Abstract
Background
Psychological therapies have been developed for parents of children and adolescents with a chronic illness. Such therapies include interventions directed at the parent only or at parent and child/adolescent, and are designed to improve parent, child, and family outcomes. This is an updated version of the original Cochrane review published in Issue 8, 2012, (Psychological interventions for parents of children and adolescents with chronic illness).
Objectives
To evaluate the efficacy of psychological therapies that include parents of children and adolescents with chronic illnesses including painful conditions, cancer, diabetes mellitus, asthma, traumatic brain injury (TBI), inflammatory bowel diseases (IBD), skin diseases, or gynaecological disorders. We also aimed to evaluate the adverse events related to implementation of psychological therapies for this population. Secondly, we aimed to evaluate the risk of bias of included studies and the quality of outcomes using the GRADE assessment.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and PsycINFO for randomised controlled trials (RCTs) of psychological interventions that included parents of children and adolescents with a chronic illness. Databases were searched to July 2014.
Selection criteria
Included studies were RCTs of psychological interventions that delivered treatment to parents of children and adolescents with a chronic illness compared to an active control, waiting list, or treatment as usual control group.
Data collection and analysis
Study characteristics and outcomes were extracted from included studies. We analysed data using two categories. First, we analysed data by each individual medical condition collapsing across all treatment classes at two time points. Second, we analysed data by each individual treatment class; cognitive behavioural therapy (CBT), family therapy (FT), problem solving therapy (PST) and multisystemic therapy (MST) collapsing across all medical conditions. For both sets of analyses we looked immediately post-treatment and at the first available follow-up. We assessed treatment effectiveness for two primary outcomes: parent behaviour and parent mental health. Five secondary outcomes were extracted; child behaviour/disability, child mental health, child symptoms, family functioning, and adverse events. Risk of bias and quality of evidence were assessed.
Main results
Thirteen studies were added in this update, giving a total of 47 RCTs. The total number of participants included in the data analyses was 2985, 804 of whom were added to the analyses in the update. The mean age of the children was 14.6 years. Of the 47 RCTs, the studies focused on the following paediatric conditions: n = 14 painful conditions, n = 13 diabetes, n =10 cancer, n = 5 asthma, n = 4 TBI, and n = 1 atopic eczema. We did not identify any studies treating parents of children with gynaecological disorders or IBD. Risk of bias assessments of included studies were predominantly unclear. Evidence quality, assessed using the GRADE criteria, was judged to be of low or very low quality.
Analyses of separate medical conditions, across all treatment types, revealed two beneficial effects of psychological therapies for our primary outcomes. First, psychological therapies led to improved adaptive parenting behaviour in parents of children with cancer post-treatment (standardised mean difference (SMD) −0.20, 95% confidence interval (CI) −0.36 to −0.04, Z = 2.44, p = 0.01). In addition, therapies also improved parent mental health at follow-up in this group (SMD = −0.18, 95% CI −0.32 to −0.04, Z = 2.58, p = 0.01). We did not find any effect of therapies for parent behaviour for parents of children with a painful condition post-treatment or at followup, or for parent mental health for parents of children with cancer, diabetes, asthma, or TBI post-treatment. For all other primary outcomes, no analysis could be conducted due to lack of data.
Across all medical conditions, three effects were found for the primary outcomes of psychological therapies. PST had a beneficial effect on parent adaptive behaviour (SMD = −0.25, 95% CI −0.39 to −0.11, Z = 3.59, p < 0.01) and parent mental health (SMD= −0.24, 95% CI −0.42 to −0.05, Z = 2.50, p = 0.01) immediately post-treatment and this effect was maintained at follow-up for parent mental health (SMD= −0.19, 95% CI −0.34 to −0.04, Z = 2.55, p = 0.01). The remaining analysis for PST on parent behaviour found no effect. No effects were found for CBT post-treatment or at follow-up for either parent outcome. For FT, only one analysis could be run on parent mental health and no effect was found. Due to lack of data, the remaining analyses of primary outcomes could not be run. For MST, no parent outcomes could be analysed due to lack of data.
Secondary outcome analyses are presented in the Results section. Five studies reported that there were no adverse events during the trial. The remaining 42 studies did not report adverse events.
Authors’ conclusions
This update includes 13 additional studies, although our conclusions have not changed from the original version. There is little evidence for the efficacy of psychological therapies that include parents on most outcome domains of functioning, for a large number of common chronic illnesses in children. However, psychological therapies are efficacious for some outcomes. CBT that includes parents is beneficial for reducing children’s primary symptoms, and PST that includes parents improved parent adaptive behaviour and parent mental health. There is evidence that the beneficial effects can be maintained at follow-up for diabetes-related symptoms in children, and for the mental health of parents of children with cancer and parents who received PST.
INDEX TERMS: Medical Subject Headings (MeSH): Chronic Disease [*psychology], Cognitive Therapy, Family Therapy, Parenting [psychology], Parents [*psychology], Problem Solving, Psychotherapy [*methods], Randomized Controlled Trials as Topic
MeSH check words: Adolescent, Child, Humans
PLAIN LANGUAGE SUMMARY
Psychological therapy for parents of children and adolescents with a longstanding or life-threatening physical illness
Background
This is an update of a previously published review published in 2012 investigating the efficacy of psychological therapies for parents of children with a longstanding or life-threatening physical illness. This review updates includes studies that have been conducted in the previous two years to give an up-to-date review of the evidence.
Parenting a child with a longstanding or life-threatening illness is very difficult, and can have a negative impact on many aspects of the parents’ life. Parents of these children often have difficulty balancing caring for their child with other responsibilities and demands. As a result, parents may experience more stress, worries, mood disturbance, family arguments, and their children may show troubling or problematic behaviour. Parents also have a major influence on their child’s well-being and adjustment, and play an important role in how their child adapts to living with an illness. Treatments for parents of children with a longstanding illness aim to improve parent distress, parenting behaviours, family conflict, child distress, child disability and the child’s medical symptoms.
Review question
To evaluate the effectiveness of psychological therapies for parents of children and adolescents with chronic illnesses including painful conditions, cancer, diabetes mellitus, asthma, traumatic brain injury (TBI), inflammatory bowel diseases (IBD), skin diseases, or gynaecological disorders. Psychological therapies will be compared to active, treatment as usual, or wait-list controls. There were two primary outcomes of interest: parent mental health and parenting behaviour. We included five secondary outcomes; child behaviour/disability, child mental health, child symptoms, family functioning, and adverse events.
Study characteristics
The search was completed in July 2014. Forty-seven studies were found in the search including 3778 participants. The average age of the children was 14.6 years. We found studies that focused on six chronic illnesses (painful conditions, cancer, diabetes, asthma, traumatic brain injury and eczema) and evaluated four types of psychological therapies (cognitive behavioural therapy, family therapy, problem solving therapy, multisystemic therapy). Outcomes were extracted from the time point immediately after the treatment and at the first available follow-up. We analysed the data in two ways: first we grouped the studies by each individual illness (across all therapies) and then we grouped the studies by each individual psychological therapy (across all chronic illnesses).
Key Results
Psychological therapies improved parenting behaviour of parents of children with cancer immediately following treatment. Parent distress also improved for parents of children with cancer. Children with painful conditions and those with symptoms of diabetes showed benefit immediately following treatment, and for diabetes the reduction in symptoms was maintained at follow-up. When analysing different psychological therapies, we found cognitive behavioural therapy can improve the child’s medical symptoms. Problem-solving therapy can improve a parent’s distress and their ability to solve problems, with the reduction in parental distress continuing long-term. Five studies reported that there were no adverse events during the study period. The remaining studies failed to report or discuss adverse events. Risk of bias assessments of included studies were predominantly unclear due to poor reporting.
Conclusion
There is evidence that psychological therapies including parent interventions can benefit parents of children with a chronic illness, particularly for parents of children with cancer. However, due to the small number of studies in this review, future studies are likely to change the findings in this review.
BACKGROUND
This is an updated version of the original Cochrane review published in Issue 8, 2012 (Eccleston 2012b).
Description of the condition
Chronic illness affects the lives of many children and their families. The prevalence of illness and disability differs by geographical and economic context. In the USA, Canada, Northern Europe, UK and Australia chronic activity-limiting conditions are reported to be frequent, with painful illness, allergy, asthma and obesity being common (McDougall 2004). The changing demographic of childhood illness in economically wealthy countries has prompted a re-analysis of the role of paediatric medicine, as chronic illness becomes more prevalent than acute (e.g. Halfon 2010; Van Cleave 2010). Other parts of the world present different clinical challenges. In Africa, for example, life expectancy is 54 years and shorter in sub-Saharan Africa where almost half the population are children and the most prevalent chronic conditions are related to communicable diseases, in particular HIV-related disease, malaria and tuberculosis (WHO 2011).
The existing published literature shows a bias towards the medical management of chronic illness related to environment or lifestyle. Chronic pain in childhood is known to have widespread negative outcomes for children and parents (Palermo 2000). Psychological intervention reviews have also been undertaken on the impact of sickle cell disease (Anie 2012), recurrent abdominal pain/irritable bowel syndrome (Huertas-Ceballos 2008), type 1 diabetes (McBroom 2009), traumatic brain injury in children (Soo 2007) and asthma (Yorke 2005).
The impact of childhood chronic illness on other family members, including parents, has been of growing interest for two reasons. First, it is now recognised that parents who have significant emotional distress of their own, and poor family functioning, can either directly or indirectly affect child outcomes by engaging in problematic responses to children’s pain behaviours (Logan 2005; Palermo 2007). Second, it is now recognised that adaptive strategies used by parents can have a positive effect on child adjustment to chronic illness (Logan 2005).
Description of the intervention
Addressing the high level of parenting stress and mental health problems of parents, while enabling parents to be agents of change in the management of their child’s chronic illness, have recently been promoted as viable components of intervention in paediatric chronic conditions (Jordan 2007; Palermo 2009b). Studies have focused on the education of parents about the specific condition or treatment (e.g. cystic fibrosis; Savage 2014), whilst others evaluate the benefit of lay- or nurse-mediated social support (e.g. Lewin 2010). In psychological science, specific treatment approaches have been developed that focus on reducing the emotional distress expressed by parents, or on altering parenting behaviours to promote better child outcomes, whether this be decreasing emotional distress, or improving physical symptoms or behaviour.
Psychological interventions of interest are defined as any psychotherapeutic treatment specifically designed to change parent cognition or behaviour, or both, with the intention of improving child outcomes. Psychological interventions are varied in their approaches and there is still debate surrounding which treatment is most effective for improving mental health and behaviour in parents and children with chronic illnesses. Such interventions include cognitive behavioural therapy (CBT), which has been found to be effective for modifying parent behaviour in children with a painful condition (e.g. Palermo 2009a; Williams 2012). Problem-solving therapy (PST) has also been used to reduce distress in parents of children with various chronic illnesses (D’Zurilla 1971; Sahler 2002). Other treatments have emerged from a family-systems approach that focuses explicitly on the family as a unit of intervention (Ellis 2005; Wysocki 2000) such as multisystemic therapy (MST) or family therapy (FT).
How the intervention might work
There are a variety of interventions described as psychological. Cognitive and cognitive behavioural therapies dominate, but therapies with a psychodynamic or systemic tradition are also represented. Family and couple therapies have also been developed. All psychological interventions include a rationale for therapy and specific goals for therapy. Education around illness and behaviour is common. Establishing the therapy and the therapist as credible is an important general stage (Nock 2001). Next, a therapeutic relationship is established that will enable a confidential, non-blaming investigation of behaviour. Then, depending on the illness and behavioural presentation, specific components may include anxiety management, problem-solving skills, cognitive therapy for depression, and relationship management. Finally, most treatments will include a maintenance component that focuses on robust behavioural change within a normal home environment outside the clinic, over time. Such components have been used in parent interventions using different therapies to improve parental functioning, child behaviour and mental health.
Cognitive behavioural interventions specifically are based on a number of foundational assumptions (Beck 2011). First, behaviour is socially and historically contingent (Skinner 1953). Second, cognition is an emergent property of behavioural context (James 1980). Third, behaviour is regulated by cognitive goals (Bandura 1989). Fourth, emotions influence both behaviour and cognition (Ashby 1999; Gilliom 2002). Fifth, most behaviour is deployed outside of conscious awareness or control (Bargh 2008). Finally, some attempts to control cognition and behaviour can have paradoxical negative effects on desired outcomes (Beck 2011; Wegner 1994).
Other interventions such as PST (D’Zurilla 1971) are based on enhancing social competence through constructive problem-solving attitudes and skills. PST is based on a model of social problem-solving (D’Zurilla 1999). Specific problem-solving skills are taught in sequential steps that typically include defining the problem, generating alternative solutions, decision making, and solution verification and implementation. PST has previously been effective with depression, anxiety and stress-related syndromes (D’Zurilla 1999) and has been implemented with caregivers in a number of contexts.
Family and systemic therapies specifically focus on a contextual and relational view of the aetiology and maintenance of behaviour. In particular, the target of health behaviour change is typically related to family functioning, or in the cognitive representation of the family, rather than on individual attitudes, beliefs or behaviour. Typically, family or systems therapy approaches will include multiple family members, and outcomes are often expressed on behalf of the family or dyad (two individuals regarded as a pair).
Why it is important to do this review
Chronic illness is experienced by children, but within the context of the family. Parents are often detrimentally affected by their child’s illness, which adds a burden of adult distress to the child’s distress and disability. Further, parent distress can impair their performance in supporting their child in adapting to a chronic illness. Psychological interventions are available which focus on helping parents to help both themselves and their children. Establishing the evidence at this stage of development can guide best practice and further treatment development.
OBJECTIVES
To evaluate the efficacy of psychological therapies that include parents of children and adolescents with chronic illnesses including painful conditions, cancer, diabetes mellitus, asthma, traumatic brain injury (TBI), inflammatory bowel diseases (IBD), skin diseases, or gynaecological disorders. We also aimed to evaluate the adverse events related to implementation of psychological therapies for this population.
To evaluate the risk of bias of included studies and the quality of outcomes using the GRADE assessment.
METHODS
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs) of psychological interventions that include a parent component, compared with attention control, other active treatment, or waiting-list control groups. The parent intervention had to be primarily psychological in nature. Studies that met the inclusion criteria consisted of the following:
RCT, published in full in a peer-reviewed journal;
Primary aim of the trial was an evaluation of a psychological intervention;
Involved parents of children who have an illness for three months or more (Van der Lee 2007);
Involved parents of children adjusting to a diagnosis of cancer;
Had a participant n of 10 or more in both the treatment and control arm at end of treatment or at follow-up.
Types of participants
Parents of a child who has endured a chronic illness for three months or more, or who was recently diagnosed with a condition (e.g., cancer) that is expected to last more than three months. We regard parents as the primary caregiver of a child or adolescent under the age of 19 years. We define parents for the purposes of this review as any adult who adopts the responsibility for the role of parenting the child (this could include biological parent, guardian, or other adult family member). There was no lower age limit for the children; however, by the definition of ’chronic illness’, the child must be three months or older. Physical illnesses that were considered for inclusion were:
Asthma;
Cancer;
Diabetes mellitus;
Gynaecological disorders (e.g. chronic dysmenorrhoea and endometriosis);
Inflammatory bowel diseases (IBD);
Painful condition (including but not exclusively limited to arthritis, back pain, complex regional pain syndrome (CRPS), fibromyalgia, headache, idiopathic pain conditions, irritable bowel syndrome (IBS), recurrent abdominal pain);
Skin diseases (e.g. eczema);
Traumatic brain injury (TBI).
Chronic illnesses were selected from the National Survey of Children with Special Health Care Needs 2009 to 2010 (Data Resource Center 2010). It was impractical to include all chronic illnesses on this list therefore we selected the most common. However, three illnesses (cancer, inflammatory bowel diseases and gynaecological disorders) were not included in the list of ‘Current Health Conditions and Functional Difficulties’ but were added for the purposes of this review. Cancer has a high incidence level, and in the UK alone 1600 0–14 year-old children are diagnosed with cancer each year (Cancer Research UK 2014). In the USA, it is estimated that 15,780 0–19 year-olds are diagnosed with cancer (National Cancer Institute 2014). Studies that delivered interventions to parents of children who have ’survived’ an illness but still experienced distress, such as childhood survivors of cancer, were also eligible for inclusion. Inflammatory bowel diseases and gynaecological disorders are also common conditions in childhood and adolescence, and are included because they are thought to be prevalent but under-represented in the academic literature.
Types of interventions
Studies were included if the interventions were primarily psychological, and had credible, recognisable psychological/psychotherapeutic content, and were specifically developed for, or included parents. Psychological interventions were defined as any psychotherapeutic treatment specifically designed to change parent cognition or behaviour, or both, and had the intention of improving parent or child outcomes. However, studies that included parents as ’coaches’ to support exclusively child-focused interventions were excluded from this review. The intervention had to aim to provide treatment to the parent rather than teach them to deliver an intervention to their child. Similarly, we also excluded health promotion therapies such as intervening with the parent to cease smoking to improve their child’s asthma. We have excluded studies that combine psychological interventions with pharmacological interventions or are qualitative in nature, because it is difficult to combine qualitative and quantitative data.
Types of outcome measures
Parent outcomes were the primary target of our review. However, if the study also reported child outcomes as stated below, we also analysed and reported these data as secondary outcomes. We analysed data at post-treatment and the first available follow-up period, where reported.
Primary outcomes for the purposes of this review include parenting behaviour and parent mental health. Secondary outcomes include child behaviour/disability, child mental health, child illness-related symptoms, family function and adverse events.
We made a judgement when studies reported multiple measures within one of the six outcome domains and did not define their primary or secondary outcome measure. The rules of this judgement were to select the most generic, reliable, and most frequently used measure within the field, and most appropriate for the given outcome category. When both parents and children reported on a measure, we extracted the self-reported item unless the non-self-reported measure was a more generic measure. For family functioning measures, we preferentially extracted parent data over child data, as the review is focused on whether interventions can help parents of children with a chronic illness.
Search methods for identification of studies
Electronic searches
Two searches have been conducted. The first, from inception to March 2012, and the second from March 2012 to July 2014. We searched four databases for studies for this update. The dates listed below state the most recent date of our search.
Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library, Issue 6, 2014;
MEDLINE via Ovid, 1946 to 30/6014;
EMBASE via Ovid, 1974 to 30/6/14;
PsycINFO via Ovid, 1806 to June week 4 2014.
We adapted the search strategies from the MEDLINE search (for all search strategies see Appendix 1). There was no language restriction imposed and no unpublished literature or grey material was included, so only the highest quality trials were included. The search strategy included four categories of words: psychological interventions, parents, children/adolescents and chronic illnesses (as stated above), and was refined by a methodological filter used to identify RCTs according to Cochrane guidance (Higgins 2011).
Searching other resources
We performed a reference list and citation search of all included studies. Relevant meta-analyses and systematic reviews were searched for additional studies. We also contacted authors of selected studies and experts in the field for further studies that had not already been identified from the search. In addition, we searched online trial repositories for additional studies including metaRegister of controlled trials (mRCT) (www.controlledtrials.com/mrct/), ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
Three review authors (EF, EL, JB) sifted through potential studies and identified those eligible to be included, with CE acting as arbiter. No blinding of study authors’ names, institutions or journals occurred during this process. We resolved any disagreements by discussion between all review authors.
We made selection of abstracts using the following criteria.
- Participants
-
◦Parents had to be referred to in the title or abstract of each study;
-
◦The parent had to be the primary caregiver of the child;
-
◦Children had to have one or more of the chronic illnesses listed above;
-
◦Children had to be in the age range three months to 19 years;
-
◦There had to be 10 or more participants in each condition at the end of the treatment assessment.
-
◦
- Intervention
-
◦The intervention had to be primarily psychological in at least one treatment arm;
-
◦Design was an RCT;
-
◦One or more parents had to be treated by the intervention;
-
◦The parents or child or both had to complete assessments at baseline and at a point in time during or after the intervention.
-
◦
- Comparison groups
-
◦Active treatment group;
-
◦Treatment-as-usual group (e.g. usual doctors’ appointments and treatment without added psychological therapy);
-
◦Waiting-list control.
-
◦
Quantitative outcomes had to be presented We then obtained the selected studies meeting the criteria in full and EF, EL and JB read and assessed them independently.
Data extraction and management
Three review authors (EF, EL and JB) carried out data extraction from studies that were identified by all review authors as appropriate for inclusion. We extracted demographics of parents and children (e.g. age, sex), characteristics of the child’s illness (e.g. diagnosis, length of illness), and characteristics of therapy (e.g. setting, components, treatment team, therapy type). Finally, we extracted relevant outcomes for analysis.
Assessment of risk of bias in included studies
We assessed risk of bias using the recommended Cochrane guidance (Higgins 2011). Of the six suggested ’Risk of bias’ categories, we judged studies on random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias). We excluded the option of ’blinding participants and personnel’ because we deemed it redundant because personnel cannot be blinded to whether they deliver a treatment.
Decisions about random sequence generation were based on whether authors gave a convincing method of randomisation. Participants being stratified by age or sex did not count as biased. Allocation concealment judgements were based on whether sufficient methods were employed for random allocation to take place. We judged risk of blinding of outcome assessment on whether the measures were administered and collected by an assessor who was blind to the treatment allocation. For attrition bias, we assigned a low risk of bias when authors gave both a description of attrition and stated that there were no significant differences between completers and non-completers. We judged there to be unclear risk of bias when there was an adequate description of attrition but authors did not report whether there were significant differences between completers and non-completers. We judged there to be a high risk of attrition bias when no description of attrition was reported.
We judged selective reporting bias on whether data were fully reported in the study or if authors later responded to data requests. We assigned a low risk of bias when all the data were reported in the paper, an unclear risk of bias when authors responded to data requests, and a high risk of bias when the data were not reported in the paper and authors did not respond to data requests. Previously we had rated the concordance between the aims, measures, and results of studies; however, we decided not to conduct this assessment for this update and deleted previous ratings.
The original version of this review included quality assessments advocated by Yates 2005. However, for this update we used the GRADE assessment of quality in accordance with Cochrane guidance (Higgins 2011). Quality of evidence using GRADE was assessed in order to determine the quality of evidence and to enable a summary of the level of confidence in the estimate of effect. First, GRADE assessments for parent outcomes combining all psychological therapies are presented. Second, GRADE assessments for all outcomes for each medical condition are presented.
There were five categories that were assessed to obtain a GRADE rating for an outcome: limitations in the design, indirectness of evidence, unexplained heterogeneity, imprecision of results, and probability of publication bias. Quality ratings could be downgraded from high to either moderate, low, or very low quality evidence.
For limitations in the design, the category was downgraded once if the majority of risk of bias ratings from included studies in an outcome were rated as ’unsure’ or ’high’ risk of bias. It was downgraded twice if there were a high proportion of high risk ratings. For indirectness of evidence, if 50% or more of studies had a waiting-list control the outcome was downgraded once, however if 75% or more had a waiting-list control, the outcome was downgraded twice. The inconsistency of results was downgraded once when the heterogeneity of the analysis was more than 45% and downgraded twice when the heterogeneity was 75% or more. For imprecision of results, we downgraded the outcome if the included studies had fewer than 500 participants. Outcomes were downgraded twice if there were fewer than 150 participants contributing to an outcome. Last, for publication bias, we downgraded outcomes where 50% or more of the contributing studies had a high risk of bias rating for publication bias.
Measures of treatment effect
We investigated four classes of psychological therapies: cognitive behavioural therapy (CBT), family therapy (FT), problem-solving therapy (PST) and multisystemic therapy (MST). CBT is based on theories of behavioural analysis (Bergin1975), cognitive theory (Beck 1979) and social learning theory (Bandura 1977). CBT therefore includes a range of strategies with the goals of modifying social/environmental and behavioural factors that may exacerbate or cause symptoms, and modifying maladaptive thoughts, feelings and behaviours to reduce symptoms and prevent relapse. FT is based on family systems theory (Haley 1976; Minuchin 1974), which emphasises the role of the family context in an individual’s emotional functioning. FT interventions typically focus on altering patterns of interactions between family members, and include structural family therapy (Minuchin 1974), strategic family therapy (Haley 1976) and behavioural systems family therapy (Robin 1989). PST is based on the D’Zurilla 1982 social problem-solving model, which defines problem solving in terms of an individual’s ability to recognise problems and use a positive orientation and problem-solving skills to solve them. PST includes didactic instruction in problem-solving skills, followed by in-session modelling, behavioural rehearsal and performance feedback, as well as homework assignments (D’Zurilla 2007). Finally, MST is an intensive family- and community-based intervention based on the Bronfenbrenner 1979 social ecological model and family systems theory (Haley 1976; Minuchin 1974). MST therefore targets the child, their family and broader systems such as the child’s school, work or medical team as needed. MST incorporates a wide range of evidence-based intervention techniques based on the individual needs of the child and family (Henggeler 2003), including cognitive-behaviour approaches, parent training and family therapies. We extracted data immediately post-treatment (i.e. immediately after the treatment programme had finished). Where data were available, we also analysed studies at follow-up, which is classed as the first available time point after post-treatment. We categorised outcomes into one of six outcome domains: parenting behaviour, parent mental health, child behaviour/disability, child mental health, child symptoms and family functioning. Where studies had more than one comparator group, we chose the ‘active control group’ over ‘standard treatment’ or ‘wait-list control’ groups.
There are four therapies (CBT, FT, PST and MST), eight conditions (asthma, cancer, diabetes mellitus, gynaecological disorders, inflammatory bowel diseases, painful conditions, skin diseases, and traumatic brain injury), two time points (post-treatment and follow-up) and six possible outcomes (parenting behaviour, parent mental health, child behaviour/disability, child mental health, child symptoms and family functioning). There are six categories by which we analysed data:
For each condition, across all types of psychological therapy, what is the efficacy for the six outcomes immediately post-treatment?
For each condition, across all types of psychological therapy, what is the efficacy for the six outcomes at follow-up?
For each psychological therapy, across all conditions, what is the efficacy for the six outcomes immediately post-treatment?
For each psychological therapy, across all conditions, what is the efficacy for the six outcomes at follow-up?
The interaction between the condition and the psychological therapy efficacy.
Investigation of characteristics of particularly effective treatments.
Analyses are presented for each of the six outcomes, however, due to the heterogeneous nature of the conditions and studies, this was not always possible. We pooled data using the standardised mean difference (SMD) and a random-effect model, as studies did not consistently use the same scales when measuring the same outcomes. Cohen’s d effect sizes can be interpreted as follows: 0.2 = small, 0.5 = medium, 0.8 = large (Cohen 1992).
Dealing with missing data
We contacted authors of studies when data were not reported fully in publications. However, when authors could not send data to the review authors or were non-responsive to emails, we excluded data.
Subgroup analysis and investigation of heterogeneity
When there were multi-arm trials or trials that compared more than one active treatment, we used the primary active treatment and compared with the least biased comparator (active control). Analyses of the following subgroups are presented where data permitted:
Parent-only interventions versus family-based interventions;
Intervention effects within specific illnesses;
Intervention effects across specific types of psychological interventions.
We also explored heterogeneity through subgroup analysis.
RESULTS
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies for a detailed description of included and excluded studies.
