ABSTRACT
Adipocytes exposed to high glucose concentrations exhibit impaired metabolic function, including an increase of oxidative and proinflammatory factors that might favor the development of insulin resistance. Caveolin-1 (Cav-1) is a key mediator of the insulin transduction pathway whose expression is significantly enhanced during adipocyte differentiation. In this work, we studied the effects of high glucose concentration on the regulation of Cav-1 expression and activation and its relation to the insulin signaling pathway during the adipogenic process and in long-term differentiated adipocytes. Both, long-term high glucose exposure during adipogenesis and short-term glucose incubation of mature adipocytes, promoted triglyceride accumulation in 3T3-L1 cells. The short-term exposure of mature adipocytes to high glucose significantly reduced the sensitivity to insulin of Cav-1, insulin receptor (IR) and potein kinase B (AKT-2) phosphorylation, as well as insulin-induced deoxyglucose uptake. Adipocytes differentiated in the presence of high glucose lost Cav-1 and IR response to insulin-stimulated phosphorylation, but maintained the insulin sensitivity of AKT-2 phosphorylation and deoxyglucose uptake. Although long-term high glucose exposure increased DNA methylation in Cav-1 promoter, Cav-1 expression was not affected. Moreover, these cells showed an increase of Cav-1, IR and AKT-2 protein content, pointing to an adaptive response induced by the long-term high glucose exposure.
KEYWORDS: 3T3-L1, adipogenesis, DNA methylation, GLUT4, glucose uptake, insulin receptor, leptin
Introduction
Prolonged or repeated exposure to elevated glucose concentrations exerts deleterious effects on the metabolic function of different cell types, which are referred to as glucotoxicity, and are commonly associated with increased risk of macro- and microvascular complications.1 Although acute hyperglycemia can be caused by several factors, including carbohydrate-rich intake and catecholamine release (i.e., stress), chronic hyperglycemia is most usually elicited as a consequence of defects in pancreatic β-cell insulin secretion and insulin utilization by peripheral tissues like muscle, pancreas, liver, endothelium and white adipose tissue.2,3 The effects of high glucose in adipocyte differentiation could explain some of the metabolic dysregulations accompanying obesity. In this sense, Chuang et al.4 demonstrated that 25 mM glucose enhanced adipogenesis and lipid accumulation in mesenchymal stem cells, and a state of high glucose has been reported to induce the process of adipogenesis in muscle-derived stem cells.5 White adipose tissue has been recognized as crucial for maintaining lipid and glucose homeostasis due to its role in energy storage, as well as for the systemic regulatory function of its secreted adipokines.6 For example, leptin, adiponectin, adipolin and other adipokines secreted by the healthy adipose tissue are linked with insulin sensitivity,7 and a dysregulation of adipocyte storage capacity, size and adipokine secretory patterns can contribute to the development of metabolic complications, including low-grade inflammation and insulin resistance.8 In adipocytes, exposure to high glucose concentrations triggers the induction of reactive oxygen species,9,10 which can result in mitochondrial dysfunction and in the activation of proinflammatory cascades, such as those of Toll-like receptors (TLR) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).2,11,12 Hyperglycemia has been also associated to insulin signaling disruption and glucose uptake alterations. In this sense, high glucose concentrations induce a reduction of insulin-stimulated glucose uptake by producing IR dephosphorylation 13 and alterations in insulin receptor substrate-1 (IRS-1) and phosphatidylinositol-3-kinase (PI3K) functions.14
Caveolin-1 (Cav-1), the main structural and functional protein of caveolae, is a key mediator of the insulin transduction pathway directly interacting with the β-subunit of IR.15 Cav-1 has indeed been proposed to be important for the integration of Glut-4 within the plasma membrane from its containing intracellular vesicles.16,17 According to this, Cav-1 knockout mice show insulin resistance and defective IR protein expression in adipose tissue.18 Caveolin 2 (Cav-2) is other isoform expressed in adipose tissue being its expression also influenced by the adipocyte differentiation process.19 In this context, it has been recently observed that Cav-2 facilitates the interaction with IRS-1 and downstream insulin signal transmission.20 Therefore, and given the importance of Cav-1 for a correct insulin transduction signaling in adipocytes, the main objective of this study was to determine the effects of high glucose concentration on the regulation of Cav-1 expression and its relation to the insulin signaling pathway, during the adipogenic process as well as in mature adipocytes. For this purpose we have used 2 experimental designs in mouse 3T3-L1 preadipocytes (Fig. 1), the exposure of these cells to 25 mM extra-glucose during the differentiation process for 21 days, and the incubation of 7-day mature 3T3-L1 adipocytes with 25 mM extra-glucose for 48 hours.
Figure 1.
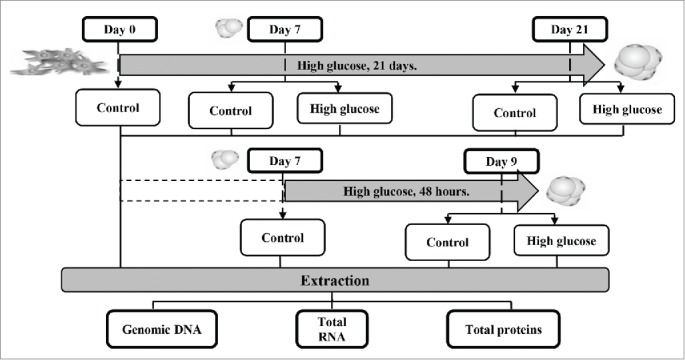
Experimental design. Two approaches were designed to determine the effects of high glucose concentration on the regulation of Cav-1 expression and its relation to the insulin signaling pathway during adipocyte differentiation and in mature adipocytes. For the first objective, 3T3-L1 preadipocytes were differentiated during 21 days in the presence of 25 mM extra-glucose. Checkpoints were established at days 0, 7 and 21 of this process. As a second method, 7-day mature 3T3-L1 adipocytes were incubated with 25 mM extra-glucose for 48 hours. Control points were set up at days 7 and 9. Genomic DNA, total RNA and total proteins were extracted at every checkpoint to carry out further determinations.
Results
Modulation of the adipokine secretion pattern by high glucose concentrations
Continuous long-term exposure of differentiating adypocytes to high glucose concentration significantly reduced leptin secretion at day 7, but this effect was not observed at day 21 (Fig. 2A). In contrast, adiponectin release was not affected by glucose at either stage of the adipogenic process (Fig. 2C). A 48-h exposure of mature adipocytes to high glucose concentration caused a significant decrease of leptin secretion with no alterations in adiponectin release (Fig. 2B, and D).
Figure 2.
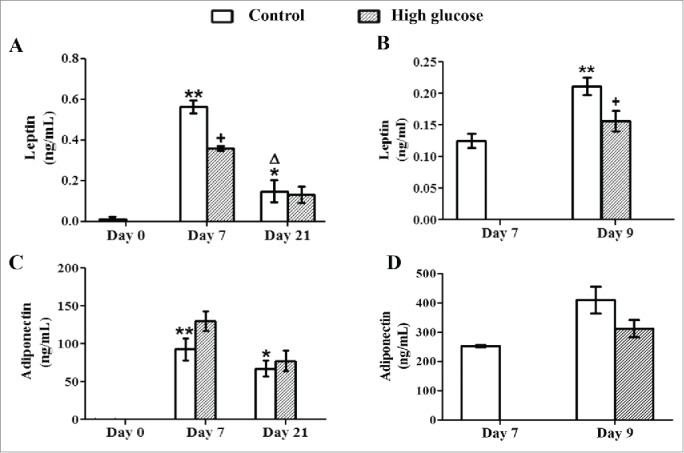
Effects of high glucose on secreted leptin and adiponectin levels during 3T3-L1 differentiation and in 3T3-L1 mature adipocytes. Culture medium samples of (A, C) 3T3-L1 preadipocytes (d0) and control /glucose-exposed (50 mM) adipocytes (d7, d21) and of (B, D) Control (d7, d9)/glucose-exposed (50 mM, 48 hours) 3T3-L1 adipocytes (d9) were obtained for adipokine ELISA assays. Data are means ± SEM of the concentration of each adipokine secreted to the cell medium. Groups were compared by using the ANOVA for a single factor and Dunnett's test. (A, C) Data from control groups are compared to day 0 *. p < 0.05; **. p < 0.01 or to day 7 Δ. p < 0.05. Glucose-exposed cells are compared to the control group from the same differentiation day +. p < 0.05. (B, D) Data from control cells at day 9 were compared to control cells at day 7 **. p < 0.01. Data from glucose-exposed cells were compared to their same day control group +. p < 0.05. The number of independent samples analyzed is ≥10 for each adipokine and condition evaluated.
