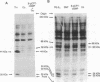
Abstract
Most platelet agonists activate and elevate the cytosolic free calcium concentration in human platelets through receptor-dependent mechanisms that are antagonized by cAMP- and cGMP-elevating agents. Nitrovasodilators such as nitroprusside and endothelium-derived relaxing factor are potent cGMP-elevating platelet inhibitors. In the present study, the role of cGMP and cGMP-dependent protein kinase in nitrovasodilator inhibition of ADP- and thrombin-evoked calcium elevation and activation of human platelets was investigated. Preincubation of platelets with 8-(4-chlorophenylthio)guanosine 3',5'-cyclic monophosphate (8-pCPT-cGMP; a membrane-permeant selective activator of the cGMP-dependent protein kinase that does not significantly affect cGMP-regulated phosphodiesterases) inhibited the thrombin-induced phosphorylation mediated by myosin light chain kinase and protein kinase C. Nitrovasodilator-induced protein phosphorylation in human platelets was distinct from that induced by cAMP-elevating prostaglandins and could be mimicked by 8-pCPT-cGMP. Preincubation of human platelets with nitrovasodilators or 8-pCPT-cGMP inhibited the ADP- and thrombin-evoked calcium elevation in the presence and absence of external calcium. Nitrovasodilators and 8-pCPT-cGMP also inhibited the agonist-induced Mn2+ influx, but stopped-flow experiments indicated that the ADP receptor-operated cation channel was not significantly inhibited. These results suggest that in human platelets nitrovasodilators inhibit the agonist-induced calcium mobilization from intracellular stores and the secondary store-related calcium influx but not the ADP receptor-operated cation channel. The results also suggest that these nitrovasodilator effects are mediated by cGMP and the cGMP-dependent protein kinase.
Full text
PDF
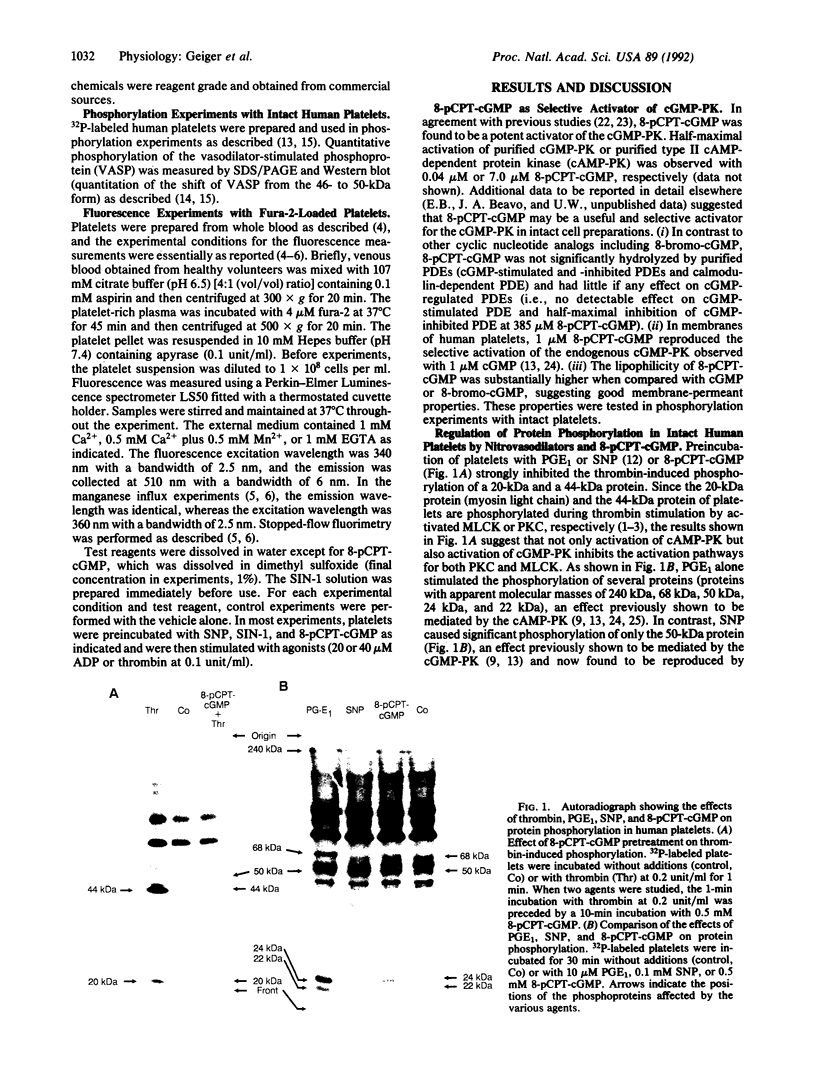
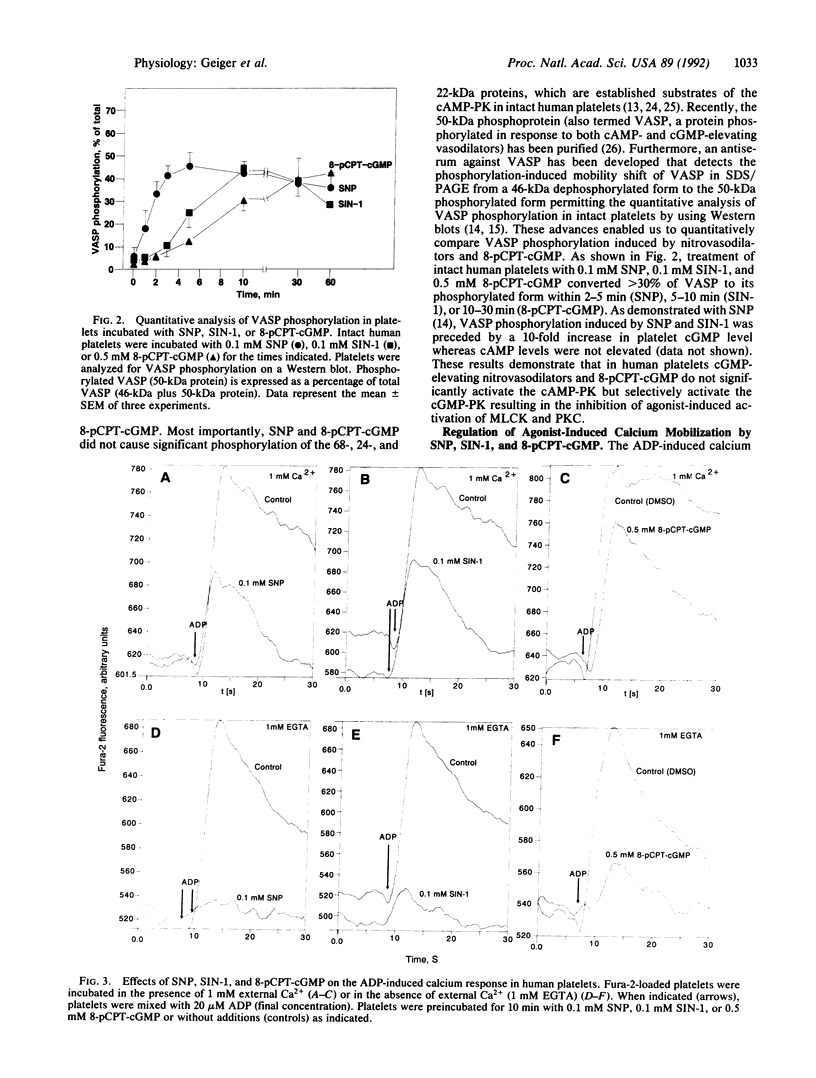


