Abstract
All living organisms must maintain equilibrium in response to internal and external challenges within their environment. Changes in neural plasticity (alterations in neuronal populations, dendritic remodeling, and synaptic turnover) are critical components of the homeostatic response to stress, which has been strongly implicated in the onset of affective disorders. However, stress is differentially perceived depending on the type of stress and its context, as well as genetic background, age and sex; therefore, an individual’s maintenance of neuronal homeostasis must differ depending upon these variables. We established Drosophila as a model to analyze homeostatic responses to stress. Sexually immature and mature females and males from an isogenic wild-type strain raised under controlled environmental conditions were exposed to four reproducible and high-throughput translatable stressors to facilitate the analysis of a large number of animals for direct comparisons. These animals were assessed in an open-field arena, in a Light-Dark Box, and in a Forced Swim Test, as well as for sensitivity to the sedative effects of ethanol. These studies establish that immature and mature females and males represent behaviorally distinct populations under control conditions as well as after exposure to different stressors. Therefore, the neural substrates mediating the stress response must be differentially expressed depending upon the hormonal status of the brain. In addition, an adaptive response to a given stressor in one paradigm was not predictive for outcomes in other paradigms.
Keywords: open field arena, light dark box, forced swim test, ethanol sedation, sexual dimorphism, temporal dimorphism
Introduction
The stress response is essential for organismal survival and is centrally mediated (McEwen, 1998). Stress is a causative factor for the onset of depression (Calabrese et al., 2009; Liu et al., 2010), and there is a strong genetic component (Fernández-Guasti et al. 2012), but the neural pathways responsible for the gene by environment interactions that determine an adaptive stress response have not been elucidated. Sexually immature animals respond differently to the same stressor than do mature animals (Argue & Neckameyer, 2013a; Cohen et al., 2013; Lau, 2013), as do males and females (Handa & Weiser 2014). Therefore, sex and sexual maturity are critical factors in determining vulnerability to, and the severity of, stress-related psychiatric disorders (Altemus et al., 2014; Fernández-Guasti et al., 2012; Bangasser & Valentino, 2014).
Paradigms inducing depressive-like behaviors have been employed (largely in rodent models) to identify factors that predispose an individual to mental illness, as well as to screen for potential antidepressant therapeutics. However, no model has been developed that fully mimics human depression (Nestler & Hyman, 2010), and most studies cannot be compared due to strain and species differences, dissimilar methodologies, and other factors.
We established Drosophila as a model to analyze homeostatic responses to stress. Our intent is not to model the human stress response, but to identify substrates critical for the development and maintenance of neuronal homeostasis. The Drosophila and mammalian nervous systems arise from common evolutionary origins, and neurobiological signaling pathways are also conserved (Hirth & Reichert, 1999; O’Kane, 2011), providing a strong rationale for this reductionist approach.
We examined stress-elicited behavior in four genetically identical and environmentally controlled populations (sexually immature and sexually mature females and males), and subjected them to control conditions and to four high-throughput stressors (starvation, oxidative stress, sleep deprivation and social isolation) to facilitate the analysis of a large number of animals for direct comparisons. Starvation stress correlates with an enhanced risk for schizophrenia and bipolar disorder (Carter, 2007; Kroll, 2007; Schmidt, 2007). Food shortage is a stress common to all animal species, and the primary response mechanisms appear conserved between flies and mammals (reviewed in Rion & Rawecki, 2007). Oxidative stress has been implicated in the development of schizophrenia (reviewed in Prabakaran et al., 2004) and other affective disorders; the brain is highly sensitive to oxidative damage (reviewed in Chopra et al., 2011), and humans suffering from depression display disturbances in oxidative response pathways. Oxidative stress can be induced by exposure to paraquat, which damages the hippocampus in mice via the generation of reactive oxygen species (Chen et al., 2010). Sleep deprivation perturbs the stress response (McEwen, 2006), adult neurogenesis and hippocampal function (Meerlo et al., 2009), and is a causative factor in affective disorders (Szklo-Coxe et al., 2007). Rodents reared in isolation display increased aggression, changes in cognitive function and perturbed sensorimotor gating, analogous to the impairments observed in schizophrenia (Fone & Porkess 2008) and decreased performance in the Forced Swim test (Brenes et al., 2009). Immature rats exposed to constant darkness display perturbed monoaminergic signaling pathways and depressive behaviors (Gonzales & Aston-Jones 2006).
We adapted classic depression and anxiety mammalian paradigms for use in Drosophila (open field arena, Light-Dark Box, Forced Swim Test, and response to ethanol), and analyzed behavioral output of the four populations under control conditions and in response to the four stressors. While juvenile females and males responded similarly to the same stressor in a given assay, sexually mature females and males displayed unique, sexually dimorphic responses. Surprisingly, negative outcomes in a given assay in response to one stress were not predictive for outcomes in other assays. Therefore, although Drosophila provides a simpler model for elucidation of the neural substrates activated in response to stress, reactions to environmental disruptions are sufficiently complex to enable meaningful comparisons with mammalian systems.
Methods
Fly Husbandry
The wild type isogenic line derived from Canton S (CSwu) was established in the laboratory of Martin Heisenberg (University of Wurzburg, Germany) in 1978 and was a gift from Dr. J. Steven de Belle (DART Neuroscience, San Diego, CA, USA). Flies were maintained at 25°C on standard agar-cornmeal-yeast food, under 12h light:12h dark conditions and ~65% relative humidity. Population density was strictly controlled to minimize any developmental effects.
Stressors
Newly eclosed male and female flies were collected and maintained separately in groups of ≤10 under identical conditions for 5 days (sexually mature) and then exposed to a stressor, or maintained under control or stress conditions immediately after collection (1 day, sexually immature). Separate groups of animals (n=30/sex/age group except for the locomotor test where n=40 was used) were exposed to a) starvation stress, b) oxidative stress, c) sleep deprivation, or d) social isolation. For control conditions, flies were placed in polystyrene vials (24×75 mm, Azer Scientific Cat. No. ES-3825) containing 2% yeast, 5% sucrose dissolved in 1mL deionized water.
For starvation stress, flies were placed in polystyrene vials containing a 2.1cm glass fiber filter disc (Fisher Scientific, USA) saturated with only water.
To establish oxidative stress conditions, flies were placed in vials containing 2% yeast, 5% sucrose, 30mM methyl viologen dichloride hydrate (paraquat) dissolved in 1mL deionized water on a 2.1cm glass fiber filter circle.
For sleep deprivation, animals were placed in vials with 2% yeast, 5% sucrose, stably attached with museum putty (Ready America, Escondido CA) to a Wheaton vibrator (IKA Vibrax VXR, Cat. No. 868806) set at 300 rpm under a constant light source (1600 – 1700 LUX).
Animals exposed to social isolation were individually placed in a 10×75 mm borosilicate glass tube (Corning, Cat. No. 99445-10) containing a glass fiber filter cut with a hole puncher and saturated with 50 µL yeast-sucrose; the tubes were capped, placed in a rack, and kept in a dark cabinet. This procedure does not result in changes in visual system anatomy or function, nor does it affect phototactic behavior (Hirsch et al. 1990). Animals were maintained under control or stress conditions for 24 hr at 25°C prior to behavioral analyses.
Behavioral Assays
Before all analyses, individual flies were assessed for normal geotaxis, a quick and easy behavioral paradigm to identify individuals with generalized physiological defects. Defective flies were excluded from the analyses.
Locomotion (Open Field Arena)
The EthoVision XT tracking system (Noldus Information Technology Leesburg, VA, USA) was used to assess locomotor behaviors under normal room lighting (1100 LUX). Individual flies were aspirated into a 60mm diameter petri dish in which the bottom portion of the dish was painted white to increase color contrast and reduce glare., we focused our analyses on basal motor activity occurring in the last 5 min of the 20 min observation period, when all populations had adapted to the novelty of the arena. Threshold velocities for the degree of mobility (stopping, walking, hopping) were determined using velocities from the control animals for each population (immature males, immature females, mature males, mature females). 40 animals were assayed for each population and control or stress condition.
Light-Dark Assay
Light-dark chambers were constructed from a 5cm L × 2cm W × 1.5cm H transparent Lucite box with cover (The Container Store). One-half of the lower and upper chambers were painted black with nail polish (Sally Hansen Insta-dri fast dry nail color, “Night Flight”). Boxes were placed on a light box (Apollo Portable Light Box Model No. LB101, 16W) in a dark room. A single fly was gently aspirated into the "light" side of the covered box and the number of transitions between dark and light sides, as well as the amount of time spent in the light half, were recorded. The fly was considered in the dark or light sections of the box when the head and at least half of the body were located within those sections. Since Drosophila have been shown to adapt to a novel environment within a 15 min period, output was determined for the last 5 min of the observation period, where it was likely that the animals would have adapted to the Box. 30 animals were assayed for each population and control or stress condition.
Forced Swim Test
Lab-Tek II 4 well Chamber slides (Cat. No. 154526) were used for the Forced Swim Test chambers. Each well was filled with 2mL 0.08% SDS (Bio-Rad, Sodium Dodecyl Sulfate, Cat. No. 161–0301), and a single fly was gently aspirated into the chamber until it settled into an individual well. Each fly was videotaped for 5 min (Canon Vixia HF M52 Camcorder), and each video was analyzed for the latency until first immobility, and for the duration and number of immobility bouts for each min of the 5 min assay period. 30 animals were assayed for each population and control or stress condition. To establish that general locomotor activity for each fly was not compromised by the treatment or assay, each animal was removed with the flat end of a spatula at the end of the test and gently flicked onto a napkin, and assessed for the ability to immediately walk off. Almost every animal assayed was capable of normal walking behavior after the assay. The few animals that were not were excluded from the final analyses. A sample video is included in the Supplementary Materials.
Ethanol sedation
Sedation chambers were constructed using a length of chenille stems (Chenille Kraft, Walmart) stably inserted into a hard cotton plug (Azer Scientific Fly Locks, Cat. No. ES-3844). The stem was submerged in 100% ethanol (200 proof, Pharmaco-AAPER, Cat. No. 111ACS200), and the excess ethanol was removed by flicking the wand. A single fly was gently aspirated into the bottom of a 24×75 mm polystyrene vial and the vial was immediately capped with the plug. Time (in sec) was determined until the fly became sedated (when a fly dropped to the bottom of the vial and remained immobile). After a two min exposure, the sedated fly was gently "flipped" into a fresh vial that was capped with a normal plug, and the time (in sec) was recorded for recovery (determined by the ability of the fly to right itself, and either walk off or initiate a grooming regimen). 30 animals were assayed for each population and control or stress condition.
