Abstract
Objective
To compare foam bubble size and bubble size distribution, stability, and degradation rate of commercially available polidocanol endovenous microfoam (Varithena®) and physician-compounded foams using a number of laboratory tests.
Methods
Foam properties of polidocanol endovenous microfoam and physician-compounded foams were measured and compared using a glass-plate method and a Sympatec QICPIC image analysis method to measure bubble size and bubble size distribution, Turbiscan™ LAB for foam half time and drainage and a novel biomimetic vein model to measure foam stability. Physician-compounded foams composed of polidocanol and room air, CO2, or mixtures of oxygen and carbon dioxide (O2:CO2) were generated by different methods.
Results
Polidocanol endovenous microfoam was found to have a narrow bubble size distribution with no large (>500 µm) bubbles. Physician-compounded foams made with the Tessari method had broader bubble size distribution and large bubbles, which have an impact on foam stability. Polidocanol endovenous microfoam had a lower degradation rate than any physician-compounded foams, including foams made using room air (p < 0.035). The same result was obtained at different liquid to gas ratios (1:4 and 1:7) for physician-compounded foams. In all tests performed, CO2 foams were the least stable and different O2:CO2 mixtures had intermediate performance. In the biomimetic vein model, polidocanol endovenous microfoam had the slowest degradation rate and longest calculated dwell time, which represents the length of time the foam is in contact with the vein, almost twice that of physician-compounded foams using room air and eight times better than physician-compounded foams prepared using equivalent gas mixes.
Conclusion
Bubble size, bubble size distribution and stability of various sclerosing foam formulations show that polidocanol endovenous microfoam results in better overall performance compared with physician-compounded foams. Polidocanol endovenous microfoam offers better stability and cohesive properties in a biomimetic vein model compared to physician-compounded foams. Polidocanol endovenous microfoam, which is indicated in the United States for treatment of great saphenous vein system incompetence, provides clinicians with a consistent product with enhanced handling properties.
Keywords: Foam drainage times, foam half time, physician-compounded foams, bubble size, bubble size distribution, varicose veins, polidocanol endovenous microfoam, sclerotherapy, biomimetic analysis method, polidocanol injectable foam
Introduction
Physician-compounded foams (PCFs) have been introduced in vein treatment with the aim of increasing efficacy and treating larger varicose veins relative to liquid sclerosants.1,2 However, foams are not all the same and, in fact, they can be dramatically different from each other. The performance of foams is highly dependent on their physical characteristics such as gas composition, the different absorption rates of nitrogen and carbon dioxide in the bloodstream, and bubble size.3–5
PCFs offer several advantages over traditional liquid sclerosants. When injected into a vein, a cohesive foam displaces the blood (rather than mixing with it), creating better contact with the vein wall. Foam treatment offers the possibility of using lower sclerosant concentrations.6 This, in turn, increases the safety of foam treatment as shown in clinical trials.7–9 Furthermore, foam is echogenic, which improves visibility and treatment accuracy.10 Also, foam treatment can be performed in an outpatient setting without need for sedation or tumescent anesthesia.11
Foam treatment also presents challenges. Room air (RA) forms stable foam, but because the nitrogen it contains does not dissolve efficiently in blood, nitrogen bubbles may persist and can cause adverse effects.12,13 Carbon dioxide (CO2) foams can be made, but the increased solubility results in foams that coarsen rapidly, leading to drastically reduced stability. PCF methods may generate large gas bubbles that may be potentially problematic in the circulation. Strategies such as using CO2 rather than RA and limiting the injected volume of PCF to less than 10 mL have been proposed to reduce the incidence of serious complications, since significant neurological events have occurred after injections of as little as 4 mL of PCF. These neurological events have been attributed to nitrogen/air.14–16 In fact, two reports have documented that all patients injected with PCF for the treatment of venous varicosities have gas bubbles visible in the right heart chambers, and some patients, such as those with patent foramen ovale (PFO) or other right-to-left shunts, have gas bubbles in the left heart chambers.17,18
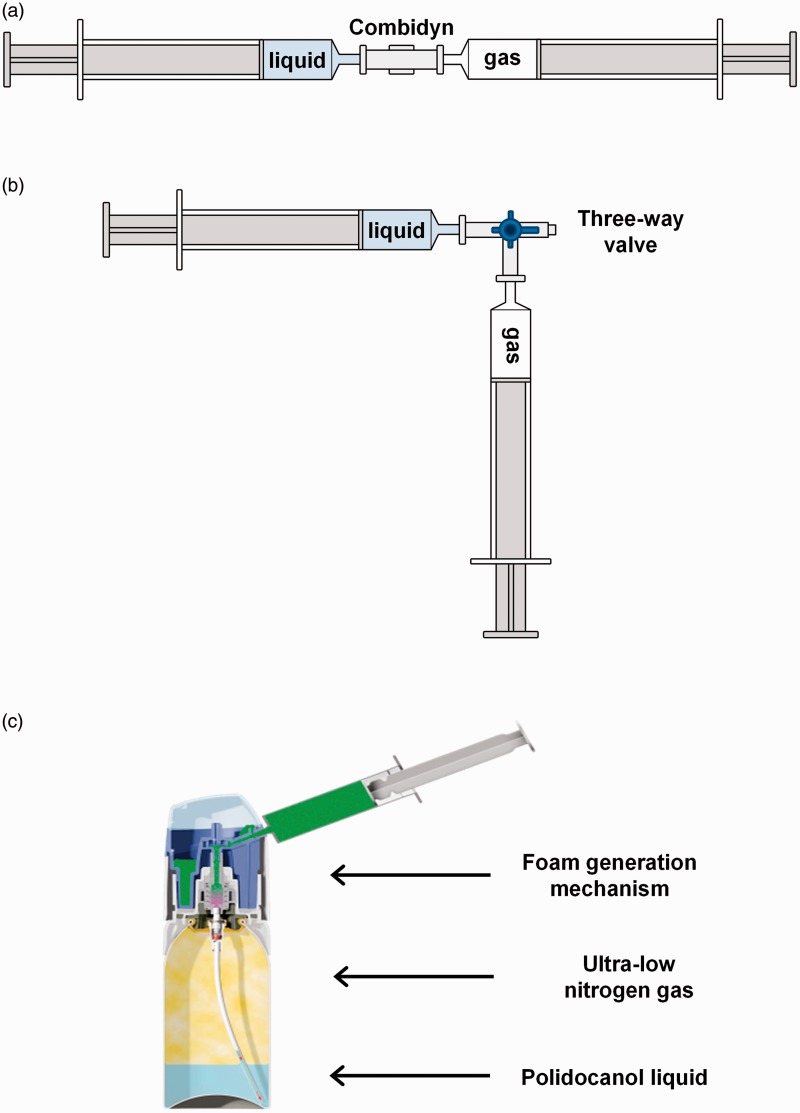
The two most popular techniques that clinicians use to generate PCFs are the double syringe system (DSS) and the Tessari method.19,20 DSS involves passing the sclerosant liquid and gas between two syringes joined by a simple straight connector (Figure 1(a)). The Tessari method is similar, but the straight connector is replaced with a three-way valve (Figure 1(b)). Although these two methods are very similar, the DSS method is felt to produce slightly better foam.21 Polidocanol endovenous microfoam (PEM) (Varithena® (polidocanol injectable foam 1%), Provensis Ltd., a BTG International group company) is a new product designed to overcome the challenges associated with PCFs. PEM is generated by a proprietary device that produces consistent, pharmaceutical-grade low-nitrogen (<0.8%), O2:CO2 (65:35) foam (Figure 1(c)). In a recent case series of 60 patients with middle cerebral artery bubble emboli during or after treatment with PEM, no evidence of subclinical cerebral injury was found on MRI.22 In addition, in two pivotal phase 3 clinical trials using PEM, there were no clinically meaningful neurologic adverse events observed.23
Figure 1.
