Abstract
Flow-induced K secretion (FIKS) in the aldosterone-sensitive distal nephron (ASDN) is mediated by large-conductance, Ca2+/stretch-activated BK channels composed of pore-forming α-subunits (BKα) and accessory β-subunits. This channel also plays a critical role in the renal adaptation to dietary K loading. Within the ASDN, the cortical collecting duct (CCD) is a major site for the final renal regulation of K homeostasis. Principal cells in the ASDN possess a single apical cilium whereas the surfaces of adjacent intercalated cells, devoid of cilia, are decorated with abundant microvilli and microplicae. Increases in tubular (urinary) flow rate, induced by volume expansion, diuretics, or a high K diet, subject CCD cells to hydrodynamic forces (fluid shear stress, circumferential stretch, and drag/torque on apical cilia and presumably microvilli/microplicae) that are transduced into increases in principal (PC) and intercalated (IC) cell cytoplasmic Ca2+ concentration that activate apical voltage-, stretch- and Ca2+-activated BK channels, which mediate FIKS. This review summarizes studies by ourselves and others that have led to the evolving picture that the BK channel is localized in a macromolecular complex at the apical membrane, composed of mechanosensitive apical Ca2+ channels and a variety of kinases/phosphatases as well as other signaling molecules anchored to the cytoskeleton, and that an increase in tubular fluid flow rate leads to IC- and PC-specific responses determined, in large part, by the cell-specific composition of the BK channels.
Keywords: kidney, mechanoregulation, potassium transport, WNK kinases, cilia
the aldosterone-sensitive distal nephron (ASDN), which is composed of the distal convoluted tubule (DCT), connecting tubule (CNT), and cortical collecting duct (CCD) (9, 95, 115, 122, 133), plays a critical role in the final renal regulation of electrolyte and water homeostasis. Increases in urinary flow rate in the ASDN, induced by volume expansion, administration of diuretics or a high K (HK) diet, subject epithelial cells therein to 1) fluid shear stress (FSS); 2) circumferential stretch acting parallel and perpendicular, respectively, to the tubular wall; and 3) drag/torque on apical cilia of principal cells and microvilli/microplicae of intercalated cells (28, 40, 54, 229). Cumulative evidence from studies performed in isolated CCDs microperfused in vitro indicate that these hydrodynamic forces are transduced into increases in net transepithelial Na absorption through the epithelial Na channel (ENaC) (137, 178), and K secretion, the latter dependent on flow-induced increases in intracellular Ca2+ concentration ([Ca2+]i) that activate apical stretch-, voltage-, and Ca2+-activated K (referred to as BK) channels (110, 236, 237). BK channels are now considered to not only mediate flow-induced K secretion (FIKS), but also play a major role in K adaptation to dietary K loading (10, 110, 112, 113, 140, 157, 158, 168, 174, 236, 237).
K Secretion in the ASDN
Total body K content depends on the balance between intake and output, the latter regulated primarily by K secretion into the urinary (tubular) fluid in the ASDN (49, 62, 64, 65, 86, 122, 124, 173). In the adult, whose homeostatic goal is to maintain a state of zero K balance, ∼90% of the K ingested daily is excreted by the kidney, with the remaining ∼10% eliminated by the gut. K secretion in the ASDN requires 1) an apical permeability to K and 2) a favorable electrochemical gradient, which is determined by apical Na entry through ENaC and its electrogenic Na-K-ATPase-mediated basolateral extrusion in exchange for the uptake of K.
In animals fed a normal K diet, net K secretion in the microperfused CCD is completely dependent on ENaC-mediated Na absorption (137, 197, 198). However, in rats fed a HK diet for as little as 18 h, a fraction of distal K secretion appears to occur via an amiloride-insensitive and thus ENaC-independent pathway (59). Indeed, basolateral Na uptake via the Na/H exchanger can sustain basolateral pump activity in principal cells and net K secretion in the isolated perfused rabbit CCD in the absence of significant Na absorption (139). Note that the paracellular permeability to Na and K is low in this segment under physiologic conditions (147).
In the fully differentiated ASDN, high tubular flow rates stimulate K secretion and urinary K excretion (49, 65, 91, 123, 173). This response is due, at least in part, to increased delivery to and reabsorption of Na via ENaC (91, 123, 137, 178), which in turn increases the driving force for passive K efflux across the apical membrane. In addition, multiple lines of evidence suggest that ENaC is a mechanoregulated channel that is directly activated by increases in FSS (4, 137, 178), which would help maintain the electrochemical driving force for K secretion under high flow states.
K secretory channels.
The high-conductance BK channel (also known as maxi-K, Slo1, or KCa1.1) was the first ion channel described, in 1984, at the single-channel level in the kidney by the patch-clamp technique (85). The pore-forming α-subunit of this channel was originally cloned from the slowpoke locus of Drosophila (slo) and is encoded by the KCNMA1 gene in humans (1, 6, 25, 213). The BK channel is activated by membrane depolarization, elevation of [Ca2+]i, hypoosmotic stress, and/or membrane stretch (57, 58, 79, 85, 90, 101, 105, 150, 153, 177, 181, 199–201, 208, 209). However, its vanishingly low open probability (Po) at the resting membrane potential and [Ca2+]i in the ASDN (57, 58, 60, 150, 177, 181, 222) called into question its physiologic relevance to urinary K secretion, and it thus received little subsequent attention over the subsequent 15 years. In contrast, the high prevalence and Po of the apical ATP-sensitive small K conductance (SK) channel, first characterized in 1989, made this the likely candidate for the primary K secretory channel in the ASDN (57, 60, 66, 176, 222, 245). The SK channel is encoded by ROMK (Kir1.1) (20, 80, 257) and, like the BK channel, has been detected in both rat and rabbit CNT/CCD.
After its initial identification, the SK/ROMK channel, considered to be the primary K secretory channel in the ASDN, was the focus of numerous studies aimed at examining its role in the renal regulation of K excretion. However, cumulative evidence from studies of ontogeny and disease over the past two decades suggested that other K channels contribute to K secretion under conditions where SK/ROMK channel-mediated K secretion was limited. The first evidence that at least two K channels mediate physiologically relevant K secretion was from studies performed to examine the molecular basis for the observations that, in contrast to the adult, growing infants and children 1) must maintain a state of positive K balance, increasing their total body K from ∼8 meq/cm body height at birth to >14 meq/cm body height by 18 years of age (26, 56), and 2) have a limited capacity for urinary K excretion (44, 205). Indeed, the notion that the growing subject is a “sink” for K was elegantly demonstrated in a study in which plasma K concentrations were compared in newborn piglets administered K loads (25 meq·kg−1·day−1) in either water or milk for the first 40 h of life. Whereas K-loaded animals provided water alone lost weight and experienced life-threatening hyperkalemia and paralysis, piglets fed the equivalent amount of K in milk grew well and remained normokalemic (127).
To address the question as to how the newborn retains K, as is necessary for growth, CCDs isolated from maturing rabbits were microperfused in vitro and the rates of net transepithelial K secretion were measured at slow (∼1) and fast (∼5 nl·min−1·mm−1) luminal flow rates. Net K secretion was absent at 2 wk of age, but the rate of K secretion in CCDs from weanling (4-wk-old) rabbits, perfused at slow flow rates, was ∼75% of that measured in adult (>6-wk-old) animals perfused at slow flow rates (173) (Fig. 1). Yet, FIKS, robust in adult animals, was absent in weanling tubules (173). Thus, there appeared to be a temporal dissociation between basal K secretion and FIKS. The absence of K secretion early in life was not due to a limited capacity of the CCD for Na absorption (identical to that in adult rabbits at all flow rates; Fig. 1) or basolateral pump activity (38, 173, 177), but instead, was due to the absence of an apical membrane permeability to K (176). Subsequent studies in maturing rabbits revealed that the postnatal appearance of conducting SK/ROMK channels and apical immunodetectable BKα subunit protein in the rabbit CCD coincided with the appearance of basal K transport at 3 wk (before weaning) and FIKS at 5 wk (postweaning) postnatal age, respectively (173, 237).
Fig. 1.
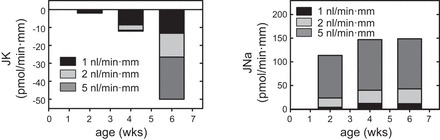
Flow stimulation of net K secretion (left) and Na absorption (right) in the maturing rabbit CCD. Net transport was measured at slow (∼1 nl·min−1·mm−1), moderate (∼2 nl·min−1·mm−1), and fast (∼5 nl·min−1·mm−1) flow rates in tubules isolated from 2-, 4-, and 6-wk-old rabbits (n = 5–6 per age group) and microperfused in vitro. Net K secretion was absent at 2 wk of age and could not be stimulated by an increase in tubular fluid flow rate. Although a 5-fold increase in flow rate stimulated net Na absorption at 4 wk to levels comparable to those observed at 6 wk of age, no flow-induced increase in net K secretion (FIKS) was detected at 1 mo of life in response to an increase in flow rate to 5 nl·min−1·mm−1. Weaning occurs in the rabbit by 4 wk of age. FIKS was clearly evident by 6 wk of age. [Adapted from Satlin (173) and Woda et al. (237).]
The second observation that questioned the primacy of the SK/ROMK channel as the K secretory channel in the CCD was that infants with antenatal Bartter syndrome due to loss-of-function mutations in ROMK (type II Bartter syndrome) are hyperkalemic soon after birth, but develop modest hypokalemia beyond the neonatal period (53, 170, 190). Hyperkalemia is not persistent, as would be expected in the absence of the presumed primary K secretory channel. An initial clue to the identity of this “other” channel was provided by the finding that the kaliuresis observed in adult ROMK knockout mice (116, 117) was sensitive to iberiotoxin (IbTX) (10), a selective blocker of the BK channel whose receptor resides in the pore of the α-subunit (10, 27, 61, 208); IbTX does not inhibit SK/ROMK (10) or Ca2+-activated SK3 channels (18). This observation provided compelling evidence that the high distal flow rates characteristic of this murine model of Bartter syndrome, due to an impaired urinary concentrating ability, activate BK channels and thus urinary K excretion.
The repertoire of K secretory channels identified in the ASDN has continued to expand over the past decade, although there appears to be significant species- and cell-specificity in their functional expression. Most recently, a small-conductance voltage-insensitive K channel, SK3 (KCa2.3), activated by a TRPV4-mediated increase in [Ca2+]i, and inhibited by apamin (136), has been functionally identified in mouse collecting duct where it localizes to the luminal border of both principal and intercalated cells (18). Yet another apical K channel, Kv1.3, present in rat intercalated cells, can be recruited to secrete K into the tubular fluid in response to dietary K loading (29).
Molecular biology of the BK channel.
Functional mammalian BK channels are composed of four pore-forming α-subunits, alone or assembled together with regulatory β-subunits (6, 47, 94, 118, 131) that modulate the Ca2+ affinity, voltage sensitivity, and pharmacology of the channel as well as its association with interacting proteins (25, 76, 130, 213, 221). Whereas both mouse and human slo homologues generate BK channels when expressed in Xenopus laevis oocytes, i.e., they are generally sensitive to voltage and Ca2+ and have large single-channel conductances (25, 46, 128, 151, 172, 213), the β-subunit does not carry current when expressed alone. Ca2+ binding is essential for physiological BK channel activity as Ca2+ shifts the depolarization required for channel opening to allow activation to occur within the physiological range of membrane potentials.
