Abstract
Schizophrenia is a clinically heterogeneous disorder that is perhaps more accurately characterized as “the schizophrenia syndrome.” This clinical heterogeneity is reflected in the heterogeneous neurobiological presentations associated with the illness. Moreover, even highly specific neural aberrations that are associated with distinct symptoms of schizophrenia are linked to a wide range of risk factors. As such, any individual with schizophrenia likely has a particular set of risk factors that interact and converge to cross the disease threshold, forming a particular etiology that ultimately generates a core pathophysiology. This core pathophysiology may then produce 1 or more symptoms of schizophrenia, leading to common symptoms across individuals in spite of disparate etiologies. As such, the schizophrenia syndrome can be considered as an equifinal entity: a state of dysfunction that can arise from different upstream etiologies. Moreover, schizophrenia etiologies are multifactorial and can involve the interactive effects of a broad range of genetic, environmental, and developmental risk factors. Through a consideration of how disparate etiologies, caused by different sets of risk factors, converge on the same net dysfunction, this paper aims to model the equifinal nature of schizophrenia symptoms. To demonstrate the equifinal model, we discuss how maternal infection and adolescent cannabis use, 2 recognized schizophrenia risk factors, may interact with other genetic, environmental, and/or developmental risk factors to cause the conserved clinical presentation of impaired working memory.
Key words: basket cell, maternal infection, cannabis, etiology, working memory, prefrontal cortex, parvalbumin, cholecystokinin
Introduction
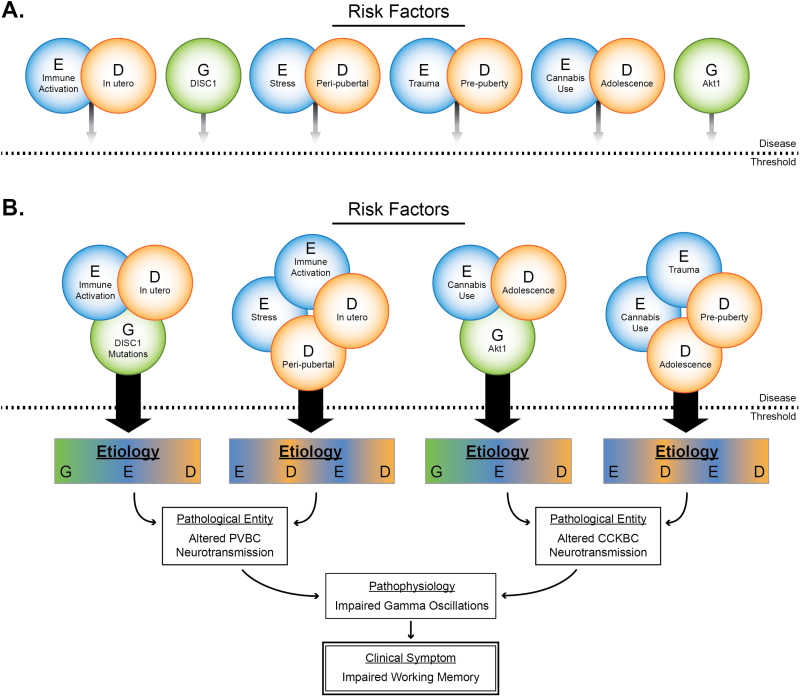
Schizophrenia is a neurodevelopmental syndrome associated with functional impairments extending across social, emotional, perceptive, and cognitive domains.1,2 The complex clinical presentation of schizophrenia is accompanied by an equally complex etiology and pathology, thought to involve genetic susceptibility that provides a vulnerable substrate upon which environmental insults can act.3,4 Many identified gene × environment interactions emerge during particular developmental periods. Thus, the etiologies that produce symptoms of schizophrenia may be thought of as the convergence of gene × environment × development (G × E × D) risk factors, which may need to be present in particular combinations to produce clinically relevant pathology. Such combinations of risk factors are likely necessary to initiate the pathogenic cascade that ultimately produces clinical symptoms.5 As there are likely many sets of G × E × D risk factors capable of ultimately producing an individual clinical symptom of schizophrenia, any given symptom of schizophrenia can represent an equifinal outcome: one that can emerge from different upstream etiologies (figure 1). One research strategy to mitigate this etiological complexity is to focus studies on symptoms that are associated with defined neuronal circuitry pathways, as even different etiologies will likely arrive at a common pathological entity.6 Impaired cognitive ability, namely working memory, is a candidate symptom to study through this type of approach.
