Abstract
Purpose
Analysis of regional wall motion of the right ventricle (RV) is primarily qualitative with large interobserver variation in clinical practice. Thus the purpose of this study was to use feature tracking to analyze regional wall motion abnormalities in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC).
Materials and Methods
We enrolled 110 subjects (39 overt ARVC [mutation+/phenotype+] (35.5%), 40 preclinical ARVC [mutation+/phenotype–] (36.3%), and 31 control subjects (28.2%)). Cine steady state free precession cardiac MR was performed with temporal resolution ≤40 msec in the horizontal long axis (HLA), axial, and short axis (SA) directions. Regional strain was analyzed using feature tracking software and reproducibility was assessed via intraclass correlation coefficient. Dunnett's test was used in univariate analysis for comparisons to control subjects; cumulative odds logistic regression was used for minimally and fully adjusted multivariate models.
Results
Strain was significantly impaired in overt ARVC compared to control subjects both globally (p<0.01) and regionally (all segments of HLA view, p<0.01). In the HLA view, regional reproducibility was excellent within (ICC=0.81) and moderate between (ICC=0.62) observers. Using a threshold of –31% subtricuspid strain in the HLA view, the sensitivity and specificity for overt ARVC were 75.0% and 78.2%, respectively. In multivariable analysis involving all three groups, subtricuspid strain less than –31% (beta=1.38, p=0.014) and RV end diastolic volume (EDV) index (beta=0.06, p=0.001) were significant predictors of disease presence.
Conclusions
RV strain can be reproducibly assessed with MR feature tracking, and regional strain is abnormal in overt ARVC compared to control subjects.
Keywords: Magnetic Resonance Imaging (MRI), Strain, Feature Tracking, Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), Cardiac Magnetic Resonance (MR)
Introduction
For the right ventricle of the heart, identification of regional disease by magnetic resonance (MR) is problematic since quantitative tools to determine regional function are not generally available. Tagged MR provides quantitative assessment of left ventricular strain, but has not generally proven to be useful to measure RV strain due to the thinness of the myocardial wall.(1)
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy with disease frequently presenting with regional dysfunction and enlargement of the right ventricle.(2) Affected patients typically present during the second to fifth decades of life, most commonly with palpitations, syncope, or sudden cardiac death.(3–5) Diagnosis is made based on the Task Force Criteria (TFC),(6) which involves assessment for major and minor criteria in six categories, including imaging assessment, to detect RV global and regional dysfunction, as well as RV enlargement.(6) To date, regional function by MR in ARVC is only analyzed qualitatively (visually); this can lead to variability between readers and false positive diagnoses.(7, 8)
Feature tracking is a technique that allows regional motion analysis for which strain information can be derived. Feature tracking is a post-processing procedure that does not inherently rely on a particular pulse sequence or modality, and has been applied in the past to echocardiographic, computed tomography, and magnetic resonance cine sequences.(9) The primary aim of this study was to determine the feasibility and reproducibility of RV myocardial strain analysis of cine MR images in ARVC patients via feature tracking in comparison to control subjects.
Materials and Methods
Study Population
The study population included 110 individuals who were evaluated for ARVC at a large, urban, tertiary medical center. All subjects prospectively provided written informed consent and the study was approved by an institutional review board. Patients were selected from a preexisting database based on availability of genetic and MR data. All selected patients underwent comprehensive clinical evaluation including MR to determine the presence or absence of diagnostic TFC for ARVC.(6) Cases included 79 ARVC-associated desmosomal mutation carriers who were divided in two groups: 1) overt ARVC (those fulfilling diagnostic criteria for ARVC, n=39); and 2) preclinical ARVC (those not fulfilling diagnostic criteria for ARVC, n=40). All overt patients were diagnosed with ARVC independently of MR, so that the diagnostic TFC provide an independent standard of reference. As a control group, we included 31 individuals who were either mutation-negative family members of mutation-positive ARVC patients (n=9), or subjects without ARVC upon comprehensive clinical evaluation (n=22) and had been referred for preemptive evaluation or to rule out ARVC.
MR Acquisition and Analysis
Cine images in the horizontal long axis (HLA), axial, and short axis (SA) planes were acquired at 1.5 Tesla (Avanto, Siemens Medical Imaging, Erlangen, Germany) using a balanced, steady state free precession (SSFP) sequence (TR/TE/FA 2.4/1.2/50-75 degrees, matrix 256-192, field of view 30-36 cm, temporal resolution ≤40 msec, slice thickness 6–8 mm).