Alternative splicing of slo in the COOH terminus generates variants that differ in their responses to changes in Ca2+ and voltage, regulation by protein phosphorylation, palmitoylation, and other signaling cascades (31, 172, 186, 187, 211, 213, 243, 247, 255, 258), as well as trafficking and cell localization (92, 98, 120, 221, 251). Among the five variants of the mouse BKα subunit COOH terminus that have been identified, three are expressed at significant levels in kidney (% of total renal BK channel mRNA levels) (31): ZERO, resulting from splicing of exon 19 to 23 (75%); e21, resulting in insertion of a 59-amino acid cysteine-rich stress-axis regulated exon (STREX) between exons 19 and 23 (10%); and Δe23, resulting from the skipping of exon 23, thereby splicing exon 19 to 24, which leads to a frameshift that introduces a premature stop codon within exon 24 (5%). The STREX variant demonstrates a left shift in the Ca2+ sensitivity of the channel compared with the ZERO variant and slower rates of deactivation (31, 172). Δe23 is not functionally expressed at the cell surface and acts as a dominant negative in terms of cell surface expression by trapping other BK channel splice variant α-subunits in the endoplasmic reticulum and perinuclear compartments (31, 98).
Four BK channel β-subunits have been identified at the mRNA and/or protein level in mammalian kidney and appear to be differentially expressed along the nephron (67, 140, 156, 214, 228). The observation, from studies in heterologous systems, that coexpression of β1 with BKα increases the Ca2+ sensitivity and charybdotoxin binding affinity of the channel (46, 130) suggests that this is the logical subunit to comprise the CCD BK channel, which exhibits robust IbTX-sensitive FIKS. However, the BKβ1-subunit (as well as the β2-subunit; see below) contains an endocytic sorting signal that promotes endocytosis of BKα and thus a reduction in surface expression of the channel (212, 252). Clearance studies in mice with targeted deletion of BKβ1 reveal attenuated FIKS (157, 158). BKβ1 KO mice are also hypertensive due to hyperaldosteronism secondary to adrenal hypersensitivity to the elevated plasma K concentration that accompanies the reduction in renal BKα/β1-mediated K secretion (67, 68). While these data provide solid support for a role of the BKα/β1 channel in mediating FIKS (157, 158), immunodetectable β1 is not expressed in the rabbit midcortical CCD (50, 140), a segment that consistently exhibits robust IbTX-sensitive FIKS (110, 112, 236), However, BKα/β1 has been identified in rabbit in a cortical segment that is most likely initial CCD (156–158). Immunodetectable BKα localizes to the apical membrane of mouse CNT cells along with β1 (156, 158), mouse CCD intercalated cells without β1 (67, 68, 158), and is also found at the apical surface of intercalated cells in rabbit CCD (50, 140).
Coexpression of β2 (214) or β3 (24, 214, 242) subunits with the BKα-subunit generally results in complete or partial, respectively, inactivation of channel currents. The inhibitory nature of the β2- and β3-subunits suggests they are unlikely candidate subunits for the BK conductance in the native mammalian CCD. Subunit β4 increases the sensitivity for voltage at Ca2+ concentrations of greater than 1.5 μM, alters the gating behavior of the expressed channels in a Ca2+-dependent manner and, if glycosylated, dramatically reduces IbTX association rates, thereby rendering the channel relatively insensitive to this toxin (24, 88, 132, 219). Immunodetectable BKα/β4 is found in the TALH, DCT, and intercalated cells (but not CNT/principal cells) of the mouse distal CNT and CCD (67). Mice lacking BKβ4 have no detectable phenotype under baseline conditions when fed a normal diet (68).
The composition of the native channel in the CCD, whether composed of an α-subunit alone, an α-subunit associated with a β1-subunit, a nonglycosylated (IbTX-sensitive) β4-subunit, or an as yet undescribed β-subunit, remains to be clarified. Recently, a second family of BK channel auxiliary subunits has been identified. These leucine-rich repeat containing subunits (LRRCs), also referred to as γ-subunits, allow BK channels to open at near-physiological Ca2+ concentration and membrane voltage in nonexcitable cells (246, 254). It is as yet uncertain as to whether this subunit is expressed in the ASDN.
The BK channel may associate with other auxiliary proteins in a macromolecular complex. Both Slob (Slowpoke-binding protein) and Slip1 (Slo-interacting protein 1) interact with the cytoplasmic tail domain of Slo to regulate channel activity by directing the localization and distribution of channels within cells (182, 241). Activity of the BK channel is also modulated (reduced) by the 14-3-3 protein, acting through Slob and signaling proteins (192, 259).
Genetic ablation studies in mice have more clearly defined the roles of the BK vs. SK/ROMK channels in K homeostasis. Targeted deletion of BKα (168), β1 (156–158), or β4 (82) subunits leads to marked attenuation of the kaliuresis induced by high-flow conditions in vivo, including, respectively, pharmacologic V2 receptor blockade, volume expansion, or HK diet. These observations provide solid support for the notion that the SK/ROMK channel mediates constitutive K secretion in animals ingesting a normal K diet whereas the BK channel mediates FIKS (110, 112, 113, 236). The BK channel also plays a critical role in the adaptation to a dietary K load (105, 140) and maintenance of circulating volume and blood pressure (BP) by regulating Na and fluid homeostasis (68, 69, 157) Mice with genetic ablation of BKα present primarily with a neurological phenotype but also exhibit hypertension, proposed to be secondary to BK channel deficiency in vascular smooth muscle (180).
Cell specificity of K channel expression.
Principal cells, the majority cell type in the ASDN, mediate Na absorption via ENaC and K secretion via the SK/ROMK channel. These cells possess a single apical cilium that projects into the lumen (51, 225). This organelle bends in response to fluid shear (183) and transduces mechanical perturbation into increases in [Ca2+]i (160–162). Intercalated cells secrete H+ via an apical vacuolar H+-ATPase (α-cells) or HCO3− via apical pendrin (β-cells) but can also, under certain conditions, reabsorb K via an apical H-K-ATPase (37, 119, 175, 184, 189). Non-A non-B intercalated cells possess both apical H+-ATPase and pendrin (48, 93). β-Intercalated cells can also absorb NaCl via a thiazide-sensitive apical Na-dependent Cl/HCO3 exchanger (NDCBE) that operates in tandem with pendrin (103). Intercalated cells lack a central cilium, at least in rabbit, but are decorated with a plethora of apical microvilli and microplicae (51). Whereas ENaC and SK/ROMK channels are restricted to principal cells in rat and rabbit ASDN, BK channels are detected by patch-clamp analysis in both principal and intercalated cells in these species (105, 150, 153, 177). However, the incidence of conducting BK channels in intercalated cells exceeds that in principal cells (105, 150, 153). Emerging evidence further suggests that the activation profiles of BK channels are cell specific and may differ between species. For example, the intercalated cell BK channel in rabbit, but not rat, has been reported to be activated by stretch, independent of Ca2+, as evidenced by patch-clamp studies performed after chelation of free Ca2+ with EGTA in the pipette or the bath solutions (150).
The identity of the specific cell(s) in the ASDN responsible for FIKS remains uncertain. Principal cells possess robust basolateral Na-K-ATPase activity and are considered to mediate transepithelial Na absorption via ENaC and K secretion via ROMK, as discussed above (57, 60, 176, 222). However, the density of conducting and immunoreactive apical BK channels is low in these cells (50, 68, 105, 140, 150, 153, 158, 177, 215, 237). In fact, it is difficult to reconcile the relative absence of immunodetectable apical BKα in principal cells of rabbit (50, 140, 237) and mouse (67, 68, 158) CCD with the electrophysiologic evidence for conducting channels therein (105, 150). While we initially proposed that BKα splice variants in principal cells were not recognized by the chicken anti-BKα antibody we raised against an epitope at the extreme COOH terminus of the protein (237), similar results were reported using a mouse anti-Slo1 antibody (StressMarq) raised against a highly conserved upstream (a.a. 690–715) epitope (135). Using an in vitro immunoperfusion approach with 3D reconstructions of confocal images, we have recently localized immunodetectable BKα to the principal cell central cilium, a structure that has been difficult to detect by conventional analysis of kidney sections (unpublished observations).
In support of a role for principal cells in BK channel-mediated K secretion are the observations that BK channel activity in CCD principal cells increases in HK-fed rats (105) and in rabbits and rats fed a normal K diet, following inhibition of PKA (112) or p38/ERK (105). These data suggest that a pool of quiescent (constitutively suppressed) BK channels in principal cells can be activated and recruited to secrete K under high flow conditions or in response to dietary K. Furthermore, FIKS in mouse kidney (10) and rabbit CCD (33, 111, 112, 237) is inhibited by IbTX. Coassembly of BKα with β1-subunits, which have been identified in an intracellular compartment and at the apical membrane of principal cells in rabbit initial CCD (156, 158), generates a channel sensitive to 50 nM IbTX whereas the β4-subunit, present in intercalated cells (but not principal cells) of the mouse distal CNT and CCD (67), renders BKα-subunits resistant to low nanomolar concentrations of IbTX if it is glycosylated (132, 144). While mice lacking BKβ4 have no detectable phenotype under baseline conditions when fed a normal diet (82), BKβ1 KO mice subject to volume expansion exhibit an attenuated kaliuretic response (157, 158). These data are consistent with the notion that FIKS is mediated by a BKα or BKα/β1 channel.
Support for a role for intercalated cells in FIKS includes the findings that the density of immunodetectable apical BKα-subunits in rabbit (50, 140) and mouse (67, 68, 158), and functional (105, 150, 153, 177) BK channel activity are greater in intercalated than in principal cells. Furthermore, apical BKα-subunit expression in intercalated cells is increased in response to a HK diet (140). In addition, the apical membrane voltage of the intercalated cell is depolarized relative to that of the principal cell (14, 96, 153), predicting that BK channel Po would be high (due to the voltage sensitivity of Po), reducing the requirement for elevation of [Ca2+]i to open the channel (25, 153). Of interest are recent studies (185) that indicate that phosphorylation of the mineralocorticoid receptor (MR) at S843, which prevents ligand binding and activation of the MR, is found exclusively in intercalated cells in kidney. Hyperkalemia increases whereas angiotensin II (AII) via the AT1R and WNK4 (both increased in volume depletion) decrease MRS843-P levels. The observation that dephosphorylation of MRS843-P, which lies downstream of AII and WNK4 signaling, inhibits K secretion implicates selective MR inactivation in intercalated cells in the adaptation to hyperkalemia. Finally, WNK4 inhibits BKα functional and protein expression in heterologous expression systems (see below) (223, 250, 260).