Statistics
Raw data were entered into Graphpad Prism. Control groups for all populations were compared by two-way ANOVA, using sex and developmental stage (sexually immature vs. mature) as independent factors, and behavioral measures as dependent factors, followed by Bonferroni post-tests. To directly compare temporal effects, immature females were compared with mature females, and immature males with mature males, by Student’s t tests. To address sexual dimorphism, immature males and females, and mature males and females, were compared to each other and analyzed for each parameter by Student’s t tests. Data for sexually immature and mature females and males were analyzed separately by one-way ANOVA with stress condition as the independent factor and behavioral measures as dependent factors. When necessary, Dunnett’s post-hoc tests were used.
Results
Immature and mature females and males represent behaviorally distinct populations when assayed in diverse behavioral paradigms
Locomotion
The first phase of data analysis focused on all four populations (immature and mature females and males) under control conditions to evaluate the effect of sex and developmental stage on behavioral parameters in each translational assay. Four components of locomotor activity were assayed in experimental subjects that had been exposed to control conditions: pattern (measured by turn angle and distance moved), speed (measured by velocity), freezing behavior and escape behavior. When comparing populations under control (no stressor) conditions, immature females and males displayed similar locomotor profiles, which were significantly altered by the onset of sexual maturity (Fig. 1, Tables 1 and 2). The profiles for pattern, freezing and highly mobile parameters were determined using output from the last 5 min of the 20 min observation period, when the flies had become adapted to the novel environment of the open field arena.
Fig. 1. Immature and mature female and male Drosophila display distinct motor patterns.
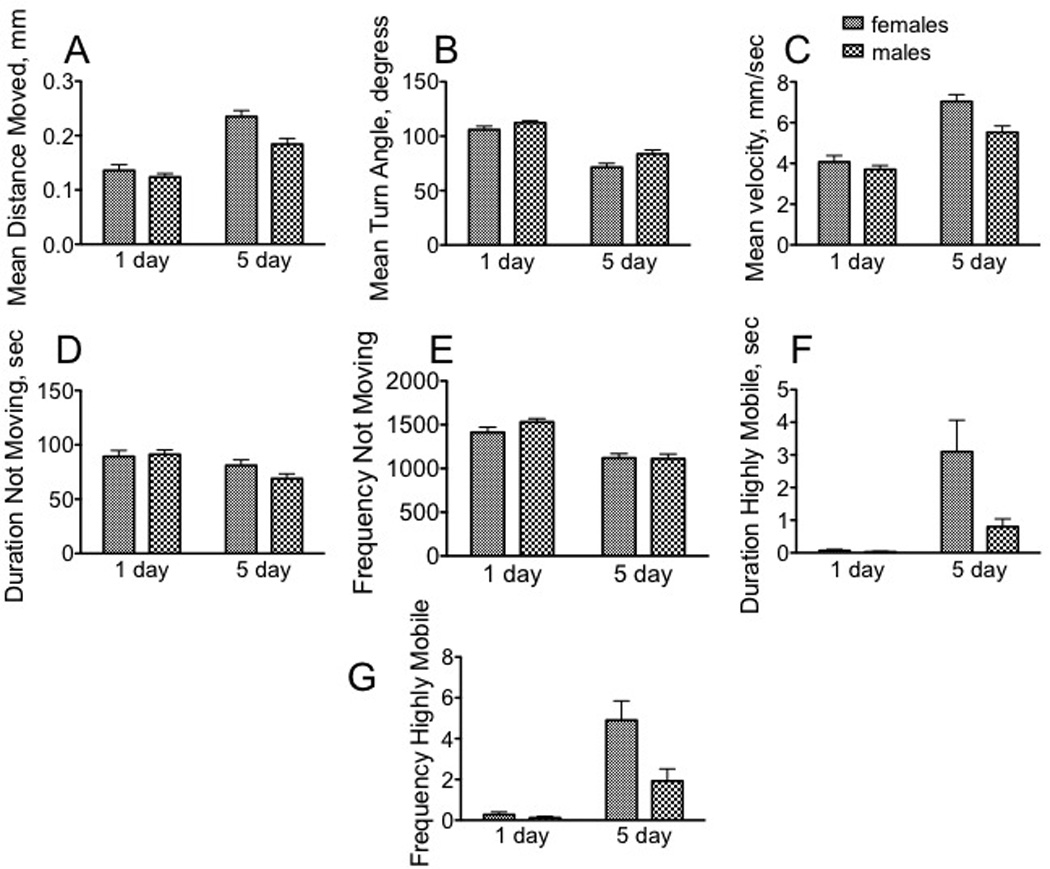
Data were collected from min 15 – 20 in an open field arena, during which time the animals had habituated to the novel environment. A – C, motor pattern. A, mean distance moved; B, mean velocity; C, mean turn angle. D –E. Freezing behavior. D, duration not moving; E, frequency not moving. F – G, escape behavior. These included hops of velocity greater than 50 mm/s, as well as short bursts of flight. F, duration highly mobile; G, frequency of high mobility * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, two-way ANOVA followed by Bonferroni post tests. n = 40 for each population..
Table 1.
Sexual maturity has a striking influence on behavioral outcomes in each paradigm.
| Behavior | Parameter | 1F : 5F | 1M : 5M |
|---|---|---|---|
| Open field arena | mean distance moved | ⇧**** | ⇧**** |
| mean turn angle | ⇩**** | ⇩**** | |
| mean velocity | ⇧**** | ⇧**** | |
| duration not moving | ⇩*** | ||
| frequency not moving | ⇩*** | ⇩**** | |
| duration escape | ⇧** | ⇧** | |
| frequency escape | ⇧**** | ⇧** | |
| Light-Dark box | time in light | ⇧*** | |
| transitions | ⇧**** | ||
| Forced Swim Test | # bouts | ⇩* | |
| duration bouts | ⇩*** | ⇩**** | |
| latency bouts | ⇧**** | ||
| Ethanol sensitivity | sedation | ⇩**** | ⇩**** |
| recovery | ⇩**** |
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001,
Student’s unpaired t tests comparing immature females and mature females (1F : 5F), and immature males and mature males (1M : 5M). Direction of arrow indicates whether the assayed behavior increased (up arrow) or decreased (down arrow) in the mature populations. n = 30 for each population (40 for locomotion).
Table 2.
Sexually mature males and females display a strongly dimorphic response in several behavioral paradigms.
| Behavior | Parameter | 1F : 1M | 5F : 5M |
|---|---|---|---|
| Open field arena | mean distance moved | ⇩** | |
| mean turn angle | ⇧* | ||
| mean velocity | ⇩** | ||
| duration not moving | |||
| frequency not moving | |||
| duration escape | ⇩* | ||
| frequency escape | ⇩** | ||
| Light-Dark box | time in light | ⇩* | |
| transitions | ⇧**** | ||
| Forced Swim Test | # bouts | ⇧* | |
| duration bouts | |||
| latency bouts | ⇩* | ||
| Ethanol sensitivity | sedation | ⇩**** | |
| recovery |
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001,
Student’s unpaired t tests, comparing immature females and males (1F : 1M) and mature females and males (5F : 5M). Direction of arrow indicates whether the assayed behavior increased (up arrow) or decreased (down arrow). n = 30 for each population (40 for locomotion).
Developmental effects
While both the mean distance moved [F(1, 156) = 64.86, p < 0.0001], and velocity [F(1, 156) = 64.86, p < 0.0001] increased in older flies (Fig. 1A, B), the mean turn angle decreased [F(1, 156) = 95.42, p < 0.0001, two way ANOVA] (Fig. , C), suggesting that mature flies sacrificed complexity for speed and distance in an open field arena. Mature males decreased their duration of time spent immobile [F(1, 156) = 10.03, p = 0.0019] (Fig. 1D), and both mature females and males decreased the frequency of immobile bouts [F(1, 156) = 48.89, p < 0.0001] (Fig. 1E). There was an interaction between age and sex for duration of short hops or bursts of flight [F(1, 156 = 5.12, p = 0.0251], and for the frequency of escape behaviors [F(1, 156 = 6.28, p = 0.0132] (Fig. 1F, G).
Sex effects
Mature females displayed greater distance travelled [F(1, 156) = 10.10, p = 0.0018] (0.235 mm/bout of activity, Fig. 1A) and greater velocity [F(1, 156 = 10.10, p = 0.0018) (7.04 mm/s, Fig. 1B), but a decreased mean turn angle [F(1, 156) = 8.22, p = 0.0047] (71.4°, Fig. 1C), relative to their male counterparts (0.184 mm/bout; 5.52 mm/s; 83.6°). Freezing behavior was not sexually dimorphic (Fig. 1D, E).
Light-Dark Box
LDB Under control conditions, immature males spent significantly less time in the light during the observation period (85.1 sec) relative to mature males (130.8 sec) [F(1, 116 = 10.85, p = 0.0013, two way ANOVA] (Fig. 2A). Sex did not affect appreciably affect time spent in the light portion of the Light-Dark Box. There was a significant interaction between age and sex for transitions [F(1, 116) = 19.01, p < 0.0001, two way ANOVA] (Fig. 2B).
Fig. 2. Sexually mature males and females display dimorphic and distinct responses in three different paradigms relative to immature flies.