Since little is known about quantitative strain in the RV in ARVC, we approached the analysis in a stepwise manner: a) global strain (average of all segments) to determine which MR cine view distinguished overt and control groups; b) segmental (local) strain in various views to determine if differences between groups were primarily driven by regional abnormalities; c) qualitative reads by radiologists in comparison to quantitative strain measurements to determine if there is any advantage of quantitative strain analysis.
Quantitative Analysis: Global Metrics
For global metrics, RV and left ventricular (LV) ejection fraction (EF), end systolic volume (ESV), and end diastolic volume (EDV) were measured with CVI42 (Circle Cardiovascular Imaging; Client Version 248, Server Version 258; Calgary, Alberta, Canada). RV ESV and EDV were indexed to body surface area (BSA) using the Dubois formula (BSA = body weight(kg)0.425 × height(cm)0.725 × 0.007184).
Quantitative Analysis: Strain Measurements
For regional analysis, we measured peak longitudinal (HLA and axial) and circumferential (SA) strain using Multimodality Tissue Tracking (MTT) software (MTT Version 6.0.4725, Toshiba Medical Systems Corporation, Tokyo, Japan).(10) On SA views, the most basal slice without right atrium through-plane motion during systole was analyzed. This position was chosen to optimize detection of abnormalities both at the inferior RV base and anteriorly at the RV outflow tract. On long axis (HLA and axial) views, the most central slice in which the valve plane was visible throughout the cardiac cycle was analyzed. Endocardial and epicardial contours were drawn in a semiautomated fashion from the apical insertion point to the mitral valve plane in the HLA and axial views and from the anterior to the inferior RV insertion points in the circumferential view. Contours were based on spline curves, the order of which is defined internally by the manufacturer. Points along the contour at end diastole (ED) were used to define a 10×10mm neighborhood, which was matched to a neighborhood of the subsequent frame within a search window by minimizing the sum of squared differences between the pixel intensities (Figures 1, 2). The quality of tracking was visually verified and manually adjusted if necessary. Of the 330 possible MR series, 55 (16.7%) were excluded due to poor image quality (poor electrocardiogram gating due to arrhythmia, patient motion).
Figure 1.
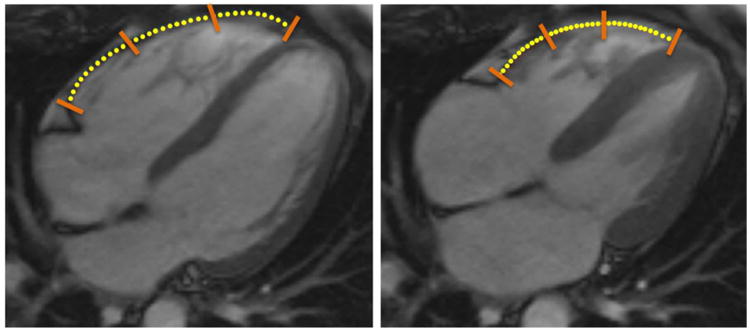
Right ventricular feature tracking of cine steady state free precession (SSFP) images in the horizontal long axis (i.e., 4-chamber) view of a 44 y/o female control subject. Contours were drawn in a semiautomated manner at end-diastole (left) and propagated throughout the cardiac cycle. The position of the propagated contours at end-systole (right) are also shown. Multimodality Tissue Tracking (MTT) points have been overlain with yellow circles to be more easily visualized; orange bars indicate demarcation between segments.
Figure 2.
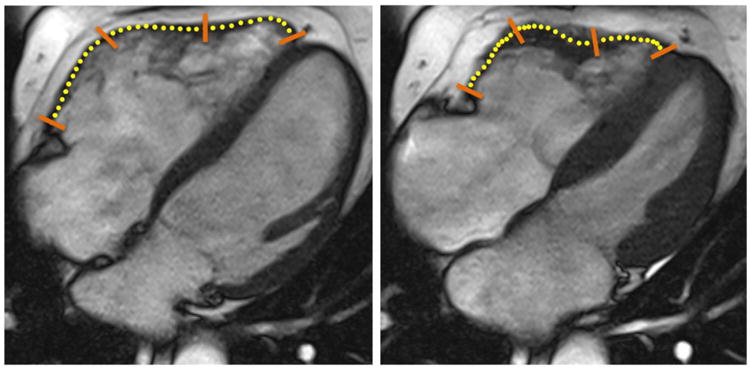
Right ventricular feature tracking of cine steady state free precession (SSFP) images in the horizontal long axis (i.e., 4-chamber) view of a 55 y/o male patient with overt ARVC. Contours were drawn in a semiautomated manner at end-diastole (left) and propagated throughout the cardiac cycle. The position of the propagated contours at end-systole (right) are also shown. Multimodality Tissue Tracking (MTT) points have been overlain with yellow circles to be more easily visualized; orange bars indicate demarcation between segments.
