Abstract
Dry eye disorders, including Sjögren’s syndrome, constitute a common problem in the aging population, with limited effective therapeutic options available. The cAMP-activated Cl− channel cystic fibrosis transmembrane conductance regulator (CFTR) is a major prosecretory channel at the ocular surface. We investigated whether compounds that target CFTR can correct the abnormal tear film in dry eye. Small-molecule activators of human wild-type CFTR identified by high-throughput screening were evaluated in cell culture and in vivo assays, to select compounds that stimulate Cl−-driven fluid secretion across the ocular surface in mice. An aminophenyl-1,3,5-triazine, CFTRact-K089, fully activated CFTR in cell cultures with EC50 ∼250 nM and produced an ∼8.5 mV hyperpolarization in ocular surface potential difference. When delivered topically, CFTRact-K089 doubled basal tear volume for 4 h and had no effect in CF mice. CFTRact-K089 showed sustained tear film bioavailability without detectable systemic absorption. In a mouse model of aqueous-deficient dry eye produced by lacrimal ablation, topical administration of 0.1 nmol CFTRact-K089 3 times daily restored tear volume to basal levels, preventing corneal epithelial disruption when initiated at the time of surgery and reversing it when started after development of dry eye. Our results support the potential utility of CFTR-targeted activators as a novel prosecretory treatment for dry eye.—Flores, A. M., Casey, S. D., Felix, C. M., Phuan, P. W., Verkman, A. S., Levin, M. H. Small-molecule CFTR activators increase tear secretion and prevent experimental dry eye disease.
Keywords: ocular surface, chloride channels, cornea, conjunctiva
Dry eye is a heterogeneous group of disorders with common features of reduced tear volume and tear fluid hyperosmolarity, which lead to inflammation at the ocular surface. The clinical consequences, which include eye discomfort and visual disturbance, represent a major public health concern in an aging population. Dry eye affects up to one-third of the global population (1), including 5 million Americans age 50 and over (2, 3). The economic burden of dry eye is substantial, with direct annual health care costs estimated at $3.84 billion in the United States (4).
Several dry eye treatment strategies have been evaluated in preclinical and human studies, including those targeting ocular surface inflammation, cellular desiccation, mitochondrial oxidation, tear/mucin secretion, and Meibomian gland dysfunction (5). However, despite considerable development efforts in dry eye therapeutics, topical cyclosporine (an immunosuppressant) remains the only U.S. Food and Drug Administration-approved drug for dry eye. Another topical anti-inflammatory drug, SAR 1118 (lifitegrast), is in late-stage development (6). In one study, 94% percent of surveyed ophthalmologists believed that additional treatments are needed for moderate-to-severe dry eye (7).
The ocular surface is a collection of anatomically continuous epithelial and glandular tissues that are functionally linked to maintain the tear film (8). Although lacrimation contributes the bulk of reflex tearing, animal studies implicate involvement of the cornea and conjunctiva in regulation of tear volume and composition by solute and water transport (9–11). The principal determinants of water movement across the ocular surface into the tear film include apical chloride (Cl−) secretion through cAMP- and calcium (Ca2+)-dependent Cl− transporters, and sodium (Na+) absorption, largely through the epithelial Na+ channel (ENaC).
With regard to prosecretory candidates for dry eye therapy, an ENaC inhibitor, P321, has recently entered phase 1/2 studies (12). Diquafosol, a uridine triphosphate analog that targets surface epithelial P2Y2 receptors and stimulates Cl− and mucin secretion by Ca2+ signaling (13), is approved for dry eye in Japan (14, 15), but failed phase 3 trials in the United States because of lack of efficacy (16).
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-activated Cl− channel expressed in some secretory epithelial cells, including those in cornea and conjunctiva (9, 17, 18). Basal CFTR activity at the ocular surface is probably minimal, as only mild tear film abnormalities are seen in patients with cystic fibrosis (CF) who have loss-of-function mutations in CFTR (19–22). However, we found substantial capacity for CFTR-facilitated Cl− transport at the ocular surface in mice (11, 23), as subsequently shown in rat conjunctiva (24), providing a rational basis for investigation of CFTR activators as a prosecretory strategy for dry eye. The only clinically approved CFTR activator, VX-770 (ivacaftor), is indicated for potentiating the channel gating of certain CFTR mutants causing CF, but only weakly activates wild-type (WT) CFTR (25, 26).
We evaluated and prioritized novel small-molecule activators of WT CFTR identified by high-throughput screening as potential topical therapy for dry eye, with the research strategy summarized in Fig. 1. The goal was to improve on our previously identified CFTR activators (27), which lack suitable potency and chemical properties to be advanced to clinical development, and to demonstrate the efficacy of newly identified CFTR activators in a mouse model of dry eye.
Figure 1.
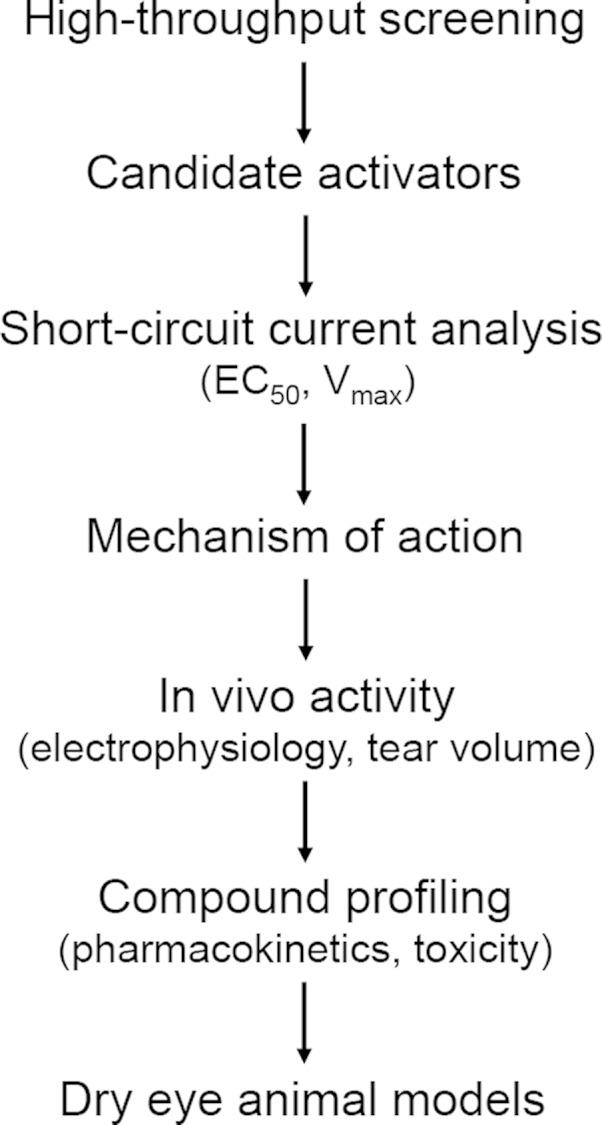
Strategy for preclinical development of CFTR activators for dry eye therapy. Human WT CFTR activators identified by high-throughput screening are confirmed and characterized by electrophysiological and biochemical assays and then tested in live mice for activity at the ocular surface by measurements of potential difference and tear fluid volume. The best compounds are then tested for pharmacokinetic properties and efficacy in a dry eye mouse model.
