Abstract
Background
Migraine is comorbid with obesity. Recent research suggests an association between migraine and adipocytokines, proteins that are predominantly secreted from adipose tissue and which participate in energy homeostasis and inflammatory processes.
Objectives
In this review, we first briefly discuss the association between migraine and obesity and the importance of adipose tissue as a neuroendocrine organ. We then present a systematic review of the extant literature evaluating circulating levels of adiponectin and leptin in those with migraine.
Methods
A search of the PubMed database was conducted using the keywords “migraine,” “adiponectin,” and “leptin.” In addition reference lists of relevant articles were reviewed for possible inclusion. English language studies published between 2005 and 2015 evaluating circulating blood concentration of adiponectin or leptin in those with migraine were included.
Conclusions
While the existing data are suggestive that adipokines may be associated with migraine, substantial study design differences and conflicting results limit definitive conclusions. Future research utilizing carefully considered designs and methodology is warranted. In particular careful and systematic characterization of pain states at the time of samples, as well as systematic consideration of demographic (eg, age, sex) and other vital covariates (eg, obesity status, lipids) are needed to determine if adipokines play a role in migraine pathophysiology and if any adipokine represents a viable, novel migraine biomarker, or drug target.
Keywords: adipokines, adiponectin, leptin, migraine, obesity
INTRODUCTION
Migraine is comorbid with obesity.1 While this observed association is not new, it is only recently that studies have focused on how obesity-related bioactive substances might be involved in migraine pathophysiology. Multiple bioactive substances (eg, serotonin, dopamine, calcitonin-gene related protein, histamine) that are targeted by migraine therapies have long been recognized to also play important roles in energy homeostasis.2,3 More recently, translational human research suggests an association between migraine and the adipokines, a class of cell-signaling proteins that participate in both energy homeostasis and inflammation and which are predominantly secreted from adipose tissue.2,3
In this review, we first briefly discuss the association between migraine and obesity and the importance of adipose tissue as a neuroendocrine organ. We then focus on the association between migraine and 2 bioactive products of adipose tissue – the adipokines adiponectin (ADP) and leptin. We present a brief summary of the role and function of each adipokine, followed by a systematic review of the human research studies that have evaluated circulating levels of ADP or leptin in migraineurs.
Obesity and Migraine
Complete reviews on the epidemiology and potential mechanisms for the migraine and obesity association have been recently published and is beyond the scope of the current manuscript.1,2,4 In brief, the prevalence of migraine is increased in individuals who are obese as compared to normal weight and increases with increasing obesity status (ie, from normal weight to overweight to obese).5–7 Additionally, the association between migraine and obesity may be more evident in women as compared to men and in those under the age of 50–55 years as compared to older individuals (Fig. 1).1,4–7 Further, although there is still uncertainty about whether the increased risk of migraine associated with obesity is stronger for chronic as compared to episodic migraine (EM),1 the prevalence of chronic migraine8,9 and the transformation from episodic to chronic daily headache10 is increased in those who are obese.
Fig. 1.
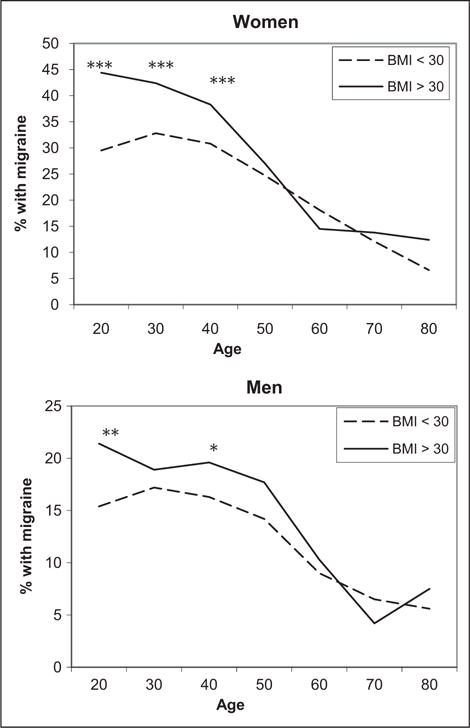
The prevalence of migraine/severe headache in obese and non-obese women and men by age. In the National Health and Examination Survey, the prevalence of migraine and severe headaches was increased in obese compared to non-obese adults under 55 years of age and not in those older than 55. ***P ≤ .001; **P ≤ .01; *P ≤ .05. Adapted with permission from Headache.5
One reason why obesity may increase susceptibility to migraine is that obesity is often (although not always) associated with changes in the function of adipose tissue and increased inflammation.2,11 Notably, with advancing age the physiological function of adipose tissue is modified and the disease risk of obesity is attenuated.12–19
Adipose Tissue and Adipokines
Since the mid to late 1990s, it has been recognized that adipose tissue is a highly functioning neuroendocrine organ that is centrally regulated by the hypothalamus and its connections. Adipose tissue itself is composed of adipocytes, embedded in a loose connective tissue meshwork that contains adipocyte precursors and other immune cells, and has a substantial vascular and nervous supply.11 With adipose tissue expansion during weight gain, macrophage recruitment increases and changes in receptor expression and in the secretion of cytokines and adipokines occur.11 In the following sections, we give a brief description of the function and roles of the adipokines, ADP, and leptin, followed by a systematic review of the human research studies evaluating circulating levels of these adipokines in those with migraine.
Adiponectin
ADP is an adipokine predominantly produced by adipocytes (subcutaneous > visceral adipose tissue) with important roles in energy homeostasis, glucose and lipid homeostasis, and inflammation.20–25 ADP exists in high concentrations in the circulation as total ADP or as one of the low to high molecular weight (HMW) multimers (HMW-ADP, middle molecular weight [MMW]-ADP, low molecular weight [LMW]-ADP).20 ADP is also present in cerebrospinal fluid, although at lower concentrations than in blood and with the notable absence of the HMW multimer.23
ADP and its multimers exhibit sex differences (HMW: women > men; LMW: women ≤ men), age differences (with increases with age in both sexes but more so in men) and are capable of modulating opposing inflammatory processes (Fig. 2A).26,27 For example, HMW-ADP is capable of activating proinflammatory pathways and is associated with increases in interleukin (IL)-6 levels whereas LMW-ADP activates anti-inflammatory pathways and is associated with reductions in IL-6 levels.26 Additionally, ADP levels correlate with obesity status (lean > obese) as well as with insulin sensitivity and serum lipids.28
Fig. 2.
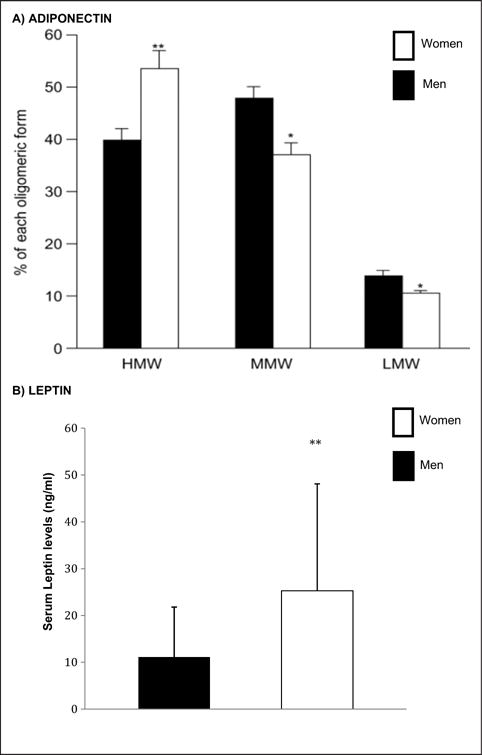
Sexual dimorphism of adiponectin (A) and leptin (B). (A) Serum levels of the adiponectin multimers from women and age- and body mass index-matched men were evaluated using an enzyme immunoassay. Adapted from Ref. 24. (B) Serum leptin levels from women and men were evaluated by enzyme immunoassay. Figure data from Ref. 25. *P < .05; **P < .01.
