Abstract
Through the direct control of infection or by providing cytokine signals to other cellular players, T cells play a central role in the orchestration of the immune response. However, in many disease states, T cells are rendered dysfunctional, unable to carry out their effector functions. As T cell activation is bioenergetically demanding, some T cell dysfunction can have metabolic underpinnings. In this review, we will discuss how T cells are programmed to fuel their effector response, and how programmed or pathologic changes can disrupt their ability to generate the energy needed to proliferate and carry out their critical functions.
Introduction
Metabolism, broadly defined, represents a vast array of catabolic and anabolic biochemical processes that promotes cellular function and survival. These pathways are highly regulated, critically important, and date back to the very origins of life. However, it has become increasingly clear that in highly differentiated cellular systems (for instance, in the nervous and immune systems), metabolic and nutrient sensing pathways have diverse functions that go beyond growth or death decisions. In recent years, studies exploring how metabolism interfaces with immune function have garnered much interest. In this article, we will review studies that have dissected the extensive crosstalk between T cell activation states and metabolism/nutrient sensing. Furthermore, we will explore what is known about the metabolic nature of T cell dysfunction and the prominent questions that remain to be explored in this field.
Activation: fueling clonal expansion and differentiation
Naïve T cells must persist for an entire lifetime, lying in wait to sense their cognate antigen. However, upon activation, T cells must proliferate rapidly in order to effectively generate enough clones to direct the immune response and clear pathogens. During this expansion phase, T cells need to be heavily biosynthetic, replicating DNA, producing new proteins, and generating membranes [1]. Memory T cells, having recovered from a robust immune response, must then again exist in a state of extreme quiescence, but be energetically primed to be reactivated with vigor and persistence. These three functional states are fundamentally distinct, and must too have distinct metabolism to support these broad cellular functions.
Highly proliferative cells are very anabolic, requiring immense biosynthetic processes to produce intermediates for cell growth. First described in cancer, very proliferative cells undergo complete glycolysis, fermenting glucose into lactate to generate ATP, even in the presence of oxygen (‘aerobic glycolysis’ or the ‘Warburg effect’) [2, 3]. T lymphocytes, too, were shown to change their metabolism in response to PHA stimulation, shifting the majority of their glucose flux into the aerobic glycolysis pathway [4].
As T cell activation pathways became clearer, so too did the appreciation that these pathways intersected with metabolic changes. T cell activation, for instance, can contribute heavily to activation of the phosphoinositol-3-kinase (PI3K) pathway [5]. These lipid signaling pathways can then contribute to the activation of Akt (Protein Kinase B), which has a central role in cellular metabolism [6]. Akt activation, downstream of T cell activation pathways such as T cell receptor, costimulatory pathways, and cytokines like IL-2 or IL-7, was shown to be crucial for maintenance of cell size and survival [7-10]. Akt can instruct metabolic changes through multiple mechanisms, including post-translational modification of glycolytic enzymes, enhancement of surface-expression of glucose transporters, and transcriptional upregulation of glycolytic regulators [9, 11-13]. As Akt is required for optimal function of T cells, its effects on glycolytic metabolism may, too, be required for T cell activation [14].
While the phenomenon of aerobic glycolysis was well described in T cell activation, its contribution to the T cell phenotype was poorly understood. However, it has been suggested that glycolysis is dispensable for T cell activation, but required for T cell effector functions like cytokine production [15]. Pearce and colleagues describe a role for the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in binding Ifng mRNA and preventing effective translation. When glycolysis is active GAPDH instead participates in metabolism, freeing Ifng mRNA from regulation. Thus, glycolysis may play a crucial role in effector function by releasing distinct mRNA transcripts from post-translational repression.