MATERIALS AND METHODS
Mice
Wild-type (WT) and CF (homozygous ΔF508-CFTR mutant) mice on a CD1 genetic background were bred at the University of California, San Francisco (UCSF) Animal Facility. Mice aged 8–12 wk (25–35 g) were used. Female BALB/c mice (7–8 wk old) were purchased from the Harlan Laboratory (Livermore, CA, USA). Animal protocols were approved by the UCSF Institutional Animal Care and Use Committee and were in compliance with the Association for Research in Vision and Opthamology Statement for the Use of Animals in Ophthalmic and Vision Research.
Short-circuit current
Fischer rat thyroid (FRT) cells stably expressing WT human CFTR were cultured on Snapwell inserts (Corning Costar, Corning NY, USA) for short-circuit current (Isc) measurements. After 6–9 d in culture, when the transepithelial resistance was >1000 Ω/cm2, the inserts were mounted in an Ussing chamber system (World Precision Instruments, Sarasota, FL, USA). The basolateral solution contained 130 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, and 10 mM Na-HEPES (pH 7.3). In the apical bathing solution, 65 mM NaCl was replaced by Na-gluconate, and CaCl2 was increased to 2 mM. Both solutions were bubbled with air and maintained at 37°C. The basolateral membrane was permeabilized with 250 μg/ml amphotericin B (27, 28). Hemichambers were connected to a DVC-1000 voltage clamp via Ag/AgCl electrodes and 3 M KCl agar bridges for Isc recording.
cAMP and cytotoxicity assays
Intracellular cAMP activity was measured in a GloSensor luminescence assay (Promega Corp., Madison, WI, USA). FRT cells stably transfected with the pGloSensor cAMP plasmid (Promega Corp., Madison, WI, USA) were cultured in white 96-well microplates (Corning Costar) overnight. The cells were then washed 3 times with PBS and incubated with 5 μM test compound for 10 min in the absence and presence of 100 nM forskolin (FSK). To assay cytotoxicity, FRT cells were cultured overnight in black 96-well Costar microplates and incubated with test compounds at up to 100 µM (the maximum solubility in PBS) for 1 or 24 h. Cytotoxicity was measured by Alamar Blue assay, according to the manufacturer’s instructions (Thermo Scientific-Invitrogen, Carlsbad, CA, USA).
Ocular surface potential difference measurements
Open-circuit transepithelial potential differences (PDs) were measured continuously in anesthetized mice in response to serial perfusions of different solutions over the ocular surface (11). Mice were anesthetized with Avertin (2,2,2-tribromoethanol, 125 mg/kg intraperitoneal; Sigma-Aldrich, St. Louis, MO, USA), and core temperature was maintained at 37°C with a heating pad. The eyes were oriented with the cornea and conjunctiva facing upward and exposed by retracting the eyelid with cross-action forceps. Solutions were isosmolar (320 ± 10 mOsM; compositions provided in ref. 11) and contained 10 µM indomethacin to prevent CFTR activation by prostaglandins. The ocular surface was perfused at 6 ml/min through plastic tubing with a multireservoir gravity pinch-valve system (ALA Scientific, Westbury, NY, USA) and variable-flow peristaltic pump (medium flow model; Thermo Fisher Scientific, Fair Lawn, NJ, USA). A probe catheter was fixed 1 mm above the cornea with a micropositioner, and a suction cannula was positioned 3 mm from the orbit. The measuring electrode was in contact with the perfusion catheter and connected to a high-impedance voltmeter (IsoMilivolt Meter; World Precision Instruments). The reference electrode was grounded via a winged 21-gauge needle filled with isosmolar saline, and inserted subcutaneously in the abdomen. Measuring and reference electrodes consisted of Ag/AgCl with 3 M KCl agar bridges.
Tear volume
To measure unstimulated tear production, phenol red threads (Zone-Quick; Oasis Medical, Glendora, CA, USA) were placed for 10 s in the lateral canthi of isoflurane-anesthetized mice using jewelers’ forceps. Tear volume was measured as the length of thread wetting, as visualized under a dissecting microscope. Serial measurements were used to evaluate compound pharmacodynamics after application of 2 μl drops of compound formulations (50–100 μM compound in PBS containing 0.5% polysorbate and 0.5% DMSO) compared with those induced by vehicle.
Lissamine green staining
To assess corneal epithelial disruption, 5 µL of lissamine green (LG) dye (1%) was applied to the ocular surface of isoflurane-anesthetized mice. Photographs of the eye were taken with a digital camera (Nikon, Melville, NY, USA) adapted to a zoom stereo microscope (Olympus, Center Valley, PA, USA). Each corneal quadrant was scored on a 3-point scale by 1 blinded, trained observer, with the extent of staining in each quadrant classified as: grade 0, no staining; grade 1, sporadic staining (involving <25% of the total surface); grade 2, diffuse punctate staining (25–75%); and grade 3, coalesced punctate staining (≥75%). The total grade is reported as the sum of scores from all 4 quadrants, ranging from 0 to 12.
Pharmacokinetics and tissue distribution
To determine the residence time of CFTR activators in the preocular mouse tear film, compounds were recovered for liquid chromatography/mass spectroscopy (LC/MS) after single-dose ophthalmic delivery. Three eye washes (3 μL PBS each) were recovered from the lateral and medial canthi with 5 μl microcapillary tubes (Drummond Scientific Co., Broomhall, PA, USA) after manual eyelid blinking (12). Pooled washes were diluted with acetonitrile/water (1:1) containing 0.1% formic acid and analyzed by LC/MS using an Xterra MS C18 column (2.1 mm × 100 mm, 3.5 μm particle size) connected to a 2695 HPLC solvent delivery system and a Micromass ZQ mass spectrometer (both from Waters, Milford, MA, USA) with positive electrospray ionization.
To study compound accumulation in systemic tissues, mouse blood, brain, kidney, and liver were analyzed after 14 d of 3-times daily topical application (doses: 0.1 nmol, 2 µl, 50 µM). Blood samples were collected from the left ventricle into K3 EDTA minitubes (Greiner, Kremsmünster, Austria) and centrifuged (29). The supernatant was extracted with an equal volume of ethyl acetate, and the extract was dried with an air stream. Organs from treated and control mice were removed after ventricular perfusion with heparinized PBS (10 U/ml), weighed, mixed with acetic acid and water (100 μl/g tissue), and homogenized (30). Ethyl acetate (10 ml/g tissue) was added, samples were vortexed and centrifuged (3000 rpm for 15 min), and the ethyl acetate-containing supernatant was evaporated. Residues obtained from organic extracts of serum and organ homogenates were then reconstituted and analyzed by LC/MS, as described above.