Characteristics of included studies [ordered by study ID]
| Allen 1998 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment, 3 months and 1 year | |
| Participants | End of treatment n = 27, 3-month follow-up = 27, 12-month follow-up = 21 Start of treatment n = 27 Sex of children: 11 M, 16 F Sex of parents: Not reported Mean age of children =12.2 Mean age of parents = Not reported Source = Referred by paediatricians and neurologists in the community and recruited by newspaper ad Diagnosis of child = Migraine headache Mean years of illness = 4.4 years |
|
| Interventions | “Thermal Biofeedback plus Parent Pain Behaviour Management” (CBT) “Thermal Biofeedback” Mode of delivery: Individual, face-to-face Intervention delivered by: Authors Training: Not reported Duration of intervention (child, hrs) = 6 × 40 minutes = 4 hours Duration of intervention (parent, hours) = not reported |
|
| Outcomes | * Extracted measures used in the analyses Child measures Pain diary* Coping Assistance Questionnaire Child Perception Abbreviated Acceptability Rating Profile Parent measures Parent Perception of Pain Interference Questionnaire* Coping Assistance Questionnaire for Parents* Abbreviated Acceptability Rating Profile |
|
| Notes | Funding: “This manuscript was supported in part by grant MCJ319152 from the Maternal and Health Bureau, Health Resources Services Administration, and by grant 90 DD 032402” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized, controlled group-outcome design, subjects were assigned to either thermal biofeedback intervention, or the same biofeedback intervention plus pain behavior management guidelines”. Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Ambrosino 2008 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 1 month (end of treatment), 3 months, 6 months and 12 months post-intervention | |
| Participants | End of treatment n = 81 children, 3-month follow-up = 79 children, 6-month follow-up = 72, 12-month follow-up = 72 Start of treatment n = 87 parents and children received intervention at start Sex of children: 34 M, 53 F Sex of parents: 5 M, 82 F Mean age of children = 9.91 (± 1.44) Mean age of parents = 40.01 (± 5.40) Source = Yale Pediatric Diabetes Program Diagnosis of child = Type 1 diabetes Mean years of illness = 3.71 ± 2.91 years |
|
| Interventions | “Coping Skills Training (CST)” (CBT) “Group Education (GE)” Mode of delivery: Groups, face-to-face, parents met separately Intervention delivered by: Mental health professionals Training: Not reported Duration of intervention (child) = 6 × 1½ = 9 hours Duration of intervention (parent) = 6 × 1½ = 9 hours |
|
| Outcomes | * Extracted measures used in the analyses Child measures Metabolic control* Child Depression Inventory (CDI)* Disease-related variables Issues in Coping with IDDM - Child scale Self-Efficacy for Diabetes Scale Diabetes Quality of Life Scale for Youth (DQOL) Diabetes Family Behavior Scale (DFBS) Parent measures Center for Epidemiologic Depression Scale (CES-D)* Family Adaptability and Cohesion Scale (FACES II)* Issues in Coping with IDDM - Parent scale Diabetes Responsibility and Conflict Scale |
|
| Notes | Funding: “This study was supported by grants funded by the National Institute for Nursing Research (National Institute of Health, 1&2R01NR004009)” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were randomised initially by a sealed envelope technique and later by computer to either the coping skills therapy of group eduction.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “Participants were randomised initially by a sealed envelope technique and later by computer to either the coping skills therapy of group eduction.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | “All follow-up data were collected by trained research assistants.” Comment: blinding unclear, probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Antonini 2014 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment = 8.16 months after start of intervention | |
| Participants | End of treatment n = 36 Start of treatment n = 40 parents and children received intervention at start Sex of children: Not reported Sex of parents: Not reported Mean age of children = 5.42 (± 2.1) Mean age of parents = Not reported Source = Hospital Diagnosis of child = TBI Mean years of illness = Not reported |
|
| Interventions | ”Online parenting skills program” “Internet resource comparison” (IRC) Mode of delivery: Home visit, online Intervention delivered by: Therapist with psychology masters degree Training: Given manual and had meetings with an advanced supervising psychologist Duration of intervention (child) = 10 core sessions, 4 supplementary sessions Duration of intervention (parent) = 10 core sessions |
|
| Outcomes | * Extracted measures used in the analyses Dyadic Parent-Child Interaction Coding System (DPICS)* Child Behaviour Checklist (CBCL)* Eyberg Child Behaviour Inventory (ECBI) |
|
| Notes | Funding: “This work was supported by the National Institute on Disability and Rehabilitation Research, Department of Education (grant numbers 133G060167, H133B090010 to SLW)” COI: “The authors declare that they have no conflicts of interest” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization scheme was generated using SAS by the medical centers Division of Biostatistics and created using permuted block sizes for each of the randomizations. ” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | “The randomization scheme was generated using SAS by the medical centers Division of Biostatistics and created using permuted block sizes for each of the randomizations. ” Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Video coders remained naive to condition.” Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition reported but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and at 3 months | |
| Participants | End of treatment n = 131 mothers, 3-month follow-up =123 mothers Start of treatment n = 197 mothers Sex of children: 103 M, 94 F Sex of parents: 0 M, 197 F Mean age of child =8.1 Mean age of parents = 36.3 Source = 4 paediatric cancer centres in USA Diagnosis of child = Cancer Mean years of illness = Average 6 weeks since diagnosis, range 2 to 16 weeks from diagnosis |
|
| Interventions | “Problem-Solving Skills Training” (PST) “Problem-Solving Skills Training + Personal Digital Assistant” Mode of delivery: Individual, face-to-face Intervention delivered by: Therapists with graduate training in Clinical Psychology Training: Special training in PSST Duration of intervention (child, hours) = 0 Duration of intervention (parent, hours) = 8 × 1 = 8 hours |
|
| Outcomes | * Extracted measures used in the analyses Parent measures Social Problem-Solving Inventory-Revised (SPSI-R)* Beck Depression Inventory-II (BDI-II)* Profile of Mood States (POMS) Impact of Event Scale-Revised (IES-R) |
|
| Notes | The comparison looks like a non inferiority trial but it was not designed in this way so we have included it despite the lack of a control group Funding: “National Institutes of Health [grants CA 65520, CA 098954, and The University of Texas M. D. Anderson core grant CA 16672 for data management at this institution” COI: “Conflict of interest: None declared” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computerized randomisation to one of the three treatment arms was performed at the data management centre.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “Computerized randomisation to one of the three treatment arms was performed at the data management centre.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Barakat 2010 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 12 months | |
| Participants | End of treatment n = 37, 12-month follow-up = 34 Start of treatment n = 42 received session 1 Sex of children: 15 M, 22 F Sex of parents: Not reported Mean age (SD) of child = 14.17 (1.75) Mean age of parents = Not reported Source = “Comprehensive sickle cell centre” Diagnosis of child = Sickle cell disease Mean years of illness = Lifetime |
|
| Interventions | “Pain Management Intervention” (CBT) “Disease Education Intervention” Mode of delivery: Individual families, face-to-face Intervention delivered by: Clinical Psychology doctoral students Training: Not reported Duration of intervention (child, hours) = 4 × 90 minutes = 6 hours Duration of intervention (parent, hours) = 4 × 90 minutes = 6 hours |
|
| Outcomes | * Extracted measures used in the analyses Child measures Pain diary (% days with pain and % interference with activities)* Coping Strategies Questionnaire Family Cohesion Scale* Health-related Hindrance Inventory Health Service Use per Medical Chart Review School Attendance Records |
|
| Notes | Funding: “This research was funded by National Heart, Lung, and Blood Institute (U54 30117 to J.R)” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A 2-group, randomised treatment design was used.” Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Barry 1997 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 3 months | |
| Participants | End of treatment n = 29, 3-month follow-up = 29 Start of treatment n = 36 Sex of children: 10 M, 19 F Sex of parents: Not reported Mean age of child =9.4 Mean age of parents = Not reported Source = Ads in elementary schools and community health centres, referrals from paediatricians and family physicians Diagnosis of child = Headache Mean years of illness = 2 headaches/month |
|
| Interventions | “Cognitive Behavioural Therapy” (CBT) “Wait-list Control” Mode of delivery: Group, face-to-face Intervention delivered by: Mental health professionals Training: Not reported Duration of intervention (child, hours) = 2 × 90 minutes = 3 hours Duration of intervention (parent, hours) = 2 × 90 minutes = 3 hours |
|
| Outcomes | * Extracted measures used in the analyses Child measures Pain diary* |
|
| Notes | Funding: No funding statement was provided in the manuscript COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Each parent-child pair was initially matched with another pair based on the child’s age, sex and headache pain as indicated by the parents’ ratings of average duration, frequency, and intensity of headaches. Subsequently, one of each of the matched parent-child pairs was randomly assigned to either the treatment condition or the waiting list control condition. ” Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | “Each parent-child pair was initially matched with another pair based on the child’s age, sex and headache pain as indicated by the parents’ ratings of average duration, frequency, and intensity of headaches.” Comment: method of concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were not fully reported |
| Celano 2012 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 6 months | |
| Participants | End of treatment n = 40, 6-month follow-up = 37 Start of treatment n = 41 Sex of children: 26 M, 15 F Sex of parents: 6M, 35 F Mean age (SD) of child = 10.5 (1.6) Mean age of parents = Not reported Source = Urban children’s hospital and residential camp for children with asthma Diagnosis of child = Asthma Mean years of illness = More than 1 year |
|
| Interventions | “Home based family intervention” “Enhanced treatment as usual” Mode of delivery: Individual families, face-to-face Intervention delivered by: Trained asthma counsellors, post-doctoral psychology fellow and respiratory therapist Training: Not reported Duration of intervention (child, hours) = 4 to 6 sessions, average 78 minutes per session Duration of intervention (parent, hours) = 4 to 6 sessions, average 78 minutes per session |
|
| Outcomes | * Extracted measures used in the analyses Child measures Family Asthma Management System Scale Metered Dose Inhaler Checklist Cotinine/creatinine ratio Number of school days missed Asthma symptom days* Urgent health care visits Medical records reviewed Parent measures Family Asthma Management System Scale Parenting Stress Index (PSI-SF) Brief Symptoms Inventory (for parent distress)* |
|
| Notes | Funding: “This research was supported by a grant from the National Heart Lung & Blood Institute” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation....by blocked randomisation within age group (8 to 10 vs. 11 to 13) .” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Trained assistants blind to group assignment.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 2 months | |
| Participants | End of treatment n = 31, 2-month follow-up = 31 Start of treatment n = 37 Sex of children: 19 M, 18 F Sex of parents: Not reported Mean age (SD) of child = 9.2 (1.7) Mean age of parents = Not reported Source = Outpatient neurology clinic at a large children’s hospital in Midwestern USA Diagnosis of child = Headache Mean years (SD) of illness = 2 years 3 months (2 years 2 months) |
|
| Interventions | “Headstrong CD ROM” (CBT) “Wait-list Control” Mode of delivery: Computer and phone calls Intervention delivered by: CD ROM Training: Not reported Duration of intervention (child, hours) = 4 × 1 hr = 4 hours Duration of intervention (parent, hours) = 1 × 1 hr = 1 hour |
|
| Outcomes | * Extracted measures used in the analyses Child measures Headache diary* Pediatric Migraine Disability Assessment* Parent measures Headache diary Pediatric Migraine Disability Assessment |
|
| Notes | Funding: “This research was supported in part by an educational grant from AstraZeneca LP” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomly assigned to one of two groups by a research assistant using a uniform random numbers table.” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | “Randomly assigned to one of two groups by a research assistant using a uniform random numbers table.” Comment: does not state whether research assistant was blind to concealment |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Study neurologists remained blind to randomisation condition throughout the study. Chance of unblinding were limited because follow-up appointments with the study neurologist were scheduled for 2 months following the initial assessment.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Duarte 2006 | ||
| Methods | RCT. 2 arms. Assessed at 1st, 2nd, 3rd and 4th session (sessions were monthly) | |
| Participants | End of treatment n = 32 children Start of treatment n = 32 children Sex of children: 10 M, 22 F Sex of parents: Not reported Mean age (SD) of children = 9.15 (2.1) Mean age of parents = Not reported Source = Pediatric Gastroenterology Reference Service Diagnosis of child = Recurrent abdominal pain Mean years of illness = 25 ± 17.5 months |
|
| Interventions | “Cognitive-behavioural family intervention” (CBT) “Control group” Mode of delivery: face-to-face (group/individual not reported) Intervention delivered by: General health professionals Training: Not reported Duration of intervention (child, hours) = 4 × 50 minutes = 3 hours, 20 minutes Duration of intervention (parent, hours) = 4 × 50 minutes = 3 hours, 20 minutes |
|
| Outcomes | * Extracted measures used in the analyses Child measures Pain diary* Visual analogue scale Pressure Pain Threshold |
|
| Notes | Funding: No funding statement was provided in the manuscript COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly allocated to 2 groups.” Comment: probably done but unclear method |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Ellis 2004 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 6 months after study entry (end of treatment) | |
| Participants | End of treatment = 25 Start of treatment n = 31 Sex of children: 14 M, 11 F Sex of parents: All female Mean age (SD) of children = 13.6 (1.6) Mean age of parents = 39.1 (7.6) Source = Endocrinology clinic within a tertiary care children’s hospital Diagnosis = Type 1 diabetes Mean years of illness = At least 1 year |
|
| Interventions | “Multisystemic Therapy” (MST) “Standard Care Control” Mode of delivery: Individual families, face-to-face and phone contact Intervention delivered by: mental health professionals Training: Completed 1 week MST training Duration of intervention (child) = Mean 6.5 months, 46 sessions Duration of intervention (parent) = Mean 6.5 months, 46 sessions |
|
| Outcomes | * Extracted measures used in the analyses Child measures Metabolic control* Twenty-Four Hour Recall Interview Frequency of blood glucose testing from blood glucose meter The Diabetes Management Scale (DMS) Health Service Use per Medical Chart Review Parent measures Satisfaction with treatment The Diabetes Management Scale (DMS) |
|
| Notes | Funding: “This project was supported by Grant #R21DK57212 from the National Institute of Diabetes, Digestive and Kidney Diseases” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomisation to treatment or control group was completed immediately after baseline data collection by the project statistician.” Comment: no description provided |
| Allocation concealment (selection bias) | Low risk | “Randomisation to treatment or control group was completed immediately after baseline data collection by the project statistician.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “All data was collected by a trained research assistant who was blind to the adolescent’s treatment status.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Ellis 2005 | ||
| Methods | RCT 2 arms. Assessed pre-treatment, 7 months after study entry (end of treatment), 12 months after study entry (6-month follow-up) | |
| Participants | End of treatment n = 110, 6-month follow-up = 85 Start of treatment n = 127 children and their families Sex of children: 62 M, 65 F Sex of parents: Not reported Mean age of children = 13.25 (± 1.95) Mean age of parents = 38.8 (± 6.8) Source = Endocrinology clinic within a tertiary care children’s hospital Diagnosis = Type 1 diabetes Mean years of illness = 5.25 (± 4.35) years |
|
| Interventions | “Multisystemic Therapy” (MST) “Standard Care Control” Mode of delivery: Individual families, face-to-face and phone contact Intervention delivered by: Mental health professional Training: 1-week training in MST and diabetes education Duration of intervention (child) = Mean 5.7 months, 48 sessions Duration of intervention (parent) = Mean 5.7 months, 48 sessions |
|
| Outcomes |
*Extracted measures used in the analyses Child measures HbA1c Levels* Diabetes Stress Questionnaire* Family Relationship Index (FRI) of the Family Environment Scale (FES)* Frequency of Blood Glucose Testing from blood glucose meter Twenty-Four Hour Recall Interview Health Service Use per Medical Chart Review (hospitalisations, emergency department visits) Diabetes Family Behavior Checklist (DFBC) Diabetes Family Responsibility Questionnaire Parental overestimation of adolescent responsibility score Parent measures Family Relationship Index (FRI) of the Family Environment Scale (FES)* Diabetes Family Behavior Checklist (DFBC) Diabetes Family Responsibility Questionnaire |
|
| Notes | Funding: “This project was supported by grant Ro1 DK59067 from the National Institute of Diabetes and Digestive and Kidney Diseases” COI: “No conflict of interest declared” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Random assignment to treatment group was completed after baseline data collection.” Comment: no method described |
| Allocation concealment (selection bias) | Unclear risk | “To ensure equivalence across treatment conditions, random assignment was stratified according to HbA1 c level at the baseline visit.” |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Ellis 2012 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, 7 month post-treatment, 6 month follow-up | |
| Participants | End of treatment n = 117, 6-month follow-up =117 Start of treatment n = 146 Sex of children: 64 M, 82 M Sex of parents: Not reported Mean age (SD) of child = 14.2 (2.3) Mean age of parents = Not reported Source = Hospital Diagnosis of child = Diabetes Mean years of illness = 4.7 years (3.0) |
|
| Interventions | “Multisystemic therapy” (MST) “Telephone support” Mode of delivery: Home/school/clinic visits Intervention delivered by: 5 masters-level therapists Training: 5-day training, phone consultation with MST expert, follow-up booster Duration of intervention (child, hours) = Minimum 2 meetings per week for 6 mths Duration of intervention (parent, hours) = Minimum 2 meetings per week for 6 mths |
|
| Outcomes |
*Extracted measures used in the analyses Metabolic control* Regimen adherence* |
|
| Notes | Funding: “This project was supported by grant #RO1DK59067 from the National institute of Diabetes, Digestive and Kidney diseases” COI: “Three of the authors are board members of Evidence Based Services, which has a licensing agreement with MST Services, which has a licensing agreement with MST Services, LLC, for dissemination of multisystemic therapy treatment technology. There are no other potential author conflicts of interest” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were randomized in a 1:1 ratio to MST or telephone support. Randomization occurred immediately after baseline data collection using a permuted block algorithm to ensure equivalence across treatment condition…” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | “The project statistician generated the randomization sequence and participants were notified of their randomization status by the project manager.” Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “All measures were collected by a trained research assistant in the participants’ homes. The research assistant was blind to treatment assignment to the extent possible in a behavioural trial.” Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Gulewitsch 2013 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, 3 months post-treatment | |
| Participants | End of treatment n = 37 Start of treatment n = 38 Sex of children: 14 M, 24 F Sex of parents: Not reported Mean age of child =9.4 Mean age of parents = Not reported Source = Public announcements in local newspapers and at paediatricians’ and community health centres, referrals from paediatricians and family physicians Diagnosis of child = Functional abdominal pain and Irritable bowel syndrome Mean months (SD) of illness = 34.61 (40.7) |
|
| Interventions | “Hypnotherapeutic behavioural treatment” “Wait-list Control group” (WCG) Mode of delivery: Group face-to-face Intervention delivered by: Psychologist Training: Not reported Duration of intervention (child, hours) = 2 × 90 minutes = 3 hours Duration of intervention (parent, hours) = 2 × 90 minutes = 3 hours |
|
| Outcomes |
*Extracted measures used in the analyses Child Behaviour Checklist (CBCL)* Pain diary Pediatric-pain disability index (P-PDI)* Abdominal pain index (AP) Health-related quality of life (HRQoL) |
|
| Notes | Funding: No funding statement was provided in the manuscript COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomly assigned following simple randomization procedures (computerized random number generator) to TG (n=20 or WCG (n=18).” Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Hicks 2006 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 1-month follow-up and 3-month follow-up | |
| Participants | End of treatment n = 37, 1 -month follow-up = 37, 3-month follow-up =32 Start of treatment n = 47 Sex of children: 17 M, 30 F Sex of parents: Not reported Mean age (SD) of children = 11.7 (2.1) Mean age of parents = Not reported Source = Media, posters in physicians offices and advertisements in school newsletters Diagnosis = Recurrent head or abdominal pain Mean years of illness = 3 years |
|
| Interventions | “Online cognitive-behavioral treatment programme” (CBT) “Wait list Control” Mode of delivery: Individual, online web programme, email and phone contact Intervention delivered by: Internet and researcher Training: Not reported Duration of intervention (child) = Mean 3 hours on the phone, duration to complete online programme not described Duration of intervention (parent) = Not reported |
|
| Outcomes |
*Extracted measures used in the analyses Child measures Pain diary* Pediatric Quality of Life Inventory Treatment expectation Treatment satisfaction Parent measures Pediatric Quality of Life Inventory Treatment expectation Treatment satisfaction |
|
| Notes | Funding: “The first author acknowledges the support received through the Peter Samuelson STARBRIGHT Foundation 2002 Dissertation Award in pediatric psychology and the Canadian Pain Society Small Grant for Local and Regional Initiatives” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The 47 participants were stratified by age and pain severity and randomly assigned by blocks to either the treatment condition or the standard medical care wait-list condition.” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | “The 47 participants were stratified by age and pain severity and randomly assigned by blocks to either the treatment condition or the standard medical care wait-list condition.” Comment: no method of concealment described |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Hoekstra-Weebers 1998 | ||
| Methods | RCT. 2 arms. Pre-treatment (at diagnosis), post-treatment, 6-month follow-up | |
| Participants | End of treatment and 6-month follow-up n = 81 parents, 41 children Start of treatment n = 120 parents, 61 children Sex of parents: 40 M, 41 F Sex of children: 23 M, 18 F Mean age (SD) of parents = 36.6 (5.4) Mean age (SD) of children = 6.4 (4.7) Source = Paediatric oncology clinic Diagnosis = Cancer Mean years of illness = 2 to 21 days post diagnosis |
|
| Interventions | “Psychoeducational and Cognitive-Behavioral Intervention” (CBT) “Standard Care Control” Mode of delivery: Individual, face-to-face Intervention delivered by: Master’s student in Psychology Training: Not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 8 × 90 minutes =12 hours |
|
| Outcomes |
*Extracted measures used in the analyses Parent measures Symptom Check List (SCL)* State-Trait Anxiety Inventory-State* Goldberg General Health Questionnaire (GHQ) Social Support List-Discrepancies (SSL-D) Intensity of emotions questionnaire designed by the authors |
|
| Notes | Funding: “This study has been funded by the Dutch Cancer Society and the Pediatric Oncology Foundation Groningen” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Parents were randomly assigned…. parents drew one of two envelopes in which a letter indicated in which group they were placed. ” Comment: method unclear |
| Allocation concealment (selection bias) | Unclear risk | “Parents were randomly assigned…. parents drew one of two envelopes in which a letter indicated in which group they were placed.” Comment: probably done but unsure whether envelopes were sealed or numbered |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Kashikar-Zuck 2005 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and post-treatment | |
| Participants | End of treatment n = 27 Start of treatment n = 30 Sex of children: 0 M, 30 F Sex of parents: 3 M, 27 F Mean age of children = 15.83 (1.26) Mean age of parents = Not reported Source = paediatric rheumatology clinic, Midwestern USA Diagnosis = Fibromyalgia syndrome Mean years of illness = Over 2 years |
|
| Interventions | “Cognitive Skills Training” (CBT) “Self Monitoring” Mode of delivery: Individual, face-to-face plus phone contact Intervention delivered by: Doctoral level paediatric psychology intern or psychology fellow Training: Trained by principal investigator Duration of intervention (child) = 6 sessions, hours not reported Duration of intervention (parent) = 3 sessions, hours not reported |
|
| Outcomes |
*Extracted measures used in the analyses Child measures Children’s Depression Inventory* (CDI) Functional Disability Inventory* (FDI) Visual analogue scale (VAS) Pain Coping Questionnaire (PCQ) Tender point examination |
|
| Notes | Funding: “Supported by grants from the Cincinnati Children’s Hospital Research Foundation and National Institutes of Health Grant 1P60AR47784-0” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer generated pseudo-random number list was used. A simple randomisation technique was used with a 1:1 allocation ratio for 30 subjects as a single block. ” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “…a research assistant who was blind to the objectives of the study enrolled the subject and opened a sealed envelope with the subject’s study identification number.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “A research assistant who was blind to the study objectives and to the subjects’ treatment assignment administered the self-report measures. The rheumatologist or occupational therapist who conducted the tender point assessments was blind to the subjects’ treatment assignment.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Kashikar-Zuck 2012 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment, 6-month follow-up | |
| Participants | End of treatment n = 106, follow-up n = 100 Start of treatment n = 114 Sex of children: 9 M, 105 F Sex of parents: Not reported Mean age (SD) of children = 15.0 (1.8) Mean age of parents = Not reported Source = 4 paediatric rheumatology centres, Midwestern USA Diagnosis = Fibromyalgia syndrome Mean years (SD) of illness = 2 years, 10 months (2 years, 6 months) |
|
| Interventions | “Cognitive behavioural therapy” (CBT) “Fibromyalgia education” Mode of delivery: Individual, face-to-face Intervention delivered by: Therapists with postdoctoral training in paediatric psychology Training: 6- to 8-hour training by principal investigator Duration of intervention (child) = 6 hours Duration of intervention (parent) = 2 hours, 15 minutes |
|
| Outcomes |
*Extracted measures used in the analyses Child measures Child Depression Inventory* (CDI) Functional Disability Inventory* (FDI) Visual analogue scale* (VAS) Pediatric Quality of Life Inventory (PedsQL) |
|
| Notes | Funding: “Supported by grants from the Cincinnati Children’s Hospital Research Foundation and National Institutes of Health Grant 1P60AR47784-0” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Eligible patients were randomly assigned to 1 of the 2 treatment arms based upon a computer-generated randomisation list. Randomisation was stratified by site.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “When a patient was enrolled, the study therapist contacted the biostatistician to obtain the subject identification number and treatment allocation.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “The principle investigator, study physicians, study coordinator, and assessment staff were all blinded to the patients’ treatment condition throughout the trial. Patients were asked not to divulge what treatment they were receiving to the study physician.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Kazak 2004 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and 3 to 5 months post-treatment | |
| Participants | End of treatment n = 116 children Start of treatment n = 150 children Sex of children: 73 M, 77 F Sex of parents: 106 M, 146 F Mean age (SD) of children = 14.61 (2.4) Mean age of parents = Not reported Source = Oncology tumour registry at the Children’s Hospital of Philadelphia Diagnosis = Childhood cancer survivor Mean years (SD) of illness = 5.30 (2.92) years post-treatment |
|
| Interventions | “Surviving Cancer Competently Intervention Program SCCIP” (CBT) “Wait-list Control” Mode of delivery: Group, face-to-face Intervention delivered by: Nurses, social workers, psychologists, graduate and post-doctoral psychology trainees Training: 12 hours Duration of intervention (child) = 5 hours direct, 2 hours informal Duration of intervention (parent) = 5 hours direct, 2 hours informal |
|
| Outcomes |
*Extracted measures used in the analyses Child measures Post-Traumatic Stress Disorder Reaction Index (PTSD-RI)* Impact of Events Scale-Revised (IES-R) Revised Children’s Manifest Anxiety Scale (RCMAS) Parent measures Post-Traumatic Stress Disorder Reaction Index (PTSD-RI)* Impact of Events Scale-Revised (IES-R) State-Trait Anxiety Inventory (STAI) |
|
| Notes | Funding: “This research was funded by a grant from the National Cancer Institute (CA63930) and a grant from the Abramson Cancer Center of The University of Pennsylvania (CA15488)” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Families were randomised to the treatment or wail-list control condition.” Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Laffel 2003 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment and 1 year | |
| Participants | End of treatment n = 100 children Start of treatment n = 105 Sex of children: 53 M, 47 F Sex of parents: Not reported Mean age (SD) of children =12.1 (2.3) Mean age of parents = Not reported Source = Joslin Diabetes Center Pediatric and Adolescent Unit Diagnosis = Type 1 diabetes Mean years of illness = 2.7 years ±1.6 years |
|
| Interventions | “Teamwork Intervention” (FT) “Standard Care” Mode of delivery: Individual families, face-to-face Intervention delivered by: Research assistant Training: Not reported Duration of intervention (child) = 4 sessions over 1 year (hours not reported) Duration of intervention (parent) = 4 sessions over 1 year (hours not reported) |
|
| Outcomes |
*Extracted measures used in the analyses Child measures Glycemic Control (A1c)* Diabetes Family Conflict Scale* Clinician Report of Adherence to Diabetes Management Tasks Diabetes Family Responsibility Questionnaire Joint structured interview to assess parental involvement in diabetes management tasks Pediatric Quality of Life Inventory (PedsQL) Parent measures Diabetes Family Conflict Scale* Diabetes Family Responsibility Questionnaire Joint structured interview to assess parental involvement in diabetes management tasks |
|
| Notes | Funding: “Supported by a grant (DK-46887) from the National Institute of Diabetes, Digestive and Kidney Diseases, the Charles H. Hood Foundation, and the Katherine Adler Astrove Youth Education Fund” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomly assigned according to age and duration.” Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition not adequately described |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Lask 1979 | ||
| Methods | RCT. Assessed at pre-treatment, post-treatment and 1 year follow-up | |
| Participants | End of treatment n = 37 children, 33 families Start of treatment n = 37 children, 33 families Sex of children: Not reported Sex of parents: Not reported Mean age of children = Range 4 to 14 years, mean not reported Mean age of parents = Not reported Source = not reported Diagnosis = Asthma Mean years of illness = Not reported |
|
| Interventions | “Family psychotherapy” (FT) Standard care control group Mode of delivery: Individual families, face-to-face Intervention delivered by: Mental health professional Training: Not reported Duration of intervention (child) = 6 × 1 hr family psychotherapy = 6 hours Duration of intervention (parent) = 6 × 1 hr family psychotherapy = 6 hours |
|
| Outcomes |
*Extracted measures used in the analyses Child measures Diary cards* Peak expiratory flow rate (PEFR) Forced expiratory volume (FEV) Thoracic gas volume (TGV) |
|
| Notes | Funding: “We are indebted… to the Sembal Trust for financial assistance” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Families were then randomly allocated to the experimental (group A) or control group (group B).” Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely |
| Lehmkuhl 2010 | ||
| Methods | RCT 2 arms. Assessed pre and post-treatment | |
| Participants | End of treatment n = 22 Start of treatment n = 32 Sex of children: 9 M, 23 F Sex of parents: 2 M, 27 F, 3 unknown Mean age (SD) of children = 13.66 (2.43) Mean age (SD) of parents = 41.53 (8.14) Source = university-affiliated paediatric endocrinology clinic Diagnosis = type 1 diabetes Mean years of illness = over 6 months |
|
| Interventions | “Telehealth Behavioral Therapy” (CBT) “Wait list control” Mode of delivery: Individual, phone calls Intervention delivered by: Psychologists and Clinical Psychology interns Training: Not reported Duration of intervention (child) = 36 phone calls, 9 to 12 hours Duration of intervention (parent) = 36 phone calls, 9 to 12 hours |
|
| Outcomes |
*Extracted measures used in the analyses Child measures A1c Now* Diabetes Family Behavior Scale, Abbreviated (DFBS)* Diabetes Self-Management Profile (DSMP) Diabetes Family Behavior checklist (DFBC) Diabetes Family Responsibility Questionnaire Parent measures Diabetes Self-Management Profile (DSMP) Diabetes Family Behavior checklist (DFBC) Diabetes Family Responsibility Questionnaire Clinician measures Clinical Global Impression Scale (CGIS) Clinical Global Improvement (CGI) |
|
| Notes | Funding: “This study was supported by the Florida Department of Health and Children’s Medical Services” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were then randomly assigned to the immediate treatment group or to a 1 month wait-list using a random numbers table.” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | “All assessments were conducted by an independent rater. The rater was a full-time research assistant.” Comment: unclear whether rater was blind |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Levy 2010 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment, 3-month follow-up, 6-month follow-up | |
| Participants | End of treatment n = 168, 3 months = 143, 6 months =154 Start of treatment n = 200 Sex of children: 55 M, 145 F Sex of parent: 12 M, 188 F Mean age (SD) of child = 11.21 (2.55) Mean age (SD) of parent = 43.75 (6.35) Source = Paediatric GI Clinics at Seattle Children’s Hospital and the Atlantic Health System in Morristown, New Jersey Seattle area participants were also recruited via local clinics and community-posted flyers Diagnosis = Functional abdominal pain Mean years of illness =3+ episodes of abdominal pain during a 3-month period |
|
| Interventions | “Cognitive-behavioural treatment” (CBT) “Educational intervention” Mode of delivery: Individual families, face-to-face Intervention delivered by: Therapists Training: not reported Duration of intervention (child) = 3 × 75 minutes = 4 hours Duration of intervention (parent) = 3 × 75 minutes = 4 hours |
|
| Outcomes |
*Extracted measures used in the analyses Child measures Functional Disability Inventory* (FDI) Faces Pain Scale-Revised* Child Depression Inventory* (CDI) Child Somatization Inventory (CSI) Multidimensional Anxiety Scale for Children (MASC) Parent measures Functional Disability Inventory (FDI) Faces Pain Scale-Revised Child Somatization Inventory (CSI) |
|
| Notes | Funding: No funding statement was provided in the manuscript COI: “Conflict of Interest Disclosures: None reported” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was then performed by a different researcher using a computerised random-number generator, stratifying by age.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “Randomisation was then performed by a different researcher using a computerised random-number generator, stratifying by age.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Nurse assessors were blind to the treatment assignment of the children.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Marsland 2013 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, 2 week post-treatment | |
| Participants | End of treatment n = 38 Start of treatment n = 49 Sex of children: 18 M, 19 F Sex of parent: 2 M, 35 F Mean age (SD) of child = 13.05 (3) Mean age (SD) of parent = 41.15 (8.35) Source = Hospital Diagnosis = Cancer Mean years (SD) of illness = 2 (14.7) |
|
| Interventions | ”Connections to coping” “Treatment as usual” (TAU) Mode of delivery: face-to-face, telephone and online. Intervention delivered by: Clinician Training: Training in the conceptual foundations of the intervention and the stressors and burden commonly experienced by families of children with cancer, along with training in conducting each of the sessions Duration of intervention (child) = 6 face-to-face sessions, 6 phone calls, Duration of intervention (parent) = 6 face-to-face sessions, 6 phone calls, web support |
|
| Outcomes |
*Extracted measures used in the analyses Beck Depression Inventory (BDI)* State-Trait Anxiety Inventory (STAI) Perceived Stress Scale (PSS) Impact of Event Scale (IES) Interpersonal Support Evaluation List (ISEL) Client Satisfaction questionnaire-8 (CSQ-8) |
|
| Notes | Funding: “Grant R21 CA04255-01 from the National Cancer Institute” COI: “Conflicts of interest: None declared” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “…before randomly assigning them in a ratio of 2:1 to the intervention (N=30) or treatment-as-usual (TAU) control condition (N=15).” Comment: no method described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was fully reported and there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Mullins 2012 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 1 month post-treatment and 3 month follow-up | |
| Participants | End of treatment n = 41 Start of treatment n = 57 Sex of children: 28 M, 24 F Sex of parents: 0 M, 52 F Mean age (SD) of child = 8.19 (4.5) Mean age (SD) of parent = 35.1 (6.9) Source = Hospital Diagnosis = Cancer Mean years of illness = 2.43 (0.98) |
|
| Interventions | “Intervention group” (IG) “Treatment as usual” (TAU) Mode of delivery: In-clinic individual, face-to-face, phone calls Intervention delivered by: Nurse interventionists, psychology interventionists Training: Manualised intervention, training by corresponding author Duration of intervention (child) = 0 Duration of intervention (parent) = Mean = 300 minutes/ 5 hours |
|
| Outcomes | * Extracted measures used in the analyses The Intensity of Treatment Rating (ITR) Parent Perception of Uncertainty Scale (PPUS) Symptom Checklist 90-Revised (SCL-90-R)* Impact of Events Scale-Revised (IES-R) Care of My Child With Cancer Scale (CMCC) |
|
| Notes | Funding: “This work was supported by a grant (R21NR010103) to L. L. Mullins, PhD. (Principal Investigator) from the National Institutes of Health (NIH/NINR)” COI: “Conflicts of interest: None declared” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ’Blocked randomization was used for participant assignment to condition’ Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported and there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Naar-King 2014 | ||
| Methods | RCT Assessed at pre-treatment, post-treatment = 7 months after baseline data collection | |
| Participants | End of treatment n = 119 Start of treatment n = 170 Sex of children: 102 M, 65 F Sex of parents: Not reported Mean age (SD) of children = 13.5 (1.3) Mean age of parents = Not reported Source = Hospital Diagnosis = Asthma Mean years of illness = Not reported |
|
| Interventions | “Multisystemic Therapy-Health Care” (MST-HC) “Family support” (FS) Mode of delivery: In-vivo observations in child’s everyday life Intervention delivered by: Therapist Training: Had training in MST Duration of intervention (child) = Mean 5.14 months, 27.09 sessions Duration of intervention (parent) = Mean 5.14 months, 27.09 sessions |
|
| Outcomes | * Extracted measures used in the analyses Family Asthma Management System Scale (FAMSS)* Daily Phone Diary (DPD) Forced expiratory volume in 1s (FEV1)* |
|
| Notes | Funding: “This research was supported by a grant from the National Institute of Health (1R01AA0 22891-01)” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomization was stratified based on (1) severity of asthma complications as indicated by the number of recent hospitalizations…. (2) receipt of asthma specialty care (…).” Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Baseline data collection, including spirometry, subsequently occurred in the home by trained research assistants. All data collectors were blind to the participant’s study condition.” Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported and data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Methods | RCT. Assessed at pre-treatment, 3 weeks after last clinic visit post-treatment | |
| Participants | End of treatment n = 116 Start of treatment n = 122 Sex of children: Not reported Sex of parents: Not reported Mean age of children =11.5 Mean age of parents = Not reported Source = Hosital Diagnosis = Diabetes Mean years of illness =5.8 |
|