Fig. 1.
Model of the equifinal nature of impaired working memory in schizophrenia. (A) Genetic (G), environmental (E), and developmental (D) risk factors are not potent enough to cross the disease threshold. (B) In specific combinations, these risk factors can cross the disease threshold to produce a multifactorial etiology. These distinct etiologies can then initiate pathogenesis to produce a pathological entity, which leads to the core pathophysiology that underlies the clinical symptom. Distinct etiologies can lead to the same pathological entity, and distinct pathological entities can lead to a common pathophysiology. This framework identifies how different, multifactorial etiologies can ultimately lead to a common clinical symptom.
Working memory, the ability to transiently hold information in mind to guide future thoughts or behaviors,7 is a fundamental cognitive function consistently impaired across individuals with schizophrenia.6,8 Indeed, working memory deficits are present before the emergence of positive symptoms,9 are pervasive and persistent,8,10 and may underlie deficits seen in other cognitive domains in schizophrenia.11 Further, cognitive ability, including working memory, is the best predictor of important functional outcomes, such as employment, reintegration into society, and relapse.12,13 Working memory ability depends upon proper activation of circuitry in the prefrontal cortex (PFC).14,15 One process thought to be essential for working memory ability is synchronized neuronal activity in the gamma frequency (30–80 Hz).16–19 Accordingly, individuals diagnosed with schizophrenia show altered PFC activation, including lower power of gamma oscillations, during tasks that recruit working memory.18,20 Thus, alterations in PFC circuitry may contribute to the cognitive impairment seen in individuals with schizophrenia.
Gamma oscillations depend upon the coordinated inhibition of pyramidal cells by perisomatic-targeting GABAergic basket cells that express the calcium-binding protein parvalbumin (PV).18,21 PV basket cells are thought to be critical for the precise, rapid pace of gamma oscillations, and direct stimulation of PV basket cells can generate gamma oscillations.22,23 Gamma oscillatory activity can be modulated by a second population of GABAergic basket cells that express the neuropeptide cholecystokinin (CCK).24 CCK basket cells are thought to be critical for fine-tuning gamma oscillations and PV basket cell activity,21,24 and they also express the cannabinoid 1 receptor (CB1R).25 In addition to distinct molecular and electrophysiological profiles, these 2 basket cell populations also have distinct developmental trajectories from birth to adulthood.26 Interestingly, molecular alterations that may impair the ability of PV and CCK basket cells to regulate gamma oscillations have been identified in studies of postmortem human PFC tissue of individuals with the schizophrenia syndrome.27 In PV basket cell axonal boutons, protein levels of PV and the cardinal GABA-synthesizing enzyme, GAD67, are lower.28,29 In CCK basket cells, mRNA levels of CCK and the CB1R are lower.30
The distinct molecular and developmental profiles of PV and CCK basket cells render them sensitive to different risk factors. However, as each population can influence gamma oscillations, disparate factors acting on either cell type may produce a core pathophysiology of dysfunctional gamma oscillations (figure 1). This paper considers the equifinal nature of working memory deficits in the schizophrenia syndrome by discussing how different multifactorial etiologies, through different neurobiological mechanisms and active at different developmental periods, could give rise to the core pathophysiology of impaired gamma oscillations and the common clinical symptom of impaired working memory.