A third state exists for conventional T cells: long-lived memory cells, resident to lymphoid organs, circulating in the blood, or resident in the tissues [16]. Like naïve T cells, these cells must persist for a lifetime, but must also respond with greater vigor upon re-activation. Naïve T cells survive mainly by oxidative phosphorylation as they require very little proliferation and are functionally quiescent [1]. After T cell expansion and contraction, memory T cells switch back from aerobic glycolysis to mainly utilizing oxidative phosphorylation, and generate energy using fatty acid oxidation [17]. Further, memory T cells maintain a vast mitochondrial reserve, which renders them bioenergetically primed for reactivation [18]. Interestingly, memory T cells have also been shown to utilize glucose to make ATP, utilize this ATP to synthesize fatty acids, and then break the same fatty acids down through fatty acid oxidation [19]. This “futile cycle” may be utilized to maintain long-term mitochondrial health [20]. Further, T cell differentiation into epigenetically-regulated and transcriptionally programmed effector lineages can also be heavily influenced by metabolic signaling and nutrient sensing, a subject which has been reviewed extensively [21-26].
Anergy: maintaining tolerance through modulation of metabolism
In 1987, Mark Jenkins and Ron Schwartz described a peculiar phenomenon that revealed a fundamental aspect of T cell recognition and activation [27]. Culture of T cell clones on chemically fixed antigen presenting cells resulted in a state of T cell hyporesponsiveness: when these T cells were re-stimulated after seeing antigen in this altered context, they did not proliferate nor produce IL-2, despite the fact that had seen antigen and proliferated initially. This phenomenon, now termed T cell clonal anergy, was assumed to be the product of non-inflammatory antigen presentation and the dominant form of peripheral tolerance [28]. After the discovery of costimulation and the two-signal hypothesis, it became abundantly clear that costimulation (CD28 ligation most prominently) provided the additional signaling required to escape anergy and become effectively activated [28]. In the absence of that CD28-derived “Signal 2”, T cells would enter into the anergic state, unable to effectively respond to future stimulations, even if they included CD28 ligation.
While CD28 was revealed to be a crucial second signal for T cell activation, additional studies revealed not only more costimulatory molecules but also molecules which dampened T cell activity, termed “co-inhibitory molecules”. Cytotoxic T Lymphocyte Antigen 4 (CTLA4) was one of the first of these coinhibitory molecules studied [29], whose genetic deletion in mice resulted in massive lymphoproliferation and lethal autoimmunity [30, 31].
However, the fundamental basis of the anergic phenotype was largely unknown. Lack of costimulation resulted in NFAT nuclear translocation in the absence of AP-1, which resulted in less transcription of IL-2 and other cytokine genes, and the stability of Il2 mRNA itself was also implicated as a mechanism by which T cells stimulated without costimulation resulted in hyporesponsiveness [32, 33]. The advent of microarray technology revealed that anergic T cells expressed a robust transcriptional profile, resulting in not only master regulators that enforced the anergic phenotype (Egr2, Egr3, Dgka, Cblb) but also downregulation of many cytokine and cell cycle genes [34, 35].
In order to study the additional signals that lead to anergy, many immunosuppressive compounds, with different mechanisms of action, were employed. For instance, cyclosporine and FK506 (tacrolimus), FKBP12-binding compounds that inhibit calcineurin (PP2B), prevent effective NFAT nuclear translocation and subsequent IL-2 production [36]. Both have been used clinically to prevent organ rejection and to treat autoimmunity [37, 38]. Its mechanism of action effectively blocks signaling downstream of TCR (Signal 1). While cyclosporine is an effective immunosuppressant, due to the blockade of TCR signaling, long term tolerance could never be achieved because anergy requires effective signal 1 in the absence of signal 2.
Around this time, another immunosuppressant was studied: rapamycin, a cyclophilin-binding, macrolide antibiotic produced by Streptomyces hygroscopicus, discovered on Easter Island (‘Rapa Nui’) [39]. This compound (also known as sirolimus) is a potent immunosuppressant that could result in tolerance and has been used extensively in transplantation [40-42]. Study of its downstream target, the mechanistic target of rapamycin (mTOR), revealed that costimulation, the net sum of positive costimulatory and negative coinhibitory signals, was routed in part through mTOR. mTOR is a protein kinase, conserved from yeast to man, which integrates environmental signals of nutrient availability, energy charge, and growth factor signals, to make cell fate decisions in terms of metabolism, ribosome biogenesis, translation initiation, and the inhibition of autophagy [43]. Interestingly, T cells have conscripted this mTOR machinery to integrate costimulatory signals as well, and T cells that receive costimulation in the context of mTOR blockade become anergic [26].