| Interventions | “WE*CAN intervention” “Usual Care Comparison” Mode of delivery: Clinic visits and telephone calls Intervention delivered by: Health advisors (College graduates) Training: Health Advisors received local and central training, and participated in monthly conference calls which could answer any issues they had with the intervention Duration of intervention (child) = 3 sessions and 3 phone calls Duration of intervention (parent) = 3 sessions and 3 phone calls |
|
| Outcomes | * Extracted measures used in the analyses HbA1c* Diabetes Self Management Profile (DSMP)* PedsQL Core Generic Module and Diabetes Module Diabetes Family Responsibility Questionnaire Diabetes Family Conflict Scale* Children’s Depression Inventory |
|
| Notes | Funding: “This research was supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development. The following institutions and investigators comprised the steering committee of the Family Management of Diabetes multi-site trial Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland: Tonja R Nansel, PhD, Bruce Simons-Morton, EdD, Ronald J. Iannotti Joslin Diabetes Center, Boston, Massachusetts: Lori Laffel, MD MPH, Korey Hood, PhD. Contract N01-HD-4-3364 Nemours Children’s Clinic, Jacksonville, Florida: Tim Wysocki, PhD, Amanda Lochrie, PhD. Contract N01- HD-4-3361 Texas Children’s Hospital, Houston, Texas: Barbara Anderson, PhD. Contract N01-HD-4-3362. Children’s Memorial Hospital, Chicago, Illinois: Jill Weissberg-Benchell, PhD, Grayson Holmbeck, PhD. Contract N01-HD-4-3363 James Bell Associates, Arlington, Virginia; Cheryl McDonnell, PhD, MaryAnn D’Elio, Contract N01-HD-3-3360” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “30 to 32 families (total of 122) meeting the eligibility criteria were recruited and randomized into intervention or usual care groups.” No method given. Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Except for biomedical data, which was obtained from medical records reviews and by interview during clinic visits, data collection occurred at home visits at baseline and follow-up by trained interviewers not employed by the clinic.” Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | No attrition flow chart and there was no description of significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Nansel 2012 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, 24 month post-treatment | |
| Participants | End of treatment n = 331 Start of treatment n = 390 Sex of children: 192 M, 198 F Sex of parents: Not reported Mean age (SD) of child = 12.5 (1.8) Mean age of parents = Not reported Source = Hospital Diagnosis of child = Diabetes Mean years of illness = 4.85 |
|
| Interventions | “WE*CAN intervention” “Usual Care Comparison” Mode of delivery: In-person, individual families Intervention delivered by: Health Advisor Training: Not reported Duration of intervention (child, hours) = 7 visits = 3½ hours Duration of intervention (parent, hours) = 7 visits = 3½ hours |
|
| Outcomes | * Extracted measures used in the analyses HbA1c Diabetes Self-Management Profile |
|
| Notes | Funding: “Supported by the intramural research program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, under the following contracts: N01-HD-4-3364, Joslin Diabetes Center, Boston, Massachusetts; N01-HD-4-3361, Nemours Children’s Clinic, Jacksonville, Florida; N01-HD-4-3362, Texas Children’s Hospital, Houston, Texas; N01-HD-4-3363, Children’s Memorial Hospital, Chicago, Illinois; and N01-HD-3-3360, James Bell Associates, Arlington, Virginia. Funded by the National Institutes of Health (NIH)” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A system of random permuted blocks within strata was prepared by the study coordinating center by a person not involved with data collection.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “A separate randomization list was prepared for each strata; lists were transferred to a sequence of sealed envelopes, each containing the assignment of intervention or usual care. Persons conducting assessments were blinded to study assignment.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Persons conducting assessments were blinded to study assignment.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were not fully reported |
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and 11 weeks follow-up | |
| Participants | End of treatment n = 27 Start of treatment n = 46 Sex of children: 25 M, 12 F Sex of parents: Not reported Mean age (SD) of child = 9.24 (1.48) Mean age of parent = Not reported Source = Paediatric chest clinic of the Prince of Wales Hospital Hong Kong Diagnosis = Asthma Mean years (SD) of illness = 5.70 (2.41) |
|
| Interventions | “We Together-We Success Parallel Group for Children with Asthma and their Parents (WTWS)” (FT) “Control Group” (wait list) Mode of delivery: Group, face-to-face Intervention delivered by: Not reported Training: Not reported Duration of intervention (child) = 11 × 2 hours = 22 hours Duration of intervention (parent) = 11 × 2 hours = 22 hours |
|
| Outcomes | * Extracted measures used in the analyses Child measures Exhaled nitric oxide (eNO)* Spirometry Parent measures Anxiety Subscale of Chinese version Hospital Anxiety and Depression Scale (HADS)* Caretakers’ perceived efficacy in the management of child’s asthma (self-constructed)* The Emotion Scale of Body-Mind-Spirit Well-Being Inventory (BMSWBI) Standard Short Form 12 (SF-12) Chinese (Hong Kong) Version 1 measuring health-related quality of life Participant’s adjustment to asthma (self-constructed)* |
|
| Notes | Funding: No funding statement was provided in the manuscript COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A randomised wait-list-controlled clinical trial design was adopted in this study”. Comment: no method described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Niebel 2000 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and post-treatment | |
| Participants | End of treatment n = 47 Start of treatment n = 57 Sex of children: 5 M, 47 F Sex of parents: 0 M, 47 F Mean age (SD) of children = 3.9 (2.43) Mean age (SD) of parents = 33.9 (1.25) Source = Not reported Diagnosis = Eczema Mean years (SD) of illness = 9.1 years (8.36) |
|
| Interventions | “Direct Behavioural Parental Education” “Standardized Video-based Parental Education” “Dermatologic Standard Treatment” Mode of delivery: Group and individual, face-to-face and video-based Intervention delivered by: Mental health professional Training: Not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 10 × 2 = 20 hours (direct), 1.67 hours (video-based) |
|
| Outcomes | * Extracted measures used in the analyses * Skin condition and subjective symptoms of dermatological assessment for children |
|
| Notes | Funding: “We thank the ministry of work, social, youth and health of the state of Schleswig-Holstein for financial support” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Olivares 1997 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 9-month follow-up | |
| Participants | End of treatment n = Not reported Start of treatment n = 36 Sex of children: 19 M, 17 F Sex of parents: 12 M, 23 F Mean age of children = Not reported Mean age (SD) of parents = Treatment group = 39.71 (5.47), control group = 40.87 (7.05) Source = Not reported Diagnosis = Diabetes Mean years (SD) of illness = treatment group = 4.76 (3.8) years, control group = 3.72 (2.22) years |
|
| Interventions | “Programme to modify parent behaviour” (CBT) “Wait list control” Mode of delivery: Group, face-to-face Intervention delivered by: Not reported Training: Not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 8 sessions × 70 min = 9 hours 20 min |
|
| Outcomes | * Extracted measures used in the analyses Knowledge about behaviour modification* Responsibility for diabetes care* Blood glucose level* |
|
| Notes | Funding: No funding statement was provided in the manuscript COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data not fully reported |
| Palermo 2009 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment and 3-month follow-up | |
| Participants | End of treatment n = 44 Start of treatment n = 48 Sex of children: 13 M, 35 F Sex of parents: 7:41 Mean age (SD) of children = 14.8 (2.0) Mean age of parents = Not reported Source = Academic health centre, Pacific Northwest USA Diagnosis = Mixed pain conditions Mean years of illness = 30 months |
|
| Interventions | “Internet-delivered family cognitive-behavioral therapy” (CBT) “Wait list control group” Mode of delivery: individual families, Internet Intervention delivered by: Internet and online coach. Online coach was a PhD level postdoctoral psychology fellow Training: 1 year of experience delivering face-to-face CBT to children with chronic pain Duration of intervention (child) = 4 hours Duration of intervention (parent) = 4 hours |
|
| Outcomes | * Extracted measures used in the analyses Child measures Pain diary* Child Activity Limitations Interview* (CALI) Revised Child Anxiety and Depression Scale* (RCADS) Treatment Evaluation Inventory - Short Form Parent measures Adult Responses to Children’s Symptoms* (ARCS) Treatment Evaluation Inventory - Short Form |
|
| Notes | Funding: “This research was supported by Grant HD050674 from the National Institutes of Health/National Institute of Child Health and Human Development (PI: Palermo) and by a grant from the Doernbecher Foundation” COI: “There are no financial relationships that might lead to a conflict of interest” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A fixed allocation randomisation scheme was used. Specifically, we used blocked randomisation with blocks of 10 to assign participants to the two treatment conditions during the course of randomisation. An online random number generator was used to produce the blocked randomisation.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “Group assignments were identified by ID number in sealed envelopes. Following completion of all pre-treatment assessments, a research coordinator opened the sealed envelope to reveal the group assignment.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | Participants completed questionnaires online |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, no significant differences between completers and non-completers was described |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Robins 2005 | ||
| Methods | RCT 2 arms. Assessed pre-treatment, post-treatment and 6 to 12 months following study entry | |
| Participants | End of treatment n = 69, follow-up = 69 Start of treatment n = 86 Child sex: 30 M, 39 F Parent sex: Not reported Mean age (SD) of children = 11.34 (2.4) Mean age of parents = Not reported Source = Community-based primary care physicians and hospital-based paediatric gastroenterologists Diagnosis = Recurrent abdominal pain Mean years of illness = 3+ episodes over 3 months |
|
| Interventions | “Standard Medical Care plus Short-Term Cognitive-Behavioral Family Treatment” (CBT) “Standard Medical Care” Mode of delivery: group, face-to-face Intervention delivered by: Psychology post-doctoral fellow or pre-doctoral intern Training: Not reported Duration of intervention (child) = 5 sessions × 40 minutes = 3 hours 20 minutes Duration of intervention (parent) = 3 sessions × 40 minutes = 2 hours |
|
| Outcomes | * Extracted measures used in the analyses Child measures Abdominal Pain Index* (API) Child Somatization Inventory* (CSI) Functional Disability Inventory Child Version* (FDI) School Absences obtained from school attendance records Parent measures Abdominal Pain Index (API) Child Somatization Inventory (CSI) Clinician measures Health service use obtained from physician offices |
|
| Notes | Funding: “This study was supported in part by a grant through the Nemours Research Programs, awarded to the first author” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The remaining sample of 86 were randomly assigned using a coin-flip method.” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented on significant differences between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Sahler 2002 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and 3-month follow-up | |
| Participants | End of treatment n = 81 Start of treatment n = 92 Sex of children: Not reported Sex of parents: 0 M, 92 F Mean age (SD) of children = 8.32 (5.5) Mean age (SD) of mothers = 35.35 (6.6) Source = 6 children’s hospitals in USA Diagnosis = Cancer Mean years of illness = 2 to 16 weeks from diagnosis |
|
| Interventions | “Problem solving therapy” (PST) “Standard psychosocial care” Mode of delivery: Individual, face-to-face Intervention delivered by: Mental health professional or doctoral candidate in psychology Training: 3-day workshop Duration of intervention (child) = 0 Duration of intervention (parent) = 8 sessions × 1 hr = 8 hours |
|
| Outcomes | * Extracted measures used in the analyses Parent measures Social Problem-Solving Inventory-Cancer* Profile of Mood States* |
|
| Notes | Funding: “This work was supported by Grant R25 CA 65520 from the National Cancer Institute, National Institutes of Health” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was performed centrally, after stratification by site, using a two-block technique that produced a unique sequence for each site, delivered as a set of consecutively numbered envelopes specifying each subject’s assignment”. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “Randomisation was performed centrally, after stratification by site, using a two-block technique that produced a unique sequence for each site, delivered as a set of consecutively numbered envelopes specifying each subject’s assignment”. Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Sahler 2005 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and 6 months after T1 | |
| Participants | End of treatment n = 407 Start of treatment n = 430 Sex of children: 219 M, 210 F Sex of parents: 0 M, 429 F Mean age of children at diagnosis = 7.6 Mean age of mothers = 35.5 Source = 7 sites is USA + 1 site in Israel Diagnosis = Cancer Mean years of illness = 2 to 16 weeks from diagnosis |
|
| Interventions | “Bright IDEAS Problem Solving Skills Training” (PSST) “Usual psychosocial care” (UPC) Mode of delivery: Individual, face-to-face Intervention delivered by: not reported Training: Not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 8 × 1 hr = 8 hours |
|
| Outcomes | * Extracted measures used in the analyses Parent measures Profile of Mood States (POMS)* Beck Depression Inventory-II (BDI-II)* Social Problem-Solving Inventory-Revised (SPSI-R)* NEO-Five Factor Inventory (NEO-FFI) Impact of Event Scale-Revised (IES-R) |
|
| Notes | Funding: “This project was supported by National Cancer Institute, National Institutes of Health Grant R25 CA65520” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomisation was performed centrally.” Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition was reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Sahler 2013 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, immediately following intervention post-treatment, 3 month follow-up | |
| Participants | End of treatment n = 204 Start of treatment n = 309 Sex of children: 165 M, 144 F Sex of parents: 0 M, 309 F Mean age (SD) of children = 8.8 (5.9) Mean age (SD) of mothers = 37.3 (8.2) Source = Hospital Diagnosis = Cancer Mean years of illness =2.6 years |
|
| Interventions | “Bright IDEAS problem-solving skills training” (PSST) “Nondirective support” (NDS) Mode of delivery: In-clinic, individual, face-to-face Intervention delivered by: Research assistants (RA) with graduate training in clinical or behavioural psychology Training: Manual given, RAs trained in groups and then given individual weekly supervision Duration of intervention (child) = In-clinic, individual, face-to-face Duration of intervention (parent) = 8 × 1-hour sessions over 16 weeks |
|
| Outcomes | * Extracted measures used in the analyses Social Problem Solving Inventory-Revised (SPSI-R)* The Profile of Mood States (POMS) Total Mood Distubrance scale (TMD) Beck Depression Inventory (BDI-II)* Impact of Event Scale Revised (IES-R) |
|
| Notes | Funding: “Supported by Grant No. R01 CA098954” COI: The author indicated no potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants completed baseline (T1) assessment and were randomly assigned to a treatment arm by using a block design of six stratified by site and language.” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “The reviewers were blinded to treatment condition.” Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition reported, but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Sanders 1994 | ||
| Methods | RCT. 2 arms. Assessed at pre-treatment, post-treatment, 6-month follow-up, 12-month follow-up | |
| Participants | End of treatment n = 44 Start of treatment n = 44 Sex of children: 16 M, 28 F Sex of parents: Not reported Mean age (SD) of children = 9.22 (1.9) Mean age (SD) of parents = 39.3 (4.9) Source = Not reported Diagnosis = Recurrent abdominal pain Mean years (SD) of illness = 44 months (37.76) |
|
| Interventions | “Cognitive-behavioral family intervention” (CBT) “Standard paediatric care” Mode of delivery: Individual, face-to-face Intervention delivered by: Clinical Psychologists Training: Not reported Duration of intervention (child) = 6 × 50 minutes = 5 hours Duration of intervention (parent) = 6 × 50 minutes = 5 hours |
|
| Outcomes | * Extracted measures used in the analyses Child measures Pain diary* Videotaped vignettes, assessment of children’s self coping Parent measures Child Behavior Checklist (CBCL)* Videotaped vignettes, assessment of maternal care giving* Parent Observation Record (POR) Treatment expectancies Measures of relapse - interview Satisfaction with treatment |
|
| Notes | Funding: “This study was supported by Grant 53091 from the National Health and Medical Research Council of Australia to Matthew R. Sanders, Ross W. Shepherd, and Geoffrey Cleghorn” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The study used a randomised group comparison design with two treatment conditions.” Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported |
| Saßman 2012 | ||
| Methods | RCT 2 arms. Assessed pre-treatment, 3 month post-treatment and 12-month after treatment follow-up | |
| Participants | End of treatment n = 31, 6-month follow-up n = NA Start of treatment n = 37 Sex of children: Not reported Sex of parents: 32 M, 33 F Mean age (SD) of children = 6.1 (2.1)Mean age (SD) of parents = 41.3 (4.78) Source = Parent conferences, posters, flyers or direct contact via medical practitioner or diabetes educator Diagnosis = Diabetes Mean length of illness = 2.6 years |
|
| Interventions | “DELFIN program” (Das Elterntraining für Eltern von Kindern mit Diabetes Typ 1; English translation: The parenting programme for parents of children with diabetes type 1) “Waitlist” Mode of delivery: In-clinic and phone calls Intervention delivered by: Psychologist Training: Not reported Duration of intervention (child) = 0 Duration of intervention (parent) = 2hr × 5 weeks = 1 0hr |
|
| Outcomes | * Extracted measures used in the analyses Parenting Scale (PS)* Questions to Education Behaviour form Depression-Anxiety-Stress Scale (DASS)* Strengths and Difficulties Questionnaire (Parent version) Satisfaction with the DELFIN programme questionnaire A1c* |
|
| Notes | Funding: “The German Diabetes Association (DDG) is gratefully acknowledged for their support (‘Menarini-Award’). Parts of this publication have been presented in 2011 at the annual meeting of the German Diabetes Association (DDG)” COI: “The authors declare that they have no competing interests” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “..randomization with a 1:1 allocation ratio.” Comment: No method described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition reported but no data were presented describing equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Seid 2010 | ||
| Methods | RCT. 3 arms. Assessed pre-treatment, post-treatment and 6-month follow-up | |
| Participants | End of treatment n = 204, 6-month follow-up n = 188 Start of treatment n = 252 Sex of children: 154 M, 98 F Sex of parents: 9 M, 244 F Mean age (SD) of children = 7.37 (3.07) Mean age of parents = Not reported Source = Federally qualified health centres, a commercial HMO, school/daycare, local asthma initiatives and self referrals in San Diego, CA, USA Diagnosis = Asthma Mean length (SD) of illness = 44 months (37.76) |
|
| Interventions | “Problem-Solving Skills Training + Care Coordination” (PST + Asthma Education) “In Home Asthma Education + Care Coordination” (Asthma Education) “Standard care wait-list control” Mode of delivery: individual families, face-to-face Intervention delivered by: Master’s level health educator (PST), paraprofessional asthma home visitors (care co-ordination) Training: 2-week training Duration of intervention (PST + Asthma Education) = 6 × 45 to 60 minutes Duration of intervention (Asthma Education) = 5 × 45 to 60 minutes |
|
| Outcomes | * Extracted measures used in the analyses Child measures Pediatric Quality of Life Inventory Asthma Module Asthma Symptoms Scale (PedsQL Asthma) Parent measures Pediatric Quality of Life Inventory (PedsQL)* Health Service Use self report |
|
| Notes | Funding: “This research was supported by a grant from the Maternal and Child Health Bureau of the Health Resources and Services Administration (R40 MC01214/08044)” COI: “Conflict of Interest: Dr Varni holds the copyright and the trademark for the PedsQL and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Blocked randomisation, stratified by site of care and disease severity was used. Prepared randomisation lists were created by the statistician and concealed until intervention assignment.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “Blocked randomisation, stratified by site of care and disease severity was used. Prepared randomisation lists were created by the statistician and concealed until intervention assignment.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Bilingual, bicultural research staff, blinded to the intervention group, administered surveys in English or Spanish in participants’ homes.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data fully reported |
| Shekarabi-Ahari 2012 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, post-treatment and 8 weeks | |
| Participants | End of treatment n = 20, 8 week follow-up = 20 Start of treatment n = 20 Sex of children: 11 M, 9 F Sex of parents: 0 M, 20 F Mean age of children = 6.15 Mean age of parents = 33.35 Source = Hospital Diagnosis of child = Cancer Mean years of illness = Not reported |
|
| Interventions | ”Hope therapy” ”Treatment as usual” (TAU) Mode of delivery: face-to-face Intervention delivered by: Not reported Training: Not reported Duration of intervention (child, hrs) = 0 Duration of intervention (parent, hours) = 8 sessions = 16 hours |
|
| Outcomes | * Extracted measures used in the analyses Beck’s Depression Inventory* Snyder’s Hope Scale Efficacy of Hope therapy Based on hope |
|
| Notes | Funding: No funding statement was provided in the manuscript COI: “The authors have no conflict of interest in this article” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Later on, they were randomly assigned into two matched control and experimental groups (each with 10 members).” Comment: Method not described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | No description found in text. Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Attrition was not adequately described |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported on request |
| Stark 2005 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment, 8 weeks after baseline post-treatment | |
| Participants | End of treatment n = 49 Start of treatment n = 65 Sex of children: 9 M, 40 F Sex of parents: Not reported Mean age (SD) of children =6.45 (2) Mean age (SD) of parents = 36.1 (5.4) Source = Rheumatology centres Diagnosis of child = Juvenile Rhuematoid Arthritis Mean years of illness = Unknown |
|
| Interventions | “Behavioral Intervention” (BI) “Enhanced Standard of Care” (ESC) Mode of delivery: In-clinic, face-to-face in groups, parents and children in separate sessions Intervention delivered by: PHD Psychologist for parents, Post-doctoral fellow with help of a trained RA for children Training: Not reported Duration of intervention (child, hours) = 4 sessions/visits lasting 60 - 90 mins Duration of intervention (parent, hours) = 4 sessions/visits lasting 60 – 90 mins |
|
| Outcomes | * Extracted measures used in the analyses Weighed food diaries |
|
| Notes | Funding: “This research was supported by a Clinical Science Grant from the Arthritis Foundation, NIH/NIDDK Grant #DK59492 to Lori J. Stark, Ph.D., and by USPHS Grant #MO1 RR 08084 from the General Clinical Research Centers Program, National Center for Research Resources, NIH. The authors thank Drs David Glass, Murray Passo, Brent Graham, Thomas Griffin, Robert Colbert, Alexei Grom, Gloria Higgins, and Suzanne Bowyer for their assistance in enrolling families and conducting treatment groups for this investigation” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Participants were stratified on an estimate of their typical Ca intake at baseline across the two conditions….After stratification by estimated Ca intake classification, a block randomization protocol was utilized with a block size of two within each strata of Ca intake.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | “The randomization sequence was generated and kept by personnel separate from the personnel conducting recruitment calls and the intervention.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “..the first two weekdays and the first weekend day, were analyzed by a registered dietician in the General Clinical Research Center (GCRC), who was unaware of the subject’s treatment condition..” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Attrition reported, there were significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias |
| Notes | Funding: “This research was supported by a Clinical Science Grant from the Arthritis Foundation, NIH/NIDDK Grant #DK59492 to Lori J. Stark, Ph.D., and by USPHS Grant #MO1 RR 08084 from the General Clinical Research Centers Program, National Center for Research Resources, NIH. The authors thank Drs David Glass, Murray Passo, Brent Graham, Thomas Griffin, Robert Colbert, Alexei Grom, Gloria Higgins, and Suzanne Bowyer for their assistance in enrolling families and conducting treatment groups for this investigation” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Participants were stratified on an estimate of their typical Ca intake at baseline across the two conditions....After stratification by estimated Ca intake classification, a block randomization protocol was utilized with a block size of two within each strata of Ca intake.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | “The randomization sequence was generated and kept by personnel separate from the personnel conducting recruitment calls and the intervention.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “..the first two weekdays and the first weekend day, were analyzed by a registered dietician in the General Clinical Research Center (GCRC), who was unaware of the subject’s treatment condition..” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition reported, there were significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported. Aims, measures and results were fully concordant. Comment: probably no reporting bias |
| Stehl 2009 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and 1 month post-treatment | |
| Participants | End of treatment n = 48 families, 92 caregivers Start of treatment n = 76 families, 152 caregivers received intervention Sex of children: 41 M, 35 F Sex of parents = Not reported Mean age of children = 6 years Mean age of primary caregiver = 36 years Source = Oncology service Diagnosis = Cancer Mean years of illness = After diagnosis |
|
| Interventions | “Surviving Cancer Competently Intervention Program-Newly Diagnosed” (CBT) “Standard Psychosocial Care” Mode of delivery: Group, face-to-face, CD-ROM based multiple family discussion groups Intervention delivered by: psychology fellows, psychology intern, Master’s level psychologist and doctoral-level nurse Training: 18 hours of didactic and experiential training Duration of intervention (children) = 3×45 minutes + 3 booster sessions Duration of intervention (parents) = 3×45 minutes + 3 booster sessions |
|
| Outcomes |
* Extracted measures used in the analyses Parent measures State Trait Anxiety Inventory* (STAI) Impact of Event Scale-Revised (IES-R) Acute Stress Disorder Scale (ASDS) Programme Evaluation Clinicians measures Social Work Activity Form Child Life Activity Form Intensity of Treatment Rating Scale (ITR-2) |
|
| Notes | Funding: “This research was supported by a grant from the National Cancer Institute (CA088828)” COI: “Conflict of interest: None declared” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was completed by a predetermined concealed random assignment list maintained by a staff member unaware of patient identity.” Comment: probably done |
| Allocation concealment (selection bias) | Low risk | “Randomization was completed by a predetermined concealed random assignment list maintained by a staff member unaware of patient identity.” Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Add data collection took place at the hospital at a time and location of convenience for the family and was conducted by research assistants.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data fully reported |
| Tsiouli 2014 | ||
| Methods | RCT. 2 arms. Assessed 8 weeks after baseline = post-treatment | |
| Participants | End of treatment n =44 Start of treatment n = 80 Sex of children: Not reported Sex of parents: 9 M, 35 F Mean age of children = Not reported Mean age of parents = 43.28 Source = Diabetes Centre Diagnosis = Type 1 diabetes Mean years of illness = Not reported |
|
| Interventions | “Stress Management and Health Promotion program” “Control group” Mode of delivery: In-clinic, phone calls Intervention delivered by: Author of progressive muscle relaxation and diaphragmatic breathing and a CD Training: Author of progressive muscle relaxation and diaphragmatic breathing Duration of intervention (child) = No treatment Duration of intervention (parent) = 37 minute session of relaxation techniques and had to use these twice a day for 8 weeks |
|
| Outcomes |
* Extracted measures used in the analyses Salivary cortisol Perceived stress scale (PSS) Parenting Stress Index-Short Form (PSI-SF)* Health Locus of Control scale |
|
| Notes | Funding: “The authors declare that no financial support was received” COI: “There are no conflicts of interest” |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “..first randomized (by using the random, number generator) and then assigned to intervention and control groups by a fellow researcher.” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | No description of allocation |
| Blinding of outcome assessment (detection bias) | Low risk | “All samples were returned to the researcher in eight weeks.” Comment: Probably done |
| All outcomes | ||
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition reported, no data reported significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Low risk | Data were fully reported |
| Wade 2006a | ||
| Methods | RCT. 2 arms. Assessed pre and post-treatment | |
| Participants | End of treatment n = 32 children and their parents Start of treatment n = 37 children and their parents Sex of children: 21 M, 11 F Sex of parents: Not reported Mean age (SD) of children = 10.83 (2.94) Mean age of parents = Not reported Source = Trauma registry at Cincinnati Children’s Hospital Medical Center Diagnosis = Traumatic brain injury Mean years (SD) of illness = 8.78 (4.53) |
|
| Interventions | “Family-centered problem-solving intervention” (PST) “Usual Care” Mode of delivery: Individual families, face-to-face Intervention delivered by: 5th year Clinical Psychology graduate student Training: 2 months Duration of intervention (children) = 7 × 75 minutes = 8 hours 45 minutes to 11 hours 40 minutes + up to 4 individualised sessions Duration of intervention (parents) = 7 × 75 minutes = 8 hours 45 minutes to 11 hours 40 minutes + up to 4 individualised sessions |
|
| Outcomes |
* Extracted measures used in the analyses Child measures Conflict Behavior Questionnaire* Treatment satisfaction Parent measures Child Behavior Checklist* (CBCL) Conflict Behavior Questionnaire* (CBQ) Brief Symptom Inventory* (BSI) Treatment satisfaction |
|
| Notes | Funding: “This work was supported by a grant (H133G990069) from the National Institute on Disability and Rehabilitation Research, Office of Special Education and Rehabilitation Services, US Department of Education” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Families were randomly assigned to the family-centred problem-solving intervention or usual care group using a random numbers table.” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | “Interviewers were also upper-level psychology graduate students who received extensive training.” Comment: no suggestion that they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported after authors responded to requests |
| Wade 2006b | ||
| Methods | RCT 2 arms. Assessed pre-treatment and at session 7 of 8 | |
| Participants | End of treatment n = 41 (40 analysed) Start of treatment n = 46 Sex of children: 23 M, 17 F Sex of parents: not reported Mean age (SD) of children = 11.00 (3.27) Mean age of parents = Not reported Source = Trauma registry at Cincinnati Children’s Hospital Medical Center Diagnosis = Traumatic brain injury Mean years (SD) of illness = 13.73 (7.10) months since injury |
|
| Interventions | “Family Problem Solving” (PST) “Internet Resources Control” Mode of delivery: Individual, online and video conferencing Intervention delivered by: Clinical Psychology graduate student Training: 2 months Duration of intervention (children) = 8 core modules, 6 supplementary modules, time not reported Duration of intervention (parents) = 8 core modules, 6 supplementary modules, time not reported |
|
| Outcomes |
* Extracted measures used in the analyses Parent outcomes Family Assessment Device (FAD) Family Burden of Injury Interview sub scales (FBII) Likert scales of global family problem solving, communication and behaviour management Child Behavior Checklist Internalizing Problems* (CBCL) Home and Community Social Behavior Scale (HCSBS) Social Problem-Solving Index (SPSI-short version) Symptom Checklist-90-Revised (SCL-90-R) Global Severity Index (GSI) Center for Epidemiologic Studies Depression Scale* (CES-D) Anxiety Inventory (AI) Online usage questionnaire Website Evaluation Questionnaire (WEQ) |
|
| Notes | Funding: Study 1: An online family intervention to reduce parental distress following pediatric brain injury - “This work was supported by National Council on Medical Rehabilitation Research, National Institutes of Health Grant HD40942” Study 2: The efficacy of an online cognitive-behavioral family intervention in improving child behavior and social competence following pediatric brain injury - “This work was supported by National Institutes of Health Grant HD40942-02, National Council on Medical Rehabilitation Research” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Families were randomly assigned to family problem-solving or internet resources comparison via a computer programme.” Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No description found in text. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Given the nature of the study, neither the participants nor the research assistant was blind to group assignment. The primary outcome measures were based on parent and child report and therefore not dependent on the judgments of the research staff. ” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was not reported, there were no significant differences between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported after authors responded to requests |
| Wade 2011 | ||
| Methods | RCT. 2 arms. Assessed pre-treatment and post-treatment | |
| Participants | End of treatment n = 35 Start of treatment n = 42 Sex of children: 17 M, 23 F Sex of parents: not reported Mean age (SD) of children = 14.25 (2.29) Mean age (SD) of parents = 41.2 (5.90) Source = Inpatient rehabilitation unit of 2 urban children’s hospitals Diagnosis = Traumatic brain injury Mean years (SD) of illness = 9.54 (4.97) months since injury |
|
| Interventions | “Teen Online Problem Solving” (PST) “Internet Resource Comparison” Mode of delivery: individual, internet and video conferencing Intervention delivered by: staff psychologist + Clinical Psychology graduate students Training: multi-day training Duration of intervention (children) = 10 core modules, 6 supplementary sessions, time not reported Duration of intervention (parents) = 10 core modules, 6 supplementary sessions, time not reported |
|
| Outcomes |
*Extracted measures used in the analyses Child measures Youth Self Report* (YSR) Interaction Behaviour Questionnaire* (IBQ) Behavioral Rating Inventory of Executive Functioning Parent measures Child Behaviour Checklist* (CBCL) Behavioral Rating Inventory of Executive Functioning (BRIEF) |
|
| Notes | Funding: “This work was supported by grant #H133G050239 from the National Institute of Disability and Rehabilitation Research, U.S. Department of Education” COI: “The authors have indicated they have no financial relationships relevant to this article to disclose” | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Families were randomly assigned to either teen online problem-solving or internet resource group by use of a randomisation scheme that stratified participants on the basis of the adolescent’s gender and race/ethnicity to ensure comparable diversity in each group.” Comment: method is not fully described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Given the nature of the study we were unable to conceal group assignment from the participants and research staff; however, the primary outcome measures were based on parent and teen report and therefore not dependent on judgments of research staff. Comment: non-blinding of participants and research staff justified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was reported, no data were presented on equivalence between completers and non-completers |
| Selective reporting (reporting bias) | Unclear risk | Data were fully reported after authors responded to requests |
| Wysocki 1999 | ||
| Methods | RCT 3 arms. Assessed pre-treatment, 3 months (post-treatment), 6-month follow-up and 12-month follow-up | |
| Participants | End of treatment n = 115 (post-treatment), 113 (6-month follow-up), 108 (12-month follow-up) Start of treatment n = 119 children Sex of children: 50 M, 69 F Sex of parents: 82 M, 117 F Mean age (SD) of children = 14.3 (1.4) Mean age of parents = Not reported Source = Missouri and Florida Diagnosis = Type 1 diabetes Mean years (SD) of illness = 5.0 (3.8) |
|
| Interventions | “Behavioral Family Systems Therapy (BFST)” (FT) “Education and Support Group” (ES) “Standard Care” Mode of delivery: Individual for BFST, group for ES, face-to-face Intervention delivered by licensed Clinical Psychologists Training: 150 hours Duration of intervention (children) = 10 sessions, time not reported Duration of intervention (parents) = 10 sessions, time not reported |
|
| Outcomes |
* Extracted measures used in the analyses Child measures Parent-Adolescent Relationship Questionnaire (PARQ)* Issues Checklist (IC) 24 Hour Recall Interview of Conflict Situations Teen Adjustment to Diabetes Scale (TADS)* Diabetes Responsibility and Conflict (DRC) 24 Hour Recall Interview of IDDM Self-Care Self-Care Inventory (SCI) Glycated haemoglobin* Parent measures Parent-Adolescent Relationship Questionnaire (PARQ)* Issues Checklist (IC) 24 Hour Recall Interview of Conflict Situations Teen Adjustment to Diabetes Scale (TADS) Diabetes Responsibility and Conflict (DRC) 24 Hour Recall Interview of IDDM Self-Care Self-Care Inventory (SCI) Parent-reported health service use |
|
| Notes | Funding: “This work was supported by grant 1-RO1-DK43802 “Behavior Therapy for Families of Diabetic Adolescents” awarded by the National Institutes of Health to the first author and by the Pediatric and General Clinical Research Centers of Washington University (RR6021 and RR00036)” COI: No conflict of interest statement included in the manuscript |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The research scientist at the opposing centre randomly assigned each family, without knowledge of the family’s baseline status on any of the outcome measures to one of three conditions.” Comment: method not fully described |
| Allocation concealment (selection bias) | Unclear risk | No description found in text |
| Blinding of outcome assessment (detection bias) All outcomes |
Unclear risk | “A research assistant administered questionnaires at evaluation sessions; the research assistant completed telephone interviews during the 2 weeks preceding each of the four evaluations.” Comment: blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was reported, but no data were presented on equivalence between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data not fully reported |
| Wysocki 2006 | ||
| Methods | RCT. 3 arms. Assessed at pre-treatment, 6 months (post-treatment), 12-month follow-up, 18-month follow-up | |
| Participants | End of treatment n = 92 (post-treatment), 88 (12-month follow-up), 85 (18-month follow-up) Start of treatment n = 104 children (number of caregivers not reported) Sex of children: 57 M, 47 F Sex of parents: Not reported Mean age (SD) of children = 14.2 (1.9) Mean age of parents = Not reported Source = 2 paediatric centres in the Southeast and Midwest USA Diagnosis = Type 1 diabetes or insulin-treated type 2 diabetes Mean years (SD) of illness = 5.5 (3.4) |
|
| Interventions | “Behavioral Family Systems Therapy for Diabetes (BFST-D)” (FT) “Educational Support Group” “Standard Care” Mode of delivery: Individual families, face-to-face Intervention delivered by: licensed Clinical Psychologist, Social Worker Training: Trained in BFST-D Duration of intervention (BFST-D) = 12 sessions, time not reported Duration of intervention (ES) = 12 × 1½ hr sessions |
|
| Outcomes |
* Extracted measures used in the analyses Child measures Parent-Adolescent Relationship Questionnaire (PARQ)* Glycosylated haemoglobin (HbA1c)* Diabetes Responsibility and Conflict (DRC) Diabetes Self-Management Profile (DSMP) Family problem solving discussions coded using Interaction Behavior Code Parent measures Parent-Adolescent Relationship Questionnaire (PARQ)* Diabetes Responsibility and Conflict (DRC) Diabetes Self-Management Profile (DSMP) Family problem-solving discussions coded using Interaction Behavior Code |
|
| Notes | Funding: “This study was supported by NIH grants 1 RO1-DK43802 and K24 DK67128 to the first author; and NIH grants P60 DK20579 and RR00036 which support the Diabetes Research and Training Center and General Clinical Research Center, respectively, at the Washington University School of Medicine” COI: No conflict of interest statement included in the manuscript | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A three-group, randomised treatments design was used.” Comment: method not described fully |
| Allocation concealment (selection bias) | Unclear risk | “Families were stratified by HbA1c”. Comment: no description of concealment described |
| Blinding of outcome assessment (detection bias) All outcomes |
Low risk | “Raters were unaware of the family’s identity or group assignment or of when the recording was made.” Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was reported, but no data were presented on equivalence between completers and non-completers |
| Selective reporting (reporting bias) | High risk | Data were incompletely reported. Aims, measures and results were partially concordant. Comment: probably some reporting bias |
CBT: cognitive behavioural therapy; GI: gastrointestinal; HMO: health maintenance organisation; MST: multisystemic therapy; PSST: problem- solving skills training; PST: problem-solving therapy; RCT: randomised controlled trial; SD: standard deviation; TBI: traumatic brain injury.