Maternal Infection-Associated Risk Factors and PV Basket Cell Dysfunction
One ExD risk factor associated with increased risk of schizophrenia is maternal infection.31–33 For example, 1 review of epidemiological studies calculates that approximately 30% fewer individuals would develop schizophrenia if the 3 most common maternal infections were completely prevented.34 However, maternal infection alone is not sufficient to cause schizophrenia. For example, while up to 50% of the general population is infected during influenza pandemics,35 the relative risk of schizophrenia increases 1- to 3-fold after such an outbreak.34,36 As such, most offspring exposed to maternal infection will not go on to develop schizophrenia. Thus, the vulnerability induced by maternal infection must interact with other genetic, environmental, and/or developmental factors to cross the disease threshold.37–40
Experimental animal models of maternal immune activation have provided insights into these multifactor relationships. For example, mice exposed to immune activation in utero show schizophrenia syndrome-relevant behavioral abnormalities in adulthood, such as impaired sensorimotor gating41–43 and cognitive ability,41,43–47 including working memory.48,49 Maternal immune activation models also show molecular changes that are identified in the PFC of individuals with schizophrenia,50 including lower expression of PV,43 GAD67,51 and GAD67 in PV axonal boutons.52
As diverse infectious agents can cause similar molecular and behavioral alterations, some shared mechanism of immune activation likely underlies this effect. One likely candidate is the maternal and/or fetal immune response. Maternal infection significantly increases the levels of immune-associated agents in the fetal brain, namely proinflammatory cytokines.53,54 Proinflammatory cytokines increase the production of reactive oxygen and reactive nitrogen species, which can lead to a state of oxidative stress. While a short period of oxidative stress can be tolerated in the stable adult cortex, the developing fetal brain may be particularly vulnerable given its lower antioxidant capacity, high rate of oxygen metabolism, and significant population of immature cells.55,56 PV basket cells appear to be particularly sensitive to the damaging effects of proinflammatory cytokine release and oxidative stress given their protracted development and the especially high energetic demands their electrophysiological and circuit properties afford.57–59
Importantly, maternal infection alone is unlikely to initiate a pathogenic cascade significant enough to cross the disease threshold. However, once PV basket cells are impaired by prenatal immune activation, additional insults acting upon these vulnerable cells may more readily tip the system over the disease threshold. Indeed, recent animal studies investigating the effects of combinatorial E × D risk factors support just such an interpretation. Studies that induce maternal immune activation followed by peripubertal stress show an additive effect on both behavioral and molecular measures. This combination produces more robust deficits in cognition and sensorimotor gating60 and GAD67 mRNA and protein levels61 than either challenge alone. Moreover, mice exposed to maternal immune activation show greater proinflammatory cytokine release during peripubertal stress.60 Together, these results suggest that maternal immune activation is a risk factor that renders the developing cortex, and especially PV basket cells, more vulnerable to the proinflammatory cytokine release initiated by peripubertal stress. The lower PV and GAD67 expression caused by these upstream insults is predicted to impair gamma oscillations,62 and subsequently working memory ability.27
Experimental animal studies have also investigated G × E × D interactions in the context of maternal immune activation. Mutations in the DISC1 gene have been linked to schizophrenia and other severe mental illnesses,63 and DISC1 mutations in mice can cause reductions in PV and GAD6764–67, as well as deficits in gamma oscillations68 and working memory ability.69 Combining maternal immune activation with a DISC1 genetic mutation (G × E × D risk factors) produces greater deficits in PV in the PFC70 and measures of cognitive ability and sensorimotor gating70–73 than the gene mutation alone. Thus, DISC1 mutations appear to render animals, and putatively humans, more susceptible to in utero immune activation. Accordingly, an epidemiological study demonstrated that in utero exposure to infection combined with genetic liability (ie, a family history of schizophrenia) conferred a significantly increased risk for developing schizophrenia.38 As such, other genetic and environmental risk factors present in an individual may determine the impact of maternal infection on cognitive function, with clinically relevant pathology emerging only when specific risk factors co-occur.