Effect of high glucose on 3T3-L1 intracellular lipid accumulation and cell viability
Intracellular triglyceride accumulation associated to the 3T3-L1 differentiation process was quantified by Oil Red O staining in control and high glucose-exposed cells. Long-term exposure to high glucose concentration induced a significant increase in stored triglycerides after 7 days of differentiation, which was not observed after 21 days (Fig. 3A and C). The short-term (48-h) glucose exposure of mature adipocytes also increased significantly the amount of triglycerides stored (Fig. 3B and D). Cell viability was not affected by long-term glucose exposure, although a slight reduction (p = 0.022) was observed after the short-term exposure (data not shown).
Figure 3.

Effects of high glucose on cell differentiation and triglyceride storage during 3T3-L1 differentiation and in 3T3-L1 mature adipocytes. (A) 3T3-L1 preadipocytes (d0) and control /glucose-exposed (50 mM) adipocytes (d7, d21) and (B) Control (d7, d9)/glucose-exposed (50 mM, 48 hours) 3T3-L1 adipocytes (d9) were photographed using a light microscope (40X magnification). (C) Intracelullar triglycerides quantification of the preadipocytes (d0) and control /glucose-exposed (50 mM) adipocytes (d7, d21) and (D) of the control (d7, d9)/glucose-exposed (50 mM, 48 hours) 3T3-L1 adipocytes (d9) using Oil Red O Staining. Data are means ± SEM of dye OD at 540 nm. (C) Data from control groups are compared to day 0**.p < 0.01 and glucose-exposed groups are compared to the control groups from the same differentiation day ++. p < 0.01. (D) Data from control group are compared to day 7**.p < 0.01 and from glucose-exposed group are compared to the control group of the same differentiation day ++. p < 0.01 .The number of independent samples analyzed is ≥10 for each day and condition evaluated.
High glucose induces caveolin-1 gene hypermethylation during adipocyte differentiation
DNA methylation levels of Cav-1 and IR genes were analyzed by MassArray Epityper. The selected region for Cav-1 included 2 CpG islands located along the promoter, the first exon and the first intron of the gene (−619 bp to +1333 from the initiation codon ATG, containing 70 CpG dinucleotides). As shown in Figure. 4, the differentiation of 3T3-L1 preadipocytes (d0) to long-term differentiated adipocytes (d21) in the presence of high glucose increased significantly the methylation percentage of 31 CpG dinucleotides of the Cav-1 gene. In contrast, short-term (48-h) exposure to high glucose slightly decreased the methylation level of 8 out of 70 CpG sites from the Cav-1 gene region under study (Fig. 5).
Figure 4.
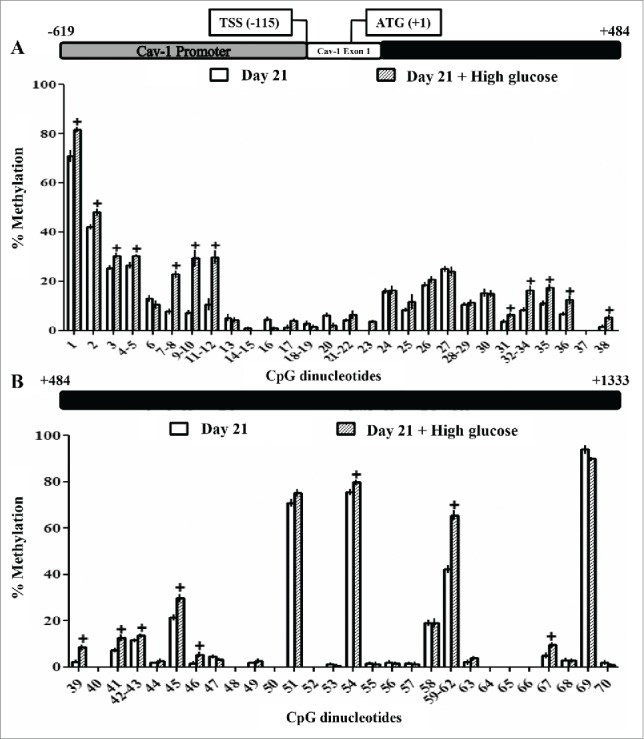
Methylation levels of CpG dinucleotides located in the caveolin-1 promoter, exon 1 and intron 1 in 3T3-L1 long-term differentiated adipocytes exposed or not to high glucose during the differentiation process. The methylation levels of 70 CpG sites in caveolin-1 promoter, exon 1 and intron 1 were compared in control /glucose-exposed (50 mM) 3T3-L1 long-term differentiated adipocytes at day 21. MassARRAY system was used for the quantitative methylation analysis. (A) CpG 1 to 38 (B) CpG 39 to 70 of the sequence under study. Data are means ± SEM of the methylation percentage of each CpG dinucleotide specified in the figure. Groups were compared by using the Mann-Whitney U test. Significant differences between control and glucose-exposed cells +. p < 0.05. Gene structure is schematized over the graphs indicating the Transcription Start Site (TSS) and the initiation codon (ATG) position. The number of independent samples analyzed is between 5–8, measured in triplicate for each CpG dinucleotide under study.
Figure 5.

Methylation levels of CpG dinucleotides in the caveolin-1 promoter, exon 1 and intron 1 in 3T3-L1 mature adipocytes exposed or not to high glucose during 48 hours. The methylation levels of 70 CpG sites in caveolin-1 promoter, exon 1 and intron 1 were compared in control (d7)/glucose-exposed (50 mM, 48 hours), 3T3-L1 mature adipocytes (d9). MassARRAY system was used for the quantitative methylation analysis. (A) CpG 1 to 38 (B) CpG 39 to 70 of the sequence under study. Data are means ± SEM of the methylation percentage of each CpG dinucleotide specified in the figure. Groups were compared by using the Mann-Whitney U test. Significant differences between control day 7 and day 9 cells*. p < 0.05. Significant differences between control and glucose-exposed cells +. p < 0.05. Gene structure is schematized over the graphs indicating the Transcription Start Site (TSS) and the initiation codon (ATG) position. The number of independent samples analyzed is between 5–8, measured in triplicate for each CpG dinucleotide under study.
The methylation status of a selected region of IR, which included part of the first exon and of the first intron (-249 bp to +545 bp from the initiation codon ATG including 40 CpG sites), was also analyzed after long-term adipocyte differentiation (d21). As long-term high glucose exposure produced no significant changes in any of the CpG sites under study, IR methylation was not analyzed when mature adipocytes were exposed to high glucose only for 48 hours (data not shown).
Effect of high glucose on the expression of caveolin-1 and insulin signaling pathway intermediates
Exposure to high glucose concentration did not modify the gene expression of Cav-1, IR, AKT-2 and Glut-4 during the adipocyte differentiation process (Fig. 6A and 9A). However, the effect of high glucose during 3T3-L1 cell adipogenesis was especially relevant at the protein level. Whereas neither Cav-1 nor IR protein expression was affected by high glucose after 7 days of differentiation, both were significantly upregulated at day 21 when compared to control cells. AKT-2 protein level was already significantly increased in response to glucose at day 7 and maintained so at day 21 of the differentiation process (Fig. 6B). In contrast, high glucose exposure reduced Glut-4 protein expression during the differentiation process (d7), but not in long-term differentiated adipocytes (d21) (Fig. 9C).
Figure 6.