Mouse model of dry eye produced by lacrimal ablation
Aqueous-deficient dry eye was achieved by lacrimal duct cautery (LDC), with or without lacrimal gland excision (LGE) (31). The extraorbital lacrimal gland was exposed on each side of WT female BALB/c mice (7–8 wk of age) by 3 mm linear skin incisions. The duct on each side was ablated with a high-temperature hand-held cautery pen, avoiding facial vessels and nerves. In some experiments the entire extraorbital gland was also removed. Comparative experiments indicated that LDC alone produced the same effect on tearing and LG staining as produced by LDC/LGE. Incisions were each closed with a single, interrupted 6-0 silk suture. Intraorbital lacrimal tissue remained intact. Eyes with reduced corneal sensation (<5% of mice studied), identified from neurotrophic corneal ulcers appearing within 1 d of surgery, were excluded. Mice were randomized to receive treatment (in both eyes) with either CFTRact-K089 (0.1 nmol) or vehicle. They were treated 3 times/d (8 am, 2 pm, and 8 pm) starting on d 1 or 5 after ablation surgery. Tear volume and LG staining were performed immediately before LGD/LGE and 1 h after the initial dose on d 4, 10, and 14.
Conjunctival goblet cell density
In some dry eye treatment experiments, the globes were freshly dissected on d 15, with the eyelids attached and the conjunctiva intact. Ocular tissues were paraffin embedded and 5 μm sections oriented through the central cornea, posterior pole, and superior and inferior fornices/eyelids were cut, stained with periodic acid-Schiff (PAS), and photographed. The number of PAS-positive goblet cells in the most densely populated 100 μm linear region of intact forniceal conjunctiva (inferior or superior, depending on quality of the section) were counted with ImageJ software (National Institutes of Health, Bethesda, MD, USA) by an observer masked to treatment group. No significant difference in the linear density of goblet cells was found when quantifying a single section versus averaging counts from 3 separate sections.
Statistics
Data are expressed as means ± sem. For direct comparisons between 2 means, the 2-sided Students’ t test was used. For longitudinal measurements of tear volume and LG scores in the dry eye treatment studies, a linear mixed-effects regression was used, adjusting for nonindependence of measurements taken on the same eye and on both eyes of the same animal. Analysis was conducted in R, ver.3.2 for Mac (R Foundation for Statistical Computing, Vienna, Austria), using packages lme4 and robustlmm.
RESULTS
Characterization of small-molecule CFTR activators
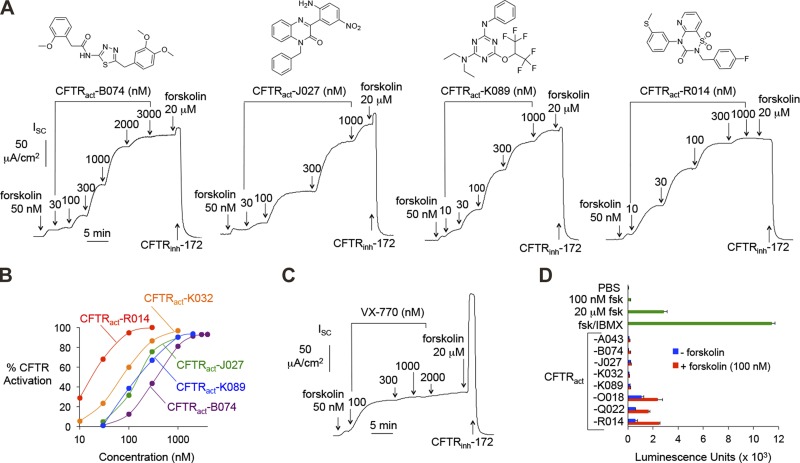
A cell-based functional high-throughput screen of 120,000 compounds at 10 µM identified 20 chemical classes of small-molecule activators of WT CFTR that produced >95% of maximum CFTR activation. The screening was performed in FRT epithelial cells coexpressing human WT CFTR and a cytoplasmic yellow fluorescent protein halide sensor in a 96-well format (27, 32, 33). Additional details of the primary screening will be reported separately. Secondary screening involved Isc measurement in CFTR-expressing FRT cells pretreated with submaximal FSK (50 nM). Twenty-one compounds from 8 chemical classes produced large increases in Isc at 1 µM (>75% of maximum current produced by 20 µM FSK). A summary of EC50 and maximum velocity (Vmax) values for each compound is provided in Supplemental Table 1.
Structures of activators from the 4 most active chemical classes are shown in Fig. 2A, along with corresponding concentration-dependence data from Isc measurements. Each compound fully activated CFTR, as a high concentration of FSK produced little further increase in Isc, and the increase in Isc was fully inhibited by a CFTR inhibitor, CFTRinh-172. EC50 values ranged from 20 to 350 nM (Fig. 2B). VX-770 showed relatively weak activity against WT CFTR (Fig. 2C). CFTRact-K032 and -K089 are structurally similar to CFTRact-11, a compound identified in a prior screen for CFTR activators (26) that had lower potency and showed incomplete CFTR activation (∼50% Vmax).
Figure 2.
In vitro characterization of CFTR activators. A) Top: chemical structures. Bottom: representative Isc measured in FRT cells expressing WT CFTR. CFTR current was stimulated by test compounds and FSK and inhibited by CFTRinh-172 (10 μM). B) Concentration-dependence of CFTR activators. Each data set derived from a single dose–response experiment as in (A) and fitted to an exponential curve. One-hundred percent CFTR activation is defined as that produced by 20 μM FSK. C) Isc measurement for VX-770, as in (A). D) Cellular cAMP concentration in FRT cells in response to incubation for 10 min with 5 µM test compounds without or with FSK (100 nM). Positive controls included FSK (100 nM and 20 μM) and FSK+IBMX (100 μM) (n = 4–8). Means ± sem.
Compounds that directly activate CFTR without causing elevation of cellular cAMP were sought to minimize potential off-target effects (Fig. 2D). Compounds producing elevations in intracellular cAMP (from classes O, Q, and R), probably by phosphodiesterase inhibition, were excluded from further consideration. Nanomolar-potency compounds from classes B, J, and K, which did not increase cAMP, were selected for further characterization in living mice.