Methods for producing PCFs and PEM. In the DSS method, syringes are connected by a Combidyn® adapter (a), while in the Tessari method, they are connected by a three-way valve (b). In both techniques, the foam was produced by passing the polidocanol solution (liquid phase) from one syringe, 10 times into and out of the other syringe initially containing the gas or gas mixture (gaseous phase). Foam was produced at room temperature (20℃–22℃). The proprietary canister system for generating PEM (Varithena®) is shown in (c).
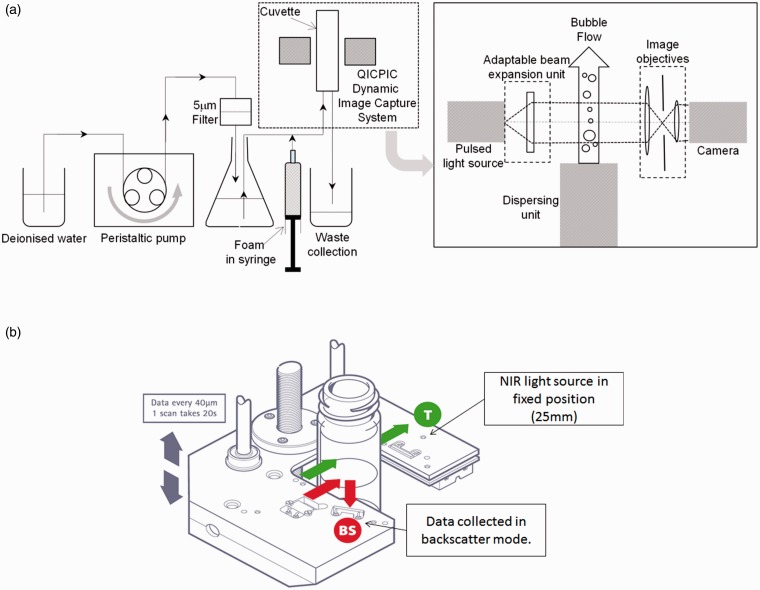
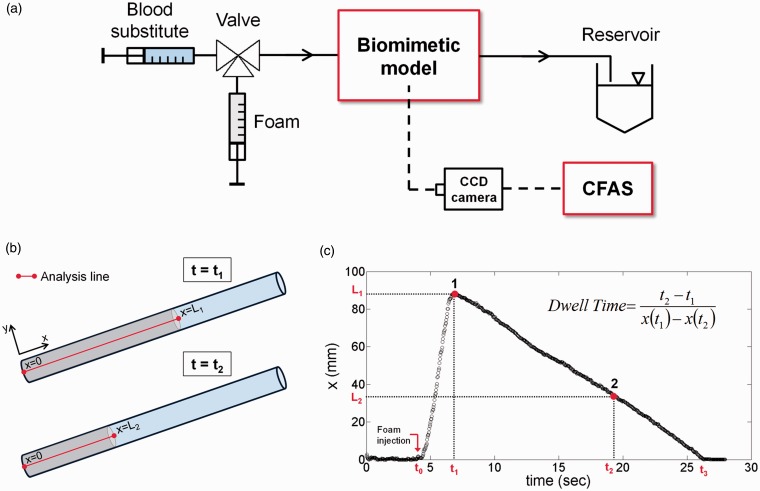
Methods for measuring bubble size and size distribution include the Sympatec image analysis sensor QICPIC (Sympatec Ltd., Bury, Lancashire, UK) and Turbiscan™ LAB apparatus (Formulaction SAS, L’Union, France) (Figure 2). Sympatec provides a bubble size distribution for microbubbles flowing in deionized water, whereas Turbiscan™ provides dynamic information on foam. The speed at which liquid separates from the body of foam has been used as a measure of foam stability.24 Foam drainage time (FDT) and rate are good measures of foam stability.21 Methods for measuring foam stability and cohesiveness include the Turbiscan™ LAB operated in the scanning detector mode for foam drainage kinetics and foam half time (FHT), and operated in the fixed detector mode for FDT determination. The novel biomimetic analysis system was developed for the quantification of foam properties under clinically relevant conditions to establish a robust method for comparative characterisation of PEM and PCFs. The high-resolution computational video analysis system allows accurate quantification of foam dynamic behaviour, including foam plug expansion rate, degradation rate (DR) and dwell time (DT) (Figure 3).25
Figure 2.
Methods for measuring bubble size distribution. Sympatec QICPIC image analysis sensor (a) and Turbiscan™ LAB apparatus (b).
Figure 3.