However, the role of mTOR is not simply to integrate costimulatory signals. Akt and mTOR signaling are crucially involved in the metabolic phenotype of T cell activation. As T cell activation is so metabolically demanding, T cells require the ability to sense the nutrient status of their microenvironment. CD28 signaling, routed in part through mTOR, is thus required to upregulate metabolic machinery including amino acid transporters, glucose transporters, transferrin receptors, and the sustained upregulation of glycolysis [44]. Importantly, rendering T cells anergic through TCR stimulation alone not only prevents upregulation of metabolic machinery, but also prevents it from being upregulated in future stimulations, even those delivered with costimulation, suggesting that these cells are “metabolically anergic” [45]. Starvation of T cells through blockade of glucose, amino acid, or energy charge sensing results in durable anergy induction, suggesting that nutrient sensing represents a key “safety” to prevent activation in metabolically dearth conditions [45]. As tightly regulated metabolism is crucial to effective T cell expansion and function, anergic T cells are transcriptionally rendered metabolically deficient. By preventing the upregulation of metabolic machinery required to carry out the effector response, the transcriptional program induced by an anergy-inducing stimulus maintains the hyporesponsive nature of the cells; even upon receiving a fully activating stimulation in the future, T cells will be unable to metabolically support efficient cytokine production and effector expansion.
Exhaustion: dysfunction through insufficient metabolism
While anergy represents T cell activation in the context of minimal signals (immature APCs delivering pMHC in the absence of costimulatory molecules, for instance), with little to no inflammation, there are other settings in which T cells receive strong and sustained signals. In chronic viral infections, evasion of the immune response results in viral persistence [46]. This effectively means that T cells responding to this virus will receive continual TCR stimulation and costimulatory molecule signaling, all in the presence of chronic, inflammatory cytokines. Zinkernagel and Ahmed described the fate of T cells responding to a chronic viral infection in mice (lymphochloriomeningitis virus clone 13; LCMVCL13) [47, 48]. T cells responding to viral epitopes in the context of LCMVCL13 were significantly hyporesponsive, compared to those responding to the same epitopes of an acute strain of the same virus. They reasoned that this dysfunctional hyporesponsiveness was driven by chronic activation, resulting in the inability to effectively rest after initial expansion, a phenotype now termed ‘T cell exhaustion’ [49]. Exhausted T cells fail to secrete cytokines, lyse target cells, or proliferate effectively [50]. Further, this chronic stimulation drives the elevated and sustained expression of co-inhibitory molecules like PD-1, LAG-3, Tim-3, and 2B4, which further act to suppress the activation of T cells [50].
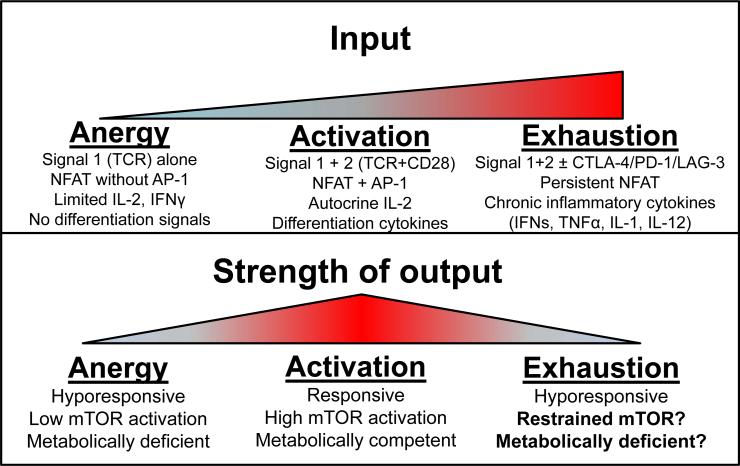
However, T cell exhaustion is not limited to chronic viral infection. Cancer, too, represents a chronically inflamed state with persistent antigen, resulting in chronic T cell activation. Unsurprisingly, T cells that infiltrate tumors exhibit dysfunction akin to exhaustion seen in chronic viral infections: an inability to lyse tumor cells or produce cytokines upon tumor recognition [51]. This, too, is associated with elevated and sustained expression of coinhibitory molecules. Indeed, blockade of coinhibitory molecule signaling results in increased antitumor T cell responses and has led to astonishing clinical responses [52]. Thus, exhaustion represents a hyporesponsive state that is phenotypically similar to anergy. However, while anergy is a transcriptionally programmed process induced by minimal signaling, exhaustion occurs, pathologically, by abundant inflammatory signals (Figure 1). However, there are conserved mechanisms that promote both anergy and exhaustion, the most well studied being strong TCR stimulation. TCR ligation promotes nuclear activity of NFAT, and dominant NFAT activity can promote both anergic and exhausted phenotypes, dependent on context [32, 53, 54]. Exhausted T cells persist, in the presence of chronic activation, unable to efficiently carry-out effector functions. Thus, it may stand to reason that some of the dysfunction in T cell metabolism may result from inefficient metabolic activity.