The segmentation of the RV for various views is illustrated in Figure 3. In the HLA and axial views, the RV was segmented into subtricuspid, anterior wall, and apical regions; in the SA view, the RV was segmented into outflow tract, free wall, angle, and inferior regions. Image quality was inadequate for feature tracking in 19 (6.9%) regions in the HLA view, 24 (9.1%) in the axial view, and 14 (3.7%) in the SA view. By convention for strain, segmental shortening is negative; segments that show greater local contraction have more negative strain.
Figure 3.
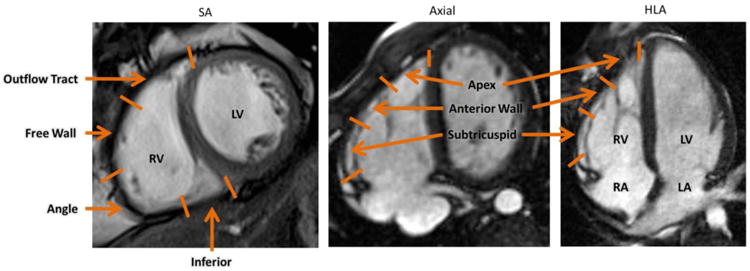
Definition of regions for the RV in the SA (left), axial (center), and HLA (right) views. Abbreviations: SA: Short axis; HLA: Horizontal Long Axis; RV: Right Ventricle; LV: Left Ventricle; RA: Right Atrium; LA: Left Atrium.
Qualitative Readings
Two readers qualitatively assessed wall motion blinded to group assignment and demographic data (DAB and SLZ, 15 and 5 years of experience in interpreting ARVC, respectively). Wall motion on the HLA cine views was scored as 1 (normal, no abnormality), 2 (possible abnormality), or 3 (definite abnormality). After independent assessment, the readers conferred over the cases and decided upon a consensus read. Interobserver agreement was assessed using the Cohen's kappa coefficient.
Statistical Analysis
Continuous and discrete data are presented as mean (±SD) and n (%), respectively. For continuous data, comparisons to the control group were performed using Dunnett's test, which inherently accounts from multiple comparisons. Categorical data were compared using Pearson's χ2 test. Univariate and multivariate cumulative odds logistic regression was used to determine predictors of clinical diagnosis based on regional strain and clinical risk factors. Model selection was performed using stepwise backward selection based on Akaike's information criterion. Intra- and inter-observer reproducibility of strain measurements was evaluated visually by Bland-Altman analysis; correlation was assessed by intraclass correlation coefficient (ICC). For ICC, a value ≥0.75 was considered excellent, <0.75 and ≥0.40 moderate, and <0.40 poor. All statistical analysis was performed using R (v3.1.0). P-values <0.05 were considered significant.
Results
Baseline Characteristics
MR images from 110 subjects (39 overt ARVC [mutation+, phenotype+] (35.5%), 40 preclinical ARVC [mutation+, phenotype–] (36.3%), and 31 control subjects (28.2%)) were evaluated. Clinical characteristics of the study population are shown in Table 1. The average age was 33.3 years (±15.8); 54 (49.1%) of patients were female. Age and gender were similar between the groups. As expected, RV EF was lower and RV EDV Index was greater in overt ARVC relative to control subjects; there were no significant differences in LV function or dimension between groups (Table 1). Patients with preclinical ARVC were similar to control subjects for all global metrics.
Table 1.
Characteristics of the Study Population.