Note - some demographic information such as the sex of participants may not match the number of participants randomised. We have extracted and reported data from studies, however, some studies have missing demographic data.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aleman 1992 | Insufficient psychotherapeutic content |
| Anderson 1999 | Insufficient psychotherapeutic content |
| Bellin 2013 | Insufficient psychotherapeutic content |
| Betancourt 2004 | Identified participants prospectively |
| Borhani 2011 | Aim of study was irrelevant to this review |
| Braga 2005 | Insufficient psychotherapeutic content |
| Bruzzese 2008 | Aim of study was irrelevant to this review |
| Burke 1997 | Insufficient psychotherapeutic content |
| Burke 2001 | Insufficient psychotherapeutic content |
| Cakan 2007 | Aim of study was irrelevant to this review |
| Canino 2008 | Aim of study was irrelevant to this review |
| Carey 2008 | Aim of study was irrelevant to this review |
| Chen 2013 | Insufficient psychotherapeutic content |
| Chernoff 2002 | Insufficient psychotherapeutic content |
| Chiang 2009 | Insufficient psychotherapeutic content |
| Ellis 2007 | Aim of study was irrelevant to this review |
| Ellis 2008 | Aim of study was irrelevant to this review |
| Evans 1999 | Insufficient psychotherapeutic content |
| Fedele 2013 | Aim of study was irrelevant to this review |
| Field 1998 | Insufficient psychotherapeutic content |
| Forsander 1995 | Aim of study was irrelevant to this review |
| Forsander 2003 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Garbutt 2010 | Insufficient psychotherapeutic content |
| Gerber 2010 | Aim of study was irrelevant to this review |
| Giallo 2008 | Insufficient psychotherapeutic content |
| Glang 2007 | Insufficient psychotherapeutic content |
| Grey 2011 | Replicated data already included in the review |
| Groß 2013 | Insufficient psychotherapeutic content |
| Gulewitsch 2012 | Aim of study was irrelevant to this review |
| Gustafsson 1986 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Harris 2001 | Aim of study was irrelevant to this review |
| Haus 1976 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Hernandez 1998 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Hommel 2012 | Aim of study was irrelevant to this review |
| Hovell 1994 | Insufficient psychotherapeutic content |
| Humphreys 2000 | Insufficient psychotherapeutic content |
| Ireys 1996 | Insufficient psychotherapeutic content |
| Ireys 2001 | Insufficient psychotherapeutic content |
| Jay 1990 | Aim of study was irrelevant to this review |
| Johnson 1987 | Insufficient psychotherapeutic content |
| Kamps 2008 | Inadequate n: the number of patients in any treatment arm was fewer than 10 |
| Kaslow 2000 | Insufficient psychotherapeutic content |
| Katz 2014 | Insufficient psychotherapeutic content |
| Kazak 1996 | Insufficient psychotherapeutic content |
| Kazak 2005 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Ketchen 2006 | Insufficient psychotherapeutic content |
| Klinnert 2005 | Insufficient psychotherapeutic content |
| Klinnert 2007 | Insufficient psychotherapeutic content |
| Kroner-Herwig 1998 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Kupfer 2010 | Insufficient psychotherapeutic content |
| Kurowski 2013 | Aim of study was irrelevant to this review |
| Lasecki 2008 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Logan 1997 | Insufficient psychotherapeutic content |
| Lyon 2013 | Aim of study was irrelevant to this review |
| Mendez 1997 | Insufficient psychotherapeutic content |
| Murphy 2012 | Insufficient psychotherapeutic content |
| Nelson 2011 | Insufficient psychotherapeutic content |
| Pérez 1999 | Insufficient psychotherapeutic content |
| Rasoli 2008 | Aim of study was irrelevant to this review |
| Sanders 1989 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Sanders 1996 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Satin 1989 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Scholten 2011 | Aim of study was irrelevant to this review |
| Sieberg 2011 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Staab 2002 | Insufficient psychotherapeutic content |
| Sullivan-Bolyai 2010 | Insufficient psychotherapeutic content |
| Szczepanski 2010 | Insufficient psychotherapeutic content |
| Van der Veek 2013 | Aim of study was irrelevant to this review |
| Wade 2010 | Aim of study was irrelevant to this review |
| Walders 2006 | Insufficient psychotherapeutic content |
| Walker 1996 | Aim of study was irrelevant to this review |
| Warner 2011 | Inadequate n: the number of participants in any treatment arm was fewer than 10 |
| Wysocki 1997 | Aim of study was irrelevant to this review |
Results of the search
The first search which was conducted from inception to March 2012 identified 35 studies for inclusion. For results of the initial search see Appendix 2. The updated search identified studies from March 2012 to July 2014. Four hundred and eighteen abstracts were identified in the database search and read for inclusion, of these 376 were excluded. See Figure 1 for a flowchart of studies.
Figure 1.
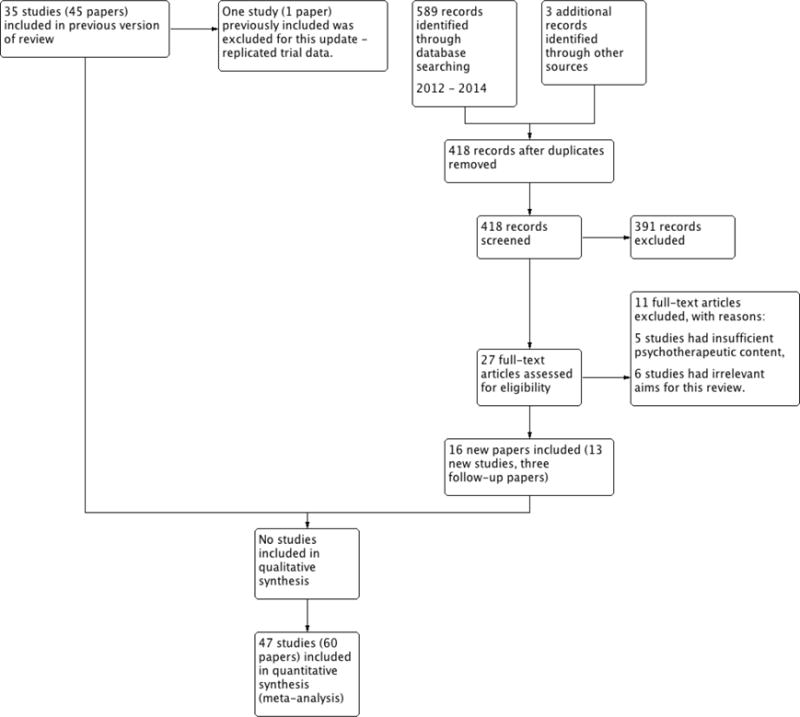
Study flow diagram.
Sixteen papers were identified in the updated search which met the inclusion criteria, three of which (Levy 2010; Stark 2005; Wade 2011) were identified as follow-up papers of already included trials. Therefore, 13 new trials are included in this update, adding to the 35 previously included studies. One previously included study was excluded for the purposes of this review (Grey 2011), as it combined data with another study already included in this review and would inflate the results if included. Therefore, in total there are 60 included papers and 47 included studies (Allen 1998; Ambrosino 2008; Antonini 2014; Askins 2009; Barakat 2010; Barry 1997; Celano 2012; Connelly 2006; Duarte 2006; Ellis 2004; Ellis 2005; Ellis 2012; Gulewitsch 2013; Hicks 2006; Hoekstra-Weebers 1998; Kashikar-Zuck 2005; Kashikar-Zuck 2012; Kazak 2004; Laffel 2003; Lask 1979; Lehmkuhl 2010; Levy 2010; Marsland 2013; Mullins 2012; Naar-King 2014; Nansel 2009; Nansel 2012; Ng 2008; Niebel 2000; Olivares 1997; Palermo 2009; Robins 2005; Sahler 2002; Sahler 2005; Sahler 2013; Sanders 1994; Saßman 2012; Seid 2010; Shekarabi-Ahari 2012; Stark 2005; Stehl 2009; Tsiouli 2014; Wade 2006a; Wade 2006b; Wade 2011; Wysocki 1999; Wysocki 2006).
Included studies
There were previously 35 studies (45 papers) included in this review. Grey 2011 was previously included in the review but is now excluded for the purposes of this update as the manuscript replicates data already included from another included trial. The updated search identified an additional 13 studies (Antonini 2014; Ellis 2012; Gulewitsch 2013; Marsland 2013; Mullins 2012; Naar-King 2014; Nansel 2009; Nansel 2012; Sahler 2013; Saßman 2012; Shekarabi-Ahari 2012; Stark 2005; Tsiouli 2014) and three follow-up papers of studies already included (Levy 2010; Stark 2005; Wade 2011), resulting in a total of 47 studies (60 papers).
Of the 47 studies included in this review, 43 had two comparator arms and four studies had three comparator arms (Niebel 2000; Seid 2010; Wysocki 1999; Wysocki 2006). Of the 43 studies that had two arms, 16 studies used active controls (e.g. education), 17 studies used ’treatment-as-usual controls’ and 10 studies used wait-list controls. The total number of participants at the end of treatment was 3778 (mean = 80 per study). The total number of participants entering treatment was 4607 (mean = 98 per study). Therefore, the completion rate for all studies was 82%, making the attrition percentage 18%. The proportion of completers across studies ranged from 55% to 100%. The age of children receiving treatment could be extracted from 30 studies (mean = 14.6, standard deviation (SD) = 2.71).
We categorised the studies by the primary illness of the children. There were 14 studies of children with painful conditions (Allen 1998; Barakat 2010; Barry 1997; Connelly 2006; Duarte 2006; Gulewitsch 2013; Hicks 2006; Kashikar-Zuck 2005; Kashikar-Zuck 2012; Levy 2010; Palermo 2009; Robins 2005; Sanders 1994; Stark 2005). Ten studies with the primary illness of cancer met the inclusion criteria (Askins 2009; Hoekstra-Weebers 1998; Kazak 2004; Marsland 2013; Mullins 2012; Sahler 2002; Sahler 2005; Sahler 2013; Shekarabi-Ahari 2012; Stehl 2009), 13 studies investigated children with diabetes (Ambrosino 2008; Ellis 2004; Ellis 2005; Ellis 2012; Laffel 2003; Lehmkuhl 2010; Nansel 2009; Nansel 2012; Olivares 1997; Saßman 2012; Tsiouli 2014; Wysocki 1999; Wysocki 2006), five investigated asthma (Celano 2012; Lask 1979; Naar-King 2014; Ng 2008; Seid 2010), four studies treated children with traumatic brain injury (Antonini 2014; Wade 2006a; Wade 2006b; Wade 2011) and one study included children with atopic eczema (Niebel 2000). No studies met the inclusion criteria for gynaecological disorders or inflammatory bowel diseases.
Similarly, we also categorised studies by the type of psychological therapy delivered. There were 22 studies that delivered cognitive behavioural therapy (CBT) (Allen 1998; Ambrosino 2008; Barakat 2010; Barry 1997; Connelly 2006; Duarte 2006; Gulewitsch 2013; Hicks 2006; Hoekstra-Weebers 1998; Kashikar-Zuck 2005; Kashikar-Zuck 2012; Laffel 2003; Levy 2010; Marsland 2013; Niebel 2000; Olivares 1997; Palermo 2009; Robins 2005; Sanders 1994; Shekarabi-Ahari 2012; Stehl 2009; Tsiouli 2014), eight studies delivered family therapy (FT) (Celano 2012; Kazak 2004; Lask 1979; Lehmkuhl 2010; Ng 2008; Saßman 2012; Wysocki 1999; Wysocki 2006), 12 studies delivered problem-solving therapy (PST) (Antonini 2014, Askins 2009; Mullins 2012; Nansel 2009; Nansel 2012; Sahler 2002; Sahler 2005; Sahler 2013; Seid 2010; Wade 2006a; Wade 2006b; Wade 2011), four studies delivered multisystemic therapy (MST) (Ellis 2004; Ellis 2005; Ellis 2012; Naar-King 2014) and one study delivered an exclusively behavioural intervention (Stark 2005).
The proportion of therapy received by parent and child varied between studies. The majority of studies gave equal attention to both parent and child (29 studies). In 12 studies only the parent received therapy, eight of which were delivered treatment to parents whose children had been diagnosed with cancer. Four further studies spent the majority of treatment time with the child. The final two studies did not specify how much therapy the parent and child respectively received (see Table 1).
Table 1.
Therapy characteristics of included studies
| Study | Patient group | Therapy type | Duration of therapy (child/parent) | Proportion of therapy (child: parent) | Mode of delivery, group/individual | Therapy delivered by | Therapist training |
|---|---|---|---|---|---|---|---|
| Shekarabi-Ahari 2012 | Cancer | CBT | 0/16 hours | 0:100 | In-person, individual | Not reported | Not reported |
| Allen 1998 | Painful condition (headache) | CBT | 4 hours/not reported | Not reported | In-person, individual | Authors | Not reported |
| Ambrosino 2008 | Diabetes | CBT | 9 hours/9 hours | 50:50 | In-person, group | Mental health professionals | Not reported |
| Antonini 2014 | TBI | PST | 10 sessions, 4 supplementary sessions/10 sessions, 4 supplementary sessions | 50:50 | Computer and home visit, individual | Therapist with psychology masters degree | Given manual & had meetings with an advanced supervising psychologist |
| Askins 2009 | Cancer | PST | 0/8 hours | 0:100 | In-person, individual | Therapists with graduate training in Clinical Psychology | Specialised training in PSST |
| Barakat 2010 | Painful condition (sickle cell disease) | CBT | 6 hours/6 hours | 50:50 | In-person, individual families | Clinical Psychology doctoral students | Not reported |
| Barry 1997 | Painful condition (headache) | CBT | 3 hours/3 hours | 50:50 | In-person, group | Mental health professionals | Not reported |
| Celano 2012 | Asthma | FT | 4 to 6 sessions/ 4 to 6 sessions | 50:50 | In-person, individual families | Mental and health care professionals | Not reported |
| Connelly 2006 | Painful condition (headache) | CBT | 4 hours/1 hour | 80:20 | Computer, phone calls, individual | CD-ROM and research staff | Not reported |
| Duarte 2006 | Painful condition (recurrent abdominal pain) | CBT | 3 hours, 20 minutes/3 hours, 20 minutes | 50:50 | In-person, not reported | General health professionals | Not reported |
| Ellis 2004 | Diabetes | MST | 48 sessions/48 sessions | 50:50 | In-person, Individual Families | Mental health professionals | Course on MST |
| Ellis 2005 | Diabetes | MST | 46 sessions/46 sessions | 50:50 | In-person + phone calls, individual families | Mental health professionals | Course on MST |
| Ellis 2012 | Diabetes | MST | Minimum 48 sessions/Minimum 48 sessions | 50:50 | In-person, Individual Families | Masters level therapist | 5-day training, phone consultation with MST expert, follow-up booster |
| Gulewitsch 2013 | Painful condition (Functional Abdominal Pain + irritable bowel syn-dome) | CBT | 3 hrs/3hrs | 50:50 | In-person, group | Trained Psychologists | Not reported |
| Hicks 2006 | Painful condition (headache + recurrent abdominal pain) | CBT | Not reported/not reported | Not reported | Online + phone calls, individual | Internet + researcher | Not reported |
| Hoekstra-Weebers 1998 | Cancer | CBT | 0/12 hours | 0:100 | In-person, individual | Master’s student in Psychology | Not reported |
| Kashikar-Zuck 2005 | Painful condition (fibromyalgia) | CBT | 6 sessions/3 sessions | 66:33 | In-person + phone calls, individual | Pre-doctoral Psychology Intern and post-doctoral Psychology Fellow | Trained by PI |
| Kashikar-Zuck 2012 | Painful condition (fibromyalgia) | CBT | 6 hours/2 hours 15 minutes | 73:27 | In-person, individual | Post-doctoral Psychology Fellow | 6 to 8 hours CBT training by PI |
| Kazak 2004 | Cancer | FT | 5 hours/5 hours | 50:50 | In-person, group | Nurses, Social Workers, Clinical Psychologists, graduate and post-doctoral Psychology Trainees | 12-hour training |
| Laffel 2003 | Diabetes | CBT | 4 sessions/4 sessions | 50:50 | In-person, individual families | Research assistant | Not reported |
| Lask 1979 | Asthma | FT | 6 hours/6 hours | 50:50 | In-person, individual families | Mental health professional | Not reported |
| Lehmkuhl 2010 | Diabetes | CBT | 9 to 12 hours/9 to 12 hours | 50:50 | Phone calls, individual families | Clinical Psychologists and pre-doctoral Psychology Interns | Not reported |
| Levy 2010 | Painful condition (recurrent abdominal pain) | CBT | 4 hours/4 hours | 50:50 | In-person, individual families | Therapists | Not reported |
| Marsland 2013 | Cancer | CBT | 0/6 face-to-face sessions, 6 phone calls | 0:100 | In-person, telephone and online, individual | Masters level clinician | Training on the conceptual foundations of the intervention |
| Mullins 2012 | Cancer | PST | 0/5 hours | 0:100 | In-person, individual, phone calls | Nurse interventionists & psychology interventionists | Manual, training by corresponding author |
| Naar-King 2014 | Asthma | MST | 27 sessions/27 sessions | 50:50 | In-vivo observations, in-person, individual families | Therapist | Not reported |
| Nansel 2009 | Diabetes | PST | 3 sessions, 3 phone calls/3 sessions, 3 phone calls | 50:50 | In-person, individual, phone calls | Health advisors (College graduates) | Specially trained college graduates |
| Nansel 2012 | Diabetes | PST | 7 visits = 3.5 hours | 50:50 | In-person, individual families | Health advisors | Not reported |
| Ng 2008 | Asthma | FT | 22 hours/22 hours | 50:50 | In-person, group | Not reported | Not reported |
| Niebel 2000 | Atopic eczema | CBT | 0/22 hours | 0:100 | In-person + video, individual families | Mental health professional | Not reported |
| Olivares 1997 | Diabetes | CBT | 0/9 hours, 20 minutes | 0:100 | In-person, group | Not reported | Not reported |
| Palermo 2009 | Painful condition (mixed pain conditions) | CBT | 4 hours/4 hours | 50:50 | Online, individual families | Online + Psychology Fellow | 1 year of experience delivering face-to-face CBT to children with chronic pain |
| Robins 2005 | Painful condition (recurrent abdominal pain) | CBT | 3 hours, 20 minutes/2 hours | 63:37 | In-person, group | Pre-doctoral Psychology Intern and post-doctoral Psychology Fellow | Not reported |
| Sahler 2002; | Cancer | PST | 0/8 hours | 0:100 | In-person, individual | Mental health professional and Psychology graduate student | 3-day workshop |
| Sahler 2005 | Cancer | PST | 0/8 hours | 0:100 | In-person, individual | Not reported | Not reported |
| Sahler 2013 | Cancer | PST | 0/ 8 hours | 0:100 | In-person, individual | Research assistants (RA) | Graduate training in clinical or behavioural psychology, manual given, RA’s trained in groups & then given individual weekly supervision |
| Saßman 2012 | Diabetes | FT | 0/10 hours | 0:100 | In-person, telephone, groups | Psychologist | Not reported |
| Sanders 1994 | Painful condition (recurrent abdominal pain) | CBT | 5 hours/5 hours | 50:50 | In-person, individual | Clinical Psychologists | Not reported |
| Seid 2010; | Asthma | PST | 11 hours/11 hours | 50:50 | In-person, individual families | Master’s level Health Educator | 2-week training |
| Stark 2005 | Juvenile Rhuematoid Arthritis | BI | 4–6 hours/4 -6 hours | 50:50 | In-person, groups | Parents - PHD Psychologist Children - Postdoctoral fellow with help of a trained RA | Not reported |
| Stehl 2009; | Cancer | CBT | 2 hours, 15 minutes/2 hours, 15 minutes | 50:50 | In-person + CD-ROM, group | Psychology Fellows, Psychology Intern, Master’s level Psychologist, doctoral-level Nurse | 18 hours training |
| Tsiouli 2014 | Diabetes | CBT | 0/9.87 hours | 0:100 | In-person, telephone, individual | Author of progressive muscle relaxation and diaphragmatic breathing & a CD | Not reported |
| Wade 2006a | TBI | PST | 10 hours, 20 minutes/10 hours, 20 minutes | 50:50 | In-person, individual families | Clinical Psychology graduate student | 2 months training |
| Wade 2006b | TBI | PST | 14 modules/14 modules | 50:50 | Online + video conferencing, individual | Clinical Psychology graduate student | 2 months training |
| Wade 2011 | TBI | PST | 16 modules/16 modules | 50:50 | Online + video conferencing, individual | Staff Psychologist, Clinical Psychology graduate students | Multi-day training |
| Wysocki 1999 | Diabetes | FT | 10 sessions/10 sessions | 50:50 | In-person, individual families | Clinical Psychologists | 150 hours training |
| Wysocki 2006 | Diabetes | FT | 12 sessions/12 sessions | 50:50 | In-person, individual families | Clinical Psychologist, Social Worker | Trained in BFST-D |
BFST-D: Behavioural Family Systems Therapy for Diabetes; BI: Behavioural intervention; CBT: cognitive behavioural therapy; FT: family therapy; MST: multisystemic therapy; PI: principal investigator; PSST: problem solving skills training; PST: problem-solving therapy; TBI: traumatic brain injury
Forty studies treated participants in-person with the therapist, six studies used online programmes to deliver part or all of the therapy to participants and one study used telephone calls. Of the 47 studies, 36 studies carried out therapy with individuals or with individual families, whilst 10 studies carried out therapy in groups, and one study did not specify how treatment was carried out. A summary of the characteristics of therapy and a narrative summary of treatment content are presented in Table 1 and Table 2 respectively.
Table 2.
Intervention content and therapy classification of included studies
| Author | Therapy summary | Therapy type |
|---|---|---|
|
Shekarabi-Ahari 2012 Cancer |
Hope Therapy. Parents received training in goal identification and definition, positive self-talk, visualizing steps to goal completion, evaluating eating and exercise habits, addressing obstacles to goal attainment, and relapse prevention. Children did not receive any intervention | CBT |
|
Allen 1998 Painful condition (migraine) |
Thermal biofeedback plus parent behaviour management. Parents were provided with pain behaviour management guidelines which focused on minimising attention to pain, encouraging the child to participate in daily activities, and praising practice of biofeedback. Children received thermal biofeedback training | CBT |
|
Ambrosino 2008 Diabetes |
Coping skills training. Parents and children received training in communication skills, social problem solving, recognising links between thoughts/feelings/behaviours, stress management and conflict resolution. The focus of this intervention was to improve participants’ general ability to manage daily problems, and did not directly address diabetes management | CBT |
|
Antonini 2014 TBI |
I-InTERACT. Parents were taught positive parenting skills, antecedent behaviour management strategies, and didactic information on cognitive and behavioral sequelae of traumatic brain injury. Families could also complete four supplemental modules on communication skills, working with the school, pain management, and guilt/grief. Training was completed via self-guided online modules and videoconferencing with a study therapist that included in vivo parent coaching where parents played with the child and received live feedback from the study therapist through an earpiece | PST |
|
Askins 2009 Cancer |
PST + PDA. Mothers received problem solving training using the Bright IDEAS framework: Be optimistic about solving problems, Identify the problem, Determine options, Evaluate options and choose one, Act and See if it worked. Mothers were also provided a personal digital assistant (PDA) device that was designed to review and practise problem solving steps and record problems and solutions encountered between sessions. Children did not receive any intervention | PST |
|
Barakat 2010 Painful condition (SCD) |
Pain management intervention. Parents and children received education about sickle cell disease (SCD) as well as training in deep breathing, progressive muscle relaxation, cognitive restructuring and guided imagery | CBT |
|
Barry 1997 Painful condition (Headache) |
Cognitive behavioural group treatment. Parents received pain education as well as training in relaxation, imagery and positive parenting strategies. Children received pain education as well as training in relaxation, imagery, distraction and cognitive restructuring | CBT |
|
Celano 2012 Asthma |
Home-based family intervention. Families received asthma education regarding trigger control resources and feedback on the child’s lung functioning and metered does inhaler (MDI)/spacer technique, as well as psychosocial modules targeting family rules and discipline, family communication and caregiver mental health | FT |
|
Connelly 2006 Painful condition (Headache) |
Headstrong programme. Using CD-ROMs, children and parents jointly completed a module on management of pain behaviours and creation of a pain-coping plan. Children received headache education and training in guided imagery, deep breathing, progressive muscle relaxation, cognitive restructuring and problem solving | CBT |
|
Duarte 2006 Painful condition (RAP) |
Cognitive-behavioural family intervention. Parents and children received education about abdominal pain as well as training in operant techniques with an emphasis on increasing adaptive behaviours when in pain, deep breathing, physical exercise, progressive muscle relaxation, thought stopping, distraction and imagery | CBT |
|
Ellis 2004 Diabetes |
Multisystemic therapy (MST). Families received an intensive, family- and community-based intervention designed to target problems related to adherence to diabetes treatment across the multiple systems within which the child and their family operated. A variety of psychological interventions were employed depending on individual need, including cognitive behavioural therapy, parent training and behavioural family systems therapy | MST |
|
Ellis 2005 Diabetes |
Multisystemic therapy (MST). See Ellis 2004 above. | MST |
|
Ellis 2012 Diabetes |
Multisystemic therapy (MST). Families received an intensive, family-centred, community-based intervention designed for adolescents with poor-self management of diabetes. Parent intervention included education about diabetes care, operant training, and communication skills training. Peer intervention included enlisting the support of peers to support regimen adherence. School interventions included problem solving with school personnel to monitor, support and communicate with the family regarding the adolescent’s diabetes care and regimen adherence. Strategies were also developed to support the adolescent’s regimen adherence in community settings, and to promote a positive working relationship with healthcare providers. Adolescent interventions focused on improving diabetes care skills and increasing motivation for completing diabetes care | MST |
|
Gulewitsch 2013 Painful condition (FAP + IBS) |
Brief hypnotherapeutic-behavioural intervention. Parents received training in operant learning mechanisms and were educated about the link between stress, anxiety and FAP’s. Children received training in hypnosis and education about the relationship between stress and FAP | CBT |
|
Hicks 2006 Painful condition (RAP) |
Online psychological treatment for pae-diatric recurrent pain. Using a website, parents received training in ways to promote healthy behaviour. Children received pain education as well as training in deep breathing, relaxation, imagery, cognitive strategies and healthy lifestyle choices. Children also received a tape of personalised relaxation exercises and a thought journal. Each week, families were contacted by a researcher via phone or email to check progress and review materials | CBT |
|
Hoekstra-Weebers 1998 Cancer |
Intervention programme for parents of paediatric cancer patients. Parents received education regarding the potential impact of the child’s illness on the child and family as well as training in emotional expression, cognitive restructuring, problem-focused coping skills, communication and assertiveness skills. Children did not receive any intervention | CBT |
|
Kashikar-Zuck 2005 Painful condition (Fibromyalgia) |
Coping skills training. Parents received operant training with a focus on encouraging active coping behaviour and independent pain management. Children received education about behavioural pain management as well as training in progressive muscle relaxation, distraction, activity pacing, cognitive techniques and problem solving | CBT |
|
Kashikar-Zuck 2012 Painful condition (Fibromyalgia) |
Cognitive behavioural therapy (CBT) for the treatment of juvenile fibromyalgia. This intervention is a revised version of the Coping Skills Training program evaluated in Kashikar-Zuck (2005). Parents received operant training with a focus on encouraging independent pain management, maintaining a normal routine, avoiding status checks and increasing their child’s use of coping skills learned in the programme. Children received education about behavioural pain management as well as training in progressive muscle relaxation, distraction, activity pacing, using self statements, problem solving and relapse prevention strategies | CBT |
|
Kazak 2004 Cancer |
Surviving Cancer Competently Intervention Programme (SCCIP). Families received education about the link between thoughts, feelings and behaviours and training in cognitive restructuring. Families also participated in discussion groups about the ways cancer has affected their family, recognising and responding to distress in other family members, and acknowledging and accepting their cancer experience | CBT |
|
Laffel 2003 Diabetes |
Teamwork intervention. Parents and children received training in communicating about diabetes and sharing blood glucose results with family members, the need for teamwork between parents and children in diabetes management during adolescence, managing family members’ responses to the child’s blood glucose levels, sharing diabetes management with family members, and using a diary to help problem solve high and low blood glucose levels | FT |
|
Lask 1979 Asthma |
Family psychotherapy. This intervention aimed to improve the psychological well-being of the family by focusing on attitudes towards asthma and its treatment, fear of death and negative emotions experienced by family members | FT |
|
Lehmkuhl 2010 Diabetes |
Telehealth behavioural therapy. Using telephone contact, families received diabetes education in addition to training in specific skills targeting diabetes care and family functioning, including problem solving, behavioural contracting, communication skills, cognitive restructuring and family structuring | FT |
|
Levy 2010 Painful condition (FAP) |
Social learning and cognitive behavioural therapy. Children and parents received pain education in addition training in deep breathing, progressive muscle relaxation, imagery, operant strategies, cognitive restructuring and relapse prevention strategies | CBT |
|
Marsland 2013 Cancer |
Connections to Coping intervention. Parents received education about stress and training in methods of relaxation, approaches to managing stress within the family (e.g., communication skills training), problem solving around increasing social support, and operant training. Children were given access to an online program designed to provide information about methods of coping with stress, guided relaxation exercises, social support via message boards and emails with study staff, and links to local resources | CBT |
|
Mullins 2012 Cancer |
Interdisciplinary intervention. Parent intervention included education about the nature of uncertainty and training in communication skills, cognitive coping skills, problem solving skills, and social support. Treatment was delivered via alternating sessions with a psychologist and a nurse, with nursing sessions focusing on reinforcing the content taught by the psychologist. Children did not receive any intervention | PST |
|
Naar-King 2014 Asthma |
Multisystemic Therapy adapted for health care settings (MST-HC). Adolescents received training in asthma education. Parents received operant training, communication skills training, and problem solving to develop family routines around the adolescent’s asthma care. School interventions included strategies to support communication between the family and the school and increasing accessibility of medications to youths while in school. Strategies were also developed to support a positive relationship between the family and healthcare providers | MST |
|
Nansel 2009 Diabetes |
WE*CAN Intervention. Parents and children jointly selected a goal for the child’s diabetes management and developed a plan to address this problem using the WE*CAN process: W - work together to set goals, E - explore possible barriers and solutions, C - choose the best solutions, A - act on your plan, N - note the results | PST |
|
Nansel 2012 Diabetes |
See Nansel 2009. | PST |
|
Ng 2008 Asthma |
We Together - We success Parallel Group for Children with Asthma and their Parents (WTWS). Parents and children received asthma education and discuss issues regarding mutual respect between family members, psychosocial factors that may impact asthma symptoms, applying concepts from traditional Chinese medicine to asthma management, and fostering the child’s independence | FT |
|
Niebel 2000 Skin Diseases (Eczema) |
Direct parental education in groups. Parents received asthma education and training in operant strategies, scratch-control techniques, stress management, progressive muscle relaxation, how to coach their children in using progressive muscle relaxation, how to conduct social skills training with their children and relapse prevention. Children did not participate in the intervention | CBT |
|
Palermo 2009 Painful condition (Mixed pain conditions) |
Web-based Management of Adolescent Pain (Web-MAP). Using an internet program, parents received education about chronic pain and training in recognising stress and negative emotions, operant strategies, modelling, sleep hygiene and lifestyle, communication and relapse prevention. Children received education about chronic pain and training in recognising stress and negative emotions, deep breathing and relaxation, distraction, cognitive skills, sleep hygiene and lifestyle, staying active and relapse prevention | CBT |
|
Robins 2005 Painful condition (RAP) |
Short-term cognitive behavioural therapy. Children and parents received education about pain and stress as well as training in deep breathing, imagery, relaxation and operant strategies. Children also training in tracking the antecedents and consequences of pain episodes and cognitive restructuring | CBT |
|
Sanders 1994 Painful condition (RAP) |
Cognitive-behavioural family intervention. Parents received education about behavioural pain management, operant training and relapse prevention. Children received education about behavioural pain management, muscle relaxation, deep breathing, imagery, cognitive restructuring, distraction and relapse prevention | CBT |
|
Sahler 2002 Cancer |
Problem solving skills training. Mothers received problem solving training using the Bright IDEAS framework: Be optimistic about solving problems, Identify the problem, Determine options, Evaluate options and choose one, Act and See if it worked. Children did not receive any intervention | PST |
|
Sahler 2005 Cancer |
Problem solving skills training. See Sahler 2002 | PST |
|
Sahler 2013 Cancer |
Problem solving skills training. See Sahler 2002 | PST |
|
Saßman 2012 Diabetes |
DELFIN intervention (Das Elterntraining für Eltern von Kindern mit Diabetes Typ 1 (The parenting program for parents of children with diabetes type 1). Parents received communication skills training and operant training focused on the child’s diabetes management as well as family conflict not related to the child’s diabetes. Children did not receive any intervention | FT |
|
Seid 2010 Asthma |
Problem solving skills training + care co-ordination. Parents received in-home asthma education, referrals to community resources, co-ordination with medical providers and problem solving training using the Bright IDEAS framework (see Sahler 2002 above). Children did not receive any intervention | PST |
|
Stark 2005 Painful condition (Juvenile Rhuematoid Arthritis) |
Behavioral Intervention (BI). Parents received nutrition education and operant training focused on gradually increasing their child’s calcium intake. Children received nutrition education and participated in a practice meal during each session where operant techniques were used to motivate children to reach their calcium goals during the meal | BI |
|
Stehl 2009 Cancer |
Surviving Cancer Competently Intervention Programme - Newly diagnosed (SCCIP-ND). Parents received education about the link between thoughts, feelings and behaviours, training in cognitive restructuring, and discussion of beliefs about the role cancer will play in the family’s future. Parents also watched a CD-ROM of other parents of children with cancer discussing their experiences and responses to diagnosis. Children did not receive any intervention | CBT |
|
Tsiouli 2014 Diabetes |
Stress management. Parents received training in relaxation methods. They were also encouraged to have a healthy lifestyle and engage in positive diet and exercise behaviours. Children did not receive any intervention | CBT |
|
Wade 2006a TBI |
Family problem solving intervention. Families received problem solving training using the ABCDE framework (Aim, Brainstorm, Choose, Do It and Evaluate) and were encouraged to have a positive attitude towards problem solving. Families also received education on the effects of TBI on child functioning as well as training in behavioural management, communication skills and handling crises | PST |
|
Wade 2006b TBI |
Family problem solving intervention. Using an internet program and videoconferencing, families received training in problem solving, communication, behaviour management skills and relapse prevention. Families could also complete supplemental sessions if needed on stress management, working with the school, sibling concerns, anger management, pain management and marital communication | PST |
|
Wade 2011 TBI |
Teen Online Problem Solving (TOPS). Using an internet program and videoconferencing, families received training in stress management, problem solving, planning and organisation, communication and self regulation. Families could also complete supplemental sessions if needed on stress management, self care, marital communication, memory difficulties, planning for after high school graduation, sibling concerns, pain management and communication between teens and parents | PST |
|
Wysocki 1999 Diabetes |
Behavioural Family Systems Therapy (BFST). Families received training in problem solving skills, communication skills and cognitive restructuring as well as functional and structural family therapy interventions targeting family systems issues that may have interfered with effective problem solving and communication skills | FT |
|
Wysocki 2006 Diabetes |
Behavioural Family Systems Therapy for Diabetes (BFST-D). This intervention is a revised version of the BFST intervention evaluated in Wysocki 1999. Families received training in problem solving, communication skills and cognitive restructuring as well as functional and structural family therapy interventions targeting family systems issues related to effective problem solving and communication. Diabetes-specific adaptations included targeting two or more barriers to diabetes management in treatment, training in behavioural contracting, education in how to improve diabetic control based on data from self monitoring of blood glucose levels, simulation of living with diabetes by parents for 1 week, and involvement of peers/teachers/extended family in treatment as needed | FT |
BFST-D: Behavioural Family Systems Therapy for Diabetes; BI: Behavioural intervention; CBT: cognitive behavioural therapy; FAP: Functional abdominal pain; FT: family therapy; IBS: Irritable bowel syndrome; MST: multisystemic therapy; PST: problem-solving therapy; RAP: Recurrent abdominal pain; TBI: traumatic brain injury.