In the brain, ADP receptors have been shown to be expressed in the cortex, hypothalamus, brainstem, and circumventricular organs (eg, subfornical organ) as well as the endothelium of the cerebral microvasculature.20,29,30 ADP signaling through its receptors is mediated by multiple pathways including some implicated in migraine such as nuclear factor kappa beta (NFkβ), AMP-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK), and endothelial nitric oxide synthase (e-NOS) as well others (eg, peroxisome proliferator-activated receptors).20,30
Leptin
Like ADP, leptin is predominantly produced by adipocytes and has roles in energy homeostasis, glucose and lipid homeostasis, and inflammation. Leptin levels are greater in reproductive age women than men (Fig. 2B). However, although leptin levels increase with age in men, age-related changes are controversial in women, with some studies reporting no change and others showing decreased levels with age.27 As with ADP, peripheral levels of leptin have been shown to be associated with obesity status, lipid levels, and insulin sensitivity.31,32 Leptin is also capable of crossing the blood-brain barrier and is present in the CSF.33
Leptin receptors are widespread throughout the body including the cortex, hypothalamus, brainstem, and endothelium of the cerebral microvasculature. There are at least 6 leptin receptor isoforms. The long isoform is abundantly expressed in the hypothalamus and to less extent in other non-CNS organs (eg, liver, kidney), whereas the short isoforms of the leptin receptor can be found in almost all tissues tested to date. Like ADP, leptin signal transduction through its receptors is mediated through multiple pathways including those implicated in migraine such as NFkB, AMPK, MAPK, and e-NOS as well as others (eg, Janus kinase/signal transducers and activators of transcription).27,31–33
METHODS
Publication Search
Given the substantial heterogeneity across the cohorts and study designs of these studies, meaningful interpretation of a formal meta-analysis was not deemed possible; and a systematic review undertaken. A systematic search of PubMed database was conducted on May 7, 2015 using the key words “migraine AND adiponectin” and “migraine AND leptin” by the first author and repeated and verified by senior author. In addition, reference lists of relevant articles and manuscripts known to any of the authors who evaluated adipokines in those with migraine but which were not otherwise identified in the search, were reviewed for possible inclusion. All English language manuscripts, published between 2005 and 2015, evaluating circulating blood concentrations of ADP or leptin in women and men with migraine as compared to controls, or before and after treatment, were included. In the case of multiple manuscripts based on the same (or overlapping) study populations, only the manuscript based on the largest cohort was included. In cases where a manuscript utilizing an overlapping cohort reported additional non-overlapping data (eg, new study with addition of men to previously utilized cohort of women) both studies were included and this was noted accordingly. Studies published only in abstract form were excluded but are presented briefly in Supporting Information Table 1 for those with interest. All included full-text manuscripts were reviewed by each of the authors and the following information extracted to standardized tables: first author, publication year, study design, sample size, population included, presence or absence of headache in controls, mean age and sex of study participants, how obesity status was assessed, study exclusions, baseline laboratories performed, as well as crude and, if available, adjusted adipokine levels.
RESULTS
Identification of Adiponectin and Migraine Studies
Nineteen manuscripts were identified through the search terms “migraine AND adiponectin” (Fig. 3). Additional searches including each of the ADP multimers (HMW, MMW, LMW-ADP) did not reveal any further studies. Review of references from these 19 manuscripts and related-review manuscripts2,34 and those known by the authors identified 3 additional manuscripts35–37 Of these 22 manuscripts, 10 were reviews or editorials, 2 did not evaluate ADP, and 1 evaluated ADP levels only in migraineurs with and without adverse childhood events38 and were thus excluded. Two studies published only as an abstract (identified from review of references from a previous review on the topic39 or known to the authors) were excluded from the current systematic review but are included in Supporting Information Table 1 for those with interest.38,40
Fig. 3.
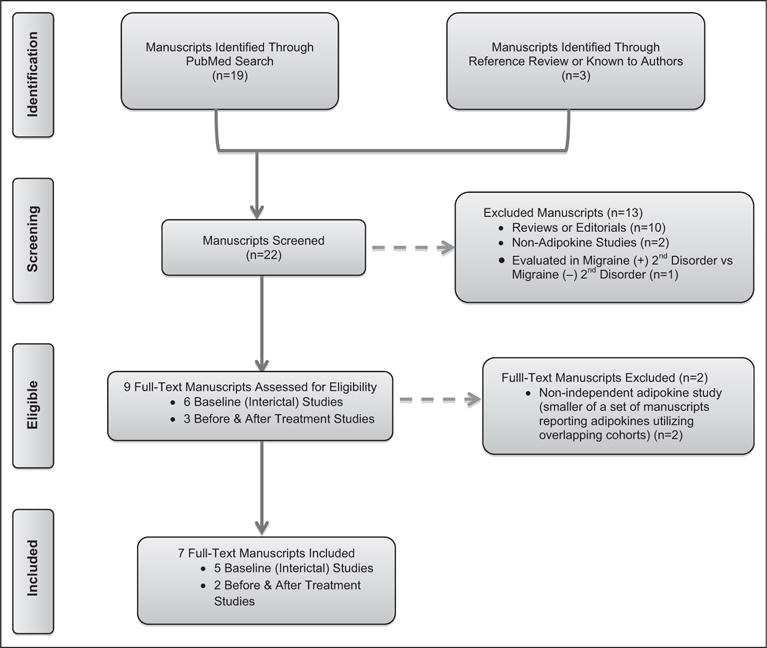
PRISMA flow diagram of manuscripts evaluating blood levels of adiponectin in those with migraine.
Of the remaining 9 manuscripts, 6 included interictal evaluations of adipokines35–37,41–43 and 3 were manuscripts evaluating blood levels of total-ADP (T-ADP) or ADP multimers before and after abortive or preventive therapy in those with migraine.30,44,45 Full review of the 6 interictal manuscripts identified 3 interictal manuscripts that utilized substantially overlapping cohorts.35–37 The manuscript reporting adipokines levels in the smallest sample of this cohort was thus excluded.35 While some overlap also occurred between the remaining two,36,37 we chose to include both as the smaller of these 2 manuscripts36 included data not available in the largest of these manuscripts by the addition of men to the original cohort of women,36 leaving a total of 5 interictal manuscripts. Finally, full review of the 3 manuscripts evaluating ADP before and after treatment identified 2 manuscripts that utilized overlapping cohorts,30,45 of which the manuscript utilizing the smaller cohort was excluded,45 leaving a total of 2 before and after treatment manuscripts.
Adiponectin and Migraine Manuscript Reviews and Discussion
Interictal ADP and Migraine
Five peer-reviewed manuscripts included evaluations of interictal peripheral blood levels of ADP or ADP multimers in those with migraine as compared to controls.36,37,41–43 Crude total ADP levels were significantly increased in migraineurs as compared to controls in 3 of these 5 manuscripts, spanning 3 distinct and separate cohorts41–43; and were not significantly different in the 2 manuscripts utilizing overlapping cohorts for differing primary aims but which included crude ADP in each (Table 1).36,37 In the following paragraphs, we first briefly review each of these manuscripts then summarize the data as a whole and discuss where future research is needed.
Table 1.
Crude Interictal Adipokine Levels in Studies Comparing Those with Migraine vs Controls
| Author and Year (Journal) | Controls | Migraine | Direction of Change | P-value |
|---|---|---|---|---|
| Crude Total-ADP Levels (μg/mL†) | ||||
| Peterlin (2008) (N) | 7.5 ± 2.4 | 10.1 ± 4 (CM)‡ | ↑ | .024 |
| 8.6 ± 3.5 (EM)‡ | ||||
| Bernecker (2011) (EJN Vol 18 page 571)§ | 16. ± 610 | 16.6 ± 7.4 | — | >.05 |
| Bernecker (2011) (EJN Vol 18 page 1233)§ | 18.3 ± 11.2 | 16.1 ± 7.6 | — | >.05 |
| Duarte (2014) (JNS) | 36.6 ± 9.7 | 43.6 ± 11.8 ng/mL | ↑ | <.0001 |
| Dearborn (2014) (N) | 7.0 ± 0.2 | 8.3 ± 0.6 (DM) | ↑ | .05 |
| 8.1 ± 0.5 (PM+DM) | ↑ | .031 | ||
| Crude Leptin Levels (ng/mL) | ||||
| Guldiken (2008) (HA) | 49 ± 25 | 40 ± 21 | ↓ | <.05 |
| Bernecker (2011) (EJN Vol. 18 p. 571)§ | 10 ± 12 | 14 ± 11 | — | >.05 |
| Bernecker (2011) (EJN Vol. 18 p. 1233)§ | 13 ± 13 | 18 ± 15 | — | >.05 |
| Dearborn (2014) (N) | 18 ± 1 | 21 ± 3 | — | .371 |
| Ligong (2015) (MSM) | 5 ± 1.8 | 5 ± 1.6 | — | >.05 |
Leptin levels have been rounded to nearest 1 ng/mL. Adiponectin levels have been rounded to nearest 0.1 μg/mL.
Units are in μg/mL except where noted otherwise.
Levels are WHR adj.
These findings are from studies which utilize overlapping cohorts.
C = Cephalalgia; CM = chronic migraine; DM = definite migraine; EM = episodic migraine; EJN = European Journal of Neurology; JNS = Journal of Neurological Sciences; MSM = Medical Science Monitor; N = Neurology; PM = Probable Migraine; Total-ADP = Total Adiponectin.