Adolescent Cannabis Use-Associated Risk Factors and CCK Basket Cell Dysfunction
Sustained cannabis use during early adolescence has repeatedly been shown to increase risk for developing schizophrenia.74 It may also potentiate schizophrenia onset: in a large meta-analysis, cannabis users developed psychotic symptoms an average of 3 years earlier than individuals who developed psychotic symptoms but did not use cannabis.75 Further, the link between heavy cannabis use during adolescence and schizophrenia is not readily explained as “self-medication,” as cannabis use almost always precedes emergence of psychotic symptoms in these individuals.74
Cannabis use during adolescence is associated with persistent impairments in cognitive ability, including working memory, which can extend for years after abstinence.74,76–80 For example, studies in rats found that when ∆9-tetrahydrocannabinol (THC), the principal psychoactive chemical in cannabis,81 is chronically administered during adolescence, it can produce spatial working memory deficits in adulthood.82 Given this lasting relationship, cannabis may exert its effects by altering the circuitry involved in regulating gamma oscillations and working memory processes. Studies in both humans and animal models support this interpretation: the power of evoked gamma oscillations is significantly reduced in chronic cannabis users,83 in human subjects acutely administered THC,84 and in adult mice that were administered THC during the pubertal period.85
CCK basket cells may be especially affected by the presence of THC in the brain. THC exerts its actions by activating the CB1R,86 a Gi/o-protein coupled receptor.87 CB1Rs are highly expressed in the PFC,88 and in primate PFC, CB1Rs are almost exclusively expressed on CCK basket cells.25 The unique sensitivity of prefrontal CCK basket cells to exogenous cannabinoids suggests that they may be a key anatomical substrate mediating the long-term effect of cannabis on both gamma oscillations and working memory performance. Indeed, during the intense pyramidal cell firing characteristic of gamma oscillations, endogenous cannabinoids (known as endocannabinoids) are retrogradely released from these pyramidal cells and bind to the CB1Rs located on CCK basket cells.89 Activation of CB1Rs suppresses GABA release from CCK basket cells,89–91 leading to reduced inhibition of pyramidal cells92,93 in a process termed depolarization-induced suppression of inhibition. Chronic activation of CB1Rs via exogenous cannabinoids during prefrontal cortical development may disrupt this usually carefully regulated system and cause persistent impairments in the ability of CCK basket cells to properly regulate gamma oscillations.94
However, despite a significant interaction with both schizophrenia onset74 and working memory impairment,95 and the existence of a plausible biological mechanism for its effects, adolescent cannabis use appears to be neither necessary nor sufficient to cause schizophrenia onset. Indeed, only a minority of adolescents who smoke cannabis develop any kind of psychosis, and not all individuals who develop schizophrenia smoke cannabis during adolescence.74 Thus, as in the case of maternal immune activation, adolescent cannabis use may represent an E × D interactive risk factor, promoting schizophrenia symptomology only in individuals specifically vulnerable due to the presence of other risk factors. Indeed, studies suggest that genetic variation may play a significant role in determining an individual’s susceptibility to the damaging effects of adolescent THC exposure. For example, carriers of the AKT1 rs2494732 C/C single nucleotide polymorphism who also used cannabis were at a 2 times greater risk of developing a psychotic disorder, compared with T/T carriers.96,97 Moreover, the influence of the C/C genotype scaled with frequency of use: C/C allele carriers who used cannabis daily had a 7-fold greater risk of developing a psychotic disorder than T/T allele carriers with equivalent cannabis use.97 Further, heavy cannabis users with the C/C genotype perform worse on cognitive tasks, even after a year of cannabis abstinence.98 As such, the AKT1 rs2494732 C/C genotype may render individuals especially sensitive to the effects of chronic CB1R stimulation during adolescence.
Finally, like maternal immune activation, sustained adolescent cannabis use may interact with other E × D risk factors to compound schizophrenia risk. Childhood trauma is an E × D risk factor99 shown to act in conjunction with adolescent cannabis use to compound risk for schizophrenia onset.100 For example, a national comorbidity study demonstrated that individuals who experienced childhood sexual trauma and used cannabis before 16 years of age were nearly 12 times more likely to develop a psychotic disorder than individuals who only experienced 1 risk factor.101 As such, adolescent cannabis use may represents a single contributory E × D “hit” that may produce the net outcome of schizophrenia only in conjunction with other genetic, environmental, and/or developmental risk factors.