We were unable to extract quantitative data from 10 of the 47 studies (Barry 1997; Celano 2012; Duarte 2006; Kazak 2004; Lask 1979; Lehmkuhl 2010; Nansel 2012; Olivares 1997; Robins 2005; Stark 2005). These studies did not present means or standard deviations. Stark 2005 delivered behavioural therapy for children with juvenile rheumatoid arthritis and provided outcome data on calcium intake. This was heterogeneous with other outcomes we extracted for this condition and therapy and therefore was not appropriate to include in the meta-analysis. Therefore 37 studies (2984 participants post-treatment) presented data that were included in at least one analysis.
Excluded studies
Eleven studies that were excluded in the update of this review (Bellin 2013; Borhani 2011; Chen 2013; Fedele 2013; Groß 2013; Gulewitsch 2012; Katz 2014; Kurowski 2013; Lyon 2013; Murphy 2012; Van der Veek 2013). One previously included study Grey 2011 was excluded in this update as it combined two sets of trial data, one of which is already included independently (Grey 2011). Therefore a total of 73 studies were excluded, as they did not meet the inclusion criteria for this review.
Thirty-six studies had insufficient psychotherapeutic content, such as instruction, education, parents trained as ’coaches’ for their children or health prevention interventions (Aleman 1992; Anderson 1999; Braga 2005; Bellin 2013; Burke 1997; Burke 2001; Chen 2013; Chernoff 2002; Chiang 2009; Evans 1999; Field 1998; Garbutt 2010; Giallo 2008; Glang 2007; Groß 2013, Hovell 1994; Humphreys 2000; Ireys 1996; Ireys 2001; Johnson 1987; Kaslow 2000; Katz 2014; Kazak 1996; Ketchen 2006; Klinnert 2005; Klinnert 2007; Kupfer 2010; Logan 1997; Mendez 1997; Murphy 2012; Nelson 2011; Pérez 1999; Staab 2002; Sullivan-Bolyai 2010; Szczepanski 2010; Walders 2006). Twenty-two studies had an aim that was irrelevant to the objectives of this review, such as fidelity studies, mixed illnesses or the intervention focusing on the parents’ communication with professionals (Borhani 2011; Bruzzese 2008; Cakan 2007; Canino 2008; Carey 2008; Ellis 2007; Ellis 2008; Fedele 2013; Forsander 1995; Gerber 2010; Gulewitsch 2012; Harris 2001; Hommel 2012; Jay 1990; Kurowski 2013; Lyon 2013; Rasoli 2008; Scholten 2011; Van der Veek 2013; Wade 2010; Walker 1996; Wysocki 1997). Thirteen studies had an insufficient number of participants (n < 10) post-treatment in one or more arms of treatment (Forsander 2003; Gustafsson 1986; Haus 1976; Hernandez 1998; Kamps 2008; Kazak 2005; Kroner-Herwig 1998; Lasecki 2008; Sanders 1989; Sanders 1996; Satin 1989; Sieberg 2011; Warner 2011), one paper recruited participants prospectively (Betancourt 2004), and as mentioned above, one combined two sets of trial data (Grey 2011). These judgements were often difficult to make and led to extended discussion between review authors.
Risk of bias in included studies
We used five ’Risk of bias’ categories: random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias) (Figure 2; Figure 3). Twenty-three studies described a convincing method of randomisation and were judged as low risk of bias, a further 24 studies did not provide an adequate description and were judged as unclear. No studies were rated as high risk of bias for randomisation. There were 15 studies that described a convincing method of allocation and we judged them to have a low risk of allocation bias, a further 32 studies did not provide an adequate description and were judged as unclear. No studies were rated as high risk of allocation bias. For detection bias, 20 studies reported outcome assessors that were blinded to treatment allocation and were judged to have a low risk of bias, a further 27 studies did not provide an adequate description and were judged as unclear. No studies were assessed as having a high risk of outcome bias. For attrition bias, 15 studies reported attrition and found no significant differences between completers and non-completers, so we judged them to have a low risk of bias. Twenty-one studies reported attrition but did not report differences between completers and non-completers and so they were judged as unclear and 11 studies did not give an adequate description of attrition and so we judged them to be of high risk. For selective reporting bias, data could be fully extracted from the published paper in 18 studies and were judged to have a low risk of selective reporting bias. A further 14 studies were unclear, meaning data could not be extracted from the published manuscript but authors responded to data requests. We found 15 studies to have a high risk of selective reporting bias because data could not be extracted and we received no response from our requests for data.
Figure 2.
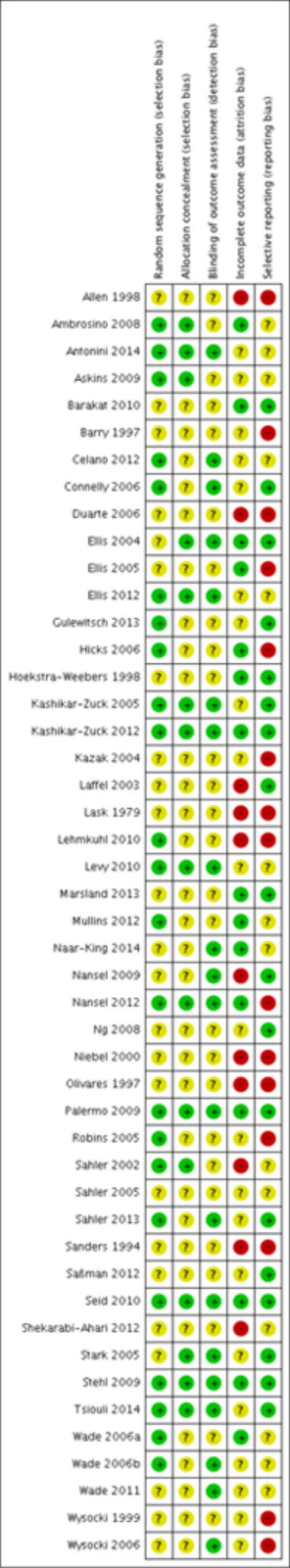
’Risk of bias’ summary: review authors’ judgements about each risk of bias item for each included study.
Figure 3.
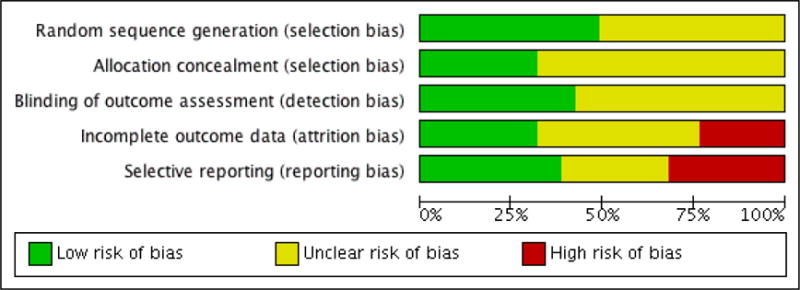
’Risk of bias’ graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Summary of findings for the main comparison Psychological therapies for parent behaviour post-treatment; Summary of findings 2 Psychological therapies for parent mental health post-treatment.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON [Explanation]
| Parenting Behaviour Post-treatment | ||||||
|---|---|---|---|---|---|---|
| Patient or population: Parents of children with a chronic illness Settings: Community Intervention: Psychological therapies | ||||||
| Outcomes | Probable outcome with control | Probable outcome with intervention | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Parenting behaviour post-treatment Pain condition (low scores indicate less adverse behaviour ratings) | The mean parenting behaviour post-treatment pain condition in the intervention groups was 0.34 standard deviations lower (1.18 lower to 0.5 higher) |
SMD −0.34 (−1.18 to 0.5) | 92 (2 studies) |
⊕○○○ very low1,2,3,4,5,6 |
||
| Parenting behaviour post-treatment Cancer (low scores indicate less adverse behaviour ratings) | The mean parenting behaviour post-treatment cancer in the intervention groups was 0.2 standard deviations lower (0.36 to 0.04 lower) |
SMD −0.2 (−0.36 to −0.04) | 836 (5 studies) |
⊕○○○ very low1,4,6 |
||
| SMD: Standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Majority of studies included in outcome have unclear or high risk of bias
Heterogeneity I² > 45%
Wide confidence intervals
Significant proportion of studies use a wait-list control
Low number of participants included in the outcome
A significant proportion of studies did not report outcomes in published paper
ADDITIONAL SUMMARY OF FINDINGS [Explanation]
| Psychological therapies for parent mental health | ||||||
|---|---|---|---|---|---|---|
|
Patient or population: Parents of children with a chronic illness Settings: Community Intervention: Psychological therapies | ||||||
| Outcomes | Probable outcome with control | Probable outcome with intervention | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Parent mental health post-treatment Diabetes (high scores indicate poor mental health) | The mean parent mental health post-treatment diabetes in the intervention groups was 0.22 standard deviations higher (0.08 lower to 0.53 higher) |
SMD 0.22 (−0.08 to 0.53) | 175 (3 studies) | ⊕⊕○○ low1,2 |
||
| Parent mental health post-treatment Cancer (high scores indicate poor mental health) | The mean parent mental health post-treatment cancer in the intervention groups was 0.22 standard deviations lower (0.46 lower to 0.01 higher) |
SMD −0.22 (−0.46 to 0.01) | 1010 (9 studies) | ⊕○○○ very low1,3,4 |
||
| Parent Mental Health TBI (high scores indicate high mental health) | The mean parent mental health tbi in the intervention groups was 0.49 standard deviations lower (1.14 lower to 0.16 higher) |
SMD −0.49 (−1.14 to 0.16) | 72 (2 studies) |
⊕○○○ very low1,2,3 |
||
| Parent Mental Health Asthma (high scores indicate poor mental health) | The mean parent mental health asthma in the intervention groups was 0.2 standard deviations lower (0.66 lower to 0.26 higher) |
SMD −0.2 (−0.66 to 0.26) | 74 (2 studies) |
⊕ ○○○ very low1,2,4 |
||
| SMD: Standardised mean difference; TBI: traumatic brain injury | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Majority of studies included in outcome have unclear or high risk of bias
Low number of participants included in the outcome
Heterogeneity I² > 45%
Majority of studies had wait-list control group
We analysed data in two categories. In the first, outcomes for each individual condition across all psychological therapies are analysed post-treatment and follow-up. For the second, outcomes for each psychological therapy across all conditions post-treatment and at follow-up are presented. No analyses could be conducted for gynaecological disorders due to lack of studies meeting the inclusion criteria, and no adverse events were reported in any study reviewed.
Individual conditions across all psychological therapies
Painful condition
Two studies of children with chronic pain (n = 92) were entered into an analysis of parent adaptive behaviour post-treatment. The analysis revealed that when all psychological therapies were combined, there was no effect for parent adaptive behaviour (Z = 0.80, p = 0.43; Analysis 1.1). Only one study could be included at follow-up, therefore no conclusions can be drawn. Seven studies of children with chronic pain (n = 457) were entered into an analysis investigating the effect of psychological therapies for reducing child behaviour/disability post-treatment, and three studies could be included at follow-up (n = 289). However, analyses revealed that therapies were not beneficial at reducing disability in children with chronic pain post-treatment (Z = 1.85, p = 0.06; Analysis 1.2), or at follow-up (Z = 0.95, p = 0.34; Analysis 2.1). We entered four studies of children with chronic pain post-treatment (n = 356) and two studies at follow-up (n = 255) into an analysis of child mental health. Combined psychological therapies did not have an effect on child mental health post-treatment (Z = 0.14, p = 0.89; Analysis 1.3) or at follow-up (Z = 0.14, p = 0.89; Analysis 2.2). Nine studies of children with chronic pain post-treatment (n = 540), and six studies at follow-up (n = 391) were entered into an analysis investigating the effects of psychological interventions at reducing child pain-related symptoms. A small beneficial effect of psychological treatments was found post-treatment (SMD = −0.39, 95% confidence interval (CI) −0.67 to −0.11; Z = 2.69, p < .01; Analysis 1.4; Figure 4). However, at follow-up no effect was found suggesting that a reduction in pain was not maintained (Z = 1.57, p = 0.12; Analysis 2.3). There was only one study of children with chronic pain that could be entered into an analysis of family functioning post-treatment and at follow-up, therefore no conclusion could be drawn.
Figure 4.
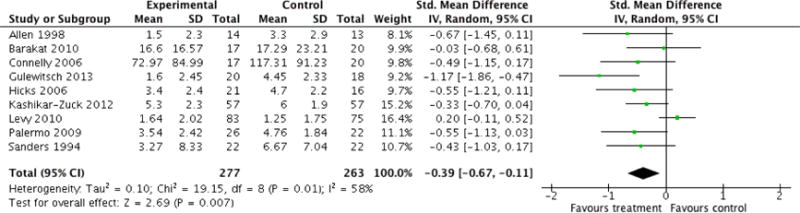
Forest plot of comparison: 1 Painful Conditions Post-treatment, outcome: 1.4 Child Symptoms.
No studies presented extractable data on parent mental health post-treatment or at follow-up.
Cancer
Five studies of parents of children with cancer were entered into an analysis of parent behaviour post-treatment (n = 836) and five studies at follow-up (n = 789) to investigate the efficacy of psychological therapies. Psychological therapies had a small beneficial effect for parenting behaviour post-treatment (SMD = −0.20, 95% CI −0.36 to −0.04, Z = 2.44, p = 0.01; Analysis 3.1; Figure 5). However, this effect was not maintained at follow-up (Z = 1.39, p = 0.16; Analysis 4.1). Nine studies of parents of children with cancer were entered into an analysis of parent mental health post-treatment (n = 1010) and six studies (n = 819) were included at follow-up. There was no effect of psychological therapies on parent mental health post-treatment (Z = 1.86, p = 0.06; Analysis 3.2). However, at follow-up psychological therapies had a small beneficial effect for improving parent mental health (SMD = −0.18, 95% CI −0.32 to −0.04, Z = 2.58, p = 0.01; Analysis 4.2; Figure 6). There was only one study of children with cancer that we could be entered into an analysis of child symptoms post-treatment, and no studies were available at follow-up, therefore no conclusions can be drawn.
Figure 5.
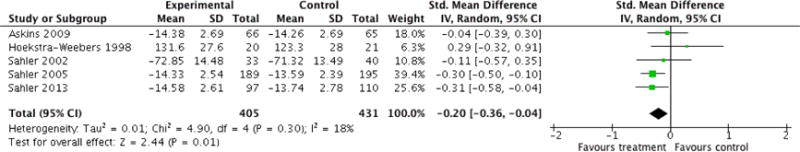
Forest plot of comparison: 3 Cancer Post-treatment, outcome: 3.1 Parent Behaviour.
Figure 6.
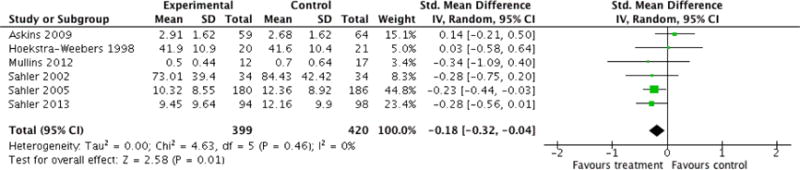
Forest plot of comparison: 4 Cancer Follow-up, outcome: 4.2 Parent Mental Health.
No studies presented extractable data on child behaviour/disability, child mental health and family functioning post-treatment or at follow-up.
Diabetes
There was only one study of children with diabetes that could be entered into an analysis of parenting behaviour post-treatment, therefore no conclusions could be drawn. Three studies of children with diabetes (n = 175) were entered into an analysis investigating the effect of psychological interventions for reducing parent mental health post-treatment. There was no beneficial effect found for psychological therapies post-treatment (Z = 1.43, p = 0.15; Analysis 5.1). Only one study could be entered at follow-up, therefore no conclusion could be drawn. Three studies of children with diabetes (n = 329) were entered into an analysis of child behaviour post-treatment, however, no effect was found (Z = 0.58, p = 0.56; Analysis 5.2). Only one study was available at followup therefore, no conclusions can be drawn. Two studies of children with diabetes (n = 198) were entered into an analysis of child mental health post-treatment, however, no effect of psychological therapies was found (Z = 0.28, p = 0.78; Analysis 5.3). Nine studies of children with diabetes post-treatment (n = 774), and four studies at follow-up (n = 385) were entered into an analysis of child diabetes-related symptoms. Psychological therapies were found to have a small beneficial effect at reducing child symptoms post-treatment (SMD = −0.19, 95% CI −0.37 to −0.01, Z = 2.12, p = 0.03; Analysis 5.4) and this effect was maintained at followup (SMD = −0.25, 95% CI to −0.45 to −0.05, Z = 2.41, p = 0.02, Analysis 6.1). We entered five studies of children with diabetes into an analysis of family functioning post-treatment (n = 422) however, no effect was found (Z = 0.12, p = 0.90; Analysis 5.5). No studies presented extractable data on parenting behaviour, child mental health and family functioning at follow-up.
Asthma
There was only one study of children with asthma that could be entered into analyses on parenting behaviour post-treatment, therefore no conclusions could be drawn. Two studies of children with asthma could be entered into an analysis of parent mental health post-treatment (n = 74). Psychological therapies were not beneficial for parent mental health (Z = 0.86, p = 0.39; Analysis 7.1). Similarly, two studies of children with asthma could be entered into an analysis of child behaviour/disability post-treatment (n = 200), however no effect was found (Z = 1.48, p = 0.14; Analysis 7.2). Four studies of children with asthma were entered into an analysis of child asthma-related symptoms post-treatment (n = 337) and two studies were entered at follow-up (n = 132). The overall effect of all psychological therapies on child symptoms was not beneficial post-treatment (Z = 1.53, p = 0.13; Analysis 7.3) or at follow-up (Z = 0.55, p = 0.58; Analysis 8.1).
No studies presented extractable data on child mental health and family functioning post-treatment or at follow-up, or parenting behaviour, parent mental health and child behaviour/disability at follow-up.
Traumatic brain injury
There was only one study of children with traumatic brain injury that could be entered into analyses on parenting behaviour post-treatment and none at follow-up, therefore no conclusions could be drawn. We entered two studies of children with traumatic brain injury into an analysis of parent mental health post-treatment (n = 72) but no effect of psychological therapies was found (Z = 1.49, p = 0.14; Analysis 9.1). Four studies of children with traumatic brain injury were entered into an analysis of child behaviour/disability post-treatment (n = 144), however, psychological therapies were not found to have an effect (Z = 1.40, p = 0.16; Analysis 9.2). Two studies of children with traumatic brain injury were entered into an analysis of family functioning post-treatment (n = 67), however, no effect was determined (Z = 0.33, p = 0.74; Analysis 9.3).
No studies presented extractable data on child mental health or child symptoms post-treatment and no data were available for any outcomes at follow-up.
Skin diseases
There was only one study of children with skin diseases that could be entered into an analysis of parenting behaviour, parent mental health, child behaviour and child symptoms post-treatment, therefore no conclusions could be drawn.
No studies presented extractable data on child mental health or family functioning post-treatment or any outcomes at follow-up.
Individual psychological therapies across all conditions
Cognitive behavioural therapy
Four studies post-treatment (n = 166) and two studies at followup (n = 85) were entered into an analysis to investigate the effects of cognitive behavioural therapy (CBT) across all conditions on parenting behaviour. The overall effect of CBT on parenting behaviour was not beneficial post-treatment (Z = 0.08, p = 0.94; Analysis 10.1) or at follow-up (Z = 0.56, p = 0.58; Analysis 11.1). Seven studies post treatment (n = 325) and two studies at followup (n = 115) presented data on parent mental health, however, no effect of CBT for parent mental health could be determined post-treatment (Z = 0.66, p = 0.51; Analysis 10.2) or at follow-up (Z = 1.26, p = 0.21; Analysis 11.2). Eight studies post-treatment (n= 487) and three studies at follow-up (n=289) were entered into an analysis to investigate the effects of CBT on child behaviour/disability. No effect could be determined post-treatment (Z = 1.34, p = 0.18; Analysis 10.3) or at follow-up (Z = 0.95, p = 0.34; Analysis 11.3). Five studies post-treatment (n = 439) and two studies at follow-up (n = 257) were entered into an analysis to investigate the effects of CBT on child mental health, however, there was no effect of CBT post-treatment (Z = 0.21, p = 0.83; Analysis 10.4) or at follow-up (Z = 0.27, p = 0.78; Analysis 11.4). We entered 12 studies post-treatment (n = 754) and seven studies at follow-up (n = 472) into an analysis on child symptoms. The overall effect of CBT on child symptoms post-treatment was beneficial (SMD= −0.32, 95% CI −0.53 to −0.11, Z = 2.98, p < .01; Analysis 10.5). However this effect was not maintained at follow-up (Z = 1.70, p = 0.09; Analysis 11.5). We entered three studies post-treatment (n = 211) and two studies at follow-up (n = 107) into an analysis of the effects on family functioning. No beneficial effect of CBT for family functioning was found post-treatment (Z = 0.40, p = 0.69; Analysis 10.6) or at follow-up (Z = 0.61, p = 0.54; Analysis 11.6).
Family therapy
Only one study could be entered into an analysis on the efficacy of family therapy (FT) across all conditions on parenting behaviour post treatment, therefore no analysis was conducted. Three studies (n = 131) were entered into an analysis of the effects of FT on parent mental health post-treatment, however, no effect was found (Z = 0.16, p = 0.88; Analysis 12.1). Only one study could be entered at follow-up, therefore no conclusions could be drawn. Two studies post-treatment (n = 107) were entered into an analysis on child behaviour/disability. The overall effect of FT was not beneficial for children with a chronic condition (Z = 1.44, p = 0.15; Analysis 12.2). We entered five studies post-treatment (n = 259) and two studies at follow-up (n = 96) into an analysis investigating the effects of FT on child symptoms. No beneficial effect was found for FT post-treatment (Z = 0.35, p = 0.73; (Analysis 12.3) or at follow-up (Z = 0.12, p = 0.91; Analysis 13.1). We entered two studies (n = 132) into an analysis on family functioning, however, no effect was found (Z = 0.45, p = 0.65; Analysis 12.4).
No studies presented extractable data on child mental health post-treatment or at follow-up. Furthermore, no data were available for parenting behaviour, child behaviour/disability and family functioning at follow-up.
Problem solving therapy
We entered five studies post-treatment (n= 832) and four studies at follow-up (n = 748) into an analysis investigating the effects of problem solving therapy (PST) across all conditions on parenting behaviour. PST had a small beneficial effect on parenting behaviour post-treatment (SMD = −0.25, 95% CI −0.39 to −0.11, Z = 3.59, p < .01; Analysis 14.1), however this was not maintained at follow-up (Z = 1.75, p = 0.08; Analysis 15.1). Seven studies post-treatment (n = 907) and five studies at follow-up (n = 778) were entered into an analysis on parent mental health. There was a small beneficial effect of PST on parent mental health post-treatment (SMD = −0.24, 95% CI −0.42 to −0.05, Z= 2.50, p = 0.01; Analysis 14.2). This beneficial effect was maintained at follow-up (SMD = −0.19, 95% CI −0.34 to −0.04, Z = 2.55, p = 0.01; Analysis 15.2). We entered five studies post-treatment (n=260) into an analysis of the effects of PST on child behaviour/disability, however no effect was found (Z = 1.21, p = 0.22; Analysis 14.3). There was only one study that could be entered at follow-up, therefore no conclusions could be drawn. We entered two studies post-treatment (n = 216) into an analysis on child symptoms. There was no beneficial effect of PST post-treatment (Z = 1.41, p = 0.16; Analysis 14.4). There was only one study that could be entered at follow-up, therefore no conclusions could be drawn. We entered three studies post-treatment (n = 183) into an analysis of the effects of PST on family functioning, however no effect was found (Z = 0.54, p = 0.59; Analysis 14.5).
No studies presented extractable data on family functioning at follow-up, or child mental health post-treatment or at follow-up.
Multisystemic therapy
We entered two studies post-treatment (n = 313) into an analysis investigating the effects of multisystemic therapy (MST) on child behaviour/disability. No effect was found at reducing child behaviour/disability (Z = 0.99, p = 0.32; Analysis 16.1). There was only one study at follow-up that could be entered into an analysis, therefore no conclusions could be drawn. Only one study post-treatment that could be entered into an analysis on the effects of MST across all conditions on child mental health; therefore no conclusions could be drawn. We entered four studies post-treatment (n = 455) and two studies at follow-up (n = 247) into an analysis of the effects of MST on child symptoms. There was no beneficial effect of MST on child symptoms post-treatment (Z = 1.52, p = 0.13; Analysis 16.2) or at follow-up (Z = 1.47, p = 0.14, Analysis 17.1).
No studies presented extractable data on parenting behaviour, parent mental health and family functioning post-treatment and at follow-up. Furthermore, no studies presented extractable data on child mental health at follow-up.
DISCUSSION
Summary of main results
There were two objectives of this review. First, we aimed to evaluate psychological therapies delivered to parents of children with a chronic condition. Second, we sought to evaluate the risk of bias and quality of evidence for the included studies. Evidence was evaluated in two ways. First, we chose to determine the efficacy across psychological therapies for individual child health conditions. Second, we chose to determine the efficacy of individual psychological therapies across condition type. Data were analysed post-treatment and at follow-up. We did not identify any studies for gynaecological disorders or inflammatory bowel diseases. The majority of studies did not report whether they encountered any adverse events due to treatment, however five studies reported that there were no adverse events (Gulewitsch 2013; Kashikar-Zuck 2012; Nansel 2009; Nansel 2012; Stark 2005). Kazak 2004 did not report any adverse events, but reported that participants reporting higher levels of distress were more likely to drop out of the treatment compared to less distressed participants, suggesting that the treatment may not be suitable for those who perhaps need it most. Because few studies reported whether or not they encountered adverse events, we are unable to comment further on the relevance of any adverse events on treatment safety. There were a number of analyses that could not be run due to missing data. This reflects the status of this developing field that has not yet produced a consensus of common core outcomes.
Combined psychological therapies for each illness condition
First, we analysed data by each medical condition across all treatment classes, giving 72 possible analyses post-treatment and at follow-up (Table 3). We were unable to conduct 48 analyses due to lack of data, 19 analyses were conducted but no effect was found, and five analyses found a beneficial effect of psychological therapies. First, psychological therapies were found to be beneficial at improving parenting behaviour for parents of children with cancer conditions post-treatment and for improving parent mental health at follow-up. Second, for children with diabetes and painful conditions, psychological therapies were beneficial at reducing medical symptoms (e.g., improving glycemic control, reducing pain intensity) for these conditions post-treatment, and these effects were maintained at follow-up for children with diabetes. We found no effect for 19 outcomes for four chronic conditions. There were insufficient data for 48 outcomes for all six conditions.
Table 3.
Scorecard of meta-analytic findings for individual chronic conditions across psychological therapies
| Combined psychological therapies for each illness condition (post Rx) | ||||||
|---|---|---|---|---|---|---|
| Parent | Child | |||||
| Behaviour | Mental health | Behaviour/disability | Mental health | Primary symptom | Family functioning | |
| Pain | No effect (2) | Unknown (0) | No effect (7) | No effect (4) | Effect found (9) | Unknown (1) |
| Cancer | Effect found (5) | No effect (9) | Unknown (0) | Unknown (0) | Unknown (1) | Unknown (0) |
| Diabetes | Unknown (1) | No effect (3) | No effect (3) | No effect (2) | Effect found (9) | No effect (5) |
| Asthma | Unknown (1) | No effect (2) | No effect (2) | Unknown (0) | No effect (4) | Unknown (0) |
| TBI | Unknown (1) | No effect (2) | Unknown (4) | Unknown (0) | Unknown (0) | No effect (2) |
| Skin diseases | Unknown (1) | Unknown (1) | Unknown (1) | Unknown (0) | Unknown (1) | Unknown (0) |
| Combined psychological therapies for each illness condition (Follow-up) | ||||||
| Parent | Child | |||||
| Behaviour | Mental health | Behaviour/disability | Mental health | Primary symptom | Family functioning | |
| Pain | Unknown (1) | Unknown (0) | No effect (3) | No effect (2) | No effect (6) | Unknown (1) |
| Cancer | No effect (5) | Effect found (6) | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) |
| Diabetes | Unknown (0) | Unknown (1) | Unknown (1) | Unknown (0) | Effect (4) | Unknown (0) |
| Asthma | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) | No effect (2) | Unknown (0) |
| TBI | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) |
| Skin diseases | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) |
Number in parentheses denotes number of studies included in analysis
Individual psychological therapies for combined illness conditions
Second, we analysed data by each treatment class across all medical conditions, giving 60 possible analyses post-treatment and at follow-up (Table 4). We were unable to conduct 21 analyses due to lack of data, 23 analyses were conducted but no effect was found, and four analyses showed a beneficial effect across conditions. Cognitive behavioural therapy (CBT) was found to be beneficial at improving the primary disease-related symptoms of children with chronic illness conditions post-treatment. Problem-solving therapy (PST) improved parenting behaviour and mental health post-treatment and the effects for mental health were maintained at follow-up.
Table 4.
Scorecard of meta-analytic findings for psychological therapies across all chronic conditions
| Individual psychological therapies for combined illness conditions (post Rx) | ||||||
|---|---|---|---|---|---|---|
| Parent | Child | |||||
| Behaviour | Mental Health | Behaviour/disability | Mental health | Primary symptom | Family functioning | |
| CBT | No effect (4) | No effect (7) | No effect (8) | No effect (5) | Effect found (12) | No effect (3) |
| FT | Unknown (1) | No effect (3) | No effect (2) | Unknown (0) | No effect (5) | No effect (2) |
| PST | Effect found (5) | Effect found (7) | No effect (5) | Unknown (0) | No effect (2) | No effect (3) |
| MST | Unknown (0) | Unknown (0) | No effect (2) | Unknown (1) | No effect (4) | Unknown (0) |
| Individual psychological therapies for combined illness conditions (Follow-up) | ||||||
| Parent | Child | |||||
| Behaviour | Mental Health | Behaviour/disability | Mental health | Primary symptom | Family functioning | |
| CBT | No effect (2) | No effect (2) | No effect (3) | No effect (2) | No effect (7) | No effect (2) |
| FT | Unknown (0) | Unknown (1) | Unknown (0) | Unknown (0) | No effect (2) | Unknown (0) |
| PST | No effect (4) | Effect found (5) | Unknown (1) | Unknown (0) | Unknown (1) | Unknown (0) |
| MST | Unknown (0) | Unknown (0) | Unknown (0) | Unknown (0) | No effect (2) | Unknown (0) |
Number in parentheses denotes number of studies included in analysis
CBT: cognitive behavioural therapy; FT: family therapy; MST: multisystemic therapy; PST: problem-solving therapy; TBI: traumatic brain injury
It was not possible to conduct planned subgroup analyses for comparisons of the interaction between condition and psychological therapy effectiveness or the characteristics of particular treatments.
Overall completeness and applicability of evidence
We were unable to identify any trials for children with gynaecological disorders or inflammatory bowel diseases therefore studies investigating these disorders are still needed. One study including children with functional abdominal pain (FAP) and irritable bowel syndrome (IBS) was included in this update, where previously there was no evidence for parents of children with IBS (Gulewitsch 2013). Despite this, IBS is a prevalent condition during adolescence (Karabulut 2013).
There were 21 studies that provided extractable data for parent outcomes. Parent outcomes were not included in the majority of trials. Only studies investigating children and adolescents with cancer had sufficient data for parent outcomes post-treatment and at follow-up. The remaining chronic conditions reported only one parent outcome post-treatment and no analyses could be conducted for parent outcomes at follow-up. Future randomised controlled trials (RCTs) of parent interventions should include relevant parent mental health and behavioural outcomes using standardised measures.
When evaluating individual psychological therapies, we were able to evaluate parent outcomes for those who had received CBT or PST. However, follow-up evaluations must be conducted, particularly for parents receiving CBT. In addition, most studies evaluating PST investigated parents of children with cancer, although two new trials for this update evaluated PST for parents of children with diabetes. It is noticeable that MST is the only therapy type that does not have data for any parent outcomes post-treatment and at follow-up. Further, at follow-up no analyses could be conducted for parent outcomes when parents were treated with family therapy (FT), due to lack of data. Therefore, we are unable to comment on the maintenance of psychological therapies for parents receiving these treatments.
The number of participants included in some studies is still small, with an average number of 80 participants entered into analyses. Although more trials have been included in this update, there are still insufficient data for many outcomes.
Quality of the evidence
Five ’Summary of findings’ tables are presented in this updated review. First, parent outcomes across all conditions post-treatment are presented (Summary of findings for the main comparison; Summary of findings 2). Due to a lack of evidence for parent outcomes at follow-up we were unable to assess these outcomes. Second, ’Summary of findings’ tables are presented for pain, cancer, and diabetes conditions (Table 5; Table 6; Table 7). Due to a lack of data we did not produce ’Summary of findings’ tables for the remaining conditions. All outcomes in these tables were judged to be of low or very low quality. Contributing reasons for this are small sample sizes that were included in analyses, unclear or high risk of bias across studies, high heterogeneity within analyses, and suspected publication bias when authors are unable or unwilling to share data.
Table 5.
Psychological therapies for parents of children and adolescents with a chronic pain condition
| Psychological therapies for parents of children and adolescents with a chronic pain condition | ||||||
|---|---|---|---|---|---|---|
| Patient or population: Parents of children with a chronic illness Settings: Community Intervention: Psychological therapies | ||||||
| Outcomes | Probable outcome with control | Probable outcome with intervention | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Parenting behaviour post-treatment (low scores indicate less adverse behaviour ratings) | The mean parent behaviour post-treatment in the intervention groups was 0.34 standard deviations lower (1.18 lower to 0. 5 higher) | SMD −0.34 (−1. 18 to 0.5) | 92 (2 studies) |
⊕○○○ very low1,2,3,4,5,6 |
||
| Child behaviour/disability post-treatment (low scores indicate indicate less adverse behaviour/disability ratings) | The mean child behaviour/disability post-treatment in the intervention groups was 0.29 standard deviations lower (0.59 lower to 0. 02 higher) | SMD −0.29 (−0. 59 to 0.02) | 457 (7 studies) |
⊕○○○ very low2,4,5 |
||
| Child behaviour/disability at follow-up (low scores indicate less adverse behaviour/disability ratings) | The mean child behaviour/disability at follow-up in the intervention groups was 0.17 standard deviations lower (0.52 lower to 0. 18 higher) | SMD −0.17 (−0. 52 to 0.18) | 289 (3 studies) |
⊕○○○ very low2,3,5,6 |
||
| Child mental health post-treatment (high scores indicate poor mental health) | The mean child mental health post-treatment in the intervention groups was 0.02 standard deviations lower (0.35 lower to 0.3 higher) | SMD −0.02 (−0.35 to 0.3) | 356 (4 studies) |
⊕⊕○○ low2,5 |
||
| Child mental health at follow-up (high scores indicate poor mental health) | The mean child mental health at follow-up in the intervention groups was 0.02 standard deviations higher (0.23 lower to 0.26 higher) | SMD 0.02 (−0.23 to 0.26) | 255 (2 studies) |
⊕○○○ very low5,6 |
||
| Child symptoms at post-treatment (low scores indicate lower pain ratings) | The mean child symptoms at post-treatment in the intervention groups was 0.39 standard deviations lower (0.67 to 0.11 lower) | SMD −0.39 (−0.67 to −0.11) | 540 (9 studies) |
⊕⊕○○ low1,2 |
||
| Child symptoms at follow-up (low scores indicate lower pain ratings) | The mean child symptoms at follow-up in the intervention groups was 0.38 standard deviations lower (0.86 lower to 0. 1 higher) | SMD −0.38 (−0.86 to 0.1) | 391 (6 studies) |
⊕○○○ very low1,2,3,5,6 |
||
| SMD: Standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Majority of studies included in outcome have unclear or high risk of bias
Heterogeneity I2 > 45%
Wide confidence intervals
Significant proportion of studies use a wait-list control
Low number of participants included in the outcome
A significant proportion of studies did not report outcomes in published paper
Table 6.
