Abstract
Objective:
To gain insight into the mechanism by which sex-determining region of Y chromosome (SRY)-related high-mobility-group box 2 (SOX2) involved in carcinogenesis and cancer stem cells (CSCs).
Data Sources:
The data used in this review were mainly published in English from 2000 to present obtained from PubMed. The search terms were “SOX2”, “cancer”, “tumor” or “CSCs”.
Study Selection:
Articles studying the mitochondria-related pathologic mechanism and treatment of glaucoma were selected and reviewed.
Results:
SOX2, a transcription factor that is the key in maintaining pluripotent properties of stem cells, is a member of SRY-related high-mobility group domain proteins. SOX2 participates in many biological processes, such as modulation of cell proliferation, regulation of cell death signaling, cell apoptosis, and most importantly, tumor formation and development. Although SOX2 has been implicated in the biology of various tumors and CSCs, the findings are highly controversial, and information regarding the underlying mechanism remains limited. Moreover, the mechanism by which SOX2 involved in carcinogenesis and tumor progression is rather unclear yet.
Conclusions:
Here, we review the important biological functions of SOX2 in different tumors and CSCs, and the function of SOX2 signaling in the pathobiology of neoplasia, such as Wnt/β-catenin signaling pathway, Hippo signaling pathway, Survivin signaling pathway, PI3K/Akt signaling pathway, and so on. Targeting towards SOX2 may be an effective therapeutic strategy for cancer therapy.
Keywords: Anticancer Therapy, Cancer, Cancer Stem Cell, Signaling, Sex-determining Region of Y Chromosome-related High-mobility-group Box 2
INTRODUCTION
Sex-determining region of Y chromosome (SRY)-related high-mobility-group box 2 (SOX2) is one of the key transcriptional factors that maintain the pluripotency and self-renewal properties of embryonic stem (ES) cells. Recent studies indicate that SOX2 is widely over-expressed in various human cancer and cancer stem cells (CSCs) and is found to contribute to tumorigenesis and recurrence of certain types of cancer.[1,2,3] However, the function of SOX2 and its underlying mechanism in cancer cells or CSCs are unclear.
Sex-determining region of Y chromosome-related high-mobility-group box 2: a member of sex-determining region of y chromosome-related high-mobility group domain proteins
The highly conserved domain of high-mobility group (HMG) SOX family member genes encode an 80 amino acid residues. There are approximately 20 different SOX genes in the vertebrate genome that are classified into eight subfamilies according to sequence homologies in their HMG domains. SOX proteins act in part by remodeling the local chromatin architecture upon DNA-binding to the HMG domain that induces DNA bending.
Virtually all members of the SOX family have been found to be deregulated in a wide variety of tumors. It has been accepted that SOX proteins themselves do not possess sufficient affinities for DNA binding, the transcription activity of SOX proteins requires recruitment of other protein partners, such as Nanog, OCT4, Sall4, and so on.[4,5,6]
Sex-determining region of Y chromosome-related high-mobility-group box 2 highly expression in human cancers
Previous clinical and experimental evidences have demonstrated that SOX2 is over-expressed and acts as an oncogene in a number of cancers such as head and neck squamous cell carcinoma,[7] pancreatic cancer,[8] breast cancer,[9] glioblastoma[10] and colorectal cancer.[11] In prostate cancer, SOX2 promotes tumorigenesis and suppresses apoptosis[12] and involved in epidermal growth factor receptor (EGFR)-mediated self-renewal of human prostate cancer stem-like cells.[13] In lung and esophagus cancer, the highly up-regulated SOX2 promotes cell migration and proliferation by driving cells squamous differentiation and pluripotency.[14,15,16] Animal experiments showed that about half of the mice expressed the highest levels of SOX2 in developed carcinoma in the lung. These tumors resemble adenocarcinoma but express the squamous marker, Trp63. In breast cancer, it has been demonstrated that SOX2 is likely to promote cell proliferation and tumorigenesis through facilitating the G1/S transition of the cell cycle.[1]