Schematic of the biomimetic vein model set-up (a). Foam is injected into the tube over time t1 to form a column of length x (mm) (b). On completion of the injection at x = L1, the foam degrades over time t2 to a length of x = L2, whereby the DR and DT may be attained (c).
CFAS: computational foam analysis system.
This paper reports the results of a number of tests performed to compare foam bubble size and bubble distribution, stability, and DR of PEM and PCFs. These foams were also investigated in the biomimetic vein model to relate stability to clinical function.
Materials and methods
Foam production and characterisation
Full details about foam production and characterisation methods are provided in the Supplementary information sections S1 and S2, respectively.
For preparation of PCFs, 1% aqueous buffered polidocanol solution was used throughout these studies. Foam densities were at a liquid to gas ratio of 1:7 for direct comparison with PEM or at 1:4 to represent commonly used formulations. DSS and Tessari methods were used to create PCFs.
PEM consists of a proprietary O2:CO2 (65:35) gas mixture with ultra-low nitrogen content (<0.8%) and 1% polidocanol solution contained within a pressurized canister and combined on discharge as uniform microfoam. Sterile canisters of the product were used to generate 5 mL of microfoam for experimentation.
Foam properties of PEM and PCFs were measured and compared using the glass-plate method and Sympatec QICPIC image analysis to measure bubble size and bubble size distribution. The Turbiscan™ LAB apparatus for FHT and FDT, and the biomimetic vein model to measure foam stability. These are summarised in Table 1, and methodological details are reported in the Supplementary information section S2.
Table 1.
Summary of the methods of foam characterisation employed in the present study.
| Equipment | Analysis | Supplier |
|---|---|---|
| Glass-plate method | Bubble size and bubble size distribution | In-house method developed at BTG |
| Sympatec QICPIC | Bubble size and bubble size distribution | Sympatec Ltd., Bury, Lancashire, UK |
| Turbiscan™ LAB | FHT and FDT (foam stability) | Formulaction SAS, L’Union, France |
| Biomimetic vein model | Foam dwell time/degradation rate (foam stability) | In-house method developed at University of Southampton |
Statistical analysis
One-way analysis of variance (ANOVA) was performed to analyse the differences between group means using OriginPro (Origin Lab Corp., Northampton, MA) software package. Pairwise comparison tests were performed using the Bonferroni method. The significance level was set to 0.05 (i.e. differences were considered to be statistically significant for p-value < 0.05). The number of experimental repeats for each test is reported in Table 2. Selected relevant p-values for comparison between PEM and PCFs using the different methods are shown in Table 3.
Table 2.
Number of experimental repeats for each foam characterisation experiment performed.
| Experiment | Number of repeats (N) |
|---|---|
| Foam bubble sizing | 5 |
| Foam half time | 5 |
| Foam drainage time | 4 |
| Foam dwell time | 4 |
Table 3.
Selected p-values obtained from pairwise statistical comparisons between PEM and PCF foams (p ≪ 0.01 indicates values lower than 0.001).
| Foam formulations | p-value |
|---|---|
| Foam drainage time (FDT, Figure 7) | |
| PEM vs. DSS (1:7) | 0.01 < p < 0.05 (=0.033) |
| PEM vs. Tessari (1:7) | p ≪ 0.01 |
| PEM vs. DSS (1:4) | 0.01 < p < 0.05 (=0.012) |
| PEM vs. Tessari (1:4) | p ≪ 0.01 |
| Foam half time (FHT, Figure 8) | |
| DSS (combined) vs Tessari (combined) | 0.01 < p < 0.05 (=0.045) |
| PEM vs DSS RA (1:7) | p ≪ 0.01 |
| PEM vs. DSS 65:35 O2:CO2 (1:7) | p > 0.05 |
| PEM vs. DSS 35:65 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. DSS 30:70 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. DSS 23:77 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. DSS 100 CO2 (1:7) | p ≪ 0.01 |
| Dwell time (DT, Figure 9) | |
| PEM vs. DSS RA (1:7) | 0.01 < p < 0.05 (=0.018) |
| PEM vs. DSS 65:35 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. DSS 35:65 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. DSS 30:70 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. DSS 23:77 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. DSS 100 CO2 (1:7) | p ≪ 0.01 |
| PEM vs. Tessari RA (1:7) | p ≪ 0.01 |
| PEM vs. Tessari O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. Tessari 35:65 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. Tessari 30:70 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. Tessari 23:77 O2:CO2 (1:7) | p ≪ 0.01 |
| PEM vs. Tessari 100 CO2 (1:7) | p ≪ 0.01 |
| PEM vs. DSS RA (1:4) | p < 0.01 (=0.0054) |
| PEM vs. DSS 65:35 O2:CO2 (1:4) | p ≪ 0.01 |
| PEM vs. DSS 35:65 O2:CO2 (1:4) | p ≪ 0.01 |
| PEM vs. DSS 100 CO2 (1:4) | p ≪ 0.01 |
Results
Comparison of methods used to measure bubble size distribution
Figure 4 provides a visual comparison of the bubble images captured using the glass plate and the Sympatec methods and the frequency of bubble size measured by these two methods for PEM. The glass-plate method shows a closely packed foam (Figure 4(a)) and a relatively tightly distributed bubble size distribution with no bubbles >∼300 µm in diameter (Figure 4(b)). The image captured from the Sympatec shows the bubbles are no longer in close contact with one another at the point of measurement, having been separated in the flowing carrier liquid (Figure 4(c)). This method over-reports the true bubble size with bubbles up to ∼600 µm, and bubbles smaller than 15 µm in diameter are not detectable because of foam coarsening during the time taken to administer the foam into the instrument, and the subsequent loss of smaller bubbles before they reach the detector in the instrument (Figure 4(d)).
Figure 4.
Comparison of glass plate and Sympatec method analyses of polidocanol endovenous microfoam. (a) Image of PEM from the optical image analysis method and (b) bubble size distribution measured for this foam; compared to (c) a single frame image of the same sample of PEM captured from the Sympatec dynamic image capture method and (d) the bubble size distribution measured by this method (over a 15 s period, corresponding to 375 image frames). Note how the Sympatec over-reports the true bubble size.
