Abstract
Cardiac myosin-binding protein C (cMyBP-C) regulates actin–myosin interaction and thereby cardiac myocyte contraction and relaxation. This physiologic function is regulated by cMyBP-C phosphorylation. In our study, reduced site-specific cMyBP-C phosphorylation coincided with increased S-glutathiolation in ventricular tissue from patients with dilated or ischemic cardiomyopathy compared to nonfailing donors. We used redox proteomics, to identify constitutive and disease-specific S-glutathiolation sites in cMyBP-C in donor and patient samples, respectively. Among those, a cysteine cluster in the vicinity of the regulatory phosphorylation sites within the myosin S2 interaction domain C1-M-C2 was identified and showed enhanced S-glutathiolation in patients. In vitro S-glutathiolation of recombinant cMyBP-C C1-M-C2 occurred predominantly at Cys249, which attenuated phosphorylation by protein kinases. Exposure to glutathione disulfide induced cMyBP-C S-glutathiolation, which functionally decelerated the kinetics of Ca2+-activated force development in ventricular myocytes from wild-type, but not those from Mybpc3-targeted knockout mice. These oxidation events abrogate protein kinase–mediated phosphorylation of cMyBP-C and therefore potentially contribute to the reduction of its phosphorylation and the contractile dysfunction observed in human heart failure.—Stathopoulou, K., Wittig, I., Heidler, J., Piasecki, A., Richter, F., Diering, S., van der Velden, J., Buck, F., Donzelli, S., Schröder, E., Wijnker, P. J. M., Voigt, N., Dobrev, D., Sadayappan, S., Eschenhagen, T., Carrier, L., Eaton, P., Cuello, F. S-glutathiolation impairs phosphoregulation and function of cardiac myosin-binding protein C in human heart failure.
Keywords: redox proteomics, post-translational modifications, cross-bridge cycling, contractile function, cardiac disease
Heart failure (HF) is a clinical syndrome arising from diverse causes and remains one of the leading causes of human mortality (1). At the sarcomeric level, disease progression is accompanied by a decreased overall phosphorylation of myofilament proteins and impaired contractile function (2–5). Cardiac myosin-binding protein C (cMyBP-C) is a thick filament–associated protein. It is involved in the regulation of contraction and relaxation and thus in in vivo cardiac performance (6, 7). Phosphorylation of cMyBP-C at the cardiac-specific M-motif within the N-terminal M-domain inhibits its binding to myosin S2, which has been causally associated with the removal of a structural constraint on myosin heads. This effect promotes actin–myosin interaction and accelerates the cross-bridge cycling kinetics necessary to enhance rates of relaxation and force generation in diastole and systole, respectively (8–11). There are 3 phosphorylation sites in the M-domain of cMyBP-C, Ser275, Ser284, and Ser304 (referring to the human sequence) that are phosphorylated in response to neurohumoral stimulation. Phosphorylation is described to follow a hierarchical pattern, where phosphorylation at Ser284 acts as a switch, rendering the other 2 sites more susceptible for protein kinase–mediated phosphorylation (12, 13). All 3 sites are phosphorylated by PKA (12, 14). Other kinases have been reported to phosphorylate cMyBP-C site specifically within the M-motif (15), some of which are thought to assume significance under disease conditions, such as Ca2+/calmodulin-dependent kinase isoform II (CaMKII) (13), p90 ribosomal S6 kinase (16), and protein kinase D (17). The importance of post-translational modification of cMyBP-C for cardiac contractile function is highlighted by the observation that phosphorylation of cMyBP-C was low in samples from patients with end-stage HF, a condition where contractility is impaired (2, 3, 5). HF is associated with increased production of reactive oxygen and nitrogen species (ROS/RNS), contributing to alterations in contractile performance by inducing oxidative post-translational modifications (OPTMs) in target proteins (18–23). S-glutathiolation of cMyBP-C was identified previously in a proteomic screen of in vitro labeled adult rat ventricular myocytes (ARVMs) with biotinylated glutathione disulfide (24) and in a study of the deoxycorticosterone acetate-salt hypertensive mouse model (25). Recent findings described 3 novel S-glutathiolation sites in mouse cMyBP-C by mass spectrometry: Cys475, Cys623, and Cys651 (referring to the human sequence) (26). Also, increased S-glutathiolation of cMyBP-C has been shown in a mouse model of hypertrophic cardiomyopathy (HCM) (27). Whether S-glutathiolation of cMyBP-C occurs in human HF and whether it affects protein kinase–mediated phosphoregulation of cMyBP-C, seems important to determine, and so these questions were the main objective of the current study.
MATERIALS AND METHODS
Materials
Anti-glutathione antibodies were provided by Dr. Ewald Schröder and Prof. Philip Eaton (King’s College London, London, United Kingdom) or purchased from ViroGen (Watertown, MA, USA). Anti-cMyBP-C antibody was a gift from Prof. Mathias Gautel (King’s College London, United Kingdom) and pSer275 and pSer304 cMyBP-C antibodies from Prof. Sakthivel Sadayappan (Loyola University, Chicago, IL, USA). pSer284 antibody was purchased from Enzo Life Sciences (Lörrach, Germany) (2), anti-calsequestrin antibody from Thermo Scientific (Waltham, MA, USA), anti-His antibody (G18) from Santa Cruz Biotechnology (Dallas, TX, USA). PKAcat was from Merck Millipore (Darmstadt, Germany), and CaMKIIα was from New England Biolabs (Ipswich, MA, USA). Glutathione disulfide (GSSG), methoxypolyethylene glycol maleimide (PEG-maleimide, 5 or 10 kDa), DTT, reduced glutathione (GSH), and H2O2 were from Sigma-Aldrich (Taufkirchen, Germany). The Monolith NT.115 Protein Labeling Kit RED-NHS was from NanoTemper Technologies (Munich, Germany).
Animals
The study was conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals, as issued by the National Institutes of Health (Bethesda, MD, USA). Experimental procedures conformed to the German Law for the Protection of Animals. Mybpc3-targeted knockout (KO) (Black Swiss) mice have been described (28). Mice were given the β-adrenoceptor antagonist propranolol (0.5 g/L) in their drinking water for 3 d before they were euthanized. ARVMs were isolated from male Wistar rats (Charles River Laboratories, Sulzfeld, Germany).
ARVM isolation and culture
ARVMs were isolated as described previously (9) and were cultured in modified M199 culture medium [mM199; M199 supplemented with (in mM): taurine 5, creatine 5, l-carnitine 2 and 100 IU/ml penicillin/streptomycin] in laminin-coated 6-well culture plates for 24 h before use (16).
Pharmacological treatment of ARVM
To induce post-translational modifications of endogenous cMyBP-C, ARVMs were treated for 10 min with vehicle (PBS), 100 µM H2O2, and isoprenaline (ISO; 3 nM) or pretreated with 100 µM H2O2 and subsequently with ISO (3 nM). ARVMs were lysed in buffer consisting of (in mM) Tris 100 (pH 7.4) and maleimide 100, with 1% (w/v) SDS, supplemented with protease inhibitors (Roche, Berlin, Germany) for PEG switch assay or directly in nonreducing sample buffer supplemented with maleimide (100 mM) or reducing Laemmli sample buffer for Western immunoblot analysis.
Site-directed mutagenesis
The cDNA for the human C1-M-C2 domain (aa residues 153–450) of cMyBP-C in pET8c (12) was used as a template for site-directed mutagenesis using the QuikChange kit (Agilent Technologies, Frankfurt am Main, Germany). Cysteines were replaced by non-S-glutathiolatable serine.
Primers (underline refers to the mutated codon): C239S, TGGCAGCTACCGCTCTGAGGTGTCCAC; C249S, CCAAGGACAAATTTGACTCCTCCAACTTCAATCTCAC; C426S, GACCATCAGCCAGTCCTCATTGGCGGACG; C436S, CGACGCAGCCTACCAGTCCGTGGTGGG; and C443S, GGTGGCGAGAAGTCTAGCACGGAGCTC.
Cloning of human β-myosin subfragment S2
The proximal 126 residues (aa 838–963) of human β-myosin subfragment S2 (S2Δ) contain the binding site for cMyBP-C (29), and were amplified by PCR from human cardiac cDNA.
Primers: CACCCCGCTGCTGAAGAGTGCAGAA; TCATTTGGCCAGTGTCAGCTCCAG
The PCR product was cloned into the pET151/D-TOPO vector (Life Technologies, Darmstadt, Germany).
Purification of recombinant human C1-M-C2 cMyBP-C and β-myosin S2Δ
Recombinant human His6-tagged β-myosin S2Δ, human wild-type (WT) and mutant His6-tagged C1-M-C2 (C249S) cMyBP-C proteins were expressed in Escherichia coli BL21 (DE3) pLysS strain and purified using Ni-NTA agarose (Qiagen, Venlo, Limburg, The Netherlands) (12, 16).
In vitro S-glutathiolation assay
For the in vitro S-glutathiolation (IVG) assay, recombinant C1-M-C2 (200 pmol) was incubated in assay buffer [(in mM) Tris 30 and MgCl2 15 (pH 7.4)] and exposed to vehicle (PBS), DTT (10 mM), GSH (1 mM), or GSSG (1 mM) for 30 min at room temperature. Nonreducing Laemmli sample buffer [2×; 125 mM Tris-HCl (pH 6.8), 4% (w/v) SDS, 20% (v/v) glycerol, 0.02% (w/v) bromophenol blue, and 100 mM maleimide] was added, and samples were analyzed by SDS-PAGE and Western immunoblot.
PEG switch assay
A polyethylene glycol (PEG) switch assay (30) was performed to assess the OPTM of C1-M-C2 or endogenous cMyBP-C. Beforehand, IVG of C1-M-C2 was performed.
Patients
Patients’ data were handled anonymously and according to the Declaration of Helsinki.Procedures to obtain specimens were approved by the local ethics committee (Ethics Committee of the Medical Association of Hamburg 532/116/9.71991). Left ventricular myocardial tissue samples were obtained from patients with dilated (DCM) or ischemic (ICM) cardiomyopathy who underwent heart transplantation. Nonfailing (NF) myocardial samples were obtained from donors who had no history of cardiac abnormalities. Patient characteristics are summarized in Table 1.