THE FUNCTION OF SEX-DETERMINING REGION OF Y CHROMOSOME-RELATED HIGH-MOBILITY-GROUP BOX 2 IN HUMAN TUMORS
Sex-determining region of Y chromosome-related high-mobility-group box 2 and cancer stem cell
The concept of CSC was raised that a small percentage of cancer cell population has the same characters as ES cells.[17] CSCs may play a key role in cancer initiation, progression, and resistance to current treatments. Further studies of CSCs isolated from human prostate cancer, lung cancer, and some other cancer cell lines indicated that SOX2 is over-expressed with other embryonic markers such as Nanog, OCT4, and the surface marker protein CD44.[18,19]
Sex-determining region of Y chromosome-related high-mobility-group box 2 is over-expressed in CSCs and plays a role in tumorigenesis and recurrence. In the study of glioma blastoma,[20] it is found that CSCs express SOX2, and the expression levels were two-fold to more than 200-fold higher in CSCs than those in adherent cells. CSCs express both tumor-associated antigen and relevant major histocompatibility complex molecules that are necessary for cytotoxic T lymphocyte (CTL) recognition and activation. It may be a novel strategy for cancer immunotherapy by using CSCs associated antigens SOX2 prolongs survival.
CD133 has been recognized as a specific cell surface marker for CSCs in various tumors.[21,22] The enhancement of SOX2 and OCT4 expression was required for hypoxia-induced CD133 expression.[23] Knockdown of OCT4 or SOX2 expression in N417 cells abolished CD133P1 activity. Thus, in the hypoxic conditions, OCT4 and SOX2 promote CD133 expression in the lung cancer cells via their direct interaction with the P1 promoter.
Sex-determining region of Y chromosome-related high-mobility-group box 2 with cancer metastasis
Previous studies suggest that SOX2 is involved in tumorigenesis and metastasis. High SOX2 expression is associated with larger tumor size and positive lymph node status. Corresponding metastatic lymph nodes showed higher SOX2 expression and were significantly more often SOX2 positive than primary tumors.[24] In colorectal carcinoma cell lines, SOX2 was validated as a target for miR-200c. Knockdown of miR-200c significantly enhanced proliferation, migration, and invasion.[25]
Sex-determining region of Y chromosome-related high-mobility-group box 2 and macrophages
Macrophages promote cancer initiation and malignant progression. They create an inflammatory environment that is mutagenic and promotes growth during tumor initiation.[26,27,28] As tumors progress, macrophages stimulate angiogenesis, enhance tumor cell migration and invasion, and suppress antitumor immunity. Macrophages promote tumor cell extravasation, survival, and subsequent growth. Specialized subpopulations of macrophages may represent important new therapeutic targets.
Macrophages have been reported to promote CSC-like phenotypes in murine breast cancer cells by upregulating their expression of SOX2.[29] Downregulation of SOX2 in tumor cells by small interfering RNA blocked the ability of macrophages to induce these CSC-like phenotypes and inhibited tumor growth in vivo. Furthermore, a novel EGFR/signal transducers and activators of transcription 3 (STAT3)/SOX2 paracrine signaling pathway has been identified. Therefore, the EGFR/STAT3/SOX2 signaling pathway may be a novel therapeutic target.
THE SIGNALING PATHWAY OF SEX-DETERMINING REGION OF Y CHROMOSOME-RELATED HIGH-MOBILITY-GROUP BOX 2 IN HUMAN TUMORS
Wnt/β-catenin signaling pathway
Activation of the Wnt/β-catenin signaling pathway plays an important role in lung cancer.[30] As previously described, SOX2 promoting tamoxifen resistance in breast cancer cells may through Wnt/β-catenin signaling pathway.