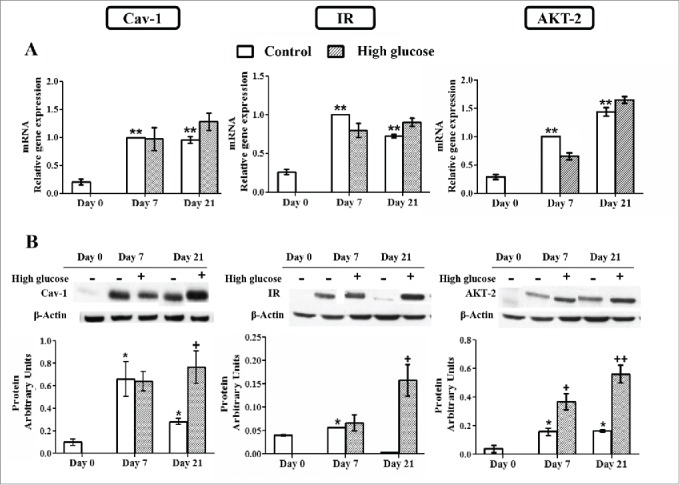
Caveolin-1 and insulin signaling intermediates expression during 3T3-L1 differentiation in the presence or in the absence of high glucose (50 mM). (A) mRNA levels. Data are means ± SEM of the ratio between each gene and cyclophilin expression at differentiation days 0, 7 and 21. Groups were compared by using the ANOVA for a single factor and Dunnett's test. (B) Protein levels. Data are means ± SEM of the ratio between each protein and β-actin expression at differentiation days 0, 7 and 21. Groups were compared by using the Wilcoxon signed-rank test. Data from control cells were compared to day 0 *. p < 0.05; **. p < 0.01. Data from glucose-exposed groups were compared to their same day control group +. p < 0.05; ++. p < 0.01. The number of independent samples analyzed for (A) is ≥10 for each determination and condition evaluated, whereas the number of independent samples analyzed for (B) is between 5–10 for each protein and condition evaluated.
Figure 9.
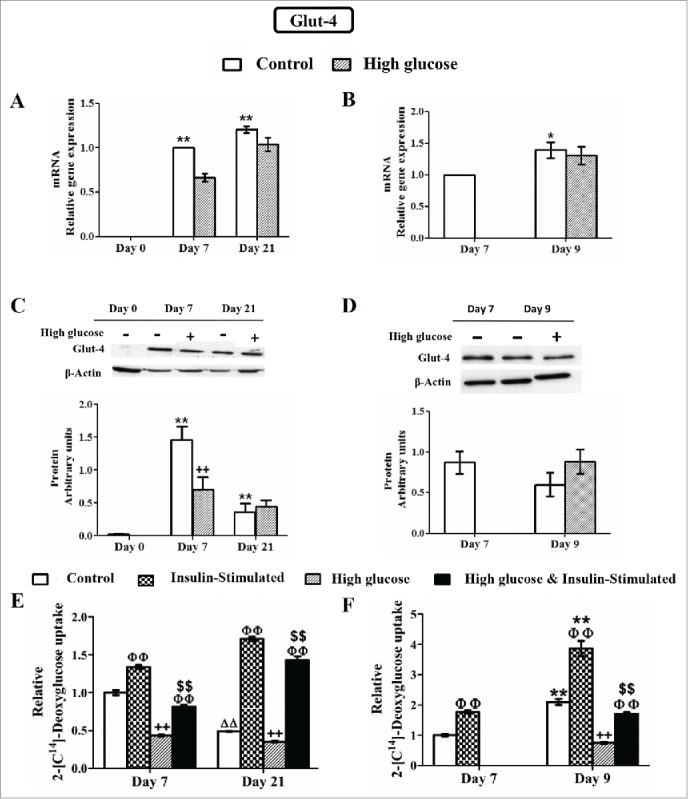
Effects of high glucose on glucose transporter type 4 (Glut-4) expression and deoxyglucose uptake during 3T3-L1 differentiation (A, C, E) and in 3T3-L1 mature adipocytes (B, D, F). Results from A, C and E correspond to preadipocytes (d0) and control /glucose-exposed (50 mM) adipocytes (d7,d21), whereas results from B, D and F correspond to control (d7, d9) /glucose-exposed (50 mM, 48 hours) adipocytes (d9). (A, B) mRNA levels. Data are means ± SEM of the ratio between Glut-4 and cyclophilin expression. Groups were compared by using the ANOVA for a single factor and Dunnett's test. (C, D) Protein levels. Data are means ± SEM of the ratio between Glut-4 and β-actin expression. Groups were compared by using the Wilcoxon signed-rank test. (E, F) Deoxyglucose uptake by control and glucose-exposed 3T3-L1 cells before and after insulin stimulation (50 nM, 10 min). Data are means ± SEM of 2-[C14]-deoxyglucose (in µmol) incorporated by cells after 10 minutes, adjusted by total protein in grams. Groups were compared by using the ANOVA for a single factor and Dunnett's test. (A, C, E) Data from control groups are referred to control day 0 **. p < 0.01 or to control day 7 ΔΔ. p < 0.01. Data from glucose-exposed groups were compared to their same day control group ++. p < 0.01. (E) Data from insulin-stimulated groups were compared to their unstimulated group, ΦΦ.p < 0.01. Data from insulin-stimulated glucose-exposed groups were compared to the same day insulin-stimulated control groups $$. p < 0.01. (B, D, F) Data from control groups at day 9 were compared to control cells at day 7 *. p < 0.05; **. p < 0.01. Data from glucose-exposed groups were compared to their same day control group ++. p < 0.01. (F) Data from insulin-stimulated groups were compared to their unstimulated group, ΦΦ. p < 0.01. Data from insulin-stimulated glucose-exposed groups were compared to the same day insulin-stimulated control groups $$. p < 0.01. The number of independent samples analyzed for (A), (B), (E) and (F) is ≥10 for each determination and condition evaluated whereas the number of independent samples analyzed for (C) and (D) is between 5 and 10 for each protein and condition evaluated.
The short-term (48-h) exposure to high glucose concentration had no effect on Cav-1, IR, AKT-2 and Glut-4 expression, neither at the mRNA nor at the protein level (Figures 7A-D).
Figure 7.

Caveolin-1 and insulin signaling intermediates expression in control and high glucose-treated (50 mM, 48 hours) 3T3-L1 mature adipocytes. (A) mRNA levels. Data are means ± SEM of the ratio between each gene and cyclophilin expression at differentiation days 7 and 9. (B) Protein levels. Data are means ± SEM of the ratio between each protein and β-actin expression at differentiation days 7 and 9. Groups from (A) and (B) were compared by using the ANOVA for a single factor and Dunnett's test. Data from control cells at day 9 were compared to control cells at day 7 *. p < 0.05. Data from glucose-exposed groups were compared to their same day control group +. p < 0.05. The number of independent samples analyzed for (A) and (B) is ≥10 for each determination and condition evaluated.
Effect of high glucose on the activation of caveolin-1 and insulin signaling pathway intermediates and on insulin-induced glucose uptake
Basal Cav-1 phosphorylation level was significantly increased after 21 days of adipocyte differentiation and, as we have previously reported,21 this differentiation-associated phosphorylation is responsible of the lack of sensitivity to insulin (Fig. 8A). On the other hand, although IR and AKT-2 activation were also induced by the adipogenic process in long-term differentiated adipocytes (day 21), they were able to show a significant response to insulin as well (Fig. 8A). Exposure to high glucose level during the adipogenesis for 21 days significantly reduced the sensitivity to insulin of Cav-1 and IR phosphorylation. On the other hand, AKT-2 insulin-dependent activation was not affected by exposure to high glucose for 21 days (Fig. 8A).
Figure 8.
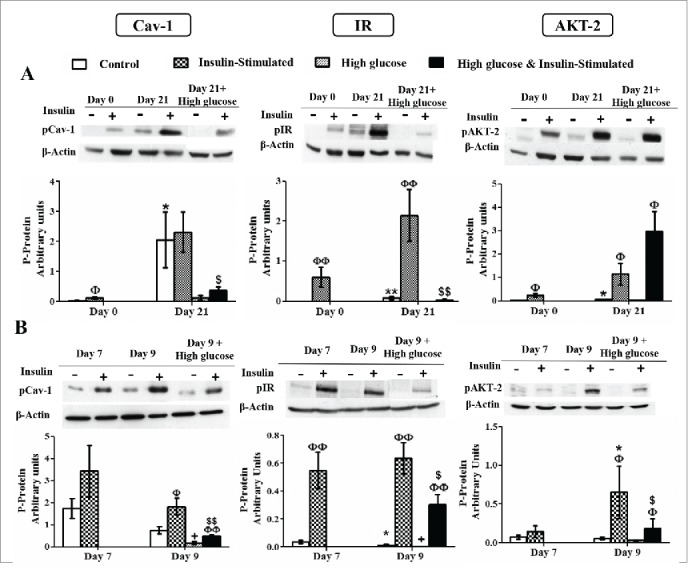
Caveolin-1 and insulin signaling intermediates activation during 3T3-L1 differentiation in the presence or in the absence of high glucose (50 mM) (A) and in control and high glucose-treated (50 mM, 48 hours) 3T3-L1 mature adipocytes (B). Results represent data from cells before and after stimulation with insulin (50 nM, 10 minutes). Data are means ± SEM of the ratio between each phospho-protein and β-actin expression at the differentiation days 0 and 21 (A) and at the differentiation days 7 and 9 (B). Groups were compared by using the Mann-Whitney test. Data from control cells at day 21 were compared to control cells at day 0 (A) and from control cells at day 9 were compared to control cells at day 7 (B) *. p < 0.05; **. p < 0.01. Data from glucose-exposed groups were compared to their same day control group +. p < 0.05. Data from insulin-stimulated groups were compared to their unstimulated control group Φ. p < 0.05, ΦΦ. p < 0.01. Data from insulin-stimulated glucose-exposed group were compared to their same day insulin-stimulated control group $. p < 0.05; $$. p < 0.01. The number of independent samples analyzed for (A) and (B) is between 5–10 for each protein and condition evaluated.