Sustained luminal K secretion by intercalated cells would require a robust basolateral K uptake pathway, such as that provided by the Na-K-ATPase, to maintain a high steady-state [K]i. Immunocytochemical studies in rat kidney, performed using antibodies directed against the Na-K pump α-subunit and an antigen retrieval technique, reveal modest labeling, at best, of intercalated cells in the CCD (171). Yet, Na-K-ATPase pump activity (13) and ouabain-sensitive currents are not detected in these cells whereas pump activity is readily detected in principal cells in rat CCDs (153). Although basolateral Na-K-ATPase antigenicity has been identified in rabbit intercalated cells (167), electron microprobe analysis of intracellular electrolyte concentrations in individual cells in isolated perfused rabbit CCDs revealed a significantly smaller increase in [Na]i in intercalated vs. principal cells exposed to basolateral ouabain (179). In sum, these immunocytochemical and functional analyses suggest that intercalated cells may have insufficient Na-K-ATPase activity to sustain high rates of luminal K secretion. Evidence now suggests that, as in the colon, K can be taken up at the basolateral membrane of the CCD not only by the pump but also by the Na-K-2Cl cotransporter NKCC1 (19). In fact, we reported that BK channel-mediated FIKS is dependent on a basolateral bumetanide-sensitive Cl-dependent transport pathway, proposed to be NKCC1 based on its immunolocalization in both intercalated and principal cells in the CCD (111). The findings that 1) mice with genetic disruption of NKCC1 exhibit higher serum K concentrations with inappropriately low urinary K excretion compared with wild-type mice (134, 218) and 2), in human subjects, the 24 h kaliuresis that follows once-daily administration of furosemide is lower than following administration of an equinatriuretic dose of a thiazide diuretic (165, 166) are consistent with an important role of NKCC1 in distal K secretion. Still uncertain is how Na, taken up at the basolateral membrane by NKCC, is extruded back out of intercalated cells. An intriguing possibility, yet to be tested, is that Na is extruded at the basolateral membrane via anion exchanger 4-mediated Na-HCO3 cotransport; using cotransporter-specific antibodies, AE4 labeling has been identified along the basolateral membrane of mouse and rabbit β-ICs (30).
Regulation of BK Channel Activity By Cell Signaling Components
An increase in luminal flow rate in the microperfused rabbit CCD is transduced into a biphasic increase in [Ca2+]i composed of an initial rapid transient spike in [Ca2+]i to >300 nM, reflecting IP3-mediated release of Ca2+ from internal stores, followed by a plateau elevation (∼150 nM) sustained for at least 20 min of high flow due to luminal Ca2+ entry into cells, presumably through store-operated and/or TRP channels (110, 113). These flow-induced changes in [Ca2+]i are apparent in both principal and intercalated cells in the CCD. Luminal IbTX inhibits the plateau elevation of [Ca2+]i but not the initial [Ca2+]i spike (110, 113). [Ca2+]i exceeding 10 μM is generally required to activate BK channels in neurons at membrane potentials of 0 mV (24). However, elevations of [Ca2+]i to this magnitude are expected to be cytotoxic and thus are likely elicited only in “Ca2+-signaling domains,” macromolecular complexes in the plasma membrane that include Ca2+ channels, that allow for temporal and spatial restriction of the Ca2+ signal (7, 16, 143). In the rat distal nephron, elevation of [Ca2+]i to the 200–500 nM range is adequate to stimulate BK channels in intercalated cells studied at a zero membrane potential (153).
FIKS, a “read-out” of BK channel activity, is critically dependent on a flow-induced increase in [Ca2+]i in the rabbit CCD. Indeed, FIKS is absent in microperfused CCDs in which the cytosolic Ca2+ signal has been eliminated by removal of luminal Ca2+, chelation of [Ca2+]i with BAPTA, inhibition of IP3-stimulated Ca2+ release from the endoplasmic reticulum (ER) by 2-aminoethyl diphenyl borate, or thapsigargin-induced depletion of ER Ca2+, treatments that do not affect flow-stimulated Na absorption (137). Among the well-described cellular processes activated by an increase in [Ca2+]i is the stimulation of exocytosis in various secretory cells (21). Pretreatment of CCDs with colchicine to disrupt microtubule function or brefeldin-A to inhibit delivery of preformed channels from the intracellular pool to the plasma membrane inhibits FIKS (but not flow-stimulated Na absorption) (137), underscoring the critical importance of exocytosis in BK channel-mediated FIKS.
An unanswered question is how a transient increase in [Ca2+]i elicited by an acute increase in luminal flow rate leads to sustained FIKS. The persistent activation of channel-mediated ion currents in response to a transient stimulus has, in other systems, been attributed to posttranslational modifications such as phosphorylation or dephosphorylation, multimerization, and the activities of cyclases, esterases, and proteases (22). In fact, the COOH terminus of BKα contains multiple kinase and phosphatase motifs that associate with partners to regulate channel gating and signaling pathways. Among these effectors are cAMP-dependent PKA (104, 163, 188, 211, 258), PKC (11, 72, 73, 164, 188, 220, 240, 258), cGMP-dependent PKG (3, 11, 196, 258), and cSrc (2, 109). The BK leucine zipper region serves as an anchor for a PKA-associated regulatory complex (118).
We and others have begun to examine whether sustained activation of the BK channel in response to a transient stimulus is due to direct phosphorylation or dephosphorylation of the channel itself or an associated accessory or regulatory protein. The results of these studies, summarized below, indicate that the BK channel in principal cells of the CCD, in the absence of luminal flow or perfused at slow luminal flow rates, is tonically inhibited by both PKA (112) and MAPK (105). These observations suggest that hydrodynamic forces generated by increases in tubular fluid flow activate/inhibit these kinase-associated cell-specific signaling pathways that in turn influence apical BK channel function. In fact, numerous studies, primarily in endothelial cells, have shown that FSS and circumferential stretch regulate multiple signaling molecules that can initiate and propagate mechanical signals through a network of pathways (reviewed in refs. 35, 41, 230).
PKA and PKC.
The α-subunit of the reconstituted BK channel is phosphorylated by PKA and PKC (104). The BK channel in the native CCD is also regulated by these kinases. Specifically, luminal perfusion of CCDs with myristoylated PKI (mPKI), a peptide inhibitor of the free catalytic subunit of PKA, increased net K secretion in rabbit CCDs perfused at a slow flow rate (112). The sensitivity of this enhanced K flux to IbTX implicated the BK channel as a target for regulation by PKA and suggested that the apical channel is constitutively inhibited by this kinase. However, patch-clamp analysis of rat CCD indicated that mPKI activated (demonstrated by an increase in NPo) BK channels solely in principal cells (112). In fact, mPKI inhibited NPo of BK channels in intercalated cells. Luminal calphostin C, which binds to the diacylglycerol (DAG) binding site, or C1 domain, in PKC and other proteins, like mPKI, increased net IbTX-sensitive K secretion in CCDs perfused at a slow flow rate. However, the target of calphostin C inhibition appeared not to be PKC as luminal bisindolylmaleimide and Gö6976, inhibitors of classical Ca2+-dependent (PKC-α, PKC-β) and novel Ca2+-independent (PKC-δ and PKC-ε) PKC isoforms, failed to enhance net K secretion at the slow flow rate (112). To the extent that the calphostin C target has not yet been identified, the remainder of this discussion will focus on PKA. Flow stimulation of net Na absorption was detected in all CCDs treated with these inhibitors.
The transport and patch-clamp results summarized above led us to conclude that the apical BK channel in the principal cell is tonically inhibited by PKA under low flow conditions. The observation that inhibition of “apical” PKA and an increase in luminal flow rate lead to comparable increases in IbTX-sensitive net K secretion suggests that flow may reduce cAMP available to activate PKA, thereby releasing the BK channel or a closely associated regulatory protein from tonic inhibition in a signaling complex at the apical membrane, as has been described for the channel in smooth muscle and brain (reviewed in ref. 118). Of note is that rat cholangiocyte cilia respond to an increase in luminal flow with a TRPP1/2 channel complex-mediated increase in [Ca2+]i that activates ciliary adenylate cyclase 6, which in turn suppresses local and global cAMP signaling (126). This cascade, if triggered by flow in native CCDs, would be expected to release the BK channel from constitutive inhibition and uncover BK channel-mediated K secretion in ciliated principal cells, but not intercalated cells, devoid of cilia (112).
The variability in functional response of the BK channels to distinct kinases (and phosphatases) has been proposed to be due to alternative splicing of the α-subunit (99, 211, 258), association of α-subunits with distinct regulatory β-subunits (46, 130) and/or associated proteins (118, 182, 241), as well as through differential assembly of BK channels with protein kinase/phosphatase signaling complexes (118, 220). In fact, we have proposed that the cell specificity of the mPKI effect in the CCD may reflect the presence of unique BKα splice variants in principal cells that are inhibited (e.g., STREX, which introduces a new PKA consensus site into the channel sequence) vs. activated (e.g., ZERO, e20, e22) by PKA (31). PKA phosphorylation of the conserved COOH-terminal PKA consensus site (RQPSS899) in all four α-subunits is required for channel activation, whereas phosphorylation of only a single STREX insert at its PKA consensus motif inhibits the channel (118, 210, 211). The cell specificity of regulation by PKA may also reflect differential expression of BKβ-subunits as it is assumed that the β-subunits in principal (β1) and intercalated (β4) cells differ. In support of the latter possibility is the observation that endogenous PKA activates channel activity in HEK293 cells heterologously expressing the human BKα-subunit but decreases channel activity when the α- and β1-subunits are coexpressed (46). Note that the precise composition of BK channels in principal and intercalated cells has yet to be defined.
MAPK.
BK channel activity, measured as NPo by patch-clamp analysis, is constitutively inhibited by p38 and ERK MAPK in rat principal but not intercalated cells (105). Specifically, single-channel recordings of principal cells in split-open CCDs from rats fed a normal K diet showed that p38 blockade with the specific inhibitor SB202190 dramatically increased the number and Po of apical BK channels. PD098059, a drug that blocks ERK activation, had no significant effect alone but had an additive effect on channel activity when applied with the p38 inhibitor (105). The cell specificity of the MAPK response, as for the PKA effects, is proposed to be due to variability in BK channel composition between the two cell types. Phosphorylation of ERK and p38 is stimulated by WNK4, which inhibits BK channel activity (250). A HK intake suppresses the phosphorylation of p38 and ERK (105), which is expected to increase BK channel activity in the CCD, enhancing BK channel-mediated net K secretion.
The patch-clamp results summarized above predict that, if principal cells are responsible for BK channel-mediated K secretion, treatment of CCDs microperfused at slow flow rates with a p38 inhibitor should increase net K secretion. However, this is not observed (unpublished observations); indeed, the rates of net K secretion and Na absorption in CCDs perfused and bathed with SB203580, an inhibitor of p38 MAPK catalytic activity that binds to the ATP binding pocket but does not inhibit phosphorylation of p38 MAPK by upstream kinases, were identical. How do we reconcile the difference between the patch-clamp data and the perfusion data? The simplest explanation is that BK channels in intercalated but not principal cells in the native CCD secrete K. Assignation of a role for intercalated cells in mediating FIKS would also resolve the conundrum raised by the finding that monolayers of mpkCCD principal-like cells, subjected to 0.4 dyn/cm2 of FSS, a force considered to approximate that experienced by an ex vivo CCD microperfused at a physiologically fast flow rate, express a ∼2-fold greater abundance of p-ERK and p-p38 compared with cells not exposed to FSS (28). FSS-induced activation of these MAPK elements would be expected to inhibit BK channel activity and FIKS if mediated by principal cells but would have no effect on BK channel-mediated K secretion if effected by intercalated cells. Note that circumferential stretch (constant 10% equibiaxial stretch) did not alter p-p38 and p-ERK expression in mpkCCD cells compared with unstretched controls (28).
