Abstract
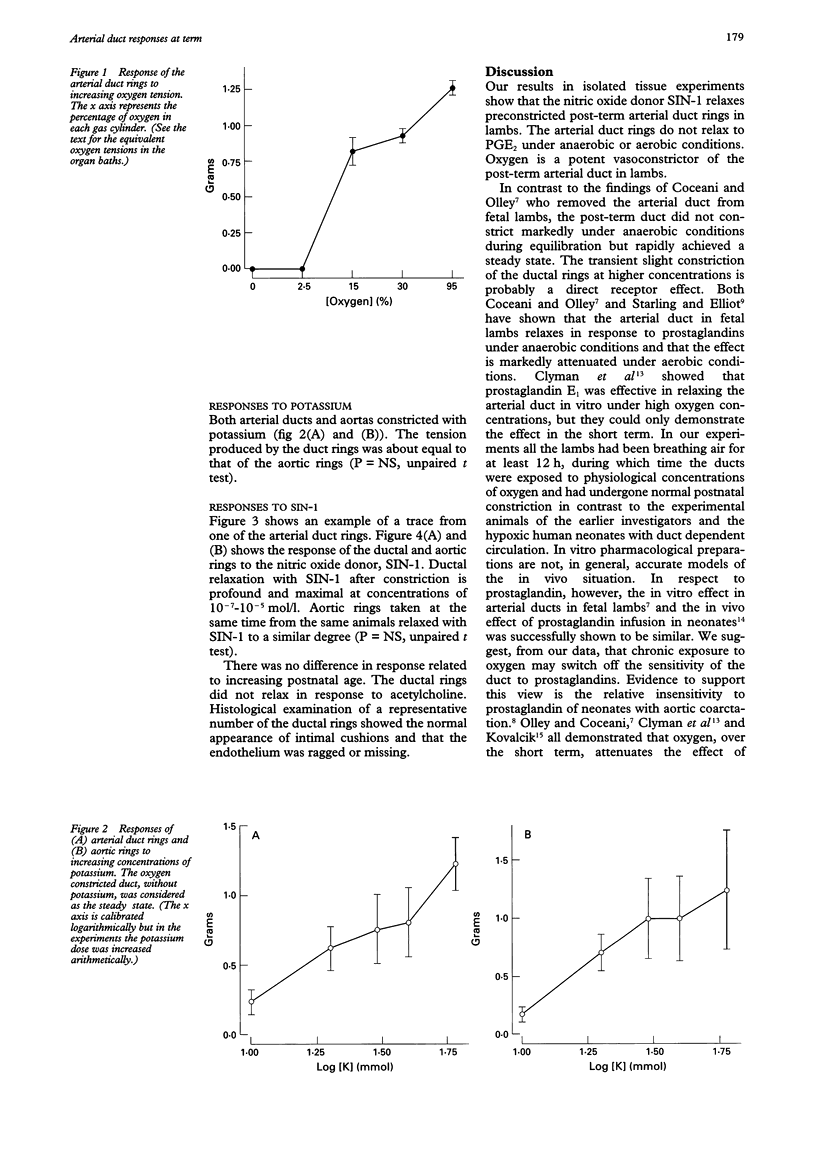
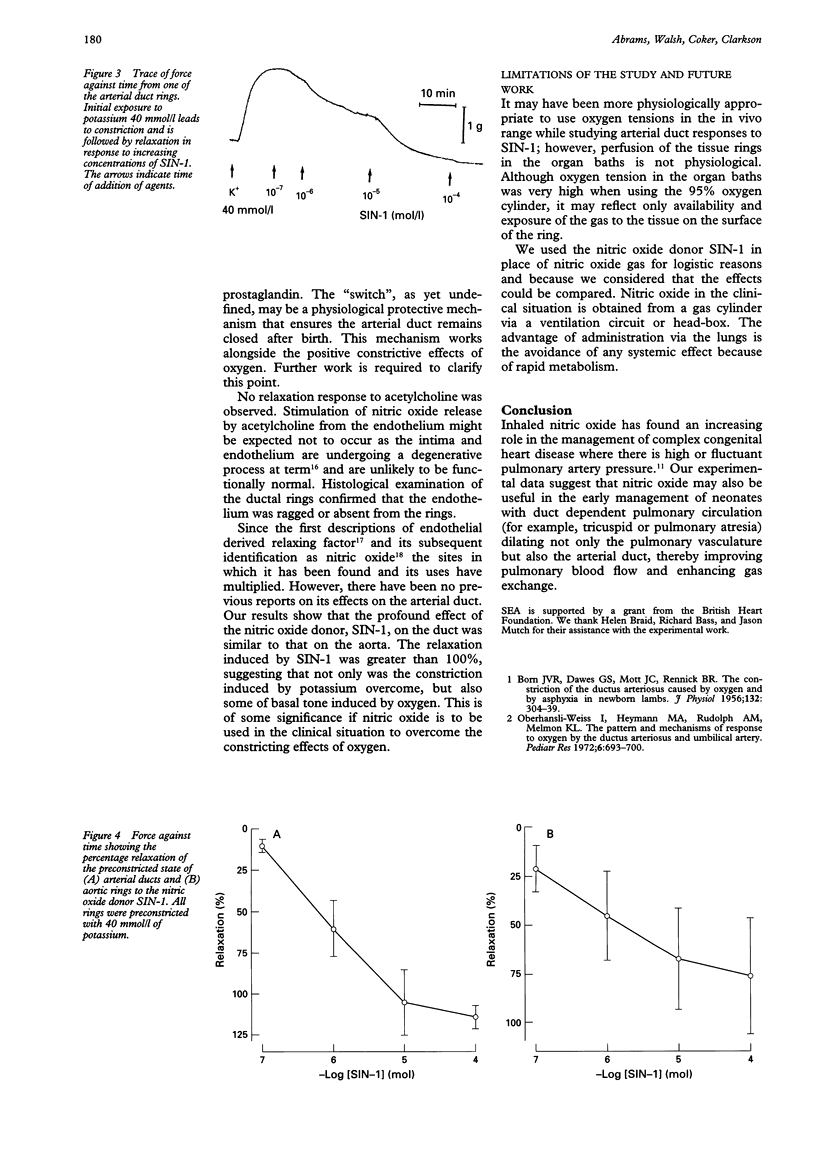
BACKGROUND--Nitric oxide is a potent dilator of the pulmonary vasculature. There have been no previous reports on the action of nitric oxide on the arterial duct. OBJECTIVES--To determine the responses of isolated post-term arterial duct rings from lambs to oxygen, prostaglandin E2 (PGE2) and the nitric oxide donor, 3-morpholinosydnonimine (SIN-1). SETTING--Experimental laboratory. SUBJECTS--Six neonatal lambs. METHODS--Lambs aged 1-5 days were killed and the arterial duct and aorta excised and cut into rings. These were mounted on tension gauges in organ baths containing Krebs-Henseleit solution. Rings were exposed to increasing concentrations of oxygen, PGE2 and after preconstriction with potassium (40 mmol/l) to SIN-1. Tension and relaxation responses were recorded. RESULTS--Increased oxygen tension resulted in increased tension in the ductal rings above 88.9 mm Hg as previously described. No response to PGE2 occurred before or after ductal rings were exposed to oxygen. SIN-1 caused relaxation of smooth muscle in the arterial duct to a similar degree as that in the aortic rings. CONCLUSIONS--As previously shown, oxygen is a potent constrictor of the arterial duct. The post-term arterial duct does not relax in response to PGE2 possibly as a result of inactivation by oxygen of the special sensitivity of the duct to PGE2. SIN-1 is a potent smooth muscle relaxing agent in the term arterial duct and may have a role in the initial management of neonates with duct dependent pulmonary circulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., DAWES G. S., MOTT J. C., RENNICK B. R. The constriction of the ductus arteriosus caused by oxygen and by asphyxia in newborn lambs. J Physiol. 