Insulin stimulation increased Cav-1, IR and AKT-2 activation in 9-day mature adipocytes (Fig. 8B). In these cells, short-term (48-h) high glucose exposure significantly decreased basal Cav-1 and IR phosphorylation and also sensitivity to insulin (Fig. 8B). In the case of AKT-2, high glucose did not change basal phosphorylation, but sensitivity to insulin was also significantly reduced (Fig. 8B).
In order to determine the activity of Glut-4, the final effector of the insulin signaling pathway, 2-deoxyglucose uptake was analyzed before and after insulin stimulation. A previous work of our group reported that basal 2-deoxyglucose uptake increased during adipocyte differentiation but decreased in long-term differentiated adipocytes (d21) (Fig. 9E).21 As expected, insulin was able to induce hexose uptake in adipocytes (d7, d21) not exposed to high glucose. Exposure to high glucose during adipocyte differentiation significantly decreased the amount of 2-deoxyglucose taken up by fat cells. However, these cells were still sensitive to insulin and able to activate Glut-4-mediated deoxyglucose transport in response to the hormone, although to a reduced level (Fig. 9E). Similar results were obtained in short-term (48-h) high glucose exposed cells, which showed significantly lower basal 2-deoxyglucose transport and also maintained insulin sensitivity to a lesser extent (Fig. 9F).
Discussion
The experimental design of the current study allowed to examine the alterations that a high glucose concentration might produce in the differentiation process of adipocytes and in their sensitivity to insulin. We extended the maturation period until 21 days after hormone induction, in an attempt to get insight into the differences between small and enlarged long-term differentiated adipocytes. The in vitro model selected, embryonic 3T3-L1 cells, maintains cells isolated from the influence of other cell types, hormones or proinflammatory factors that might be present in an in vivo situation. As a consequence, caution must be exercised when extrapolating results to the pathophysiological conditions normally associated to hyperglycemia.
Exposure to high glucose increased lipid storage in mature adipocytes (after 48 h) and during the differentiation process (after the first 7 days). However, this high glucose-induced lipid accumulation was not observed in long-term differentiated adipocytes (d21) (Fig. 3C-D). These results confirm that glucose promotes the formation of lipid droplets and accelerates adipocyte differentiation, as has been reported in similar models.22 In this sense, Chuang et al. demonstrated that hyperglycemia enhances lipid accumulation in mesenchymal stem cells (MSCs) through an increase of ERK-mediated PI3K/AKT pathway. Indeed, this cascade ends with the overexpression of PPARγ, a key regulator of adipogenesis. 4 Similar results have been obtained in human osteoblastic MG-63 cells 23 and in primary rat osteoblasts, 24 where high glucose induced ROS-mediated lipid drop accumulation and overexpression of adipogenic markers such as PPARγ, aP2 and adipsin. Long-term differentiated hypertrophic adipocytes have probably saturated their lipid storage capacity, causing that exposure to high glucose concentrations is not longer able to stimulate an increase in their lipid content.
Aberrant secretion of adipokines plays a central role in the development of inflammation and in the pathogenesis of metabolic diseases.25,26 In our model, the expression of adiponectin and leptin increased with adipocyte differentiation as expected (d7), but was lower in long-term differentiated cells (d21) (Fig. 2A, C). This decrease could be related to the cell hypertrophy and senescence related to prolonged maturation, which has been previously associated to metabolic alterations.27 A significant decrease in leptin secretion was observed after 7 days of differentiation in the presence of high glucose or after exposing mature adipocytes for 48 hours to the same high glucose concentration (Fig. 2A, B), despite the slightly higher lipid content found in all these cells. Although high glucose has been usually considered a proinflammatory condition 28 and some authors have reported that it stimulates leptin secretion, 29 our result is more in accordance with the lower circulating leptin levels found in untreated STZ-diabetic rats.30 Indeed, our observation might be also related to the results obtained by Mueller et al. in rat adipocytes, where leptin secretion was associated directly with the amount of glucose taken up by the cells and its metabolism.31 In 21-day differentiated adipocytes, long-term high glucose did not affect the reduced leptin secretion observed (Fig. 2A), suggesting that the effect of glucose on leptin secretion could depend on the duration of the stimulus and the maturation moment of the adipocytes. In this sense, it has been suggested that the effect of uncontrolled diabetes to lower leptin levels is a contributing factor for diabetic hyperphagia.32 Adiponectin release was not affected by high glucose exposure during the 21 days of differentiation process (Fig. 2C). However, similarly to the results obtained by other authors in mature adipocytes,33,34 adiponectin levels tended to decrease in these cells after high glucose exposure during 48 hours, although it did not reach statistical significance (Fig. 2D).
Hyperglycemia and diabetes have been associated with changes in the DNA methylation levels of many genes in different tissues.35 Cav-1 is a gene whose expression is regulated by DNA methylation in different cell types and processes, such as 3T3-L1 adipocyte differentiation21 and growing tumors.36 In the current study, exposure to a high glucose concentration during the whole differentiation process (d21) significantly increased the methylation levels of 31 CpG sites out of the 70 CpG sites located in the proximal promoter and along the first intron of the Cav-1 gene (Fig. 4). However, this higher methylation did not correlate with changes in the expression of the gene, which was not altered (Fig. 6A). On the other hand, only 3 CpG sites showed a significant decrease in methylation after the short-term (48-h) glucose exposure (d9) (Fig. 5), a change that also did not associate with any change in the expression of the gene (Fig. 7A). These results indicate that the modifications in the Cav-1 gene methylation pattern induced by high glucose exposure do not have an effect on its expression. In addition, high glucose exposure during the differentiation process (d21) did not induce modifications neither in the expression nor in the methylation of the IR gene (data not shown), a key intermediate in insulin signaling. In a previous study, Burdge et al.37 found a decrease in IR gene methylation after supplementing the diet of juvenile-prepuberal rats with folate, but no study up to date has reported changes in the methylation of this gene as a result of hyper or hypoglycemic conditions.
Cav-1 has been recognized as an important mediator of the insulin signaling pathway and its expression is significantly enhanced during adipocyte differentiation, as occurs with IR, AKT-2 and Glut-4.21,38 Interestingly, the positive modulation of the adipogenic program produced by long-term high glucose exposure during the first stage of this process (d7) did not involve any difference in Cav-1, IR, AKT-2 and Glut-4 mRNA expression (Fig. 6A and 9A). However, it is important to note that, the increase of Glut-4 protein level associated to adipocyte maturation (day 7) is significantly diminished in the presence of high glucose, but this effect is not observed in long-term differentiated adipocytes (d21), since these cells show lower Glut-4 protein level even in the absence of added glucose (Fig. 9C). High glucose presence could partially prevent the increase in Glut-4 protein pool, an event that will in turn also limit the capacity of glucose uptake to respond to insulin (Fig. 9E). Indeed, reduced Glut-4 protein expression is a common characteristic of adipose tissue in animal models of diabetes and insulin resistance 39 and in diabetic and obese subjects.40,41
We also observe that high glucose induces a reduction of basal (Glut-1-mediated) non-insulin-sensitive glucose uptake as early as day 7, that is maintained at day 21 (Fig. 9E). Apparently, differentiation in the presence of a high glucose concentration makes sugar transport less active. High glucose presence seems to reduce also Glut-1 content, 42 although we have not measured it. This effect of high glucose is also evident when added to mature adipocytes for 2 days (Fig. 9F). In contrast to this interpretation, it has been described that, in hyperglycemic diabetic rats, skeletal-muscle Glut-4 reduction is partially counter-balanced by an increase in Glut-1 expression 43. Nonetheless, this was only true for skeletal muscle but not for adipocytes because their metabolic functions are very different. In our model, high glucose presence induces a reduction of both, insulin-sensitive and basal non-insulin-sensitive glucose uptake.