WNKs.
WNK (with-no-lysine) kinases play important roles in the regulation of Na absorption and K secretion in the ASDN, especially in response to changes in dietary K intake (102, 231). Mutations in full-length kinase-active WNK1 (L-WNK1) or WNK4, both expressed in the ASDN, cause familial hyperkalemic hypertension (FHHt; also referred to as pseudohypoaldosteronism type II), a hereditary disease characterized by hypertension, hyperkalemia, and hyperchloremic metabolic acidosis with normal or slightly elevated aldosterone levels (155, 232). L-WNK1 and WNK4 kinases regulate Na transporters in the ASDN, including NCC and ENaC (78, 83, 89, 100, 169, 203, 232, 244, 248). L-WNK1 stimulates ENaC via activation of serum and glucocorticoid-inducible kinase 1 (sgk1) (244), whereas WNK4, expressed in principal cells and intercalated cells of C57/B6 mice (185), inhibits ENaC by stimulating clathrin-dependent endocytosis (89, 169). It is well established that L-WNK1 and WNK4 inhibit ROMK expression by enhancing channel endocytosis (39, 77, 89, 102, 169, 216). FHHt-causing WNK4 mutations enhance the inhibition of ROMK and are associated with a further reduction in ROMK surface density and K secretion beyond that mediated by wild-type WNK4 (89).
Mechanisms by which WNK1 regulates transporters are complex, as an alternatively spliced WNK1 isoform lacking the NH2 terminus and kinase domain and functioning in a dominant interfering mode is specifically expressed in the kidney (kidney-specific WNK1, or KS-WNK1) (43, 149). KS-WNK1 antagonizes the effects of L-WNK1 and WNK4 on ROMK endocytosis (102, 204, 216). A high ratio of L-WNK1 to KS-WNK1 in the CCD enhances ENaC-mediated Na reabsorption and limits ROMK-mediated K secretion, resulting in dissociation of the two cation fluxes thought to be tightly coupled to the basolateral Na-K-ATPase. Acute (102, 216) and chronic (148) dietary K loading of the rat and mouse, respectively, increases the expression of KS-WNK1 at the message and protein level in whole kidney, although increases in KS-WNK1 mRNA expression appear to be restricted to the TALH and DCT and not CCD (34).
In contrast to the hyperkalemia observed in FHHt, loss-of-function ROMK mutations in Bartter syndrome are associated with hypokalemia (190). The kaliuresis observed in this setting is mediated by BK channels (10) and highlights the importance of this channel in renal K secretion. The effects of WNK kinases on BK channel activity are now being elucidated. WNK4 reduces whole cell and surface expression of BKα in HEK293 cells by a process that is dependent on its kinase activity, sensitive to inhibitors of lysosomal degradation, and also dependent on MAPKs. We also found that WNK4 enhances ubiquitination of BKα, consistent with a role for WNK4 in targeting the channel for internalization and/or degradation via an ubiquitin-dependent pathway in these cells (223). These results are consistent with the notion that WNK4 enhances the routing of BK channels to lysosomes in a ubiquitin-dependent manner (260) that is also dependent on activation of MAPKs (250). In contrast to its effect on ROMK, L-WNK1 significantly increases BKα whole cell and functional expression in HEK293 cells (114, 224). L-WNK1 expression reduced ERK phosphorylation, whereas knockdown of WNK1 increased the ubiquitination of BKα, effects that are opposite to the effects seen with WNK4 (114). Surprisingly, both KS-WNK1 and kinase dead L-WNK1 increased BKα whole cell expression in HEK293 cells, suggesting that WNK1 kinase activity is not required to enhance BKα expression (224).
KS-WNK1 KO mice exhibit an increase in BKα expression (2-fold in α and β1 and 50% decrease in β4) in kidney (71), consistent with a role of L-WNK1 in increasing BK channel expression. As BK channels are expressed at multiple sites in the nephron, it will be important to define whether there are changes in BK channel expression in specific cell types in the KS-WNK1 KO mice. It was also reported that loss-of-function mutations in KS-WNK1 lead to a reduced capacity of the CCD for basal K secretion but not FIKS in microperfused CCDs (33). These findings are consistent with a role for L-WNK1 in selectively inhibiting ROMK channels in the ASDN, while not inhibiting BK channels (33).
Although it is known that L-WNK1 and WNK4 show distinct subcellular distribution patterns (232), emerging evidence has revealed cell type-specific expression of WNK kinases. WNK-1 expression in the CCD is low in rabbits fed a LK diet but is selectively upregulated in β intercalated cells in HK-fed animals, where it is localized to the apical membrane and subapical region of this specific cell population (224). This finding has important implications regarding the adaptive response to K loading, where urinary flow rates are increased (12, 23, 52, 193, 215). The selective upregulation of L-WNK1 in β intercalated cells would promote BK channel-mediated K secretion to maintain K balance. A lack of upregulation of L-WNK1 in principal cells would prevent L-WNK1-dependent inhibition of ROMK expression.
If L-WNK1 levels are increased in some individuals with FHHt, why do they present with hyperkalemia? L-WNK1-dependent downregulation of ROMK expression should reduce K secretion by principal cells. While L-WNK1 should increase BK channel expression in β intercalated cells, reduced distal Na delivery and tubular flow due to enhanced NaCl absorption in the early DCT should blunt BK channel-mediated K secretion in latter aspects of the ASDN. Furthermore, thiazide diuretics correct hyperkalemia in patients with FHHt, consistent with the notion that BK channel-mediated FIKS can be observed in FHHt if distal flow rates are increased.
Regulation of BK Channel Function By Extrinsic Factors
Flow rate.
Apical BK channels, normally quiescent at slow urinary flow rates, are activated in the ASDN at high flows by mechanoinduced increases in [Ca2+]i, as described above. Primary cilia in principal cells function as flow sensors, whose mechanical deflection by urinary flow leads to Ca2+ entry into cilia through cilia TRPP2 channels, likely associated with TRPV4 as heteromeric channels, with downstream effects including a global increase in cytoplasmic [Ca2+]i (17, 36, 45, 63, 74, 97, 110, 142, 145, 146, 161, 162, 239). However, initial studies in MDCK cells, later extended to microperfused tubules, suggest that flow-induced cytosolic [Ca2+]i transients are not due exclusively to direct Ca2+ entry through ciliary TRP channels into the cell body but involve release of autocrine/paracrine factors such as ATP (87, 191) and PGE2 (55) that activate purinergic (81, 84, 87, 125, 159) and EP (55) receptor-associated signaling cascades. Indeed, the primary cilium is functionally distinct from the cytoplasm and represents only a tiny fraction of the total cell volume (45, 202). Mechanoactivation of Ca2+ channels in cilia would be expected to provide only a small “rivulet” of Ca2+ at its base into the large cytoplasmic volume, without significant perturbation of global [Ca2+]i (45). However, the apical cilium has been proposed to be essential for ATP secretion and serve as a chemosensor for secreted ATP; an increase in [Ca2+]i presumably primes “releasable” pools of ATP beneath the cell surface for secretion in response to chemical, osmotic, and mechanical stimuli which, in turn, activates autocrine/paracrine purinergic signaling, transduced by nearby receptors (84). Note that the ATP-permeable hemichannel connexin 30, localized to the apical membrane of β intercalated cells, is an important route for release of ATP into the urine, particularly in response to changes in Na balance (129, 191). Pannexin-1 (Panx1) channels, expressed along the apical membranes of intercalated cells, may also participate in ATP secretion as Panx1-deficient mice excrete less ATP than do wild-type animals (75). Luminal flow stimulates PGE2 release in the CCD, which inhibits Na absorption and enhances FIKS. As expected, indomethacin enhances flow-stimulated Na absorption but dampens FIKS (55). Studies by Gueutin et al. (70) have implicated β intercalated cells as critical for ATP release, which, via purinergic P2Y2 receptor activation, leads to production and release of PGE2 in the CCD.
Dietary K.
Chronic dietary K supplementation enhances renal K secretion (195, 238), due to an aldosterone-induced increase in driving force favoring K secretion in the ASDN, as well as aldosterone-independent mechanisms. Aldosterone rapidly induces sgk1 in the ASDN (32) that, in turn, stimulates Na reabsorption, in part by inhibiting retrieval of ENaCs from the luminal membrane (42). Aldosterone also rapidly induces expression of GILZ in mpkCCD cells that stimulates ENaC-mediated Na transport by inhibiting ERK signaling (194).
A HK diet is associated with increases in apical membrane expression and activity of both BK and SK/ROMK channels (105, 140, 154, 222). Expression of ROMK but not BK channels may be enhanced by increases in circulating levels of aldosterone (50, 217, 249). An increase in dietary K intake for as little as 6 h increases SK/ROMK channel density in rat CCD, an adaptation well described after 10–14 d of HK intake (152, 222). While this effect appears to require an increase in plasma K, the observation that adrenalectomized rats fail to exhibit an increase in SK channel density in response to HK intake suggests that circulating steroid levels play a permissive role in this process, at least in this species (152). In contrast, microperfused CCDs isolated from K adapted rabbits exhibit enhanced K secretion even after adrenalectomy (235). Furthermore, the apical K conductance of the CCD is increased in both control and adrenalectomized rabbits fed a HK diet (138). These data suggest that aldosterone may be necessary for K adaptation in rat, but not necessarily in rabbit.
Chronic (∼10 day) dietary K loading enhances FIKS in the rabbit CCD (140), a functional adaptation accompanied by changes in BKα- and β-subunit mRNA expression, as well as BKα localization in intercalated cells (140). Specifically, steady-state abundance of mRNA encoding BKα and β2–4 subunits in single CCDs from HK fed animals exceeds that detected in control K-fed (CK) rabbit. Immunofluorescence microscopy revealed a predominantly intracellular distribution of BKα in kidneys from animals fed a LK diet whereas robust apical labeling was detected in α intercalated cells in HK kidneys, consistent with redistribution from an intracellular pool to the plasma membrane.
Dietary K loading (10% K) increases IbTX-sensitive K secretion in in vivo microperfused mouse distal nephron (10), consistent with the finding by others (168) that a 5% HK diet for 6 days increases expression of BK channel protein in kidney. Mice with genetic ablation of BKβ1 exhibit, at baseline, reduced urinary K and Na clearances, conditions that are exacerbated when the animals are fed a HK (5% K) diet (69).
The effect of dietary K loading on BK channel activity in rat appears to be inconsistent. Whereas some patch-clamp studies have found that K loading fails to stimulate BK channel activity in rat CCD (79, 153, 222), others report that the probability of detecting apical BK channels and channel activity in the CCD is greater in rats fed a HK compared with a standard K diet (105). These discrepancies may be related to the use, in some studies, of a high KCl (vs. NaCl with only 5 mM K) bath solution that depolarizes the cell membrane potential and thereby inactivates BK channels (105). Additionally, as channel activation appears to require an increase in [Ca2+]i to at least 200 nM (153), channel activity may not be readily detected by standard cell-attached patch-clamp analysis in which the channels within the pipette are protected from hydrodynamic forces expected to generate a localized increase in [Ca2+]i (113).