In 2008, Peterlin et al conducted a small clinical study that evaluated T-ADP and ADP multimers in non-diabetic, normotensive reproductive-aged (<50) women with EM (n = 13), chronic daily headache with either chronic or transformed migraine (n = 12), and non-headache controls (n = 12).41 Those with lipid and thyroid disorders were excluded and migraineurs were matched to non-headache controls by age and BMI (Table 2). Those with EM were pain free and those with chronic or transformed migraine (CM/TM) were at baseline level of pain at the time of blood draws. Crude levels of T-ADP in those with CM/TM, EM, and control subjects, respectively, were 10.1 (±4), 8.6 (±3.5), 7.5 (±2.4) and after adjusting for WHR, these levels varied significantly across the three headache groups (P = .024). Notably, the increase in T-ADP was largely due to increases in the HMW and MMW multimers of ADP (Fig. 4).
Table 2.
Interictal Migraine Studies Evaluating Adiponectin (ADP) in Those with Migraine as Compared to Controls
| Author (Year and Journal) | Design and Sample Size | Migraine State at Sample Time and HA Frequency | Controls | Age (Mean) | Sex | Obesity Control | Baseline Labs | Exclusions | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Peterlin (2008 N) | CS-Clin Case-Con eval ADP and ADP multimers in CM vs EM vs Con Con: n = 12 EM: n = 13 CDH (CM + TM): n = 12 |
Interictal EM HA freq: 1–9 HA/mo CM HA Freq: 15+ days/mo |
HA-Free | Con: 33 ± 9 EM: 35 ± 10 CM: 34 ± 9 |
W | m-BMI matched WHR Adj Mean m-BMI: Con: 25 EM: 24 CM: 24 |
Glucose | Diabetes Thyroid disease CV disease Infection Autoimmune Renal disease Cholesterol | In this selected patient group of non-diabetic, normotensive Caucasian women, after adjusting for WHR, T-ADP was increased in those with migraine vs controls (CM 10 ± 4 vs EM 9 ± 3 vs Con 8 ± 2 μg/mL, P = .002) Increase in T-ADP in migraineurs due to increases in HMW and MMW-ADP. |
| Bernecker† (2011 EJN Volume 18 page 571–576) | CS-Clin Case-Con eval MMP and TIMPS in non-obese EM vs non-obese Con. Given study design only crude levels reported for variables (eg. ADP) other than MMP. Con: n = 74 EM: n = 50 |
Interictal EM: ~1 ± 2 HA days/mo |
HA-Free | Con: 36 ± 9 EM: 37 ± 12 |
W and M comb | Not BMI Adj Include overweight with normal weighted individuals vs obese Mean m-BMI:Con: 23 EM: 23 |
Glucose Cholesterol Lipid panel Insulin (measured and with group differences; ADP not adj given study design) | Diabetes Thyroid CV disease Infection Metabolic disorders Chronic illness Depression Obesity (BMI >29.9) | In this selected patient group of women and men with low frequency EM and without depression who were non-obese, crude T-ADP ns different in non-obese EM 17 ± 7 vs non-obese Con 16 ± 10; P-value reported as NS. AdjustedT-ADP levels were not reported by study design. Note: The women in this study cohort overlap with those reported in Bernecker (2011) EJN Vol. 18 p. 1233. |
| Bernecker† (2011 EJN Volume 18 page 1233–1239) | CS-Clin Case-Con Eval oxidative stress markers in women with migraine Given study design only crude levels reported for ADP. Con: n = 48 EM: n = 48 |
Interictal Median HA Freq: 1 day/mo EM HA Freq Range = 0.2–10 HA days/mo |
HA-Free | Con: 22 ± 4 EM: 24 ± 5 |
W | Not BMI Adj Included normal, overweight, obese Mean m-BMI: Con: 22 EM: 24 |
Glucose Cholesterol Lipid panel Insulin (measured and with group differences; ADP not adj given study design) | Diabetes Thyroid CV disease Infection Metabolic disorders Chronic illness Depression Obesity (BMI >29.9) | In this selected patient group of women with low frequency EM and without depression crude T-ADP NS different in EM 16.1 ± 7.6 vs non-obese Con 18.3 ± 11.2; P-value reported as NS. Adjusted T-ADP levels were not reported by study design. Levels of 4-hydroxy-2-nonenalmation (an oxidative stress marker) were reported to be significantly increased in those with migraine and to correlate with T-ADP levels and BMI. |
| Dearborn (2014 N) | CS-Gen Pop Case-Cohort eval T-ADP and ADP multimers in older mig vs older Con. Con: n = 850 All Mig: n = 131 Def Mig: n = 72 p-Mig: n = 59 |
Mixed State Note: EM + CM comb; thus some likely CM and in pain at sample time. HA Freq: Not reported |
Mig-Free (non-migraine headache may have been included in Con). | Con: 53 ± 0.3 Mig: 51 ± 0.7 |
W and M Comb and Strat | m-BMI Adj Mean m-BMI: Con: 27 Mig: 27 |
Glucose Cholesterol | Diabetes Races other than black and white | In this general population cohort of age 45 + men and women, unadjusted T-ADP was increased in migraineurs (EM + CM) as compared to con, (Mig: 8 ± 0.6 vs Con 7 ± 0.2 μg/mL, P = .03 for All-Mig and P = .05 for definitive Mig). After adj (age, race, BMI, and glucose), OR of mig increased with increasing T-ADP (OR 1.86; CI: 1.15, 3.01; P = .01) and HMW-ADP (OR 1.96; CI: 1.11, 3.48; P=.02) in men and not women. |
| Duarte (2014 JNS) | CS-Clin Case-Con to eval T-ADP and ADP multimers in Mig vs Con Con: n = 65 EM: n = 45 CM: n = 23 |
Interictal and Mixed State HA Freq: ~11 ± 8 HA days/mo |
HA-Free | Con: 45 ± 16 Mig: 41 ±15 |
W and M Comb and Strat | m-BMI Adj Mean m-BMI: Con: 26 Mig: 25 |
Infection Inflammatory disease Allergic disorders Autoimmune Disorders Hepatic disease Neurodegen disorders Tumors | In this selected patient group of women and men, T-ADP increased in those with migraine (EM + CM) vs controls. (T-ADP: Mig 44 ± 12 vs 37 ± 10 ng/mL HA-free Con, P < .0001) and remained sig different after adj (BMI), P = .005. |
Adj = adjusted; C = Cephalalgia; clin = clinical; CM = chronic migraine; CDH = chronic daily headache; Con = control; Comb = combined; CS = cross-sectional; EJN = European Journal of Neurology; EM = episodic migraine; freq = frequency; HA-free Con = HA-free control; HMW = high molecular weight; JNS = Journal of Neurological Sciences; LMW = low molecular weight; mig = migraine; MMW = middle molecular weight; mig = migraine; m-BMI = measure body mass index; mo = month; N = Neurology; non-HA-Free Con = non-HA-free control; ns = no signifigance; sr-BMI = self-reported body mass index; strat = stratified; T-ADP = total adiponectin; TM = transformed migraine; W = women; WHR = waist to hip ratio.
These findings are from studies utilizing overlapping cohorts and do not represent entirely unique or separate cohorts and should be interpreted together.
Fig. 4.
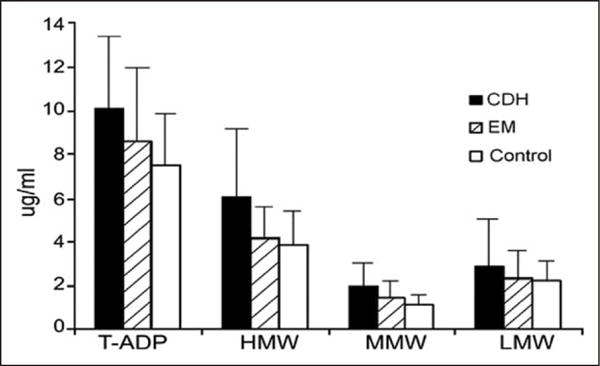
Serum total adiponectin (T-ADP) and ADP multimers in non-diabetic, normotensive reproductive-aged (<50) women with episodic migraine (EM, n = 13), chronic daily headache (CDH) with either chronic or transformed migraine (n = 12), and non-headache controls (n = 12). After adjusting for WHR, levels varied significantly across the three headache groups (P = .024). Adapted from Ref. 39.