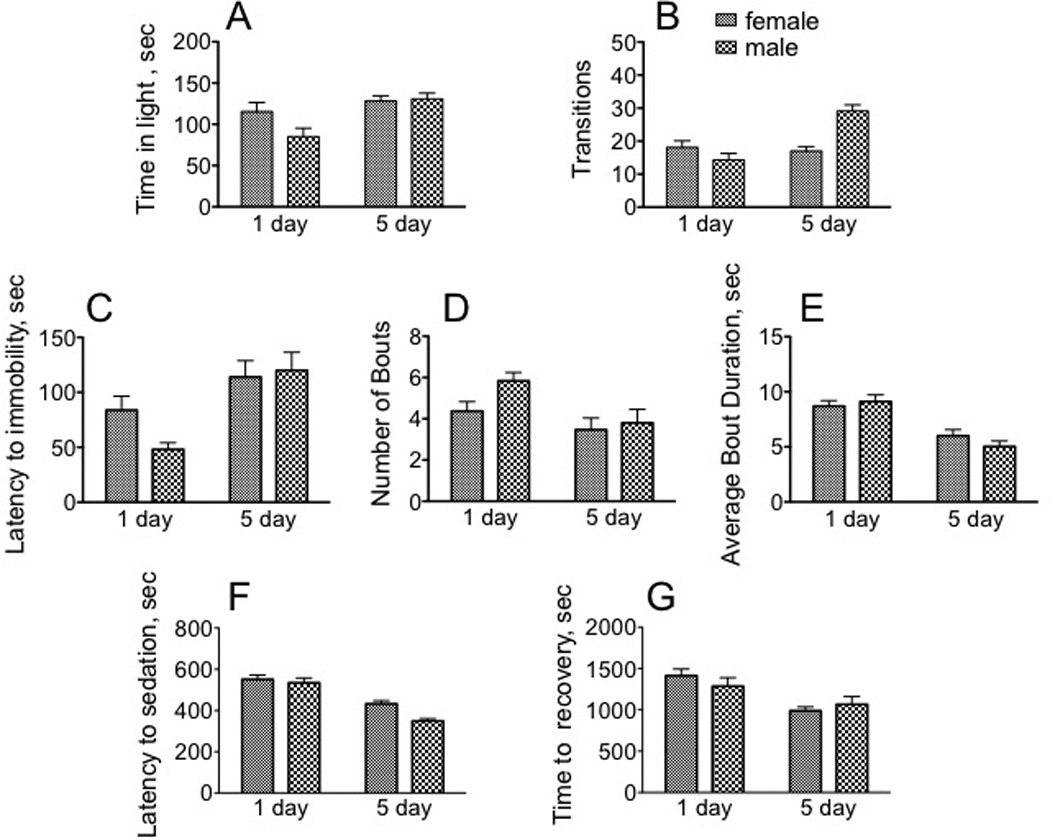
A - B, light - dark box. A, Amount of time spent in light (min 10–15 of the 15 min observation period). B, Number of transitions between the light and dark sides of the arena during min 10–15 of the 15 min observation period. C – E, Forced Swim Test. C, latency to first bout of immobility; D, number of bouts; E, average bout duration. F – G, response to the sedative effects of ethanol. F, sec until sedation; G, sec until recovery from sedation. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, two-way ANOVA followed by Bonferroni post tests. n = 30 for each population.
Forced Swim Test
As observed for locomotor parameters in the open field arena and in the Light-Dark Box, sexually immature animals responded very differently than did sexually mature animals. Mature males significantly increased the time until first bout of immobility (immature versus mature males, 48.1 and 120.0 sec, respectively, Fig. 2C) [F(1, 116 = 14.98, p = 0.0002, two way ANOVA]. Older males also displayed a decrease in the number of bouts (immature males, 5.8; mature males 3.8, Fig 2D) [F(1, 116 = 7.46, = < 0.0073]. Older animals also decreased their average bout duration (immature females, 8.7 sec; mature females, 6.0 sec; immature males, 9.1 sec; mature males 5.0 sec, F(1, 116) = 37.50, p < 0.0001, Fig. 2E). We interpret this behavior to be indicative of the motivation of the animals for escape from an unpleasant environment, and our results suggest that mature animals display a more positive outcome. There were no significant differences between males and females in any of the test parameters (latency to immobility, bout number and duration) in this assay.
Response to Ethanol
Immature flies became sedated more easily than mature adults after exposure to ethanol vapor (9.2 min and 8.9 min, respectively, immature females and males, compared with 7.2 and 5.8 min, Fig 2F) [F(1, 116) = 65.70, p < 0.0001, two way ANOVA]. Immature females also took longer to recover (23.5 min) than their mature counterparts (16.5 min, Fig. 2G) [F(1, 116) = 14.58, p = 0.0002], suggesting that older females were more resistant to the sedative effects of ethanol. While there was no difference between males and females when assaying recovery from ethanol sedation, there was a significant difference in their initial sensitivity to ethanol vapors. Males became sedated in less time than did females [F(1, 116) = 7.10, p = 0.0088].
Summary of effects
Temporal dimorphism
Table I summarizes the differences observed in each assay when comparing immature females with mature females, or immature males with mature males. Sexual maturity had a profound influence on almost every parameter in the open field arena. Sexually mature females increased their mean distance moved [t(78) = 3.253, p = 0.0017], mean velocity [t(78) = 6.417), p< 0.0001], and frequency [t(78) = 4.828, p < 0.0001] and duration [t(78) = 3.127, p = 0.0025] of escape behaviors relative to sexually immature females. Mature females decreased the frequency of immobile periods [t(78) = 3.744), p = 0.0003] and the mean turn angle [t(78) = 7.078), p < 0.0001] (Students t test), suggesting that they have a more robust motor pattern than sexually immature females, but with a simpler trajectory. Sexually mature males also increased their mean distance moved [t(78) = 4.872, p < 0.0001], mean velocity [t(78) = 4.872, p < 0.0001], and duration [t(78) = 3.164), p = 0.0022] and frequency [t(78) = 3.028, p = 0.0033] of escape behaviors relative to immature males. They also decreased their mean turn angle [t(78) = 6.748, p <0.0001], and duration [t(78) =3.740, p = 0.00103] and frequency [t(78) = 6.426), p < 0.0001] of immobility compared with immature males.
There was no temporal dimorphism when comparing immature and mature females for the measured parameters of the Light-Dark Box, but mature males spent more time in light [t(58) = 3.693), p = 0.0005] and displayed a greater number of transitions [t(58) = 5.388), p < 0.0001] than did immature males.
Mature males were also more active than immature males in the Forced Swim Test, displaying a decrease both in the number [t(58) =2.608), p = 0.0116] and average duration of each bout [t(58) = 5.108), p < 0.0001], as well as an increase in time until the first bout of immobility [t(58) = 4.10), p < 0.0001] relative to immature males. Sexually mature females displayed a significant decrease in bout duration compared to their immature counterparts [t(58) = 3.520, p = 0.0008].
The response to the sedative actions of ethanol was also highly temporally dimorphic, since sexually mature flies were significantly less sensitive to sedation than their immature counterparts (female: t(58) = 4.411), p < 0.0001; male: t(58) = 7.097), p < 0.0001, Student’s t test]. Sexually mature females also recovered more quickly than immature females [t(58) = 4.537, p < 0.0001].
Sexual dimorphism
Overall, sexually immature females and males under control conditions displayed similar outcomes for all parameters in the open field arena as well as in response to the sedative effects of ethanol, and mature animals displayed similar responses in the Forced Swim Test (Table 2). A minimal difference for immature animals was observed for the light dark box: males decreased their time spent in the light part of the box, but no other parameters were affected [t(58) = 2.017), p = 0.0483]. Sexual dimorphism between immature males and females was also observed in the Forced Swim Test, where males displayed an increase in the number of bouts [t(58) = 2.356, p = 0.0219], and a decrease in latency [t(58) = 2.527, p = 0.0142].
Sexually mature males and females, however, displayed considerable dimorphism in their behavior in every locomotor parameter except for parameters reflecting periods of immobility. Compared to their female counterparts, males tended to move a shorter distance [t(78) = 3.263, p = 0.0016] at decreased velocity [t(78) = 3.263, p = 0.0016], with more complex trajectories (measured by an increase in turn angle, [t(78) = 2.364, , p = 0.0206], and decreased both the frequency [t(78) = 2.662, p = 0.0094] and duration [t(78) = 2.305, p < 0.0238] of escape behavior (hops, short bursts of flight). In the light-dark box, males significantly increased the number of transitions [t(58) = 5.306), p < 0.0001]. Thus, while sexually mature females appeared to be more active in an open field arena, sexually mature males displayed increased exploratory activity in the Light-Dark Box. Conversely, mature males displayed a greater sensitivity to the sedative effects of ethanol [t(58) = 4.250), p < 0.0001], although recovery was similar for both populations.
The four populations display unique responses to stress
We chose four physiological stressors (starvation, oxidative stress, sleep deprivation and social isolation) with these common attributes: (a) a rich history in both the vertebrate and invertebrate literature; (b) demonstrated effects as stressors in mammalian systems with direct correlative evidence for a role in the etiology of affective disorders; (c) simple, high-throughput and easily reproducible conditions to facilitate the analysis of a large number of animals; (d) verified in our hands as producing changes in neurotransmitter signaling pathways in flies; and (e) sufficiently diverse in origin to elicit a wide complement of genomic responses. Multiple stressors were used, because they differ in their physiological effects (Mason, 1971). Flies were stressed for 24 hours, since we have demonstrated this resulted in neurochemical changes predictive for changes in behavioral output (Neckameyer & Weinstein, 2005), and served more as an acute rather than chronic stress.
Locomotion
Immature females and males did not appreciably alter locomotor pattern parameters in response to stress (Table 3). Starvation was the only stressor with any effect, and for both populations, velocity [female: F (4, 195) = 8.693, p < 0.0001, Dunnett’s post-hoc q = 3.722, p < 0.01; male: F (4, 195) = 6.501, p < 0.0001, Dunnett’s post-hoc q = 3.549, p < 0.01, one way ANOVA] and distance moved increased [female: F (4, 195) = 8.693, p < 0.0001, Dunnett’s post-hoc q = 3.722; male: F(4, 195) = 6.501, p < 0.0001, Dunnett’s post-hoc q = 3.549, p < 0.01, one way ANOVA], but the complexity of the pattern (measured by turn angle) decreased [female: F(4, 195) = 8.540, p < 0.0001, Dunnett’s post-hoc q = 4.459, p < 0.001; male: F(4, 195) = 9.716, p < 0.0001, Dunnett’s post-hoc q = 4.622, p < 0.001, one way ANOVA], as did the frequency of immobility [female: F(4, 195) = 8.927, p < 0.0001, Dunnett’s post-hoc q = 4.247, p < 0.001; male: F(4, 195) = 5.424, p = 0.0004, Dunnett’s post-hoc q = 3.133, p < 0.01, one way ANOVA). This was not true for sexually mature populations, since paraquat treatment and social isolation affected mature females, and starvation, oxidative stress, and social isolation affected males. In several cases, the response of sexually mature males was the reverse of that for mature females; for example, treatment with paraquat decreased the mean distance moved [F(4, 195) = 25.58, p < 0.0001, Dunnett’s post-hoc q = 3.549, p < 0.01] and increased the degree of the average turn angle [F(4, 195) = 31.86, p < 0.0001, Dunnett’s post-hoc q = 3.255, p < 0.01] in sexually mature females, but the opposite was observed in males [F (4, 195) = 43.94, p < 0.0001, Dunnett’s post-hoc q = 4.595, p < 0.001 and F(4, 195) = 30.08, p < 0.0001, Dunnett’s post-hoc q = 3.288, p < 0.01] (one way ANOVA). A noticeable difference was that females increased both the frequency and duration of freezing behavior in response to paraquat treatment (frequency: [F(4, 195) = 16.24, p < 0.0001, Dunnett’s post-hoc q = 2.902, p < 0.05]; duration: [F(4, 195) = 28.76, p < 0.0001, Dunnett’s post-hoc q = 2.802, p < 0.05] and social isolation [F(4, 195) = 16.24, p < 0.0001, Dunnett’s post-hoc q = 6.754, p < 0.001] and [F(4, 195) = 28.76, p < 0.0001, Dunnett’s post-hoc q = 9.037, p < 0.001]. Generally, starvation, oxidative stress, and sleep deprivation increased escape behavior in mature males, while social isolation increased the frequency of immobility (as it did for mature females).
