated cleavage of the adaptor protein MAVS. J Virol 81:964–976. doi: 10.1128/JVI.02076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Liang Y, Qu L, Chen Z, Yi M, Li K, Lemon SM. 2007. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc Natl Acad Sci U S A 104:7253–7258. doi: 10.1073/pnas.0611506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Xi X, Lei X, Zhang X, Cui S, Wang J, Jin Q, Zhao Z. 2013. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog 9:e1003231. doi: 10.1371/journal.ppat.1003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee A, Morosky SA, Delorme-Axford E, Dybdahl-Sissoko N, Oberste MS, Wang T, Coyne CB. 2011. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog 7:e1001311. doi: 10.1371/journal.ppat.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong X, Feng H, Sun Q, Li H, Wu TT, Sun R, Tibbetts SA, Chen ZJ, Feng P. 2010. Murine gamma-herpesvirus 68 hijacks MAVS and IKKbeta to initiate lytic replication. PLoS Pathog 6:e1001001. doi: 10.1371/journal.ppat.1001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong X, Feng P. 2011. Murine gamma herpesvirus 68 hijacks MAVS and IKKbeta to abrogate NFkappaB activation and antiviral cytokine production. PLoS Pathog 7:e1002336. doi: 10.1371/journal.ppat.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong X, He Z, Durakoglugil D, Arneson L, Shen Y, Feng P. 2012. Murine gammaherpesvirus 68 evades host cytokine production via replication transactivator-induced RelA degradation. J Virol 86:1930–1941. doi: 10.1128/JVI.06127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He S, Zhao J, Song S, He X, Minassian A, Zhou Y, Zhang J, Brulois K, Wang Y, Cabo J, Zandi E, Liang C, Jung JU, Zhang X, Feng P. 2015. Viral pseudo-enzymes activate RIG-I via deamidation to evade cytokine production. Mol Cell 58:134–146. doi: 10.1016/j.molcel.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling PD, Tan J, Sewatanon J, Peng R. 2008. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. J Virol 82:8000–8012. doi: 10.1128/JVI.02752-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai K, Thikmyanova N, Wojcechowskyj JA, Delecluse HJ, Lieberman PM. 2011. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS Pathog 7:e1002376. doi: 10.1371/journal.ppat.1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Full F, Jungnickl D, Reuter N, Bogner E, Brulois K, Scholz B, Sturzl M, Myoung J, Jung JU, Stamminger T, Ensser A. 2014. Kaposi's sarcoma associated herpesvirus tegument protein ORF75 is essential for viral lytic replication and plays a critical role in the antagonization of ND10-instituted intrinsic immunity. PLoS Pathog 10:e1003863. doi: 10.1371/journal.ppat.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anand R, Hoskins AA, Stubbe J, Ealick SE. 2004. Domain organization of Salmonella typhimurium formylglycinamide ribonucleotide amidotransferase revealed by X-ray crystallography. Biochemistry 43:10328–10342. doi: 10.1021/bi0491301. [DOI] [PubMed] [Google Scholar]
- 40.Gaspar M, Gill MB, Losing JB, May JS, Stevenson PG. 2008. Multiple functions for ORF75c in murid herpesvirus-4 infection. PLoS One 3:e2781. doi: 10.1371/journal.pone.0002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guy HI, Schmidt B, Herve G, Evans DR. 1998. Pressure-induced dissociation of carbamoyl-phosphate synthetase domains. The catalytically active form is dimeric. J Biol Chem 273:14172–14178. [DOI] [PubMed] [Google Scholar]
- 42.Mycek MJ, Waelsch H. 1960. The enzymatic deamidation of proteins. J Biol Chem 235:3513–3517. [PubMed] [Google Scholar]
- 43.Robinson NE, Robinson AB. 2001. Prediction of protein deamidation rates from primary and three-dimensional structure. Proc Natl Acad Sci U S A 98:4367–4372. doi: 10.1073/pnas.071066498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson NE, Robinson AB. 2001. Molecular clocks. Proc Natl Acad Sci U S A 98:944–949. doi: 10.1073/pnas.98.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson D, Bleskan J, Gardiner K, Bowersox J. 1999. Human phosphoribosylformylglycineamide amidotransferase (FGARAT): regional mapping, complete coding sequence, isolation of a functional genomic clone, and DNA sequence analysis. Gene 239:381–391. doi: 10.1016/S0378-1119(99)00378-9. [DOI] [PubMed] [Google Scholar]
- 46.Massière F, Badet-Denisot MA. 1998. The mechanism of glutamine-dependent amidotransferases. Cell Mol Life Sci 54:205–222. doi: 10.1007/s000180050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonavia A, Franti M, Pusateri Keaney E, Kuhen K, Seepersaud M, Radetich B, Shao J, Honda A, Dewhurst J, Balabanis K, Monroe J, Wolff K, Osborne C, Lanieri L, Hoffmaster K, Amin J, Markovits J, Broome M, Skuba E, Cornella-Taracido I, Joberty G, Bouwmeester T, Hamann L, Tallarico JA, Tommasi R, Compton T, Bushell SM. 2011. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV). Proc Natl Acad Sci U S A 108:6739–6744. doi: 10.1073/pnas.1017142108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang QY, Bushell S, Qing M, Xu HY, Bonavia A, Nunes S, Zhou J, Poh MK, Florez de Sessions P, Niyomrattanakit P, Dong H, Hoffmaster K, Goh A, Nilar S, Schul W, Jones S, Kramer L, Compton T, Shi PY. 2011. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. J Virol 85:6548–6556. doi: 10.1128/JVI.02510-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann HH, Kunz A, Simon VA, Palese P, Shaw ML. 2011. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc Natl Acad Sci U S A 108:5777–5782. doi: 10.1073/pnas.1101143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucas-Hourani M, Dauzonne D, Jorda P, Cousin G, Lupan A, Helynck O, Caignard G, Janvier G, Andre-Leroux G, Khiar S, Escriou N, Despres P, Jacob Y, Munier-Lehmann H, Tangy F, Vidalain PO. 2013. Inhibition of pyrimidine biosynthesis pathway suppresses viral growth through innate immunity. PLoS Pathog 9:e1003678. doi: 10.1371/journal.ppat.1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LP, Stoye JP, Crow YJ, Taylor IA, Webb M. 2011. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 52.List F, Vega MC, Razeto A, Hager MC, Sterner R, Wilmanns M. 2012. Catalysis uncoupling in a glutamine amidotransferase bienzyme by unblocking the glutaminase active site. Chem Biol 19:1589–1599. doi: 10.1016/j.chembiol.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 54.Lerm M, Selzer J, Hoffmeyer A, Rapp UR, Aktories K, Schmidt G. 1999. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect Immun 67:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugai M, Hatazaki K, Mogami A, Ohta H, Peres SY, Herault F, Horiguchi Y, Masuda M, Ueno Y, Komatsuzawa H, Suginaka H, Oswald E. 1999. Cytotoxic necrotizing factor type 2 produced by pathogenic Escherichia coli deamidates a gln residue in the conserved G-3 domain of the rho family and preferentially inhibits the GTPase activity of RhoA and rac1. Infect Immun 67:6550–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buetow L, Flatau G, Chiu K, Boquet P, Ghosh P. 2001. Structure of the Rho-activating domain of Escherichia coli cytotoxic necrotizing factor 1. Nat Struct Biol 8:584–588. doi: 10.1038/89610. [DOI] [PubMed] [Google Scholar]
- 57.Chao X, Muff TJ, Park SY, Zhang S, Pollard AM, Ordal GW, Bilwes AM, Crane BR. 2006. A receptor-modifying deamidase in complex with a signaling phosphatase reveals reciprocal regulation. Cell 124:561–571. doi: 10.1016/j.cell.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 58.Cui J, Yao Q, Li S, Ding X, Lu Q, Mao H, Liu L, Zheng N, Chen S, Shao F. 2010. Glutamine deamidation and dysfunction of ubiquitin/NEDD8 induced by a bacterial effector family. Science 329:1215–1218. doi: 10.1126/science.1193844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanada T, Kim M, Mimuro H, Suzuki M, Ogawa M, Oyama A, Ashida H, Kobayashi T, Koyama T, Nagai S, Shibata Y, Gohda J, Inoue J, Mizushima T, Sasakawa C. 2012. The Shigella flexneri effector OspI deamidates UBC13 to dampen the inflammatory response. Nature 483:623–626. doi: 10.1038/nature10894. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 61.Orth JH, Preuss I, Fester I, Schlosser A, Wilson BA, Aktories K. 2009. Pasteurella multocida toxin activation of heterotrimeric G proteins by deamidation. Proc Natl Acad Sci U S A 106:7179–7184. doi: 10.1073/pnas.0900160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, Krachler AM, Broberg CA, Li Y, Mirzaei H, Gilpin CJ, Orth K. 2012. Type III effector VopC mediates invasion for Vibrio species. Cell Rep 1:453–460. doi: 10.1016/j.celrep.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cruz-Migoni A, Hautbergue GM, Artymiuk PJ, Baker PJ, Bokori-Brown M, Chang CT, Dickman MJ, Essex-Lopresti A, Harding SV, Mahadi NM, Marshall LE, Mobbs GW, Mohamed R, Nathan S, Ngugi SA, Ong C, Ooi WF, Partridge LJ, Phillips HL, Raih MF, Ruzheinikov S, Sarkar-Tyson M, Sedelnikova SE, Smither SJ, Tan P, Titball RW, Wilson SA, Rice DW. 2011. A Burkholderia pseudomallei toxin inhibits helicase activity of translation factor eIF4A. Science 334:821–824. doi: 10.1126/science.1211915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deverman BE, Cook BL, Manson SR, Niederhoff RA, Langer EM, Rosova I, Kulans LA, Fu X, Weinberg JS, Heinecke JW, Roth KA, Weintraub SJ. 2002. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell 111:51–62. doi: 10.1016/S0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]
- 65.Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM. 1998. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 66.Wang H, Piatkov KI, Brower CS, Varshavsky A. 2009. Glutamine-specific N-terminal amidase, a component of the N-end rule pathway. Mol Cell 34:686–695. doi: 10.1016/j.molcel.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bidinosti M, Ran I, Sanchez-Carbente MR, Martineau Y, Gingras AC, Gkogkas C, Raught B, Bramham CR, Sossin WS, Costa-Mattioli M, DesGroseillers L, Lacaille JC, Sonenberg N. 2010. Postnatal deamidation of 4E-BP2 in brain enhances its association with raptor and alters kinetics of excitatory synaptic transmission. Mol Cell 37:797–808. doi: 10.1016/j.molcel.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Washington EJ, Banfield MJ, Dangl JL. 2013. What a difference a Dalton makes: bacterial virulence factors modulate eukaryotic host cell signaling systems via deamidation. Microbiol Mol Biol Rev 77:527–539. doi: 10.1128/MMBR.00013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weintraub SJ, Deverman BE. 2007. Chronoregulation by asparagine deamidation. Sci STKE 2007:re7. [DOI] [PubMed] [Google Scholar]
- 70.Tsai K, Messick TE, Lieberman PM. 2015. Disruption of host antiviral resistances by gammaherpesvirus tegument proteins with homology to the FGARAT purine biosynthesis enzyme. Curr Opin Virol 14:30–40. doi: 10.1016/j.coviro.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]