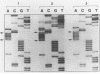
Abstract
Stromelysin is a member of the family of metalloproteinases that degrade extracellular matrix. In situ hybridisation and histopathological studies suggest that stromelysin activity may be important in the connective tissue remodelling processes associated with atherogenesis and plaque rupture. Single strand conformation polymorphism analysis identified a common polymorphism in the stromelysin gene promoter located 1171 bp upstream from the start of transcription in which one allele has a run of six adenosines (6A) and another has five adenosines (5A). 72 men with coronary heart disease, were genotyped. They were participants in the St Thomas' Atherosclerosis Regression Study who were randomised to receive usual care (UC), dietary intervention (D), or diet plus cholestyramine (DC), with angiography at baseline and at 39 months. In these patients the frequency of the 5A allele was 0.49 (95% CI from 0.41 to 0.57) and was not significantly different from that in a sample of 354 healthy UK men. In the UC group, patients who were homozygous for the 6A allele showed greater progression of angiographic disease than those with other genotypes: the minimum absolute width of coronary segments decreased by 0.04 (SEM 0.10) mm for 5A5A, 0.20 (0.07) mm for 5A6A, and 0.67 (0.19) mm for 6A6A (P < 0.01). The findings were similar but slightly less significant for the change in mean absolute width of coronary segments (P < 0.05). No significant associations were seen in patients in the D or DC groups. In data pooled from the three treatment groups, the 6A6A genotype was significantly associated with greater progression of coronary atherosclerosis than other genotypes in patients with baseline percentage diameter stenosis less than 20% (P < 0.05), but not in those with baseline percentage diameter stenosis greater than or equal to 20%. These results provide the first evidence of a link between genetic variation in stromelysin and progression of coronary atherosclerosis and support the hypothesis that connective tissue remodeling mediated by metalloproteinases contribute to the pathogenesis of atherosclerosis.
Full text
PDF

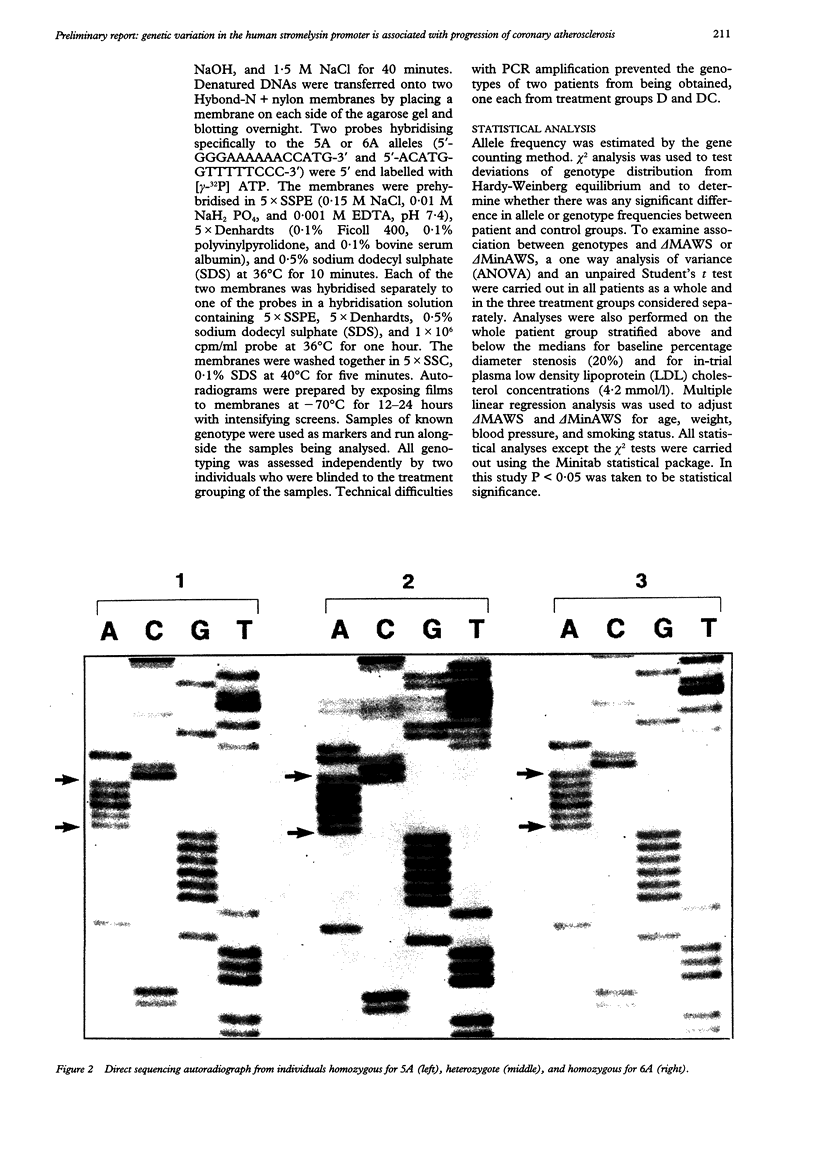
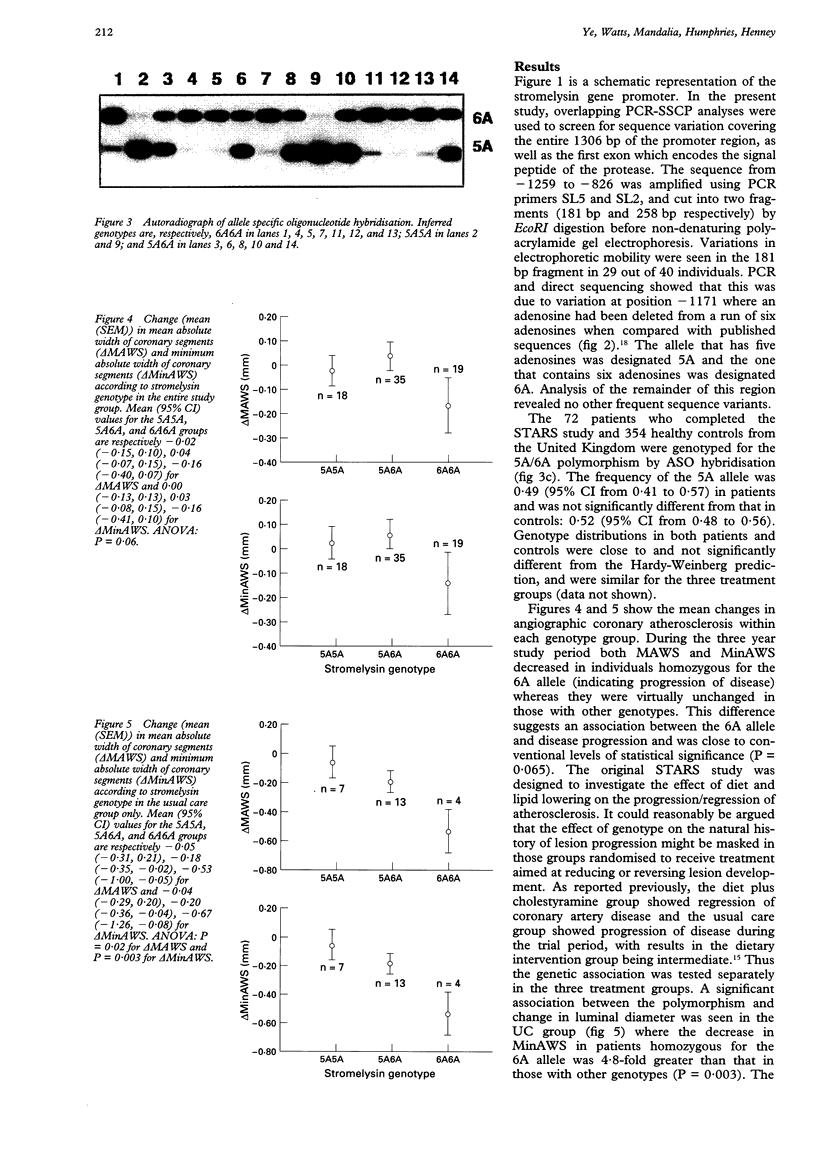



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose J. A., Winters S. L., Arora R. R., Eng A., Riccio A., Gorlin R., Fuster V. Angiographic evolution of coronary artery morphology in unstable angina. J Am Coll Cardiol. 1986 Mar;7(3):472–478. doi: 10.1016/s0735-1097(86)80455-7. [DOI] [PubMed] [Google Scholar]
- Arntzenius A. C. Regression of atherosclerosis. Benefit can be expected from low LDL-C and high HDL-C levels. Acta Cardiol. 1991;46(4):431–438. [PubMed] [Google Scholar]