Conclusions
Maternal immune activation and adolescent cannabis use represent just 2 examples, selected from a pool of schizophrenia syndrome risk factors, which can interact with other genetic, environmental, and developmental risk factors to create a schizophrenia etiology102–104 (figure 1). Throughout their lifetimes, individuals are invariably exposed to a wide range of schizophrenia-related risk factors. The studies discussed in this article suggest that these risk factors must occur in particular combinations to cross the disease threshold. It is important to recognize that while these examples outline a useful model of the disease progression, the precise neurobiological underpinnings of disease are invariably more complex. Indeed, impaired PFC gamma oscillations are neither the only mechanism through which the risk factors discussed in this article may confer symptomatology nor the only neurobiological substrate of impaired working memory.
However, a consideration of how particular symptoms can emerge from different etiologies105,106 may be valuable for producing effective and potent treatments for individuals with a mental illness, and preventative measures for individuals at risk for a mental illness.5 The efficacy of such a personalized approach has already been demonstrated in medical conditions ranging from breast cancer107 to cystic fibrosis,108 and identification of individual etiological routes may inform psychiatric treatment as well.109,110 For example, drugs that target CCK basket cell functioning may be uniquely effective in individuals with schizophrenia who have a particular genotype and had heavy use of cannabis during adolescence. Such a technique is highly individualized, with treatments targeting individuals who have experienced specific risk factors, and preventative measures targeting individuals with a high likelihood of experiencing specific risk factors. Indeed, with such an approach, the equifinality of schizophrenia symptoms, and the multifactorial nature of schizophrenia etiologies, may become a useful facet of treatment and prevention, rather than an obstacle in its path.
Acknowledgments
The authors gratefully acknowledge the excellent graphic design skills of Mary Brady, as well as the thought-provoking discussion and valuable feedback of Drs David Lewis, Allison Marin, Joshua Buckholtz, and Mr Kenneth Allen. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Association AP. DSM-V: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 2. Piper M, Beneyto M, Burne TH, Eyles DW, Lewis DA, McGrath JJ. The neurodevelopmental hypothesis of schizophrenia: convergent clues from epidemiology and neuropathology. Psychiatr Clin North Am. 2012;35:571–584. [DOI] [PubMed] [Google Scholar]
- 3. Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baddeley A. Working memory. Science. 1992;255:556–559. [DOI] [PubMed] [Google Scholar]
- 8. Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. [DOI] [PubMed] [Google Scholar]
- 9. Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373–390. [DOI] [PubMed] [Google Scholar]
- 11. Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. [DOI] [PubMed] [Google Scholar]
- 12. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 13. Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67 Suppl 9:3–8; discussion 36-42. [PubMed] [Google Scholar]
- 14. Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. [DOI] [PubMed] [Google Scholar]
- 15. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 16. Howard MW, Rizzuto DS, Caplan JB, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. [DOI] [PubMed] [Google Scholar]
- 17. Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 2012;32:12411–12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Senkowski D, Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry. 2015;77:1010–1019. [DOI] [PubMed] [Google Scholar]
- 21. Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. [DOI] [PubMed] [Google Scholar]
- 22. Cardin JA, Carlén M, Meletis K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. [DOI] [PubMed] [Google Scholar]
- 25. Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull. 2011;37:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014;19:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curley AA, Arion D, Volk DW, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J Physiol. 2012;590:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. [DOI] [PubMed] [Google Scholar]
- 33. Brown AS, Cohen P, Harkavy-Friedman J, et al. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. [DOI] [PubMed] [Google Scholar]
- 34. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glass RJ, Glass LM, Beyeler WE, Min HJ. Targeted social distancing design for pandemic influenza. Emerg Infect Dis. 2006;12:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Selten JP, Frissen A, Lensvelt-Mulders G, Morgan VA. Schizophrenia and 1957 pandemic of influenza: meta-analysis. Schizophr Bull. 2010;36:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clarke MC, Tanskanen A, Huttunen M, et al. Increased risk of schizophrenia from additive interaction between infant motor developmental delay and obstetric complications: evidence from a population-based longitudinal study. Am J Psychiatry. 2011;168:1295–1302. [DOI] [PubMed] [Google Scholar]