CFTR activators increase ocular surface chloride secretion and tear volume
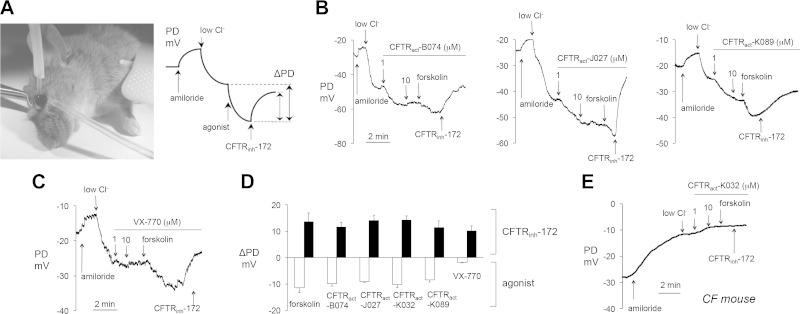
An open-circuit PD method developed in our lab was used to evaluate compound activity at the ocular surface in vivo, as depicted in Fig. 3A (11). Cl− channel function was quantified by measuring PD during continuous perfusion of the ocular surface with a series of solutions that imposed a transepithelial Cl− gradient and contained various channel agonists, inhibitors, or both. The ocular surface was first perfused with isosmolar saline to record the baseline PD. Amiloride was then added to the perfusate, followed by exchange to a low Cl− solution in which most Cl− was replaced with an impermeant anion, gluconate. These maneuvers enabled direct visualization of CFTR activation upon addition of candidate CFTR activators.
Figure 3.
PD measurements of CFTR activators at the ocular surface in live mice. A) Left: photograph of an anesthetized mouse demonstrating ocular surface perfusion for PD measurement. The perfusion catheter, attached to the measuring electrode, is oriented perpendicular to the ocular surface. Cross-clamping forceps retract the upper eyelid to expose cornea and bulbar/palpebral conjunctiva for perfusion. The reference electrode is grounded via subcutaneous butterfly needle. Right: Idealized PD tracing for a typical experiment testing CFTR activity. B) Representative ocular surface PD measurements in WT mice. (Solution compositions are detailed in ref. 11). Concentrations: amiloride, 100 μM; FSK and CFTRinh-172, 10 μM; test compounds, 1–10 μM, as indicated. C) Study as in B, but with VX-770, 1–10 μM, as indicated. D) Summary of ΔPD in WT mice produced by FSK (20 μM), or test compounds or VX-770 (each 1 μM). PDs were recorded in the presence of 100 μM amiloride and an outward apical Cl− gradient. Means ± sem (n = 8–20 eyes per agonist tested). E) Representative ocular surface PD measurements in CF mouse. Study as in (B, C) CFTRact-K032 (1–10 μM, as indicated).
Large hyperpolarizations were observed after exposure to CFTRact-B074, -J027, and -K089, which were increased relatively little by FSK and were reversed by CFTRinh-172 (Fig. 3B). In comparison, VX-770 produced minimal changes in ocular surface PD (Fig. 3C). Figure 3D summarizes PD data for indicated activators, with data for additional compounds reported in Supplemental Table 1. Control studies conducted in CF mice lacking functional CFTR showed no changes in PD after addition of each of the compounds tested, with a representative curve shown for CFTRact-K032 (Fig. 3E).
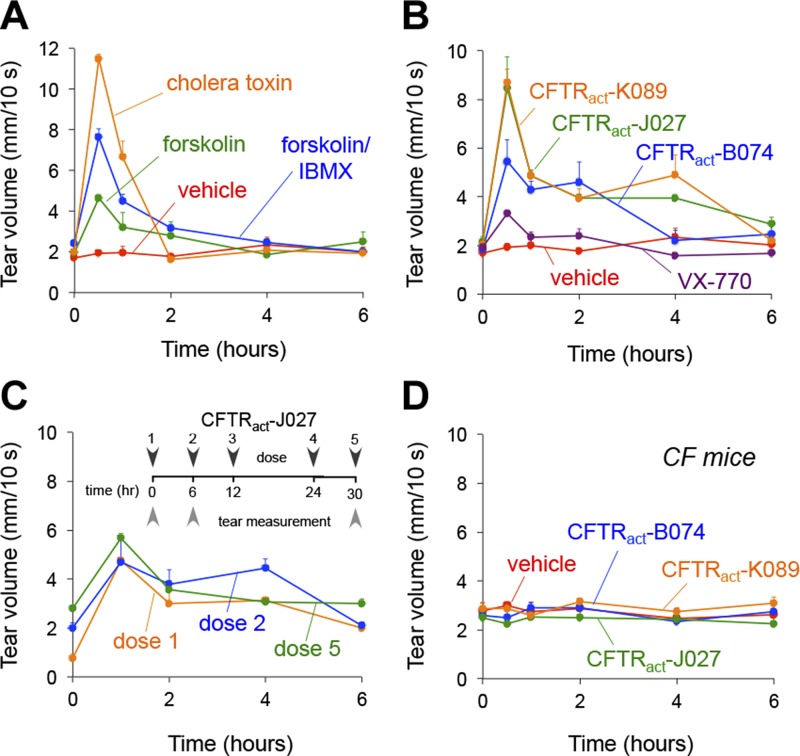
CFTR activators were next tested for their efficacy in augmenting tear volume in mice. Preliminary experiments identified a standard ophthalmic formulation (0.5% polysorbate) that increased compound solubility and duration of action. After a single topical dose, the indirect CFTR activators cholera toxin, FSK, and 3-isobutyl-1-methylxanthine (IBMX) substantially increased basal tear volume at 30 min, but these effects were transient and undetectable after 2 h (Fig. 4A). By comparison, the direct CFTR activators identified herein, CFTRact-B074, -J027, and -K089, increased tear fluid volume by ∼2-fold for at least 4 h. VX-770 produced little increase in tear volume (Fig. 4B). Repeated topical administrations (3 times daily for up to 2 wk) augmented tear volume in a sustained fashion without tachyphylaxis (Fig. 4C). CFTR activators did not increase tear volume in CF mice, demonstrating selective CFTR targeting (Fig. 4D).
Figure 4.
Tear fluid volume measurement of CFTR activators in living mice. A) Tear volume was measured just before and at the indicated times after single-dose topical application of vehicle (PBS, 0.5% polysorbate, 0.5% DMSO), cholera toxin (0.1 μg/ml), FSK (20 μM), or FSK+IBMX (250 μM). The effect of cholera toxin was measured after preanesthetizing the ocular surface with 4% lidocaine to suppress irritation and reflex tear secretion (n = 6–10 eyes per condition). Means ± sem. B) Time course of tear volume after topical delivery of the indicated compound. Concentrations: CFTRact-B074, 100 μM; CFTRact-J027, 50 μM; CFTRact-K089, 50 μM; and VX-770, 10 μM (n = 6–18 eyes). Mean ± sem. C) Effect of repeated application. CFTRact-J027 (0.1 nmol) was topically applied 3 times/d for 2 d. Tear volume measurements were obtained after doses 1 and 2 on d 1 and dose 5 on d 2 (n = 6 eyes). Means ± sem. D) Lack of effect of CFTR activators on tear volume in CF mice, with compounds tested at the same concentrations as in (B).