Bubble size distributions of various foam formulations
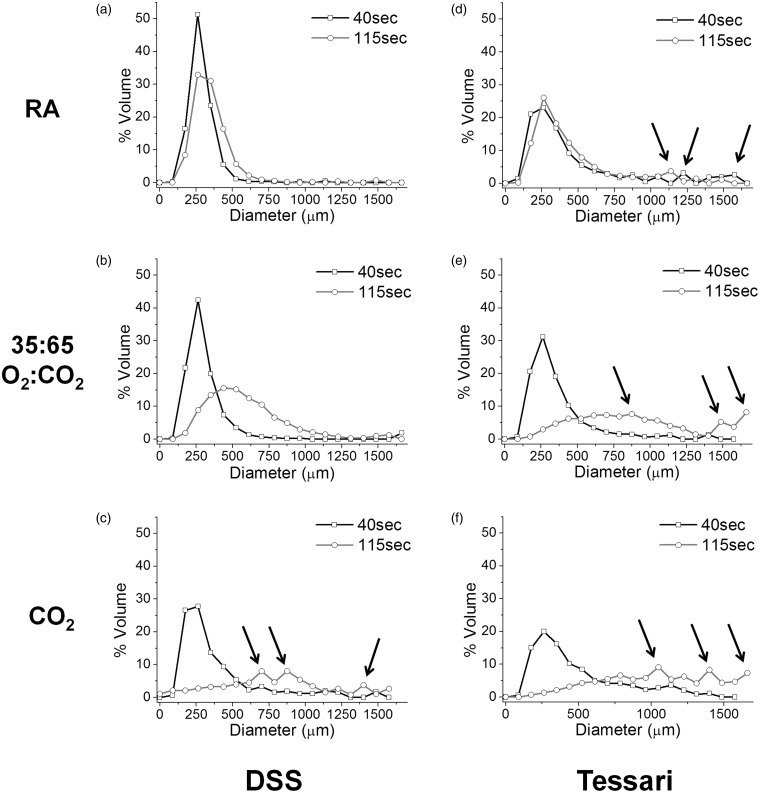
Figure 5 shows the distribution of bubble sizes (in terms of volume fraction) generated using the Sympatec method for PCFs (liquid:gas ratio of 1:7) produced using RA, O2:CO2 (35:65) and CO2, and the DSS and Tessari foam preparation methods. Initial bubble size distribution for prepared foams is narrowest for RA < O2:CO2 < CO2 for PCFs produced by either preparation method. The Tessari method clearly generates more large bubbles compared to the DSS method regardless of the gas mixture used.
Figure 5.
Size distributions of physician-compounded foams (DSS vs. Tessari) with liquid:gas ratio 1:7, obtained using the Sympatec method. Bubble size distribution curves for PCFs using different gas formulations (a + d RA; b + e: O2:CO2 of 35:65; c + f 100% CO2) for both the DSS and Tessari methods 40 s and 115 s after foam preparation. Arrows highlight existence of larger bubbles in the PCF (n = 5).
RA: room air.
When PEM bubble size distribution is compared to a PCF made with the same gas mixture (O2:CO2 (65:35)) and liquid:gas ratio (1:7) using either the DSS or Tessari methods, there is clearly a narrower bubble size distribution for PEM when measured using the Sympatec method 40 and 115 s after foam preparation (Figure 6). Generally, the contrast is more pronounced when PEM is compared with the other O2:CO2 PCFs made with higher CO2 content (Figures 6(a) vs. 5(b) to (f)). A greater number of larger bubbles were present for PCFs, particularly using the Tessari method. At 40 s, PEM bubble size distribution is similar to that of RA foam (maximum bubble size <500 µm by this method) and at 115 s only slightly broader than that of RO foams produced using the DSS method (Figures 6(a) vs. 5(a)) and without the large bubbles present for RA foam made by the Tessari method (Figures 6(a) vs. 5(d)).
Figure 6.
Comparison of bubble size distributions at 40 s and 115 s for PEM (a) compared to PCF O2:CO2 (65:35) made by DSS (b) and Tessari (c) methods. Arrows highlight existence of large bubbles in the foam (n = 5).
Foam drainage time (FDT)
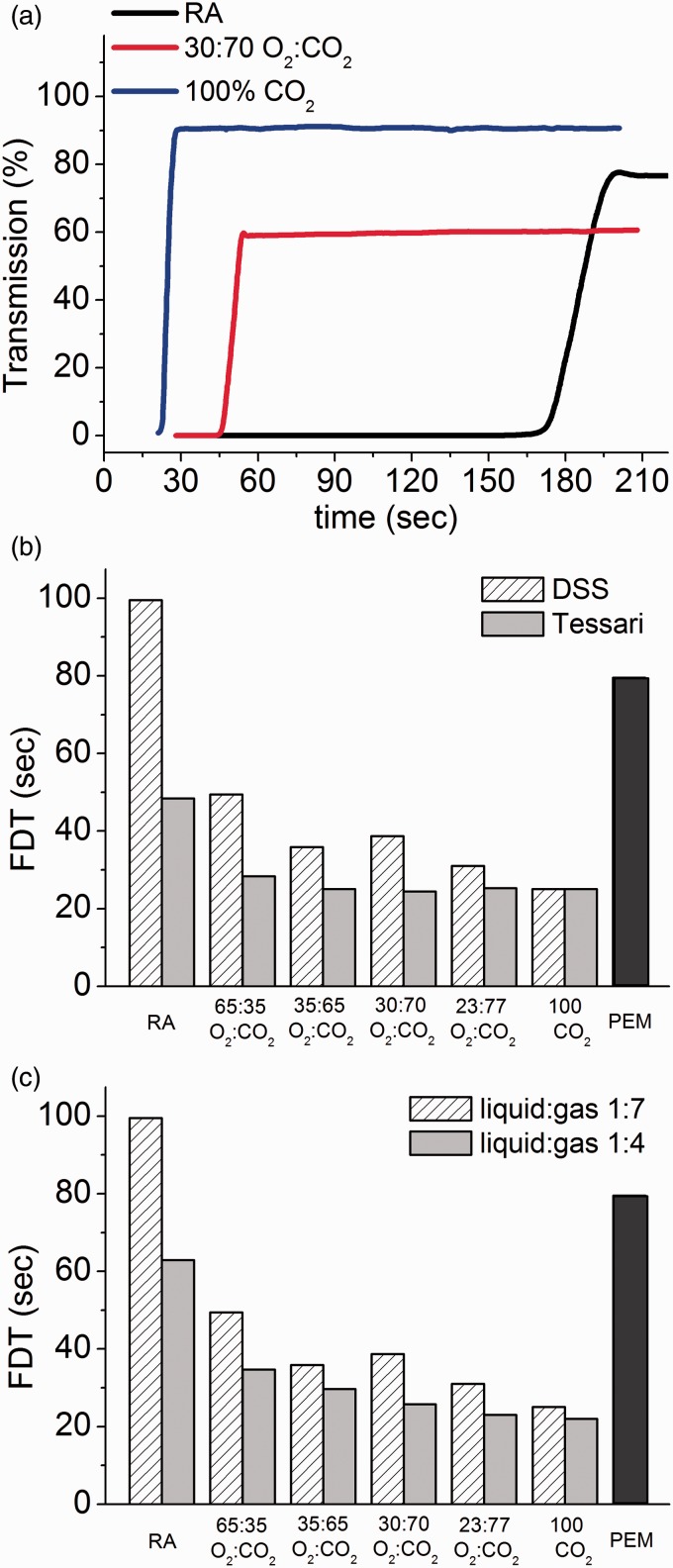
FDT is an important indicator of foam stability.21 Essentially, if the foam drains rapidly, it will coarsen and degrade more quickly; the cohesiveness of the foam will be of short duration and, thus, there will be less time for the drug to be in contact with the vessel wall because the degrading foam will not be able to properly displace the blood in the vessel lumen. In this test method, the FDT is the time at which light transmission is detected at