- 38. Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–1030. [DOI] [PubMed] [Google Scholar]
- 39. Horváth S, Mirnics K. Schizophrenia as a disorder of molecular pathways. Biol Psychiatry. 2015;77:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blomstrom A, Karlsson H, Gardner R, Jorgensen L, Magnusson C, Dalman C. associations between maternal infection during pregnancy, childhood infections and the risk of subsequent psychotic disorder-a Swedish cohort study of nearly 2 million individuals. Schizophr Bull. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. [DOI] [PubMed] [Google Scholar]
- 42. Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wischhof L, Irrsack E, Osorio C, Koch M. Prenatal LPS-exposure–a neurodevelopmental rat model of schizophrenia–differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:17–30. [DOI] [PubMed] [Google Scholar]
- 44. Vorhees CV, Graham DL, Braun AA, et al. Prenatal immune challenge in rats: altered responses to dopaminergic and glutamatergic agents, prepulse inhibition of acoustic startle, and reduced route-based learning as a function of maternal body weight gain after prenatal exposure to poly IC. Synapse. 2012;66:725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meyer U, Nyffeler M, Engler A, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. 2006;173:243–257. [DOI] [PubMed] [Google Scholar]
- 47. Ibi D, Nagai T, Kitahara Y, et al. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64:297–305. [DOI] [PubMed] [Google Scholar]
- 48. Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. [DOI] [PubMed] [Google Scholar]
- 49. Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res. 2012;140:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75:307–315. [DOI] [PubMed] [Google Scholar]
- 51. Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull. 2014;40:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dickerson DD, Overeem KA, Wolff AR, Williams JM, Abraham WC, Bilkey DK. Association of aberrant neural synchrony and altered GAD67 expression following exposure to maternal immune activation, a risk factor for schizophrenia. Transl Psychiatry. 2014;4:e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miller SL, Wallace EM, Walker DW. Antioxidant therapies: a potential role in perinatal medicine. Neuroendocrinology. 2012;96:13–23. [DOI] [PubMed] [Google Scholar]
- 56. Ikonomidou C, Kaindl AM. Neuronal death and oxidative stress in the developing brain. Antioxid Redox Signal. 2011;14:1535–1550. [DOI] [PubMed] [Google Scholar]
- 57. Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Do KQ, Cuenod M, Hensch TK. Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr Bull. 2015;41:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang Z, Cowell RM, Nakazawa K. Convergence of genetic and environmental factors on parvalbumin-positive interneurons in schizophrenia. Front Behav Neurosci. 2013;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giovanoli S, Engler H, Engler A, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. [DOI] [PubMed] [Google Scholar]
- 61. Deslauriers J, Larouche A, Sarret P, Grignon S. Combination of prenatal immune challenge and restraint stress affects prepulse inhibition and dopaminergic/GABAergic markers. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:156–164. [DOI] [PubMed] [Google Scholar]
- 62. Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci. 2011;31:18137–18148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Porteous DJ, Thomson PA, Millar JK, et al. DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol Psychiatry. 2014;19:141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee FH, Zai CC, Cordes SP, Roder JC, Wong AH. Abnormal interneuron development in disrupted-in-schizophrenia-1 L100P mutant mice. Mol Brain. 2013;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ayhan Y, Abazyan B, Nomura J, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2011;16:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shen S, Lang B, Nakamoto C, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hikida T, Jaaro-Peled H, Seshadri S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA. 2007;104:14501–14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sauer JF, Struber M, Bartos M. Impaired fast-spiking interneuron function in a genetic mouse model of depression. ELife 2015;4:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Johnstone M, Thomson PA, Hall J, McIntosh AM, Lawrie SM, Porteous DJ. DISC1 in schizophrenia: genetic mouse models and human genomic imaging. Schizophr Bull. 2011;37:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ibi D, Nagai T, Koike H, et al. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav Brain Res. 2010;206:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nagai T, Kitahara Y, Ibi D, Nabeshima T, Sawa A, Yamada K. Effects of antipsychotics on the behavioral deficits in human dominant-negative DISC1 transgenic mice with neonatal polyI:C treatment. Behav Brain Res. 2011;225:305–310. [DOI] [PubMed] [Google Scholar]