| ARVC subjects | Control Subjects n=31 | ||
|---|---|---|---|
|
| |||
| Overt ARVC n=39 | Preclinical ARVC n=40 | ||
| Male | 17 (44) | 22 (55) | 17 (55) |
| Age (yrs) | 32.3 ± 13.5 | 31.3 ± 18.1 | 37.2 ± 14.9 |
| Global MR parameters | |||
| RV EDV Index | 88.3 ± 25.6† | 68.4 ± 14.4 | 69.7 ± 12.9 |
| RV ESV Index | 47.4 ± 24.9† | 31.1 ± 9.8 | 30.0 ± 8.6 |
| RV EF | 48.3 ± 11.7† | 54.9 ± 9.6 | 56.9 ± 9.7 |
| LV EDV Index | 77.4 ± 12.1 | 69.1 ± 12.9 | 73.5 ± 9.6 |
| LV ESV Index | 30.6 ± 9.5 | 25.2 ± 7.0 | 30.9 ± 15.1 |
| LV EF | 62.7 ± 6.3 | 63.2 ± 11.7 | 58.9 ± 13.3 |
| Clinical Phenotype | |||
| Repolarization criteria | 36 (92) | 4 (10) | - |
| T-wave inversion V1-3 | 33 (85) | 0 (0) | - |
| T-wave inversion V1-2 | 3 (8) | 4 (10) | - |
| T-wave inversion V1-4 w/CRBBB | 0 (0) | 0 (0) | - |
| Depolarization criteria | 22 (56) | 13 (33) | - |
| Epsilon wave | 2 (5) | 0 (0) | - |
| Prolonged TAD | 17 (44) | 5 (13) | - |
| Late potentials | 15/34 (44) | 12/38 (32) | - |
| Arrhythmia criteria | 25 (64) | 2 (5) | - |
| LBBB superior axis VT | 6 (15) | 0 (0) | - |
| LBBB inferior axis VT | 9 (23) | 0 (0) | - |
| >500 PVCs/24 hrs | 21/32 (66) | 2/35 (6) | - |
| Structural criteria | 23 (59) | 0 (0) | - |
| Major | 16 (41) | 0 (0) | - |
| Minor | 7 (18) | 0 (0) | - |
| TFC fulfillment: number of criteria (median) | 6 (IQR 5-7) | 2 (IQR 2-3) | - |
p<0.05 compared to control;
p<0.01 compared to control.
Abbreviations: ARVC: arrhythmogenic right ventricular cardiomyopathy, BSA: body surface area, CRBBB: complete right bundle branch block, EDV: end-diastolic volume, EF: ejection fraction, ESV: end-systolic volume, LBBB: left bundle branch block, LV: left ventricular, PVC: premature bentricular complex, RV: right ventricular, TAD: terminal activation duration, TFC: task force criteria, VT: ventricular tachycardia.
Global (Average) Strain Analysis
As shown in Table 2, global strain magnitude was lower in overt ARVC compared to control subjects in all MR views (p<0.0001, p=0.017, and p<0.01 for HLA, axial, and SA views, respectively). However, the greatest strain differences between overt ARVC subjects and control subjects were in the HLA view (−19.3% versus −27.7%, respectively, p<0.0001), corresponding to 30.3% ((27.7% – 19.3%)/27.7%*100%) lower strain in overt subjects. Global strain was similar between control subjects and patients with preclinical ARVC. Global strain was not related to either gender or age (p=NS).
Table 2.
Global strain values stratified by diagnostic group.
| ARVC subjects | Control n=31 | ||
|---|---|---|---|
|
| |||
| Overt n=39 | Preclinical n=40 | ||
| Global HLA (εll) | −19.3 (±6.2)† | −26.2 (±5.0) | −27.7 (±5.5) |
| Global Axial (εll) | −23.3 (±8.1)* | −28.6 (±7.2) | −28.5 (±5.2) |
| Global SA (εcc) | −11.0 (±5.6)† | −16.3 (±4.7) | −14.8 (±3.2) |
p<0.05 compared to control;
p<0.01 compared to control.
Abbreviations: HLA: horizontal long axis; SA: short axis
Regional Strain Analysis
Table 3 shows strain by region for the HLA, axial, and SA views. We began by comparing the overt ARVC and control groups, since regional strain was expected to be most different for those groups. In the HLA view (Table 3), strain magnitude was lower in overt ARVC compared to control subjects for the subtricuspid (p<0.001), anterior wall (p<0.01), and apical (p<0.01) regions. In the axial view, only the apical segment showed differences in strain for the overt versus the control subjects (p=0.04). In the short axis view, only the free wall (p<0.001) and angle (p=0.045) segments showed lower strain for the overt versus the control subjects. Thus, we concluded that the HLA view was most likely to discriminate overt ARVC from control subjects. Using a cutoff of greater (more negative) than −31% as normal subtricuspid strain in the HLA view, overt ARVC patients were separated from control subjects with 75.0% sensitivity and 78.2% specificity.
Table 3.
Regional strain values stratified by diagnostic group.
| ARVC subjects | Control n=31 | ||
|---|---|---|---|
|
| |||
| Overt n=39 | Preclinical n=40 | ||
| HLA (εll) view | |||
| Subtricuspid | −24.4 (±10.8)† | −33.4 (±10.9) | −36.9 (±10.5) |
| Anterior wall | −17.7 (±6.4)† | −23.0 (±6.1) | −22.8 (±6.3) |
| Apex | −18.6 (±8.8)† | −23.3 (±7.8) | −25.5 (±9.6) |
| Axial (εll) view | |||
| Subtricuspid | −31.0 (±13.2) | −38.2 (±11.8) | −31.4 (±13.7) |
| Anterior wall | −22.1 (±13.1) | −24.1 (±8.5) | −25.9 (±5.9) |
| Apex | −22.4 (±10.4)* | −29.0 (±14.9) | −30.6 (±12.7) |
| SA (εcc) view | |||
| Outflow tract | −11.3 (±6.8) | −15.3 (±6.8) | −14.4 (±6.4) |
| Free wall | −10.7 (±4.2)† | −16.7 (±5.8) | −15.7 (±3.0) |
| Angle | −11.6 (±6.7)* | −17.0 (±6.4) | −15.2 (±3.9) |
| Inferior | −13.8 (±7.9) | −20.4 (±8.7) | −17.9 (±5.5) |
p<0.05 compared to control;
p<0.01 compared to control.