the bottom of the foam column. Figure 7(a) shows the percent transmission of light through the foam over a period of time. For 100% CO2, the foam drains very quickly and so light passes through the liquid layer almost immediately as indicated by the blue curve in the image. At a 30:70 O2:CO2 composition (red curve), the resulting foam also drains very quickly, but the curve does not inflect upward until the 45 s mark, as the foam is more stable and, therefore, drains more slowly than foam made with 100% CO2. RA foam (black curve) is most stable and takes approximately 165 s before light begins to transmit through the draining liquid. FDT is shown for PEM compared with the DSS vs. Tessari foams produced with different gas compositions and 1:7 liquid:gas ratio (Figure 7(b)). In general, FDT is longer for DSS-prepared PCFs when compared against the Tessari, with the difference becoming greater as the CO2 levels in the foams decrease and the foams become more stable. The FDT of PEM is greater than any combination of gas mixture and method of preparation, with the exception of RA using the DSS method. Figure 7(c) shows the FDT for PEM and PCFs produced using the DSS method with two different liquid-to-gas ratios (1:7 and 1:4). The FDT increases in the order RA > PEM > O2:CO2 ≫ CO2-only, with foams of a liquid:gas ratio of 1:7 consistently more stable (longer FDTs) than those prepared with a 1:4 ratio. PEM has a statistically significantly higher FDT (close to that of RA) when compared to all CO2-containing PCFs at either liquid:gas ratio, prepared by either the DSS or Tessari method (p < 0.035).
Figure 7.
Example foam drainage time curves used to measure FDT (a); FDT for DSS versus Tessari, and compared with PEM (b); FDT for different PCFs made using the DSS method at 1:4 and 1:7 liquid to gas ratios, and compared with PEM (c). Standard deviation ranged from 0.37% to 5.58% of the mean (n = 4).
RA: room air; PEM: polidocanol endovenous microfoam; FDT: foam drainage time.
Foam Half Time (FHT)
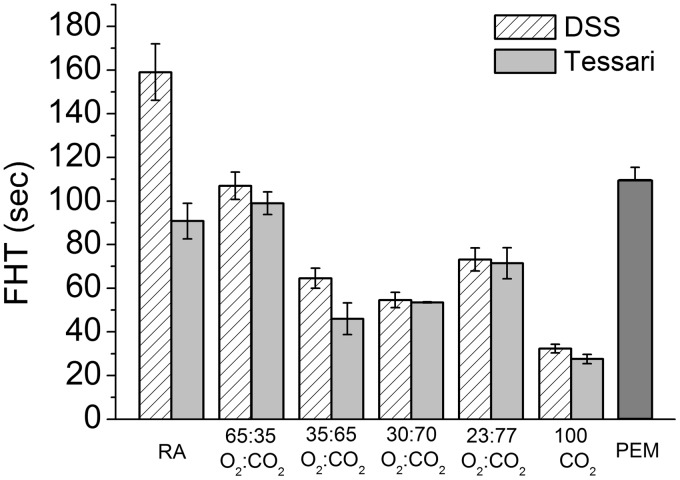
The comparison of FHT for various PCF formulations made using the DSS and Tessari methods (1:7 liquid:gas ratio) relative to PEM was generated by the Turbiscan™. PEM displayed longer FHT compared to all CO2-containing PCFs, indicating that PEM had greater stability (Figure 8).
Figure 8.
Comparison of the foam half time (Turbiscan™) for various PCF formulations made using DSS and Tessari methods (1:7 liquid:gas ratio) and foam half time for PEM. PEM displayed a longer FHT than CO2-containing PCFs (n = 5).
FHT: foam half time; RA: room air; PEM: polidocanol endovenous microfoam.