- 72. Nagai T, Ibi D, Yamada K. Animal model for schizophrenia that reflects gene-environment interactions. Biol Pharm Bull. 2011;34:1364–1368. [DOI] [PubMed] [Google Scholar]
- 73. Lipina TV, Zai C, Hlousek D, Roder JC, Wong AH. Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J Neurosci. 2013;33:7654–7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to Pot - a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. [DOI] [PubMed] [Google Scholar]
- 76. Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–319. [DOI] [PubMed] [Google Scholar]
- 77. Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. [DOI] [PubMed] [Google Scholar]
- 78. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA. Repeated Δ9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am J Psychiatry. 2014;171:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Verrico CD, Liu S, Bitler EJ, et al. Delay- and dose-dependent effects of delta(9)-tetrahydrocannabinol administration on spatial and object working memory tasks in adolescent rhesus monkeys. Neuropsychopharmacology. 2012;37:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 82. Rubino T, Realini N, Braida D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. [DOI] [PubMed] [Google Scholar]
- 83. Skosnik PD, D’Souza DC, Steinmetz AB, et al. The effect of chronic cannabinoids on broadband EEG neural oscillations in humans. Neuropsychopharmacology. 2012;37:2184–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cortes-Briones J, Skosnik PD, Mathalon D, et al. Delta-THC disrupts gamma (gamma)-band neural oscillations in humans. Neuropsychopharmacology. 2015;232:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Raver SM, Haughwout SP, Keller A. Adolescent cannabinoid exposure permanently suppresses cortical oscillations in adult mice. Neuropsychopharmacology. 2013;38:2338–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hoffman AF, Lupica CR. Synaptic targets of Δ9-tetrahydrocannabinol in the central nervous system. Cold Spring Harb Perspect Med. 2013;3:a012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 88. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. [DOI] [PubMed] [Google Scholar]
- 89. Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. [DOI] [PubMed] [Google Scholar]
- 90. Földy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee SH, Földy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30:7993–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. [DOI] [PubMed] [Google Scholar]
- 93. Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. [DOI] [PubMed] [Google Scholar]
- 94. Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508. [DOI] [PubMed] [Google Scholar]
- 95. Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1:81–98. [DOI] [PubMed] [Google Scholar]
- 96. van Winkel R. Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry. 2011;68:148–157. [DOI] [PubMed] [Google Scholar]
- 97. Di Forti M, Iyegbe C, Sallis H, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry. 2012;72:811–816. [DOI] [PubMed] [Google Scholar]
- 98. van Winkel R, van Beveren NJ, Simons C. AKT1 moderation of cannabis-induced cognitive alterations in psychotic disorder. Neuropsychopharmacology. 2011;36:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wilkinson ST, Radhakrishnan R, D’Souza DC. Impact of cannabis use on the development of psychotic disorders. Curr Addict Rep. 2014;1:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Houston JE, Murphy J, Adamson G, Stringer M, Shevlin M. Childhood sexual abuse, early cannabis use, and psychosis: testing an interaction model based on the National Comorbidity Survey. Schizophr Bull. 2008;34:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40:190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. McOmish CE, Burrows EL, Hannan AJ. Identifying novel interventional strategies for psychiatric disorders: integrating genomics, ‘enviromics’ and gene-environment interactions in valid preclinical models. Br J Pharmacol. 2014;171:4719–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. van Os J, Rutten BP, Myin-Germeys I, et al. Identifying gene-environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull. 2014;40:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Morris SE, Insel TR. Reconceptualizing schizophrenia. Schizophr Res. 2011;127:1–2. [DOI] [PubMed] [Google Scholar]
- 106. Keshavan MS, Nasrallah HA, Tandon R. Schizophrenia, “Just the Facts” 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophr Res. 2011;127:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nandy A, Gangopadhyay S, Mukhopadhyay A. Individualizing breast cancer treatment-The dawn of personalized medicine. Exp Cell Res. 2014;320:1–11. [DOI] [PubMed] [Google Scholar]
- 108. Clancy JP, Jain M. Personalized medicine in cystic fibrosis: dawning of a new era. Am J Respir Crit Care Med. 2012;186:593–597. [DOI] [PubMed] [Google Scholar]
- 109. McMahon FJ, Insel TR. Pharmacogenomics and personalized medicine in neuropsychiatry. Neuron. 2012;74:773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]