Abbreviations: HLA: horizontal long axis; SA: short axis
For evaluation of the preclinical (mutation+/phenotype–) group, some patients with early disease may have subtle signs of ARVC such as regional wall motion abnormality while other patients may never develop a full ARVC phenotype. Thus, for a sufficiently large sample size, RV strain may be on average intermediate between control and overt ARVC subjects. Indeed, in the HLA view, regional strain in the preclinical group was intermediate between control and overt groups (Table 3 (middle column) and Figure 4 (green bars)). Using the −31% cutoff described above, 13/34 (38.2%) patients in the preclinical group showed reduced (less negative) subtricuspid strain. Preclinical patients with abnormal strain were more often male (n=10/13 [77%] vs. 10/21 [48%], p=0.092) and older (30.5 ± 18.6 vs. 27.5 ± 15.4 years, p=0.621) than preclinical subjects with normal strain. Interestingly, compared to individuals with normal strain, subjects with abnormal strain were significantly more likely to have other minor clinical abnormalities in addition to their genetic predisposition (n=10/13 [77%] vs. n=7/21 [33%], p=0.013).
Figure 4.
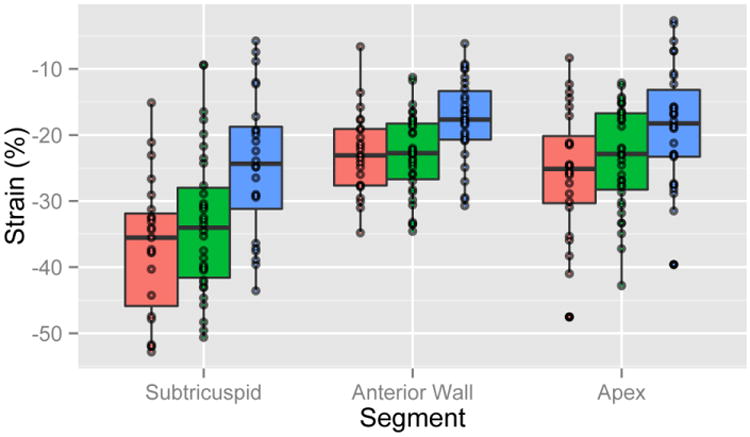
Regional strain by group in the horizontal long axis view. Red, green, and blue boxplots correspond to control subjects, preclinical ARVC, and overt ARVC, respectively.
We performed multivariable analyses to determine the independent predictive value of regional strain for presence or absence of disease. Abnormal HLA strain in the subtricuspid region (defined as less negative than −31% subtricuspid strain) was considered the predictor variable of interest in the multivariable analysis. As shown in Table 4, abnormal subtricuspid strain was an independent predictor of ARVC diagnosis (beta 1.38, p=0.014) in multivariable analysis controlling for gender, RV EF and RV EDV index, while RV EF was not significant in the multivariable model.
Table 4.
Multivariable analysis using cumulative logistic regression for the prediction of group assignment (control, preclinical ARVC, and overt ARVC) by abnormal subtricuspid strain and relevant clinical factors.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Beta (95% CI) | P-value | Beta (95% CI) | P-value | |
| Age (yr) | −0.01 (−0.03, 0.01) | 0.248 | - | - |
| Female | 0.35 (−0.34, 1.04) | 0.319 | 1.46 (0.36, 2.55) | 0.009 |
| Abnormal subtricuspid strain (<|−31%|) | 1.67 (0.77, 2.58) | <0.001 | 1.38 (0.28, 2.47) | 0.014 |
| RV EF (%) | −0.10 (−0.15, -0.06) | <0.001 | −0.06 (−0.12, 0.01) | 0.080 |
| RV EDV Index (m2/mL) | 0.06 (0.04, 0.09) | 0.001 | 0.06 (0.02, 0.10) | 0.001 |
Comparison of Strain with Qualitative Radiologist Interpretation
Cohen's kappa coefficient for DAB vs SLZ was 0.78, indicating moderate interobserver reproducibility. A consensus wall motion abnormality rating of ‘Definitely Abnormal’ was specific but not sensitive for overt ARVC (specificity 95.7% versus sensitivity 44.4%). A consensus wall motion abnormality rating of either ‘Definitely Abnormal’ or ‘Possibly Abnormal’ was moderately sensitive and specific for overt ARVC (sensitivity 55.6% versus specificity 63.8%).