Psychological therapies for parents of children and adolescents with diabetes
| Psychological therapies for parents of children and adolescents with diabetes | ||||||
|---|---|---|---|---|---|---|
| Patient or population: Parents of children with diabetes Settings: Community Intervention: Psychological therapies | ||||||
| Outcomes | Probable outcome with control | Probable outcome with intervention | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Parent mental health post-treatment (high scores indicate poor mental health) | The mean parent mental health post-treatment in the intervention groups was 0.22 standard deviations higher (0.08 lower to 0. 53 higher) | SMD 0.22 (−0.08 to 0.53) | 175 (3 studies) |
⊕⊕○○ low1,2 |
||
| Child behaviour/disability post-treatment (low scores indicate less adverse behaviour ratings) | The mean child behaviour/disability post-treatment in the intervention groups was 0.06 standard deviations higher (0.15 lower to 0.28 higher) | SMD 0.06 (−0.15 to 0.28) | 329 (3 studies) |
⊕○○○ very low1,2,3 |
||
| Child mental health post-treatment (high scores indicate poor mental health) | The mean child mental health post-treatment in the intervention groups was 0.08 standard deviations lower (0.63 lower to 0.47 higher) | SMD −0.08 (−0.63 to 0.47) | 198 (2 studies) |
⊕○○○ very low1,2,3,4,5 |
||
| Child symptoms post-treatment (low scores indicate lower HbA1 c) | The mean child symptoms post-treatment in the intervention groups was 0.19 standard deviations lower (0.37 to 0.01 lower) | SMD −0.19 (−0.37 to −0.01) | 774 (9 studies) |
⊕○○○ very low1,3,6 |
||
| Child symptoms at follow-up (low scores indicate lower HbA1c) | The mean child symptoms at follow-up in the intervention groups was 0.25 standard deviations lower (0.45 to 0.05 lower) | SMD −0.25 (−0.45 to −0.05) | 385 (4 studies) |
⊕○○○ very low1,2,3 |
||
| Family functioning post-treatment (low scores indicate better family functioning) | The mean family functioning post-treatment in the intervention groups was 0.01 standard deviations lower (0.2 lower to 0. 18 higher) | SMD −0.01 (−0.2 to 0.18) | 422 (5 studies) |
⊕○○○ very low1,2,3 |
||
| SMD: Standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Majority of studies included in outcome have unclear or high risk of bias
Low number of participants included in the outcome
A significant proportion of studies did not report outcomes in published paper
Heterogeneity I2 > 45%
Wide confidence intervals
Majority of studies did not use an active comparator
Table 7.
Psychological therapies for parents of children and adolescents with cancer
| Psychological therapies for parents of children and adolescents with cancer | ||||||
|---|---|---|---|---|---|---|
| Patient or population: Parents of children with cancer Settings: Community Intervention: Psychological therapies | ||||||
| Outcomes | Probable outcome with control | Probable outcome with intervention | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Parenting behaviour post-treatment (low scores indicate less adverse behaviour ratings) | The mean parent behaviour post-treatment in the intervention groups was 0.2 standard deviations lower (0.36 to 0.04 lower) | SMD −0.2 (−0.36 to −0.04) | 836 (5 studies) |
⊕○○○ very low1,2,3 |
||
| Parenting behaviour at follow-up (low scores indicate less adverse behaviour ratings) | The mean parent behaviour at follow-up in the intervention groups was 0.12 standard deviations lower (0.29 lower to 0. 05 higher) | SMD −0.12 (−0. 29 to 0.05) | 789 (5 studies) |
⊕○○○ very low1,2,3 |
||
| Parent mental health post-treatment (high scores indicate poor mental health) | The mean parent mental health post-treatment in the intervention groups was 0.22 standard deviations lower (0.46 lower to 0. 01 higher) | SMD −0.22 (−0. 46 to 0.01) | 1010 (9 studies) |
⊕○○○ very low1,2,4 |
||
| Parent mental health at follow-up (high scores indicate poor mental health) | The mean parent mental health at follow-up in the intervention groups was 0.18 standard deviations lower (0.32 to 0.04 lower) | SMD −0.18 (−0. 32 to −0.04) | 819 (6 studies) |
⊕○○○ very low1,2,3 |
||
| SMD: Standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Majority of studies included in outcome have unclear or high risk of bias
Majority of studies did not have an active comparator
A significant proportion of studies did not report outcomes in published paper
Heterogeneity I2 > 45%
Risk of bias was also assessed to determine the quality of individual studies. The majority of assessments were unclear or high risk across studies, highlighting issues with conducting and reporting of trials. However, assessments for the updated studies were mostly judged to be low or unclear risk of bias, indicating that risk of bias is improving in more recent trials. Random sequence generation, blinding of outcome assessors, and selective reporting bias had almost 50% of ratings judged as low risk. Incomplete outcome data and selective reporting were the only categories to contain any high risk of bias ratings. The following points contribute to lower quality of individual studies and evaluation of outcomes, and remain prominent issues within this field.
First, multiple measurement tools within a given domain are often employed in individual studies and there is little agreement as to the preferred measurement tool across studies. This increases the heterogeneity of analyses. In some cases measurement is relatively homogeneous (e.g. pain intensity), whereas in others there is greater variety (e.g. family functioning scales in diabetes). These trials do not routinely identify a-priori the primary outcome, and there is unusual variety of outcome reporting. For example, one study discussed parent judgement of child outcome when the more valid measure, but non-significant finding, of child report was available (Levy 2010). A posteriori selection of outcome measures is a significant problem in this field. As per our protocol, we were uninfluenced by the primary reporting of measures and focused on the best measure available in each domain. This field needs to take account of reporting biases and establish standards to improve the reporting of a-priori decisions regarding measurement.
Second, we attempted to review trials with a parent intervention component. Therefore, trials inevitably included varied amounts of parent-directed content. Although we planned subgroup analyses, the data were not of sufficient quantity and quality to enable such an investigation. For some analyses we combined studies that were designed specifically with parents as the sole focus, and in others they were part of a combined treatment. Further, the philosophy of some treatments (e.g. MST) was antithetical to our strategy of determining an individual as a treatment target, however, we have included them in this review. It should be noted that significant findings in this review emerged when there was homogeneity of approach, homogeneity of outcome measurements, and a larger number of participants.
Third, it should be noted that we had some difficulties in data retrieval due to incomplete and partial data reporting. Data were sometimes reported graphically, and participant means or standard deviations or both were often missing. As a result, complete or partial data were available to extract from 37 (n = 2985) of the 47 studies (n = 3778) included in this review. Twenty-eight trials produced complete outcome data in their published paper (Allen 1998; Barakat 2010; Connelly 2006; Ellis 2004; Ellis 2005; Gulewitsch 2013; Hicks 2006; Hoekstra-Weebers 1998; Kashikar-Zuck 2005; Kashikar-Zuck 2012; Laffel 2003; Levy 2010; Marsland 2013; Nansel 2009; Ng 2008; Palermo 2009; Sahler 2013; Sanders 1994; Saßman 2012; Seid 2010; Shekarabi-Ahari 2012; Stehl 2009; Tsiouli 2014; Wade 2006a; Wade 2006b; Wade 2011; Wysocki 1997; Wysocki 2006) and nine authors responded to our requests for data (Ambrosino 2008; Antonini 2014; Askins 2009; Ellis 2012; Mullins 2012; Naar-King 2014; Niebel 2000; Sahler 2002; Sahler 2005). Other authors were unable or unwilling to provide additional data or did not respond. The non-production of data is a problem in science (Nature 2009), and has been particularly discussed in psychology (Wicherts 2006; Wicherts 2011). We support the general move toward central registries for all trial data. Fourth, piecemeal and repeat publication was found in eight cases where multiple manuscripts were published from the same trial. In particular, one study (Ellis 2005) was reported six times in five different journals while another trial (Wysocki 1999) was reported five times in four different journals, with variable citation of previous publications in later publications. Such practices are unhelpful, create confusion and increase unnecessary labour (American Psychological Association 2011). Many journals now have policies regarding publication of multiple manuscripts from the same trial, including a detailed description of previous publications from that trial and a statement regarding the unique contribution of the present manuscript (e.g. Drotar 2010).
Finally, replication by other research teams independent to the therapy progenitors is uncommon. For example, Ellis and colleagues are the only group who have evaluated MST in young people with diabetes (Ellis 2004; Ellis 2005; Ellis 2012). Similarly, PST for children and adolescents with traumatic brain injury (TBI) has not been evaluated by any research team outside of Wade and colleagues (Antonini 2014; Wade 2006a; Wade 2006b; Wade 2011).
Agreements and disagreements with other studies or reviews
Combined psychological therapies for each illness condition
Only a handful of reviews have also investigated psychological interventions for parents of children with a chronic illness. The results are consistent with a previous meta-analysis regarding the effectiveness of CBT in reducing child symptoms in young people with chronic pain (Eccleston 2014), and are in agreement with the finding that children with painful conditions have reduced symptoms after psychological interventions (Fisher 2014). The results are somewhat consistent with a meta-analysis of psychological paediatric oncology interventions, which showed no effects on child behaviour or child mental health, but positive effects for parent mental health and parenting behaviour (Pai 2006). The results are consistent with previous reviews of psychological interventions that included parents of children with diabetes, which reported positive effects on child symptoms (Armour 2005); however, they are not consistent with a review on families of children with diabetes, which found that psychological interventions improved family functioning (McBroom 2009). Previous reviews of psychological interventions that included parents of children with asthma or skin diseases were inconclusive due to a lack of trials that met the inclusion criteria (Ersser 2014; Yorke 2005). Notably, disagreements between the present meta-analysis and previous reviews may be attributable to differences in inclusion criteria, selection of outcome measures and/or selection of comparator group.
Individual psychological therapies for combined illness conditions
The results are consistent with a systematic review and meta-analysis which found that PST has a beneficial effect on parent mental health and parenting behaviour post-treatment and at followup (Law 2014), and consistent with the Law 2014 findings for other therapy types. One prior review indicated that psychological interventions which include coping skills training for adolescents and young adults with chronic illness (cancer, diabetes, juvenile idiopathic arthritis, sickle cell disease and asthma) and their parents/families had mixed effects on child psychosocial functioning and family functioning (Sansom-Daly 2012). We were unable to find any previous reviews that compared results from individual psychological therapies across chronic illness conditions for parent outcomes or child symptoms. Therefore, we cannot draw any conclusions regarding the consistency of our results with previous reviews by treatment type for parent outcomes or child symptoms.
AUTHORS’ CONCLUSIONS
Implications for practice
1. For parents of children with a chronic illness
There is little evidence available to guide parents as to the most effective intervention expected to produce changes in their own mental health or behavioural functioning. Limited data are available. Problem-solving therapy (PST) produced change in parenting behaviour and mental health, in part by reducing parenting stress. However, there are positive findings to suggest that cognitive behavioural therapy (CBT) that include parents are beneficial for reducing children’s primary symptoms such as pain.
2. For clinicians
The only therapy that was expressly developed to reduce parent distress and was delivered to parents of children with a chronic illness was PST, which we found led to improvements in parent mental health and parenting behaviour. These trials were predominantly delivered to parents of children with cancer, but have also been trialled in parents of children with diabetes. Although CBT, family therapy (FT), and multisystemic therapy (MST) also treated parents, no beneficial effect of therapy was found, although CBT did reduce child symptoms post-treatment. These therapies include parents but remain focused on the child as the agent of behaviour change, and do not purposefully target strategies to improve parent mental health or behaviour.
3. For policy makers
It is surprising how few trials have targeted parent behaviour or mental health, given the longstanding interest of psychologists in understanding the relationship between child and parent adjustment to chronic illness. When combining all therapies for parenting outcomes, we concluded that the quality of evidence was low to very low, meaning further research is very likely to change the estimates of effects. This is primarily due to the low number of studies that reported parenting outcomes. Thus, little guidance is currently available to understand the most effective interventions to implement with parents of youth with chronic health conditions.
Implications for research
Since the first version of this review, there have been 13 new studies. However, many of the suggestions we made in 2012 still stand (Eccleston 2012b). There are relatively few studies of psychological interventions that target parents of children with a chronic illness and further work is needed to develop and evaluate additional parent-focused interventions that aim to alter parent mental health or parenting behaviour. For example, there were no studies of children with gynaecological disorders or inflammatory bowel diseases that met the inclusion criteria, and only one with children with skin diseases, meaning we were not able to conduct any meta-analyses for these conditions. The next generation of trials should improve by taking account of the limitations identified in this review, including:
Larger sample sizes;
Following CONSORT guidelines (Schulz 2010);
The clearer identification of primary outcomes;
Designing treatment content to specifically target change in the primary outcomes;
More consistency of measurement and greater consensus within the field around appropriate measures to use within and across illness groups;
Placing treatment manuals and data in a shared database to facilitate replication of intervention trials and re-analysis of results;
Measuring and reporting parent-specific outcomes;
Inclusion of fathers as trial and intervention participants;
Reporting of adverse events.
This review has also highlighted several future directions for research that examines interventions targeting parents of children with chronic illness. Problem-solving therapy (PST) improved parent mental health and parenting behaviour. Previously, we suggested that studies were needed to evaluate PST in populations other than cancer and traumatic brain injury. In this update, two studies delivered PST to parents of children with diabetes, however more PST studies on a broader range of chronic illnesses are still required (e.g. parents of children with pain). Replication studies are also needed for interventions that have been evaluated by only one research team, such as multisystemic therapy for families of children with diabetes, and PST for families of children with traumatic brain injury. We recognise that this goal may be difficult to achieve given the high degree of competition for funding and lack of interest among funding agencies for replication studies. Research is also needed to evaluate interaction effects such as the impact of changes in parent outcomes on child outcomes, as well as evaluation of specific treatment characteristics such as the intensity of intervention delivered to children versus parents. Fathers have also not been included in most trials and we do not know anything about the effects of the psychological interventions included in this review if delivered to fathers, which is a common critique of the field of paediatric psychology.
Finally, in regards to duplication and piecemeal publication. Editorial policies are needed to inform authors regarding reporting standards for multiple publications from the same trial. Editors play a crucial role in creating and enforcing these policies, and need to take a pro-active approach to identifying such papers during the review process (Committee on Publication Ethics 2011; World Association of Medical Editors 2012).
Acknowledgments
We would like to thank Dr Zeinab Mousavi, Dr Gerrit Hirschfeld, Dr Anna Huguet, Dr Rikard Wicksell, Dr Jordi Miro, Megan Walker, and Nils Niederstrasser for their time in helping to translate papers and extract data.
We would like to thank all authors who responded to emails requesting further studies or data.
We would also like to thank Jane Hayes and Joanne Abbot for all their help designing and carrying out the search strategies.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
SOURCES OF SUPPORT
Internal sources
University of Bath, UK.
External sources
National Institutes of Health/National Institutes for Child Health and Human Development, USA. Grant number: R21HD065180 (PI: Palermo)
APPENDICES
Appendix 1. Search strategies for 2014 update
CENTRAL
#1 MeSH descriptor: [Psychotherapy] explode all trees
#2 MeSH descriptor: [Problem Solving] 2 tree(s) exploded
#3 psychotherap*:ti,ab,kw (Word variations have been searched)
#4 ((cogniti* or family or behavior* or behaviour* or psychological*) near/5 (intervention* or treatment* or therap*)):ti,ab,kw (Word variations have been searched)
#5 (problem* near/5 solv*):ti,ab,kw (Word variations have been searched)
#6 CBT:ti,ab,kw (Word variations have been searched)
#7 (#1 or #2 or #3 or #4 or #5 or #6)
#8 MeSH descriptor: [Parents] explode all trees
#9 MeSH descriptor: [Family] explode all trees
#10 MeSH descriptor: [Caregivers] this term only
#11 (parent* or mother* or father* or family or families or caregiver* or care-giver*):ti,ab,kw (Word variations have been searched)
#12 (#8 or #9 or #10 or #11)
#13 MeSH descriptor: [Child] explode all trees
#14 MeSH descriptor: [Infant] explode all trees
#15 MeSH descriptor: [Adolescent] explode all trees
#16 (child* or infant* or adolesc* or baby or babies or toddler* or teenager* or youth*):ti,ab,kw (Word variations have been searched)
#17 (#13 or #14 or #15 or #16)
#18 MeSH descriptor: [Pain] explode all trees
#19 MeSH descriptor: [Complex Regional Pain Syndromes] explode all trees
#20 MeSH descriptor: [Rheumatic Diseases] explode all trees
#21 MeSH descriptor: [Neoplasms] explode all trees
#22 MeSH descriptor: [Diabetes Mellitus] explode all trees
#23 MeSH descriptor: [Asthma] explode all trees
#24 MeSH descriptor: [Brain Injuries] explode all trees
#25 MeSH descriptor: [Inflammatory Bowel Diseases] explode all trees
#26 MeSH descriptor: [Anemia, Sickle Cell] explode all trees
#27 MeSH descriptor: [Skin Diseases] explode all trees
#28 MeSH descriptor: [Genital Diseases, Female] explode all trees
#29 MeSH descriptor: [Menstruation Disturbances] explode all trees
#30 (pain* or headache*):ti,ab,kw (Word variations have been searched)
#31 (rheumat* or arthriti* or fibromyalgia):ti,ab,kw (Word variations have been searched)
#32 (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinoma*):ti,ab,kw (Word variations have been searched)
#33 diabet*:ti,ab,kw (Word variations have been searched)
#34 asthma*:ti,ab,kw (Word variations have been searched)
#35 (brain near/5 (trauma* or injur*)):ti,ab,kw (Word variations have been searched)
#36 (bowel* near/5 inflammatory near/5 (condition* or disease* or illness*)):ti,ab,kw (Word variations have been searched)
#37 (sickle cell near/5 (disease* or disorder* or anemia*)):ti,ab,kw (Word variations have been searched)
#38 ((skin near/5 (disease* or disorder*)) or eczema*):ti,ab,kw (Word variations have been searched)
#39 ((gynecologic* or gynaecologic*) near/5 (disease* or disorder*)):ti,ab,kw (Word variations have been searched)
#40 dysmenorrh*:ti,ab,kw (Word variations have been searched)
#41 endometriosis:ti,ab,kw (Word variations have been searched)
#42 MeSH descriptor: [Chronic Disease] this term only
#43 ((chronic* or longterm or long-term) near/5 (condition* or ill* or disease*)):ti,ab,kw (Word variations have been searched)
#44 (#18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43)
#45 (#7 and #12 and #17 and #44) Publication Year from 2012 to 2014
MEDLINE (OVID)
exp Psychotherapy/ (150715)
Problem Solving/ (21235)
psychotherap*.mp. (68147)
((cogniti* or family or behavior* or behaviour* or psychological*) adj5 (intervention* or treatment* or therap*)).mp. (107111)
(problem* adj5 solv*).mp. (54029)
CBT.mp. (5158)
or/1–6 (265435)
exp Parents/ (72641)
exp Family/ (236222)
Caregivers/ (21502)
(parent* or mother* or father* or family or families or caregiver* or care-giver*).mp. (1252254)
or/8–11 (1284976)
exp Child/ (1543748)
exp Infant/ (934743)
Adolescent/ (1611500)
(child* or infant* or adolesc* or baby or babies or toddler* or teenager* or youth*).mp. (3174766)
or/13–16 (3174766)
exp Pain/ (306198)
exp Complex Regional Pain Syndromes/ (4336)
exp Rheumatic Diseases/ (175661)
exp Neoplasms/ (2561658)
exp Diabetes Mellitus/ (314181)
exp Asthma/ (105870)
exp Brain Injuries/ (49346)
exp Inflammatory Bowel Diseases/ (60373)
exp Anemia, Sickle Cell/ (17799)
exp Skin Diseases/ (810983)
exp Genital Diseases, Female/ (358725)
exp menstruation disturbances/ (24223)
(pain* or headache*).mp. (582940)
(rheumat* or arthriti* or fibromyalgia).mp. (232887)
(cancer* or neoplas* or tumor* or tumour* or malignan* or carcinoma*).mp. (2952920)
diabet*.mp. (471099)
asthma*.mp. (140539)
(brain adj5 (trauma* or injur*)).mp. (68497)
(bowel* adj5 inflammatory adj5 (condition* or disease* or illness*)).mp. (31393)
(sickle cell adj5 (disease* or disorder* or anemia*)).mp. (19955)
((skin adj5 (disease* or disorder*)) or eczema*).mp. (105706)
((gynecologic* or gynaecologic*) adj5 (disease* or disorder*)).mp. (5081)
dysmenorrh*.mp. (5152)
endometriosis.mp. (20182)
Chronic Disease/ (219791)
((chronic* or longterm or long-term) adj5 (condition* or ill* or disease*)).mp. (418976)
or/18–43 (5469388)
randomized controlled trial.pt. (376608)
controlled clinical trial.pt. (88576)
randomized.ab. (297316)
placebo.ab. (155203)
drug therapy.fs. (1708731)
randomly.ab. (214956)
trial.ab. (308738)
groups.ab. (1366808)
45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 (3363144)
exp animals/ not humans.sh. (3954113)
53 not 54 (2885271)
7 and 12 and 17 and 44 and 55 (1119)
(201203* or 201204* or 201205* or 201206* or 201207* or 201208* or 201209* or 201210* or 201011* or 201212* or 2013* or 2014*).ed. (2350810)
56 and 57 (193)
EMBASE (OVID)
exp Psychotherapy/ (188395)
Problem Solving/ (26195)
psychotherap*.mp. (96298)
((cogniti* or family or behavior* or behaviour* or psychological*) adj5 (intervention* or treatment* or therap*)).mp. (213343)
(problem* adj5 solv*).mp. (62494)
CBT.mp. (7776)
or/1–6 (385750)
exp Parents/ (155722)
exp Family/ (335975)
Caregivers/ (39951)
(parent* or mother* or father* or family or families or caregiver* or care-giver*).mp. (1393196)
or/8–11 (1438650)
exp Child/ (2083396)
exp Infant/ (860556)
Adolescent/ (1219788)
(child* or infant* or adolesc* or baby or babies or toddler* or teenager* or youth*).mp. (2974509)
or/13–16 (3192664)
exp Pain/ (829233)
exp Complex Regional Pain Syndromes/ (7323)
exp Rheumatic Diseases/ (188325)
exp Neoplasms/ (3394555)
exp Diabetes Mellitus/ (598732)
exp Asthma/ (188864)
exp Brain Injuries/ (122563)
exp Inflammatory Bowel Diseases/ (3120)
exp Anemia, Sickle Cell/ (26998)
exp Skin Diseases/ (1197204)
exp Genital Diseases, Female/ (490160)
exp menstruation disturbances/ (51427)
(pain* or headache*).mp. (971422)
(rheumat* or arthriti* or fibromyalgia).mp. (348433)
(cancer* or neoplas* or tumor* or tumour* or malignan* or carcinoma*).mp. (3603831)
diabet*.mp. (709575)
asthma*.mp. (213871)
(brain adj5 (trauma* or injur*)).mp. (124711)
(bowel* adj5 inflammatory adj5 (condition* or disease* or illness*)).mp. (43873)
(sickle cell adj5 (disease* or disorder* or anemia*)).mp. (26819)
((skin adj5 (disease* or disorder*)) or eczema*).mp. (145349)
((gynecologic* or gynaecologic*) adj5 (disease* or disorder*)).mp. (18632)
dysmenorrh*.mp. (9538)
endometriosis.mp. (27781)
Chronic Disease/ (149689)
((chronic* or longterm or long-term) adj5 (condition* or ill* or disease*)).mp. (512959)
or/18–43 (7140484)
random$.tw. (892218)
factorial$.tw. (23405)
crossover$.tw. (49675)
cross over$.tw. (22262)
cross-over$.tw. (22262)
placebo$.tw. (203743)
(doubl$ adj blind$).tw. (147609)
(singl$ adj blind$).tw. (14595)
assign$.tw. (241607)
allocat$.tw. (84725)
volunteer$.tw. (181433)
Crossover Procedure/ (39305)
double-blind procedure.tw. (219)
Randomized Controlled Trial/ (347009)
Single Blind Procedure/ (18444)
or/45–59 (1428276)
(animal/ or nonhuman/) not human/ (4623513)
60 not 61 (1262127)
7 and 12 and 17 and 44 and 62 (952)
(201203* or 201204* or 201205* or 201206* or 201207* or 201208* or 201209* or 201210* or 201011* or 201212* or 2013* or 2014*).dd. (3247786)
63 and 64 (263)
limit 65 to embase (204)
PsycINFO (OVID)
exp Psychotherapy/ (175503)
Problem Solving/ (22845)
psychotherap*.mp. (154000)
((cogniti* or family or behavior* or behaviour* or psychological*) adj5 (intervention* or treatment* or therap*)).mp. (139698)
(problem* adj5 solv*).mp. (55470)
CBT.mp. (7754)
or/1–6 (353096)
exp Parents/ (70852)
exp Family/ (37598)
Caregivers/ (18965)
(parent* or mother* or father* or family or families or caregiver* or care-giver*).mp. (493810)
or/8–11 (493876)
(child* or infant* or adolesc* or baby or babies or toddler* or teenager* or youth*).mp. (737112)
exp Pain/ (41502)
exp Rheumatoid Arthritis/ (1552)
exp Neoplasms/ (34777)
exp Diabetes Mellitus/ (3778)
exp Asthma/ (3706)
exp traumatic brain injury/ (11272)
exp Sickle Cell Disease/ (714)
exp skin disorders/ (3350)
exp gynecological disorders/ (1464)
(pain* or headache*).mp. (95554)
(rheumat* or arthriti* or fibromyalgia).mp. (7775)
(cancer* or neoplas* or tumor* or tumour* or malignan* or carcinoma*).mp. (54682)
diabet*.mp. (19914)
asthma*.mp. (6035)
(brain adj5 (trauma* or injur*)).mp. (20229)
(bowel* adj5 inflammatory adj5 (condition* or disease* or illness*)).mp. (524)
(sickle cell adj5 (disease* or disorder* or anemia*)).mp. (1016)
((skin adj5 (disease* or disorder*)) or eczema*).mp. (2250)
((gynecologic* or gynaecologic*) adj5 (disease* or disorder*)).mp. (569)
dysmenorrh*.mp. (335)
endometriosis.mp. (166)
((chronic* or longterm or long-term) adj5 (condition* or ill* or disease*)).mp. (36179)
or/14–35 (220042)
7 and 12 and 13 and 36 (3218)
clinical trials/ (7688)
(randomis* or randomiz*).tw. (48845)
(random$ adj3 (allocat$ or assign$)).tw. (30187)
((clinic$ or control$) adj trial$).tw. (41268)
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (19858)
(crossover$ or “cross over$”).tw. (7095)
random sampling/ (601)
Experiment Controls/ (727)
Placebo/ (3797)
placebo$.tw. (31173)
exp program evaluation/ (16454)
treatment effectiveness evaluation/ (16706)
((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. (59056)
or/38–50 (186590)
37 and 51 (314)
limit 52 to yr=“2012 -Current” (87)
Appendix 2. Search results, 2012
We conducted the initial search from inception to June 2012. We extracted a total of 114 papers to identify whether they met the full inclusion criteria; we found 107 papers in the initial search, and a further seven studies later in an updated search before publication. Of these 114 papers, we found 99 from the search of databases, six papers from the citation search, four papers from reference searches and five papers from authors of included studies. We deemed 35 studies (45 papers) to meet the inclusion criteria for the review, whilst we excluded 61 studies (69 papers).
DATA AND ANALYSES
Comparison 1. Painful Conditions Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Behaviour | 2 | 92 | Std. Mean Difference (IV, Random, 95% CI) | −0.34 [−1.18, 0.50] |
| 2 Child Behaviour/Disability | 7 | 457 | Std. Mean Difference (IV, Random, 95% CI) | −0.29 [−0.59, 0.02] |
| 3 Child Mental Health | 4 | 356 | Std. Mean Difference (IV, Random, 95% CI) | −0.02 [−0.35, 0.30] |
| 4 Child Symptoms | 9 | 540 | Std. Mean Difference (IV, Random, 95% CI) | −0.39 [−0.67, −0.11] |
Comparison 2. Painful Conditions Follow-up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Child Behaviour/Disability | 3 | 289 | Std. Mean Difference (IV, Random, 95% CI) | −0.17 [−0.52, 0.18] |
| 2 Child Mental Health | 2 | 255 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [−0.23, 0.26] |
| 3 Child Symptoms | 6 | 391 | Std. Mean Difference (IV, Random, 95% CI) | −0.38 [−0.86, 0.10] |
Comparison 3. Cancer Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Behaviour | 5 | 836 | Std. Mean Difference (IV, Random, 95% CI) | −0.20 [−0.36, −0.04] |
| 2 Parent Mental Health | 9 | 1010 | Std. Mean Difference (IV, Random, 95% CI) | −0.22 [−0.46, 0.01] |
Comparison 4. Cancer Follow-up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Behaviour | 5 | 789 | Std. Mean Difference (IV, Random, 95% CI) | −0.12 [−0.29, 0.05] |
| 2 Parent Mental Health | 6 | 819 | Std. Mean Difference (IV, Random, 95% CI) | −0.18 [−0.32, −0.04] |
Comparison 5. Diabetes Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Mental Health | 3 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [−0.08, 0.53] |
| 2 Child Behaviour/Disability | 3 | 329 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [−0.15, 0.28] |
| 3 Child Mental Health | 2 | 198 | Std. Mean Difference (IV, Random, 95% CI) | −0.08 [−0.63, 0.47] |
| 4 Child Symptoms | 9 | 774 | Std. Mean Difference (IV, Random, 95% CI) | −0.19 [−0.37, −0.01] |
| 5 Family Functioning | 5 | 422 | Std. Mean Difference (IV, Random, 95% CI) | −0.01 [−0.20, 0.18] |
Comparison 6. Diabetes Follow-up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Child Symptoms | 4 | 385 | Std. Mean Difference (IV, Random, 95% CI) | −0.25 [−0.45, −0.05] |
Comparison 7. Asthma Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Mental Health | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | −0.20 [−0.66, 0.26] |
| 2 Child Behaviour/Disability | 2 | 200 | Std. Mean Difference (IV, Random, 95% CI) | −0.87 [−2.02, 0.28] |
| 3 Child Symptoms | 4 | 337 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [−0.05, 0.38] |
Comparison 8. Asthma Follow-up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Child Symptoms | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | −0.16 [−0.72, 0.40] |
Comparison 9. Traumatic Brain Injury Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Mental Health | 2 | 72 | Std. Mean Difference (IV, Random, 95% CI) | −0.49 [−1.14, 0.16] |
| 2 Child Behaviour/Disability | 4 | 144 | Std. Mean Difference (IV, Random, 95% CI) | −0.27 [−0.64, 0.11] |
| 3 Family Functioning | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | −0.14 [−0.94, 0.67] |
Comparison 10. Cognitive Behavioural Therapy Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Behaviour | 4 | 166 | Std. Mean Difference (IV, Random, 95% CI) | −0.02 [−0.41, 0.38] |
| 2 Parent Mental Health | 7 | 325 | Std. Mean Difference (IV, Random, 95% CI) | −0.14 [−0.56, 0.28] |
| 3 Child Behaviour/Disability | 8 | 487 | Std. Mean Difference (IV, Random, 95% CI) | −0.21 [−0.51, 0.10] |
| 4 Child Mental Health | 5 | 439 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [−0.23, 0.29] |
| 5 Child Symptoms | 12 | 754 | Std. Mean Difference (IV, Random, 95% CI) | −0.32 [−0.53, −0.11] |
| 6 Family Functioning | 3 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [−0.22, 0.33] |
Comparison 11. Cognitive Behavioural Therapy Follow-up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Behaviour | 2 | 85 | Std. Mean Difference (IV, Random, 95% CI) | −0.28 [−1.26, 0.70] |
| 2 Parent Mental Health | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [−0.18, 0.82] |
| 3 Child Behaviour/Disability | 3 | 289 | Std. Mean Difference (IV, Random, 95% CI) | −0.17 [−0.52, 0.18] |
| 4 Child Mental Health | 2 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [−0.21, 0.28] |
| 5 Child Symptoms | 7 | 472 | Std. Mean Difference (IV, Random, 95% CI) | −0.34 [−0.73, 0.05] |
| 6 Family Functioning | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | −0.16 [−0.66, 0.35] |
Comparison 12. Family Therapy Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Mental Health | 3 | 131 | Std. Mean Difference (IV, Random, 95% CI) | −0.03 [−0.41, 0.35] |
| 2 Child Behaviour/Disability | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | −0.87 [−2.05, 0.31] |
| 3 Child Symptoms | 5 | 259 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [−0.20, 0.29] |
| 4 Family Functioning | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | −0.08 [−0.42, 0.26] |
Comparison 13. Family Therapy Follow-up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Child Symptoms | 2 | 96 | Std. Mean Difference (IV, Random, 95% CI) | −0.02 [−0.43, 0.38] |
Comparison 14. Problem Solving Therapy Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Behaviour | 5 | 832 | Std. Mean Difference (IV, Random, 95% CI) | −0.25 [−0.39, −0.11] |
| 2 Parent Mental Health | 7 | 907 | Std. Mean Difference (IV, Random, 95% CI) | −0.24 [−0.42, −0.05] |
| 3 Child Behaviour/Disability | 5 | 260 | Std. Mean Difference (IV, Random, 95% CI) | −0.17 [−0.45, 0.11] |
| 4 Child Symptoms | 2 | 216 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [−0.08, 0.46] |
| 5 Family Functioning | 3 | 183 | Std. Mean Difference (IV, Random, 95% CI) | −0.10 [−0.48, 0.27] |
Comparison 15. Problem Solving Therapy Follow-up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent Behaviour | 4 | 748 | Std. Mean Difference (IV, Random, 95% CI) | −0.15 [−0.31, 0.02] |
| 2 Parent Mental Health | 5 | 778 | Std. Mean Difference (IV, Random, 95% CI) | −0.19 [−0.34, −0.04] |
Comparison 16. Multisystemic Therapy Post-treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Child Behaviour/Disability | 2 | 313 | Std. Mean Difference (IV, Random, 95% CI) | −0.17 [−0.50, 0.17] |
| 2 Child Symptoms | 4 | 455 | Std. Mean Difference (IV, Random, 95% CI) | −0.24 [−0.56, 0.07] |
Comparison 17. Multisystemic therapy Follow-up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Child symptoms | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | −0.19 [−0.44, 0.06] |
Analysis 1.1.