Figure 1. T cell function and dysfunction are linked with metabolism.
During normal T cell activation, cells receive TCR and CD28 stimulation, produce autocrine levels of IL-2, and respond to APC-produced differentiation cytokines, resulting in a switch to anabolic metabolism during their highly proliferative effector phase. When cells are rendered anergic, they receive only TCR stimulation with little accessory signaling, resulting in low autocrine levels of IL-2, a failure to commit to anabolic metabolism, and are transcriptionally programmed to downregulate metabolic machinery required to engage future effector responses. In situations of chronic activation, T cells also receive heightened inflammatory stimuli including interferons, TNF, and other cytokines, and begin to upregulate co-inhibitory molecules in an attempt to feedback on the immune response. These signals (and others) may restrain mTOR activation and anabolic metabolism, potentially creating a pathologic phenotype of metabolic insufficiency.
Co-inhibitory molecule signaling (elevated in exhausted T cells) has had many ties to metabolic signaling pathways. CTLA-4 has been shown to interact with PP2A [55, 56], a negative regulator of Akt, mTOR, and MAPK signaling, while PD-1 can stimulate PTEN and SHP2 phosphatase activity, which negatively regulate PI3K signaling upstream of Akt [57]. Thus, metabolic pathways downstream of Akt/mTOR may be impacted by coinhibitory molecule signaling, and coinhibitory molecule signaling enforces the “exhausted” phenotype of T cells in part by modulating metabolism. There are some hints in recent studies to this reasoning. CTLA-4 signaling, for instance, has been shown to modulate metabolic signaling and inhibit the generation of mitochondrial reserve [58]. Studies of PD-1 signaling have revealed that it, too, can modify the metabolism of T cells. PD-1 signaling has been shown to inhibit glycolytic metabolism and promote metabolism of lipids [59]. Acting through Akt and Foxo, PD-1 can promote its own expression during chronic viral infection and consequent T cell exhaustion [60]. This promotes their persistence, but also inhibits their effector function. Thus, the metabolic insufficiencies induced by PD-1 signaling may be due to the need to promote long-term persistence, but, in the case of chronic infections or cancer, result in escape of transformed/infected cells. As such, exhausted T cells may be metabolically deficient, but it is still unclear if this underlies or is merely associated with their dysfunctional phenotype. It may be that in the face of limiting nutrients, chronic activation, and coinhibitory molecule signaling, the majority of metabolic flux is spent merely maintaining cell survival, with little in reserve to promote effector function.
Concluding remarks
Metabolism and nutrient sensing are ancient signaling pathways that are critically important for the function of all cells. However, in cells of immune origin, it is of paramount importance to maintain the survival and recall of every clone that persists through selection into the periphery. These pathways have been conscripted by T cells to make cell fate decisions rather than whether to divide or die. Metabolism thus represents a key node of regulation for T cell function. Sufficient, and sometimes distinct, fuels are required throughout their functional states as naïve, effector, or memory T cells. T cells can enter into alternative, dysfunctional states when these needs are not met, including anergy (metabolically inert) and exhaustion (metabolically insufficient). Future elaboration of how metabolic fitness underlies healthy and dysfunctional T cells has the potential to reveal novel therapeutic modalities for the treatment of a variety of diseases.