This study was followed by 2 manuscripts by Bernecker et al evaluating non-adipokine blood markers in non-obese individuals but which included crude ADP evaluations.36,37 In both manuscripts, those with EM had a median monthly headache frequency of approximately 1 to 2 headache days per month (Table 2). The first of these studies included overweight or normal weighted women and men (controls: 28 men, 46 women; migraine: 8 men, 42 women); the second included an expanded cohort of women (48 controls; 48 migraine participants) building on the cohort of women included in the previous manuscript. Crude ADP levels were not different in women and men with EM vs controls36 (Table 1) and remained insignificant in the subsequent manuscript using the same cohort but excluding the men and including an additional 8 women (Table 1).37 As sex was not controlled for, confounding ADP levels in the first of these manuscripts, this second manuscript helped to establish that the exclusion of men did not change the lack of significance in this cohort. However, ADP levels were not adjusted for age and BMI in either of these manuscripts as these studies were not designed to evaluate adipokines. Additionally, models adjusting ADP levels for insulin or glucose and lipids were also not reported; and insulin and lipids were significantly different in those with EM as compared to controls in those with migraine vs controls in both manuscripts (Table 1a).36,37 Notably the lead author of these manuscripts (C.B.), noted that when only the unique individuals from each these studies were combined and stratified by sex, crude T-ADP levels were not different in women/men with migraine compared to women/men without migraine (Supporting Information Table 2) and results remained similar after adjusting for age and BMI (data unpublished, personal communication).
The Bernecker et al manuscripts were followed by a study conducted by Duarte et al to evaluate interictal ADP in migraineurs.42 In this study, 133 predominantly reproductive aged women and men were evaluated. T-ADP was increased in those with migraine (EM and CM combined; n = 68) as compared to non-headache controls (n = 65); and there was no significant difference between those with EM and those with CM (Tables 1 and 2).42 Additionally, the significance of the findings did not change when men were excluded. Neither serum lipids nor glucose levels were controlled for in this study.
More recently, Dearborn et al reported a general population case-cohort study from the Atherosclerosis Risk in Communities study evaluating T-ADP and HMW-ADP in older (≥45 years) non-diabetic migraineurs (EM and CM combined; probable/definitive migraine: n = 131, definite migraine: n = 72) as compared to non-migraine controls (ie, including those who had non-migraine headaches, n = 850).43 In this study, crude T-ADP was increased in migraineurs (EM and CM) as compared to controls (Table 1). However, after adjustments, notably including for BMI and plasma glucose levels, the relative odds of migraine increased with increasing T-ADP and HMW-ADP in men but not in women (Table 2).
Interictal ADP and Migraine Summary and Interpretation
Two manuscripts, which utilized partly overlapping cohorts and evaluated arguably less severe EM (participants were required to have no depression and had a median headache frequency of 1 headache day per month) were negative. The other 3 published studies reported increased crude ADP levels in those with migraine as compared to controls. Taken together this suggests crude ADP levels may be increased in those with migraine. However, several notable methodology differences present challenges in drawing firm conclusions from this body of literature on interictal ADP levels in migraineurs (Tables 1 and 2). Two of these studies only evaluated T-ADP in women and thus are not generalizable to men.37,41 One study used non-migraine (ie, not headache-free) controls.43 The 2 manuscripts based on similar cohorts may not be generalizable to obese individuals as obese individuals were excluded and overweight individuals were included with normal weighted individuals without adjustments for BMI.36,38 Additionally given the age-related changes in migraine prevalence and in ADP levels, the general population study evaluating T-ADP and HMW-ADP in age 45+ migraineurs is not necessarily generalizable to younger migraineurs.43 Further, impaired insulin sensitivity has been described in those with migraine46 and only 2 of the studies evaluated and controlled for glucose and/or insulin levels.41,43 This may be particularly relevant given ADP’s role in insulin sensitivity and glucose regulation (and which cannot be fully attributed to ADP’s role in obesity).47–49
In sum, the interictal studies evaluating ADP levels in migraineurs suggest it is possible that ADP levels may be increased in those with migraine but substantial methodological differences and limitations across studies limit firm conclusions. Carefully designed studies which are appropriately controlled for demographic (eg, age, sex) factors and that consider related metabolic factor that may act as mediators or confounders (eg, obesity status, serum glucose, lipid levels) remain warranted.
Ictal ADP and Migraine
Chai et al evaluated ictal T-ADP and ADP multimers in participants with EM (Table 3).30 Although relatively small (n = 34), this study was the largest of all similar longitudinal ictal studies evaluating blood markers in episodic migraineurs to date. Peripheral serum levels of T-ADP and ADP multimers were evaluated in a total of 34 episodic migraineurs (women and men of all obesity status levels) before and after treatment with oral sumatriptan/naproxen vs placebo. There were no significant differences in demographics including sex and BMI, headache characteristics, or baseline laboratories including glucose, insulin, and cholesterol in those treated with active drug vs placebo.30 Notably, treatment responders had a higher BMI and lower glucose level compared to non-responders. In all participants (n = 34) pretreatment pain severity increased with every 1 μg/mL and quartile increase in the HMW: T-ADP ratio after adjustments for potential confounders including age, sex, race, BMI, glucose, and treatment arm (Table 3). In addition, T-ADP was decreased in responders as early as 30 minutes after treatment and remained decreased at 120 minutes after treatment as compared to onset of pain. Notably in responders HMW-ADP was decreased and LMW-ADP increased (both in an anti-inflammatory direction) at 120 minutes after treatment as compared to onset. In non-responders only LMW-ADP was decreased (in a pro-inflammatory direction) at 120 minutes.30
Table 3.
Migraine Studies Designed to Evaluate Changes in Adiponectin (ADP) and Leptin Concentrations Before and After Treatment
| Author (Year) | Design and Sample Size | Migraine State at Sample Time | Age | Sex | Levels Controlled for Obesity Status and BMI | Findings |
|---|---|---|---|---|---|---|
| Before and After Acute Abortive Therapy | ||||||
| Chai (2015) | Multicenter, longitudinal clinical study evaluating T-ADP and ADP multimers before and 30–120 min after acute abortive TX with sumatriptan/naproxen sodium in those with EM. n = 34 (Responders: n = 17; Non-Responders: n = 17) [Note: This manuscript reports on the final and expanded cohort from the initial report on ictal adiponectin in migraineurs before and after treatment by Peterlin et al in 2013.45] |
Ictal | Median: 34 ± 16 | W and M | m-BMI Adj mean m-BMI: 25 | In this selected patient group of women and men presenting during an acute attack: Adiponectin
|
| Before and After Migraine Prevention | ||||||
| Berilgen (2005) | Clinical study evaluating weight gain with preventive migraine TX with 25 mg/day amitriptyline and 10 mg/day flunarazine at baseline, 4 and 12 weeks after treatment in those with migraine >3 days/mo with disability. Amitriptyline: n = 19 Flunarazine: n = 20 |
Not reported | Mean: 33 ± 10 | W and M combined | Not BMI Adj Amitriptyline Mean m-BMI: 24–26 Flunarazine Mean m-BMI: 24–26 |
In this this selected patient group of women and men, body mass index increased in both groups from baseline (BMI 24) to after 12 weeks (BMI 26) of TX, (amitriptyline: P < .05, flunarazine: P< .01). Amitriptyline: Leptin levels increased from baseline (7.2 + 1.1 ng/mL) vs after 12 weeks of TX (16.9 + 2.4 ng/mL), P < .05. Flunarazine: Leptin levels increased from baseline (6.6 + 1.4 ng/mL) vs after 12 weeks of TX (13.1+2.2), P<.01. |
| Hirfanoglu (2009) | Clinical study evaluating cyotokines and leptin before and 4 months after migraine prevention with 0.2 mg/kg/day cyproheptadine (n = 17) in children <12 yrs and 0.5 mg/kg/day amitriptyline (n = 21). 10–40 mg propranololol (n = 20), or 5–10 mg/day flunarazine (n = 19) in children >12 yrs with EM. | Interictal (at least 4 days after an attack) | At TX start: 10 ± 3 At TX end: 13 ± 3 |
Boys and Girls | Not BMI Adj BMI not reported | In this selected pediatric patient group, monthly HA frequency and HA severity was reduced in all groups. Leptin increased only in those treated with cyproheptadine (Before: 3 ± 1 ng/mL vs After: 7 ± 3 ng/mL, P = .001) and flunarazine (Before: 3 ± 1 ng/mL vs After: 6 ± 4 ng/mL, P = .001) and not in those treated with amitriptyline (Before: 3 ± 1 ng/mL vs After: 4 ± 3 ng/mL, P = .17) or propranolol (Before: 4 ± 3 ng/mL vs After: 4 ± 2 ng/mL, P = .24). |
| Schutt (2010) | Clinical Case-series evaluating BMI and metabolic changes before and after 20–44 weeks of topiramate (TPM) in those with migraine (n = 6) (Monthly HA freq was not reported). | Not reported | ~47.2 | W and M Combined | Not BMI Adj Mean m-BMI: 31 BMI range: 21–32 |
In this selected patient group of women and men: Adiponectin
|
| Verrotti (2015) | Clinical study to evaluate weight regain after discontinuation of topiramate (TPM) in normal weight migraineurs (EM + CM). Leptin evaluated preTX. 3 and 6 months after TX. and 6 months after TPM withdrawal. No weight loss: n = 154 Weight loss: n = 87 |
Not reported | TX start: 24 ± 6 TX end: 25 ± 6 |
W and M Combined | Not BMI Adj Mean m-BMI: PreTX BMI: 23 6 mo after TX BMI: 19 6 mo after stop TPM BMI: 22 |
In this selected patient group of women and men, there was no change in all study parameters (including leptin) in migraineurs (EM + CM) without weight loss. In the 36% who experienced weight loss on TPM at 6 mo, HA frequency and leptin levels were decreased (Pre: 4.1 ± 1.2 ng/mL vs Post: 3.0±1.5, P<.01). After stopping TPM, although HA frequency remained decreased from prior to starting TX (Pre:13 ± 5 HA days/mo vs Post: 2 ± 1 HA days/mo, P < .01), leptin and BMI were not different (Leptin: [Pre] 4.1 ± 1.2 ng/mL vs [Post]: 3.7 ± 1.9 ng/mL; BMI: [Pre] 23 ± 5 vs [Post] 22 ± 4). |
ADP = adiponectin; EM = episodic migraine; M = men; HMW = high molecular weight; hrs = hours; LMW = low molecular weight; min = minutes; mo = month; TPM = topiramate; W = woman; TX = treatment; yrs = years
Ictal ADP and Migraine Summary and Interpretation
Although limited by sample size, the one ictal ADP study in migraineurs suggests that ADP and its multimers may be modulated due to the presence and resolution of active pain in those with migraine. Further studies validating these initial findings are warranted.