We compared the diagnostic accuracy of abnormal qualitative wall motion (defined by radiologists as “Possibly” or “Definitely” abnormal) to abnormal subtricuspid strain (less negative than −31%) between overt ARVC and control subjects. As shown in Figure 5, abnormal subtricuspid strain reduced the false positive rate (FPR) from 50% (n=15/30) to 22% (n=5/23), while simultaneously reducing the false negative rate (FNR) (44% [n=16/36] vs. 25% [n=6/24]). In addition, accuracy increased from 53% (n=35/66) to 77% (n=36/47) (Figure 5).
Figure 5.
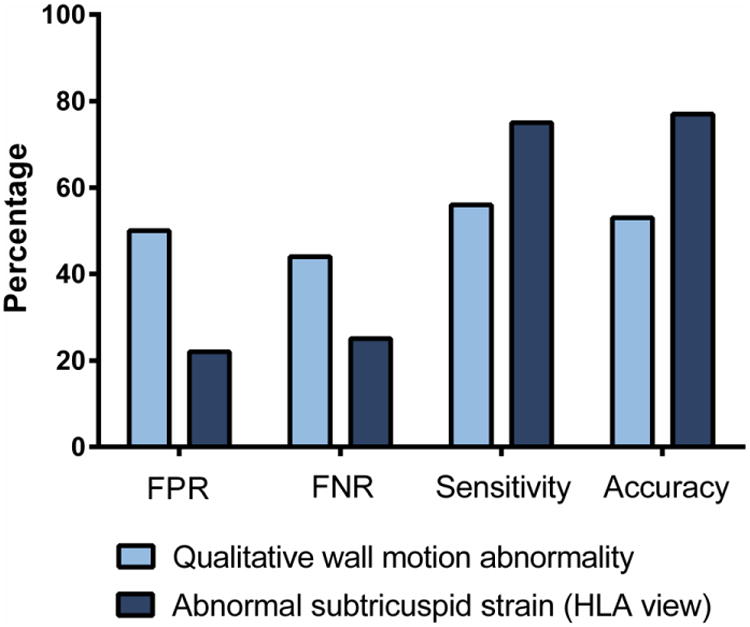
Diagnostic performance of abnormal strain (defined as more positive than −31% subtricuspid strain in the HLA view) compared to qualitative wall motion abnormality. Abbreviations: FPR: false positive rate; FNR: false negative rate, HLA: horizontal long axis.
Strain Reproducibility
Forty randomly selected cases were reanalyzed for intra- and inter-observer reproducibility. As shown in Supplemental Figure 1, intraobserver agreement was excellent in the HLA view (ICC=0.81) and moderate in the axial (ICC=0.66) and SA view (ICC=0.70). Interobserver agreement was moderate in HLA, axial, and SA views (ICC=0.62, 0.60, and 0.61, respectively).
Discussion
Qualitative determination of abnormal contraction patterns in the RV is currently a diagnostic problem for imaging physicians, due to the unusual shape and variations in contraction patterns of the RV. Using quantitative metrics, we assessed regional RV myocardial strain through MR feature tracking in a cohort including control, preclinical, and overt ARVC subjects. We showed that quantitative strain measurements were moderately reproducible within and between observers. Globally, strain magnitude was significantly lower in overt ARVC compared to control subjects in all MR views. However, regional strain measured in the HLA view showed the greatest differences between control and overt ARVC subjects. Regionally, subtricuspid strain separated overt ARVC from control subjects with high sensitivity and specificity. Of note, abnormal strain had higher diagnostic accuracy with lower false positive and false negative diagnoses compared to qualitative wall motion assessment. These results suggest that MR feature tracking may have clinical utility in ARVC evaluation.
In ARVC, a basic, yet unanswered question is the ideal plane of imaging. For feature tracking, the HLA view provided (a) the most reproducible measurements, (b) the greatest separation between overt and control, particularly in the subtricuspid region, and (c) a continuous downward trend in strain magnitude between control subjects, preclinical ARVC, and overt ARVC. The superior reproducibility and separation seen in HLA measurements may be due to less through-plane motion compared to axial and SA views.