Table 3.
Effects of diverse stressors on different locomotor parameters in immature and mature male and female Drosophila.
| Movement Pattern | 1d female | 1d male | 5d female | 5d male |
|---|---|---|---|---|
| turn, angle, degree | ||||
| starved | ⇩*** | ⇩*** | ⇩*** | |
| paraquat | ⇧** | ⇩** | ||
| sleep deprivation | ||||
| social isolation | ⇧*** | ⇧*** | ||
| mean distance moved, mm | ||||
| starved | ⇧** | ⇧** | ⇧*** | |
| paraquat | ⇩** | ⇧*** | ||
| sleep deprivation | ||||
| social isolation | ⇩*** | ⇩** | ||
| mean velocity | ||||
| starved | ⇧** | ⇧** | ⇧*** | |
| paraquat | ⇧** | ⇧*** | ||
| sleep deprivation | ||||
| social isolation | ⇧*** | ⇩** | ||
| Freezing Behavior | 1d female | 1d male | 5d female | 5d male |
| duration not moving, sec | ||||
| starved | ⇩*** | |||
| paraquat | ⇧* | ⇩*** | ||
| sleep deprivation | ||||
| social isolation | ⇧*** | |||
| frequency not moving | ||||
| starved | ⇩*** | ⇩** | ⇩*** | |
| paraquat | ⇧* | ⇩*** | ||
| sleep deprivation | ||||
| social isolation | ⇧*** | ⇧*** | ||
| Escape Behavior | 1d female | 1d male | 5d female | 5d male |
| highly mobile duration, sec | ||||
| starved | ⇧*** | |||
| paraquat | ⇧** | |||
| sleep deprivation | ⇧* | |||
| social isolation | ⇩** | |||
| frequency highly mobile | ||||
| starved | ⇧*** | |||
| paraquat | ⇧*** | |||
| sleep deprivation | ⇧** | |||
| social isolation | ⇩** |
Data were collected from min 15 – 20 in an open field arena, during which time the animals had habituated to the novel environment.
p < 0.05,
p < 0.01,
p < 0.001, one-way ANOVA followed by Dunnett’s post test. Direction of arrow indicates whether the assayed behavior increased (up arrow) or decreased (down arrow) after exposure to a given stress relative to control conditions. n = 40 for each population and control or stress condition.
Light-Dark Box
Stress only minimally affected the time spent in light for any population (Fig. 3). Social isolation affected only females, increasing their preference for the dark side of the arena (1d females: [F(4, 145) = 4.361, p = 0.0023, Dunnett’s post-hoc q = 3.595, p < 0.01]; 5d females: [F(4, 145) = 6.32100,Dunnett’s post-hoc q = 3.454, p < 0.01, one way ANOVA, Fig. 3A, C). Neither immature not mature males altered responses in this assay after exposure to stress (Fig. 3B, D).
Fig. 3. The effect of stressors on time spent in the lit arena in the Light-Dark Box.
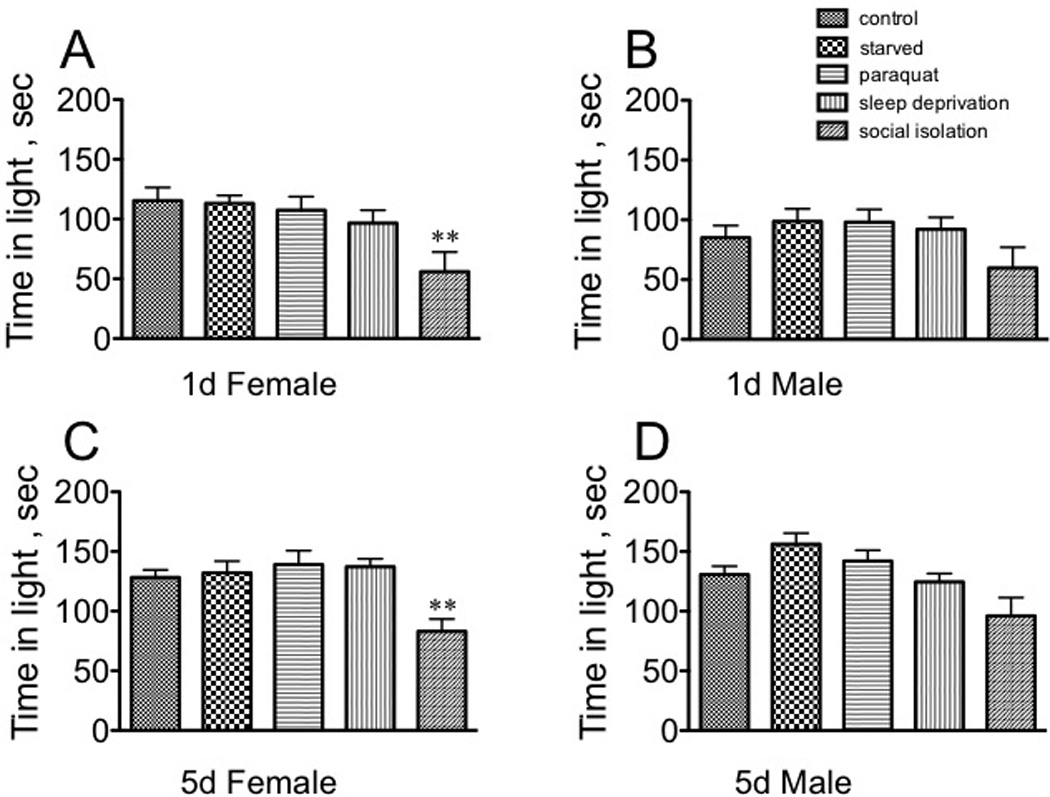
A, sexually immature females; B, sexually immature males; C, sexually mature females; D, sexually mature males. Statistical comparisons between control and stress conditions within a population, ** p < 0.01, one-way ANOVA followed by Dunnet’s post test.
Social isolation decreased the number of transitions for every population (immature females: F(4, 145) = 14.14, p < 0.0001, Dunnett’s post-hoc q = 5.302, p < 0.001; immature males: F(4, 145) = 9.043, p < 0.0001, Dunnett’s post-hoc q = 3.888, p < 0.001; mature females: F(4, 145) = 15.66, p < 0.0001, Dunnett’s post-hoc q = 4.075, p < 0.001; mature males [F(4, 145) = 19.33, p < 0.0001, Dunnett’s post-hoc q = 7.356, p < 0.001 one way ANOVA, Fig 4). Starvation and sleep deprivation increased the number of transitions for mature females [F (4, 145) = 15.66, p < 0.0001, Dunnett’s post-hoc q = 2.886, p < 0.05 for starvation and q = 2.509, p< 0.05 for sleep deprivation, 4C). Paraquat decreased the number of transitions in mature males [F(1, 145) = 19.33, p < 0.0001, Dunnett’s post-hoc q = 2.859, p < 0.05, Fig. 4D). These data suggest that the light-seeking behavior in this assay is separable from general activity/exploration as quantitated by number of transitions between the two arenas.
Fig. 4. The effect of stressors on number of transitions in the Light-Dark Box.
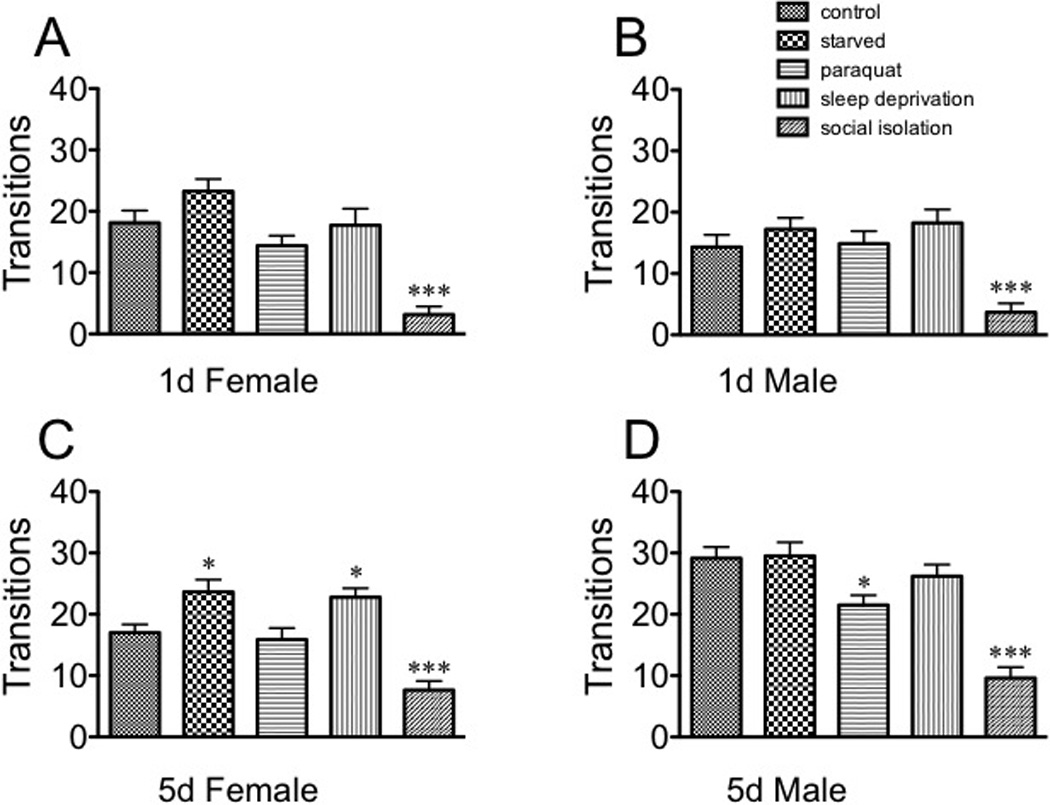
A, sexually immature females; B, sexually immature males; C, sexually mature females; D, sexually mature males. Statistical comparisons between control and stress conditions within a population, * p < 0.05, *** p < 0.001, one-way ANOVA followed by Dunnet’s post test.