ADP Levels Before and After Migraine Prevention
To date, only one study has attempted to evaluate ADP levels before and after migraine prevention treatment (Table 3).44 This study was a small case-series of migraineurs (n = 6) designed to evaluate BMI and metabolic parameters after 20–44 weeks of topiramate treatment. In this study, T-ADP increased by 70 ± 17.3% after treatment in those with migraine, P = .02. While these findings are interesting, it is not possible to determine if the ADP changes were related to changes in obesity, migraine status, or both. However, these results would support the speculation that chronic increases in total ADP (or LMW ADP) levels may be protective.44
ADP Levels Before and After Migraine Prevention Summary and Interpretation
One small case-series suggests ADP levels may be modulated by preventative treatment with topiramate in those with migraine. Future research on the effect of migraine prevention on ADP and ADP multimers, controlling for body composition and the impact of weight loss, are needed.
Identification of Leptin and Migraine Studies
Nineteen manuscripts were identified through the search terms “migraine AND leptin” (Fig. 5). Review of references in these manuscripts and related-reviews identified 2 additional studies.36,37 Of these 21 manuscripts, 6 were reviews, 3 were non-adipokine studies, and 1 utilized an animal model of aura50 and were thus excluded, leaving 12 studies which evaluated blood levels of leptin in those with migraine.30,35–37,43,44,51–55 Of these 11 manuscripts, 6 included crude interictal leptin evaluations in those with migraine as compared to controls and 5 evaluated leptin levels before and after abortive or preventive therapy in those with migraine.
Fig. 5.
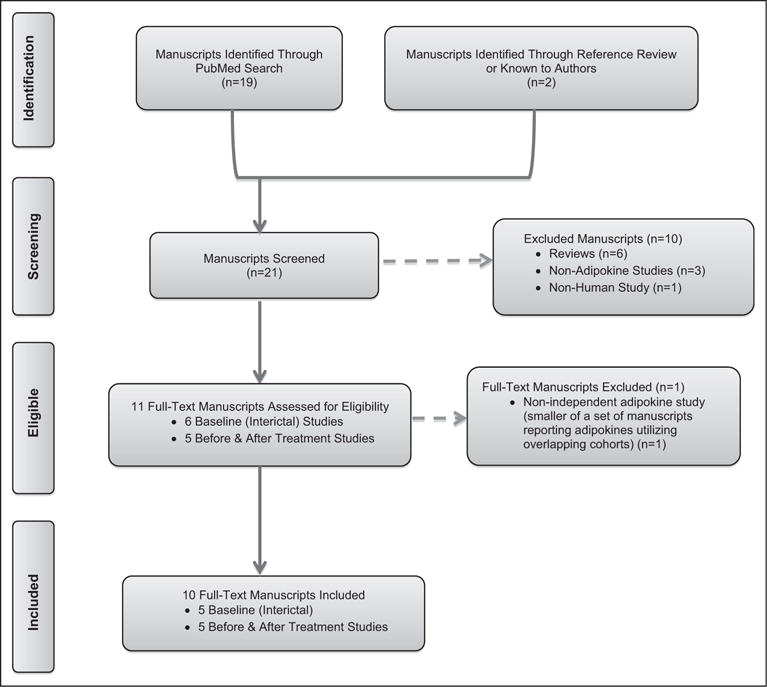
PRISMA flow diagram of manuscripts evaluating blood levels of leptin in those with migraine.
Of the 6 case-control studies that included evaluations of crude interictal leptin concentrations in those with migraine vs controls,35–37,43,52 full review of these manuscripts identified 3 manuscripts which utilized overlapping cohorts.35–37 Of these 3 manuscripts using overlapping cohorts, the smallest cohort was excluded35; 2 were included because one of the remaining 2 overlapping manuscripts included new data from men,36 leaving a total of 5 interictal manuscripts. Full review of the 5 manuscripts which evaluated leptin levels before and after abortive or preventive therapy in those with migraine, determined that none utilized overlapping cohorts and all were included.30,44,51,53,54
Leptin and Migraine Manuscript Reviews and Discussion
Interictal Leptin and Migraine Manuscripts
Five manuscripts reporting on interictal circulating blood levels of leptin in those with migraine as compared to controls were identified.36,37,43,52,55 Crude leptin levels were significantly decreased in one52 and not significantly different in 4 of these manuscripts (Table 1).36,37,43,55
In the first of these studies, Guldiken et al compared serum levels of leptin in a group of 61 migraineurs (14 men, 47 women) to 64 non-migraine controls (22 men, 42 women).52 Headache frequency was not reported and it is unclear if chronic migraineurs were included with episodic migraineurs. Crude levels of leptin were lower in individuals with migraine as compared to controls (Table 1); however, these differences were not significant after adjusting for body composition. Models were not further adjusted by age or sex or by glucose levels – which were significantly different across groups (Table 4).
Table 4.