TABLE 1.
Patient characteristics
| Sample | Diagnosis | Sex | Age | NYHA Class | Drug | Analysis |
|---|---|---|---|---|---|---|
| NF1 | NF (SAH) | M | 44 | – | nd | WB/IB/MS |
| NF2 | NF (ICH) | M | 52 | – | OS | WB/IB/MS |
| NF3 | NF (ICH) | M | 37 | – | DLO | WB/IB |
| NF4 | NF (CIC) | F | 49 | – | DOSV | WB/IB |
| NF5 | NF (nd) | F | 46 | – | nd | WB/IB |
| NF6 | NF (T) | F | 27 | – | Dihydrocodein | WB/IB |
| NF7 | NF (T) | M | 19 | – | nd | WB/IB |
| NF8 | NF (SAH) | F | 50 | – | nd | WB/IB |
| DCM1 | DCM | M | 55 | IV | ADGNR | WB/IB |
| DCM2 | DCM | M | 50 | III | ADNR | WB/IB |
| DCM3 | DCM | M | 60 | IV | ADGN | WB/IB/MS |
| DCM4 | DCM | F | 22 | III–IV | ADG | WB/IB/MS |
| DCM5 | DCM | M | 47 | IV | ACDGN | WB/IB |
| DCM6 | DCM | M | 43 | IV | CN | WB/IB |
| DCM7 | DCM | M | 36 | III–IV | ADGNR | WB/IB |
| DCM8 | DCM | M | 37 | nd | nd | WB/IB |
| ICM1 | ICM | M | 50 | IV | ADGR | WB/IB |
| ICM2 | ICM | M | 52 | III | ACDGN | WB/IB |
| ICM3 | ICM | M | 49 | III–IV | DGNR | WB/IB |
| ICM4 | ICM | M | 65 | III–IV | ADCN | WB/IB |
| ICM5 | ICM | M | 55 | IV | ADGN | WB/IB |
| ICM6 | ICM | M | 53 | III–IV | ADG | WB/IB |
| ICM7 | ICM | M | 65 | III | ADGNR | WB/IB/MS |
| ICM8 | ICM | M | 64 | IV | DNR | WB/IB/MS |
Diagnosis: nd, not determined; T, trauma; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; CIC, cerebral ischemia; NYHA, New York Heart Association classes. Drugs: A, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; C, calcium channel blocker; D, diuretics; G, cardiac glycosides; N, nitrates; R, antiarrhythmics (except β-blockers); O, dopamine, dobutamine. Analysis: WB/IB: Western immunoblot analysis.
Preparation of human myocardial tissue homogenates
Tissue samples were homogenized in a buffer consisting of (in mM) Tris 100 (pH 7.4), maleimide 100, and protease inhibitors (Roche). Laemmli sample buffer (3×) supplemented with 100 mM maleimide (nonreducing) or 9% (v/v) β-mercaptoethanol (reducing) was added.
Oxyblot
Protein carbonylation was assessed with the Oxyblot Protein Oxidation Detection Kit (Millipore, Darmstadt, Germany), according to the manufacturer’s instructions. NF, DCM, and ICM samples [10% homogenate (in mM) Tris 100 (pH 7.4), DTT 50, and protease inhibitors (Roche)] were subjected to derivatization by dinitrophenyl hydrazine (DNPH) and also served as a negative control by substituting the control solution for the DNPH solution.
Redox proteomics analysis of cMyBP-C from human and mouse ventricular tissue
Ventricular human heart tissue from NF donors or patients with DCM or ICM were homogenized in 100 mM Tris (pH 7.4) and 1% Triton X-100 (10% homogenate w/v). Mouse hearts were homogenized in 100 mM Tris (pH 7.4) and 1% Triton X-100 (10% homogenate w/v), exposed to 1 mM GSSG in a final reaction volume of 100 µl for 30 min at room temperature. Samples were centrifuged at 16,000 rpm for 10 min, and pellets were resolved in 100 mM Tris (pH 7.4) containing 50 mM N-ethylmaleimide. One set of mouse samples was alkylated with 50 mM maleimide. After 10 min incubation at room temperature proteins were solubilized in SDS (1% final concentration), supplemented with glycerol (5% v/v), and resolved by 6% nonreducing SDS-PAGE. The gel was stained with colloidal Coomassie blue, and the 150 kDa band corresponding to cMyBP-C was excised and destained in 60% methanol and 50 mM acetonitrile. Samples were digested for 16 h with chymotrypsin or trypsin (sequencing grade; Promega, Mannheim, Germany) at room temperature or 37°C, respectively, in 50 mM NH4HCO3, 0.01% Protease Max (Promega) and 1 mM CaCl2. Peptides were eluted in 30% acetonitrile and 3% formic acid, dried, and resolved in 1% acetonitrile and 0.5% formic acid. Liquid chromatography/mass spectrometry (LC/MS) was performed on a Q Exactive Plus system coupled to an ultra-high-performance LC unit (Dionex Ultimate 3000) via a Nanospray Flex Ion-Source (all from Thermo Scientific). Peptides were loaded on a C18 reversed-phase precolumn (Zorbax 300SB-C18; Agilent Technologies), followed by separation on an in-house–packed 2.2 µm (mouse samples) and 2.4 µm (human samples) Reprosil C18 resin (Dr. Maisch GmbH, Ammerbuch-Entringen, Germany) picotip emitter tip [diameter, 75 µm (mouse samples), 10 µm (human samples) 15 cm long; New Objective, Woburn, MA, USA], with a gradient for mouse samples from mobile phase A (4% acetonitrile and 0.1% formic acid) to 44% mobile phase B (80% acetonitrile and 0.1% formic acid) for 75 min with a flow rate of 300 nl/min. For human samples, a 2-step gradient was used, 70 min from 5 to 30% and for another 20 min from 30 to 60% mobile phase B. Each run was finished by washout with 99% B for 5 min and column equilibration for 10 min with 99% A. MS data were recorded by data dependent Top10 acquisition (selecting the 10 most abundant precursor ions in positive mode for high-energy collision dissociation fragmentation). The full MS scan range was 250-2000 m/z (mouse samples), 300-2000 m/z (human samples), with a resolution of 70,000 at m/z 200 and an automatic gain control value of 3 × 106 total ion counts with a maximum ion injection time of 160 ms. Only higher charged ions (2+) were selected for tandem MS (MS/MS) scans with a resolution of 17,500, an isolation window of 2 m/z, and an automatic gain control value set to 1 × 105 ions with a maximum ion injection time of 150 ms. After the fragmentation event, all selected ions were excluded in a time frame of 30 s. Data were acquired in centroid mode (mouse samples) or in profile mode (human samples) by Xcalibur software (Thermo Scientific). The LC unit was controlled by Chromeleon Xpress software (Thermo Scientific). Performance of both units, LC and MS, was integrated by DCMSLink. Xcalibur raw files were analyzed by Peaks7 Studio software for proteomics (www.bioinfor.com; Bioinformatics Solutions, Waterloo, ON, Canada). The enzyme specificity was set to chymotrypsin or trypsin, respectively. Missed cleavages were limited to 3. Nonspecific cleavage at one end of peptides was tolerated for mouse samples. Initial monoisotopic precursor mass error tolerance was set to 10 ppm, and fragment ion tolerance was set to 0.05 Da. After variable modifications were selected: at N-terminal acetylation (+42.01), oxidation of methionine (+15.99), deamidation on asparagine and glutamine, N-ethylmaleimide (+125.05) and S-glutathiolation (+305.07) on cysteines. For mouse samples, maleimide (+97.02) was used for the alkylation. After de novo sequencing of spectra, the reviewed human reference proteome set (download from Uniprot, July 27, 2014; 20203 entries; www.uniprot.org) or the reviewed mouse proteome set (download from Uniprot, August 12, 2014; 16670 entries) was used to identify peptides and proteins. The false discovery rate (FDR) was set to 1%. For quantification (FDR 5%) of human samples, average of extracted-ion chromatograms (XIC) from best 3 unmodified peptides were used to normalize all peptide XICs. Nonidentified features were quantified by peptide matching. To gain a survey on the relative abundance of S-glutathiolation between human samples, all peptides were normalized to the maximum appearance. If more than one peptide for an S-glutathiolation site was identified, only the peptide with the best quality score was selected.
MS analysis of recombinant C1-M-C2
C1-M-C2 was subjected to IVG and resolved by SDS-PAGE. The gel was Colloidal Coomassie-stained, and the band corresponding to C1-M-C2 was excised from the gel, treated with iodoacetamide (55 mM, ambient temperature, 20 min, in the dark), and in-gel digested by trypsin or chymotrypsin [conditions: 5 ng trypsin/µl (sequencing grade modified trypsin; Promega] or 20 ng chymotrypsin/µl (sequencing grade chymotrypsin; Roche) in 50 mM NH4HCO3, 37°C, 16 h). Proteins were extracted (50% acetonitrile/5% formic acid), vacuum-dried, and redissolved in 15 µl 0.1% formic acid. LC/MS runs were performed on an Orbitrap Fusion (Thermo Scientific) or on a quadripole time-of-flight (QTOF) tandem mass spectrometer. Peptide identifications were made on an Orbitrap Fusion Tribrid instrument (LC-ESI-OT-MS, Orbitrap Fusion; Thermo Scientific) equipped with an HPLC system [Ultimate 3000; Thermo Scientific; LC parameter: RP C18 column (Acclaim PepMap RSLC, Thermo, 75 µm × 250 mm, 2 µm, 100Å), flow: 0.3 µl/min, solvent A: H2O/0.1% formic acid, solvent B: acetonitrile/0.1% formic acid, gradient: 2–30% B in 30 min]. The Orbitrap was operated with a resolution of 120,000 in positive ion mode. Precursor ions were selected by using the data-dependent acquisition mode and fragmented with a normalized high-energy collision dissociation energy of 35%. The fragment ions were detected in the linear ion trap (rapid mode). The LC-ESI-OT-MS data were processed with Proteome Discoverer, v1.4.1.14 (Thermo Scientific) using the following parameters: precursor mass tolerance 10 ppm, fragment mass tolerance 0.2 Da, 1 missed cleavage (trypsin), or 3 missed cleavages (chymotrypsin). All peptide assignments were verified by manual inspection. Quantification of Cys-modified peptides was based on measurements on a QTOF Premier mass spectrometer equipped with a nanoAquity Ultra Performance Liquid Chromatography (UPLC; Waters, Milford, MA, USA). Samples were applied to a trapping column (nanoAquity UPLC column, C18, 180 µm × 20 mm). The columns were washed for 10 min with 5% acetonitrile and 0.1% formic acid (5 µl/min) and eluted onto the separation column (nanoAquity UPLC column, C18, 75 µm × 100 mm, 200 nl/min) with a gradient (A: 0.1% formic acid; B: 0.1% formic acid in acetonitrile, 2–50% B). First, peptides were identified by the MSE technique. Alternating scans (0.95 s, 0.05 s interscan delay) with low (4 eV) and high (ramp from 22–36 eV) collision energy were recorded. Second, the identifications were confirmed by MS/MS measurements. Data were evaluated with the software package Protein Lynx Global Server, version 2.5.2 (Waters).