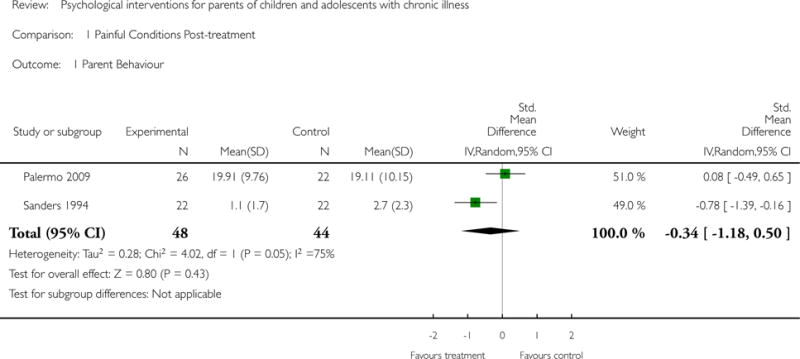
Comparison 1 Painful Conditions Post-treatment, Outcome 1 Parent Behaviour.
Analysis 1.2.
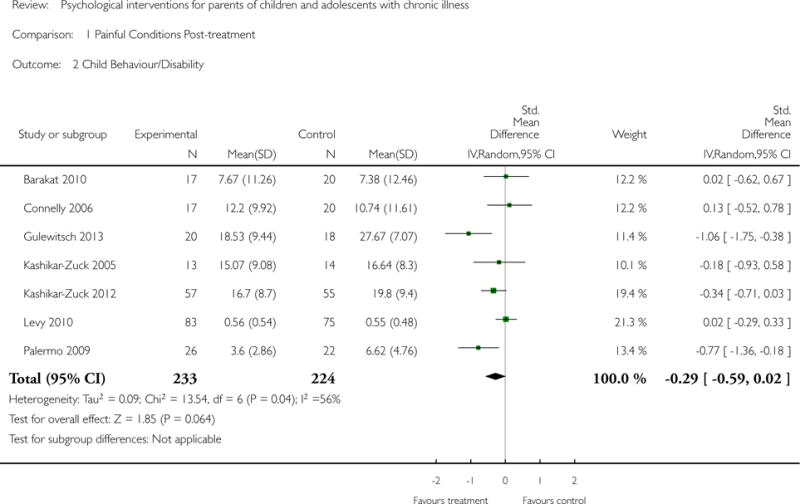
Comparison 1 Painful Conditions Post-treatment, Outcome 2 Child Behaviour/Disability.
Analysis 1.3.
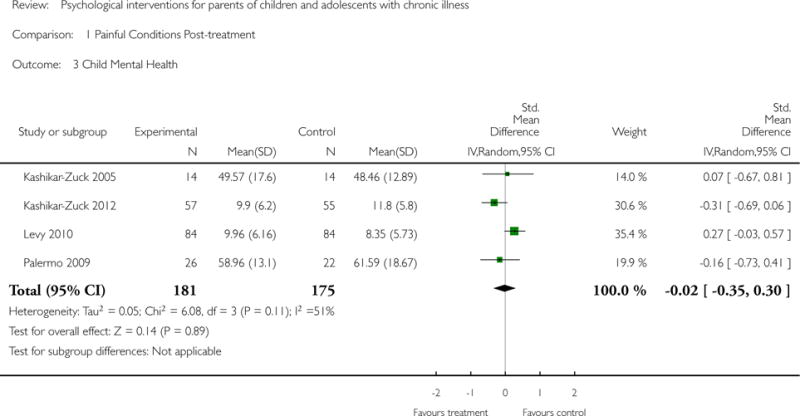
Comparison 1 Painful Conditions Post-treatment, Outcome 3 Child Mental Health.
Analysis 1.4.
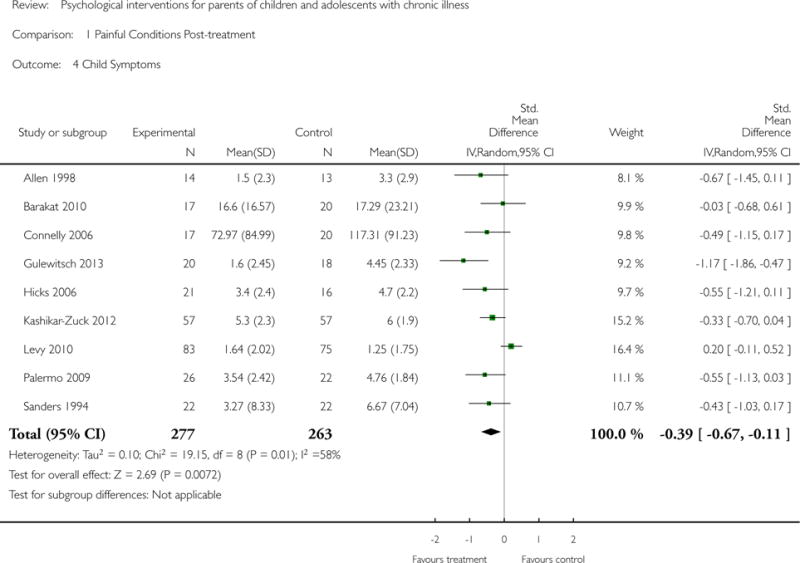
Comparison 1 Painful Conditions Post-treatment, Outcome 4 Child Symptoms.
Analysis 2.1.
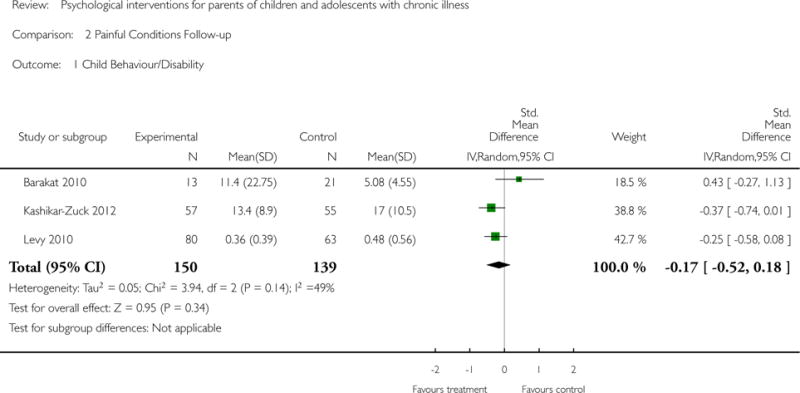
Comparison 2 Painful Conditions Follow-up, Outcome 1 Child Behaviour/Disability.
Analysis 2.2.
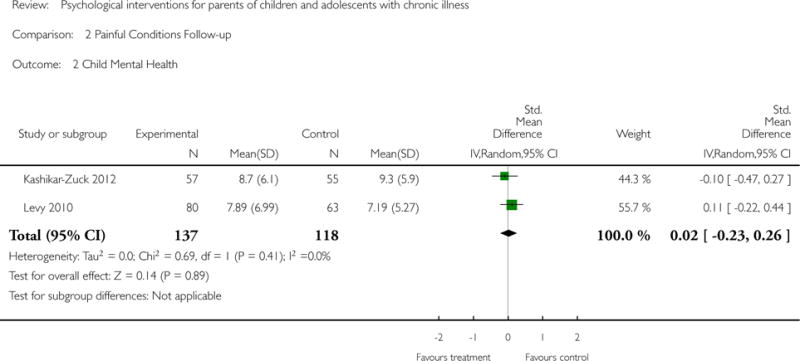
Comparison 2 Painful Conditions Follow-up, Outcome 2 Child Mental Health.
Analysis 2.3.
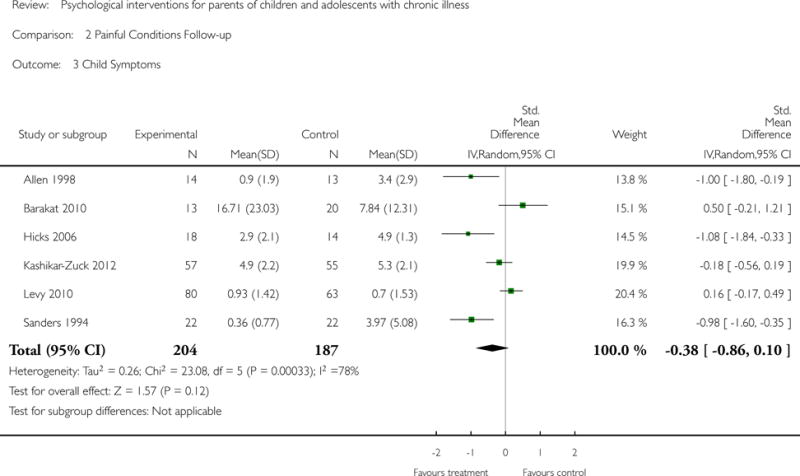
Comparison 2 Painful Conditions Follow-up, Outcome 3 Child Symptoms.
Analysis 3.1.
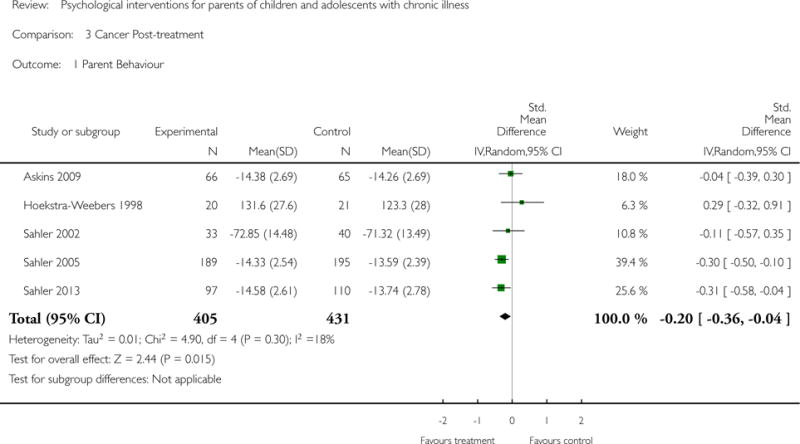
Comparison 3 Cancer Post-treatment, Outcome 1 Parent Behaviour.
Analysis 3.2.
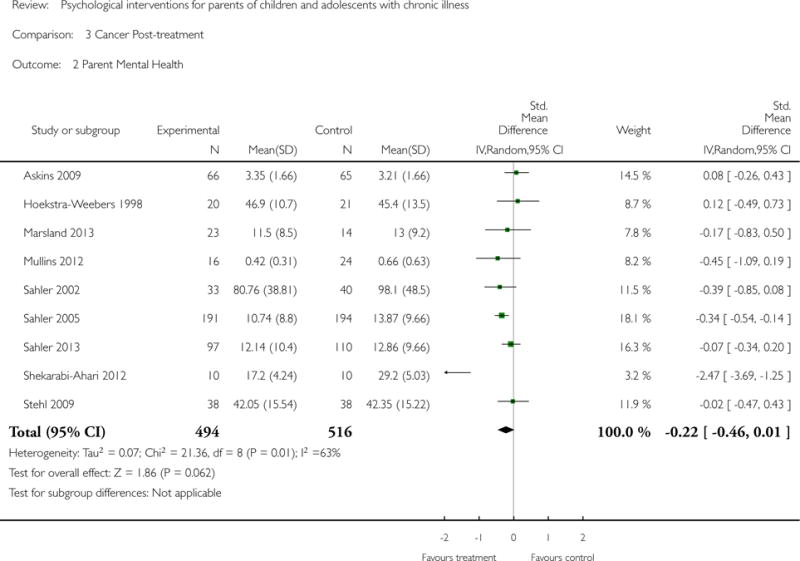
Comparison 3 Cancer Post-treatment, Outcome 2 Parent Mental Health.
Analysis 4.1.
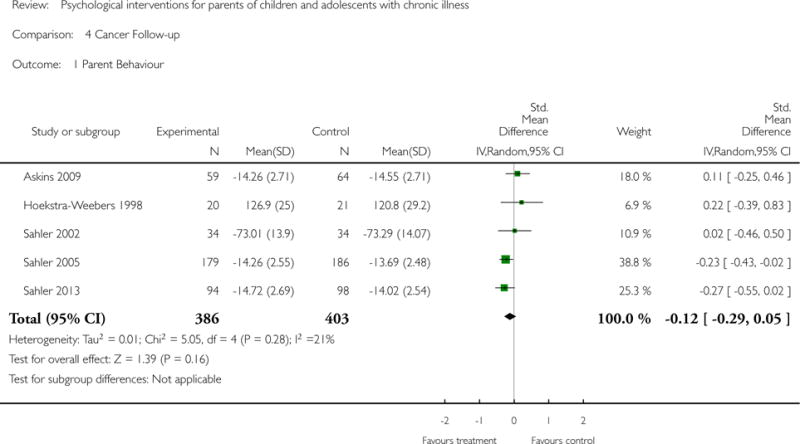
Comparison 4 Cancer Follow-up, Outcome 1 Parent Behaviour.
Analysis 4.2.
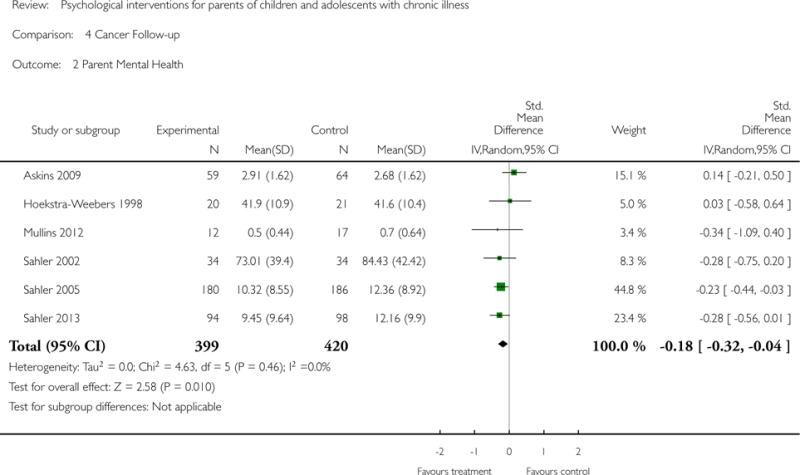
Comparison 4 Cancer Follow-up, Outcome 2 Parent Mental Health.
Analysis 5.1.
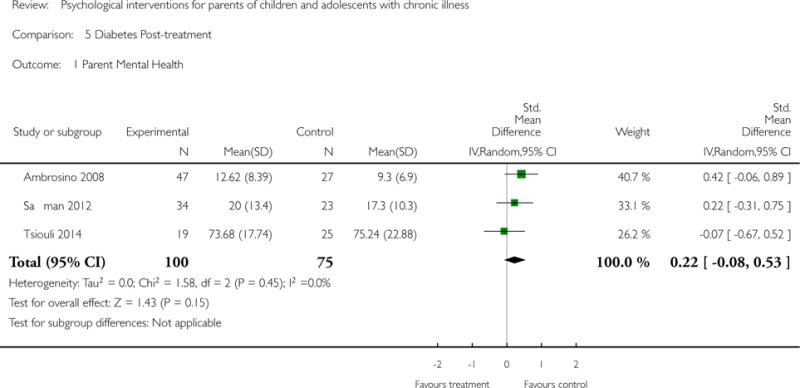
Comparison 5 Diabetes Post-treatment, Outcome 1 Parent Mental Health.
Analysis 5.2.
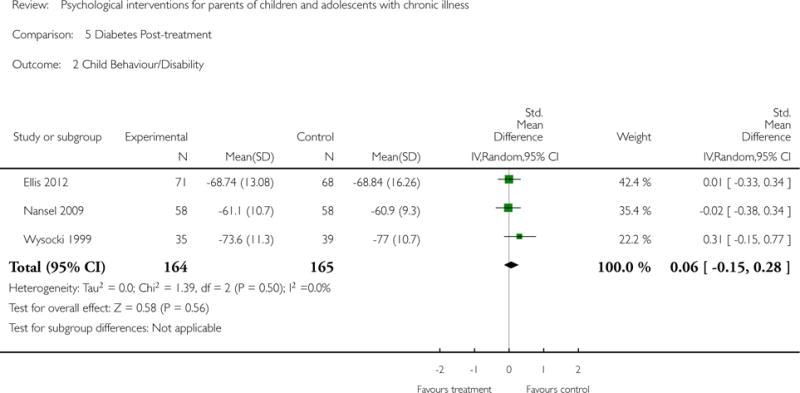
Comparison 5 Diabetes Post-treatment, Outcome 2 Child Behaviour/Disability.
Analysis 5.3.
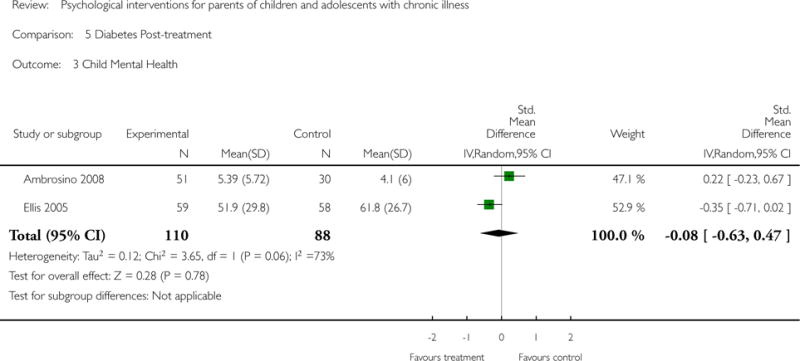
Comparison 5 Diabetes Post-treatment, Outcome 3 Child Mental Health.
Analysis 5.4.
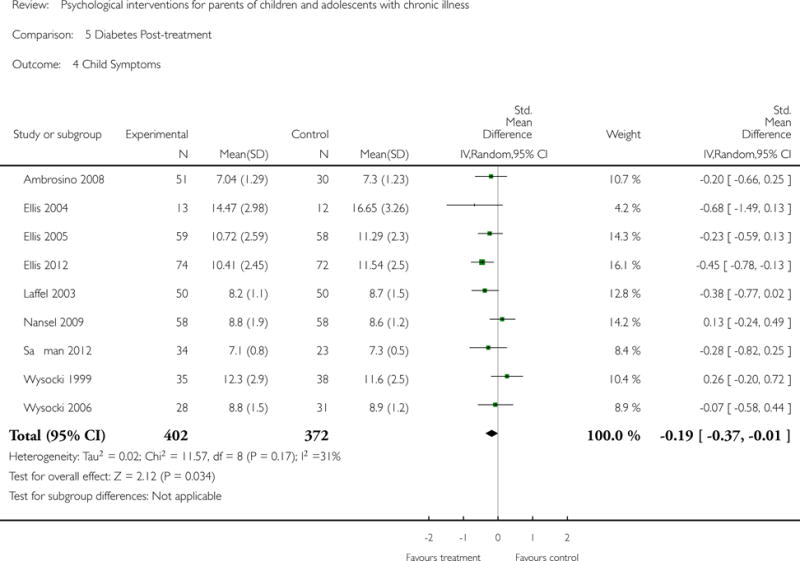
Comparison 5 Diabetes Post-treatment, Outcome 4 Child Symptoms.
Analysis 5.5.
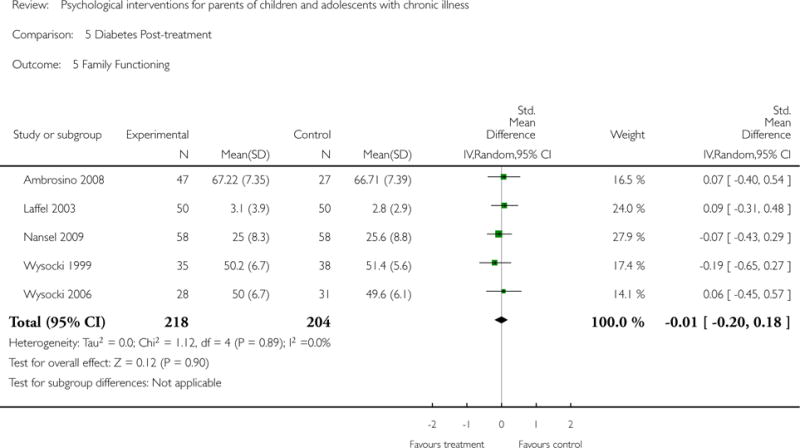
Comparison 5 Diabetes Post-treatment, Outcome 5 Family Functioning.
Analysis 6.1.
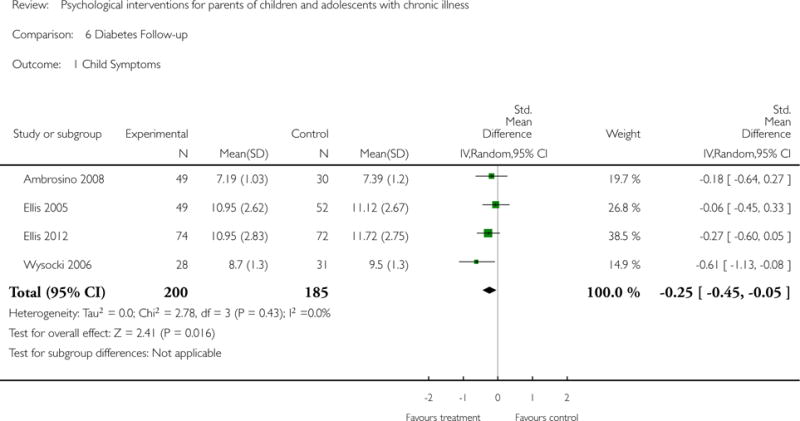
Comparison 6 Diabetes Follow-up, Outcome 1 Child Symptoms.
Analysis 7.1.
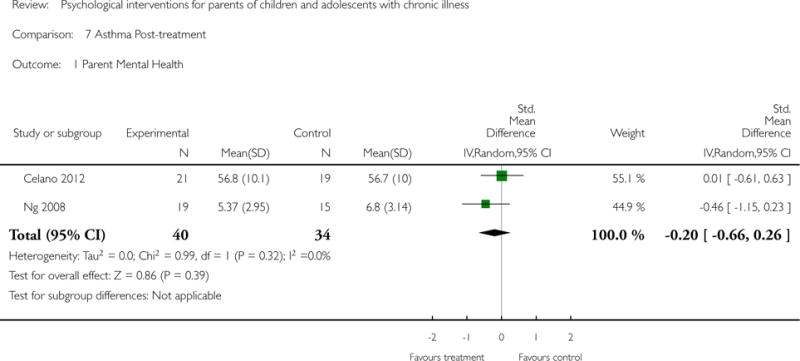
Comparison 7 Asthma Post-treatment, Outcome 1 Parent Mental Health.
Analysis 7.2.
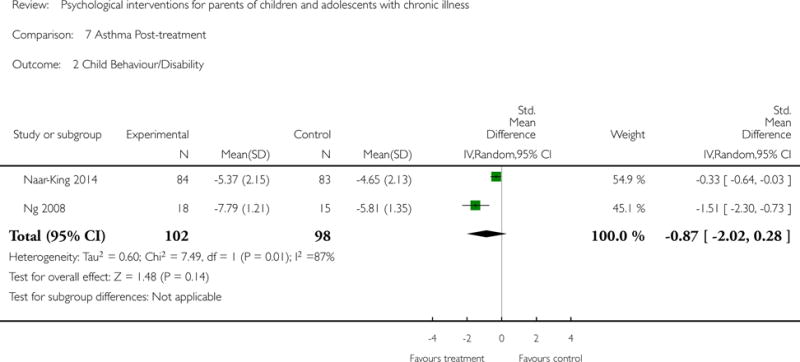
Comparison 7 Asthma Post-treatment, Outcome 2 Child Behaviour/Disability.
Analysis 7.3.
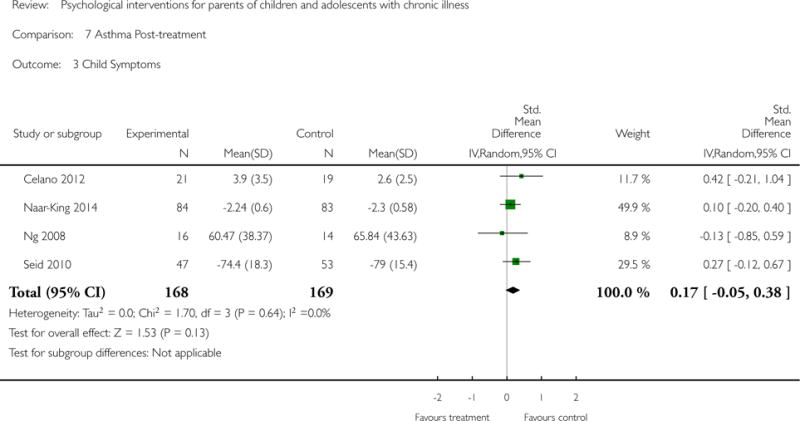
Comparison 7 Asthma Post-treatment, Outcome 3 Child Symptoms.
Analysis 8.1.
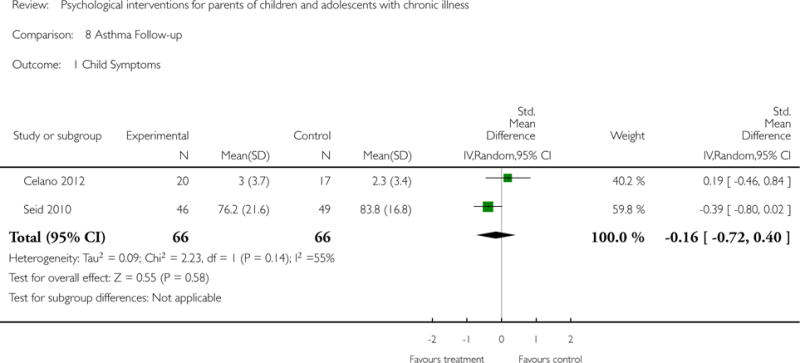
Comparison 8 Asthma Follow-up, Outcome 1 Child Symptoms.
Analysis 9.1.
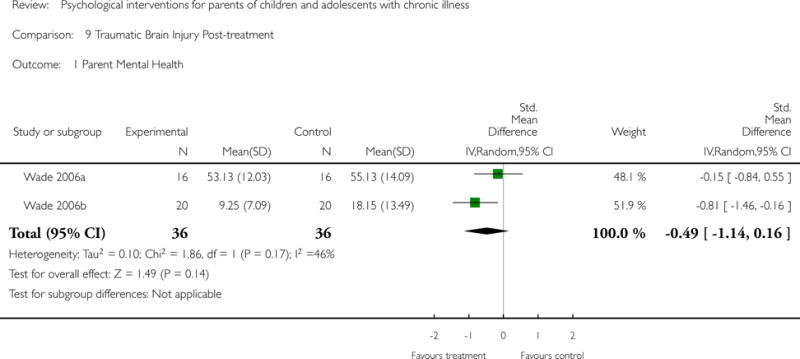
Comparison 9 Traumatic Brain Injury Post-treatment, Outcome 1 Parent Mental Health.
Analysis 9.2.
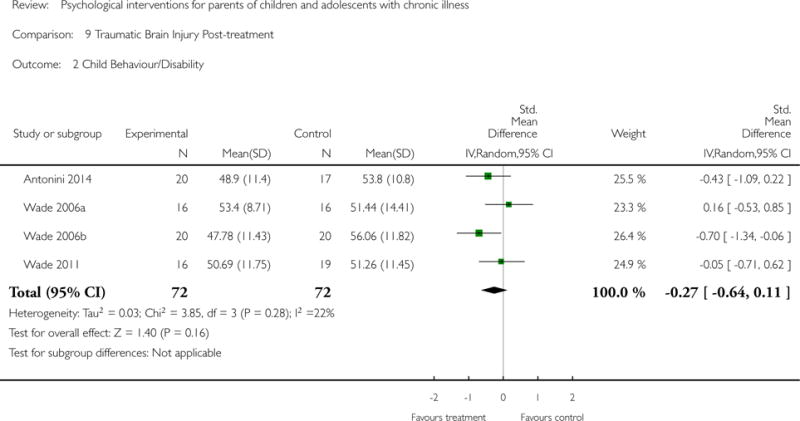
Comparison 9 Traumatic Brain Injury Post-treatment, Outcome 2 Child Behaviour/Disability.
Analysis 9.3.
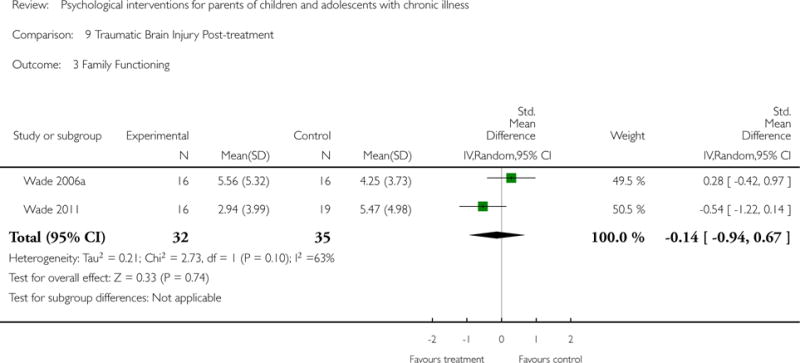
Comparison 9 Traumatic Brain Injury Post-treatment, Outcome 3 Family Functioning.
Analysis 10.1.
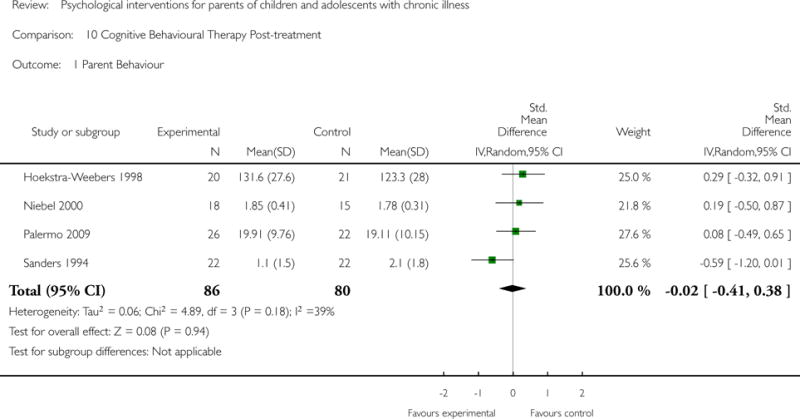
Comparison 10 Cognitive Behavioural Therapy Post-treatment, Outcome 1 Parent Behaviour.
Analysis 10.2.
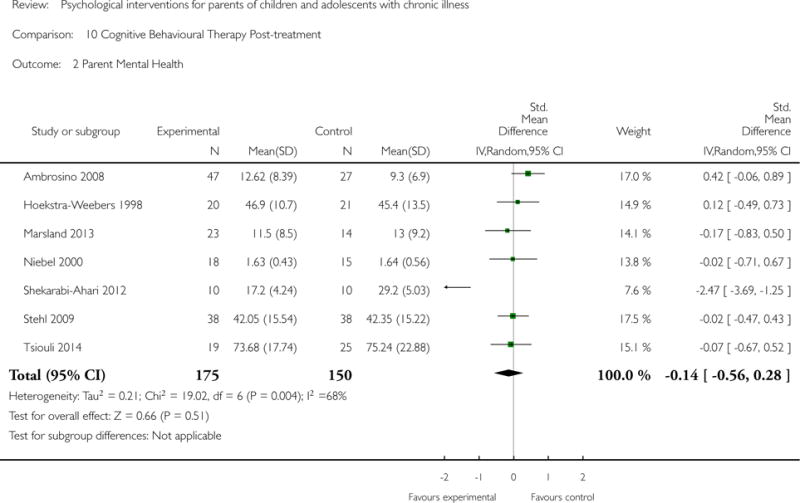
Comparison 10 Cognitive Behavioural Therapy Post-treatment, Outcome 2 Parent Mental Health.
Analysis 10.3.
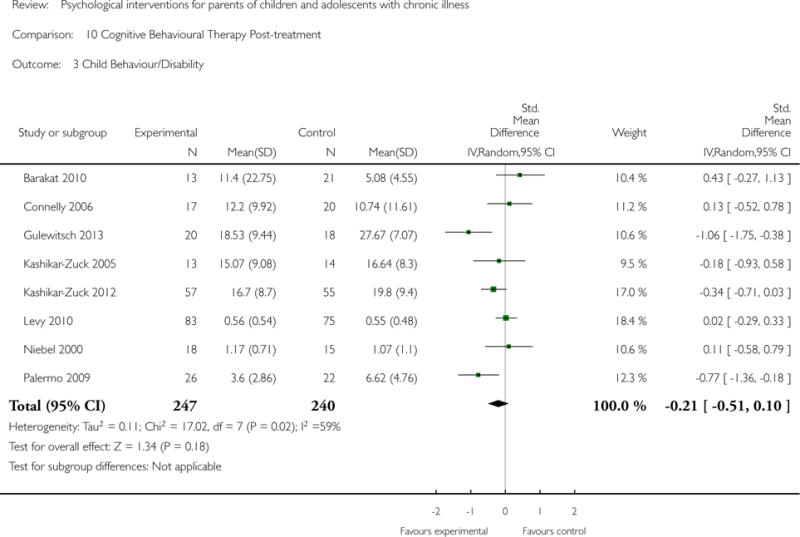
Comparison 10 Cognitive Behavioural Therapy Post-treatment, Outcome 3 Child Behaviour/Disability.
Analysis 10.4.
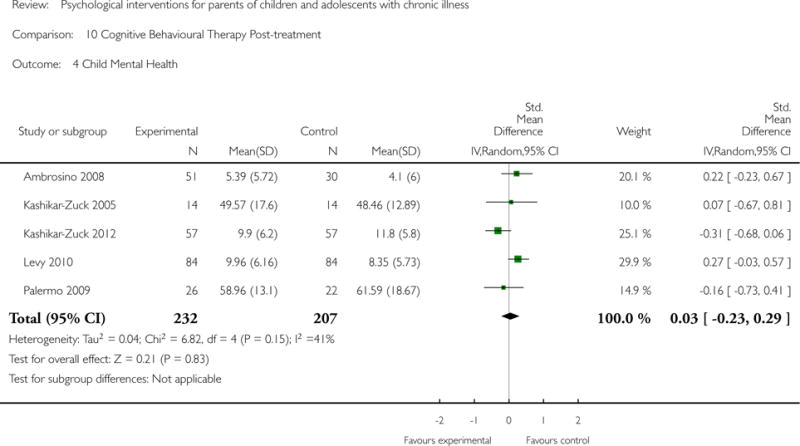
Comparison 10 Cognitive Behavioural Therapy Post-treatment, Outcome 4 Child Mental Health.