Studies Evaluating Interictal Leptin Levels in Those with Migraine as Compared to Controls
| Author (Year) Journal | Design and Sample Size | Mig State Time of Sample | Control | Age (mean) Sex | Ob Control and BMI (mean) | Baseline Labs Chol/Lipid Glucose | Exclusions | Findings |
|---|---|---|---|---|---|---|---|---|
| Guldiken (2008 HA) | CS-Clin, Case-Cohort to Eval Leptin in EM vs Non-Mig Con Con: n = 64 (22 M/42 W) EM: n = 61 (14 M/47 W) |
Not reported 1–5 HA days/mo; thus most likely interictal at time of sample | Mig-Free (non-migraine HA may have been included in Con) | Con: W 34 ± 10 M 34 ± 7 EM: W 39±9 M 39 ±2 W and M |
Adj body composition using formula based on BMI Mean m-BMI EM: 26 ± 6 Con: 26 ± 5 |
Glucose Cholesterol Lipid panel | CV disease Infection Metabolic disorders Renal disease Psychiatric disorders Meds interfering with leptin metab | In this selected patient group of women and men combined together, crude leptin levels (ng/mL) were decreased in EM (40 ± 21) vs Con (49 ± 25), P < .05. Body composition adjusted leptin levels ns different between EM vs Con. |
| Berneckerf† (2011 EJN Vol 18 pp. 571–76) | CS-Clin, Case Cohort to Eval MMP and TIMPS in Non-Obese (ie. Nland Over-Wt) EM vs Non-Obese Con Con: n = 74 (28 M/46 W) EM: n = 50 (8 M/42 W) |
Interictal Median Monthly HA Freq: 2 ± 2 HA days/mo |
HA-Free | Con: 36 ± 9 EM: 37 ± 12 W and M |
BMI Not Adj Included Overweight and NL together Mean m-BMI EM: 23 ± 3 Con 23 ± 3 |
Glucose Cholesterol Lipid panel Insulin | Diabetes Thyroid CV disease Infection Metabolic disorders Chronic illness Depression Obesity (BMI >29.9) | In this selected patient group of women and men with low frequency EM and without depression who were non-obese, crude leptin levels (ng/mL) were not significantly increased in non-ob mig (14 ± 11) vs non-ob controls (10 ± 12 ng/mL), ns; nor was leptin receptor (EM: 23 ± 8 vs Con: 24 ± 9), ns. Adjusted leptin levels were not reported by study design. |
| Bernecker† (2011 EJN Vol. 18 pp. 1233–1239) | CS-Clin Case Cohort to Eval Markers of Oxidative Stress in Non Obese (ie, Nl, and Over-Wt) EM vs Con Con: n = 48 EM: n = 48 |
Interictal Median Monthly HA Freq: 1 HA day/mo |
HA-Free | Con: 35 ± 8 EM: 37 ± 11 W |
BMI Not Adj Included Overweight and NL together Mean m-BMI EM: 24 ± 5 Con 22 ± 4 |
Glucose Cholesterol Lipid panel Insulin | Diabetes Thyroid CV disease Infection Metabolic disorders Chronic illness Depression Obesity (BMI >29.9) | In this selected patient group of women with low frequency EM and without depression who were non-obese, crude leptin levels (ng/mL) were not significantly increased in non-ob mig (18 ± 15) vs non-ob controls (13 ± 13 ng/mL), ns; nor was leptin receptor (EM: 21 ± 8 vs Con: 24 ± 9), ns. Adjusted leptin levels were not reported by study design. Note: The women in this study cohort overlap with those reported in Bernecker (2011) EJN Vol. 18 p. 571. |
| Dearborn (2014 N) | CS-Gen Pop, Case-Cohort of Mig vs Non-Mig Con to Eval Leptin and Adiponectin in Older Mig vs Older Non-HA-Free Con Con: n = 850 All Mig: n = 131 (72 Def Mig/59 prob-Mig) |
Mixed Note: All mig (EM + CM) eval together; thus some CM likely in pain at sample time |
Mig-Free (non-migraine HA may have been included in Con) | Con: 53 ± 0 Mig: 51 ± 1 Wand M | m-BMI Adj Mean m-BMI: Con: 27 ± 0 Mig: 27 ± 1 |
Glucose Cholesterol | Diabetes Race other than black and white | In this general population cohort of age 45 + men and women, crude leptin levels (ng/mL) were not significantly increased in mig (21 ± 3) vs Con (18 ± 1 μg/mL), P = .37 The adjusted (age, race, BMI, glucose) risk of mig was not modified by increasing leptin levels in men or women (women: OR 1.10; CI: 0.74, 1.63; P = .651; men: OR 3.96; CI: 0.28, 56.3; P = .308) |
| Ligong (2015 MSM) | CS-Clin, Case Cohort of Mig vs Non-HA Con to Eval Leptin in Mig vs Con Con: n = 52 (17 M/35 W) EM: n = 52 (17 M/35 W) |
Mixed Note: EM + CM eval together; thus some CM likely in pain at sample time |
HA-Free | Not Reported Range: 18–55 | BMI Adj (Not reported if BMI self-reported or measured) |
Not done | Primary HA other than migraine Secondary HA Rheumatic disease Note: diabetes was not an exclusion criteria. |
In this selected patient group of age, sex, BMI-matched, women and men, crude leptin levels (ng/mL) were not significantly increased in those with migraine (5 ± 1.8 vs controls (5 ± 1.6 ng/mL). Multivariate logistic regression analyses (including age and sex) showed that leptin did not have a significant association with migraine. Note: Although samples were drawn in a.m. it is not stated samples were fasting; and diabetes was not an exclusion criteria and glucose levels were not controlled for in analyses. |
Note: All studies were fasting and used EIA to evaluate leptin levels. EM = episodic migraine; Def-Mig = definitive migraine; Eur J Neuro = European Journal of Neurology; HA = headache; Mig = migraine; HA-free Con = headache-free control; IA = immunoassay; m-BMI = measure body mass index; mig = migraine; mo = monthly; MSM = Medical Science Monitor; non-HA-Free Con = non-headache-free control and included those with HA disorders other than migraine; NR =not reported; p-Mig = probable migraine; sr-BMI = self-reported body mass index; ob = obese; T-ADP = total adiponectin; TPM = topiramate; W = women.
These findings are from studies which utilize overlapping cohorts.
In contrast to Guldiken et al’s study, 2 subsequent manuscripts by Bernecker et al, which utilized an overlapping cohort, one with women and men and one with women alone, found no significant difference in crude leptin levels between those with migraine and controls (Tables 1 and 4).36,37 Models adjusted for BMI, age, or glucose were not reported. Notably the lead author of these manuscripts (C.B.), noted that when only the unique individuals from these studies were combined and stratified by sex, crude leptin levels were significantly increased in women but not men (Supporting Information Table 2). However, after adjustments for age and BMI, leptin levels were significantly increased in both men and women with migraine as compared to controls (data unpublished, personal communication).
Dearborn et al evaluated leptin levels in older (age 45+) migraineurs as compared to non-migraine controls in a general population cohort of 850 controls and 131 participants with probable/definite migraine (of whom 72 had definite migraine).43 Crude leptin levels were not significantly different in individuals with definite migraine (20.6 ± 3.1 ng/mL, P = .37) or all migraine (18.4 ± 2.1 ng/mL; P = .76) as compared to nonmigraine controls (17.7 ± 0.9 ng/mL) (Table 1a). In addition, after adjustments for age, race, body mass index, and glucose, the odds of migraine were not increased with increasing leptin levels (Table 4).
Most recently, Ligong et al evaluated leptin levels in women and men with migraine (women: n = 35, men: n = 17) vs age, sex, BMI matched controls (n = 52).55 Sampling was done between 6 and 8 a.m. in the morning, although it is not stated if participants were fasting. Insulin and glucose levels were not reported and diabetes was not an exclusion criteria. Crude leptin levels were not significantly different in those with migraine (5 ± 1.8 μg/L, P = .37) as compared to controls (4.9 ± 1.6 μg/L), P > .05).53 The authors reported that multiple logistic regression analyses were performed to evaluate the effects of leptin on those with migraine and that leptin levels caused no significant difference in the headache frequency, duration, or severity between groups. However, it is not clear if models evaluated the association between leptin and disease state (ie, those with migraine [positive disease state] vs those without migraine [negative disease state]). Additionally no model controlled for a medical history of diabetes or serum glucose or insulin levels.
Interictal Leptin and Migraine Summary and Interpretation
Crude leptin levels have been reported to be the same in subjects with migraine compared to controls in four out of the five studies considered. Notable and substantial study methodology differences and/or limitations present challenges in interpretation of this body of literature on interictal leptin levels in migraineurs (Tables 1a and 1c).36,37,43,52 Three of these studies did or may have included chronic migraineurs with episodic migraineurs.43,52,55 The inclusion of episodic and chronic migraineurs together and the inability to determine if a substantial proportion of the migraineurs had chronic migraine, limits the interpretability of these findings if the relationships are related to disease state or active pain. One of these studies evaluated leptin levels only in older (45+ years) migraineurs, most of whom were older than 50, and thus was not generalizable to younger migraineurs.43 Of the manuscripts looking at reproductive age individuals, none provided models fully controlled for age, sex, and insulin and/or glucose.36,37,52,55 Although the weight of the current data suggests interictal leptin levels are not different in those with migraine as compared to controls, future studies are warranted with careful consideration of the migraine state at the time samples are drawn as well as of important covariates such as age, sex, glucose, and body mass index.
Ictal Leptin and Migraine Manuscripts
One study evaluated ictal leptin levels in those with EM and was summarized in the ictal ADP section.30 This study reported that leptin levels were not modulated by treatment response. It also showed that a linear relationship between ictal migraine pain severity and leptin levels was not present in episodic migraineurs. However, in analyses including pain severity across all time points (pre and post treatment), pain severity decreased with increasing quartiles of leptin levels after adjustments, suggesting that leptin may have an inverse relationship with acute migraine pain severity.30
Ictal Leptin and Migraine Summary and Interpretation
Although limited by sample size, the one ictal leptin study in migraineurs suggest that leptin may be inversely associated with pain severity and that leptin levels are not modulated by treatment response. Although firm conclusions cannot be drawn, when the interictal data are considered in conjunction with the ictal data from Chai et al.30 it is possible that leptin levels are decreased with active pain (ictally) and are increased interictally (when pain-free) as initially reported by Bernecker et al.35 Further research is needed.