Moreover, it is disclosed that SOX2 improves metastasis of breast and prostate cancer cells by promoting epithelial-to-mesenchymal transition (EMT) through Wnt/β-catenin. Dual luciferase assay and chromatin immunoprecipitation revealed activation and binding of SOX2 on the promoter region of β-catenin.[31] In addition, SOX2 affects the protein expression levels of DKK3, DVL1 and DVL3, which are regulators or downstream molecules of Wnt signaling. This suggests that β-catenin is one of the vital downstream molecules that mediate the EMT induced by SOX2.
Hippo signaling pathway
Yes-associated protein (YAP) is a potent transcription coactivator acting via binding to the TEAD transcription factor. In addition to that, there are one or two WW domains in the central region of YAP, depending on alternative splicing. Gene expression profiling and chromatin immunoprecipitation revealed that Hippo signaling negatively regulates a subset of Wnt target genes. The Hippo effector YAP interacts with β-catenin on SOX2 and Snai2 genes.[32]
In another study,[33] Western blotting for ES cell markers also showed that SOX2 and OCT protein levels were high in YAP-overexpressing cells. In line with this ChIP-PCR data, the SOX2 protein level was changed upon YAP over-expression and knockdown conditions. It indicates that SOX2 could be YAP target gene.
Survivin signaling pathway
Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis.[34] In the study of NSCs, it is found that SOX2 directly up-regulates the expression of survivin, which inhibits the mitochondria-dependent apoptotic pathway.[35] Although over-expression of SOX2 elevates survivin expression, knockdown of SOX2 results in a decrease in survivin expression, thereby initiating the mitochondria-dependent apoptosis related to caspase 9 activation. In short, SOX2/surviving pathway regulates neural stem cell survival and homeostasis.
Recent studies have found that SOX2 regulates apoptosis through MAP4K4-survivin signaling pathway in human lung cancer cells.[36] Down-regulation of SOX2 leads to activation of MAP4K4, tumor necrosis factor α, p53, and inhibition of survivin. Rescue experiments revealed that SOX2 silencing mediated killing was blocked by ectopic expression of survivin, or by reduction of MAP4K4 expression.
PI3K/Akt signaling pathway
In the study of prostate cancer,[12] it is found that SOX2 targets cyclin E, p27 and survivin to regulate androgen-independent human prostate cancer cell proliferation and apoptosis. Moreover, transforming growth factor-alpha up regulated SOX2 and survivin protein expression via the EGFR/PI3K/Akt pathway.
In another study of laryngeal squamous cell carcinoma (LSCC),[37] over-expression of SOX2 induced phosphorylation of Akt and mammalian target of rapamycin (mTOR), which are downstream effectors of the PI3K pathway. LY294002, an inhibitor of PI3K, also markedly abolished SOX2-induced activation of the Akt/mTOR pathway and increased cell invasion and matrix metalloproteinase-2 expression.
Hedgehog signaling pathway
The Hedgehog (Hh) signaling pathway is a signaling pathway that transmits information to embryonic cells required for proper development. It was found that many diseases are associated with the malfunction of this pathway, for example, basal cell carcinoma.[38] Protein kinase CI (PKCI) phosphorylates SOX2. PKCI directly phosphorylates and recruits SOX2 to the HHAT promoter. PRKCI and SOX2 are coamplified and coordinately over-expressed in LSCC tumors. PKCI and SOX2 activate autocrine Hh signaling to maintain LSCC stem-like cells.[39]
Using the cytokeratin five promoter, Sonic Hh signaling was activated in both transient and stable transgenic fish. Transcriptions of radial glia genes cyp19a1b, s100b, blbp, gfap and the stem/progenitor genes Nestin and SOX2 were significantly upregulated.[40] Inhibition of Hh signaling, therefore, represents a promising approach toward novel anticancer therapies.