Toxicity and pharmacokinetics
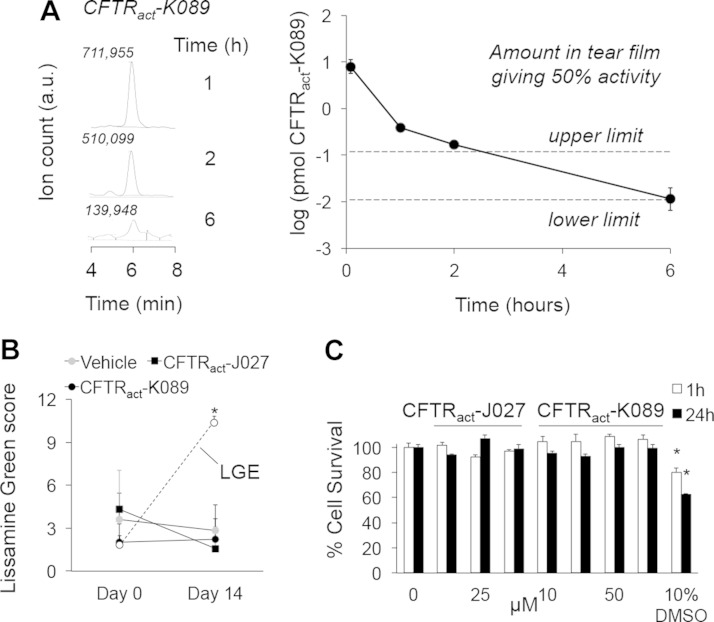
Tear collection methods were validated by demonstrating reproducible recovery of tetramethylrhodamine dextran (3 kDa) from the ocular surface up to 6 h after instillation. The pharmacokinetics of CFTRact-K089 at the ocular surface was determined by LC/MS of recovered tear washes. After instillation of 0.1 nmol of CFTRact-K089 (2 µL, 50 µM) on the ocular surface, 7.9 ± 2.4 and 0.011 ± 0.004 pmol were recovered at 5 min and 6 h, respectively (Fig. 5A). The amount of CFTRact-K089 required for 50% CFTR activation (EC50 ∼250 nM) lies between the dashed lines, reflecting concentrations calculated from the highest and lowest reported normal tear volumes in mice (34, 35). The quantity of CFTRact-K089 recovered from tear fluid predicts therapeutic levels for at least 6 h. Tear film pharmacokinetics of CFTRact-J027 could not be measured because of the lower LC/MS sensitivity for this compound.
Figure 5.
Compound pharmacology. A) LC/MS determination of CFTRact-K089 amount in tear fluid at indicated times after single-dose (0.1 nmol) administration. Representative background-subtracted peak areas from tear washes (left) and corresponding amounts recovered (right) (n = 4 eyes/time point). Means ± sem. Dashed lines: highest and lowest calculated quantities of CFTRact-K089 necessary to achieve EC50 concentration. B) Lissamine green staining of corneas in BALB/c mice, measured on a 12-point scale after 14 d of 3 times/d treatment with CFTR activators (0.1 nmol) or vehicle (n = 6 eyes per group). Means ± sem. Shown as a positive control are scores from vehicle-treated mice after LGE on d 0 (n = 11 eyes). *P < 0.001 compared with other groups. C) Cytotoxicity measured by Alamar Blue assay in FRT cells incubated with test compounds for 1 or 24 h (10% DMSO as positive control (n = 4). Means ± SEM. *P < 0.05 compared to untreated cells; P = 0.02 and 0.0006 for 1 and 24 h, respectively.
After 2 wk of 3 doses/d, the amounts of CFTRact-K089 and -J027 were below the limits of detection (∼10 and ∼700 fmol, respectively) in mouse blood, brain, liver, and kidney, indicating minimal systemic accumulation. The mice that underwent prolonged treatment showed no signs of ocular toxicity, as assessed by slit-lamp evaluation for conjunctival hyperemia, anterior chamber inflammation, and lens clarity. LG staining showed no corneal or conjunctival epithelial disruption (Fig. 5B). Also, there was no apparent acute ocular irritation, as evidenced by a lack of excessive blinking or altered behavior after administration. In cell cultures in vitro, the compounds showed no significant cytotoxicity at concentrations up to 100 µM (Fig. 5C).
CFTR activator prevents and reverses dry eye in lacrimal ablation mouse models
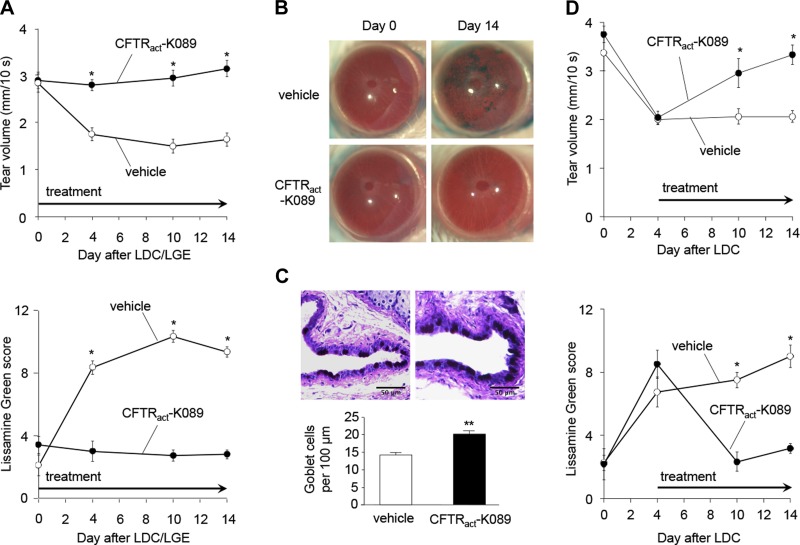
On the basis of its favorable tear film pharmacokinetics and structural characteristics, CFTRact-K089 was selected for testing in a mouse model of aqueous-deficient dry eye produced by lacrimal ablation. After LDC and extraorbital LGE in BALB/c mice, CFTRact-K089-treated mice (0.1 nmol, administered 3 ×/d) maintained basal tear volume, whereas the tear volume of vehicle-treated mice was significantly reduced at all subsequent time points, and for at least 30 d. Similar to what was reported in C57BL/6 mice (31), decreased lacrimation in vehicle-treated BALB/c mice was associated with progressive epithelial disruption from d 0 to 14 (Fig. 6A, B). CFTRact-K089 restored tear volume and prevented ocular surface epithelial disruption at all time points (Fig. 6A, B). Vehicle-treated eyes developed diffuse, progressive corneal epitheliopathy (LG score increase of 7.3 ± 0.6 by d 14), whereas eyes treated with CFTRact-K089 had minimal LG staining at all time points (LG score change, −0.6 ± 0.6). After this 2-wk period, CFTRact-K089-treated eyes maintained a significantly higher density of conjunctival goblet cells (19.9 ± 1.5 cells/100 μm) compared with vehicle-treated eyes (14.8 ± 10.8 cells/100 μm; Fig. 6C). To evaluate the efficacy of CFTRact-K089 to reverse already-established dry eye, therapy was started on d 5 after LDC, when ocular surface lissamine staining was near its maximum. CFTRact-K089 treatment, started at this later time point, reversed clinical signs of experimental dry eye (Fig. 6D).