In vitro kinase assays
In vitro kinase (IVK) assays were conducted as has been described (16). Before the assays, recombinant proteins were subjected to IVG and dialyzed [in mM: Tris 30, MgCl2 15 (pH 7.4)]. IVK was started by addition of 100 μM ATP spiked with γ[32P]-ATP at 30°C and terminated with 3× reducing Laemmli sample buffer. Proteins were resolved by 10% SDS-PAGE, colloidal Coomassie–stained, and subjected to autoradiography. In parallel, experiments were performed with cold ATP and analyzed by Western immunoblot with phosphospecific cMyBP-C antibodies.
Microscale thermophoresis
The effect of post-translational modification on the interaction of C1-M-C2 with S2Δ was investigated by microscale thermophoresis (MST) (31, 32). Fluorescence labeling of S2Δ with NT-647-NHS was performed with the Monolith NT.115 Protein Labeling Kit RED-NHS (NanoTemper Technologies) according to the manufacturer’s instructions. The His6-tag on S2Δ has been shown not to interfere with C1-M-C2 interaction (29) and was therefore not removed. For experiments, C1-M-C2 was subjected to IVG or IVK. To assure equal buffer composition between the phosphorylated and the control group, heat-inactivated PKAcat was added to the control and named “PKA dead.” Serial dilutions of C1-M-C2 (40 μM to 1.22 nM) were mixed with a fixed concentration of labeled S2Δ in each experiment. The samples were analyzed with the Monolith NT.115 at 25°C (LED power, 20%; IR laser power, 80%; laser-on time, 30 s). Results were analyzed by Prism 5 (GraphPad, La Jolla, CA, USA) and NanoTemper Analysis 1.2.101 software and the dissociation constant (KD) was calculated from the independent thermophoresis measurements (31). Normalized fluorescence (Fnorm) of the unbound state was subtracted as a baseline value from all other values; results are depicted as the ΔFnorm/mil (‰).
Force measurements
To assess the effect of S-glutathiolation on kinetics of sarcomere force development, the rate of force redevelopment (ktr) was measured before (pre) and after (post) exposure of Triton X-100-permeabilized ventricular cells from WT or Mybpc3-targeted KO mice to GSSG (1 mM) or vehicle in relaxing solution [pH 7.0 in mM: Na phosphate 50, NaCl 300, imidazole 150, PMSF 0.1, and glycerol 25% (v/v); 30 min, 20°C] (33). Force measurements were performed in activating solution (pCa 4.5) and at a sarcomere length of 2.2 µm. ktr was determined by exponential curve fit of force development after a slack test in activating solution. Passive force (Fpas) development was determined in relaxing solution (pCa 9).
Immunoblot analysis
Immunoblot analysis was performed as described elsewhere (34). Protein bands were quantified with Gene Tools software (Syngene, Cambridge, United Kindom).
Statistical analysis
Statistical comparisons were performed by unpaired (HF samples) or paired (force measurements) 2-tailed Student’s t test. IVK assays were analyzed by unpaired 2-tailed Student’s t test comparing samples with the same incubation time. Data from MST measurements were compared by using the extra sum-of-squares F test. Quantitative data are given as the means ± sem, and results reaching P < 0.05 are significant.
RESULTS
In vivo phosphorylation of cMyBP-C in human HF
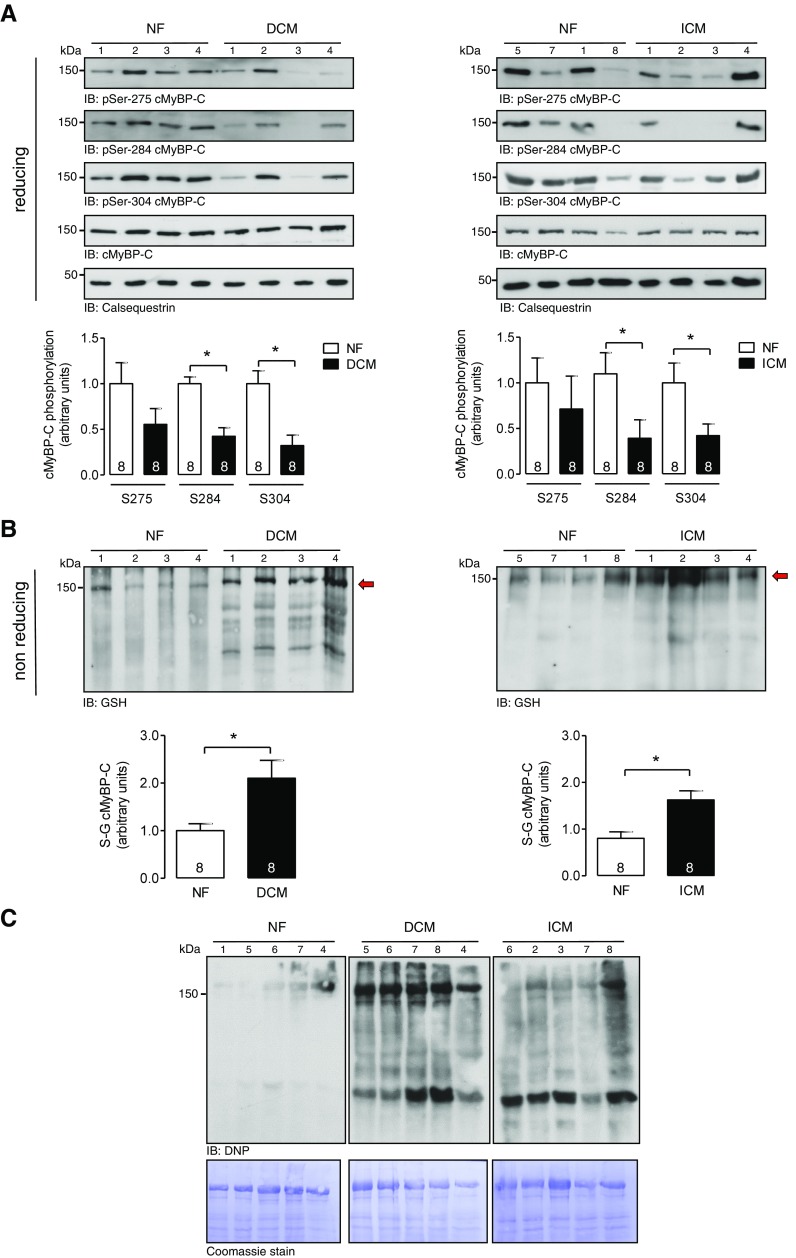
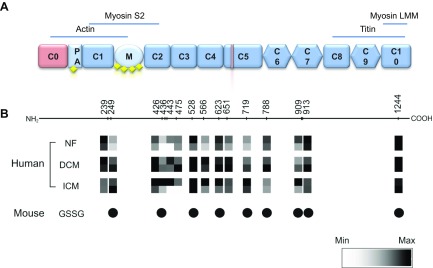
We investigated the S-glutathiolation status of cMyBP-C in myocardial tissue samples from donor and failing human hearts in parallel to the level of cMyBP-C phosphorylation at Ser275, Ser284, and Ser304. Heart homogenates from patients with DCM or ICM (Fig. 1A, B), conditions with increased ROS/RNS formation (35, 36), were analyzed and compared to NF donor heart samples. Immunoblot analysis revealed significantly increased S-glutathiolation of a protein migrating at the same molecular mass as cMyBP-C in the myofilament fractions of DCM and ICM samples compared to NF donor samples (Fig. 1B). Furthermore, enhanced protein carbonylation, as a marker of irreversible protein oxidation was detectable in the DCM and ICM samples compared to NF donors (Fig. 1C). The increase in S-glutathiolation signal in DCM and ICM patient samples was paralleled by a reduction in the phosphorylation status of cMyBP-C at Ser275, Ser284, and Ser304 (Fig. 1A), suggesting that S-glutathiolation sites nearby could negatively affect protein kinase–mediated phosphorylation. To map cysteines in cMyBP-C that are modified by S-glutathiolation, human heart samples were analyzed by redox proteomics. In human heart samples, a total of 15 S-glutathiolated cysteines were identified and quantified (Cys239, Cys249, Cys426, Cys436, Cys443, Cys475, Cys528, Cys566, Cys623, Cys651, Cys719, Cys788, Cys909, Cys913, and Cys1244) (Fig. 2; Supplemental Data 1). As expected, in patient samples, some regions of cMyBP-C show increased amounts of S-glutathiolated cysteines (Cys249, Cys426, Cys436, Cys443, Cys475, Cys566, Cys651, and Cys719), reflecting higher levels of ROS/RNS generation (Fig. 2B), whereas others showed levels comparable to those in NF donor samples.
Figure 1.