1956 May 28;132(2):304–342. doi: 10.1113/jphysiol.1956.sp005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyman R. I., Heymann M. A., Rudolph A. M. Ductus arteriosus responses to prostaglandin E1 at high and low oxygen concentrations. Prostaglandins. 1977 Feb;13(2):219–223. doi: 10.1016/0090-6980(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Coceani F., Breen C. A., Lees J. G., Falck J. R., Olley P. M. Further evidence implicating a cytochrome P-450-mediated reaction in the contractile tension of the lamb ductus arteriosus. Circ Res. 1988 Mar;62(3):471–477. doi: 10.1161/01.res.62.3.471. [DOI] [PubMed] [Google Scholar]
- Coceani F., Kelsey L., Seidlitz E. Evidence for an effector role of endothelin in closure of the ductus arteriosus at birth. Can J Physiol Pharmacol. 1992 Jul;70(7):1061–1064. doi: 10.1139/y92-146. [DOI] [PubMed] [Google Scholar]
- Coceani F., Olley P. M. The response of the ductus arteriosus to prostaglandins. Can J Physiol Pharmacol. 1973 Mar;51(3):220–225. doi: 10.1139/y73-031. [DOI] [PubMed] [Google Scholar]
- Coceani F., Wright J., Breen C. Ductus arteriosus: involvement of a sarcolemmal cytochrome P-450 in O2 constriction? Can J Physiol Pharmacol. 1989 Nov;67(11):1448–1450. doi: 10.1139/y89-232. [DOI] [PubMed] [Google Scholar]
- Freed M. D., Heymann M. A., Lewis A. B., Roehl S. L., Kensey R. C. Prostaglandin E1 infants with ductus arteriosus-dependent congenital heart disease. Circulation. 1981 Nov;64(5):899–905. doi: 10.1161/01.cir.64.5.899. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ho S. Y., Anderson R. H. Anatomical closure of the ductus arteriosus: a study in 35 specimens. J Anat. 1979 Jun;128(Pt 4):829–836. [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVALCIK V. THE RESPONSE OF THE ISOLATED DUCTUS ARTERIOSUS TO OXYGEN AND ANOXIA. J Physiol. 