Degradation rate/dwell time (DR/DT)
DR was evaluated using a biomimetic vein model to assess the ability of the foam to displace a blood substitute. DT is a more clinically meaningful expression of DR as it represents the amount of time that the foam is in contact with the vein wall and can act on the endothelium. DT is derived from DR and is calculated as the inverse of the DR using the following mathematical expression (refer to Figure 3(c))
| (1) |
The experimental set-up consisted of a segment of polytetrafluoroethylene (PTFE) tubing (either 4 mm or 10 mm in diameter) (Thermo Scientific Inc., USA) filled with a blood substitute and fixed to a platform with an adjustable inclination angle (Figure 3(a)). On initial foam injection, a foam plug was formed, which displaced the blood substitute as it travelled upwards along the tubing (plug expansion phase) (Figure 3(b)), while real-time video images were captured simultaneously. Individual foam plugs were transiently stable and then entered the plug degradation phase, during which the plug interface receded towards the initial injection site (Figure 3(c)), ending in complete plug degradation. Videos obtained from both plug expansion and degradation phases were analysed computationally (see Supplementary section S2.4).25
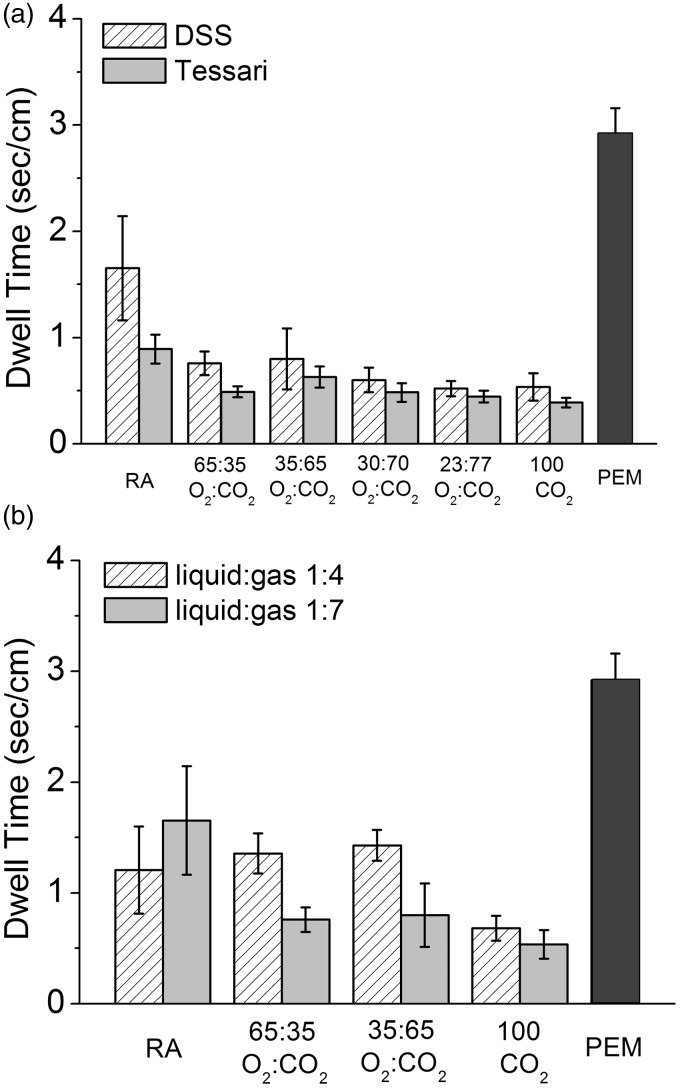
PCFs of various gas formulations were prepared by DSS or Tessari methods and introduced into the biomimetic vein model. The performance of PCFs (1:7 liquid:gas ratio) was compared with PEM, which demonstrated that CO2-containing PCFs, prepared by either method and regardless of gas formulation, had DRs faster (12.51 ± 4.49 to 25.81 ± 3.09 mm/s) than that of PEM (3.43 ± 0.29 mm/s, p < 0.05) (Figure S3.1a). Foams prepared using RA were also less cohesive and degraded more rapidly (6.05 ± 1.79 to 11.22 ± 1.72 mm/s) than PEM, although for RA made using DSS, this trend was not statistically significant (Figure S3.1a). PEM was also compared with selected PCF formulations produced using the DSS method at liquid:gas ratios of 1:4 and 1:7 (Figure S3.1b). Again, PEM had a statistically lower DR compared with all other CO2-based PCFs regardless of gas ratio (p < 0.04) and a non-statistical trend for lower DR than the RA PCFs. In general, in this model, the PCFs made with 1:4 ratios had a trend to lower DR than the corresponding PCFs made at 1:7 ratios. This is contrary to the findings for FDT for the equivalent PCFs. PEM had the longest DT, almost twice that of PCFs using RA and approximately eight times better than PCFs prepared using equivalent gas mixtures (Figure 9(a) and (b)).
Figure 9.
PEM had the longest calculated DT, almost twice that of PCFs using RA and approximately eight times better than PCFs prepared using equivalent gas mixtures in a biomimetic model (n = 4).
RA: room air; PEM: polidocanol endovenous microfoam.
Discussion
For many years, physicians have compounded RA foams. However, lack of foam homogeneity can affect the viscosity and stability of these products. Broad bubble size distributions promote foam coarsening and degradation, whilst the lack of solubility of nitrogen in the blood results in isolated bubbles persisting in the circulation. Foams with smaller and more uniform bubble size possess a lower DR, indicative of a more cohesive and stable foam that should ensure better contact with the endothelium of the vessel wall when injected into the vein. The ideal foam, then, should be durable enough to allow injection before separating into its gas and liquid components, yet short-lived enough to break down once injected. In this study, we compared methods for determining bubble size and bubble size distribution of foam formulations commonly used in vein treatment as well as methods to establish how the foam bubble size characteristics are related to the stability and cohesive properties of the foam. The optical image analysis method is an established method for static foam bubble sizing and bubble size distribution measured for freshly generated foam within its delivery syringe. The Sympatec method is a convenient tool for generating foam bubble size distribution and permits multiple measurements with differing time delays, while Turbiscan™ permits the measurement of foam coarsening on a continuous basis.
Regardless of measurement technique, we noted consistent differences between foam formulations. RA PCFs produce foams with smaller bubbles, which are inherently more stable but due to the insolubility of nitrogen carry a higher risk of transient ischemic attack (TIA).26 Replacement of RA with CO2 results in PCFs with larger initial bubble size distribution and increasingly rapid coarsening with increasing CO2 content. PEM produces foams of smaller bubble size and narrower distribution, which is more comparable with RA than CO2-containing PCFs made by either the DSS or Tessari method (Figures 5 and 6). Therefore, PEM may have the safety benefit of absorbability because of the absence of nitrogen and the efficacy of stable small-bubble foam. PEM produces no large bubbles (Figure 6), unlike the DSS and Tessari methods. The neurologic disturbances reported with foam sclerotherapy might be related to gas embolisms that originate from the foam,12,15,27 although this might not be the only factor, as recent evidence suggests a role for the release of endothelin-128 and histamine29 in these disturbances, but this role remains hypothetical. In contrast, when CO2 replaced air the frequency of bubbles seen on transcranial Doppler did not reduce but the symptoms were almost eliminated,27 and in the few reported cases where urgent CT scanning was performed following onset of neurological incident, gas was found replacing the contents of the vertebral or middle cerebral artery and in the cerebral venous drainage;15,16 the causal relationship seems inescapable. Similarly, in a study by Regan et al. where O2:CO2 foam was used in patients with proven right-to-left shunt, despite many patients with bubble emboli, no significant neurological events occurred22 illustrating the benign nature of small rapidly absorbing bubbles. To complete the picture, a similar study needs to be conducted using air-based foam.