Glucose transporter expression at protein level does not always correlate with cellular glucose uptake modulation and, as a matter of fact, we have observed a significant reduction of insulin-stimulated glucose uptake in long-term (21-days), as well as in 48-hours high glucose-exposed adipocytes (Fig. 9E-F), with no alterations in Glut-4 protein level in either case (Fig. 9C-D). In addition, it is well known that the regulation of Glut-4 activity is mediated by its translocation from specific intracellular vesicles to the plasma membrane, 44 a mechanism that has been found to be disrupted in some pathological conditions. The high-glucose-mediated inhibition of Glut-4 protein and glucose-uptake observed at day 7, could be related to the later increase in Cav-1, IR (d21) and AKT-2 (d7, d21) protein expression (Fig. 6B), as part of compensatory mechanisms triggered by the adipocytes in response to hyperglycemia-induced insulin resistance.
IR phosphorylation is necessary for insulin signaling, and a decreased phosphorylation of IR has been observed in adipocytes from insulin resistant and diabetic conditions.45,46 Insulin also induces Cav-1 tyrosine phosphorylation directly through IR kinase activity.47 High glucose exposure has been proposed to induce insulin resistance in human adipocytes,48,49 presumably affecting IR autophosphorylation at a first stage throughout glucose-induced PKC activity.50,51 In our study, adipocytes exposed to high glucose during the whole adipogenic process have lost the associated phosphorylation of Cav-1, together with its sensitivity to insulin stimulation (Fig. 8A). Similar results were obtained for pCav-1 and pIR in insulin resistant endometrial tissue of Polycystic Ovary Syndrome (PCOS) patients, despite an increase in non-activated protein levels.52. Cav-1 dephosphorylation might be mediated by tyrosine phosphatase 1B (PTP1B),53 which has been reported to be overexpressed in the presence of high glucose concentration.54 Interestingly, in these cells differentiated in the presence of high glucose, the sensitivity to insulin of IR phosphorylation is also abolished, while that of AKT-2 is maintained, suggesting that other factors might be participating in the regulation of AKT-2 phosphorylation (Fig. 8A). In support of these data, other authors have reported that insulin can induce AKT-2 phosphorylation in skeletal muscle of insulin-resistant humans, despite a complete inability to stimulate IRS-1 activation.55 These observations may lead to hypothesize the existence of an alternative insulin-dependent pathway able to promote AKT-2 activation in response of prolonged high glucose exposure.
It is remarkable that in the cells exposed to high glucose concentration during the adipocytic differentiation, where neither Cav-1 nor IR are phosphorylated in response to insulin, AKT-2 response seems to be enough to maintain insulin effect of deoxyglucose uptake (Fig. 9E). Whether the increased protein expression of Cav-1, IR and AKT-2 observed in these cells could be part of a compensatory mechanism attempting to overcome the high glucose mediated desensitization, and whether this could be related to the preservation of insulin-dependent glucose uptake through an AKT-2-regulated mechanism, are questions that need to be further investigated.
When mature adipocytes were exposed to high glucose for 2 days, basal phosphorylation of Cav-1 and IR was lower. However, in this case, sensitivity to insulin, although significantly reduced, was not completely abrogated (Fig. 8B). Noticeably, and in contrast of what occurred after long-term exposure to high glucose during the adipogenic process, activation of AKT-2 by insulin was also significantly reduced in this case (Fig. 8B). These reductions correlated positively with a significantly lower insulin-stimulated deoxyglucose uptake (Fig. 9F), suggesting also a certain degree of glucose-induced impairment in the insulin signaling pathway. Nevertheless, the mechanism must be different in this case, since there are not variations in the expression level of the signaling intermediates, the stimulation of Cav-1 and IR in response to insulin is not lost and, in contrast, the activation of AKT-2 is less prominent.
It is noteworthy to point out that the effects observed in this study could be different from those in white adipocytes. Adipocytes derived from 3T3-L1 cells tend to be multilocular, in contrast with those found in white adipose tissue that are mostly unilocular, and show lower insulin-dependent glucose uptake due to reduced translocation of Glut-4 to the plasma membrane. This has been attributed to the dysregulation of actin remodeling in hypertrophic adipocytes.56 Unilocular lipid-droplet structure might also influence insulin-induced Cav-1 trafficking at the intracellular level.57 Nevertheless, we did not find previous studies which analyzed the differences between unilocular and multilocular adipocytes in regard to the effect of insulin on Cav-1 trafficking in relation to glucose metabolism (particularly glucose uptake).
In summary, we have shown that both, long-term exposure to a high glucose concentration during adipocyte differentiation (21 days) and short-term (48-h) incubation of mature adipocytes with a high glucose concentration, promote proadipogenic mechanisms. Short-term exposure of mature adipocytes to a high glucose concentration reduces Cav-1, IR and AKT-2 phosphorylation without affecting their mRNA or protein content, resulting in a lower glucose uptake that maintains also a reduced sensitivity to insulin stimulation. Long-term high glucose presence during adipocyte differentiation drives to the development of fat cells with blocked insulin-induced Cav-1 and IR phosphorylation, but that maintain insulin sensitivity of AKT-2 phosphorylation and of glucose uptake. In addition, these cells also show an increase of Cav-1, IR and AKT-2 protein content, pointing to an adaptive response induced by long-term high glucose exposure.
Material and methods
Cell culture and high glucose conditions
3T3-L1 preadipocytes (ATCC) were seeded in 6-wells plates and grown in Dulbeco's modified Eagle's medium (DMEM, 419660-029 Invitrogen) supplemented with 10% calf blood serum (CBS, 16170-078 Invitrogen) and 1% penicillin/streptomycin (15140-122 Invitrogen) at 37°C, 5% CO2 and 95% humidity until reaching the second day post-confluence (d0). Adipocyte differentiation was then induced by incubating the cells with DMEM supplemented with 10% fetal bovine serum (FBS, 10270-106 Invitrogen), insulin (1 mg/mL, I9278 Sigma Aldrich), dexamethasone (1 mM, D-2915 Sigma Aldrich) and 3-isobutyl-1-methylxanthine (IBMX I-5879 Sigma Aldrich) (0.5 mM) for 48 hours. The medium was then replaced with DMEM containing 10% FBS and insulin (1 mg/mL) for an additional 48 hours. After that point, cells were maintained in DMEM containing 10% FBS with the medium being changed every 48 hours until they were harvested for further analysis.
Two experimental designs were performed to evaluate the effect of high glucose (D (+) glucose, G-5400, Sigma-Aldrich) concentration in the 3T3-L1 adipocyte differentiation process and in mature adipocytes (Fig. 1). In the first approach, 3T3-L1 cells were chronically exposed to glucose at a concentration of 25 mM (added over the 25 mM concentration already present in the DMEM culture medium) since the induction of the differentiation process on the second day post-confluence (d0), when they are still preadipocytes, until reaching maturation at days 7 and 21. 25 mM glucose was added to the renewal media every 48 hours. In the second protocol, adipocytes differentiated during 7 days were exposed to high glucose levels (25 mM added over the 25 mM concentration already present in the DMEM culture medium) for 48 hours, with the media being replaced after the first 24 hours with fresh media containing extra-25 mM glucose. We have chosen 50 mM glucose concentration in order to reach a high glucose level that does not induce a marked decrease in lipid accumulation and adipogenesis markers.22
Oil Red O staining
The amount of intracellular triglycerides accumulated by control and glucose-exposed 3T3-L1 cells in each stage of the differentiation process was estimated by the specific dye Oil Red O (O0625 Sigma Aldrich). This method is considered a useful way to measure the degree of adipocytic maturation achieved by the cells. The checkpoints selected for the long-term protocol were days 0, 7 and 21, while for the short-term exposure the determinations were performed at days 7 and 9. Cells were washed twice with phosphate buffered saline (PBS) and fixed with formaldehyde 3.7% for 2 hours. After being washed 3 times with isopropanol 60%, cells were stained with Oil Red O (0.5% in isopropanol) diluted to 40% with water, for 30 minutes at room temperature. Excess stain was removed by washing the cells 4 times with ethanol 70%. After drying, cells were photographed under a light microscope (Olympus Ck2, 40 X magnifications). Oil Red dye was dissolved in isopropanol and the resulting solution was spectrophotometrically quantified at 540 mM (Multiskan Spectrum, Thermo Electron Corporation, MA, USA).