Post-translational modification of cMyBP-C in human HF. A) Western immunoblot analysis of tissue homogenates from NF donors (n = 8) and failing hearts from patients with DCM (n = 8) or ICM (n = 8). The numbers correspond with patients listed in Table 1. Site-specific phosphorylation of cMyBP-C at Ser275, Ser284, and Ser304 was assessed with phosphospecific antibodies under reducing conditions. Protein loading was assessed by immunoblot for cMyBP-C and calsequestrin. B) Western immunoblot analysis of tissue homogenates from NF (n = 8) and failing hearts from patients with DCM (n = 8) or ICM (n = 8). S-glutathiolation of cMyBP-C was assessed with a pan-specific anti-glutathione antibody under nonreducing conditions. A, B) Bar charts represent quantitative data as the means ± sem. *P < 0.05 vs. NF. C) Protein carbonylation analysis of tissue homogenates from NF (n = 5) and failing hearts from patients with DCM (n = 5) or ICM (n = 5) with an anti-dinitrophenyl antibody. Protein loading was demonstrated by Coomassie staining.
Figure 2.
Post-translational modification of cMyBP-C in human HF. A) Key features of cMyBP-C. Pink: cardiac-specific domains. Yellow squares: sites of phosphorylation. B) Positions of cysteines modified by S-glutathiolation in cMyBP-C in human (NF samples 1, 2; DCM samples 3, 4; ICM samples 7, 8) or mouse ventricular tissue identified and quantified by MS are indicated in squares. Quantification of S-glutathiolated cysteines in human samples is shown as a heatmap.
S-glutathiolation of cMyBP-C
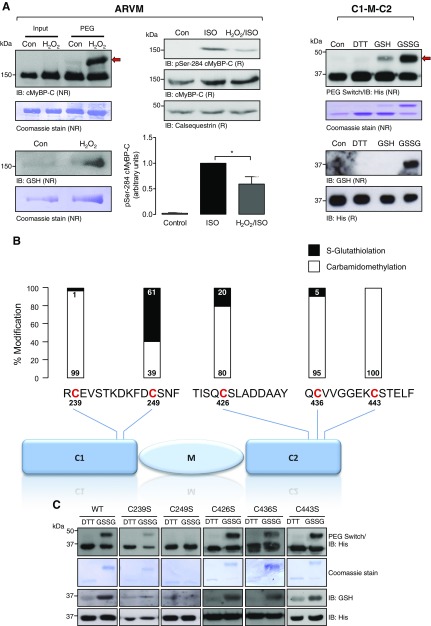
To investigate whether S-glutathiolation of cMyBP-C can be induced by exposure to an exogenous oxidant, ARVMs were exposed to vehicle or H2O2. Oxidation of cMyBP-C was analyzed by PEG switch assay (30) followed by immunoblot using a cMyBP-C antibody (Fig. 3A). In the starting material of vehicle and H2O2-treated ARVM samples, a single band was detected at 150 kDa. After the PEG switch analysis, however, an upward shift of the band corresponding to cMyBP-C occurred only in the H2O2-treated ARVM samples, indicating oxidative modification. This was accompanied by higher S-glutathiolation of cMyBP-C in the H2O2-treated ARVM samples compared with vehicle-treated samples, as assessed by the anti-glutathione antibody. This finding confirmed that cMyBP-C S-glutathiolation results from an increased oxidant load of the cell.
Figure 3.
S-glutathiolation of cMyBP-C. A) Detection of S-glutathiolation of cMyBP-C. ARVMs were exposed to vehicle (PBS) or H2O2 (100 μM) for 10 min, and cell lysates were subjected to PEG switch analysis followed by Western immunoblot analysis with a total cMyBP-C antibody. The red arrow indicates the assay-induced molecular mass increment of the oxidized protein. S-glutathiolation of cMyBP-C was assessed by Western immunoblot with the anti-glutathione antibody under nonreducing conditions. Protein loading was demonstrated by Coomassie blue staining. Phosphorylation of cMyBP-C at Ser284 was analyzed in ARVMs exposed to vehicle (Con), ISO (3 nM), or H2O2 (100 µM) for 10 min, followed by ISO (3 nM) for 10 min. Protein loading was shown by antibodies for cMyBP-C and calsequestrin. The bar chart summarizes the results of 6 experiments. Recombinant human WT His6-tagged C1-M-C2 region of cMyBP-C was exposed in vitro to DTT (10 mM), GSH (1 mM), or GSSG (1 mM) for 30 min, followed by PEG switch analysis and Western immunoblot using an anti-His antibody. S-glutathiolation was assessed by Western immunoblot using the anti-glutathione antibody under nonreducing conditions (bottom right panel). Protein loading was demonstrated by Coomassie staining or an anti-His antibody. Data are representative of at least 3 independent experiments. NR, nonreducing; R, reducing. *P < 0.05. B) Analysis of C1-M-C2 S-glutathiolation by MS. Recombinant human C1-M-C2 was exposed in vitro to GSSG (1 mM) for 30 min and analyzed by MS. A scheme of the domain structure of C1-M-C2 with the identified peptides covering the 5 candidate cysteines is illustrated. Bar charts represent a quantitation of the modification by S-glutathiolation or carbamidomethylation for each cysteine. C) Analysis of cMyBP-C (C1-M-C2) S-glutathiolation by site-directed mutagenesis. Recombinant C1-M-C2 of human cMyBP-C, in WT form or carrying single Cys/Ser substitutions (C239S, C249S, C426S, C436S, and C443S), was used in IVG assays. S-glutathiolation was detected by PEG switch analysis followed by Western immunoblot with anti-His antibody or the anti-glutathione antibody under nonreducing conditions. Equal protein loading between reducing (DTT) and oxidizing (GSSG) assay conditions was confirmed by Coomassie blue staining or the anti-His antibody. Data are representative of results in 3 independent experiments.
Next, ARVMs were exposed to H2O2, to induce S-glutathiolation, and then ISO, to induce protein kinase–mediated phosphorylation. Significantly reduced phosphorylation of cMyBP-C was observed at Ser284 when compared to that in ARVMs treated with ISO alone (Fig. 3A), suggesting crosstalk of post-translational modifications. We decided to focus our investigations on the C1-M-C2 domain of cMyBP-C (aa 153–450) (Fig. 2A), in which we identified a cluster of 5 putative S-glutathiolation sites: Cys239 and Cys249 in the C1 domain and Cys426, Cys436, and Cys443 in C2. These domains have been extensively characterized, as they contain the cardiac-specific phosphorylation-regulated M-motif. Human C1-M-C2 was expressed as a recombinant His6-tagged fusion protein. PEG switch analysis was performed (30) (Fig. 3A) to investigate oxidative modification of C1-M-C2 after exposure to DTT, GSH, or GSSG. As expected, subsequent immunoblot analysis did not reveal a PEG-tag–induced molecular mass shift under reducing conditions (DTT, GSH). In contrast, a molecular mass shift was observed, when C1-M-C2 was incubated with reactive GSSG (Fig. 3A), which is anticipated to induce S-glutathiolation via a thiol-disulfide exchange reaction. As shown by Coomassie staining, ∼80% of the recombinant protein underwent modification. Immunoblot analysis of the input samples with the anti-glutathione antibody showed a signal corresponding to S-glutathiolated C1-M-C2 only after GSSG treatment. The data confirm S-glutathiolation of cMyBP-C within the C1-M-C2 region in vitro. To identify the cysteines that are modified by in vitro S-glutathiolation, C1-M-C2 was subjected to MS analysis after exposure to DTT or GSSG. The analysis revealed S-glutathiolation of Cys239, Cys249, Cys426, and Cys436, with Cys249 as the main S-glutathiolation acceptor site as indicated by the black bars (Fig. 3B; Supplemental Data 2). Supplemental Data 2 highlights the equivalent peptide region covering Cys249 with post-translational modification in human cMyBP-C. To further validate the data acquired by MS, site-directed mutagenesis was performed. Cys239, Cys249, Cys426, Cys436, or Cys443 was replaced singly by serine, which cannot be modified by S-glutathiolation. WT and mutant C1-M-C2 were incubated with DTT or GSSG, followed by PEG switch analysis (Fig. 3C). In WT C1-M-C2, the molecular mass shift was reproduced as previously shown. Similarly, proteins with replacement of Cys239, Cys426, Cys436, or Cys443 by serine also underwent a molecular mass shift similar to the WT when exposed to GSSG. In contrast, replacement of Cys249 by serine largely reduced the shift after incubation with GSSG. Western immunoblot analysis performed with the anti-S-glutathione antibody detected an increase in signal intensity only when WT, C239S, C426S, C436S, or C443S C1-M-C2 proteins were exposed to GSSG. The signal was attenuated in the C249S mutant. Overall, these data validated the results obtained by tandem mass spectrometry of in vitro S-glutathiolated C1-M-C2.