- Buttice G., Kurkinen M. A polyomavirus enhancer A-binding protein-3 site and Ets-2 protein have a major role in the 12-O-tetradecanoylphorbol-13-acetate response of the human stromelysin gene. J Biol Chem. 1993 Apr 5;268(10):7196–7204. [PubMed] [Google Scholar]
- Buttice G., Quinones S., Kurkinen M. The AP-1 site is required for basal expression but is not necessary for TPA-response of the human stromelysin gene. Nucleic Acids Res. 1991 Jul 11;19(13):3723–3731. doi: 10.1093/nar/19.13.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case J. P., Lafyatis R., Kumkumian G. K., Remmers E. F., Wilder R. L. IL-1 regulation of transin/stromelysin transcription in rheumatoid synovial fibroblasts appears to involve two antagonistic transduction pathways, an inhibitory, prostaglandin-dependent pathway mediated by cAMP, and a stimulatory, protein kinase C-dependent pathway. J Immunol. 1990 Dec 1;145(11):3755–3761. [PubMed] [Google Scholar]
- Davies M. J., Bland J. M., Hangartner J. R., Angelini A., Thomas A. C. Factors influencing the presence or absence of acute coronary artery thrombi in sudden ischaemic death. Eur Heart J. 1989 Mar;10(3):203–208. doi: 10.1093/oxfordjournals.eurheartj.a059467. [DOI] [PubMed] [Google Scholar]
- Dawson S. J., Wiman B., Hamsten A., Green F., Humphries S., Henney A. M. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J Biol Chem. 1993 May 25;268(15):10739–10745. [PubMed] [Google Scholar]
- Diaz-Meco M. T., Quiñones S., Municio M. M., Sanz L., Bernal D., Cabrero E., Saus J., Moscat J. Protein kinase C-independent expression of stromelysin by platelet-derived growth factor, ras oncogene, and phosphatidylcholine-hydrolyzing phospholipase C. J Biol Chem. 1991 Nov 25;266(33):22597–22602. [PubMed] [Google Scholar]
- Frisch S. M., Ruley H. E. Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem. 1987 Dec 5;262(34):16300–16304. [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J. J., Chesebro J. H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992 Jan 23;326(4):242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Henney A. M., Wakeley P. R., Davies M. J., Foster K., Hembry R., Murphy G., Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8154–8158. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries S. E., Lane A., Green F. R., Cooper J., Miller G. J. Factor VII coagulant activity and antigen levels in healthy men are determined by interaction between factor VII genotype and plasma triglyceride concentration. Arterioscler Thromb. 1994 Feb;14(2):193–198. doi: 10.1161/01.atv.14.2.193. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Magun B. E., Matrisian L. M. The role of C-Fos in growth factor regulation of stromelysin/transin gene expression. Matrix Suppl. 1992;1:176–183. [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- La Ville A. E., Seddon A. M., Shaikh M., Rowles P. M., Woolf N., Lewis B. Primary prevention of atherosclerosis by lovastatin in a genetically hyperlipidaemic rabbit strain. Atherosclerosis. 1989 Aug;78(2-3):205–210. doi: 10.1016/0021-9150(89)90224-4. [DOI] [PubMed] [Google Scholar]