Leptin Levels Before and After Migraine Prevention
To date, three studies have evaluated leptin levels before and after migraine prevention treatment in adults (Table 3).44,51,54 and one in children.53 The first of these studies was conducted by Berilgen et al in 2005.51 In this study, migraineurs with 3 or more severe headaches with disability in a month were included. Leptin levels were evaluated before and after treatment with amitriptyline 25 mg/day and flunarizine 10 mg/day in 39 women and men migraine patients. In both groups, a significant increase in BMI and serum leptin was found at 12 weeks after treatment (Table 3). Additionally a positive correlation was detected between BMI, serum leptin, C-peptide, and insulin values. Results were not adjusted or stratified by sex.51
Leptin levels were also evaluated before and after 20 weeks of treatment with topiramate in adult migraineurs by Schutt et al in the case-series (n = 6) described in the ADP section.44 In these 6 patients (5 women, 1 man), along with a significant decrease in their body mass indices and homeostatic model assessment (HOMA) insulin resistance index, leptin levels decreased 39.2 ± 6.5%, P = .013 (Table 3). Changes in headache frequency and severity were not reported.44
More recently Verrotti et al evaluated the effect of topiramate treatment and withdrawal on weight and metabolic markers in predominantly normal weighted episodic and chronic migraineurs without aura. Samples were drawn prior to treatment, 3 months and 6 months after starting topiramate and then repeated 6 months after cessation of topiramate.54 Weight loss was defined as a loss of ≥5% of pretreatment body weight. Baseline (eg, pretreatment) levels of leptin did not differ between those with (n = 87) and without (n = 154) weight loss. Although glucose levels were not reported, the homeostatic model assessment of insulin resistance (HOMA-IR) at baseline was increased in those without weight loss (3.2 + 0.5) as compared to those with weight loss (2.4 + 0.7, P = .002). In those without weight loss, there was no significant change in any parameter (including leptin) during the study period (Table 4). In the 36% (n = 87) of patients who experienced weight loss on topiramate at 3 and 6 months, headache frequency and leptin levels were significantly decreased (pretreatment: 4.1 ± 1.2 ng/mL vs 6 months post-treatment: 3.0 ± 1.5, P < .01). By six months after withdrawal of treatment, leptin and BMI had returned to pretreatment levels although migraine attack frequency remained significantly reduced (Table 3).54
The only study to evaluate adipokines in children (boys and girls) before and after migraine prevention treatment was conducted by Hirfanoglu et al.53 Baseline (pretreatment) and 4-month post-treatment interictal leptin levels were measured in 77 children about to start preventive migraine therapy (cyproheptadine, n = 17; amitriptyline, n = 21; propranolol, n = 20; flunarizine, n = 19) In these children, all groups showed a significant reduction in monthly headache frequency and headache severity. However, leptin levels increased only in those treated with cyproheptadine and flunarazine and not in those treated with amitriptyline or propranolol. Body composition and glucose levels were not evaluated.53
Leptin Levels Before and After Prevention Summary
Although methodological differences and study limitations exist for each of these studies, taken together the existing data support that leptin levels are modulated by changes in obesity status (increasing with weight gain and decreasing with weight loss). No conclusion can be drawn regarding leptin levels and migraine disease state independent of weight loss. Further research is warranted.
CONCLUSION
Recent advancements in our understanding of the association between migraine and obesity have led to the hypothesis that adipokines may contribute to the neurogenic inflammation of migraine.26 While the weight of the existing data is suggestive that the adipokines ADP and leptin may be associated with migraine in some manner, substantial study design differences in consideration of demographic (eg, age, sex) and other confounders or mediators (eg, obesity status, lipids, insulin sensitivity) limit definitive conclusions. It is also possible that other yet to be identified or fully established mediators and confounders (eg, socioeconomic status, psychiatric disorders) play a role. Future research utilizing carefully considered designs and methodology is warranted. In particular careful and systematic characterization of pain and fasting states at the time of samples (particularly with leptin), as well as systematic consideration of vital confounders and mediators are needed to determine if adipokines play a role in migraine pathophysiology and if any adipokine represents a viable, novel migraine biomarker or drug target.
Supplementary Material
Table 1. Abstracts Reporting Crude Interictal Total Adiponectin Levels (μg/mL) in Those with Migraine vs Controls.
Table 2. Summary of Bernecker et al Studies Utilizing Overlapping Cohorts Reporting Crude Adiponectin and Leptin Levels in Those with Migraine vs Controls.
Acknowledgments
Funding: Dr. Peterlin receives salary support through grant support from the National Institute of Health/National Institute of Neurological Disorders and Stroke (K23-NS078345). Dr. Scher serves on an advisory board and as a consultant for Allergan. The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or the U.S. government.
Footnotes
STATEMENT OF AUTHORSHIP
-
Conception and DesignB. Lee Peterlin
-
Acquisition of DataB. Lee Peterlin, Simona Sacco, Claudia Bernecker, Ann I. Scher
-
Analysis and Interpretation of DataB. Lee Peterlin, Simona Sacco, Claudia Bernecker, Ann I. Scher
-
Drafting the ManuscriptB. Lee Peterlin
-
Revising it for Intellectual ContentSimona Sacco, Claudia Bernecker, Ann I. Scher
-
Final Approval of the Completed ManuscriptB. Lee Peterlin, Simona Sacco, Claudia Bernecker, Ann I. Scher
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
References
- 1.Chai NC, Scher AI, Moghekar A, Bond DS, Peterlin BL. Obesity and headache: Part I–a systematic review of the epidemiology of obesity and headache. Headache. 2014;54:219–234. doi: 10.1111/head.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cindy Chai N, Bond DS, Moghekar A, Scher AI, Peterlin BL. Obesity and headache: Part II–potential mechanism and treatment considerations. Headache. 2014;54:459–471. doi: 10.1111/head.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterlin BL, Rapoport AM, Kurth T. Migraine and obesity: Epidemiology, mechanisms, and implications. Headache. 2010;50:631–648. doi: 10.1111/j.1526-4610.2009.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ornello R, Ripa P, Pistoia F, et al. Migraine and body mass index categories: A systematic review and meta-analysis of observational studies. J Headache Pain. 2015;16 doi: 10.1186/s10194-015-0510-z. 27-015-0510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterlin BL, Rosso AL, Rapoport AM, Scher AI. Obesity and migraine: The effect of age, gender and adipose tissue distribution. Headache. 2010;50:52–62. doi: 10.1111/j.1526-4610.2009.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterlin BL, Rosso AL, Williams MA, et al. Episodic migraine and obesity and the influence of age, race, and sex. Neurology. 2013;81:1314–1321. doi: 10.1212/WNL.0b013e3182a824f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vo M, Ainalem A, Qiu C, Peterlin BL, Aurora SK, Williams MA. Body mass index and adult weight gain among reproductive age women with migraine. Headache. 2011;51:559–569. doi: 10.1111/j.1526-4610.2010.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006;67:252–257. doi: 10.1212/01.wnl.0000225052.35019.f9. [DOI] [PubMed] [Google Scholar]
- 9.Schramm SH, Obermann M, Katsarava Z, Diener HC, Moebus S, Yoon MS. Epidemiological profiles of patients with chronic migraine and chronic tension-type headache. J Headache Pain. 2013;14 doi: 10.1186/1129-2377-14-40. 40-2377-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. 2003;106:81–89. doi: 10.1016/s0304-3959(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 11.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 12.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 13.Van Itallie TB. Health implications of overweight and obesity in the United States. Ann Intern Med. 1985;103(Pt. 2):983–988. doi: 10.7326/0003-4819-103-6-983. [DOI] [PubMed] [Google Scholar]
- 14.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 15.Costanzo P, Cleland JG, Pellicori P, et al. The obesity paradox in type 2 diabetes mellitus: Relationship of body mass index to prognosis: A cohort study. Ann Intern Med. 2015;162:610–618. doi: 10.7326/M14-1551. [DOI] [PubMed] [Google Scholar]
