Abstract
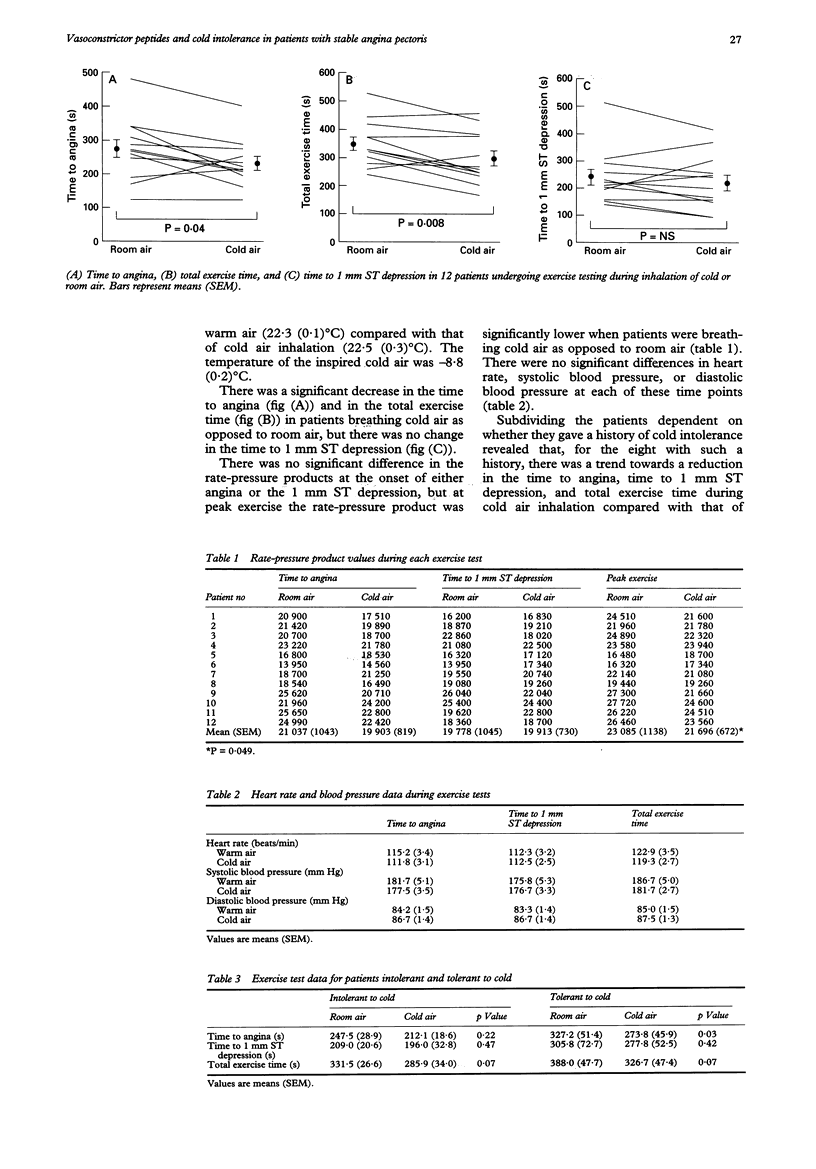
BACKGROUND--The exact mechanism that explains the phenomenon of cold intolerance in patients with angina remains controversial. Although the response to the effects of a cold environment has been examined in these patients, their response to cold air inhalation has produced conflicting results. In addition, the possible role of vasoactive peptides in the pathophysiology has not been explored. OBJECTIVES--The aims of this study were to examine the response of patients with stable angina to the effects of cold air inhalation during exercise testing, and to investigate the possible role played by the vasoconstrictor peptides endothelin-1 (ET-1) and angiotensin-II (AT-II) in the pathophysiology. METHODS--In a randomised order, 12 men with stable angina, whose medication had been stopped, underwent two separate symptom limited treadmill exercise tests. At one visit the patients exercised while breathing room air and at the other visit they exercised while breathing cold air from a specially adapted freezer. Serial peripheral venous blood samples were taken for ET-1 and AT-II estimations during each visit. RESULTS--Cold air inhalation resulted in a significant reduction in the mean time to angina (232.7 (20.4) s v 274.1 (26.9) s, P = 0.04) and the mean total exercise time (299.5 (27.0) s v 350.3 (23.9) s, P = 0.008), but no significant change in the time to 1 mm ST depression (223.3 (29.0) s v 241.3 (29.2) s, P = 0.25). There was no significant difference between the rate-pressure products at the onset of angina (P = 0.13) and the time to 1 mm ST depression (P = 0.85), but at peak exercise the rate-pressure product was significantly lower in patients breathing cold air as opposed to room air (P = 0.049). There was an equivalent significant decrease in ET-1 concentrations at peak exercise compared with that at rest at both visits (room air 5.0 (0.7) pmol/l v 4.3 (0.7) pmol/l, P = 0.03; cold air 4.4 (0.6) pmol/l v 3.8 (0.5) pmol/l, P = 0.02). There was a significant increase in AT-II concentrations 10 min after peak exercise in patients breathing room air (39.2 (6.1) pmol/l v 32.1 (4.8) pmol/l, P = 0.01) which was not repeated during cold air inhalation (36.6 (3.4) pmol/l v 28.3 (3.4) pmol/l, P = 0.07). CONCLUSIONS--Cold air inhalation in patients with stable angina results in an earlier onset of angina and a reduction in exercise capacity. Both peripheral and central reflex mechanisms appear to contribute to the phenomenon of cold intolerance. Peripheral ET-1 and AT-II do not appear to play a significant role in the pathophysiology.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allevard A. M., Gauquelin G., Gharib C. Endothelin and atrial natriuretic peptide after exercise performed until exhaustion in the rat. Life Sci. 1991;49(24):1803–1808. doi: 10.1016/0024-3205(91)90481-p. [DOI] [PubMed] [Google Scholar]
- Anderson T. W., Rochard C. Cold snaps, snowfall and sudden death from ischemic heart disease. Can Med Assoc J. 1979 Dec 22;121(12):1580–1583. [PMC free article] [PubMed] [Google Scholar]
- Chester A. H., Dashwood M. R., Clarke J. G., Larkin S. W., Davies G. J., Tadjkarimi S., Maseri A., Yacoub M. H. Influence of endothelin on human coronary arteries and localization of its binding sites. Am J Cardiol. 1989 Jun 1;63(18):1395–1398. doi: 10.1016/0002-9149(89)91055-2. [DOI] [PubMed] [Google Scholar]
- Epstein S. E., Stampfer M., Beiser G. D., Goldstein R. E., Braunwald E. Effects of a reduction in environmental temperature on the circulatory response to exercise in man. Implications concerning angina pectoris. N Engl J Med. 1969 Jan 2;280(1):7–11. doi: 10.1056/NEJM196901022800102. [DOI] [PubMed] [Google Scholar]
- Fyhrquist F., Saijonmaa O., Metsärinne K., Tikkanen I., Rosenlöf K., Tikkanen T. Raised plasma endothelin-I concentration following cold pressor test. Biochem Biophys Res Commun. 1990 May 31;169(1):217–221. doi: 10.1016/0006-291x(90)91456-3. [DOI] [PubMed] [Google Scholar]