The signals responsible for upregulation of BK channel activity in response to dietary K loading remain to be fully identified. BK channels in smooth muscle and endothelial cells are activated by epoxyeicosatrienoic acid (EET), a product of CYP-epoxygenase dependent arachidonic acid metabolism (5, 15). CYP-epoxygenases, including CYP2C23, are expressed in the CNT and CCD (121, 141, 206) and are upregulated in response to a HK diet, which also leads to an increase in 11,12-EET expression in isolated CCDs (207). Inhibition of CYP-epoxygenase in isolated microperfused rabbit CCDs with M-methylsulfonyl-6-(propargyloxyphenyl)hexanamide (MS-PPOH) abolished IbTX-sensitive and thus BK channel-mediated FIKS but not ROMK-mediated baseline net K secretion (207). These data suggest that a CYP-epoxygenase-dependent arachidonic acid pathway, stimulated in the kidney in response to dietary K loading, contributes to BK channel-mediated K secretion in the CCD.
HK diets increase tubular fluid flow rate in the ASDN in vivo via a reduction in fluid reabsorption in the proximal tubule (23) and NCC activity in the DCT (12, 52, 193, 215). High tubular flow rates will increase [Ca2+]i (see above), which, in turn, should activate BK channels directly but may activate the phosphatase calcineurin, which has been reported to inactivate p38 MAPK (106).
Chronic dietary K restriction leads to a reduction in renal K secretion due to inhibition of K secretion by ROMK and BK channels (105, 140, 227) and enhanced H-K-ATPase mediated K absorption in the CCD (189, 233, 234). Administration of a LK diet to rabbit for 10 days eliminates FIKS in the CCD, a functional adaptation accompanied by suppression of steady-state abundance of mRNA encoding BKα- and β2–4 subunits in single CCDs isolated from these animals (140).
Protein tyrosine kinase (PTK)-mediated phosphorylation of ROMK enhances endocytosis of the channels in the rat CCD under conditions of dietary K restriction (107, 108) via a dynamic process involving clathrin-coated pits (253). Tyrosine phosphorylation of ROMK channels decreases with a HK diet, and it increases in the face of dietary K restriction (227). K restriction suppresses the expression of protein phosphatase 2B (PP2B) catalytic subunits but increases expression of PP2B regulatory subunit in both rat and mouse kidney, without an effect on expression of PP1 and PP2A. Inhibition of PP2B decreases ROMK channel activity through stimulation of p38 and ERK in the CCD (256). Activation of PTK and MAPKs reduces activity (and vice versa) of not only ROMK, but also BK channel activity in mouse and rat CCD (8, 105, 226). The increase in BK channel activity detected after 7 days of a HK diet is associated with a reduction in the phosphorylation of p38 and ERK (105).
Emerging Picture of Mechanoregulation of K Secretion in the ASDN
Cumulative evidence garnered over the past 15 years reveals that the BK channel in the ASDN mediates FIKS and is thus likely the channel responsible for the kaliuresis associated with administration of diuretics or induced by volume expansion. Furthermore, this channel plays a major role in the renal adaptation to dietary K loading. The data presented in this review, reported by ourselves and others, provide compelling evidence that the BK channel in the ASDN is localized in a macromolecular complex at the apical membrane, composed of mechanosensitive apical Ca2+ channels and a variety of kinases/phosphatases as well as other signaling molecules (Fig. 2). This channel is present in both principal cells, traditionally considered to mediate Na absorption and K secretion, and intercalated cells, long considered to effect acid-base transport but not K secretion. However, the composition of the BK channel appears to be cell-specific as is the regulation of channel activity by endogenous effectors. In principal cells, BK channels, likely to be a BKα-STREX/β1 in composition, are constitutively inhibited by PKA and MAPK elements (Fig. 2). Physiologic stimuli, such as an increase in urinary flow rate or dietary K loading, may be able to activate these silent pools of apical BK channels by suppressing endogenous inhibitors. The intercalated cell BK channel, likely to be composed of α-subunits and possibly β4 or another accessory subunit, is activated by PKA and not affected by phosphorylation of p38 and ERK MAPK (Fig. 2). Emerging evidence suggests that BK channels are regulated by WNKs, but the effects of the distinct kinases in this family may be cell type-specific. The discrete cell type mediating FIKS in the ASDN, especially after dietary K loading, remains to be precisely identified, as do the specific mechanical forces and mechanoinduced signaling cascades activated by an increase in luminal fluid flow.
Fig. 2.
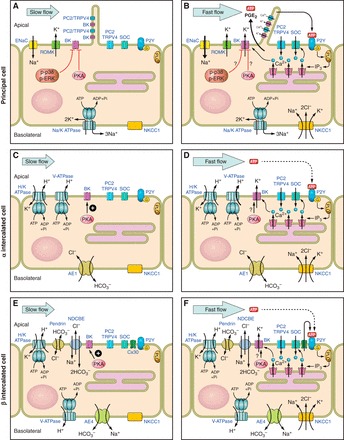
Cell-specific mechanoregulation of BK channel-mediated K secretion in the CCD. BK channels, present in principal cell cilia and at the apical membranes of both principal (A and B) and intercalated (C and D, E and F) cells in the CCD, are closed at slow physiologic flow rates (A, C, and E). An increase in tubular flow rate (B, D, and F) induces BK channel-mediated K secretion (FIKS), which requires ENaC-mediated apical Na entry, an increase in intracellular Ca2+ concentration (reflecting internal store release and Ca2+ entry into cells), and basolateral NKCC1 activity. Emerging evidence indicates that BK channel activity in this nephron segment is regulated by autocrine/paracrine factors released into the tubular fluid as well as cell-specific macromolecular complexes that include kinase signaling. Note that we do not know as yet whether principal or intercalated cells mediate FIKS. See manuscript for details.
GRANTS
This work was supported by National Institutes of Health grants from the National Institute of Diabetes and Digestive and Kidney Diseases, including R01 DK038470 (L. M. Satlin and T. R. Kleyman), R01 DK084184 (M. D. Carattino), R37 DK051391 (T. R. Kleyman and L. M. Satlin), and P30 DK079307 (Pittsburgh Center for Kidney Research). R. Carrisoza-Gaytan was partially supported by a postdoctoral scholarship from CONACYT (Mexico).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.C.-G., M.D.C., and L.M.S. performed experiments; R.C.-G., M.D.C., T.R.K., and L.M.S. analyzed data; R.C.-G., M.D.C., T.R.K., and L.M.S. interpreted results of experiments; R.C.-G. and L.M.S. prepared figures; R.C.-G., M.D.C., T.R.K., and L.M.S. drafted manuscript; R.C.-G., M.D.C., T.R.K., and L.M.S. edited and revised manuscript; R.C.-G., M.D.C., T.R.K., and L.M.S. approved final version of manuscript; T.R.K. and L.M.S. conception and design of research.
Glossary
- AII
Angiotensin II
- AE4
Anion exchanger 4
- ASDN
Aldosterone-sensitive distal nephron
- AT1R
Angiotensin type 1 receptor
- ATP
Adenosine triphosphate
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- BK
High-conductance K [channel]
- BP
Blood pressure
- [Ca2+]i
Intracellular Ca2+ concentration
- cAMP
Cyclic adenosine monophosphate
- cGMP
Cyclic guanosine monophosphate
- CCD
Cortical collecting duct
- CNT
Connecting tubule
- cSRC
Proto-oncogene tyrosine-protein kinase
- DAG
Diacylglycerol
- DCT
Distal convoluted tubule
- EET
Epoxyeicosatrienoic acid
- ENaC
Epithelial sodium channel
- ER
Endoplasmic reticulum
- FHHt
Familial hyperkalemic hypertension
- FIKS
Flow-induced K secretion
- FSS
Fluid shear stress
- GILZ
Glucocorticoid-induced leucine zipper protein
- HK
High K
- IbTX
Iberiotoxin
- IC
Intercalated cell
- IP3
Inositol trisphosphate
- KO
Knockout
- KS-WNK1
Kidney-specific with-no-lysine kinase 1
- Kv
Voltage-gated K [channel]
- MAPK
Mitogen-activated protein kinase
- mPKI
Myristoylated protein kinase inhibitor
- MR
Mineralocorticoid receptor
- MS-PPOH
M-methylsulfonyl-6-(propargyloxyphenyl)hexanamide
- NCC
Sodium chloride cotransporter
- NKCC
Sodium-potassium-2 chloride cotransporter
- Panx1
Pannexin-1
- Po
Open probability
- PC
Principal cell
- PGE2
Prostaglandin E2
- PKA
Protein kinase A
- PKC
Protein kinase C
- PKG
Protein kinase G
- PP1 and 2
Protein phosphatase 1 and 2
- PTK
Protein tyrosine kinase
- ROMK
Rat outer medullary K [channel]
- sgk
Serum-and glucocorticoid-induced protein kinase
- SK
Small-conductance K [channel]
- slo
Drosophila slowpoke
- Slip
Slo-interacting protein
- Slob
Slowpoke-binding protein
- STREX
Stress-axis regulated exon
- TALH
Thick ascending limb of Henle
- TRP
Transient receptor potential [cation channel]
- WNK
With-no-lysine [kinase]
- V2
Vasopressin 2 [receptor]
REFERENCES
- 1.Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 9: 209–216, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci USA 99: 14560–14565, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem 273: 32950–32956, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J 21: 2389–2399, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation 107: 769–776, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science 253: 551–555, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron 40: 331–346, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Babilonia E, Li D, Wang Z, Sun P, Lin DH, Jin Y, Wang WH. Mitogen-activated protein kinases inhibit the ROMK (Kir 1.1)-like small conductance K channels in the cortical collecting duct. J Am Soc Nephrol 17: 2687–2696, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann S. Cell localization and ontogeny of sodium transport pathways in the distal nephron: perspectives in function and failure. Curr Opin Nephrol Hypertens 8: 31–38, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 286: L1275–L1281, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Battilana CA, Dobyan DC, Lacy FB, Bhattacharya J, Johnston PA, Jamison RL. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest 62: 1093–1103, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck FX, Dorge A, Blumner E, Giebisch G, Thurau K. Cell rubidium uptake: a method for studying functional heterogeneity in the nephron. Kidney Int 33: 642–651, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Bello-Reuss E. Electrophysiological identification of cell types in cortical collecting duct monolayers. Renal Physiol Biochem 14: 1–11, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Benoit C, Renaudon B, Salvail D, Rousseau E. EETs relax airway smooth muscle via an EpDHF effect: BKCa channel activation and hyperpolarization. Am J Physiol Lung Cell Mol Physiol 280: L965–L973, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314: 615–620, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Berrout J, Jin M, Mamenko M, Zaika O, Pochynyuk O, O'Neil RG. Function of transient receptor potential cation channel subfamily V member 4 (TRPV4) as a mechanical transducer in flow-sensitive segments of renal collecting duct system. J Biol Chem 287: 8782–8791, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berrout J, Mamenko M, Zaika OL, Chen L, Zang W, Pochynyuk O, O'Neil RG. Emerging role of the calcium-activated, small conductance, SK3 K+ channel in distal tubule function: regulation by TRPV4. PLos One 9: e95149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: Physiology of the GI Tract (3rd Ed), edited by Johnson LR. New York: Raven Press, 1994, p. 2133–2171. [Google Scholar]
- 20.Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1132–F1140, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling–an overview. Semin Cell Dev Biol 12: 3–10, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Borodinsky LN, Spitzer NC. Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci STKE 2006: pe22, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Brandis M, Keyes J, Windhager EE. Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol 222: 421–427, 1972. [DOI] [PubMed] [Google Scholar]