Effect of S-glutathiolation on protein kinase–mediated phosphorylation
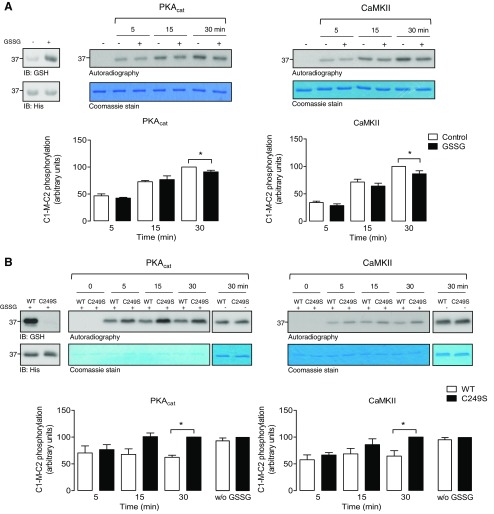
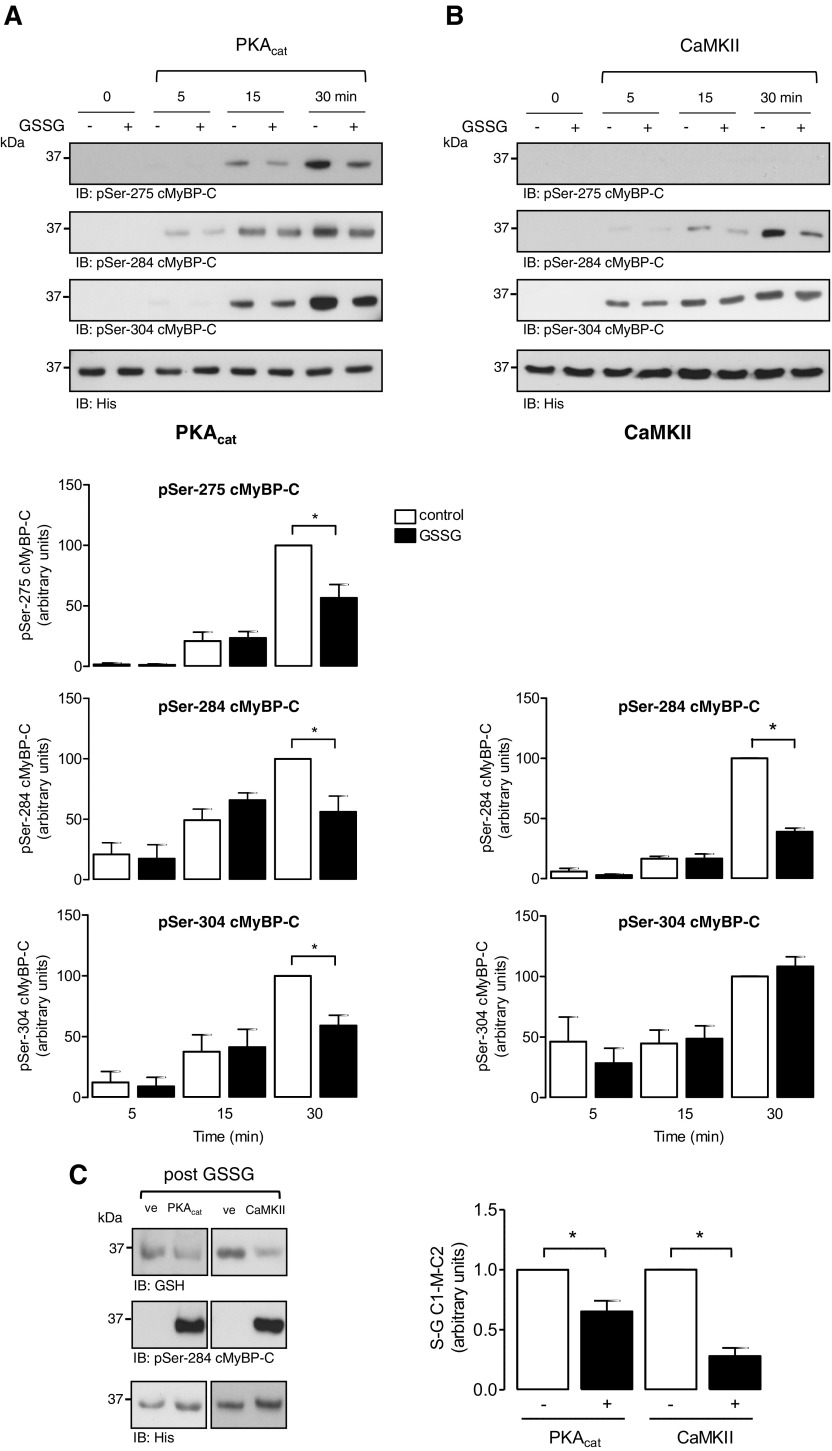
To explore potential crosstalk between S-glutathiolation and phosphorylation of cMyBP-C, IVKs in the presence of radiolabeled γ[32P]-ATP were performed in WT or a non-S-glutathiolatable serine mutant (C249S) C1-M-C2 (Fig. 4). Initially, WT protein was exposed to vehicle or GSSG to induce S-glutathiolation, which was corroborated by immunoblot analysis with the anti-glutathione antibody (Fig. 4A). In vitro phosphorylation by PKAcat and CaMKII was subsequently performed in the presence of γ[32P]-ATP and detected by autoradiography. Vehicle-treated C1-M-C2 was phosphorylated by both protein kinases in a time-dependent manner. S-glutathiolated protein displayed slower and significantly reduced maximal phosphate incorporation after 30 min, compared with vehicle-treated WT C1-M-C2 for both kinases. Additional IVK assays were performed comparing pre-S-glutathiolated WT C1-M-C2 with the non-S-glutathiolatable mutant C249S (Fig. 4B). S-glutathiolation of WT protein was demonstrated by immunoblot analysis with the anti-glutathione antibody. As expected, there was a reduced signal detectable after incubation of C249S with GSSG, caused by the loss of the main S-glutathiolation site in the C1-M-C2 cysteine cluster. In vitro phosphorylation by PKAcat and CaMKII was then performed in the presence of γ[32P]-ATP and detected by autoradiography. C249S was phosphorylated by both kinases in a time-dependent manner. In contrast, S-glutathiolated WT protein displayed markedly reduced phosphate incorporation compared to mutant C249S for both kinases. To ensure that mutation of Cys249 to serine does not alter phosphorylation by PKAcat or CaMKII, control IVK experiments were performed without prior S-glutathiolation of both substrate proteins. The results revealed no differences in the phosphate incorporation into WT or the C249S mutant by both kinases.
Figure 4.
Effect of S-glutathiolation of C1-M-C2 cMyBP-C on protein kinase-mediated phosphorylation. A) Time-course experiments of PKAcat- or CaMKII-mediated phosphorylation of WT C1-M-C2 were performed. Recombinant proteins were exposed to GSSG or vehicle. S-glutathiolation status was confirmed. Subsequently, IVK assays were performed in the presence of γ[32P]-ATP under nonreducing conditions. Phosphorylation by PKAcat or CaMKII was detected by autoradiography. Gels were Coomassie-stained to demonstrate protein loading. Data are expressed as the percentage of maximum phosphorylation under control conditions (30 min). Bar charts summarize the data of 6 independent experiments. *P < 0.05 vs. con. B) Time-course experiments of PKAcat- or CaMKII-mediated phosphorylation of WT C1-M-C2 or mutant non-S-glutathiolatable C249S C1-M-C2 were performed. Recombinant proteins were exposed to GSSG or vehicle. S-glutathiolation status was confirmed. Subsequently, IVK assays were performed in the presence of γ[32P]-ATP under nonreducing conditions. Phosphorylation by PKAcat or CaMKII was detected by autoradiography. No difference in the phosphorylation of vehicle-treated WT or C249S by PKA or CaMKII was detected (w/o GSSG). Gels were Coomassie-stained to demonstrate protein loading. Data are expressed as a percentage of C249S C1-M-C2 phosphorylation (30 min). Bar charts summarize the data of 3 independent experiments. *P < 0.05 vs. con.
To investigate the contribution of the single phosphorylation sites Ser275, Ser284, or Ser304 to the differences observed in the overall phosphorylation of C1-M-C2 after S-glutathiolation, we performed IVK experiments in the presence of nonradiolabeled ATP. Western immunoblot analysis was performed with site-phosphospecific antibodies recognizing Ser275, Ser284, and Ser304. PKAcat phosphorylated WT C1-M-C2 at all 3 sites in a time-dependent manner, an effect equally attenuated by S-glutathiolation (Fig. 5A). CaMKII phosphorylated C1-M-C2 time dependently at Ser284 and Ser304. S-glutathiolation significantly reduced CaMKII-mediated phosphorylation at Ser284, whereas Ser304 phosphorylation displayed no reduction compared to unmodified protein (Fig. 5B). When experiments were performed with the C249S C1-M-C2 mutant, no difference in the phosphorylation was detectable between vehicle-treated and S-glutathiolated protein by both kinases (Supplemental Data 4A, B). This result suggests that C249S is largely responsible for the loss in phosphorylation of S-glutathiolated WT C1-M-C2. Furthermore, the data indicate that S-glutathiolation of C1-M-C2 affects the ability of kinases to phosphorylate their substrate. This effect may mechanistically explain the reduction in cMyBP-C phosphorylation observed under HF conditions, when cellular ROS levels are increased inducing S-glutathiolation of cMyBP-C. Phosphorylation of cMyBP-C has been described to protect hearts from ischemic injury. Therefore, we investigated whether prephosphorylation protects C1-M-C2 from S-glutathiolation. Recombinant C1-M-C2 was prephosphorylated by PKAcat or CaMKII (Fig. 5C), as demonstrated by increased Ser284 phosphorylation by Western immunoblotting. Subsequent exposure of the control-treated samples to GSSG resulted in a robust increase of S-glutathiolation of C1-M-C2, shown with the anti-glutathione antibody. Kinase-mediated prephosphorylation prevented subsequent S-glutathiolation of C1-M-C2. This result suggests that kinase-mediated phosphorylation of cMyBP-C protects the protein from oxidation.
Figure 5.
Effect of S-glutathiolation of C1-M-C2 cMyBP-C on site-specific phosphorylation. A) Time-course experiments of PKAcat-mediated phosphorylation of WT C1-M-C2 with or without S-glutathiolation were performed. Recombinant proteins were exposed to GSSG or vehicle and subsequently used in IVK assays performed in the presence of nonradiolabeled ATP. B) Time-course experiments of CaMKII-mediated phosphorylation of WT C1-M-C2, with or without S-glutathiolation were performed. Recombinant proteins were exposed to GSSG or vehicle and subsequently used in IVK assays performed in the presence of nonradiolabeled ATP. Phosphorylation was detected by Western immunoblot with phosphospecific antibodies recognizing cMyBP-C when phosphorylated at Ser275, Ser284, or Ser304. Protein loading was confirmed by anti-His antibody. Data are normalized to anti-His loading control and expressed as a percentage of maximum phosphorylation under control conditions (30 min). Bar charts represent data of 5 independent experiments. *P < 0.05 vs. con. C) The effect of phosphorylation of C1-M-C2 by PKAcat or CaMKII on S-glutathiolation was investigated. Protein kinase–mediated phosphorylation of C1-M-C2 was determined by Western immunoblot with a phosphospecific antibody recognizing cMyBP-C when phosphorylated at Ser284. Subsequent S-glutathiolation was determined with the anti-glutathione antibody under nonreducing conditions. Protein loading was confirmed by the anti-His antibody. Data are normalized to anti-His loading control and expressed as a percentage of maximum S-glutathiolation under control conditions. Bar charts representing data of at least 3 independent experiments. *P < 0.05 vs. con.