Importantly, there was no correlation between the effect of a given stressor on basal locomotor activity, as measured by distance moved in the open field arena, and performance in the Light-Dark Box. Starvation increased distance moved in immature males (measured by activity in the last 5 min of a 20 min observation period in the open field arena, Table 1), yet had no effect on time spent in light or number of transitions in the Light-Dark assay. Paraquat decreased distance moved in the open field arena in mature females but did not affect behavioral outcomes in the Light-Dark Box. Starvation and paraquat treatment both increased distance moved in the open field arena in mature males; starvation had no effect and paraquat actually decreased the number of transitions in the Light-Dark assay. Social isolation had no effect on distance moved in the open field arena for immature animals, although it decreased transition number in the Light-Dark Box.
Forced Swim Test
Similarly to the locomotor assays, each population responded very differently to the different stressors in the forced swim test. Starvation [F(4, 145) = 4.341, p = 0.0024, Dunnett’s post-hoc q = 3.218, p < 0.01, one way ANOVA] and sleep deprivation [q = 2.732, p <0.05] negatively impacted the latency to the first immobile bout in immature females (Fig. 5A), but had no effect on the number of bouts, or bout duration (Fig. 5E, I). Stress did not affect bout duration for immature males (Fig. 5J); however, treatment with paraquat decreased the latency to immobility [F(4, 145) = 3.079, p = 0.0181, Dunnett’s post-hoc q = 2.660, p < 0.05, one way ANOVA] as well as the number of bouts [F (4, 145)= 2.676, p < 0.05, Dunnett’s post-hoc q = 2.751, p < 0.05, one way ANOVA, Fig 5B, F). Starvation increased bout number [F(4, 145) = 9.622, p < 0.0001, Dunnett’s post-hoc q = 2.870, p < 0.05] for mature females (Fig. 5G), and paraquat increased the average bout duration [F(4, 145) = 11.67, p < 0.0001, Dunnett’s post-hoc q = 5.372, p < 0.001] (Fig. 5K), but social isolation enhanced performance in this assay by increasing the latency to the first immobile bout [F(4, 145) = 7.990, p < 0.0001, Dunnett’s post-hoc q = 3.401, p < 0.01] (Fig. 5C) and decreasing bout number [F (4, 145) = 9.622, p < 0.0001, Dunnett’s post-hoc q = 2.822, p < 0.05] (one way ANOVA, Fig. 5G). An even stronger response after social isolation was observed in mature males (latency, Fig. 5D [F (4, 145) = 22.39, p < 0.0001, Dunnett’s post-hoc q = 4.477, p < 0.001], and number, Fig. 5H [F (4, 145) = 17.91, p < 0.0001, Dunnett’s post-hoc q = 3.572, p < 0.01]. Starvation [F (4, 145) = 22.39, p < 0.0001, Dunnett’s post-hoc q = 2.688, p < 0.05] and sleep deprivation [q = 2.563, p < 0.05] decreased latency (Fig. 5D) without affecting bout number or duration (Fig. 5H, L). However, in direct contrast to immature males, mature males exposed to paraquat displayed significantly decreased latency to the first bout of immobility [F (4, 145) = 22.39, p < 0.0001, Dunnett’s post-hoc q = 3.917, p < 0.001] and a dramatic increase in the number of bouts and bout duration (number: F(4, 145) = 17.91, p < 0.0001, Dunnett’s post-hoc q = 4.528, p < 0.001; duration: F(4, 145) = 14.37, p < 0.0001, Dunnett’s post-hoc q = 6.046, p < 0.001, one way ANOVA).
Fig. 5. The effect of stressors on behavioral parameters of the Forced Swim Test.
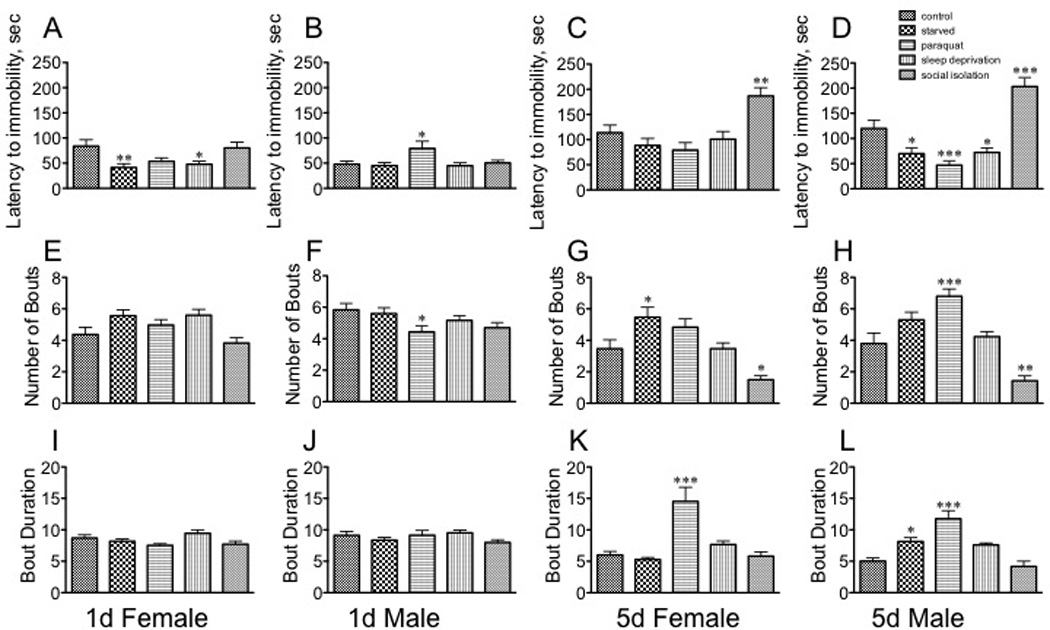
A – D, latency to first bout of immobility; E – H, number of bouts; I – L, bout duration. A, E, I sexually immature females; B, F, J sexually immature males; C, G, K sexually mature females; D, H, L, sexually mature males. Statistical comparisons between control and stress conditions within a population, * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA followed by Dunnet’s post test. n = 30 for each population.
Overall, the immobility of immature and mature females and males increased after the initial timepoint (min 0 −1), but that rate stayed relatively constant through the end of the assay (five min total duration). The pattern of immobility of immature females was not affected by exposure to stress (Fig.6A), although immature males decreased the time spent immobile after exposure to starvation for min 1–2 of the assay F(4, 145) = 4.232, p = 0.0012, Dunnett’s post-hoc q = 2.965, p < 0.05, one way ANOVA, Fig. 6B). However, the mobility of sexually mature females exposed to paraquat continued to deteriorate at every timepoint relative to controls (min 0 −1, F(4, 145) = 10.36, p < 0.0001, Dunnett’s post-hoc q = 5.005, p < 0.001; min 1–2, F(4, 145) = 11.99, p < 0.0001, Dunnett’s post-hoc q = 5.321, p < 0.0001; min 2–3, F(4, 145) = 12.50, p < 0.0001, Dunnett’s post-hoc q = 5.138, p < 0.001; min 3–4, F(4, 145) = 13.43, p < 0.0001, Dunnett’s post-hoc q = 5.883, p < 0.001; min 4–5, F(4, 145) = 13.80, p < 0.0001, Dunnett’s post-hoc q = 5.873, p < 0.001, one way ANOVA, Fig. 6C). The same was observed for mature males (min 0 −1, F(4, 145) = 11.31, p < 0.0001, Dunnett’s post-hoc q = 5.163, p < 0.001; min 1–2, F(4, 145) = 25.78, p < 0.0001, Dunnett’s post-hoc q = 7.665, p < 0.001; min 2–3, F(4, 145) = 16.19, p < 0.0001, Dunnett’s post-hoc q = 6.049, p < 0.001; min 3–4, F(4, 145) = 20.04, p < 0.0001, Dunnett’s post-hoc q = 6.766, p < 0.001; min 4–5, F(4, 145) = 11.89, p < 0.0001, Dunnett’s post-hoc q = 5.589, p < 0.001, one way ANOVA, Fig. 6D. Starvation also affected the rate of immobility in mature males, but to a much lesser extent than did paraquat (1,q = 2.542, p < 0.05;q = 3.225, p < 0.01; min 4–5, q = 3.436, p < 0.01, one way ANOVA, Dunnett’s post-hoc tests, Fig. 6D).
Fig. 6. The effect of stressors on immobility with increasing time during the Forced Swim Test.