Strain has previously been quantified in ARVC using speckle tracking echocardiography.(11–14) These studies have been limited by a combination of small numbers of patients and lack of regional analysis, genetic characterization, and a preclinical ARVC group. Prior studies report global longitudinal RV strain of approximately −30% in control subjects, and varying study to study from −10% to −25% in overt ARVC. These values are in agreement with our observations. Impairment of regional function was primarily in the basal region of the RV, consistent with our finding that subtricuspid strain allowed for the greatest discrimination between control subjects and overt ARVC. These findings are also in agreement with prior work showing that structural abnormalities most frequently localize to the subtricuspid region in ARVC.(15) Heermann et al (16) evaluated feature tracking of cine MR in ARVC, including 20 patients with ARVC and 10 control subjects. In that study, the HLA view was not evaluated, so that long axis strain was evaluated only on axial views. Heermann et al found that strain evaluated in the short axis views and ejection fraction provided the greatest difference between ARVC patients and normal subjects. Since the orientation of the heart within the chest cavity varies considerably between subjects, the angular variation between the true HLA long axis view and the axial view may be up to 35 degrees.(17) This variation may explain our results that the HLA view provided greater separation between ARVC patients and control subjects. In our multivariable model, regional subtricuspid strain was superior to global ejection fraction for prediction of the presence of disease, consistent with the concept of ARVC as a regional disease.
The present study suggests MR feature tracking has the potential to improve the reliability of ARVC diagnosis and detection of regional wall motion abnormalities. Although MR may be considered a standard of reference for ARVC, a long-standing limitation is lack of quantitative metrics for regional wall motion abnormality of the RV.(8, 18) Although patients with overt ARVC have global changes in RV ejection fraction and volumes, preclinical ARVC patients who have known desmosomal mutations may have normal or only subtle RV motion abnormalities. Thus, a major focus of clinical effort is based on accurate phenotyping of this early ARVC group in order to accurately triage therapy. In this study, subjects with preclinical ARVC had strain abnormalities that were intermediate between control and overt subjects. Further studies are warranted to follow these patients over time to determine if they indeed represent a high-risk group who may progress to overt disease or have adverse cardiovascular events.
The results of this study should be interpreted in light of its strengths and limitations. Although ARVC is a relatively common reason for heart evaluation by MR, the disease is quite rare and the sample size is therefore small. While this study is among the largest reported in the literature, a larger multicenter trial may provide further insight. An important strength of this study is that clinical characterization of study subjects was extensive and included genetic testing. In addition, ARVC diagnosis was made independently of MR findings, so that the diagnostic TFC provide an independent standard of reference. While it would be interesting to correlate strain values by MR feature tracking to those obtained by echocardiographic deformation imaging, reliable echocardiographic data was not available in part due to limitations of that technique for reliable identification of the RV. Diagnostic accuracy improved with the addition of quantitative measures compared to radiologist interpretation alone. However, false positive and false negative diagnoses remain nontrivial. Though intraobserver reproducibility was good in the HLA view, improvement to the algorithm and increased standardization of the procedure is necessary to improve interobserver reproducibility. Finally, we utilized standard MR steady state free precession (SSFP) images without further optimization for feature tracking. It seems likely that improved temporal and/or spatial resolution of cine MR may result in better feature tracking, possibly improving differences between patient groups and control subjects.
In conclusion, MR feature tracking of the RV has the potential to provide reliable quantification of regional wall motion abnormalities in ARVC. The MR approach identified abnormalities in ARVC most reliably in the HLA view. Compared to qualitative analysis, the approach provided incremental value in confirming the presence of wall motion abnormalities that were most commonly present in the subtricuspid region.
Supplementary Material
Supplemental Figure 1. Bland-Altman plots for intraobserver (left) and intraobserver (right) reproducibility in of strain measurements in the (a) horizontal long axis (top), (b) axial (middle), and (c) circumferential (bottom) views.
Acknowledgments
The authors would like to acknowledge the study participants, without whom this work would not have been possible.
Grant Support: Davis Vigneault's contributions were funded by the NIH intramural research program and the Oxford-Cambridge NIH Training Program. This work was performed during Anneline te Riele's tenure as the Mark Josephson and Hein Wellens research fellow of the Heart Rhythm Society. The Johns Hopkins ARVD/C Program is supported by the Leyla Erkan Family Fund for ARVD Research, the Dr. Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Dr. Francis P. Chiaramonte Private Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments.