Cell viability
Cell viability and proliferation were determined by the MTT tetrazolium-based colorimetric assay. The cells were seeded and differentiated in 96-well plates, following the instructions previously described for the long-term (d0, d7 and d21) and short-term (d7 and d9) glucose treatments. Cells were washed twice with PBS and incubated for an hour with free serum DMEM (419660-029 Invitrogen) containing 1 mg/mL of MTT (M5655 Sigma Aldrich) in a humidified incubator at 37°C, 5% CO2. The medium was then removed and incorporated MTT was dissolved by adding 100 μL of DMSO. The solution obtained was quantified spectrophotometrically at 540 nM (Multiskan Spectrum, Thermo Electron Corporation).
Methylation analysis of Cav-1 and IR genes
The region of the Cav-1 gene under study encompasses from 619 bp 5′ to 1333 bp 3′ of the ATG codon and includes 2 CpG islands (70 CpG sites) located in the proximal promoter, exon 1 and the first intron of the gene. On the other hand, the selected region of the IR gene includes 40 CpG dinucleotides distributed along a zone of the first exon and the first intron (249 bp 5′ to 545 bp 3′ of the ATG codon). Both, Cav-1 and IR gene sequences have been spelled in a previous work of our group, highlighting and numbering all the CpG sites located in these regions.21 Genomic DNA was extracted and purified from 3T3-L1 cells using the QIAamp DNA kit (51304 Qiagen) at every checkpoint established for the long-term (d0 and d21) and the short-term (d7 and d9) high glucose treatment. DNA concentration and quality were measured by Picogreen reagent (P11496 Invitrogen). The methylation percentage of each CpG dinucleotide under study was determined by the MassArray Epityper technique (Sequenom Inc..), performed in the Central Research Unit of the School of Medicine (UCIM) of the University of Valencia (Spain), as previously reported.21 For technical reasons, 9 CpG sites were excluded from the analysis of the Cav-1 gene. All the measurements were performed in triplicate.
Real Time RT-PCR
Total RNA from 3T3-L1 preadipocytes (d0) and control/glucose-exposed adipocytes harvested during the long-term protocol (d7, d21), was extracted from each sample using DNA/RNA/Protein Mini Kit (80004 Qiagen) according to the manufacturer's instructions. In the case of the short-term control/glucose-exposed mature adipocytes (d7 and d9), the total RNA was isolated using TRIzol® reagent (15596-026 Invitrogen). RNA concentration and quality were determined with a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Each RNA sample was subsequently treated with DNase I (DNA-Free Kit, AM 1906 Ambion) to remove possible contaminating genomic DNA before proceeding to reverse transcription. Two micrograms of purified total RNA were employed for first-strand cDNA synthesis using the enzyme M-MLV reverse transcriptase (28025-013 Invitrogen) and random hexamers as primers (N8080-127 Invitrogen), according to the manufacturer's instructions. Predesigned TaqMan® probes (Applied Biosystems) were used to perform real-time PCR for caveolin-1 (Cav-1, Mm_00483057_m1), insulin receptor (IR, Mm_01211875_m1), protein kinase B (AKT-2, Mm02026778_g1) and glucose transporter type 4 (Glut-4, Mm00436615_m1). The cDNA was amplified in an ABI Prism 7300 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA), using the TaqMan Universal PCR Master Mix (4304437 Applied Biosystems) according to standard conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. A probe for cyclophilin A (Ppia, Mm02342430_g1) was used as invariant internal control for RT-PCR and subsequent normalization. Relative gene quantification was calculated by the 2-ΔΔCt method. Data from long-term glucose conditions were referenced to day 0 whereas data from shot-term glucose treatment were referenced to day 7 as the calibrator sample.
Western Blot analysis
In the case of the long-term glucose protocol, proteins were extracted from 3T3-L1 preadipocytes (d0) and control/glucose-exposed adipocytes (d7, d21) using the DNA/RNA/Protein Mini Kit (80004 Qiagen) according to the manufacturer's instructions. After washing cells with ice-cold PBS, proteins from 3T3-L1 control/glucose-exposed mature adipocytes following the short-term protocol (d7, d9) were extracted by cell lysis with an specific protein extraction buffer containing glycerol 87%, Tris-HCl 2M, NaCl 5M, Triton 10%, NaF 1M and sodium orthovanadate 0.2M supplemented with protease inhibitor cocktail, phosphatase inhibitor cocktail and phenylmethanesulfonyl fluoride (36978 Pierce). Proteins were purified from the lysate by centrifugation at 13,000 rpm, 4 °C for 5 minutes. All protein samples were quantified by the bicinchoninic acid (BCA) assay (23225 Pierce) before subjecting them to Western Blot analysis. Total proteins were fractionated by SDS-PAGE and electrotransferred onto a Hybond-C Extra nitrocellulose membrane (RPN303E Amersham GE-Health). After blocking the membrane with 5% milk in 0.05% Tris buffered saline with Tween 20 (TBST) for 2 hours, it was incubated overnight with the suitable primary antibody dilution at 4°C. Next, the membrane was washed 3 times with TBST before being incubated with an appropriate dilution of the horseradish peroxidase (HRP)-conjugated secondary antibody. Finally, after 3 more washes, specific immunoreactive bands were detected by a chemiluminescent ECL assay kit (Amersham-Pharmacia). Primary antibodies specific for Cav-1 (sc-894, Santa Cruz Biotechnology), IR (sc-711, Santa Cruz Biotechnology), AKT (# 9272, Cell Signaling) and Glut-4 (G4048, Sigma-Aldrich) were used. Specific secondary HRP-labeled rabbit (170–6515, Bio-Rad) or mouse (NXA931, GE Healthcare) antibodies were employed depending on the primary antibody origin. β-Actin (A1978, Sigma-Aldrich) was used as an invariant internal control for sample normalization.
The same methodology was used to measure relative protein activation by phosphorylation, before and after stimulating cells with 50 nm insulin (91077C Sigma Aldrich) for 10 min. Specific primary anti-phospho protein antibodies for Cav-1 (Tyr14, 611339, BD Transduction Laboratories), IR (Tyr1146, # 3021, Cell Signaling) and AKT (Ser473, # 9271, Cell Signaling) were used for this purpose.
2-[C14]-Deoxyglucose uptake
For both experimental designs, long-term (d7 and d21) and short-term (d7 and d9) high glucose treatments, baseline and insulin-induced 2-[C14]-deoxyglucose uptake was measured. At first, cells were incubated in serum and glucose-free DMEM (11966-025 Invitrogen) for 2 hours in an incubator at 37 °C, 5% CO2, 95% humidity. Subsequently, cells were incubated in the presence or absence of insulin (50 nM, 91077C Sigma Aldrich) for 10 min before 2-[C14]-deoxyglucose uptake assay was initiated. Serum and glucose-free DMEM containing 2-deoxyglucose (50 μM) and 2-[C14]-deoxyglucose (0.075 μCi /mL, ARC0111, American Radiolabeled) was then added to the cells and they were incubated for another 10 min at 37 °C, 5% CO2, 95% humidity. The reaction was terminated by washing 3 times with pre-cold PBS containing 0.05 M glucose. Next, cells were incubated with a lysis buffer containing 0.1 M NaOH and 0.1 % SDS at 37°C for 2 hours and the cell lysate (100 μL) was transferred to a tube containing 2 mL of scintillation liquid for radioactivity counting using a scintillation counter (Wallac 1214 Rackbeta Conter, Perkin Elmer Life Sciences, Waltham, MA, USA). 2-Deoxyglucose uptake was reported as [C14] radioactivity, normalized to protein content from the remaining cell lysate as determined by BCA analysis (23225 Pierce). Measurements were performed in triplicate under conditions where hexose uptake was linear.
Secreted adipokines in the supernatant
ELISA kits from Millipore were employed to quantify leptin (Cat. # EZML-82K) and adiponectin (Cat. # EZMAKP-60K) secreted to the culture media, following the manufacturer's instructions.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Depending on the number of samples analyzed, statistical significance of differences among the groups was checked by using the ANOVA for a single factor and Dunnett's test (n ≥ 10) or applying the Wilcoxon signed rank test (n ≤10). All analyses were performed using SPSS version 15.0 for Windows (SPSS Inc.).