1963 Nov;169:185–197. doi: 10.1113/jphysiol.1963.sp007249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurphy D. M., Heymann M. A., Rudolph A. M., Melmon K. L. Developmental changes in constriction of the ductus arteriosus: responses to oxygen and vasoactive agents in the isolated ductus arteriosus of the fetal lamb. Pediatr Res. 1972 Apr;6(4):231–238. doi: 10.1203/00006450-197204000-00004. [DOI] [PubMed] [Google Scholar]
- Oberhänsli-Weiss I., Heymann M. A., Rudolph A. M., Melmon K. L. The pattern and mechanisms of response to oxygen by the ductus arteriosus and umbilical artery. Pediatr Res. 1972 Sep;6(9):693–700. doi: 10.1203/00006450-197209000-00001. [DOI] [PubMed] [Google Scholar]
- Olley P. M., Coceani F., Bodach E. E-type prostaglandins: a new emergency therapy for certain cyanotic congenital heart malformations. Circulation. 1976 Apr;53(4):728–731. doi: 10.1161/01.cir.53.4.728. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Jr, Chen T. Y., Kawai N., Wain J., Dupuy P., Shimouchi A., Bloch K., Polaner D., Zapol W. M. Inhaled nitric oxide reverses pulmonary vasoconstriction in the hypoxic and acidotic newborn lamb. Circ Res. 1993 Feb;72(2):246–254. doi: 10.1161/01.res.72.2.246. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Jr, Lang P., Bigatello L. M., Vlahakes G. J., Zapol W. M. Inhaled nitric oxide in congenital heart disease. Circulation. 1993 Feb;87(2):447–453. doi: 10.1161/01.cir.87.2.447. [DOI] [PubMed] [Google Scholar]
- Starling M. B., Elliott R. B. The effects of prostaglandins, prostaglandin inhibitors, and oxygen on the closure of the ductus arteriosus, pulmonary arteries and umbilical vessels in vitro. Prostaglandins. 1974 Nov 10;8(3):187–203. doi: 10.1016/0090-6980(74)90042-2. [DOI] [PubMed] [Google Scholar]