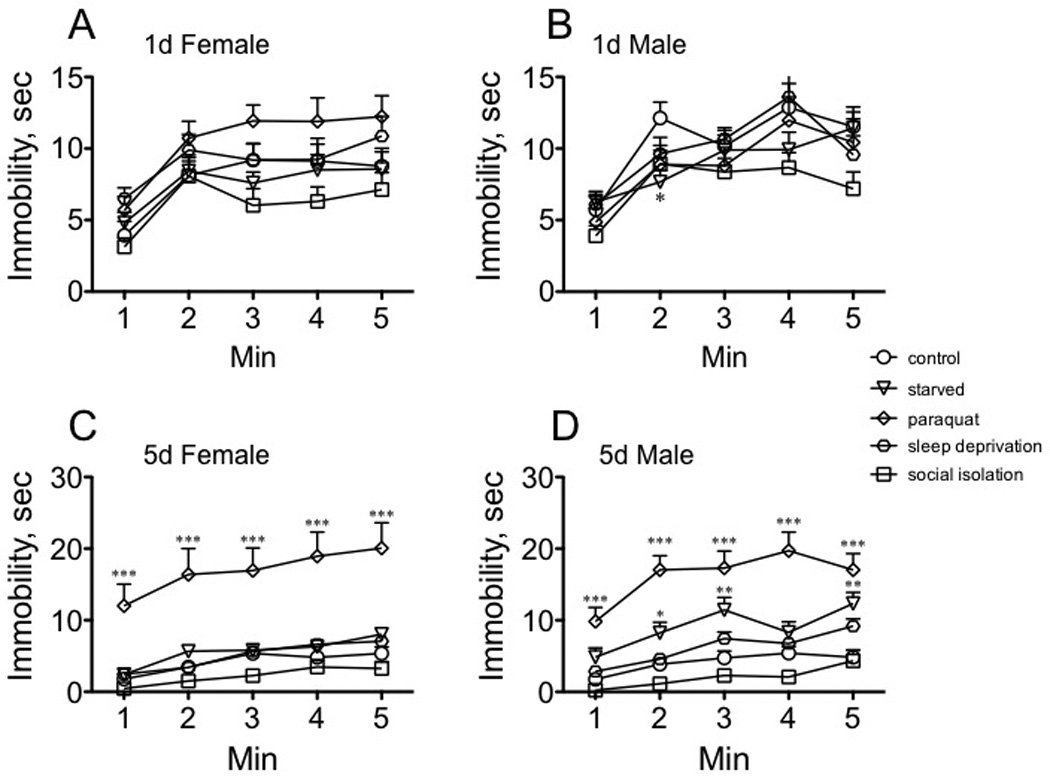
A, sexually immature females; B, sexually immature males; C, sexually mature females; D, sexually mature males. Statistical comparisons between control and stress conditions for each timepoint within a population, * p < 0.05, ** p < 0.01, *** p < 0.001, oneway ANOVA followed by Dunnet’s post test. n = 30 for each population.
Response to, and recovery from, the sedative effects of ethanol
Stress did not affect time to sedation for either immature males or females or mature males (Fig. 7A, B, D). However, both sleep deprivation and social isolation increased resistance to sedation for mature females [F(4, 145) = 4.411, p = 0.0021; Dunnett’s post-hoc sleep deprivation, q = 3.292, p < 0.01. and social isolation, q = 3.177, p < 0.01] (Fig. 7C).
Fig. 7. The effect of stressors on the sensitivity to the sedative effects of ethanol.
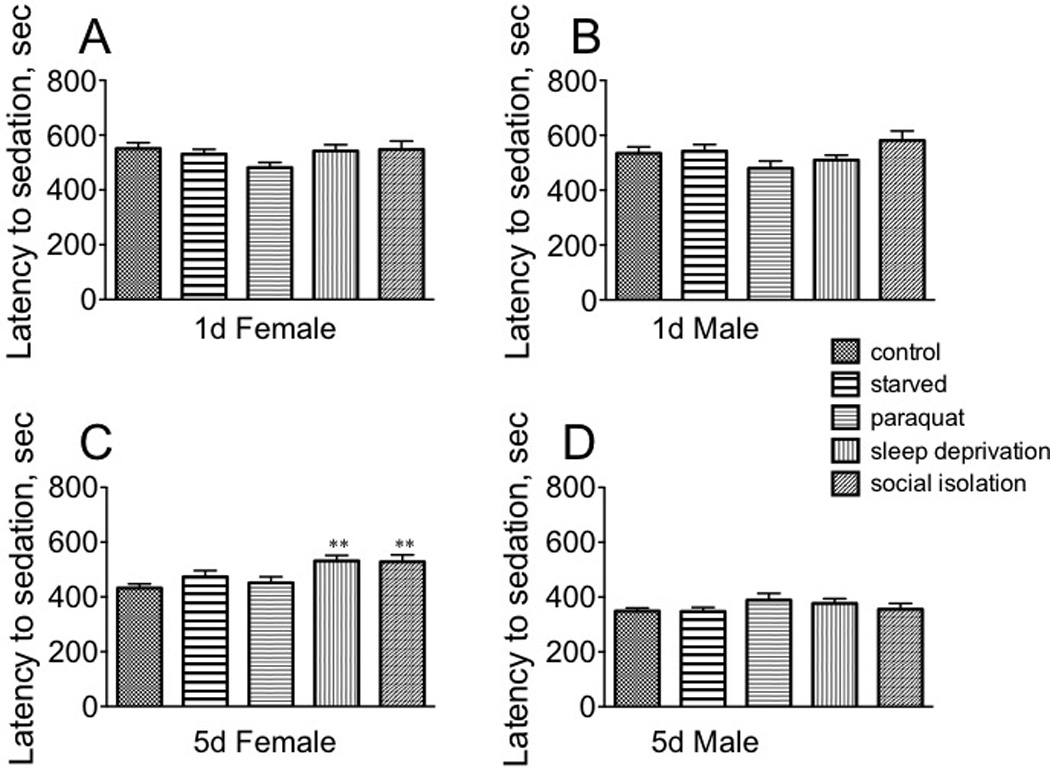
A, sexually immature females; B, sexually immature males; C, sexually mature females; D, sexually mature males. Statistical comparisons between control and stress conditions within a population, ** p < 0.01, one-way ANOVA followed by Dunnet’s post test. n = 30 for each population.
Stress affected recovery from the sedative effects of ethanol in a far more dynamic manner than for sedation (Fig. 8). Each population responded differently to different stressors, although recovery times all increased in response to a given stressor. Starvation had a profound effect on the recovery of immature females [F(1, 145) = 8.160, p < 0.0001, Dunnett’s post-hoc q = 5.659, p < 0.001], although both paraquat and sleep deprivation also increased recovery time (paraquat: q = 2.795, p < 0.05; sleep deprivation: q = 2.641, p < 0.05, Fig. 8A). While starvation similarly affected recovery for immature males [F(4, 145) = 9.436, p < 0.0001, Dunnett’s post-hoc q = 4.402, p < 0.001], paraquat also had a highly significant effect [q = 5.075, p < 0.001] (one way ANOVA, Fig. 8B). Only paraquat impacted mature female recovery rate [F(4, 145) = 11.63, p < 0.0001, one way ANOVA, Dunnett’s post-hoc q = 4.551, p < 0.001] (Fig. 8C). Mature males displayed a similar profile as their immature counterparts: both starvation [F(4, 145) = 16.56, p < 0.0001, Dunnett’s post-hoc q = 6.912, p < 0.001] and paraquat q = 3.748, p < 0.01] increased recovery time, but social isolation also had a smaller, but still significant, effect [q = 2.947, p < 0.05 (Fig. 8D). Overall, these results suggest that sexually mature females are less vulnerable to the sedative effects of ethanol after specific stressors, and their recovery from sedation was less affected by stress relative to the other populations.
Fig. 8. The effect of stressors on the rate of recovery from ethanol sedation.
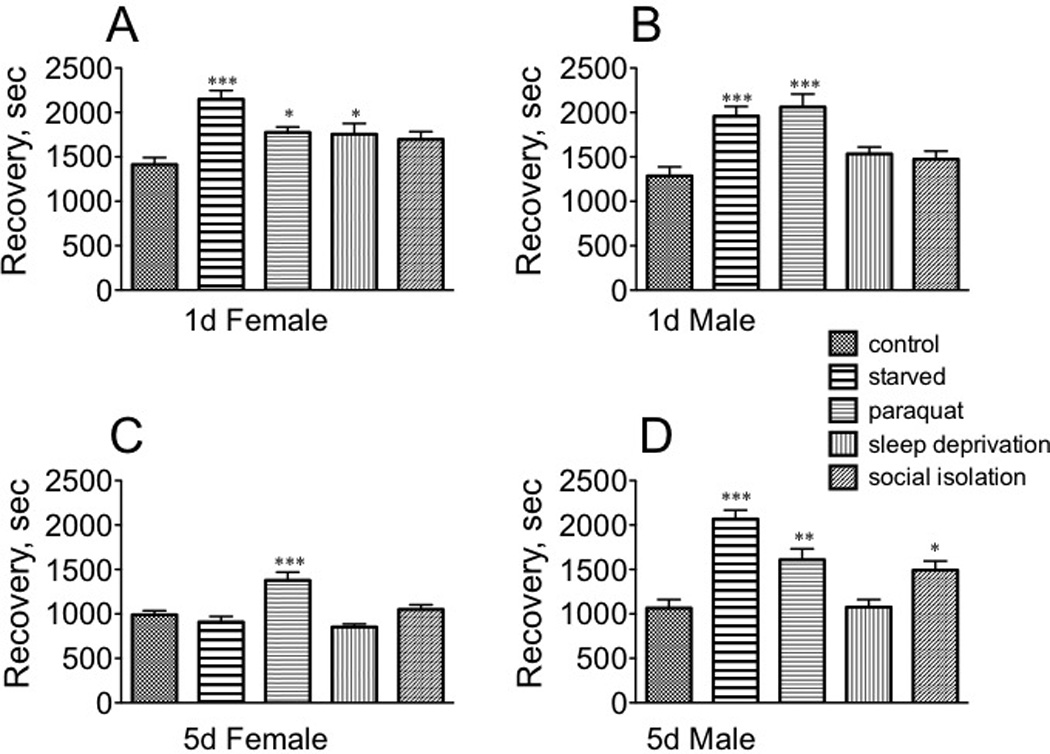
A, sexually immature females; B, sexually immature males; C, sexually mature females; D, sexually mature males. * p < 0.05, ** p < 0.01, ***, p < 0.001, one-way ANOVA followed by Dunnet’s post test. n = 30 for each population.
Discussion
We exposed sexually immature and mature female and male Drosophila melanogaster from an isogenic wild-type strain raised under controlled environmental conditions to four different stressors (starvation, oxidative stress, sleep deprivation and social isolation) to facilitate the analysis of a large number of animals for direct comparisons. These animals were assessed in an open-field arena, in a Light-Dark Box, and in a Forced Swim Test, as well as for sensitivity to the sedative effects of ethanol. While previous work by ourselves and others have described behavior of Drosophila in an open field arena and after exposure to ethanol vapor, no studies have assessed these behaviors in response to a variety of stressors, nor have they compared temporal and sex effects within a population. The Light-Dark Box and Forced Swim Test are novel adaptations of rodent paradigms. These studies establish that immature and mature females and males represent behaviorally distinct populations under control conditions as well as after exposure to different stressors.