Abbreviations
- AKT-2
Protein kinase B
- BCA
Bicinchoninic acid
- Cav-1
Caveolin-1
- CBS
Calf blood serum
- FBS
Fetal bovine serum
- Glut-4
Glucose transporter 4
- IBMX
3-isobutyl-1-methylxanthine
- IL-6
Interleukin 6
- IR
Insulin receptor
- IRS-1
Insulin receptor substrate 1
- MSCs
Mesenchymal stem cells
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PCOS
Polycystic Ovary Syndrome
- PI3K
Phosphatidylinositol-3-kinase
- PTP1B
Tyrosine phosphatase 1B
- TLR
Toll-like receptors
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
SPO and MVG have a predoctoral fellowship from the Asociación de Amigos of the University of Navarra.
Funding
This study was supported by CIBERobn and Nutrigenio project (ref. AGL2013-45554-R, MINECO, Spain).
References
- 1.Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of type 2 diabetes mellitus and emerging treatment strategies. Diabet Med 2009; 26:1185-92; PMID:20002468; http://dx.doi.org/ 10.1111/j.1464-5491.2009.02847.x [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414:813-20; PMID:11742414; http://dx.doi.org/ 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- 3.Richter EA, Hansen BF, Hansen SA. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochem J 1988; 252:733-7; PMID:3421919; http://dx.doi.org/ 10.1042/bj2520733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang CC, Yang RS, Tsai KS, Ho FM, Liu SH. Hyperglycemia enhances adipogenic induction of lipid accumulation: Involvement of extracellular signal-regulated protein kinase 1/2, phosphoinositide 3-kinase/akt, and peroxisome proliferator-activated receptor gamma signaling. Endocrinology 2007; 148:4267-75; PMID:17540722; http://dx.doi.org/ 10.1210/en.2007-0179 [DOI] [PubMed] [Google Scholar]
- 5.Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, et al.. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A 2008; 105:1226-31; PMID:18212116; http://dx.doi.org/ 10.1073/pnas.0711402105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010; 316:129-39; PMID:19723556; http://dx.doi.org/ 10.1016/j.mce.2009.08.018 [DOI] [PubMed] [Google Scholar]
- 7.Knights AJ, Funnell AP, Pearson RC, Crossley M, Bell-Anderson KS. Adipokines and insulin action: A sensitive issue. Adipocyte 2014; 3:88-96; PMID:24719781; http://dx.doi.org/ 10.4161/adip.27552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bluher M. Clinical relevance of adipokines. Diabetes Metab J 2012; 36:317-27; PMID:23130315; http://dx.doi.org/ 10.4093/dmj.2012.36.5.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 2011; 50:567-75; PMID:21163346; http://dx.doi.org/ 10.1016/j.freeradbiomed.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, et al.. The hyperglycemia-induced inflammatory response in adipocytes: The role of reactive oxygen species. J Biol Chem 2005; 280:4617-26; PMID:15536073; http://dx.doi.org/ 10.1074/jbc.M411863200 [DOI] [PubMed] [Google Scholar]
- 11.Gao CL, Zhu C, Zhao YP, Chen XH, Ji CB, Zhang CM, Zhu JG, Xia ZK, Tong ML, Guo XR. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol 2010; 320:25-33; PMID:20144685; http://dx.doi.org/ 10.1016/j.mce.2010.01.039 [DOI] [PubMed] [Google Scholar]
- 12.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005; 115:1111-9; PMID:15864338; http://dx.doi.org/ 10.1172/JCI200525102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang S, Le-Tien H, Goldstein BJ, Shin P, Lai R, Fantus IG. Decreased in situ insulin receptor dephosphorylation in hyperglycemia-induced insulin resistance in rat adipocytes. Diabetes 2001; 50:83-90; PMID:11147799; http://dx.doi.org/ 10.2337/diabetes.50.1.83 [DOI] [PubMed] [Google Scholar]
- 14.Gagnon A, Sorisky A. The effect of glucose concentration on insulin-induced 3T3-L1 adipose cell differentiation. Obes Res 1998; 6:157-63; PMID:9545023; http://dx.doi.org/ 10.1002/j.1550-8528.1998.tb00330.x [DOI] [PubMed] [Google Scholar]
- 15.Nystrom FH, Chen H, Cong LN, Li Y, Quon MJ. Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected cos-7 cells and rat adipose cells. Mol Endocrinol 1999; 13:2013-24; PMID:10598578; http://dx.doi.org/ 10.1210/mend.13.12.0392 [DOI] [PubMed] [Google Scholar]
- 16.Karlsson M, Thorn H, Parpal S, Stralfors P, Gustavsson J. Insulin induces translocation of glucose transporter GLUT4 to plasma membrane caveolae in adipocytes. FASEB J 2002; 16:249-51; PMID:11744627 [DOI] [PubMed] [Google Scholar]
- 17.Yuan T, Hong S, Yao Y, Liao K. Glut-4 is translocated to both caveolae and non-caveolar lipid rafts, but is partially internalized through caveolae in insulin-stimulated adipocytes. Cell Res 2007; 17:772-82; PMID:17846641; http://dx.doi.org/ 10.1038/cr.2007.73 [DOI] [PubMed] [Google Scholar]
- 18.Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 2003; 285:C222-35; PMID:12660144; http://dx.doi.org/ 10.1152/ajpcell.00006.2003 [DOI] [PubMed] [Google Scholar]
- 19.Zhu LL, Cui Y, Chang YS, Fang FD. A second protein marker of caveolae: Caveolin-2. Chin Med Sci J 2010; 25:119-24; PMID:20598236; http://dx.doi.org/ 10.1016/S1001-9294(10)60034-X [DOI] [PubMed] [Google Scholar]
- 20.Kwon H, Lee J, Jeong K, Jang D, Pak Y. Fatty acylated caveolin-2 is a substrate of insulin receptor tyrosine kinase for insulin receptor substrate-1-directed signaling activation. Biochim Biophys Acta 2015; 1853:1022-34; PMID:25667086; http://dx.doi.org/ 10.1016/j.bbamcr.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 21.Palacios-Ortega S, Varela-Guruceaga M, Milagro FI, Martinez JA, de Miguel C. Expression of caveolin 1 is enhanced by DNA demethylation during adipocyte differentiation. status of insulin signaling. PLoS One 2014; 9:e95100; PMID:24751908; http://dx.doi.org/ 10.1371/journal.pone.0095100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shilpa K, Dinesh T, Lakshmi BS. An in vitro model to probe the regulation of adipocyte differentiation under hyperglycemia. Diabetes Metab J 2013; 37:176-80; PMID:23807920; http://dx.doi.org/ 10.4093/dmj.2013.37.3.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Zhang X, Zheng J, Yang J. High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol Cell Biochem 2010; 338:115-22; PMID:19949837; http://dx.doi.org/ 10.1007/s11010-009-0344-6 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Yang JH. Activation of the PI3K/akt pathway by oxidative stress mediates high glucose-induced increase of adipogenic differentiation in primary rat osteoblasts. J Cell Biochem 2013; 114:2595-602; PMID:23757055; http://dx.doi.org/ 10.1002/jcb.24607 [DOI] [PubMed] [Google Scholar]
- 25.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11:85-97; PMID:21252989; http://dx.doi.org/ 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trujillo ME, Scherer PE. Adipose tissue-derived factors: Impact on health and disease. Endocr Rev 2006; 27:762-78; PMID:17056740; http://dx.doi.org/ 10.1210/er.2006-0033 [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Eliasson B, Smith U, Cushman SW, Sherman AS. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity 2012; 20:932-8; PMID:22240722; http://dx.doi.org/ 10.1038/oby.2011.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Proinflammatory effects of glucose and anti-inflammatory effect of insulin: Relevance to cardiovascular disease. Am J Cardiol 2007; 99:15B-26B; PMID:17307055; http://dx.doi.org/ 10.1016/j.amjcard.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 29.Levy JR, Stevens W. Plasma hyperosmolality stimulates leptin secretion acutely by a vasopressin-adrenal mechanism. Am J Physiol Endocrinol Metab 2004; 287:E263-8; PMID:15068959; http://dx.doi.org/ 10.1152/ajpendo.00514.2003 [DOI] [PubMed] [Google Scholar]