Signal transducer and activator of transcription 3 signaling pathway
Signal transducer and activator of transcription 3, a member of a family of DNA-binding molecules, is a potential target in the treatment of cancer.[41] In cancer cells, the highly phosphorylated STAT3 contributes to numerous physiological and oncogenic signaling pathways.[42,43,44]
In recent studies,[45] it is reported a novel small molecule inhibitor of STAT3 dimerization, STX-0119, as a cancer therapeutic. Notably, STX-0119 demonstrated strong inhibition of the expression of STAT3 target genes (c-myc, survivin, cyclin D1, HIF-1α and vascular endothelial cell growth factor) and stem cell-associated genes (SOX2, CD44, Nanog, Nestin and CD133) as well as the induction of apoptosis in one stem-like cell line. STAT3 may regulate the expression of SOX2.
On the other hand, SOX2 regulates the expression of STAT3 through JAK-STAT signaling pathway.[46] In mouse embryonic fibroblast cells, n-butylidenephthalide triggers the up-regulation of SOX2 and OCT4 gene expression levels. Microarray analysis data identified peroxisome proliferator-activated receptor, extracellular matrix, and JAK-STAT signaling as the top three deregulated pathways. SOX2 can promote the activation of JAK-STAT signaling pathway.
Sex-determining region of Y chromosome-related high-mobility-group box 2 and Nanog
Nanog, SOX2, and OCT4 are transcription factors all essential to maintaining the pluripotent ES cell phenotype.[47,48] Using chromatin immunoprecipitation, OCT4 and SOX2 bind to the Nanog promoter in living mouse and human ES cells.[49] Furthermore, by specific knockdown of OCT4 and SOX2 mRNA by RNA interference in ES cells, we provide genetic evidence for a link between OCT4, SOX2, and the Nanog promoter.
Sex-determining region of Y chromosome-related high-mobility-group box 2 and Nanog, Sall4, OCT4 jointly involved in the maintenance of ES cells without differentiation.[50] Furthermore, the data uncovered an extensive usage of autoregulatory, feed-forward connections in ES cells [Figure 1]. The morphologies of cells after Nanog or Sall4 depletion appeared similar and are distinct from OCT4 or SOX2 depletion cells.
Figure 1.
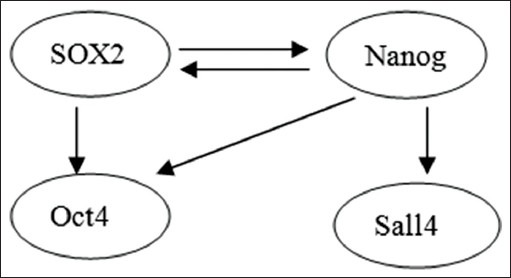
Sex-determining region of Y chromosome-related high-mobility-group box 2 and Nanog, Sall4, OCT4 jointly involved in embryonic stem.
Sex-determining region of Y chromosome-related high-mobility-group box 2 and Nestin
Nestin, a class VI intermediate filament, is known to be a CSC marker. Nestin is thought to regulate tumor cell proliferation, migration, invasion and CSC properties.[51,52] Akt inhibitor IV effectively decreased Nestin expression via SOX2 downregulation and overcame the enhanced sphere formation induced by Nestin upregulation.[52] Thus, regulation of Nestin via Akt/SOX2 is a promising candidate for novel therapeutic approaches to eradicate CSCs in lung cancer.
SEX-DETERMINING REGION OF Y CHROMOSOME-RELATED HIGH-MOBILITY-GROUP BOX 2 AND ANTICANCER THERAPY
Sex-determining region of Y chromosome-related high-mobility-group box 2 with chemo-and radiotherapy
Cancer stem cells have been shown to play roles in resistance to chemo-and radiotherapy. SOX2 is reported to be involved in tumor resistance, for example, tamoxifen resistance of breast cancer.[53] Examination of patient tumors indicated that SOX2 levels are higher in patients after endocrine therapy failure. Tamoxifen-resistant cells that were enriched for stem/progenitors express high levels of the stem cell marker SOX2. Silencing of the SOX2 gene reduced the size of the stem/progenitor cell population and restored sensitivity to tamoxifen. Gene expression analysis indicated that profiling revealed activation of the Wnt signaling pathway in SOX2-expressing cells, and inhibition of Wnt signaling sensitized resistant cells to tamoxifen. Thus, the development of tamoxifen resistance is driven by SOX2-dependent activation of Wnt signaling in cancer stem/progenitor cells.