- Gobel F. L., Norstrom L. A., Nelson R. R., Jorgensen C. R., Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978 Mar;57(3):549–556. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- Hattenhaur M., Neill W. A. The effect of cold air inhalation on again pectoris and myocardial oxygen supply. Circulation. 1975 Jun;51(6):1053–1058. doi: 10.1161/01.cir.51.6.1053. [DOI] [PubMed] [Google Scholar]
- Hayward J. M., Holmes W. F., Gooden B. A. Cardiovascular responses in man to a stream of cold air. Cardiovasc Res. 1976 Nov;10(6):691–696. doi: 10.1093/cvr/10.6.691. [DOI] [PubMed] [Google Scholar]
- Juneau M., Johnstone M., Dempsey E., Waters D. D. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation. 1989 May;79(5):1015–1020. doi: 10.1161/01.cir.79.5.1015. [DOI] [PubMed] [Google Scholar]
- Lassvik C. T., Areskog N. H. Angina in cold environment. Reactions to exercise. Br Heart J. 1979 Oct;42(4):396–401. doi: 10.1136/hrt.42.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassvik C., Areskog N. H. Angina pectoris during inhalation of cold air. Reactions to exercise. Br Heart J. 1980 Jun;43(6):661–667. doi: 10.1136/hrt.43.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon D. F., Amidi M., Leonard J. J. Left heart work and temperature responses to cold exposure in man. Am J Cardiol. 1970 Jul;26(1):38–45. doi: 10.1016/0002-9149(70)90756-3. [DOI] [PubMed] [Google Scholar]
- Miyauchi T., Tomobe Y., Shiba R., Ishikawa T., Yanagisawa M., Kimura S., Sugishita Y., Ito I., Goto K., Masaki T. Involvement of endothelin in the regulation of human vascular tonus. Potent vasoconstrictor effect and existence in endothelial cells. Circulation. 1990 Jun;81(6):1874–1880. doi: 10.1161/01.cir.81.6.1874. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Ganz P., Gordon J. B., Alexander R. W., Selwyn A. P. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988 Jan;77(1):43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- Peart I., Bullock R. E., Albers C., Hall R. J. Cold intolerance in patients with angina pectoris: effect of nifedipine and propranolol. Br Heart J. 1989 Jun;61(6):521–528. doi: 10.1136/hrt.61.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizner A. E., Chahine R. A., Ishimori T., Verani M. S., Zacca N., Jamal N., Miller R. R., Luchi R. J. Provocation of coronary artery spasm by the cold pressor test. Hemodynamic, arteriographic and quantitative angiographic observations. Circulation. 1980 Nov;62(5):925–932. doi: 10.1161/01.cir.62.5.925. [DOI] [PubMed] [Google Scholar]
- Ray S. G., McMurray J. J., Morton J. J., Dargie H. J. Circulating endothelin in acute ischaemic syndromes. Br Heart J. 1992 May;67(5):383–386. doi: 10.1136/hrt.67.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds T. B., Campra J. L. Portal hypertension. Postgrad Med J. 1983;59 (Suppl 4):55–63. [PubMed] [Google Scholar]
- Rose G. Cold weather and ischaemic heart disease. Br J Prev Soc Med. 1966 Apr;20(2):97–100. doi: 10.1136/jech.20.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechtman O., Fregly M. J., van Bergen P., Papanek P. E. Prevention of cold-induced increase in blood pressure of rats by captopril. Hypertension. 1991 Jun;17(6 Pt 1):763–770. doi: 10.1161/01.hyp.17.6.763. [DOI] [PubMed] [Google Scholar]
- Staessen J., Fagard R., Hespel P., Lijnen P., Vanhees L., Amery A. Plasma renin system during exercise in normal men. J Appl Physiol (1985) 1987 Jul;63(1):188–194. doi: 10.1152/jappl.1987.63.1.188. [DOI] [PubMed] [Google Scholar]
- Zamora M. R., O'Brien R. F., Rutherford R. B., Weil J. V. Serum endothelin-1 concentrations and cold provocation in primary Raynaud's phenomenon. Lancet. 1990 Nov 10;336(8724):1144–1147. doi: 10.1016/0140-6736(90)92766-b. [DOI] [PubMed] [Google Scholar]