Effect of S-glutathiolation on sarcomere function
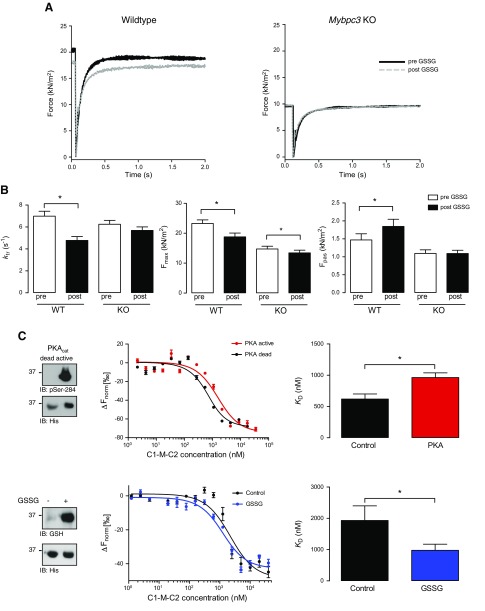
The effects of S-glutathiolation on sarcomere function were studied in membrane-permeabilized (“skinned”) ventricular myocytes from WT or Mybpc3-targeted KO mice lacking endogenous cMyBP-C. Phosphorylatable serines and S-glutathiolatable cysteines in cMyBP-C are highly conserved among mouse, rat, and human. Initially, we investigated the S-glutathiolation status of cMyBP-C in WT mice following exposure to exogenous GSSG by MS (Fig. 2A, mouse; Supplemental Data 3) and identified 9 S-glutathiolation sites (Cys249, Cys436, Cys528, Cys566, Cys623, Cys719, Cys788, Cys909, Cys913, and Cys1244) (referring to the human sequence) with a distribution of S-glutathiolated cysteines mimicking the reported pattern in failing human samples. The effect of S-glutathiolation on cross-bridge cycling kinetics was analyzed by measuring ktr after a release-restretch protocol with or without incubation with GSSG. Representative recordings of force redevelopment were made in WT or KO mice before and after GSSG treatment (Fig. 6A). Notably, the baseline level of developed force was lower in KO than in WT cells, which has been reported (37). After incubation with GSSG, ktr was significantly reduced in ventricular cells from WT mice. In contrast, GSSG treatment had no effect on ktr in KO mice, suggesting that S-glutathiolation of cMyBP-C negatively affects cross-bridge cycling kinetics (Fig. 6B). Because ktr is a major determinant of the level of active force development, we next investigated the role of S-glutathiolation of cMyBP-C on maximum force (Fmax). In ventricular cells from WT and KO mice, Fmax was significantly reduced after exposure to GSSG, with a smaller effect observed in the latter, suggesting that S-glutathiolation of cMyBP-C partially accounts for the decline in Fmax observed in WT cells. Finally, we determined the effect of S-glutathiolation on Fpas assessed in relaxing solution (pCa 9). In ventricular cells from WT mice, Fpas was significantly increased after GSSG exposure. In contrast, there was no effect of GSSG treatment on Fpas in KO mice. Control experiments without GSSG treatment showed no significant differences in ktr, Fmax, and Fpas before or after the release–restretch protocol (Supplemental Data 5), suggesting that S-glutathiolation of myofilament proteins is responsible for the observed functional effects. The above data indicate that S-glutathiolation of cMyBP-C has functional consequences distinct to phosphorylation by kinases (16, 17, 38). We identified several S-glutathiolated cysteines in cMyBP-C in ventricular human tissue, which could contribute to the observed functional consequences. In addition, sarcomeric proteins other than cMyBP-C can be S-glutathiolated and affect alterations in contractile function (39). Therefore, to investigate the role of the cysteine cluster in C1-M-C2 in the regulation of cMyBP-C function, MST experiments were performed. Alterations in the interaction between recombinant C1-M-C2 with NT-647-NHS-labeled S2Δ in response to S-glutathiolation of C1-M-C2 were assessed. Phosphorylation of C1-M-C2 by PKA has been shown to attenuate the interaction with S2Δ by isothermal titration calorimetry and cosedimentation (29). Therefore, as positive control, MST experiments were performed with PKAcat-phosphorylated C1-M-C2. Phosphorylation of C1-M-C2 was demonstrated by immunoblot with the pSer-284 cMyBP-C antibody (Fig. 6C). Thermophoresis is very sensitive to small differences in the buffer composition, and therefore a heat-inactivated PKAcat control sample (PKA dead) was studied as the control. Binding curves were assessed under control conditions or after phosphorylation. The KD of phosphorylated C1-M-C2 (926 ± 75 nM) was increased compared with that of control C1-M-C2 (618 ± 82 nM), confirming that phosphorylation attenuates the association of C1-M-C2 with S2Δ. To investigate the effect of S-glutathiolation on the interaction of C1-M-C2 with S2Δ, we performed in vitro S-glutathiolation of C1-M-C2. Binding curves were assessed under control conditions or after exposure to GSSG. Differences in control KD between phosphorylation and S-glutathiolation experiments were caused by differences in buffer composition. KD of S-glutathiolated C1-M-C2 (975.7 ± 197.6 nM) was significantly reduced compared to vehicle-treated C1-M-C2 (1932.0 ± 466.1 nM), suggesting that S-glutathiolation increases the interaction of C1-M-C2 with S2Δ. This observation may account for the significant reduction in ktr and subsequently Fmax in the skinned WT ventricular myocyte experiments after GSSG treatment.
Figure 6.
Effect of S-glutathiolation on sarcomere function in skinned ventricular myocytes from WT and Mybpc3-targeted KO mice. A) Representative traces of force redevelopment in skinned ventricular myocytes from WT and KO mouse pre- and posttreatment with GSSG. B) Rate of force redevelopment (ktr) and Ca2+-activated force (Fmax) was determined in activating solution (pCa 4.5) and passive force (Fpas) in relaxing solution (pCa 9) in Triton-permeabilized ventricular myocytes from WT (n = 3; 10 ventricular myocytes) and KO (n = 3; 12 ventricular myocytes) mice. Bar charts represent data obtained before and after incubation with GSSG (1 mM for 30 min), as indicated. *P < 0.05 vs. pre-GSSG. C) MST analysis was performed with NT-647-NHS-labeled recombinant human myosin S2Δ and PKAcat-phosphorylated or S-glutathiolated recombinant C1-M-C2. Phosphorylation and S-glutathiolation were assessed by Western immunoblotting with a phosphospecific Ser284 cMyBP-C-antibody or a glutathione-antibody, respectively. Protein loading was demonstrated with anti-His antibody. Binding curves were assessed by titrating PKAcat- or S-glutathiolated (GSSG) C1-M-C2 (40 µM to 1.2 nM) against a fixed concentration of S2Δ. Binding curves were compared against their respective controls containing similar protein and buffer composition. Bar charts represent dissociation constants (KD). Data are representative of 6–9 independent experiments. *P < 0.05 vs. PKA dead or control.
DISCUSSION
Phosphorylation of sarcomeric proteins is a key physiologic mechanism through which cardiac myofilament function is regulated in response to neurohumoral stimuli (40). In HF, a reduced overall phosphorylation status of sarcomeric proteins has been causally linked to an impaired contractile function of the heart (2, 41–43). This effect is mainly ascribed to the desensitized β1-adrenoceptor (β-AR) pathway leading to a diminished activation of the associated PKA signaling cascade (44) and increased phosphatase activity (45, 46). The role of ROS/RNS released under disease conditions (18, 23), inducing OPTMs in target proteins (19–22) that potentially contribute to alterations in contractile performance has just started to emerge. The present study provides novel evidence that S-glutathiolation of cMyBP-C is increased in human HF and that this parallels, potentially causatively, the decreased overall phosphorylation of cMyBP-C; in vitro S-glutathiolation within C1-M-C2, particularly at Cys249, prevents protein kinase–mediated phosphorylation of cMyBP-C; and S-glutathiolation of cMyBP-C negatively affects myofilament kinetics by enhancing interaction with myosin S2.
A considerable amount of evidence has accumulated to suggest that the phosphorylation status of cMyBP-C is an important determinant of cardiac function in health and disease (15, 47). Studies in transgenic mice that express mutated cMyBP-C with replacement of Ser273, Ser282, and Ser302 (referring to the mouse sequence) by non-phosphorylatable alanine (48–50) or phosphomimetic aspartate (49, 51), showed the importance of cMyBP-C phosphorylation for uncompromised cardiac function. Phosphorylation of cMyBP-C preserved sarcomeric integrity and myocardial contraction under basal conditions as well as after β-AR stimulation or in response to ischemia/reperfusion (48–51). In contrast, inability to phosphorylate cMyBP-C led to enhanced injury and subsequently to dysfunction. Studies in patients with HCM have already reported a decrease in the global phosphorylation status of cMyBP-C (52). In particular, a reduction in the phosphorylation of “the switch” Ser284 was shown in myocardial tissue samples of patients with end-stage HF and in patients with atrial fibrillation (2). These data support our observations in samples from patients with DCM or ICM that showed a reduction in overall phosphorylation of cMyBP-C.