Figure 6.
Topical CFTRact-K089 restores tear volume, preventing and reversing corneal epithelial disruption after lacrimal ablation. A) Left: basal tear volume after extraorbital LDC and LGE in BALB/c mice, comparing eyes treated with 0.1 nmol CFTRact-K089 (n = 15 eyes) to vehicle (n = 11 eyes). Means ± sem. Tear volume was measured immediately before LDC/LGE, and then 1 h after the first daily dose on d 4, 10, and 14 after LGE. *P < 0.001. Right: corneal epithelial disruption after LDC/LGE measured by LG scoring on a 12-point scale in the same eyes. Means ± sem. *P < 0.001. B) Representative photographs of eyes before LDC/LGE (left) and on d 14 after LDC/LGE in vehicle-treated and CFTRact-K089-treated eyes (right). C) Top: PAS staining in conjunctival fornices of vehicle-treated (left) and CFTRact-K089-treated mice (right). Bottom: mean linear conjunctival goblet cell densities comparing mice treated with CFTRact-K089 (n = 11 eyes) or vehicle (n = 10 eyes) for 14 d after LDC/LGE. Mean ± sem. **P < 0.01. D) Left: basal tear volume following LDC with CFTRact-K089 (0.1 nmol; n = 6 eyes) or vehicle (n = 4 eyes) starting at d 5 after LDC. Means ± sem. *P < 0.001. Right: Corneal epithelial disruption after LDC in the same eyes. Means ± sem. *P < 0.001.
DISCUSSION
The goal of this study was to investigate the potential utility of small-molecule activators of CFTR for dry eye therapy. After several prior development failures, dry eye remains an unmet need in ocular disease. Our previous experimental data and computational modeling predicted that CFTR-targeted prosecretory compounds could normalize tear film volume and ocular surface properties in dry eye (11, 23). In dry eye disorders, tear film hyperosmolarity stimulates proinflammatory signaling, secretion of cytokines and metalloproteinases, and disruption of corneal epithelial cell integrity (36–39). By minimizing tear film hyperosmolarity, CFTR activation is predicted to prevent these downstream ocular surface changes.
High-throughput screening identified small-molecule CFTR activators that produced sustained Cl−-driven aqueous fluid secretion across the ocular surface by a mechanism involving direct CFTR activation rather than upstream cAMP signaling. The rationale to choose compounds that activate CFTR directly was to minimize potential off-target effects of generalized cAMP stimulation and to reduce the likelihood of tachyphylaxis for compounds that target signaling receptors. These compounds had a low-nanomolar EC50 for activation of human CFTR in vitro and produced full activation at higher concentrations. Large CFTR-dependent PD hyperpolarizations and tear hypersecretion were demonstrated in mice. Substantial compound activities in both mice and humans will facilitate translation of data to humans.
We found that CFTRact-K089 restored tear volume and prevented corneal epithelial disruption in an experimental mouse model of lacrimal insufficiency. CFTRact-K089 also prevented conjunctival goblet cell loss, which is a feature of dry eye in humans and various mouse models (40–42). Topical CFTRact-K089 was effective in reversing clinical signs of dry eye when initiated after onset of disease. CFTR activators may be particularly suited for disorders of the lacrimal gland, such as primary Sjögren’s syndrome, by stimulating fluid transport across the intact corneal and conjunctival epithelia. CFTR activators probably exert their major prosecretory effect at the ocular surface, although there is indirect evidence for CFTR expression and function in lacrimal gland (43–46). We cannot rule out direct stimulation of lacrimal (or Harderian) secretion by CFTR activators because of possible limited penetration of topically delivered compound to intraorbital glandular tissues. At the ocular surface, the conjunctiva probably contributes the bulk of fluid secretion, given its much larger surface area compared to the cornea (47). Additional mechanistic studies are needed to confirm the relative tissue contributions (corneal vs. conjunctival vs. lacrimal) to CFTR-dependent tear secretion.
Alternative prosecretory therapies targeting different ocular surface ion channels have been considered. The only U.S. Food and Drug Administration -approved CFTR activator, VX-770, was developed as a potentiator to treat CF by correcting the channel gating of certain CFTR mutations (48). However, in agreement with prior studies (25, 26), VX-770 showed relatively little activity against WT CFTR in cell cultures and in mice. Chronic application of VX-770 may also diminish CFTR functional expression (25) and cause cataracts [seen in juvenile rats (46)], which is likely an off-target effect, because CFTR is not expressed in lens.
An indirect agonist of Ca2+-activated Cl− channels, diquafosol, augments both aqueous and mucin secretion. Given the importance of mucin for tear film adherence, this approach has a theoretical advantage over CFTR activation, which selectively restores the aqueous tear component. However, diquafosol failed phase 3 trials, most likely because of transient induced Ca2+ elevation and Cl− channel activation, producing minimal net fluid secretion. CFTR activators, which produce sustained tear secretion, overcome this limitation. CFTRact-K089 and -J027 showed favorable pharmacodynamics and could be conveniently administered topically several times per day in a standard ophthalmic formulation. More extensive structure–activity analysis may further improve compound potency and pharmacodynamics.
Another theoretical limitation of a CFTR-targeted approach to drive net fluid secretion is that CFTR-dependent apical Cl− secretion requires activity of a basolateral membrane potassium (K+) conductance (23, 49–51). Our data show that CFTR activation alone facilitates sustained outward Cl− flux and fluid secretion, suggesting that basal K+ conductance, without augmented cyclic nucleotide or Ca2+ signaling, is sufficient to support ocular surface fluid transport. Still, the potential synergy of a CFTR agonist and a K+ channel activator or an ENaC inhibitor could be explored to further increase tear secretion for dry eye therapy.
In conclusion, the efficacy of CFTRact-K089 in a clinically relevant mouse model of aqueous-deficient dry eye disease provides proof of principle for topical, prosecretory CFTR activator therapy to restore basal tear volume and prevent ocular surface pathology. Compared with immunosuppressive approaches, CFTR activation has the advantage of addressing an early event in dry eye pathogenesis. Our data thus support the development potential of CFTR activators as first-in-class dry eye therapy.