Abbreviations
- ARVC
Arrhythmogenic Right Ventricular Cardiomyopathy
- BSA
Body Surface Area
- MR
Magnetic Resonance
- ED
End Diastole
- EDV
End Diastolic Volume
- EF
Ejection Fraction
- ESV
End Systolic Volume
- FNR
False Negative Rate
- FPR
False Positive Rate
- HLA
Horizontal Long Axis
- ICC
Intraclass Correlation Coefficient
- LV
Left Ventricular
- MTT
Multimodality Tissue Tracking
- NS
Not Significant
- RV
Right Ventricular
- SA
Short Axis
- SD
Standard Deviation
- SSFP
Steady State Free Precession
- TFC
Task Force Criteria
References
- 1.Venkatesh BA, Schiros CG, Gupta H, Lloyd SG, Dell'Italia L, Denney TS. Three-dimensional plus time biventricular strain from tagged MR images by phase-unwrapped harmonic phase. J Magn Reson Imaging. 2011;34:799–810. doi: 10.1002/jmri.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tandri H, Uk MRCP, Calkins H, et al. Magnetic Resonance Imaging Findings in Patients Meeting Task Force Criteria for Arrhythmogenic Right Ventricular Dysplasia. 2003;143(March):476–482. doi: 10.1046/j.1540-8167.2003.02560.x. [DOI] [PubMed] [Google Scholar]
- 3.Marcus FI, Fontaine GH, Guiraudon G, et al. Right ventricular dysplasia: A report of 24 adult cases. Circulation. 1982:384–98. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 4.Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823–32. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 5.Groeneweg JA, Bhonsale A, James CA, et al. Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members. Circ Cardiovasc Genet. 2015;8:437–46. doi: 10.1161/CIRCGENETICS.114.001003. [DOI] [PubMed] [Google Scholar]
- 6.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–41. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus FI, Zareba W, Calkins H, et al. Arrhythmogenic right ventricular cardiomyopathy/dysplasia clinical presentation and diagnostic evaluation: results from the North American Multidisciplinary Study. Heart Rhythm. 2009;6:984–992. doi: 10.1016/j.hrthm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bomma C, Rutberg J, Tandri H, et al. Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2004;15:300–6. doi: 10.1046/j.1540-8167.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 9.Tee M, Noble JA, Bluemke DA. Imaging techniques for cardiac strain and deformation: comparison of echocardiography, cardiac magnetic resonance and cardiac computed tomography. Expert Rev Cardiovasc Ther. 2013;11:221–31. doi: 10.1586/erc.12.182. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa K, Hozumi T, Sugioka K, et al. Usefulness of automated quantitation of regional left ventricular wall motion by a novel method of two-dimensional echocardiographic tracking. Am J Cardiol. 2006;98:1531–7. doi: 10.1016/j.amjcard.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 11.Astrom Aneq M, Engvall J, Brudin L, Nylander E. Evaluation of right and left ventricular function using speckle tracking echocardiography in patients with arrhythmogenic right ventricular cardiomyopathy and their first degree relatives. Cardiovasc Ultrasound. 2012;37 doi: 10.1186/1476-7120-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakasa KR, Wang J, Tandri H, et al. Utility of tissue Doppler and strain echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2007;100:507–12. doi: 10.1016/j.amjcard.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 13.Teske AJ, Cox MG, De Boeck BW, Doevendans PA, Hauer RN, Cramer MJ. Echocardiographic tissue deformation imaging quantifies abnormal regional right ventricular function in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Soc Echocardiogr. 2009;22:920–7. doi: 10.1016/j.echo.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Iacoviello M, Forleo C, Puzzovivo A, et al. Altered two-dimensional strain measures of the right ventricle in patients with Brugada syndrome and arrhythmogenic right ventricular dysplasia/cardiomyopathy. Eur J Echocardiogr. 2011;12:773–81. doi: 10.1093/ejechocard/jer139. [DOI] [PubMed] [Google Scholar]
- 15.Te Riele ASJM, James CA, Philips B, et al. Mutation-positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced. J Cardiovasc Electrophysiol. 2013;24:1311–20. doi: 10.1111/jce.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heermann P, Hedderich DM, Paul M, et al. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2014;16:75. doi: 10.1186/s12968-014-0075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz J, Solaiyappan M, Tandri H, et al. Right ventricle shape and contraction patterns and relation to magnetic resonance imaging findings. J Comput Assist Tomogr. 2005;29:725–733. doi: 10.1097/01.rct.0000179596.86221.38. [DOI] [PubMed] [Google Scholar]
- 18.Tandri H, Castillo E, Ferrari VA, et al. Magnetic Resonance Imaging of Arrhythmogenic Right Ventricular Dysplasia. Sensitivity, Specificity, and Observer Variability of Fat Detection Versus Functional Analysis of the Right Ventricle. J Am Coll Cardiol. 2006;48:2277–2284. doi: 10.1016/j.jacc.2006.07.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Bland-Altman plots for intraobserver (left) and intraobserver (right) reproducibility in of strain measurements in the (a) horizontal long axis (top), (b) axial (middle), and (c) circumferential (bottom) views.