Highlights.
- T cells dramatically change their metabolism to support effector cell expansion
- T cell clonal anergy induces programmed changes in metabolism which can underlie hyporesponsiveness
- Dysfunction in T cell exhaustion may be linked with an inability to meet these metabolic requirements
ACKNOWLEDGEMENTS
This work was supported in part by the Sidney Kimmel Foundation for Cancer Research (SKF-015-039 to GMD) and the National Institutes of Health, NIAID (R01AI077610 and R01AI091481 to JDP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science (New York, N.Y.) 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer research. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 3.Warburg O. On the origin of cancer cells. Science (New York, N.Y.) 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Roos D, Loos JA. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Experimental cell research. 1973;77:127–135. doi: 10.1016/0014-4827(73)90561-2. [DOI] [PubMed] [Google Scholar]
- 5.Genot EM, Arrieumerlou C, Ku G, Burgering BM, Weiss A, Kramer IM. The T-cell receptor regulates Akt (protein kinase B) via a pathway involving Rac1 and phosphatidylinositide 3-kinase. Molecular and cellular biology. 2000;20:5469–5478. doi: 10.1128/mcb.20.15.5469-5478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fruman DA. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Current opinion in immunology. 2004;16:314–320. doi: 10.1016/j.coi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. European journal of immunology. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Stokoe D, Kane LP, Weiss A. The inducible expression of the tumor suppressor gene PTEN promotes apoptosis and decreases cell size by inhibiting the PI3K/Akt pathway in Jurkat T cells. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 2002;13:285–296. [PubMed] [Google Scholar]
- 9.Masse GX, Corcuff E, Decaluwe H, Bommhardt U, Lantz O, Buer J, Di Santo JP. gamma(c) cytokines provide multiple homeostatic signals to naive CD4(+) T cells. European journal of immunology. 2007;37:2606–2616. doi: 10.1002/eji.200737234. [DOI] [PubMed] [Google Scholar]
- 10.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Molecular biology of the cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentley J, Itchayanan D, Barnes K, McIntosh E, Tang X, Downes CP, Holman GD, Whetton AD, Owen-Lynch PJ, Baldwin SA. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. The Journal of biological chemistry. 2003;278:39337–39348. doi: 10.1074/jbc.M305689200. [DOI] [PubMed] [Google Scholar]
- 12.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Molecular biology of the cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantrell D. Protein kinase B (Akt) regulation and function in T lymphocytes. Seminars in immunology. 2002;14:19–26. doi: 10.1006/smim.2001.0338. [DOI] [PubMed] [Google Scholar]
- 15.Chang CH, Curtis JD, Maggi LB, Jr., Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nature immunology. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, Hsu FF, Birnbaum MJ, Pearce EJ, Pearce EL. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aon MA, Bhatt N, Cortassa SC. Mitochondrial and cellular mechanisms for managing lipid excess. Frontiers in physiology. 2014;5:282. doi: 10.3389/fphys.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Yang H, Chen X, Lu Y, Zhang Z, Wang J, Zhang M, Xue L, Xue F, Liu G. Cellular metabolism modulation in T lymphocyte immunity. Immunology. 2014 doi: 10.1111/imm.12321. DOI 10.1111/imm.12321. [DOI] [PubMed] [Google Scholar]
- 22.Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends in immunology. 2015;36:81–91. doi: 10.1016/j.it.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay G, Cantrell D. Environmental and metabolic sensors that control T cell biology. Frontiers in immunology. 2015;6:99. doi: 10.3389/fimmu.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng H, Chi H. mTOR signaling and transcriptional regulation in T lymphocytes. Transcription. 2014;5:e28263. doi: 10.4161/trns.28263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nature reviews. Immunology. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. The Journal of experimental medicine. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz RH. T cell anergy. Annual review of immunology. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 29.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 30.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 31.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science (New York, N.Y.) 