Supplementary Material
Acknowledgments
The authors thank Dr. N. A. McNamara, Dr. D. N. Stephens, and F.Y.T. Chen [Francis I. Proctor Foundation, UCSF (N.A.M., D.N.S., and F.Y.T.C.); and Departments of Anatomy and Ophthalmology, UCSF, and University of California, Berkeley School of Optometry and Vision Science Graduate Group (N.A.M.)] for expert advice in evaluation of rodent dry eye models and Dr. T. Porco (Frances I. Proctor Foundation and Departments of Ophthalmology and Epidemiology & Biostatistics, UCSF) for statistical assistance. This project was supported by U.S. National Institutes of Health (NIH)/National Center for Advancing Translational Sciences–UCSF–Clinical and Translational Science Institute Grant UL1 TR000004 (to M.H.L. and A.S.V), a Research to Prevent Blindness Career Development Award and NIH National Eye Institute Grant EY023981 (to M.H.L); NIH National Eye Institute Grant EY13574, National Institute of Biomedical Imaging and Bioengineering Grant EB00415, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK72517, DK35124, and DK101373 (to A.S.V.); and NIH National Eye Institute Grant EY002162 (to the UCSF Core Grant for Vision Research). Provisional U.S. patents have been filed on the use of the CFTR activators reported here for dry eye therapy and alternative applications. The authors declare no additional conflicts of interest.
Glossary
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- ENaC
epithelial sodium channel
- FRT
Fischer rat thyroid
- FSK
forskolin
- IBMX
3-isobutyl-1-methylxanthine
- ISC
short-circuit current
- LC/MS
liquid chromatography/mass spectroscopy
- LDC
lacrimal duct cautery
- LG
lissamine green
- LGE
lacrimal gland excision
- PAS
periodic acid-Schiff
- PD
potential difference
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Dry Eye WorkShop (DEWS) Definition and Classification Subcommittee (2007) The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop 2007 Ocul. Surf. 5, 75–92 [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg D. A., Dana R., Buring J. E., Sullivan D. A. (2009) Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 127, 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaumberg D. A., Sullivan D. A., Buring J. E., Dana M. R. (2003) Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 136, 318–326 [DOI] [PubMed] [Google Scholar]
- 4.Yu J., Asche C. V., Fairchild C. J. (2011) The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 30, 379–387 [DOI] [PubMed] [Google Scholar]
- 5.Alves M., Fonseca E. C., Alves M. F., Malki L. T., Arruda G. V., Reinach P. S., Rocha E. M. (2013) Dry eye disease treatment: a systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul. Surf. 11, 181–192 [DOI] [PubMed] [Google Scholar]
- 6.OPUS-1 Study Group (2014) Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology 121, 475–483 [DOI] [PubMed] [Google Scholar]
- 7.Asbell P. A., Spiegel S. (2010) Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey. Eye Contact Lens 36, 33–38 [DOI] [PubMed] [Google Scholar]
- 8.Dry Eye WorkShop (DEWS) Epidemiology Subcommittee (2007) The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop 2007. Ocul. Surf. 5, 93–107 [DOI] [PubMed] [Google Scholar]
- 9.Shiue M. H., Gukasyan H. J., Kim K. J., Loo D. D., Lee V. H. (2002) Characterization of cyclic AMP-regulated chloride conductance in the pigmented rabbit conjunctival epithelial cells. Can. J. Physiol. Pharmacol. 80, 533–540 [DOI] [PubMed] [Google Scholar]
- 10.Levin M. H., Verkman A. S. (2004) Aquaporin-dependent water permeation at the mouse ocular surface: in vivo microfluorimetric measurements in cornea and conjunctiva. Invest. Ophthalmol. Vis. Sci. 45, 4423–4432 [DOI] [PubMed] [Google Scholar]
- 11.Levin M. H., Verkman A. S. (2005) CFTR-regulated chloride transport at the ocular surface in living mice measured by potential differences. Invest. Ophthalmol. Vis. Sci. 46, 1428–1434 [DOI] [PubMed] [Google Scholar]
- 12.Thelin W. R., Johnson M. R., Hirsh A. J., Kublin C. L., Zoukhri D. (2012) Effect of topically applied epithelial sodium channel inhibitors on tear production in normal mice and in mice with induced aqueous tear deficiency. J. Ocul. Pharmacol. Ther. 28, 433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols K. K., Yerxa B., Kellerman D. J. (2004) Diquafosol tetrasodium: a novel dry eye therapy. Expert Opin. Investig. Drugs 13, 47–54 [DOI] [PubMed] [Google Scholar]
- 14.Koh S., Ikeda C., Takai Y., Watanabe H., Maeda N., Nishida K. (2013) Long-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eye. Jpn. J. Ophthalmol. 57, 440–446 [DOI] [PubMed] [Google Scholar]
- 15.Diquafosol Ophthalmic Solution Phase 3 Study Group (2012) A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br. J. Ophthalmol. 96, 1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tauber J., Davitt W. F., Bokosky J. E., Nichols K. K., Yerxa B. R., Schaberg A. E., LaVange L. M., Mills-Wilson M. C., Kellerman D. J. (2004) Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea 23, 784–792 [DOI] [PubMed] [Google Scholar]
- 17.Al-Nakkash L., Reinach P. S. (2001) Activation of a CFTR-mediated chloride current in a rabbit corneal epithelial cell line. Invest. Ophthalmol. Vis. Sci. 42, 2364–2370 [PubMed] [Google Scholar]
- 18.Turner H. C., Bernstein A., Candia O. A. (2002) Presence of CFTR in the conjunctival epithelium. Curr. Eye Res. 24, 182–187 [DOI] [PubMed] [Google Scholar]
- 19.Ansari E. A., Sahni K., Etherington C., Morton A., Conway S. P., Moya E., Littlewood J. M. (1999) Ocular signs and symptoms and vitamin A status in patients with cystic fibrosis treated with daily vitamin A supplements. Br. J. Ophthalmol. 83, 688–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botelho S. Y., Goldstein A. M., Rosenlund M. L. (1973) Tear sodium, potassium, chloride, and calcium at various flow rates: children with cystic fibrosis and unaffected siblings with and without corneal staining. J. Pediatr. 83, 601–606 [DOI] [PubMed] [Google Scholar]
- 21.Morkeberg J. C., Edmund C., Prause J. U., Lanng S., Koch C., Michaelsen K. F. (1995) Ocular findings in cystic fibrosis patients receiving vitamin A supplementation. Graefes Arch. Clin. Exp. Ophthalmol. 233, 709–713 [DOI] [PubMed] [Google Scholar]