It is of note that heart disease has been widely associated with an imbalanced redox state and elevated levels of ROS/RNS. This raises the question of whether oxidant-induced OPTMs in myofilament proteins contribute to the molecular alterations resulting in HF. In this context, S-glutathiolation of cMyBP-C has been described previously in vitro (24) and in a mouse model of hypertension (25) and HCM (27). The recently identified S-glutathiolation sites in murine cMyBP-C induced after exposure of homogenized mouse hearts to GSSG are localized in domains C3, C4, and C5 of cMyBP-C (30). All sites were also identified in our mass spectrometry analysis. Potentially, due to limited sequence coverage of 45–50% in this study, the N terminus of cMyBP-C, particularly Cys249 was not among the identified S-glutathiolation sites. To our knowledge, our study is the first to detect high S-glutathiolation levels of cMyBP-C in human HF, to map novel disease-specific S-glutathiolation sites in human cMyBP-C, and to correlate them with decreased site-specific phosphorylation. Besides detecting constitutively modified cysteines in NF donor tissue, we identified additional disease-specific enhanced S-glutathiolated sites in cMyBP-Cys249, Cys426, Cys436, Cys443, Cys475, Cys566, Cys651, and Cys719 in DCM and ICM. This confirms enhanced oxidant production, supported also by increased protein carbonylation and thus a disease-specific oxidation fingerprint of cMyBP-C. To test the cause-and-effect relationship for a potential redox-phosphorylation crosstalk, we functionally characterized one novel S-glutathiolation cluster, containing particularly one site that we mapped to Cys249 in C1-M-C2 of cMyBP-C. We initially validated the MS data obtained in human ventricular tissue samples by using recombinant C1-M-C2 protein by MS and site-directed mutagenesis. The result suggested that Cys249 is a functionally important S-glutathiolation site in C1-M-C2. S-glutathiolation has been reported to alter protein function by modifying protein–protein interactions or inducing changes in the structural conformation of a modified protein (53). S-glutathiolation at Cys249 occurs near the phosphorylation sites and is therefore likely to have an impact on protein kinase interaction with cMyBP-C and subsequent phosphorylation. We observed that S-glutathiolation of WT C1-M-C2, but not C249S C1-M-C2, reduced overall and site-specific in vitro phosphate incorporation by PKAcat or CaMKII compared with vehicle-treated C1-M-C2. This observation suggests that S-glutathiolation particularly at Cys249 modulates C1-M-C2 conformation and hinders subsequent phosphorylation by protein kinases. Extrapolating our in vitro findings to the results obtained in human HF samples suggests that reduced phosphorylation of cMyBP-C may not exclusively be the result of reduced PKA activity and increased phosphatase activity, but is also attributable to enhanced S-glutathiolation of cMyBP-C. In cardiac myocytes from patients with end-stage HF, addition of PKA was shown to restore phosphorylation and function of cMyBP-C (4, 52). This advocates for reduced PKA activity as the main culprit leading to a reduction in cMyBP-C phosphorylation. However, experiments are often carried out under reducing buffer conditions, involving DTT supplementation in the millimolar range, neutralizing OPTMs. In light of our findings, experimental conditions should perhaps be adjusted to account for the presence of OPTMs, which may be crucial to basal function or the development of disease. Our data revealed that S-glutathiolation translated functionally into reduced cross-bridge cycling kinetics. This effect contributed to a reduction in developed force and increased Fpas, which are characteristics of the HF phenotype. S-glutathiolation of cMyBP-C within C1-M-C2 is likely to affect the interaction with the myosin S2 domain, actin, and the regulatory light chains, because of its position within cMyBP-C. However, contributions from the other identified S-glutathiolation clusters to the observed functional consequences cannot be excluded. Particularly, the S-glutathiolation cluster near the C terminus of cMyBP-C with Cys909, Cys913, Cys1124, and Cys1244 near the C-terminal titin-interaction domain of cMyBP-C could potentially explain the significant increase in Fpas observed in WT cells after exposure to GSSG in our experiments. It was important to establish, whether there is a role of site-specific S-glutathiolation of C1-M-C2 for cMyBP-C function. Therefore, MST experiments were performed, which demonstrated that S-glutathiolation of C1-M-C2 enhances the interaction with myosin S2Δ in contrast to phosphorylation. This could explain the reduction in ktr and subsequently Fmax and also the increase in Fpas in WT myocytes after pretreatment with GSSG. Taken together, S-glutathiolation at Cys249 impairs protein kinase–mediated phosphorylation. This effect is paralleled by significant changes in key parameters of myofilament function, such as cross-bridge cycling kinetics and force development and may therefore represent a causative mechanism in the pathogenesis of human HF.
Supplementary Material
Acknowledgments
The authors thank Prof. Dieter Braun and Susanne Seidel (Department of Systems Biophysics Ludwig Maximilian University of Munich, Munich, Germany) for introducing them to MST technology. The authors also thank Jana Meisterknecht (Goethe University, Frankfurt am Main, Germany) for excellent technical assistance with redox proteomics. F.C., K.S., Si.D., A.P., So.D., L.C., T.E. are supported by the German Center for Cardiovascular Research (DZHK) and the German Ministry of Research and Education (BMBF). This study was supported by Deutsche Forschungsgemeinschaft (DFG) Grant CU 53/2-1, the Faculty of Medicine–University medical Center Hamburg-Eppendorf, and the Werner Otto Foundation. I.W. was supported by DFG Grant SFB815, project Z01. S.S. was supported by U.S. National Institutes of Health, National Heart, Lung and Blood Institute Grants R01HL105826 and K02HL114749). The authors declare no conflicts of interest.
Glossary
- ARVM
adult rat ventricular myocyte
- β-AR
β1-adrenoceptor
- cMyBP-C
cardiac myosin-binding protein C
- CaMK
Ca2+/calmodulin-dependent protein kinase
- DCM
dilated cardiomyopathy
- DNPH
dinitrophenyl hydrazine
- FDR
false discovery rate
- Fmax
maximal force
- Fnorm
normalized fluorescence
- Fpas
passive force
- GSH
glutathione reduced
- GSSG
glutathione disulphide
- HCM
hypertrophic cardiomyopathy
- HF
heart failure
- ICM
ischemic cardiomyopathy
- ISO
isoprenaline
- IVG
in vitro S-glutathiolation assay
- IVK
In vitro kinase assay
- KO
knockout
- ktr
rate of force redevelopment
- LC/MS
liquid chromatography-coupled mass spectrometry
- MST
microscale thermophoresis
- NF
nonfailing
- OPTM
oxidative post-translational modification
- PEG
polyethylene glycol
- QTOF
quadripole time-of-flight (mass spectrometer)
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- S2Δ
β-myosin subfragment S2
- UPLC
Ultra Performance Liquid Chromatography
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Ertl G., Ruschitzka F. (2014) The Year in Cardiology 2013: heart failure. Eur. Heart J. 35, 470–473 [DOI] [PubMed] [Google Scholar]
- 2.El-Armouche A., Pohlmann L., Schlossarek S., Starbatty J., Yeh Y. H., Nattel S., Dobrev D., Eschenhagen T., Carrier L. (2007) Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J. Mol. Cell. Cardiol. 43, 223–229 [DOI] [PubMed] [Google Scholar]
- 3.Copeland O., Sadayappan S., Messer A. E., Steinen G. J., van der Velden J., Marston S. B. (2010) Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J. Mol. Cell. Cardiol. 49, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 4.Kuster D. W., Bawazeer A. C., Zaremba R., Goebel M., Boontje N. M., van der Velden J. (2012) Cardiac myosin binding protein C phosphorylation in cardiac disease. J. Muscle Res. Cell Motil. 33, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuster D. W., Sequeira V., Najafi A., Boontje N. M., Wijnker P. J., Witjas-Paalberends E. R., Marston S. B., Dos Remedios C. G., Carrier L., Demmers J. A., Redwood C., Sadayappan S., van der Velden J. (2013) GSK3β phosphorylates newly identified site in the proline-alanine-rich region of cardiac myosin-binding protein C and alters cross-bridge cycling kinetics in human: short communication. Circ. Res. 112, 633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korte F. S., McDonald K. S., Harris S. P., Moss R. L. (2003) Loaded shortening, power output, and rate of force redevelopment are increased with knockout of cardiac myosin binding protein-C. Circ. Res. 93, 752–758 [DOI] [PubMed] [Google Scholar]
- 7.Carrier L., Mearini G., Stathopoulou K., Cuello F. (2015) Cardiac myosin-binding protein C (MYBPC3) in cardiac pathophysiology. Gene 573, 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfuhl M., Gautel M. (2012) Structure, interactions and function of the N-terminus of cardiac myosin binding protein C (MyBP-C): who does what, with what, and to whom? J. Muscle Res. Cell Motil. 33, 83–94 [DOI] [PubMed] [Google Scholar]
- 9.Pohlmann L., Kröger I., Vignier N., Schlossarek S., Krämer E., Coirault C., Sultan K. R., El-Armouche A., Winegrad S., Eschenhagen T., Carrier L. (2007) Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ. Res. 101, 928–938 [DOI] [PubMed] [Google Scholar]
- 10.Stelzer J. E., Dunning S. B., Moss R. L. (2006) Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ. Res. 98, 1212–1218 [DOI] [PubMed] [Google Scholar]
- 11.Stelzer J. E., Fitzsimons D. P., Moss R. L. (2006) Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys. J. 90, 4119–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautel M., Zuffardi O., Freiburg A., Labeit S. (1995) Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: a modulator of cardiac contraction? EMBO J. 14, 1952–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadayappan S., Gulick J., Osinska H., Barefield D., Cuello F., Avkiran M., Lasko V. M., Lorenz J. N., Maillet M., Martin J. L., Brown J. H., Bers D. M., Molkentin J. D., James J., Robbins J. (2011) A critical function for Ser-282 in cardiac Myosin binding protein-C phosphorylation and cardiac function. Circ. Res. 109, 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamed A. S., Dignam J. D., Schlender K. K. (1998) Cardiac myosin-binding protein C (MyBP-C): identification of protein kinase A and protein kinase C phosphorylation sites. Arch. Biochem. Biophys. 358, 313–319 [DOI] [PubMed] [Google Scholar]