Radioresistance of tumor cells remains a major therapeutic problem. In advanced cervical cancer, the percentage of overexpression of SOX2 and OCT4 in the radiation-resistant group was much higher than that in the radiation-sensitive group (P < 0.001 and P < 0.001).[54] And the patients with high expression of SOX2 and OCT4 showed a shorter progression-free survival than those with low expression. High expression of SOX2 and OCT4 indicates radiation resistance and an independent negative prognosis in cervical squamous cell carcinoma. However, in small (T1/T2) oral squamous cell carcinoma, patients with higher nuclear SOX2 expression had a remarkable longer disease-free period if they received adjuvant post-operative radiotherapy (P = 0.001).[55]
Sex-determining region of Y chromosome-related high-mobility-group box 2 with epidermal growth factor receptor tyrosine kinase inhibitors resistance
Epidermal growth factor receptor tyrosine kinase inhibitors (TKIs) have shown favorable efficacy in nonsmall-cell lung cancer (NSCLC) patients with EGFR mutation. However, resistance to EGFR TKIs is always seen in 1–2 years. SOX2 play an important role in EGFR TKIs resistance. It is showed that knockdown of SOX2 decreased proliferation in cell lines and increased sensitivity to erlotinib in HCC827 cells.[56] And SOX2 expression can be decreased by PI3K/Akt inhibitors. PI3K/Akt inhibitors might be useful to inhibit SOX2 in EGFR TKI resistant tumors. Targeting SOX2 may provide therapeutic benefit in the EGFR-mutant tumors with high levels of SOX2.
Sex-determining region of Y chromosome-related high-mobility-group box 2-based immunotherapy
Immunotherapy has become the fourth therapeutic strategy of cancer. Targeting to CSCs immunotherapy is especially promising. SOX2 has been showed to be a newly identified cancer antigen that was found to be expressed in a wide variety of human CSCs.[57] It has been proved that SOX2 can stimulate the body to produce an effective CTL and humoral immune response effects.[58,59,60,61] It is showed that nearly 50% of a cohort of NSCLC patients mounted both CD4+ and CD8+ T-cell responses against SOX2, and monoclonal gammopathies of undetermined significance patients frequently mount a humoral and cellular immune response against SOX2.[60] SOX2-based immunotherapy will be a novel strategy which will effectively kill the CSCs.
A prognostic marker for detection of early recurrence
Expression of SOX2 is often used to imply stemness and poor prognosis in cancer. In breast cancer, SOX2 expression was independently associated with increased risk of recurrence (heart rate [HR] = 2.99; P = 0.004) as well as nodes status (HR = 2.44; P = 0.009) and T-size > 1 (HR = 1.77; P = 0.035)[62] according to multivariate analysis. A meta-analysis of 926 gastric cancer patients from 9 studies showed that patients with SOX2 expression had a significantly worse 5-year overall survival compared with those with low expression (relative risk = 2.38; 95% confidence interval = 1.10–5.15; P = 0.03).[63] What is more, SOX2 overexpression was closely correlated with tumor T stage, lymph node metastasis, and TNM stage.