- 22.Mrugacz M., Kaczmarski M., Bakunowicz-Lazarczyk A., Zelazowska B., Wysocka J., Minarowska A. (2006) IL-8 and IFN-gamma in tear fluid of patients with cystic fibrosis. J. Interferon Cytokine Res. 26, 71–75 [DOI] [PubMed] [Google Scholar]
- 23.Levin M. H., Kim J. K., Hu J., Verkman A. S. (2006) Potential difference measurements of ocular surface Na+ absorption analyzed using an electrokinetic model. Invest. Ophthalmol. Vis. Sci. 47, 306–316 [DOI] [PubMed] [Google Scholar]
- 24.Yu D., Thelin W. R., Rogers T. D., Stutts M. J., Randell S. H., Grubb B. R., Boucher R. C. (2012) Regional differences in rat conjunctival ion transport activities. Am. J. Physiol. Cell Physiol. 303, C767–C780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cholon D. M., Quinney N. L., Fulcher M. L., Esther C. R. Jr., Das J., Dokholyan N. V., Randell S. H., Boucher R. C., Gentzsch M. (2014) Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci. Transl. Med. 6, 246ra96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VX08-770-102 Study Group (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 365, 1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma T., Vetrivel L., Yang H., Pedemonte N., Zegarra-Moran O., Galietta L. J., Verkman A. S. (2002) High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J. Biol. Chem. 277, 37235–37241 [DOI] [PubMed] [Google Scholar]
- 28.Galietta L. J., Springsteel M. F., Eda M., Niedzinski E. J., By K., Haddadin M. J., Kurth M. J., Nantz M. H., Verkman A. S. (2001) Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J. Biol. Chem. 276, 19723–19728 [DOI] [PubMed] [Google Scholar]
- 29.Esteva-Font C., Cil O., Phuan P. W., Su T., Lee S., Anderson M. O., Verkman A. S. (2014) Diuresis and reduced urinary osmolality in rats produced by small-molecule UT-A-selective urea transport inhibitors. FASEB J. 28, 3878–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao C., Anderson M. O., Zhang J., Yang B., Phuan P. W., Verkman A. S. (2012) Triazolothienopyrimidine inhibitors of urea transporter UT-B reduce urine concentration. J. Am. Soc. Nephrol. 23, 1210–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson W., Chen Y., Lee S. M., Lee H. S., Hua J., Dohlman T., Shiang T., Dana R. (2014) Extraorbital lacrimal gland excision: a reproducible model of severe aqueous tear-deficient dry eye disease. Cornea 33, 1336–1341 [DOI] [PubMed] [Google Scholar]
- 32.Galietta L. J., Haggie P. M., Verkman A. S. (2001) Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 499, 220–224 [DOI] [PubMed] [Google Scholar]
- 33.Galietta L. V., Jayaraman S., Verkman A. S. (2001) Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am. J. Physiol. Cell Physiol. 281, C1734–C1742 [DOI] [PubMed] [Google Scholar]
- 34.Sullivan D. A., Krenzer K. L., Sullivan B. D., Tolls D. B., Toda I., Dana M. R. (1999) Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest. Ophthalmol. Vis. Sci. 40, 1261–1265 [PubMed] [Google Scholar]
- 35.Villareal A. L., Farley W., Pflugfelder S. C. (2006) Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens 32, 272–276 [DOI] [PubMed] [Google Scholar]
- 36.Lemp M. A., Bron A. J., Baudouin C., Benítez Del Castillo J. M., Geffen D., Tauber J., Foulks G. N., Pepose J. S., Sullivan B. D. (2011) Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 151, 792–798.e1 [DOI] [PubMed] [Google Scholar]
- 37.Luo L., Li D. Q., Corrales R. M., Pflugfelder S. C. (2005) Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 31, 186–193 [DOI] [PubMed] [Google Scholar]
- 38.Liu H., Begley C., Chen M., Bradley A., Bonanno J., McNamara N. A., Nelson J. D., Simpson T. (2009) A link between tear instability and hyperosmolarity in dry eye. Invest. Ophthalmol. Vis. Sci. 50, 3671–3679 [DOI] [PubMed] [Google Scholar]
- 39.Gilbard J. P., Carter J. B., Sang D. N., Refojo M. F., Hanninen L. A., Kenyon K. R. (1984) Morphologic effect of hyperosmolarity on rabbit corneal epithelium. Ophthalmology 91, 1205–1212 [DOI] [PubMed] [Google Scholar]
- 40.Ralph R. A. (1975) Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest. Ophthalmol. 14, 299–302 [PubMed] [Google Scholar]
- 41.Chen Y. T., Li S., Nikulina K., Porco T., Gallup M., McNamara N. (2009) Immune profile of squamous metaplasia development in autoimmune regulator-deficient dry eye. Mol. Vis. 15, 563–576 [PMC free article] [PubMed] [Google Scholar]
- 42.Sung M. S., Li Z., Cui L., Choi J. S., Choi W., Park M. J., Park S. H., Yoon K. C. (2015) Effect of topical 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside in a mouse model of experimental dry eye. Invest. Ophthalmol. Vis. Sci. 56, 3149–3158 [DOI] [PubMed] [Google Scholar]
- 43.Ratcliff R., Evans M. J., Cuthbert A. W., MacVinish L. J., Foster D., Anderson J. R., Colledge W. H. (1993) Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat. Genet. 4, 35–41 [DOI] [PubMed] [Google Scholar]
- 44.Lu M., Ding C. (2012) CFTR-mediated Cl(−) transport in the acinar and duct cells of rabbit lacrimal gland. Curr. Eye Res. 37, 671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nandoskar P., Wang Y., Wei R., Liu Y., Zhao P., Lu M., Huang J., Thomas P., Trousdale M. D., Ding C. (2012) Changes of chloride channels in the lacrimal glands of a rabbit model of Sjögren syndrome. Cornea 31, 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vertex Pharmaceuticals (2012) Kalydeco [product monograph], Vertex Pharmaceuticals (Canada) Inc., Laval, QC, Canada
- 47.Watsky M. A., Jablonski M. M., Edelhauser H. F. (1988) Comparison of conjunctival and corneal surface areas in rabbit and human. Curr. Eye Res. 7, 483–486 [DOI] [PubMed] [Google Scholar]
- 48.Van Goor F., Hadida S., Grootenhuis P. D. J. (2008) Pharmacological rescue of mutant CFTR function for the treatment of cystic fibrosis. Top. Med. Chem 3, 91–120 [Google Scholar]
- 49.Wolosin J. M., Candia O. A. (1987) Cl− secretagogues increase basolateral K+ conductance of frog corneal epithelium. Am. J. Physiol. 253, C555–C560 [DOI] [PubMed] [Google Scholar]
- 50.Kompella U. B., Kim K. J., Lee V. H. (1993) Active chloride transport in the pigmented rabbit conjunctiva. Curr. Eye Res. 12, 1041–1048 [DOI] [PubMed] [Google Scholar]
- 51.Turner H. C., Alvarez L. J., Candia O. A. (2000) Cyclic AMP-dependent stimulation of basolateral K(+)conductance in the rabbit conjunctival epithelium. Exp. Eye Res. 70, 295–305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.