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 32.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 33.Powell JD, Ragheb JA, Kitagawa-Sakakida S, Schwartz RH. Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunological reviews. 1998;165:287–300. doi: 10.1111/j.1600-065x.1998.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 34.Collins S, Lutz MA, Zarek PE, Anders RA, Kersh GJ, Powell JD. Opposing regulation of T cell function by Egr-1/NAB2 and Egr-2/Egr-3. European journal of immunology. 2008;38:528–536. doi: 10.1002/eji.200737157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, Powell JD. Egr-2 and Egr-3 are negative regulators of T cell activation. Nature immunology. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 36.Siekierka JJ, Sigal NH. FK-506 and cyclosporin A: immunosuppressive mechanism of action and beyond. Current opinion in immunology. 1992;4:548–552. doi: 10.1016/0952-7915(92)90024-9. [DOI] [PubMed] [Google Scholar]
- 37.Halloran PF, Madrenas J. The mechanism of action of cyclosporine: a perspective for the 90's. Clinical biochemistry. 1991;24:3–7. doi: 10.1016/0009-9120(91)90063-k. [DOI] [PubMed] [Google Scholar]
- 38.Macleod AM, Thomson AW. FK 506: an immunosuppressant for the 1990s? Lancet. 1991;337:25–27. doi: 10.1016/0140-6736(91)93341-6. [DOI] [PubMed] [Google Scholar]
- 39.Paghdal KV, Schwartz RA. Sirolimus (rapamycin): from the soil of Easter Island to a bright future. Journal of the American Academy of Dermatology. 2007;57:1046–1050. doi: 10.1016/j.jaad.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Lo YC, Lee CF, Powell JD. Insight into the role of mTOR and metabolism in T cells reveals new potential approaches to preventing graft rejection. Current opinion in organ transplantation. 2014;19:363–371. doi: 10.1097/MOT.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annual review of immunology. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 42.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. The Journal of biological chemistry. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 43.Albert V, Hall MN. mTOR signaling in cellular and organismal energetics. Current opinion in cell biology. 2015;33:55–66. doi: 10.1016/j.ceb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 45.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. Journal of immunology (Baltimore, Md. : 1950) 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 47.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 48.Matloubian M, Somasundaram T, Kolhekar SR, Selvakumar R, Ahmed R. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. The Journal of experimental medicine. 1990;172:1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doherty PC. Immune exhaustion: driving virus-specific CD8+ T cells to death. Trends in microbiology. 1993;1:207–209. doi: 10.1016/0966-842x(93)90133-c. [DOI] [PubMed] [Google Scholar]
- 50.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 51.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends in immunology. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Topalian SL, Drake CG, Pardoll DM. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez GJ, Pereira RM, Aijo T, Kim EY, Marangoni F, Pipkin ME, Togher S, Heissmeyer V, Zhang YC, Crotty S, Lamperti ED, Ansel KM, Mempel TR, Lahdesmaki H, Hogan PG, Rao A. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soto-Nieves N, Puga I, Abe BT, Bandyopadhyay S, Baine I, Rao A, Macian F. Transcriptional complexes formed by NFAT dimers regulate the induction of T cell tolerance. The Journal of experimental medicine. 2009;206:867–876. doi: 10.1084/jem.20082731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chuang E, Fisher TS, Morgan RW, Robbins MD, Duerr JM, Vander Heiden MG, Gardner JP, Hambor JE, Neveu MJ, Thompson CB. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 56.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley JL. PD-1 signaling in primary T cells. Immunological reviews. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedicord VA, Cross JR, Montalvo-Ortiz W, Miller ML, Allison JP. Friends not foes: CTLA-4 blockade and mTOR inhibition cooperate during CD8+ T cell priming to promote memory formation and metabolic readiness. Journal of immunology (Baltimore, Md. : 1950) 2015;194:2089–2098. doi: 10.4049/jimmunol.1402390. [DOI] [PubMed] [Google Scholar]
- 59.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nature communications. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, Cui G, Li MO, Kaech SM. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]