CONCLUSION
Sex-determining region of Y chromosome-related high-mobility-group box 2 expression in cancer cells affects different patterns of malignant cellular behavior, such as metastasis, tumor cell apoptosis, and tumor cell proliferation, depending on its functional diversity. Numerous research findings indicate that SOX2 is a promising tumor biomarker. Expression of SOX2 is often used to imply stemness and poor prognosis in cancer. Moreover, the aforementioned studies indicate that SOX2 may be a potential therapeutic target in the treatment of malignant disease. Targeting signals upstream or downstream of SOX2 may prove beneficial in cancer therapy. However, the precise functions of SOX2 in different cancers are still poorly understood. The signaling pathways involved are still not very clear [Figure 2]. The in-depth study of SOX2 features will help understanding the mechanism of tumor invasion and tumor metastasis, and help to open up new paths for the prevention and treatment of malignant tumors.
Figure 2.
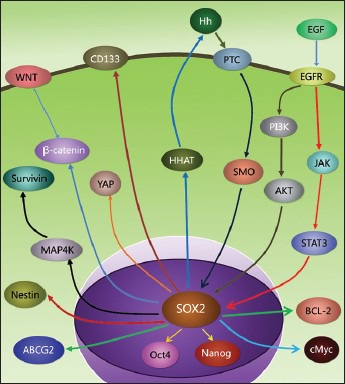
The signaling pathways of sex-determining region of Y chromosome-related high-mobility-group box 2: WNT/β-catenin signaling pathway, Hippo signaling pathway, Survivin signaling pathway, signal transducers and activators of transcription 3 (STAT3) signaling pathway, Hedgehog signaling pathway. Downstream regulatory molecules: Nanog, cMyc, STAT3, Nestin, ABCG2, Survivin, Bcl-2, Oct4, HHAT, CD133.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: This study was supported by grants from the National Natural Science Foundation of China (No. 81172234) and the Fundamental Research Funds for the Central Universities of China.
Conflict of Interest: All authors declare that there are not any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
REFERENCES
- 1.Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem. 2008;283:17969–78. doi: 10.1074/jbc.M802917200. [DOI] [PubMed] [Google Scholar]
- 2.Santini R, Pietrobono S, Pandolfi S, Montagnani V, D’Amico M, Penachioni JY, et al. SOX2 regulates self-renewal and tumorigenicity of human melanoma-initiating cells. Oncogene. 2014;33:4697–708. doi: 10.1038/onc.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia X, Li X, Xu Y, Zhang S, Mou W, Liu Y, et al. SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J Mol Cell Biol. 2011;3:230–8. doi: 10.1093/jmcb/mjr002. [DOI] [PubMed] [Google Scholar]
- 4.Otsubo T, Akiyama Y, Yanagihara K, Yuasa Y. SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer. 2008;98:824–31. doi: 10.1038/sj.bjc.6604193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: With partners in the regulation of embryonic development. Trends Genet. 2000;16:182–7. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- 6.Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093–108. doi: 10.1089/scd.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho YS, et al. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br J Cancer. 2014;111:2122–30. doi: 10.1038/bjc.2014.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanada Y, Yoshida K, Ohara M, Oeda M, Konishi K, Tsutani Y. Histopathologic evaluation of stepwise progression of pancreatic carcinoma with immunohistochemical analysis of gastric epithelial transcription factor SOX2: comparison of expression patterns between invasive components and cancerous or nonneoplastic intraductal components. Pancreas. 2006;32:164–70. doi: 10.1097/01.mpa.0000202947.80117.a0. [DOI] [PubMed] [Google Scholar]
- 9.Leis O, Eguiara A, Lopez-Arribillaga E, Alberdi MJ, Hernandez-Garcia S, Elorriaga K, et al. Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene. 2012;31:1354–65. doi: 10.1038/onc.2011.338. [DOI] [PubMed] [Google Scholar]