- 30.Sivitz WI, Walsh S, Morgan D, Donohoue P, Haynes W, Leibel RL. Plasma leptin in diabetic and insulin-treated diabetic and normal rats. Metabolism 1998; 47:584-91; PMID:9591751; http://dx.doi.org/ 10.1016/S0026-0495(98)90244-X [DOI] [PubMed] [Google Scholar]
- 31.Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Stern JS, Havel PJ. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology 1998; 139:551-8; PMID:9449624 [DOI] [PubMed] [Google Scholar]
- 32.Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes 1999; 48:1275-80; PMID:10342816; http://dx.doi.org/ 10.2337/diabetes.48.6.1275 [DOI] [PubMed] [Google Scholar]
- 33.Pan Z, Wang H, Liu Y, Yu C, Zhang Y, Chen J, Wang X, Guan Q. Involvement of CSE/ H2S in high glucose induced aberrant secretion of adipokines in 3T3-L1 adipocytes. Lipids Health Dis 2014; 13:155; PMID:25277804; http://dx.doi.org/ 10.1186/1476-511X-13-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Xu Y, Dai Z, Sun Y. Intermittent high glucose stimulate MCP-l, IL-18, and PAI-1, but inhibit adiponectin expression and secretion in adipocytes dependent of ROS. Cell Biochem Biophys 2009; 55:173-80; PMID:19756411; http://dx.doi.org/ 10.1007/s12013-009-9066-3 [DOI] [PubMed] [Google Scholar]
- 35.Martinez JA, Milagro FI, Claycombe KJ, Schalinske KL. Epigenetics in adipose tissue, obesity, weight loss, and diabetes. Adv Nutr 2014; 5:71-81; PMID:24425725; http://dx.doi.org/ 10.3945/an.113.004705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patra SK, Bettuzzi S. Epigenetic DNA-methylation regulation of genes coding for lipid raft-associated components: A role for raft proteins in cell transformation and cancer progression (review). Oncol Rep 2007; 17:1279-90; PMID:17487380 [PubMed] [Google Scholar]
- 37.Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr 2009; 139:1054-60; PMID:19339705; http://dx.doi.org/ 10.3945/jn.109.104653 [DOI] [PubMed] [Google Scholar]
- 38.Palacios-Ortega S, Varela-Guruceaga M, Algarabel M, Milagro FI, Martinez JA, de Miguel C. Effect of TNF-α on caveolin-1 expression and insulin signaling during adipocyte differentiation and in mature adipocytes. Cell Physiol Biochem 2015; 36:1499-516; PMID:26159107; http://dx.doi.org/ 10.1159/000430314 [DOI] [PubMed] [Google Scholar]
- 39.Li J, Houseknecht KL, Stenbit AE, Katz EB, Charron MJ. Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice. FASEB J 2000; 14:1117-25; PMID:10834933 [DOI] [PubMed] [Google Scholar]
- 40.Garvey WT, Huecksteadt TP, Matthaei S, Olefsky JM. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. J Clin Invest 1988; 81:1528-36; PMID:3366906; http://dx.doi.org/ 10.1172/JCI113485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karnieli E, Barzilai A, Rafaeloff R, Armoni M. Distribution of glucose transporters in membrane fractions isolated from human adipose cells. relation to cell size. J Clin Invest 1986; 78:1051-5; PMID:3531236; http://dx.doi.org/ 10.1172/JCI112660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oleszczak B, Szablewski L, Pliszka M. The effect of hyperglycemia and hypoglycemia on glucose transport and expression of glucose transporters in human lymphocytes B and T: An in vitro study. Diabetes Res Clin Pract 2012; 96:170-8; PMID:22257417; http://dx.doi.org/ 10.1016/j.diabres.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 43.Dimitrakoudis D, Vranic M, Klip A. Effects of hyperglycemia on glucose transporters of the muscle: Use of the renal glucose reabsorption inhibitor phlorizin to control glycemia. J Am Soc Nephrol 1992; 3:1078-91; PMID:1482748 [DOI] [PubMed] [Google Scholar]
- 44.Satoh T. Molecular mechanisms for the regulation of insulin-stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. Int J Mol Sci 2014; 15:18677-92; PMID:25325535; http://dx.doi.org/ 10.3390/ijms151018677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. central role of tumor necrosis factor-α. J Clin Invest 1994; 94:1543-9; PMID:7523453; http://dx.doi.org/ 10.1172/JCI117495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 1996; 271:665-8; PMID:8571133; http://dx.doi.org/ 10.1126/science.271.5249.665 [DOI] [PubMed] [Google Scholar]
- 47.Kimura A, Mora S, Shigematsu S, Pessin JE, Saltiel AR. The insulin receptor catalyzes the tyrosine phosphorylation of caveolin-1. J Biol Chem 2002; 277:30153-8; PMID:12036959; http://dx.doi.org/ 10.1074/jbc.M203375200 [DOI] [PubMed] [Google Scholar]
- 48.Renstrom F, Buren J, Svensson M, Eriksson JW. Insulin resistance induced by high glucose and high insulin precedes insulin receptor substrate 1 protein depletion in human adipocytes. Metabolism 2007; 56:190-8; PMID:17224332; http://dx.doi.org/ 10.1016/j.metabol.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 49.Buren J, Lindmark S, Renstrom F, Eriksson JW. In vitro reversal of hyperglycemia normalizes insulin action in fat cells from type 2 diabetes patients: Is cellular insulin resistance caused by glucotoxicity in vivo? Metabolism 2003; 52:239-45; PMID:12601640; http://dx.doi.org/ 10.1053/meta.2003.50041 [DOI] [PubMed] [Google Scholar]
- 50.Muller HK, Kellerer M, Ermel B, Muhlhofer A, Obermaier-Kusser B, Vogt B, Haring HU. Prevention by protein kinase C inhibitors of glucose-induced insulin-receptor tyrosine kinase resistance in rat fat cells. Diabetes 1991; 40:1440-8; PMID:1657668; http://dx.doi.org/ 10.2337/diab.40.11.1440 [DOI] [PubMed] [Google Scholar]
- 51.Berti L, Mosthaf L, Kroder G, Kellerer M, Tippmer S, Mushack J, Seffer E, Seedorf K, Haring H. Glucose-induced translocation of protein kinase C isoforms in rat-1 fibroblasts is paralleled by inhibition of the insulin receptor tyrosine kinase. J Biol Chem 1994; 269:3381-6; PMID:7508912 [PubMed] [Google Scholar]
- 52.Ormazabal P, Romero C, Gabler F, Quest AF, Vega M. Decreased phosphorylation of Y(1)(4)caveolin-1 in endometrial tissue of polycystic ovary syndrome patients may be related with an insulin resistant state in this tissue. Horm Metab Res 2013; 45:291-6; PMID:23225242 [DOI] [PubMed] [Google Scholar]
- 53.Lee H, Xie L, Luo Y, Lee SY, Lawrence DS, Wang XB, Sotgia F, Lisanti MP, Zhang ZY. Identification of phosphocaveolin-1 as a novel protein tyrosine phosphatase 1B substrate. Biochemistry 2006; 45:234-40; PMID:16388599; http://dx.doi.org/ 10.1021/bi051560j [DOI] [PubMed] [Google Scholar]
- 54.Popov D, Nemecz M, Dumitrescu M, Georgescu A, Bohmer FD. Long-term high glucose concentration influences akt, ERK1/2, and PTP1B protein expression in human aortic smooth muscle cells. Biochem Biophys Res Commun 2009; 388:51-5; PMID:19647719; http://dx.doi.org/ 10.1016/j.bbrc.2009.07.141 [DOI] [PubMed] [Google Scholar]
- 55.Storgaard H, Song XM, Jensen CB, Madsbad S, Bjornholm M, Vaag A, Zierath JR. Insulin signal transduction in skeletal muscle from glucose-intolerant relatives of type 2 diabetic patients Diabetes 2001; 50:2770-8; PMID:11723060; http://dx.doi.org/ 10.2337/diabetes.50.12.2770 [DOI] [PubMed] [Google Scholar]
- 56.Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, Jo A, Park SB, Han W, Kim JB. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol 2015; 35:1686-99; PMID:25733684; http://dx.doi.org/ 10.1128/MCB.01321-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller G. Take-over: Multiple mechanisms of inter-adipocyte communication. J Mol Cell Biol 2011; 3:81-90; PMID:21459887; http://dx.doi.org/ 10.1093/jmcb/mjr003 [DOI] [PubMed] [Google Scholar]