- 24.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261: 221–224, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res 47: 578–585, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophys J 63: 583–590, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrisoza-Gaytan R, Liu Y, Flores D, Else C, Lee HG, Rhodes G, Sandoval RM, Kleyman TR, Lee FY, Molitoris B, Satlin LM, Rohatgi R. Effects of biomechanical forces on signaling in the cortical collecting duct (CCD). Am J Physiol Renal Physiol 307: F195–F204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrisoza-Gaytan R, Salvador C, Satlin LM, Liu W, Zavilowitz B, Bobadilla NA, Trujillo J, Escobar LI. Potassium secretion by voltage-gated potassium channel Kv1.3 in the rat kidney. Am J Physiol Renal Physiol 299: F255–F264, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci USA 110: 7928–7933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J Biol Chem 280: 33599–33609, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96: 2514–2519, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng CJ, Baum M, Huang CL. Kidney-specific WNK1 regulates sodium reabsorption and potassium secretion in mouse cortical collecting duct. Am J Physiol Renal Physiol 304: F397–F402, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng CJ, Truong T, Baum M, Huang CL. Kidney-specific WNK1 inhibits sodium reabsorption in the cortical thick ascending limb. Am J Physiol Renal Physiol 303: F667–F673, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension 31: 162–169, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8: 510–521, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Constantinescu A, Silver RB, Satlin LM. H-K-ATPase activity in PNA-binding intercalated cells of newborn rabbit cortical collecting duct. Am J Physiol Renal Physiol 272: F167–F177, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Constantinescu AR, Lane JC, Mak J, Zavilowitz B, Satlin LM. Na+-K+-ATPase-mediated basolateral rubidium uptake in the maturing rabbit cortical collecting duct. Am J Physiol Renal Physiol 279: F1161–F1168, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Cope G, Murthy M, Golbang AP, Hamad A, Liu CH, Cuthbert AW, O'Shaughnessy KM. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol 17: 1867–1874, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Cortell S, Gennari FJ, Davidman M, Bossert WH, Schwartz WB. A definition of proximal and distal tubular compliance. Practical and theoretical implications. J Clin Invest 52: 2330–2339, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol 23: 9208–9221, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgado MM, Rohatgi R, Khan S, Holzman IR, Satlin LM. Sodium and potassium clearances by the maturing kidney: clinical-molecular correlates. Pediatr Nephrol 18: 759–767, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dworetzky SI, Boissard CG, Lum-Ragan JT, McKay MC, Post-Munson DJ, Trojnacki JT, Chang CP, Gribkoff VK. Phenotypic alteration of a human BK (hSlo) channel by hSlobeta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci 16: 4543–4550, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elkins T, Ganetzky B, Wu CF. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci USA 83: 8415–8419, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emmons C, Kurtz I. Functional characterization of three intercalated cell subtypes in the rabbit outer cortical collecting duct. J Clin Invest 93: 417–423, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engbretson BG, Stoner LC. Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 253: F896–F903, 1987. [DOI] [PubMed] [Google Scholar]
- 50.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evan AP, Satlin LM, Gattone VH, Connors B 2nd, Schwartz GJ. Postnatal maturation of rabbit renal collecting duct. II. Morphological observations. Am J Physiol Renal Fluid Electrolyte Physiol 261: F91–F107, 1991. [DOI] [PubMed] [Google Scholar]
- 52.Field MJ, Stanton BA, Giebisch GH. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest 74: 1792–1802, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finer G, Shalev H, Birk OS, Galron D, Jeck N, Sinai-Treiman L, Landau D. Transient neonatal hyperkalemia in the antenatal (ROMK defective) Bartter syndrome. J Pediatr 142: 318–323, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Fitzgibbons JP, Gennari FJ, Garfinkel HB, Cortell S. Dependence of saline-induced natriuresis upon exposure of the kidney to the physical effects of extracellular fluid volume expansion. J Clin Invest 54: 1428–1436, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flores D, Liu Y, Liu W, Satlin LM, Rohatgi R. Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct. Am J Physiol Renal Physiol 303: F632–F638, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flynn MA, Woodruff C, Clark J, Chase G. Total body potassium in normal children. Pediatr Res 6: 239–245, 1972. [DOI] [PubMed] [Google Scholar]
- 57.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Frindt G, Palmer LG. Ca-activated K channels in apical membrane of mammalian CCT, and their role in K secretion. Am J Physiol Renal Fluid Electrolyte Physiol 252: F458–F467, 1987. [DOI] [PubMed] [Google Scholar]
- 59.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol 297: F389–F396, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F143–F151, 1989. [DOI] [PubMed] [Google Scholar]
- 61.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990. [PubMed] [Google Scholar]
- 62.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998. [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez-Perret S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol Renal Fluid Electrolyte Physiol 236: F192–F205, 1979. [DOI] [PubMed] [Google Scholar]
- 65.Grantham JJ, Burg MB, Orloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest 49: 1815–1826, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol 289: F117–F126, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Grimm PR, Irsik DL, Liu L, Holtzclaw JD, Sansom SC. Role of BKβ1 in Na+ reabsorption by cortical collecting ducts of Na+-deprived mice. Am J Physiol Renal Physiol 297: F420–F428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA 106: 11800–11805, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal beta-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hadchouel J, Soukaseum C, Busst C, Zhou XO, Baudrie V, Zurrer T, Cambillau M, Elghozi JL, Lifton RP, Loffing J, Jeunemaitre X. Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci USA 107: 18109–18114, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hagen BM, Bayguinov O, Sanders KM. β1-Subunits are required for regulation of coupling between Ca2+ transients and Ca2+-activated K+ (BK) channels by protein kinase C. Am J Physiol Cell Physiol 285: C1270–C1280, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Hall SK, Armstrong DL. Conditional and unconditional inhibition of calcium-activated potassium channels by reversible protein phosphorylation. J Biol Chem 275: 3749–3754, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol 303: F1454–F1459, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanner M, Schmalhofer WA, Munujos P, Knaus HG, Kaczorowski GJ, Garcia ML. The beta subunit of the high-conductance calcium-activated potassium channel contributes to the high-affinity receptor for charybdotoxin. Proc Natl Acad Sci USA 94: 2853–2858, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest 117: 1078–1087, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem 285: 25161–25167, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirsch J, Frobe U, Schlatter E. Regulation and possible physiological role of the Ca2+-dependent K+ channel of cortical collecting ducts of the rat. Pflügers Arch 422: 492–498, 1993. [DOI] [PubMed] [Google Scholar]
- 80.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993. [DOI] [PubMed] [Google Scholar]
- 81.Holtzclaw JD, Cornelius RJ, Hatcher LI, Sansom SC. Coupled ATP and potassium efflux from intercalated cells. Am J Physiol Renal Physiol 300: F1319–F1326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-α/β4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol 21: 634–645, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoorn EJ, Nelson JH, McCormick JA, Ellison DH. The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 22: 605–614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155–170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hunter M, Lopes AG, Boulpaep EL, Giebisch GH. Single channel recordings of calcium-activated potassium channels in the apical membrane of rabbit cortical collecting tubules. Proc Natl Acad Sci USA 81: 4237–4239, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imai M, Nakamura R. Function of distal convoluted and connecting tubules studied by isolated nephron segments. Kidney Int 22: 465–472, 1982. [DOI] [PubMed] [Google Scholar]
- 87.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Jin P, Weiger TM, Levitan IB. Reciprocal modulation between the alpha and beta 4 subunits of hSlo calcium-dependent potassium channels. J Biol Chem 277: 43724–43729, 2002. [DOI] [PubMed] [Google Scholar]
- 89.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003. [DOI] [PubMed] [Google Scholar]
- 90.Kawahara K, Matsuzaki K. Activation of calcium channel by shear-stress in cultured renal distal tubule cells. Biochem Biophys Res Commun 184: 198–205, 1992. [DOI] [PubMed] [Google Scholar]
- 91.Khuri RN, Strieder WN, Giebisch G. Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975. [DOI] [PubMed] [Google Scholar]
- 92.Kim EY, Ridgway LD, Zou S, Chiu YH, Dryer SE. Alternatively spliced C-terminal domains regulate the surface expression of large conductance calcium-activated potassium channels. Neuroscience 146: 1652–1661, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem 269: 17274–17278, 1994. [PubMed] [Google Scholar]
- 95.Knepper MA, Kim GH, Masilamani S. Renal tubule sodium transporter abundance profiling in rat kidney: response to aldosterone and variations in NaCl intake. Ann NY Acad Sci 986: 562–569, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Koeppen BM. Electrophysiological identification of principal and intercalated cells in the rabbit outer medullary collecting duct. Pflügers Arch 409: 138–141, 1987. [DOI] [PubMed] [Google Scholar]
- 97.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwon SH, Guggino WB. Multiple sequences in the C terminus of MaxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci USA 101: 15237–15242, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lagrutta A, Shen KZ, North RA, Adelman JP. Functional differences among alternatively spliced variants of Slowpoke, a Drosophila calcium-activated potassium channel. J Biol Chem 269: 20347–20351, 1994. [PubMed] [Google Scholar]
- 100.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. WNK4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006. [DOI] [PubMed] [Google Scholar]
- 101.Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol 51: 385–399, 1989. [DOI] [PubMed] [Google Scholar]
- 102.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol 56: 193–212, 1994. [DOI] [PubMed] [Google Scholar]
- 105.Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+-dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim HW, New L, Han J, Molkentin JD. Calcineurin enhances MAPK phosphatase-1 expression and p38 MAPK inactivation in cardiac myocytes. J Biol Chem 276: 15913–15919, 2001. [DOI] [PubMed] [Google Scholar]
- 107.Lin DH, Sterling H, Wang WH. The protein tyrosine kinase-dependent pathway mediates the effect of K intake on renal K secretion. Physiology (Bethesda) 20: 140–146, 2005. [DOI] [PubMed] [Google Scholar]
- 108.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol 286: F881–F892, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ling S, Woronuk G, Sy L, Lev S, Braun AP. Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src tyrosine kinase. J Biol Chem 275: 30683–30689, 2000. [DOI] [PubMed] [Google Scholar]
- 110.Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007. [DOI] [PubMed] [Google Scholar]