Translational validity of the assays
We assayed four components of locomotor activity in response to stress: pattern, freezing behavior, escape behavior, and adaptation to a novel environment. Pattern was measured by the mean distance moved, turn angle, and velocity. Wild-type mice explore all parts of an arena and engage in both circumscribed and straight movements; similar behavior is observed in Drosophila (Liu et al., 2007). Escape behavior is an attempt to avoid predation; flight behavior displays a pharmacological response profile similar to that of panic disorder (Blanchard et al., 2011). Typically, escape responses consist of high accelerations, often accompanied by a change in direction, aimed at displacing the prey away from the threat. The arenas in these studies were designed to minimize flying and encourage walking, and short hops or limited bursts of flying were considered escape behavior.
Altered locomotor activity is a hallmark of several affective disorders (Lawrence et al., 1992); patients with unipolar depression display hypoactivity, and those with bipolar disorder are generally hyperactive (Teicher, 1995). Immobility in an open field arena has been used to model anxiety disorders (Brandeo et al., 2008; Katz et al., 1980), and alterations in motor activity are an indicator of outcome after therapeutic treatment (Bennabi et al., 2013). However, the variety in methodology and populations in these studies have confounded direct comparisons of differences between males and females, and between adolescent and adults (Kulesskaya & Voikar, 2014).
The light-dark paradigm was originally developed to assess the anxiolytic effects of benzodiazepines (Crawley & Goodwin, 1980), and tested with only male rodents (Bourin & Hascoet, 2003). Postpartum and diestrous virgin females differ in their responses dependent upon light intensity and on the strain of rat (Miller et al., 2011). Both the open field arena and Light-Dark Box assays assess innate behaviors not correlated with learning a task or associated with a punishment; these paradigms induce both fear/avoidance as well as exploratory/approach behaviors (Montgomery, 1955). Like humans, and unlike rodents, Drosophila are positively phototactic and more active during diurnal periods. In our Light-Dark Box assay, all populations preferred the dark side of the arena, suggesting that the novelty of the environment and the innate desire to escape predation present conflicting paradigms in Drosophila as they do in rodents, and the extent of the conflict is similarly affected by the hormonal status of the brain (as determined by sex, level of sexual maturity, and presumably, reproductive status).
We adapted the mouse Forced Swim Test assay for use in Drosophila, since it requires only a single observation period (see Castagne et al., 2011). Immobility may represent an adaptive attempt to conserve energy (Holmes, 2003); it may also reflect fatigue. However, while paraquat treatment increased bout duration in mature flies, it had almost no effect on transition number in the Light-Dark Box, and it increased the mean velocity in the open field arena, evidence that general physiology was not compromised at this dosage. Similarly, social isolation decreased bout number and increased latency to the first bout of immobility in the FST, yet significantly depressed transitions in the Light-Dark Box. Therefore, we interpret the active motions of the fly in the FST chamber as representing escape behavior.
We also assayed time until sedation and recovery from sedation after exposure to ethanol, since alterations in these parameters could more clearly be designated "adaptive" or "maladaptive." In rodents and flies, sufficient exposure to ethanol results in a transient increase in motor activity, followed by a depression in activity and sedation. Drosophila respond comparably to humans when exposed to ethanol (Bainton et al., 2000; Guarnieri & Heberlein, 2003). Studies in rodents have demonstrated that neural pathways mediating the stress response also control the transition to alcohol abuse and dependence, and acute exposure to alcohol has been shown to stimulate the hypothalamic pituitary adrenal axis (Lu & Richardson, 2014). Devineni & Heberlein (2012) reported that male Drosophila displayed an increased resistance to ethanol sedation, and a faster recovery; males in our study displayed a significant decrease in the time to sedation relative to mature females. However, their studies used a mixed, largely non-virgin population (males and females, 3 −5 days old) from a different strain, underscoring the effects that reproductive status and genotype may have on behavioral responses (e.g., Neckameyer & Weinstein, 2005).
Under control conditions, both immature male and female populations became sedated within 10 min after exposure to ethanol; mature flies displayed increased sensitivity, (5.8 versus 7.2 min until sedation), and both age and sex effects were observed (Tables 1 and 2). This is consistent with what has been observed in rats (Acevedo et al., 2013), and opposite to that observed for the FST, where immature adults displayed increased periods of immobility, which can be interpreted as a greater sensitivity to this assay.
Most importantly, the validity of the four paradigms does not lie in their modeling of "depressive-like" behaviors, since these assays do not serve this function even in rodent models, but in their sensitivity to differences in the hormonal and homeostatic state of the brain in individual animals, which would be reflective of differences in neural circuitry and signaling.
Sexual and temporal dimorphism
Only mature adults displayed sexually dimorphic responses in multiple quantitative parameters from each behavioral paradigm. Like most animals, mature Drosophila have evolved sexually divergent roles: the male seeks receptive females to inseminate, whereas females choose their mate and identify an appropriate place to lay their eggs. This is consistent with our observations that mature females displayed greater motor activity and a heightened level of escape behavior (measured in both the open field arena and the FST), and mature males engaged in greater exploratory activity in a novel environment (as measured in the Light-Dark Box and by an increased mean turn angle in the open field arena). Since sexually immature animals do not attempt copulation, their behavioral responses are more likely aimed at survival (e.g., food seeking), and would be similar in both sexes. This would also explain the increase in exploratory behavior in the light-dark box for mature males compared with immature males. Escape behavior (as measured in the FST) was not affected by either sex or level of sexual maturity, which is not unexpected, since all populations are expected to have a natural inclination for survival.
The underlying etiology for sex differences in affective disorders remains unknown. Obviously, the hormonal milieu of the brain changes over time in both sexes; this is true of humans, rodents and flies (see Argue & Neckameyer, 2013a, b; Argue & Neckameyer, 2014; Argue et al., 2014), and elucidating the roles of gonadotropic hormones in the different populations is a critical step in understanding how they influence the stress response, adaptive behavior, and ultimately, the development of affective disorders.
Adaptive versus maladaptive and predictive outcomes after stress
It is difficult to interpret whether a more complicated locomotor pattern (greater turn angle, greater distance moved) is more "adaptive" in an open field arena than a simpler pattern. Only starvation impacted the behavior of immature animals in this assay, and resulted in increased velocity and distance moved, decreased pattern complexity, and decreased freezing behavior. This may reflect an adaptive response in still-maturing populations to seek food sources after starvation. Sexually mature flies displayed greater velocity and distance traveled but a simpler trajectory than immature flies, and this effect was stronger in mature males relative to mature females, and may reflect the necessity for sexually mature males to seek out and inseminate conspecific females. Sleep deprivation had no effect on this behavior, while starvation and oxidative stress enhanced it. Starvation, oxidative stress, and sleep deprivation all increased male escape behavior, and starvation and oxidative stress decreased the duration and extent of freezing behavior, suggesting that these stressors may have elicited an adaptive response in this population. Conversely, social isolation had the opposite effect on these parameters, and thus may have negatively impacted male "seeking" behavior. Social isolation (and, in direct contrast to males, oxidative stress) elicited a similar response in sexually mature females (decreasing pattern complexity and escape behavior, and increasing freezing behavior).
Adaptive outcome in the Light-Dark Box is also somewhat difficult to interpret. The time spent in light and number of transitions were largely resistant to stress effects; only social isolation elicited a strong response, causing both immature and mature females, but not males, to increase the time spent in the dark area of the box. Since even extended periods of light deprivation do not affect visual acuity in Drosophila, abnormal visual physiology is unlikely to be the cause of this behavior. Social isolation strongly decreased the number of transitions for all populations, suggesting that this stressor negatively impacted exploratory behavior.
Mature animals displayed a greater latency to the first bout of immobility, and a reduction in the number and lengths of immobile bouts in the FST compared to immature flies. In contrast to analyses of locomotor patterns in an open field arena, social isolation elicited an adaptive response, since it increased latency to immobility and decreased bout number in sexually mature flies. Conversely, oxidative stress increased bout duration in mature males and females, and bout number in mature males. It had no impact on immature females, and enhanced the performance of immature males in this assay. Social isolation, which decreased velocity and distance moved in mature males, resulted in an enhanced performance in the FST. Exposure to paraquat increased velocity and distance moved in sexually mature animals, an indication of general motor activity, yet significantly impaired their activity in the FST. These data strongly suggest that a given stressor may negatively impact what is considered adaptive behavior in one paradigm, and positively impact in another paradigm, demonstrating that behavioral outcomes in one are not predictive for the other.
Generally, exposure to stress increased recovery time for all populations after ethanol sedation. Mature females seemed to display greater adaptive responses in this assay, since sleep deprivation and social isolation increased resistance to the sedative effects of ethanol in this population. The differential responses to stress in the ethanol assay strongly suggest that sedation and recovery from sedation are distinct behavioral pathways, which may arise from differences in the neural circuitry that mediate these behaviors.
Conclusions
Both the onset of sexual maturity and the sex of the individual have profound influences on behavioral outcomes, regardless of the assay. This is true not only under control conditions, but in response to a diverse array of stressors. Moreover, positive outcomes in one paradigm in response to one stressor are not predictive for outcomes in other paradigms, suggesting that response to the same stress may be both maladaptive and adaptive, depending upon the assay and the population. Even in age-matched populations reared and maintained under the same conditions, there was appreciable individual variability. Therefore, to elucidate key factors critical for adaptive maintenance of neuronal homeostasis in response to environmental disruptions, models must be developed that can be controlled for genetics and environment, and which provide sufficient numbers to account for high individual variability.
The open field arena and the Light-Dark Box were established as models to test anxiolytic behavior in rodents, and the FST was developed as a behavioral screen to assess the activity of antidepressant therapeutics in decreasing the periods of immobility. While the validity of these assays has been challenged, they are still a reliable index of general exploratory or escape behavior in rodents and in Drosophila, and serve as indicators of centrally mediated behavioral changes in response to a novel environment or challenge. Our results establish that immature and mature female and male Drosophila represent behaviorally distinct populations, evidence that the neural substrates mediating the perception of, and response to, a stress must be differentially expressed depending upon the hormonal status of the brain. The extensive genetic and transgenic toolbox of Drosophila allows for unbiased genetic screens to identify novel factors that, due to pleiotropy or lethality, would otherwise not be uncovered in mammalian models. These, in conjunction with extensive studies demonstrating strong conservation of neuronal developmental and signaling pathways, a genome with limited redundancy, and the feasibility of generating large numbers of genetically identical populations maintained under the same environment, make this model system an appealing and useful addition to current mammalian models of neuronal homeostasis.