In the foam drainage studies, PEM performed similarly to RA PCFs (Figure 7(b) and 7(c)), consistent with previous observations of similarities in bubble size and distribution for both foam types.25 For PCFs containing higher proportions of CO2, initial foam drainage was rapid (Figure 7(a)). This leads to initial high percentages of liquid drainage (first phase) and rapid attainment of an equilibrium position (just tens of seconds to reach the slower phase), whereas the relatively dry foam consisting of large bubbles has an inability to sustain the higher drainage rates warranted by the larger bubble growth. Figure 7(c) shows how FDT compares between PEM versus DSS PCF created with various gas compositions and liquid:gas ratios (1:4 and 1:7). Drier foams (1:7 liquid:gas ratio) take longer to drain than corresponding wetter foams (1:4 ratio), which are most frequently used clinically. Wetter foams will contain bigger fluid channels between the bubbles, which pose less resistance to fluid flow under gravity; capillary forces will also be lower, resulting in faster drainage.
FHT results showed relative consistency with FDT. The influential variables again were the methods used to generate the foams (PEM vs. DSS vs. Tessari) and the foam gas compositions, which followed the same trends observed for foam drainage, i.e. reduced stability with increasing CO2 content (Figure 8). The reproducibility of the results using Turbiscan™ was good, with relatively small standard deviation in the data. This validates the Turbiscan™ as a useful and convenient tool for generating FHT data in addition to dynamic foam drainage data. Foam stability measurements using a vertically standing column of foam, however, only partly convey the physical requirements for useful foam. When injected into an incompetent vein, the sclerosing foam must ensure good contact with the vessel endothelial lining while displacing blood volume.
It is recognised that the characteristics of sclerosing foams for the treatment of varicose veins may be a major determinant of efficacy and safety3; a unique in vitro biomimetic model was therefore developed to determine the behaviour of foam under clinically relevant conditions. This model allows for an assessment of the liquid-displacing capability of the foam and its subsequent rate of degradation within the vessel. In other measures of stability, RA foam performed best, but in the biomimetic model, PEM had the slowest DR, almost half that of RA and eight times better than DSS- and Tessari-equivalent gas mixtures (Figure S3.1). DT, a more meaningful expression of these data, characterizes the length of time the foam plug stays in contact with the vein wall. PEM had a DT twice as long as PCF generated with RA (Figure 9). In a previous report, sufficient practical details were disclosed so that this method, whether manually performed or with the aid of computerized image analysis, could be reproduced, introducing a new parameter – DR – to be used as a standard to quantify the cohesiveness of foams.25 In addition, the biomimetic analysis system may be of value to researchers and clinicians to gain a deeper understanding of the physical parameters governing foam performance, ultimately leading to the determination of optimal foam for differing vein diameters and venous disorders.
Technical limitations and future perspectives
In the present study, we performed a range of experiments to compare the performance of PCFs with PEM. There were some practical limitations to the study due to the time required to manipulate the foam and place it into the instrument before taking a measurement. This was generally 30–40 s for most techniques, which meant the foam had already undergone some degradation; this is, however, likely to be the time it would take a physician to administer the foam into the vein in clinical practice. Despite the wide range of experimental conditions investigated, the effect of several parameters has not been examined and could be the subject of future investigations. One such area involves the effect of the physical properties of carrier fluids on the stability/cohesiveness of sclerosing foams. Experiments using fluids of varying viscosity or physiological fluids (i.e. plasma or whole blood) may be performed and could be of interest due to the deactivation effects of biological fluids on sclerosants. However, this would require optimization of existing techniques for characterizing foam stability. Another area of possible investigation involves the effect of clinically relevant parameters such as foam injection rate on foam stability/cohesiveness. A third avenue of research could involve a more extensive investigation of the effect of different sclerosing agents (i.e. such as sodium tetradecyl sulphate or alcohol) and their concentration on foam stability/cohesiveness.
Conclusion
Polidocanol foams are not all the same, and it is difficult to compare clinical results unless characteristics are known and reproducible. Air foams have good performance but have associated risks, with persistent nitrogen bubbles in the circulation. Small bubbles and narrow bubble size distribution, with slow drainage and separation times, improve foam performance by enhancing stability. The biomimetic vein test produces a new measure of foam performance that demonstrates the low DR and longer DT of PEM compared to PCFs. The PEM made with O2:CO2, low nitrogen-gas composition and proprietary foam generation device results in better overall performance than PCF in a variety of tests, without the associated risk of high-nitrogen RA bubbles.
Dedication
In loving memory of our friend and colleague Vincent O’Byrne who recently lost his battle with cancer. His dedication and spirit were an inspiration and an example to us all.
Supplementary Material
Acknowledgment
The authors acknowledge the writing assistance provided by Dorothy Tengler, Thomas King and Tuli Ahmed, editorial support from Ellen Evans, and the technical support provided by Phill Keefe.
Contributorship
DC, VOB: protocol design and development, execution of experiments and data analysis; DNA, JH, XueZ, MA: execution of experiments; MH, XunZ, ALL, DDIW: conceived the study, critical revisions, and data analysis.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JH and MA worked for Biocompatibles Ltd. (a BTG International group company) on the development of PEM. VOB and ALL are employees at Biocompatibles Ltd. (a BTG International group company) and they are working on the development of PEM, although they were not directly paid by this research project.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: BTG International Ltd. provided materials (including chemicals, consumables and foam generation equipment) and funding for the execution of this research.