- 16.Barba R, Marco J, Ruiz J, et al. The obesity paradox in stroke: Impact on mortality and short-term readmission. J Stroke Cerebrovasc Dis. 2015;24:766–770. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Andersen KK, Olsen TS. The obesity paradox in stroke: Lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke. 2015;10:99–104. doi: 10.1111/ijs.12016. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Ptacek S, Kareholt I, Farahmand B, Cuadrado ML, Religa D, Eriksdotter M. Body-mass index and mortality in incident dementia: A cohort study on 11,398 patients from SveDem, the Swedish Dementia registry. J Am Med Dir Assoc. 2014;15:447.e1–447.e7. doi: 10.1016/j.jamda.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: A meta-analysis. Eur J Epidemiol. 2014;29:801–812. doi: 10.1007/s10654-014-9961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165:313–327. doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 2004;219:9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 23.Kusminski CM, McTernan PG, Schraw T, et al. Adiponectin complexes in human cerebrospinal fluid: Distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 24.Xu A, Chan KW, Hoo RL, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 25.Rafique N, Afzal MN. Relationship of serum leptin levels with body mass index and gender. RMJ. 2009;34:164–166. [Google Scholar]
- 26.Peterlin BL, Bigal ME, Tepper SJ, Urakaze M, Sheftell FD, Rapoport AM. Migraine and adiponectin: Is there a connection? Cephalalgia. 2007;27:435–446. doi: 10.1111/j.1468-2982.2007.01306.x. [DOI] [PubMed] [Google Scholar]
- 27.Schautz B, Later W, Heller M, Peters A, Muller MJ, Bosy-Westphal A. Impact of age on leptin and adiponectin independent of adiposity. Br J Nutr. 2012;108:363–370. doi: 10.1017/S0007114511005605. [DOI] [PubMed] [Google Scholar]
- 28.Plaisance EP, Grandjean PW, Judd RL, Jones KW, Taylor JK. The influence of sex, body composition, and nonesterified fatty acids on serum adipokine concentrations. Metabolism. 2009;58:1557–1563. doi: 10.1016/j.metabol.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alim I, Fry WM, Walsh MH, Ferguson AV. Actions of adiponectin on the excitability of subfornical organ neurons are altered by food deprivation. Brain Res. 2010;1330:72–82. doi: 10.1016/j.brainres.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 30.Chai NC, Gelaye B, Tietjen GE, et al. Ictal adipokines are associated with pain severity and treatment response in episodic migraine. Neurology. 2015;84:1409–1418. doi: 10.1212/WNL.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donahue RP, Prineas RJ, Donahue RD, et al. Is fasting leptin associated with insulin resistance among nondiabetic individuals? The Miami community health study. Diabetes Care. 1999;22:1092–1096. doi: 10.2337/diacare.22.7.1092. [DOI] [PubMed] [Google Scholar]
- 32.Sattar N, Wannamethee G, Sarwar N, et al. Leptin and coronary heart disease: Prospective study and systematic review. J Am Coll Cardiol. 2009;53:167–175. doi: 10.1016/j.jacc.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 33.Kurrimbux D, Gaffen Z, Farrell CL, Martin D, Thomas SA. The involvement of the blood-brain and the blood-cerebrospinal fluid barriers in the distribution of leptin into and out of the rat brain. Neuroscience. 2004;123:527–536. doi: 10.1016/j.neuroscience.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 34.Tietjen GE, Khubchandani J. Vascular biomarkers in migraine. Cephalalgia. 2015;35:95–117. doi: 10.1177/0333102414544976. [DOI] [PubMed] [Google Scholar]
- 35.Bernecker C, Pailer S, Kieslinger P, et al. GLP-2 and leptin are associated with hyperinsulinemia in non-obese female migraineurs. Cephalalgia. 2010;30:1366–1374. doi: 10.1177/0333102410364674. [DOI] [PubMed] [Google Scholar]
- 36.Bernecker C, Pailer S, Kieslinger P, et al. Increased matrix metalloproteinase activity is associated with migraine and migraine-related metabolic dysfunctions. Eur J Neurol. 2011;18:571–576. doi: 10.1111/j.1468-1331.2010.03205.x. [DOI] [PubMed] [Google Scholar]
- 37.Bernecker C, Ragginer C, Fauler G, et al. Oxidative stress is associated with migraine and migraine-related metabolic risk in females. Eur J Neurol. 2011;18:1233–1239. doi: 10.1111/j.1468-1331.2011.03414.x. [DOI] [PubMed] [Google Scholar]
- 38.Tietjen G, Khubchandani J, Khan M, Herial N. Adiponectin and inflammatory cytokines in young women with migraine. Headache. 2010;50:71. [Google Scholar]
- 39.Lippi G, Meschi T, Mattiuzzi C, Borghi L, Targher G. Adiponectin and migraine: Systematic review of clinical evidence. Neurol Sci. 2014;35:1167–1171. doi: 10.1007/s10072-014-1719-3. [DOI] [PubMed] [Google Scholar]
- 40.Toprak MK, Ure S. Serum adiponectin levels in migraineurs. Neurology. 2013;80:06.151. Meeting Abstracts 1. [Google Scholar]
- 41.Peterlin BL, Alexander G, Tabby D, Reichenberger E. Oligomerization state-dependent elevations of adiponectin in chronic daily headache. Neurology. 2008;70:1905–1911. doi: 10.1212/01.wnl.0000312278.40250.6e. [DOI] [PubMed] [Google Scholar]
- 42.Duarte H, Teixeira AL, Rocha NP, Domingues RB. Increased serum levels of adiponectin in migraine. J Neurol Sci. 2014;342:186–188. doi: 10.1016/j.jns.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 43.Dearborn JL, Schneider AL, Gottesman RF, et al. Adiponectin and leptin levels in migraineurs in the atherosclerosis risk in communities study. Neurology. 2014;83:2211–2218. doi: 10.1212/WNL.0000000000001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schutt M, Brinkhoff J, Drenckhan M, Lehnert H, Sommer C. Weight reducing and metabolic effects of topiramate in patients with migraine – An observational study. Exp Clin Endocrinol Diabetes. 2010;118:449–452. doi: 10.1055/s-0030-1248289. [DOI] [PubMed] [Google Scholar]
- 45.Peterlin BL, Tietjen GE, Gower BA, et al. Ictal adiponectin levels in episodic migraineurs: A randomized pilot trial. Headache. 2013;53:474–490. doi: 10.1111/head.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rainero I, Limone P, Ferrero M, et al. Insulin sensitivity is impaired in patients with migraine. Cephalalgia. 2005;25:593–597. doi: 10.1111/j.1468-2982.2005.00928.x. [DOI] [PubMed] [Google Scholar]
- 47.Goldfine AB, Kahn CR. Adiponectin: Linking the fat cell to insulin sensitivity. Lancet. 2003;362:1431–1432. doi: 10.1016/S0140-6736(03)14727-7. [DOI] [PubMed] [Google Scholar]
- 48.Storgaard H, Poulsen P, Ling C, Groop L, Vaag AA. Relationships of plasma adiponectin level and adiponectin receptors 1 and 2 gene expression to insulin sensitivity and glucose and fat metabolism in monozygotic and dizygotic twins. J Clin Endocrinol Metab. 2007;92:2835–2839. doi: 10.1210/jc.2006-1812. [DOI] [PubMed] [Google Scholar]
- 49.Gao H, Fall T, van Dam RM, et al. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: A mendelian randomization study. Diabetes. 2013;62:1338–1344. doi: 10.2337/db12-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamura E, Kanazawa N, Hamada J. Hyperleptinemia increases the susceptibility of the cortex to generate cortical spreading depression. Cephalalgia. 2015;35:327–334. doi: 10.1177/0333102414540813. [DOI] [PubMed] [Google Scholar]
- 51.Berilgen MS, Bulut S, Gonen M, Tekatas A, Dag E, Mungen B. Comparison of the effects of amitriptyline and flunarizine on weight gain and serum leptin, C peptide and insulin levels when used as migraine preventive treatment. Cephalalgia. 2005;25:1048–1053. doi: 10.1111/j.1468-2982.2005.00956.x. [DOI] [PubMed] [Google Scholar]
- 52.Guldiken B, Guldiken S, Demir M, Turgut N, Tugrul A. Low leptin levels in migraine: A case control study. Headache. 2008;48:1103–1107. doi: 10.1111/j.1526-4610.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 53.Hirfanoglu T, Serdaroglu A, Gulbahar O, Cansu A. Prophylactic drugs and cytokine and leptin levels in children with migraine. Pediatr Neurol. 2009;41:281–287. doi: 10.1016/j.pediatrneurol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 54.Verrotti A, Parisi P, Agostinelli S, et al. Weight regain after discontinuation of topiramate treatment in patients with migraine: A prospective observational study. CNS Drugs. 2015;29:163–169. doi: 10.1007/s40263-015-0229-z. [DOI] [PubMed] [Google Scholar]
- 55.Ligong Z, Jinjin Q, Chunfu C, Congcong L, Xiaojun D. Effect of obesity and leptin level on migraineurs. Med Sci Monit. 2015;21:3270–3274. doi: 10.12659/MSM.894666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Abstracts Reporting Crude Interictal Total Adiponectin Levels (μg/mL) in Those with Migraine vs Controls.
Table 2. Summary of Bernecker et al Studies Utilizing Overlapping Cohorts Reporting Crude Adiponectin and Leptin Levels in Those with Migraine vs Controls.