Supplementary Material
Acknowledgments
We would like to acknowledge the technical assistance of Kelly Hainz who assisted in the ethanol sedation and recovery assays. This paper is dedicated to the memory of Gertrude Neckameyer.
This work was supported by National Institute of Mental Health R01 MH083771.
Footnotes
Declaration of Interest
The authors declare no completing interests.
References
- Acevedo M, Pautassi R, Spear N, Spear L. Age-dependent effects of stress on ethanol-induced motor activity in rats. Psychopharmacol. 2013;230:389–398. doi: 10.1007/s00213-013-3163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemuss M, Sarvaiya N, Epperson N. Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology. 2014;35:320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue K, Neckameyer W. Temporally dimorphic recruitment of dopamine neurons into stress response circuitry in Drosophila . Behav Neurosci. 2013a;127:725–733. doi: 10.1037/a0033602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue K, Neckameyer W. Sexually dimorphic recruitment of dopamine neurons into the stress response circuitry. Behav Neurosci. 2013b;127:734–743. doi: 10.1037/a0033807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue K, Neckameyer W. Altering the sex determination pathway in Drosophila fat body modifies sex-specific stress responses. Am J Physiol Regul Integr Comp Physiol. 2014;307:R82–R92. doi: 10.1152/ajpregu.00003.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton R, Tsai L, Singh C, Moore M, Neckameyer W, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila . Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Bangasser D, Valentino R. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus C. Genetic Mouse Models of Depression. Curr Topics Behav Neurosci. 2013;14:55–78. doi: 10.1007/7854_2012_224. [DOI] [PubMed] [Google Scholar]
- Bennabi D, Vandel P, Papaxanthis C, Pozzo T, Haffen E. Psychomotor retardation in depression: A systematic review of diagnostic, pathophysiologic, and therapeutic implications. BioMed Research International. 2013:158746. doi: 10.1155/2013/158746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D, Griebel G, Pobbe R, Blanchard R. Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev. 2011;35:991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Bogdanova O, Kanekar S, D’Anci K, Renshaw P. Factors influencing behavior in the forced swim test. Physiology & behavior. 2013;118:227–239. doi: 10.1016/j.physbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Brandeo M, Zanoveli J, Ruiz-Martinez R, Oliveira L, Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behav Brain Res. 2008;188:1–13. doi: 10.1016/j.bbr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Brenes J, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav Brain Res. 2009;197:125–137. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni, Racagni G, Riva M. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;345:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Carter C. EIF2B and oligodendrocyte survival: where nature and nuture meet in bipolar disorder and schizophrenia? Schizophrenia Bull. 2007;33:1343–1353. doi: 10.1093/schbul/sbm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne V, Moser P, Roux S, Porsolt R. Rodent Models of Depression: Forced Swim and Tail Suspension Behavioral Despair Tests in Rats and Mice. Curr Protoc Neurosci. 2011;55:8.10A.1–8:10A.14. doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- Chen Q, Niu Y, Zhang R, Guo H, Gao Y, Li Y, Liu R. The toxic influence of paraquat on hippocampus of mice: involvement of oxidative stress. Neuro Toxicol. 2010;31:310–316. doi: 10.1016/j.neuro.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Cohen M, Tottenham N, Casey B. Translational developmental studies of stress on brain and behavior: implications for adolescent mental health and illness? Neurosi. 2013;249:53–62. doi: 10.1016/j.neuroscience.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opin Ther Targets. 2011;15:379–400. doi: 10.1517/14728222.2011.553603. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin F. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Devineni A, Heberlein U. Acute ethanol responses in Drosophila are sexually dimorphic. Proc Natl Acad Sci USA. 2012;109:21087–21092. doi: 10.1073/pnas.1218850110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A. Tests of unconditioned anxiety — Pitfalls and disappointments. Physiology and behavior. 2013;135:55–71. doi: 10.1016/j.physbeh.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Fiedler J, Herrera L, Handa R. Sex, Stress, and Mood Disorders: At the Intersection of Adrenal and Gonadal Hormones. Horm Metab Res. 2012;44:607–618. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone K, Porkess M. Behavioral and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32(32):1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Aston-Jones G. Light deprivation damages monoamine neurons and produces a behavioral phenotype in rats. Proc Natl Acad Sci USA. 2006;105:4898–4903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri D, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Handa R, Weiser M. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Frontiers Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder E. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2011;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hirsch H, Potter D, Zawierucha D, Choudhri T, Glasser A, Murphey R, Byers D. Rearing in darkness changes visually-guided choice behavior in Drosophila . Vis Neurosci. 1990;5:281–289. doi: 10.1017/s0952523800000353. [DOI] [PubMed] [Google Scholar]
- Hirth F, Reichert H. Conserved genetic programs in insect and mammalian brain development. Bioessays. 1999;21:677–684. doi: 10.1002/(SICI)1521-1878(199908)21:8<677::AID-BIES7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Holmes P. Rodent models of depression: reexamining validity without anthropomorphic influence. Crit Rev Neurobiol. 2003;15:143–174. doi: 10.1615/critrevneurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- Iliadi K. The Genetic Basis of Emotional Behavior: Has the Time Come for a Drosophila Model? J Neurogenet. 2009;23:136–146. doi: 10.1080/01677060802471650. [DOI] [PubMed] [Google Scholar]
- Karatsoreos I, McEwen B. Psychobiological allostasis: resistance, resilience and vulnerability. Trends in Cog Sci. 2011;15:576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Katz R, Roth K, Carroll B. Implications for a model of depression. Neurosci Biobehav Rev. 1980;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Kroll J. New directions in the conceptualization of psychotic disorders. Curr Opin Psych. 2007;20:573–577. doi: 10.1097/YCO.0b013e3282f08759. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N, Voikar V. Assessment of mouse anxiety-like behavior in the light-dark box andopen-field arena: Role of equipment and procedure. Physiology and Behavior. 2014;133:30–38. doi: 10.1016/j.physbeh.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Lau J. Developmental Aspects of Mood Disorders. Curr Topics Behav Neurosci. 2013;14:15–27. doi: 10.1007/7854_2012_214. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Teicher M, Finklestein S. Quantitative assessment of locomotor activity in psychiatry and neurology. Boston: Blackwell Scientific; c1992. pp. 449–462. [Google Scholar]
- Liu L, Davis R, Roman G. Exploratory activity in Drosophila requires the kurtz non visual arrestin. Genetics. 2007;175:1197–1212. doi: 10.1534/genetics.106.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Alloy L. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clinical Psychology Review. 2010;30:582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Richardson H. Alcohol, stress hormones, and the prefrontal cortex: A proposed pathway to the dark side of addiction. Neurosci. 2014;277:139–151. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E, Burgess N, O’Keefe J. Human spatial navigation: cognitive maps, sexual dimorphism, and neural substrates. Curr Opin Neurobiol. 1999;9:171–177. doi: 10.1016/s0959-4388(99)80023-3. [DOI] [PubMed] [Google Scholar]
- Martin J. A portrait of locomotor behavior in Drosophila determined by a video-tracking paradigm. Behav Proc. 2004;67:207–219. doi: 10.1016/j.beproc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Martin E, Ressler K, Binder E, Nemeroff C. The neurobiology of anxiety disorders: brain imaging, genetics and psychoneuroendocrininology. Psychiatr Clin N Am. 2009;32:549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. Organization of psychoendocrine mechanisms. Psychosom Med. 1967;5:565–791. [Google Scholar]
- McEwen B. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen B. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism Clinical and Experimental. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Mistlberger R, Jacobs B, Heller H, McGinty D. New neurons in the adult brain: The role of sleep and the consequences of sleep loss. Sleep Med Rev. 2009;13:187–194. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Piasecki C, Lonstein J. Use of the light-dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol Biochem Behav. 2011;100:130–137. doi: 10.1016/j.pbb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Jackson M. Effect of stress on prefrontal cortex function. Neurotox Res. 2004;6:73–78. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254–60. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Kim H. Involvement of genetic and environmental factors in the onset of depression. Exp Neurobiol. 2013;22:235–243. doi: 10.5607/en.2013.22.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W, Weinstein J. Stress affects dopaminergic signaling pathways in Drosophila melanogaster . Stress. 2005;8:117–132. doi: 10.1080/10253890500147381. [DOI] [PubMed] [Google Scholar]
- Neckameyer W, Matsuo H. Distinct neural circuits reflect sex, sexual maturity, and reproductive status in response to stress in Drosophila melanogaster. Neuroscience. 2008;156:841–856. doi: 10.1016/j.neuroscience.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Nestler E, Barrot M, DiLeone R, Eisch A, Gold S, Monteggia L. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nestler E, Hyman S. Animal Models of Neuropsychiatric Disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kane C. Drosophila as a model for the study of neuropsychiatric disorders. Curr Top Behav Neurosci. 2011;7:37–60. doi: 10.1007/7854_2010_110. [DOI] [PubMed] [Google Scholar]
- Porsolt R, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton J, Ryan M, Huffaker S, Huang J, Griffin J, Wayland M, Freeman T, Dudbrudge F, Lilley K, Karp N, Hester S, Tkachev D, Mimmack M, Yolken R, Webster M, Torrey E, Bahn S. Mitochondrial dysfunction in scizophrenia: evidence for compromised brain metabolism and oxidative stress. Molec Psych. 2004;9:684–697. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Rion S, Rawecki J. Evolutionary biology of starvation resistance: what we have learned from Drosophila . J Evol Biol. 2007;20:1655–1664. doi: 10.1111/j.1420-9101.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- Schmidt C. A deeper look into mental illness. Envt Health Persp. 2007;8:A404–A410. doi: 10.1289/ehp.115-a404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklo-Coxe M, Young T, Finn L, Mignot E. Depression: relationships to sleep paralysis and other sleep disturbances in a community sample. J Sleep Res. 2007;16:297–312. doi: 10.1111/j.1365-2869.2007.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Techier M. Actigraphy and motion analysis: new tools for psychiatry. Harvard Review of Psychiatry. 1995;3:18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