- 10.Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–8. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 11.Neumann J, Bahr F, Horst D, Kriegl L, Engel J, Luque RM, et al. SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer. 2011;11:518. doi: 10.1186/1471-2407-11-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin F, Lin P, Zhao D, Chen Y, Xiao L, Qin W, et al. Sox2 targets cyclinE, p27 and survivin to regulate androgen-independent human prostate cancer cell proliferation and apoptosis. Cell Prolif. 2012;45:207–16. doi: 10.1111/j.1365-2184.2012.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rybak AP, Tang D. SOX2 plays a critical role in EGFR-mediated self-renewal of human prostate cancer stem-like cells. Cell Signal. 2013;25:2734–42. doi: 10.1016/j.cellsig.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembelé D, et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5:e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–42. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hütz K, Mejías-Luque R, Farsakova K, Ogris M, Krebs S, Anton M, et al. The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis. 2014;35:942–50. doi: 10.1093/carcin/bgt410. [DOI] [PubMed] [Google Scholar]
- 17.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 18.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67:4807–15. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 19.Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4:193–201. doi: 10.1007/s12015-008-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27:1734–40. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–45. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 22.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–50. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 23.Iida H, Suzuki M, Goitsuka R, Ueno H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int J Oncol. 2012;40:71–9. doi: 10.3892/ijo.2011.1207. [DOI] [PubMed] [Google Scholar]
- 24.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–98. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 25.Lu YX, Yuan L, Xue XL, Zhou M, Liu Y, Zhang C, et al. Regulation of colorectal carcinoma stemness, growth, and metastasis by an miR-200c-Sox2-negative feedback loop mechanism. Clin Cancer Res. 2014;20:2631–42. doi: 10.1158/1078-0432.CCR-13-2348. [DOI] [PubMed] [Google Scholar]
- 26.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Sica A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA, Sarukhan A. Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues. Front Immunol. 2014;5:127. doi: 10.3389/fimmu.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, et al. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248–58. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 30.Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene. 2003;22:7218–21. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T, et al. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/ß-catenin signal network. Cancer Lett. 2013;336:379–89. doi: 10.1016/j.canlet.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, et al. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91:2026–32. doi: 10.1002/1097-0142(20010601)91:11<2026::aid-cncr1228>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 35.Feng R, Zhou S, Liu Y, Song D, Luan Z, Dai X, et al. Sox2 protects neural stem cells from apoptosis via up-regulating survivin expression. Biochem J. 2013;450:459–68. doi: 10.1042/BJ20120924. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Li X, Lu D, Xu Y, Mou W, Wang L, et al. SOX2 regulates apoptosis through MAP4K4-survivin signaling pathway in human lung cancer cells. Carcinogenesis. 2014;35:613–23. doi: 10.1093/carcin/bgt371. [DOI] [PubMed] [Google Scholar]
- 37.Yang N, Hui L, Wang Y, Yang H, Jiang X. SOX2 promotes the migration and invasion of laryngeal cancer cells by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep. 2014;31:2651–9. doi: 10.3892/or.2014.3120. [DOI] [PubMed] [Google Scholar]
- 38.Hong Z, Bi A, Chen D, Gao L, Yin Z, Luo L. Activation of hedgehog signaling pathway in human non-small cell lung cancers. Pathol Oncol Res. 2014;20:917–22. doi: 10.1007/s12253-014-9774-x. [DOI] [PubMed] [Google Scholar]
- 39.Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25:139–51. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ju B, Chen W, Spitsbergen JM, Lu J, Vogel P, Peters JL, et al. Activation of Sonic hedgehog signaling in neural progenitor cells promotes glioma development in the zebrafish optic pathway. Oncogenesis. 2014;3:e96. doi: 10.1038/oncsis.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong Z, Wen Z, Darnell JE., Jr Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 42.Yu H, Jove R. The STATs of cancer – New molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 43.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–9. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 44.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–54. [PubMed] [Google Scholar]