References
- 1.Redondo P, Cabrera J. Microfoam sclerotherapy. Semin Cutan Med Surg 2005; 24: 175–183. [DOI] [PubMed] [Google Scholar]
- 2.Hamel-Desnos C, Desnos P, Wollmann JC, et al. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatol Surg 2003; 29: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 3.Frullini A, Cavezzi A. Sclerosing foam in the treatment of varicose veins and telangiectases: history and analysis of safety and complications. Dermatol Surg 2002; 28: 11–15. [DOI] [PubMed] [Google Scholar]
- 4.Hsu T-S, Weiss RA. Foam sclerotherapy: a new era. Arch Dermatol 2003; 139: 1494–1496. [DOI] [PubMed] [Google Scholar]
- 5.Geroulakos G. Foam sclerotherapy for the management of varicose veins: a critical reappraisal. Phlebolymphology 2006; 13: 202–206. [Google Scholar]
- 6.Stücker M, Kobus S, Altmeyer P, et al. Review of published information on foam sclerotherapy. Dermatol Surg 2010; 36: 983–992. [DOI] [PubMed] [Google Scholar]
- 7.Bunke N, Brown K, Bergan J. Foam sclerotherapy: techniques and uses. Perspect Vasc Surg Endovasc Ther 2009; 21: 91–93. [DOI] [PubMed] [Google Scholar]
- 8.Bountouroglou D, Geroulakos G. Ultrasound-guided foam sclerotherapy for the treatment of primary varicose veins. Phlebology 2004; 19: 107–108. [Google Scholar]
- 9.Eckmann DM, Kobayashi S, Li M. Microvascular embolization following polidocanol microfoam sclerosant administration. Dermatol Surg 2005; 31: 636–643. [DOI] [PubMed] [Google Scholar]
- 10.King T, Coulomb G, Goldman A, et al. Experience with concomitant ultrasound-guided foam sclerotherapy and endovenous laser treatment in chronic venous disorder and its influence on health related quality of life: interim analysis of more than 1000 consecutive procedures. Int Angiol 2009; 28: 289–297. [PubMed] [Google Scholar]
- 11.Gibson KD, Ferris BL, Pepper D. Foam sclerotherapy for the treatment of superficial venous insufficiency. Surg Clin North Am 2007; 87: 1285–1295. [DOI] [PubMed] [Google Scholar]
- 12.Forlee MV, Grouden M, Moore DJ, et al. Stroke after varicose vein foam injection sclerotherapy. J Vasc Surg 2006; 43: 162–164. [DOI] [PubMed] [Google Scholar]
- 13.Guex J. Complications and side-effects of foam sclerotherapy. Phlebology 2009; 24: 270–274. [DOI] [PubMed] [Google Scholar]
- 14.Breu F, Guggenbichler S, Wollmann J. Duplex ultrasound and efficacy criteria in foam sclerotherapy from the 2nd European consensus meeting on foam sclerotherapy 2006, Tegernsee, Germany. Vasa 2008; 37: 90–95. [DOI] [PubMed] [Google Scholar]
- 15.Bush R, Derrick M, Manjoney D. Major neurological events following foam sclerotherapy. Phlebology 2008; 23: 189–192. [DOI] [PubMed] [Google Scholar]
- 16.Asbjornsen C, Rogers C, Russell B. Middle cerebral air embolism after foam sclerotherapy. Phlebology 2012; 27: 430–433. [DOI] [PubMed] [Google Scholar]
- 17.Ceulen RP, Sommer A, Vernooy K. Microembolism during foam sclerotherapy of varicose veins. N Engl J Med 2008; 358: 1525–1526. [DOI] [PubMed] [Google Scholar]
- 18.Rush JE, Wright D. More on microembolism and foam sclerotherapy. N Engl J Med 2008; 359: 656–657. [DOI] [PubMed] [Google Scholar]
- 19.Rao J, Goldman MP. Stability of foam in sclerotherapy: differences between sodium tetradecyl sulfate and polidocanol and the type of connector used in the double-syringe system technique. Dermatol Surg 2005; 31: 19–22. [DOI] [PubMed] [Google Scholar]
- 20.Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg 2001; 27: 58–60. [PubMed] [Google Scholar]
- 21.Wollmann J. Sclerosant foams: stabilities, physical properties and rheological behavior. Phlebologie 2010; 39: 208–208. [Google Scholar]
- 22.Regan JD, Gibson KD, Rush JE, et al. Clinical significance of cerebrovascular gas emboli during polidocanol endovenous ultra-low nitrogen microfoam ablation and correlation with magnetic resonance imaging in patients with right-to-left shunt. J Vasc Surg 2011; 53: 131–137. [DOI] [PubMed] [Google Scholar]
- 23.Todd KL, Wright D. The VANISH-2 study: a randomized, blinded, multicenter study to evaluate the efficacy and safety of polidocanol endovenous microfoam 0.5% and 1.0% compared with placebo for the treatment of saphenofemoral junction incompetence. Phlebology 2014; 29: 608–618. [DOI] [PubMed] [Google Scholar]
- 24.Van Deurzen B, Ceulen RP, Tellings SS, et al. Polidocanol concentration and time affect the properties of foam used for sclerotherapy. Dermatol Surg 2011; 37: 1448–1455. [DOI] [PubMed] [Google Scholar]
- 25.Carugo D, Ankrett DN, O’Byrne V, et al. A novel biomimetic analysis system for quantitative characterisation of sclerosing foams used for the treatment of varicose veins. J Mater Sci Mater Med 2013; 24: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 26.Sarvananthan T, Shepherd AC, Willenberg T, et al. Neurological complications of sclerotherapy for varicose veins. J Vasc Surg 2012; 55: 243–251. [DOI] [PubMed] [Google Scholar]
- 27.Morrison N, Neuhardt D, Rogers C, et al. Incidence of side effects using carbon dioxide–oxygen foam for chemical ablation of superficial veins of the lower extremity. Eur J Vasc Endovasc Surg 2010; 40: 407–413. [DOI] [PubMed] [Google Scholar]
- 28.Frullini A, Barsotti MC, Santoni T, et al. Significant endothelin release in patients treated with foam sclerotherapy. Dermatol Surg 2012; 38: 741–747. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara F, Ferrara G. The chemical mediators of some sclerotherapy complications. Phlebolo Ann Vasc 2012; 65: 27–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.