- 111.Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu W, Wei Y, Sun P, Wang WH, Kleyman TR, Satlin LM. Mechanoregulation of BK channel activity in the mammalian cortical collecting duct (CCD): role of protein kinases A and C. Am J Physiol Renal Physiol 297: F904–F915, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003. [DOI] [PubMed] [Google Scholar]
- 114.Liu Y, Song X, Shi Y, Shi Z, Niu W, Feng X, Gu D, Bao HF, Ma HP, Eaton DC, Zhuang J, Cai H. WNK1 activates large-conductance Ca2+-activated K+ channels through modulation of ERK1/2 signaling. J Am Soc Nephrol 26: 844–854, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Loffing J, Kaissling B. Sodium and calcium transport pathways along the mammalian distal nephron: from rabbit to human. Am J Physiol Renal Physiol 284: F628–F643, 2003. [DOI] [PubMed] [Google Scholar]
- 116.Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, Manning PA, Liu LH, Miller ML, Shull GE. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter's syndrome. J Biol Chem 277: 37871–37880, 2002. [DOI] [PubMed] [Google Scholar]
- 117.Lu M, Wang T, Yan Q, Yang X, Dong K, Knepper MA, Wang W, Giebisch G, Shull GE, Hebert SC. Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter's) knockout mice. J Biol Chem 277: 37881–37887, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol 570: 65–72, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lynch IJ, Greenlee MM, Gumz ML, Rudin A, Xia SL, Wingo CS. Heterogeneity of H-K-ATPase-mediated acid secretion along the mouse collecting duct. Am J Physiol Renal Physiol 298: F408–F415, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett 581: 1000–1008, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma J, Qu W, Scarborough PE, Tomer KB, Moomaw CR, Maronpot R, Davis LS, Breyer MD, Zeldin DC. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J Biol Chem 274: 17777–17788, 1999. [DOI] [PubMed] [Google Scholar]
- 122.Madsen KM, Clapp WL, Verlander JW. Structure and function of the inner medullary collecting duct. Kidney Int 34: 441–454, 1988. [DOI] [PubMed] [Google Scholar]
- 123.Malnic G, Berliner RW, Giebisch G. Flow dependence of K+ secretion in cortical distal tubules of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 256: F932–F941, 1989. [DOI] [PubMed] [Google Scholar]
- 124.Malnic G, Klose RM, Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol 211: 529–547, 1966. [DOI] [PubMed] [Google Scholar]
- 125.Mamenko M, Zaika O, Jin M, O'Neil RG, Pochynyuk O. Purinergic activation of Ca2+-permeable TRPV4 channels is essential for mechano-sensitivity in the aldosterone-sensitive distal nephron. PLos One 6: e22824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131: 911–920, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCance RA, Widdowson EM. The response of the newborn piglet to an excess of potassium. J Physiol 141: 88–96, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCobb DP, Fowler NL, Featherstone T, Lingle CJ, Saito M, Krause JE, Salkoff L. A human calcium-activated potassium channel gene expressed in vascular smooth muscle. Am J Physiol Heart Circ Physiol 269: H767–H777, 1995. [DOI] [PubMed] [Google Scholar]
- 129.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005. [DOI] [PubMed] [Google Scholar]
- 130.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 14: 645–650, 1995. [DOI] [PubMed] [Google Scholar]
- 131.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc Natl Acad Sci USA 94: 14066–14071, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593–F601, 2004. [DOI] [PubMed] [Google Scholar]
- 134.Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na+-K+-2Cl− cotransporter. Am J Physiol Heart Circ Physiol 283: H1846–H1855, 2002. [DOI] [PubMed] [Google Scholar]
- 135.Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol 496: 289–302, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moczydlowski E, Lucchesi K, Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol 105: 95–111, 1988. [DOI] [PubMed] [Google Scholar]
- 137.Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006. [DOI] [PubMed] [Google Scholar]
- 138.Muto S, Sansom S, Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting ducts from adrenalectomized rabbits. J Clin Invest 81: 376–380, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muto S, Tsuruoka S, Miyata Y, Fujimura A, Kusano E, Wang W, Seldin D, Giebisch G. Basolateral Na+/H+ exchange maintains potassium secretion during diminished sodium transport in the rabbit cortical collecting duct. Kidney Int 75: 25–30, 2009. [DOI] [PubMed] [Google Scholar]
- 140.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005. [DOI] [PubMed] [Google Scholar]
- 141.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E Jr, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest 116: 1696–1702, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 143.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20: 389–399, 1998. [DOI] [PubMed] [Google Scholar]
- 144.Nehrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol 284: C535–C546, 2003. [DOI] [PubMed] [Google Scholar]
- 145.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. [DOI] [PubMed] [Google Scholar]
- 146.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflügers Arch 451: 193–203, 2005. [DOI] [PubMed] [Google Scholar]
- 147.O'Neil RG, Sansom SC. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol 82: 281–295, 1984. [DOI] [PubMed] [Google Scholar]
- 148.O'Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17: 2402–2413, 2006. [DOI] [PubMed] [Google Scholar]
- 149.O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol 14: 2447–2456, 2003. [DOI] [PubMed] [Google Scholar]
- 150.Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991. [DOI] [PubMed] [Google Scholar]
- 151.Pallanck L, Ganetzky B. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum Mol Genet 3: 1239–1243, 1994. [DOI] [PubMed] [Google Scholar]
- 152.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007. [DOI] [PubMed] [Google Scholar]
- 154.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999. [DOI] [PubMed] [Google Scholar]
- 155.Pathare G, Hoenderop JG, Bindels RJ, San-Cristobal P. A molecular update on pseudohypoaldosteronism type II. Am J Physiol Renal Physiol 305: F1513–F1520, 2013. [DOI] [PubMed] [Google Scholar]
- 156.Pluznick JL, Sansom SC. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006. [DOI] [PubMed] [Google Scholar]
- 157.Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-β1−/− mice. Am J Physiol Renal Physiol 284: F1274–F1279, 2003. [DOI] [PubMed] [Google Scholar]
- 158.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005. [DOI] [PubMed] [Google Scholar]
- 159.Praetorius HA, Frokiaer J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol 288: F133–F141, 2005. [DOI] [PubMed] [Google Scholar]
- 160.Praetorius HA, Leipziger J. Primary cilium-dependent sensing of urinary flow and paracrine purinergic signaling. Semin Cell Dev Biol 24: 3–10, 2013. [DOI] [PubMed] [Google Scholar]
- 161.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001. [DOI] [PubMed] [Google Scholar]
- 162.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003. [DOI] [PubMed] [Google Scholar]
- 163.Reinhart PH, Chung S, Martin BL, Brautigan DL, Levitan IB. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci 11: 1627–1635, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reinhart PH, Levitan IB. Kinase and phosphatase activities intimately associated with a reconstituted calcium-dependent potassium channel. J Neurosci 15: 4572–4579, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reyes AJ. Effects of diuretics on outputs and flows of urine and urinary solutes in healthy subjects. Drugs 41, Suppl 3: 35–59, 1991. [DOI] [PubMed] [Google Scholar]
- 166.Reyes AJ, Taylor SH. Diuretics in cardiovascular therapy: the new clinicopharmacological bases that matter. Cardiovasc Drugs Ther 13: 371–398, 1999. [DOI] [PubMed] [Google Scholar]
- 167.Ridderstrale Y, Kashgarian M, Koeppen B, Giebisch G, Stetson D, Ardito T, Stanton B. Morphological heterogeneity of the rabbit collecting duct. Kidney Int 34: 655–670, 1988. [DOI] [PubMed] [Google Scholar]
- 168.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int 72: 566–573, 2007. [DOI] [PubMed] [Google Scholar]
- 169.Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc Natl Acad Sci USA 104: 4020–4024, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rodriguez-Soriano J. Bartter and related syndromes: the puzzle is almost solved. Pediatr Nephrol 12: 315–327, 1998. [DOI] [PubMed] [Google Scholar]
- 171.Sabolic I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10: 913–922, 1999. [DOI] [PubMed] [Google Scholar]
- 172.Saito M, Nelson C, Salkoff L, Lingle CJ. A cysteine-rich domain defined by a novel exon in a slo variant in rat adrenal chromaffin cells and PC12 cells. J Biol Chem 272: 11710–11717, 1997. [DOI] [PubMed] [Google Scholar]
- 173.Satlin LM. Postnatal maturation of potassium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 266: F57–F65, 1994. [DOI] [PubMed] [Google Scholar]
- 174.Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol 291: F923–F931, 2006. [DOI] [PubMed] [Google Scholar]
- 175.Satlin LM, Matsumoto T, Schwartz GJ. Postnatal maturation of rabbit renal collecting duct. III. Peanut lectin-binding intercalated cells. Am J Physiol Renal Fluid Electrolyte Physiol 262: F199–F208, 1992. [DOI] [PubMed] [Google Scholar]
- 176.Satlin LM, Palmer LG. Apical K+ conductance in maturing rabbit principal cell. Am J Physiol Renal Physiol 272: F397–F404, 1997. [DOI] [PubMed] [Google Scholar]
- 177.Satlin LM, Palmer LG. Apical Na+ conductance in maturing rabbit principal cell. Am J Physiol Renal Fluid Electrolyte Physiol 270: F391–F397, 1996. [DOI] [PubMed] [Google Scholar]
- 178.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001. [DOI] [PubMed] [Google Scholar]
- 179.Sauer M, Flemmer A, Thurau K, Beck FX. Sodium entry routes in principal and intercalated cells of the isolated perfused cortical collecting duct. Pflügers Arch 416: 88–93, 1990. [DOI] [PubMed] [Google Scholar]
- 180.Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA 101: 9474–9478, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schlatter E, Bleich M, Hirsch J, Markstahler U, Frobe U, Greger R. Cation specificity and pharmacological properties of the Ca2+-dependent K+ channel of rat cortical collecting ducts. Pflügers Arch 422: 481–491, 1993. [DOI] [PubMed] [Google Scholar]
- 182.Schopperle WM, Holmqvist MH, Zhou Y, Wang J, Wang Z, Griffith LC, Keselman I, Kusinitz F, Dagan D, Levitan IB. Slob, a novel protein that interacts with the Slowpoke calcium-dependent potassium channel. Neuron 20: 565–573, 1998. [DOI] [PubMed] [Google Scholar]
- 183.Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol Renal Physiol 272: F132–F138, 1997. [DOI] [PubMed] [Google Scholar]
- 184.Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985. [DOI] [PubMed] [Google Scholar]
- 185.Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shipston MJ. Alternative splicing of potassium channels: a dynamic switch of cellular excitability. Trends Cell Biol 11: 353–358, 2001. [DOI] [PubMed] [Google Scholar]
- 187.Shipston MJ. S-acylation dependent post-translational cross-talk regulates large conductance calcium- and voltage- activated potassium (BK) channels. Front Physiol 5: 281, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shipston MJ, Armstrong DL. Activation of protein kinase C inhibits calcium-activated potassium channels in rat pituitary tumour cells. J Physiol 493: 665–672, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Silver RB, Soleimani M. H+-K+-ATPases: regulation and role in pathophysiological states. Am J Physiol Renal Physiol 276: F799–F811, 1999. [DOI] [PubMed] [Google Scholar]
- 190.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996. [DOI] [PubMed] [Google Scholar]