- 45.Ashizawa T, Miyata H, Iizuka A, Komiyama M, Oshita C, Kume A, et al. Effect of the STAT3 inhibitor STX-0119 on the proliferation of cancer stem-like cells derived from recurrent glioblastoma. Int J Oncol. 2013;43:219–27. doi: 10.3892/ijo.2013.1916. [DOI] [PubMed] [Google Scholar]
- 46.Liu SP, Harn HJ, Chien YJ, Chang CH, Hsu CY, Fu RH, et al. n-Butylidenephthalide (BP) maintains stem cell pluripotency by activating Jak2/Stat3 pathway and increases the efficiency of iPS cells generation. PLoS One. 2012;7:e44024. doi: 10.1371/journal.pone.0044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 48.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–8. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 49.Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–7. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 50.Wu Q, Chen X, Zhang J, Loh YH, Low TY, Zhang W, et al. Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem. 2006;281:24090–4. doi: 10.1074/jbc.C600122200. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda Y, Kure S, Ishiwata T. Nestin and other putative cancer stem cell markers in pancreatic cancer. Med Mol Morphol. 2012;45:59–65. doi: 10.1007/s00795-012-0571-x. [DOI] [PubMed] [Google Scholar]
- 52.Narita K, Matsuda Y, Seike M, Naito Z, Gemma A, Ishiwata T. Nestin regulates proliferation, migration, invasion and stemness of lung adenocarcinoma. Int J Oncol. 2014;44:1118–30. doi: 10.3892/ijo.2014.2278. [DOI] [PubMed] [Google Scholar]
- 53.Pival M, Domenicil G, Iriondo O, Bruno M, Comaills V, Barredo I, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2013;5:1–14. doi: 10.1002/emmm.201303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen L, Huang X, Xie X, Su J, Yuan J, Chen X. High expression of SOX2 and OCT4 indicates radiation resistance and an independent negative prognosis in cervical squamous cell carcinoma. J Histochem Cytochem. 2014;62:499–509. doi: 10.1369/0022155414532654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Attramadal CG, Halstensen TS, Dhakal HP, Ulekleiv CH, Boysen ME, Nesland JM, et al. High nuclear SOX2 expression is associated with radiotherapy response in small (T1/T2) oral squamous cell carcinoma. J Oral Pathol Med. 2014 doi: 10.1111/jop.12261. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Dogan I, Kawabata S, Bergbower E, Gills JJ, Ekmekci A, Wilson W., 3rd SOX2 expression is an early event in a murine model of EGFR mutant lung cancer and promotes proliferation of a subset of EGFR mutant lung adenocarcinoma cell lines. Lung Cancer. 2014;85:1–6. doi: 10.1016/j.lungcan.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inoda S, Hirohashi Y, Torigoe T, Morita R, Takahashi A, Asanuma H, et al. Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells. Am J Pathol. 2011;178:1805–13. doi: 10.1016/j.ajpath.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Güre AO, Stockert E, Scanlan MJ, Keresztes RS, Jäger D, Altorki NK, et al. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci U S A. 2000;97:4198–203. doi: 10.1073/pnas.97.8.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV. SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. Oncoimmunology. 2013;2:e25205. doi: 10.4161/onci.25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–40. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polakova I, Duskova M, Smahel M. Antitumor DNA vaccination against the Sox2 transcription factor. Int J Oncol. 2014;45:139–46. doi: 10.3892/ijo.2014.2402. [DOI] [PubMed] [Google Scholar]
- 62.Finicelli M, Benedetti G, Squillaro T, Pistilli B, Marcellusi A, Mariani P, et al. Expression of stemness genes in primary breast cancer tissues: the role of SOX2 as a prognostic marker for detection of early recurrence. Oncotarget. 2014;5:9678–88. doi: 10.18632/oncotarget.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W, Li B, Wang R, Huang D, Jin W, Yang S. SOX2 as prognostic factor in head and neck cancer: a systematic review and meta-analysis. Acta Otolaryngol. 2014;134:1101–8. doi: 10.3109/00016489.2014.913311. [DOI] [PubMed] [Google Scholar]