- 15.Bardswell S. C., Cuello F., Kentish J. C., Avkiran M. (2012) cMyBP-C as a promiscuous substrate: phosphorylation by non-PKA kinases and its potential significance. J. Muscle Res. Cell Motil. 33, 53–60 [DOI] [PubMed] [Google Scholar]
- 16.Cuello F., Bardswell S. C., Haworth R. S., Ehler E., Sadayappan S., Kentish J. C., Avkiran M. (2011) Novel role for p90 ribosomal S6 kinase in the regulation of cardiac myofilament phosphorylation. J. Biol. Chem. 286, 5300–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardswell S. C., Cuello F., Rowland A. J., Sadayappan S., Robbins J., Gautel M., Walker J. W., Kentish J. C., Avkiran M. (2010) Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J. Biol. Chem. 285, 5674–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg S. F. (2013) Oxidative stress and sarcomeric proteins. Circ. Res. 112, 393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L., Eu J. P., Meissner G., Stamler J. S. (1998) Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279, 234–237 [DOI] [PubMed] [Google Scholar]
- 20.Adachi T., Pimentel D. R., Heibeck T., Hou X., Lee Y. J., Jiang B., Ido Y., Cohen R. A. (2004) S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 279, 29857–29862 [DOI] [PubMed] [Google Scholar]
- 21.Adachi T., Weisbrod R. M., Pimentel D. R., Ying J., Sharov V. S., Schöneich C., Cohen R. A. (2004) S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 10, 1200–1207 [DOI] [PubMed] [Google Scholar]
- 22.Lancel S., Zhang J., Evangelista A., Trucillo M. P., Tong X., Siwik D. A., Cohen R. A., Colucci W. S. (2009) Nitroxyl activates SERCA in cardiac myocytesvia glutathiolation of cysteine 674. Circ. Res. 104, 720–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafstad A. D., Nabeebaccus A. A., Shah A. M. (2013) Novel aspects of ROS signalling in heart failure. Basic Res. Cardiol. 108, 359 [DOI] [PubMed] [Google Scholar]
- 24.Brennan J. P., Miller J. I., Fuller W., Wait R., Begum S., Dunn M. J., Eaton P. (2006) The utility of N,N-biotinyl glutathione disulfide in the study of protein S-glutathiolation. Mol. Cell. Proteomics 5, 215–225 [DOI] [PubMed] [Google Scholar]
- 25.Lovelock J. D., Monasky M. M., Jeong E. M., Lardin H. A., Liu H., Patel B. G., Taglieri D. M., Gu L., Kumar P., Pokhrel N., Zeng D., Belardinelli L., Sorescu D., Solaro R. J., Dudley S. C. Jr (2012) Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ. Res. 110, 841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel B. G., Wilder T., Solaro R. J. (2013) Novel control of cardiac myofilament response to calcium by S-glutathionylation at specific sites of myosin binding protein C. Front. Physiol. 4, 336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flenner F., Friedrich F. W., Ungeheuer N., Christ T., Geertz B., Reischmann S., Wagner S., Stathopoulou K., Sohren K. D., Weinberger F., Schwedhelm E., Cuello F., Maier L. S., Eschenhagen T., Carrier L. (2016) Ranolazine antagonizes catecholamine-induced dysfunction in isolated cardiomyocytes, but lacks long-term therapeutic effects in vivo in a mouse model of hypertrophic cardiomyopathy. Cardiovasc. Res. 109, 90–102 [DOI] [PubMed] [Google Scholar]
- 28.Carrier L., Knöll R., Vignier N., Keller D. I., Bausero P., Prudhon B., Isnard R., Ambroisine M. L., Fiszman M., Ross J. Jr., Schwartz K., Chien K. R. (2004) Asymmetric septal hypertrophy in heterozygous cMyBP-C null mice. Cardiovasc. Res. 63, 293–304 [DOI] [PubMed] [Google Scholar]
- 29.Gruen M., Gautel M. (1999) Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J. Mol. Biol. 286, 933–949 [DOI] [PubMed] [Google Scholar]
- 30.Burgoyne J. R., Oviosu O., Eaton P. (2013) The PEG-switch assay: a fast semi-quantitative method to determine protein reversible cysteine oxidation. J. Pharmacol. Toxicol. Methods 68, 297–301 [DOI] [PubMed] [Google Scholar]
- 31.Wienken C. J., Baaske P., Rothbauer U., Braun D., Duhr S. (2010) Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 1, 100 [DOI] [PubMed] [Google Scholar]
- 32.Seidel S. A., Dijkman P. M., Lea W. A., van den Bogaart G., Jerabek-Willemsen M., Lazic A., Joseph J. S., Srinivasan P., Baaske P., Simeonov A., Katritch I., Melo F. A., Ladbury J. E., Schreiber G., Watts A., Braun D., Duhr S. (2013) Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods 59, 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Velden J., Papp Z., Zaremba R., Boontje N. M., de Jong J. W., Owen V. J., Burton P. B., Goldmann P., Jaquet K., Stienen G. J. (2003) Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc. Res. 57, 37–47 [DOI] [PubMed] [Google Scholar]
- 34.Cuello F., Bardswell S. C., Haworth R. S., Yin X., Lutz S., Wieland T., Mayr M., Kentish J. C., Avkiran M. (2007) Protein kinase D selectively targets cardiac troponin I and regulates myofilament Ca2+ sensitivity in ventricular myocytes. Circ. Res. 100, 864–873 [DOI] [PubMed] [Google Scholar]
- 35.Shimano M., Shibata R., Inden Y., Yoshida N., Uchikawa T., Tsuji Y., Murohara T. (2009) Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm 6, 935–940 [DOI] [PubMed] [Google Scholar]
- 36.Neuman R. B., Bloom H. L., Shukrullah I., Darrow L. A., Kleinbaum D., Jones D. P., Dudley S. C. Jr (2007) Oxidative stress markers are associated with persistent atrial fibrillation. Clin. Chem. 53, 1652–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecarpentier Y., Vignier N., Oliviero P., Guellich A., Carrier L., Coirault C. (2008) Cardiac Myosin-binding protein C modulates the tuning of the molecular motor in the heart. Biophys. J. 95, 720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haworth R. S., Cuello F., Herron T. J., Franzen G., Kentish J. C., Gautel M., Avkiran M. (2004) Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ. Res. 95, 1091–1099 [DOI] [PubMed] [Google Scholar]
- 39.Passarelli C., Di Venere A., Piroddi N., Pastore A., Scellini B., Tesi C., Petrini S., Sale P., Bertini E., Poggesi C., Piemonte F. (2010) Susceptibility of isolated myofibrils to in vitro glutathionylation: potential relevance to muscle functions. Cytoskeleton (Hoboken) 67, 81–89 [DOI] [PubMed] [Google Scholar]
- 40.Solaro R. J. (2008) Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J. Biol. Chem. 283, 26829–26833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacques A. M., Copeland O., Messer A. E., Gallon C. E., King K., McKenna W. J., Tsang V. T., Marston S. B. (2008) Myosin binding protein C phosphorylation in normal, hypertrophic and failing human heart muscle. J. Mol. Cell. Cardiol. 45, 209–216 [DOI] [PubMed] [Google Scholar]
- 42.Zaremba R., Merkus D., Hamdani N., Lamers J. M., Paulus W. J., Dos Remedios C., Duncker D. J., Stienen G. J., van der Velden J. (2007) Quantitative analysis of myofilament protein phosphorylation in small cardiac biopsies. Proteomics Clin. Appl. 1, 1285–1290 [DOI] [PubMed] [Google Scholar]
- 43.Hamdani N., Borbély A., Veenstra S. P., Kooij V., Vrydag W., Zaremba R., Dos Remedios C., Niessen H. W., Michel M. C., Paulus W. J., Stienen G. J., van der Velden J. (2010) More severe cellular phenotype in human idiopathic dilated cardiomyopathy compared to ischemic heart disease. J. Muscle Res. Cell Motil. 31, 289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bristow M. R., Sandoval A. B., Gilbert E. M., Deisher T., Minobe W., Rasmussen R. (1988) Myocardial alpha- and beta-adrenergic receptors in heart failure: is cardiac-derived norepinephrine the regulatory signal? Eur. Heart J. 9(Suppl H), 35–40. [DOI] [PubMed] [Google Scholar]
- 45.Neumann J., Eschenhagen T., Jones L. R., Linck B., Schmitz W., Scholz H., Zimmermann N. (1997) Increased expression of cardiac phosphatases in patients with end-stage heart failure. J. Mol. Cell. Cardiol. 29, 265–272 [DOI] [PubMed] [Google Scholar]
- 46.Wittköpper K., Dobrev D., Eschenhagen T., El-Armouche A. (2011) Phosphatase-1 inhibitor-1 in physiological and pathological β-adrenoceptor signalling. Cardiovasc. Res. 91, 392–401 [DOI] [PubMed] [Google Scholar]
- 47.Barefield D., Sadayappan S. (2010) Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J. Mol. Cell. Cardiol. 48, 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadayappan S., Gulick J., Osinska H., Martin L. A., Hahn H. S., Dorn G. W. II, Klevitsky R., Seidman C. E., Seidman J. G., Robbins J. (2005) Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ. Res. 97, 1156–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadayappan S., Gulick J., Klevitsky R., Lorenz J. N., Sargent M., Molkentin J. D., Robbins J. (2009) Cardiac myosin binding protein-C phosphorylation in a β-myosin heavy chain background. Circulation 119, 1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong C. W., Stelzer J. E., Greaser M. L., Powers P. A., Moss R. L. (2008) Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ. Res. 103, 974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadayappan S., Osinska H., Klevitsky R., Lorenz J. N., Sargent M., Molkentin J. D., Seidman C. E., Seidman J. G., Robbins J. (2006) Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc. Natl. Acad. Sci. USA 103, 16918–16923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Dijk S. J., Paalberends E. R., Najafi A., Michels M., Sadayappan S., Carrier L., Boontje N. M., Kuster D. W., van Slegtenhorst M., Dooijes D., dos Remedios C., ten Cate F. J., Stienen G. J., van der Velden J. (2012) Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ. Heart Fail. 5, 36–46 [DOI] [PubMed] [Google Scholar]
- 53.Janssen-Heininger Y. M., Nolin J. D., Hoffman S. M., van der Velden J. L., Tully J. E., Lahue K. G., Abdalla S. T., Chapman D. G., Reynaert N. L., van der Vliet A., Anathy V. (2013) Emerging mechanisms of glutathione-dependent chemistry in biology and disease. J. Cell. Biochem. 114, 1962–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.