- 191.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sokolowski B, Orchard S, Harvey M, Sridhar S, Sakai Y. Conserved BK channel-protein interactions reveal signals relevant to cell death and survival. PLos One 6: e28532, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. [DOI] [PubMed] [Google Scholar]
- 194.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem 280: 39970–39981, 2005. [DOI] [PubMed] [Google Scholar]
- 195.Stanton BA, Giebisch GH. Potassium transport by the renal distal tubule: effects of potassium loading. Am J Physiol Renal Fluid Electrolyte Physiol 243: F487–F493, 1982. [DOI] [PubMed] [Google Scholar]
- 196.Stockand JD, Sansom SC. Mechanism of activation by cGMP-dependent protein kinase of large Ca2+-activated K+ channels in mesangial cells. Am J Physiol Cell Physiol 271: C1669–C1677, 1996. [DOI] [PubMed] [Google Scholar]
- 197.Stokes JB. Potassium secretion by cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am J Physiol Renal Fluid Electrolyte Physiol 241: F395–F402, 1981. [DOI] [PubMed] [Google Scholar]
- 198.Stoner LC, Burg MB, Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol 227: 453–459, 1974. [DOI] [PubMed] [Google Scholar]
- 199.Stoner LC, Morley GE. Effect of basolateral or apical hyposmolarity on apical maxi K channels of everted rat collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 268: F569–F580, 1995. [DOI] [PubMed] [Google Scholar]
- 200.Stoner LC, Viggiano SC. Elevation of basolateral K+ induces K+ secretion by apical maxi K+ channels in Ambystoma collecting tubule. Am J Physiol Regul Integr Comp Physiol 276: R616–R621, 1999. [DOI] [PubMed] [Google Scholar]
- 201.Stoner LC, Viggiano SC. Environmental KCl causes an upregulation of apical membrane maxi K and ENaC channels in everted Ambystoma collecting tubule. J Membr Biol 162: 107–116, 1998. [DOI] [PubMed] [Google Scholar]
- 202.Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods 10: 1105–1107, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284: 18471–18480, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 290: F619–F624, 2006. [DOI] [PubMed] [Google Scholar]
- 205.Sulyok E, Nemeth M, Tenyi I, Csaba IF, Varga F, Gyory E, Thurzo V. Relationship between maturity, electrolyte balance and the function of the renin-angiotensin-aldosterone system in newborn infants. Biol Neonate 35: 60–65, 1979. [DOI] [PubMed] [Google Scholar]
- 206.Sun P, Lin DH, Wang T, Babilonia E, Wang Z, Jin Y, Kemp R, Nasjletti A, Wang WH. Low Na intake suppresses expression of CYP2C23 and arachidonic acid-induced inhibition of ENaC. Am J Physiol Renal Physiol 291: F1192–F1200, 2006. [DOI] [PubMed] [Google Scholar]
- 207.Sun P, Liu W, Lin DH, Yue P, Kemp R, Satlin LM, Wang WH. Epoxyeicosatrienoic acid activates BK channels in the cortical collecting duct. J Am Soc Nephrol 20: 513–523, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998. [DOI] [PubMed] [Google Scholar]
- 209.Taniguchi J, Takeda M, Yoshitomi K, Imai M. Pressure- and parathyroid-hormone-dependent Ca2+ transport in rabbit connecting tubule: role of the stretch-activated nonselective cation channel. J Membr Biol 140: 123–132, 1994. [DOI] [PubMed] [Google Scholar]
- 210.Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Knaus HG, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci USA 101: 11897–11902, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem 276: 7717–7720, 2001. [DOI] [PubMed] [Google Scholar]
- 212.Toro B, Cox N, Wilson RJ, Garrido-Sanabria E, Stefani E, Toro L, Zarei MM. KCNMB1 regulates surface expression of a voltage and Ca2+-activated K+ channel via endocytic trafficking signals. Neuroscience 142: 661–669, 2006. [DOI] [PubMed] [Google Scholar]
- 213.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron 13: 1315–1330, 1994. [DOI] [PubMed] [Google Scholar]
- 214.Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB, Swanson R. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem 275: 23211–23218, 2000. [DOI] [PubMed] [Google Scholar]
- 215.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013. [DOI] [PubMed] [Google Scholar]
- 216.Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA 103: 8558–8563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wald H, Garty H, Palmer LG, Popovtzer MM. Differential regulation of ROMK expression in kidney cortex and medulla by aldosterone and potassium. Am J Physiol Renal Physiol 275: F239–F245, 1998. [DOI] [PubMed] [Google Scholar]
- 218.Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 290: F409–F416, 2006. [DOI] [PubMed] [Google Scholar]
- 219.Wang B, Rothberg BS, Brenner R. Mechanism of beta4 subunit modulation of BK channels. J Gen Physiol 127: 449–465, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang J, Zhou Y, Wen H, Levitan IB. Simultaneous binding of two protein kinases to a calcium-dependent potassium channel. J Neurosci 19: RC4, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang SX, Ikeda M, Guggino WB. The cytoplasmic tail of large conductance, voltage- and Ca2+-activated K+ (MaxiK) channel is necessary for its cell surface expression. J Biol Chem 278: 2713–2722, 2003. [DOI] [PubMed] [Google Scholar]
- 222.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990. [DOI] [PubMed] [Google Scholar]
- 223.Wang Z, Subramanya AR, Satlin LM, Pastor-Soler NM, Carattino MD, Kleyman TR. Regulation of large-conductance Ca2+-activated K+ channels by WNK4 kinase. Am J Physiol Cell Physiol 305: C846–C853, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Webb TN, Carrisoza-Gaytán R, Montalbetti N, Rued A, Roy A, Socovich AM, Subramanya AR, Satlin LM, Kleyman TR, Carattino MD. Cell-specific regulation of L-WNK1 by dietary K+. Am J Physiol Renal Physiol 310: F15–F26, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Webber WA, Lee J. Fine structure of mammalian renal cilia. Anat Rec 182: 339–343, 1975. [DOI] [PubMed] [Google Scholar]
- 226.Wei Y, Bloom P, Gu R, Wang W. Protein-tyrosine phosphatase reduces the number of apical small conductance K+ channels in the rat cortical collecting duct. J Biol Chem 275: 20502–20507, 2000. [DOI] [PubMed] [Google Scholar]
- 227.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001. [DOI] [PubMed] [Google Scholar]
- 228.Weiger TM, Holmqvist MH, Levitan IB, Clark FT, Sprague S, Huang WJ, Ge P, Wang C, Lawson D, Jurman ME, Glucksmann MA, Silos-Santiago I, DiStefano PS, Curtis R. A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J Neurosci 20: 3563–3570, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Weinbaum S, Duan Y, Thi MM, You L. An integrative review of mechanotransduction in endothelial, epithelial (renal) and dendritic cells (osteocytes). Cell Mol Bioeng 4: 510–537, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Welling PA, Chang YP, Delpire E, Wade JB. Multigene kinase network, kidney transport, and salt in essential hypertension. Kidney Int 77: 1063–1069, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. [DOI] [PubMed] [Google Scholar]
- 233.Wingo CS. Potassium transport by medullary collecting tubule of rabbit: effects of variation in K intake. Am J Physiol Renal Fluid Electrolyte Physiol 253: F1136–F1141, 1987. [DOI] [PubMed] [Google Scholar]
- 234.Wingo CS, Armitage FE. Rubidium absorption and proton secretion by rabbit outer medullary collecting duct via H-K-ATPase. Am J Physiol Renal Fluid Electrolyte Physiol 263: F849–F857, 1992. [DOI] [PubMed] [Google Scholar]
- 235.Wingo CS, Seldin DW, Kokko JP, Jacobson HR. Dietary modulation of active potassium secretion in the cortical collecting tubule of adrenalectomized rabbits. J Clin Invest 70: 579–586, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. [DOI] [PubMed] [Google Scholar]
- 237.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003. [DOI] [PubMed] [Google Scholar]
- 238.Wright FS, Strieder N, Fowler NB, Giebisch G. Potassium secretion by distal tubule after potassium adaptation. Am J Physiol 221: 437–448, 1971. [DOI] [PubMed] [Google Scholar]
- 239.Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 293: F1699–F1713, 2007. [DOI] [PubMed] [Google Scholar]
- 240.Wu SN, Wang YJ, Lin MW. Potent stimulation of large-conductance Ca2+-activated K+ channels by rottlerin, an inhibitor of protein kinase C-delta, in pituitary tumor (GH3) cells and in cortical neuronal (HCN-1A) cells. J Cell Physiol 210: 655–666, 2007. [DOI] [PubMed] [Google Scholar]
- 241.Xia X, Hirschberg B, Smolik S, Forte M, Adelman JP. dSLo interacting protein 1, a novel protein that interacts with large-conductance calcium-activated potassium channels. J Neurosci 18: 2360–2369, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xia XM, Ding JP, Zeng XH, Duan KL, Lingle CJ. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel beta subunit. J Neurosci 20: 4890–4903, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science 280: 443–446, 1998. [DOI] [PubMed] [Google Scholar]
- 244.Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert SC. Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol Renal Physiol 273: F739–F748, 1997. [DOI] [PubMed] [Google Scholar]
- 246.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466: 513–516, 2010. [DOI] [PubMed] [Google Scholar]
- 247.Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, Aldrich RW, Fakler B, Trimmer JS. Profiling the phospho-status of the BKCa channel alpha subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics 7: 2188–2198, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem 278: 23066–23075, 2003. [DOI] [PubMed] [Google Scholar]
- 250.Yue P, Zhang C, Lin DH, Sun P, Wang WH. WNK4 inhibits Ca2+-activated big-conductance potassium channels (BK) via mitogen-activated protein kinase-dependent pathway. Biochim Biophys Acta 1833: 2101–2110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zarei MM, Eghbali M, Alioua A, Song M, Knaus HG, Stefani E, Toro L. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc Natl Acad Sci USA 101: 10072–10077, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zarei MM, Song M, Wilson RJ, Cox N, Colom LV, Knaus HG, Stefani E, Toro L. Endocytic trafficking signals in KCNMB2 regulate surface expression of a large conductance voltage and Ca2+-activated K+ channel. Neuroscience 147: 80–89, 2007. [DOI] [PubMed] [Google Scholar]
- 253.Zeng WZ, Babich V, Ortega B, Quigley R, White SJ, Welling PA, Huang CL. Evidence for endocytosis of ROMK potassium channel via clathrin-coated vesicles. Am J Physiol Renal Physiol 283: F630–F639, 2002. [DOI] [PubMed] [Google Scholar]
- 254.Zhang J, Yan J. Regulation of BK channels by auxiliary gamma subunits. Front Physiol 5: 401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang Y, Joiner WJ, Bhattacharjee A, Rassendren F, Magoski NS, Kaczmarek LK. The appearance of a protein kinase A-regulated splice isoform of slo is associated with the maturation of neurons that control reproductive behavior. J Biol Chem 279: 52324–52330, 2004. [DOI] [PubMed] [Google Scholar]
- 256.Zhang Y, Lin DH, Wang ZJ, Jin Y, Yang B, Wang WH. K restriction inhibits protein phosphatase 2B (PP2B) and suppression of PP2B decreases ROMK channel activity in the CCD. Am J Physiol Cell Physiol 294: C765–C773, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhou H, Tate SS, Palmer LG. Primary structure and functional properties of an epithelial K channel. Am J Physiol Cell Physiol 266: C809–C824, 1994. [DOI] [PubMed] [Google Scholar]
- 258.Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem 276: 43239–43245, 2001. [DOI] [PubMed] [Google Scholar]
- 259.Zhou Y, Schopperle WM, Murrey H, Jaramillo A, Dagan D, Griffith LC, Levitan IB. A dynamically regulated 14-3-3, Slob, and Slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron 22: 809–818, 1999. [DOI] [PubMed] [Google Scholar]
- 260.Zhuang J, Zhang X, Wang D, Li J, Zhou B, Shi Z, Gu D, Denson DD, Eaton DC, Cai H. WNK4 kinase inhibits Maxi K channel activity by a kinase-dependent mechanism. Am J Physiol Renal Physiol 301: F410–F419, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