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Bowen R., Haslam R. J. Effects of nitrovasodilators on platelet cyclic nucleotide levels in rabbit blood; role for cyclic AMP in synergistic inhibition of platelet function by SIN-1 and prostaglandin E1. J Cardiovasc Pharmacol. 1991 Mar;17(3):424–433. doi: 10.1097/00005344-199103000-00011. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Noblett B. D., Todd B. W., Wells J. N., Corbin J. D. Relaxation of vascular and tracheal smooth muscle by cyclic nucleotide analogs that preferentially activate purified cGMP-dependent protein kinase. Mol Pharmacol. 1988 Oct;34(4):506–517. [PubMed] [Google Scholar]
- Halbrügge M., Friedrich C., Eigenthaler M., Schanzenbächer P., Walter U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem. 1990 Feb 25;265(6):3088–3093. [PubMed] [Google Scholar]
- Halbrügge M., Walter U. Purification of a vasodilator-regulated phosphoprotein from human platelets. Eur J Biochem. 1989 Oct 20;185(1):41–50. doi: 10.1111/j.1432-1033.1989.tb15079.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., Bushfield M., Shaw A. M. Regulation of platelet cytosolic free calcium by cyclic nucleotides and protein kinase C. FEBS Lett. 1985 Sep 2;188(2):383–388. doi: 10.1016/0014-5793(85)80407-5. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Sage S. O., Rink T. J. Receptor-activated single channels in intact human platelets. J Biol Chem. 1990 Jun 25;265(18):10479–10483. [PubMed] [Google Scholar]
- Maurice D. H., Haslam R. J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990 May;37(5):671–681. [PubMed] [Google Scholar]
- Morgan R. O., Newby A. C. Nitroprusside differentially inhibits ADP-stimulated calcium influx and mobilization in human platelets. Biochem J. 1989 Mar 1;258(2):447–454. doi: 10.1042/bj2580447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte C., Eigenthaler M., Schanzenbächer P., Walter U. Endothelial cell-dependent phosphorylation of a platelet protein mediated by cAMP- and cGMP-elevating factors. J Biol Chem. 1991 Aug 5;266(22):14808–14812. [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reden J. Molsidomine. Blood Vessels. 1990;27(2-5):282–294. doi: 10.1159/000158820. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Calcium signaling in human platelets. Annu Rev Physiol. 1990;52:431–449. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Reast R., Rink T. J. ADP evokes biphasic Ca2+ influx in fura-2-loaded human platelets. Evidence for Ca2+ entry regulated by the intracellular Ca2+ store. Biochem J. 1990 Feb 1;265(3):675–680. doi: 10.1042/bj2650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Sandberg M., Butt E., Nolte C., Fischer L., Halbrügge M., Beltman J., Jahnsen T., Genieser H. G., Jastorff B., Walter U. Characterization of Sp-5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole- 3',5'-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem J. 1991 Oct 15;279(Pt 2):521–527. doi: 10.1042/bj2790521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989 Jan;69(1):58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- Waldmann R., Bauer S., Göbel C., Hofmann F., Jakobs K. H., Walter U. Demonstration of cGMP-dependent protein kinase and cGMP-dependent phosphorylation in cell-free extracts of platelets. Eur J Biochem. 1986 Jul 1;158(1):203–210. doi: 10.1111/j.1432-1033.1986.tb09739.x. [DOI] [PubMed] [Google Scholar]
- Waldmann R., Nieberding M., Walter U. Vasodilator-stimulated protein phosphorylation in platelets is mediated by cAMP- and cGMP-dependent protein kinases. Eur J Biochem. 1987 Sep 15;167(3):441–448. doi: 10.1111/j.1432-1033.1987.tb13357.x. [DOI] [PubMed] [Google Scholar]
- Waldmann R., Walter U. Cyclic nucleotide elevating vasodilators inhibit platelet aggregation at an early step of the activation cascade. Eur J Pharmacol. 1989 Jan 17;159(3):317–320. doi: 10.1016/0014-2999(89)90165-9. [DOI] [PubMed] [Google Scholar]
- Walter U. Physiological role of cGMP and cGMP-dependent protein kinase in the cardiovascular system. Rev Physiol Biochem Pharmacol. 1989;113:41–88. doi: 10.1007/BFb0032675. [DOI] [PubMed] [Google Scholar]
- Walter U., Waldmann R., Nieberding M. Intracellular mechanism of action of vasodilators. Eur Heart J. 1988 Jun;9 (Suppl H):1–6. doi: 10.1093/eurheartj/9.suppl_h.1. [DOI] [PubMed] [Google Scholar]
- Wolfe L., Corbin J. D., Francis S. H. Characterization of a novel isozyme of cGMP-dependent protein kinase from bovine aorta. J Biol Chem. 1989 May 5;264(13):7734–7741. [PubMed] [Google Scholar]