- Little W. C., Constantinescu M., Applegate R. J., Kutcher M. A., Burrows M. T., Kahl F. R., Santamore W. P. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988 Nov;78(5 Pt 1):1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- Mackay A. R., Ballin M., Pelina M. D., Farina A. R., Nason A. M., Hartzler J. L., Thorgeirsson U. P. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis. 1992;12(3-4):168–184. [PubMed] [Google Scholar]
- Mauviel A., Kähäri V. M., Kurkinen M., Evans C. H., Uitto J. Leukoregulin, a T-cell derived cytokine, upregulates stromelysin-1 gene expression in human dermal fibroblasts: evidence for the role of AP-1 in transcriptional activation. J Cell Biochem. 1992 Sep;50(1):53–61. doi: 10.1002/jcb.240500110. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Martin J. F., Higgs A. Symposium on regression of atherosclerosis. Eur J Clin Invest. 1993 Jul;23(7):385–398. doi: 10.1111/j.1365-2362.1993.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ward R., Gavrilovic J., Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224–230. [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones S., Saus J., Otani Y., Harris E. D., Jr, Kurkinen M. Transcriptional regulation of human stromelysin. J Biol Chem. 1989 May 15;264(14):8339–8344. [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rouis M., Nigon F., Lafuma C., Hornebeck W., Chapman M. J. Expression of elastase activity by human monocyte-macrophages is modulated by cellular cholesterol content, inflammatory mediators, and phorbol myristate acetate. Arteriosclerosis. 1990 Mar-Apr;10(2):246–255. doi: 10.1161/01.atv.10.2.246. [DOI] [PubMed] [Google Scholar]
- Sirum-Connolly K., Brinckerhoff C. E. Interleukin-1 or phorbol induction of the stromelysin promoter requires an element that cooperates with AP-1. Nucleic Acids Res. 1991 Jan 25;19(2):335–341. doi: 10.1093/nar/19.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts G. F., Lewis B., Brunt J. N., Lewis E. S., Coltart D. J., Smith L. D., Mann J. I., Swan A. V. Effects on coronary artery disease of lipid-lowering diet, or diet plus cholestyramine, in the St Thomas' Atherosclerosis Regression Study (STARS) Lancet. 1992 Mar 7;339(8793):563–569. doi: 10.1016/0140-6736(92)90863-x. [DOI] [PubMed] [Google Scholar]
- Wissler R. W., Vesselinovitch D. Studies of regression of advanced atherosclerosis in experimental animals and man. Ann N Y Acad Sci. 1976;275:363–378. doi: 10.1111/j.1749-6632.1976.tb43368.x. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]