Analysis 10.5.
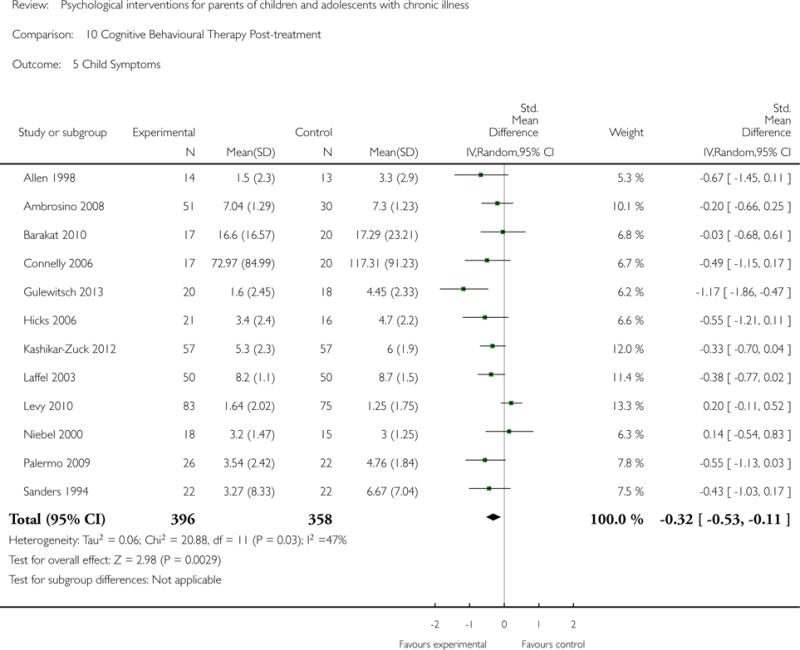
Comparison 10 Cognitive Behavioural Therapy Post-treatment, Outcome 5 Child Symptoms.
Analysis 10.6.
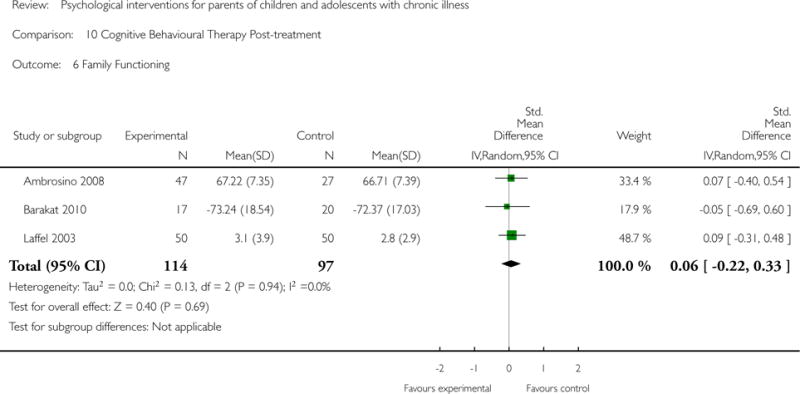
Comparison 10 Cognitive Behavioural Therapy Post-treatment, Outcome 6 Family Functioning.
Analysis 11.1.
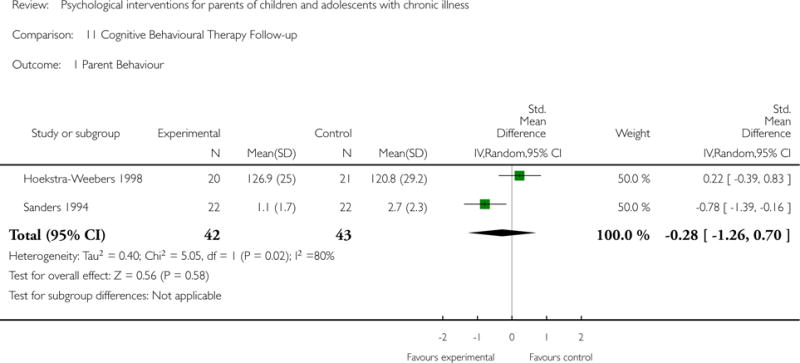
Comparison 11 Cognitive Behavioural Therapy Follow-up, Outcome 1 Parent Behaviour.
Analysis 11.2.
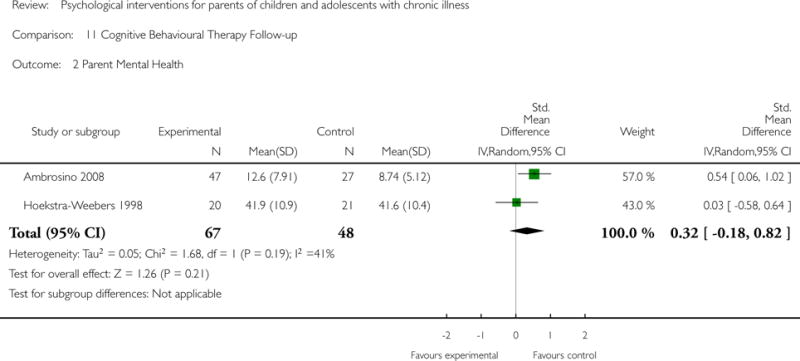
Comparison 11 Cognitive Behavioural Therapy Follow-up, Outcome 2 Parent Mental Health.
Analysis 11.3.
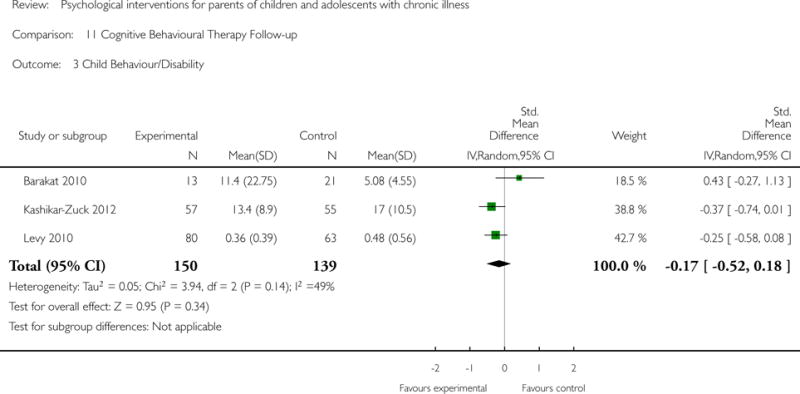
Comparison 11 Cognitive Behavioural Therapy Follow-up, Outcome 3 Child Behaviour/Disability.
Analysis 11.4.
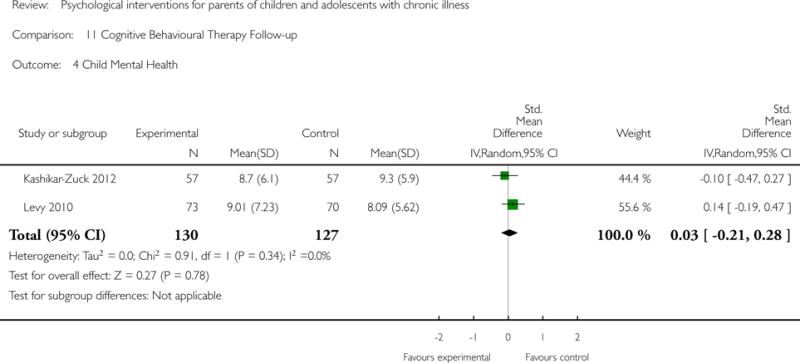
Comparison 11 Cognitive Behavioural Therapy Follow-up, Outcome 4 Child Mental Health.
Analysis 11.5.
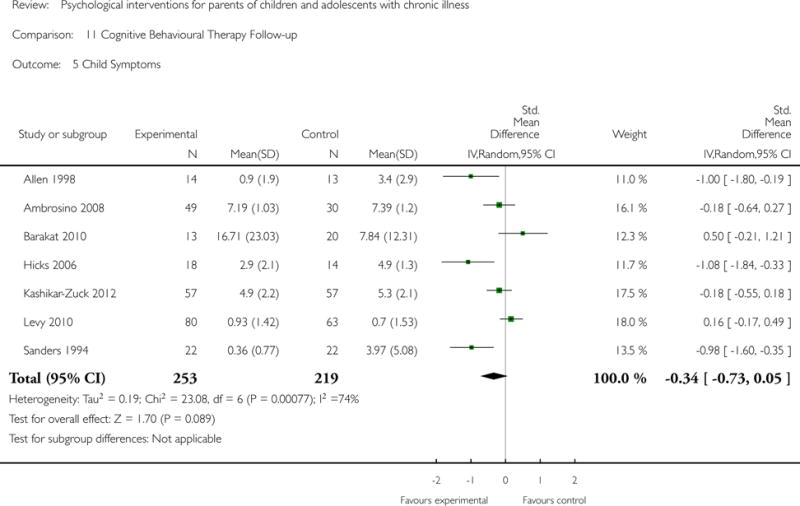
Comparison 11 Cognitive Behavioural Therapy Follow-up, Outcome 5 Child Symptoms.
Analysis 11.6.
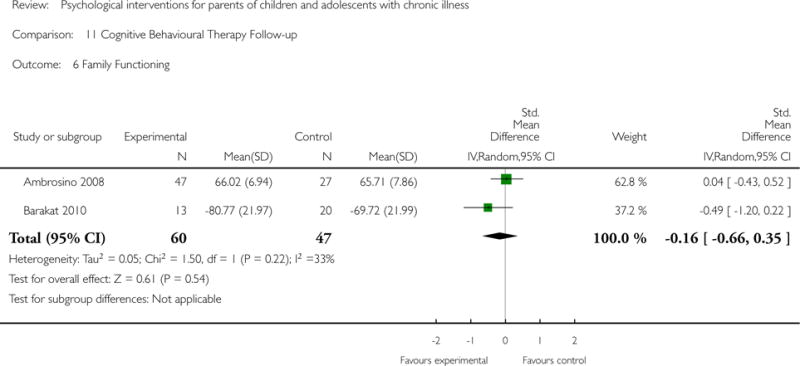
Comparison 11 Cognitive Behavioural Therapy Follow-up, Outcome 6 Family Functioning.
Analysis 12.1.
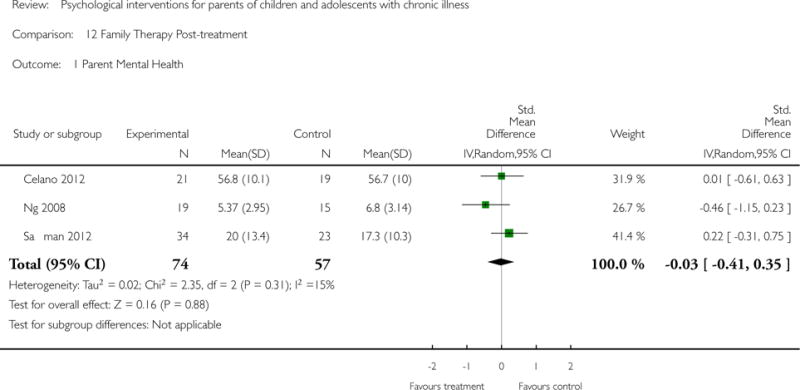
Comparison 12 Family Therapy Post-treatment, Outcome 1 Parent Mental Health.
Analysis 12.2.
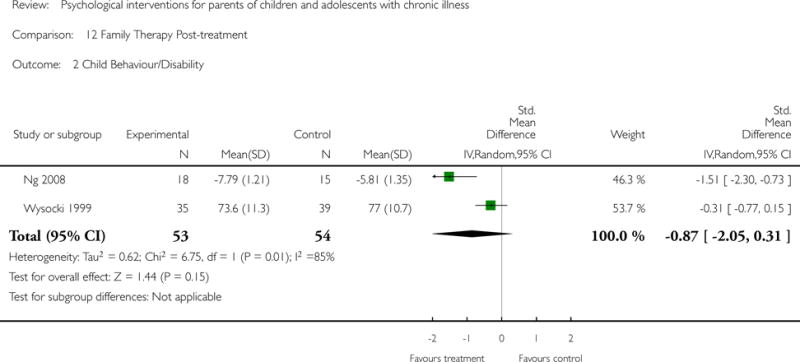
Comparison 12 Family Therapy Post-treatment, Outcome 2 Child Behaviour/Disability.
Analysis 12.3.
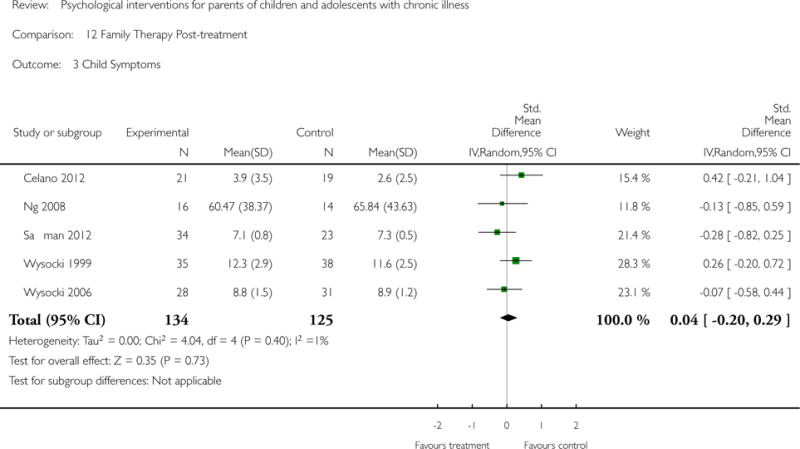
Comparison 12 Family Therapy Post-treatment, Outcome 3 Child Symptoms.
Analysis 12.4.
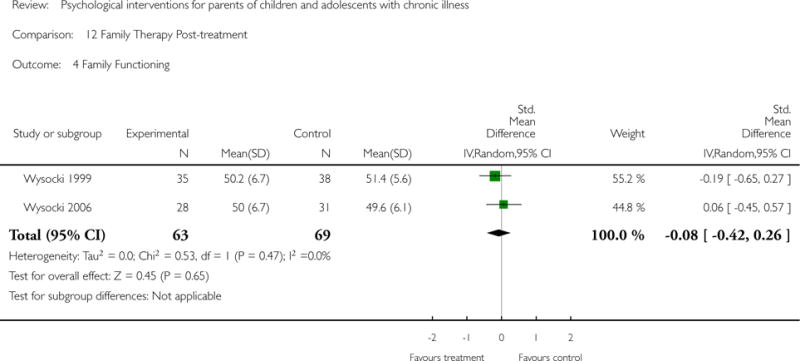
Comparison 12 Family Therapy Post-treatment, Outcome 4 Family Functioning.
Analysis 13.1.
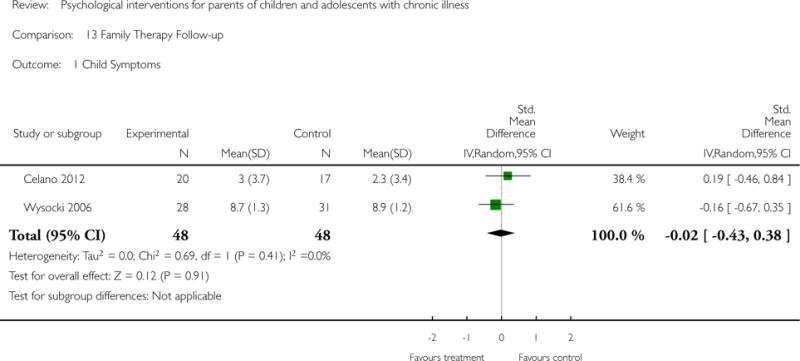
Comparison 13 Family Therapy Follow-up, Outcome 1 Child Symptoms.
Analysis 14.1.
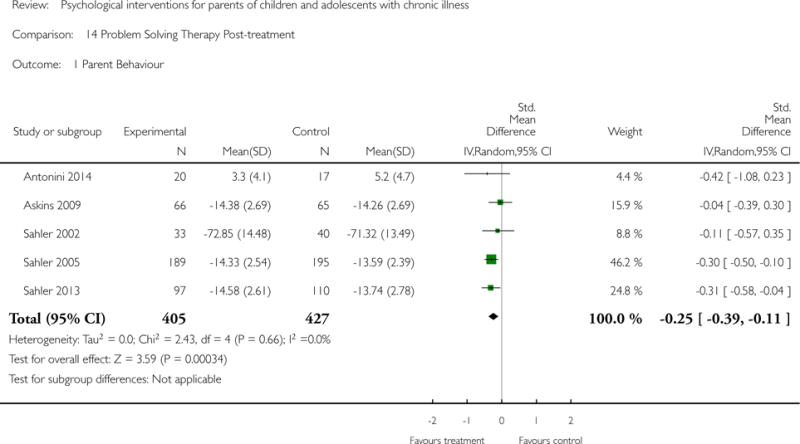
Comparison 14 Problem Solving Therapy Post-treatment, Outcome 1 Parent Behaviour.
Analysis 14.2.
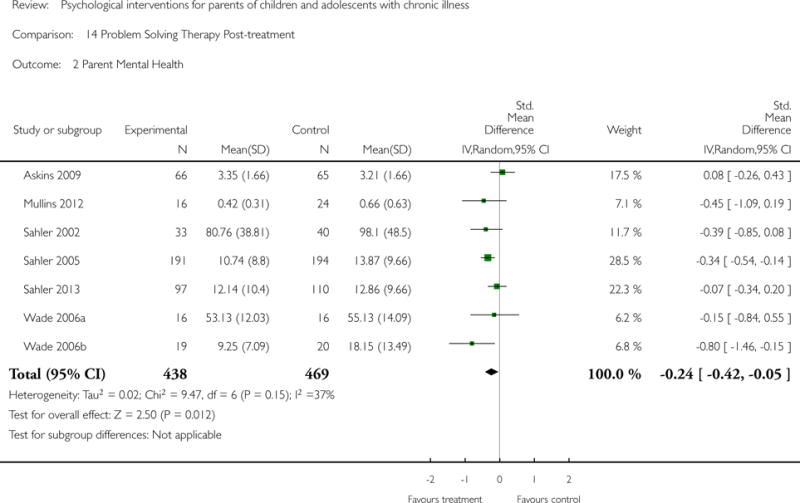
Comparison 14 Problem Solving Therapy Post-treatment, Outcome 2 Parent Mental Health.
Analysis 14.3.
Comparison 14 Problem Solving Therapy Post-treatment, Outcome 3 Child Behaviour/Disability.
Analysis 14.4.
Comparison 14 Problem Solving Therapy Post-treatment, Outcome 4 Child Symptoms.
Analysis 14.5.
Comparison 14 Problem Solving Therapy Post-treatment, Outcome 5 Family Functioning.
Analysis 15.1.
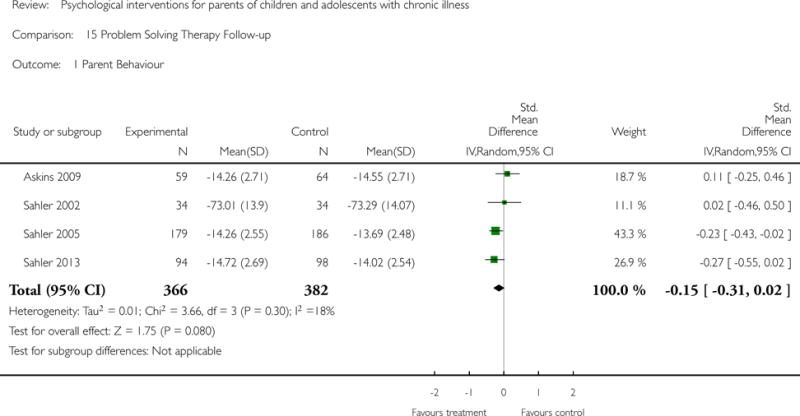
Comparison 15 Problem Solving Therapy Follow-up, Outcome 1 Parent Behaviour.
Analysis 15.2.
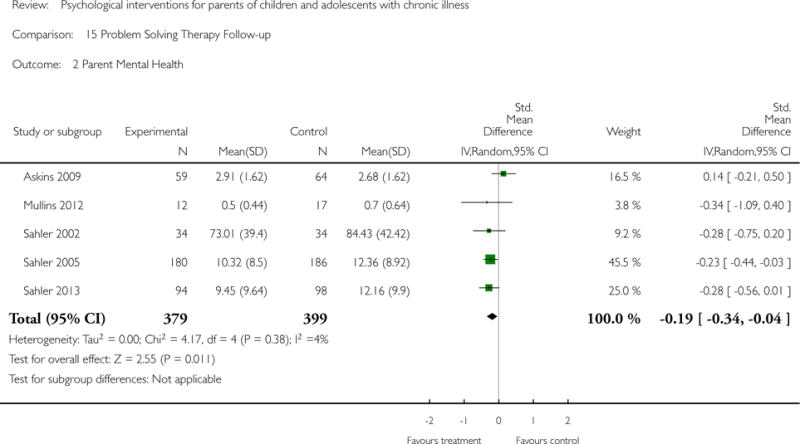
Comparison 15 Problem Solving Therapy Follow-up, Outcome 2 Parent Mental Health.
Analysis 16.1.
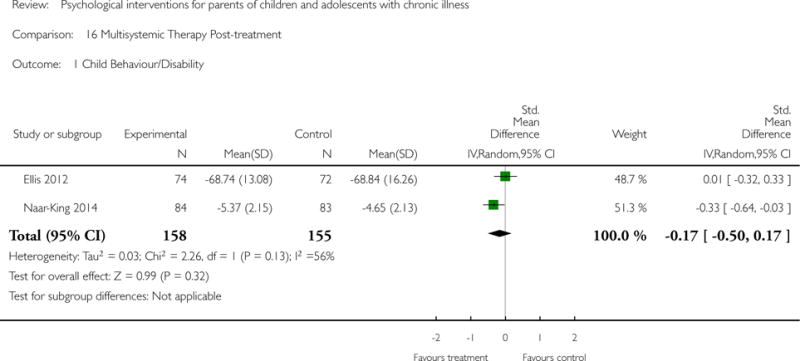
Comparison 16 Multisystemic Therapy Post-treatment, Outcome 1 Child Behaviour/Disability.
Analysis 16.2.
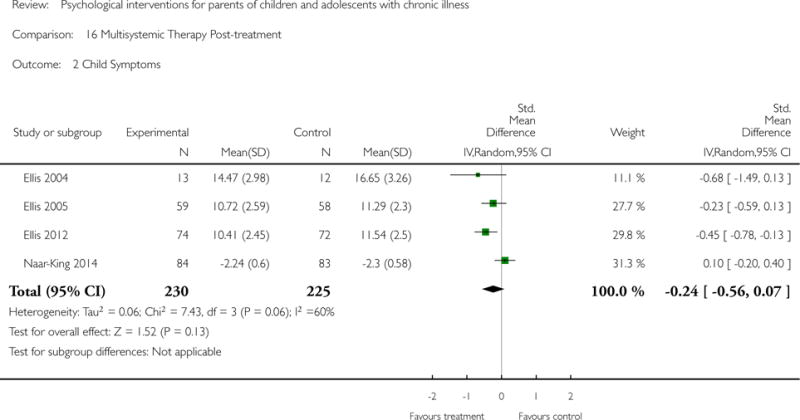
Comparison 16 Multisystemic Therapy Post-treatment, Outcome 2 Child Symptoms.
Analysis 17.1.
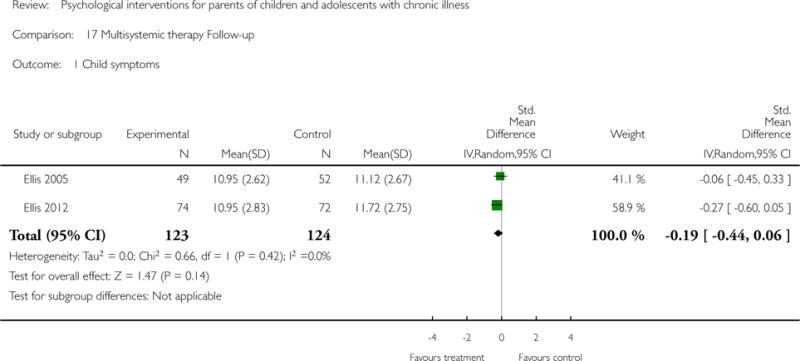
Comparison 17 Multisystemic therapy Follow-up, Outcome 1 Child symptoms.
Footnotes
CONTRIBUTIONS OF AUTHORS
CE oversaw authoring of the manuscript, arbitrated the selection of studies, interpreted the analyses, was responsible for the methodology, and will be responsible for updating the review in the future.
TP interpreted the analyses, drafted the final manuscript and will update the review in the future.
EF obtained studies, searched reference lists, selected studies for inclusion, extracted data and entered data into RevMan (RevMan 2014), interpreted the analyses and drafted the review.
EL selected the studies to include and extracted data, interpreted the analyses and drafted the review.
JB obtained studies, searched reference lists, selected studies for inclusion, extracted data, entered data into RevMan (RevMan 2014), interpreted the analyses, and drafted the review.
DECLARATIONS OF INTEREST
CE, EF, EL, JB, and TP have no relevant declarations of interest.
For transparency we declare that we have received research support from charity, government, and industry sources at various times, but none relate to this review.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
For the 2014 update, we have included GRADE assessments for the quality of evidence. We have removed concordance ratings and quality of evidence using the Yates scale, following Cochrane guidance (Higgins 2011).
- Language throughout the protocol has been altered to improve the flow and increase the accuracy.
- The tense of the language used in the methodology has been changed to past in line with Cochrane guidelines.
- Measures of treatment effect: this section has been added to provide a clearer description of intended analyses.
- The order of the four main analyses has been re-worded for a clearer understanding of the analysis plan. Parent outcomes have been listed before child outcomes as this is the focus of the review. Appendices were added for other search strategies.
- Assessment of risk of bias in included studies: this has been expanded to include a fuller description.
- Quality of trials (Yates 2005) has been deleted. Quality of evidence has now been included using GRADE ratings.
- Consistency between aims, measures, and results has been removed for this updated review.
REFERENCES
* Indicates the major publication for the study
References to studies included in this review
- Allen 1998 {published data only}.Allen KD, Shriver MD. Role of parent-mediated pain behavior management strategies in biofeedback treatment of childhood migraines. Behaviour Therapy. 1998;29:477–90. [Google Scholar]
- Ambrosino 2008 {published data only} *.Ambrosino JM, Fennie K, Whittemore R, Jaser S, Dowd MF, Grey M. Short-term effects of coping skills training in school-age children with type 1 diabetes. Pediatric Diabetes. 2008;9(3 Part 2):74–82. doi: 10.1111/j.1399-5448.2007.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey M, Whittermore R, Jaser S, Ambrosino J, Lindemann E, Liberti L, et al. Effects of coping skills training in school-age children with type 1 diabetes. Research in Nursing & Health. 2009;32(4):405–18. doi: 10.1002/nur.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini 2014 {published data only}.Antonini TN, Raj SP, Oberjohn KS, Cassedy A, Makoroff KL, Fouladi M, et al. Pilot randomized trial of an online parenting skills program for pediatric traumatic brain injury: improvements in parenting and child behavior. Behavior Therapy. 2014;45(4):455–68. doi: 10.1016/j.beth.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Askins 2009 {published data only}.Askins MA, Sahler OJ, Sherman SA, Fairclough DL, Butler RW, Katz ER, et al. Report from a multi-institutional randomized clinical trial examining computer-assisted problem-solving skills training for English- and Spanish-speaking mothers of children with newly diagnosed cancer. Journal of Pediatric Psychology. 2008;34(5):551–63. doi: 10.1093/jpepsy/jsn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat 2010 {published data only}.Barakat LP, Schwartz LA, Salaom KS, Radcliffe J. A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease. Journal of Pediatric Hematology Oncology. 2010;32(7):540–7. doi: 10.1097/MPH.0b013e3181e793f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry 1997 {published data only}.Barry J, Von Baeyer CL. Brief cognitive-behavioral group treatment for child’s headache. Clinical Journal of Pain. 1997;13(3):215–20. doi: 10.1097/00002508-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Celano 2012 {published data only}.Celano MP, Holsey CN, Kobrynski LJ. Home-based family intervention for low-income children with asthma: a randomized controlled pilot study. Journal of Family Psychology. 2012;26(2):171–8. doi: 10.1037/a0027218. [DOI] [PubMed] [Google Scholar]
- Connelly 2006 {published data only}.Connelly M, Rapoff MA, Thompson N, Connelly W. Headstrong: a pilot study of a CD-ROM intervention for recurrent pediatric headache. Journal of Pediatric Psychology. 2006;31(7):737–47. doi: 10.1093/jpepsy/jsj003. [DOI] [PubMed] [Google Scholar]
- Duarte 2006 {published data only}.Duarte MA, Penna FJ, Andrade EM, Cancela CS, Neto JC, Barbosa TF. Treatment of nonorganic recurrent abdominal pain: cognitive-behavioral family intervention. Journal of Pediatric Gastroenterology and Nutrition. 2006;43(1):59–64. doi: 10.1097/01.mpg.0000226373.10871.76. [DOI] [PubMed] [Google Scholar]
- Ellis 2004 {published data only}.Ellis DA, Naar-King S, Frey M, Templin T, Rowland M, Greger N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in poor metabolic control: a pilot investigation. Journal of Clinical Psychology in Medical Settings. 2004;11(4):315–24. [Google Scholar]
- Ellis 2005 {published data only} *.Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. The effects of multisystemic therapy on diabetes stress among adolescents with chronically poorly controlled type 1 diabetes: findings from a randomized, controlled trial. Pediatrics. 2005;116(6):e826–32. doi: 10.1542/peds.2005-0638. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control. Diabetes Care. 2005;28(7):1604–10. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Templin T, Naar-King S, Frey MA, Cunningham PB, Podolski CL, et al. Multisystemic therapy for adolescents with poorly controlled type 1 diabetes: stability of treatment effects in a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2007;75(1):168–74. doi: 10.1037/0022-006X.75.1.168. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Yopp J, Templin T, Naar-King S, Frey MA, Cunningham PB, et al. Family mediators and moderators of treatment outcomes among youths with poorly controlled type 1 diabetes: results from a randomized controlled trial. Journal of Pediatric Psychology. 2007;32(2):194–205. doi: 10.1093/jpepsy/jsj116. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Ellis DA, Idalski A, Frey MA, Cunningham P. Multisystemic therapy decreases parental overestimation of adolescent responsibility for type 1 diabetes management in urban youth. Families, Systems & Health. 2007;25(2):178–89. [Google Scholar]
- Ellis 2012 {published data only}.Ellis DA, Naar-King S, Chen X, Moltz K, Cunningham P, Idalski-Carcone A. Multisystemic therapy compared to telephone support for youth with poorly controlled diabetes: findings from a randomized controlled trial. Annals of Behavioural Medicine. 2012;44(2):207–15. doi: 10.1007/s12160-012-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulewitsch 2013 {published data only}.Gulewitsch MD, Müller J, Hautzinger M, Schlarb AA. Brief hypnotherapeutic-behavioural intervention for functional abdominal pain and irritable bowel syndrome in childhood: a randomized controlled trial. European Journal of Pediatrics. 2013;172(8):1043–51. doi: 10.1007/s00431-013-1990-y. [DOI] [PubMed] [Google Scholar]
- Hicks 2006 {published data only}.Hicks CL, Von Baeyer CL, McGrath PJ. Online psychological treatment for pediatric recurrent pain: a randomized evaluation. Journal of Pediatric Psychology. 2006;31(7):724–36. doi: 10.1093/jpepsy/jsj065. [DOI] [PubMed] [Google Scholar]
- Hoekstra-Weebers 1998 {published data only}.Hoekstra-Weebers JE, Heuvel F, Jaspers JP, Kamps WA, Klip EC. Brief report: an intervention program for parents of pediatric cancer patients: a randomized controlled trial. Journal of Pediatric Psychology. 1998;23(3):207–14. doi: 10.1093/jpepsy/23.3.207. [DOI] [PubMed] [Google Scholar]
- Kashikar-Zuck 2005 {published data only}.Kashikar-Zuck S, Swain N, Jones BA, Graham TB. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. Journal of Rheumatology. 2005;32(8):1594–602. [PubMed] [Google Scholar]
- Kashikar-Zuck 2012 {published data only}.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia. Arthritis and Rheumatism. 2012;64(1):297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak 2004 {published data only}.Kazak AE, Alderfer MA, Streisand R, Simms S, Rourke MT, Barakat LP, et al. Treatment of posttraumatic stress symptoms in adolescent survivors of childhood cancer and their families: a randomized clinical trial. Journal of Family Psychology. 2004;18(3):493–504. doi: 10.1037/0893-3200.18.3.493. [DOI] [PubMed] [Google Scholar]
- Laffel 2003 {published data only}.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. Journal of Pediatrics. 2013;142(4):409–16. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- Lask 1979 {published data only}.Lask B, Matthew D. Childhood asthma. A controlled trial of family psychotherapy. Archives of Disease in Childhood. 1979;54(2):116–9. doi: 10.1136/adc.54.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmkuhl 2010 {published data only}.Lehmkuhl HD, Storch EA, Cammarata C, Meyer K, Rahman O, Silverstein J, et al. Telehealth behavior therapy for the management of type 1 diabetes in adolescents. Journal of Diabetes Science and Technology. 2010;4(1):199–208. doi: 10.1177/193229681000400125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy 2010 {published data only} *.Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, et al. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. American Journal of Gastroenterology. 2010;105(4):946–56. doi: 10.1038/ajg.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, et al. Twelve-month follow-up of cognitive behavioural therapy for children with functional abdominal pain. JAMA Pediatric. 2013;167(2):178–84. doi: 10.1001/2013.jamapediatrics.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland 2013 {published data only}.Marsland AL, Long KA, Howe C, Thompson AL, Tersak J, Ewing LW. A pilot trial of a stress management intervention for primary caregivers of children newly diagnosed with cancer: preliminary evidence that perceived social support moderates the psychosocial benefit of intervention. Journal of Pediatric Psychology. 2013;38(4):449–61. doi: 10.1093/jpepsy/jss173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins 2012 {published data only}.Mullins LL, Fedele DA, Chaffin M, Hullmann SE, Kenner C, Eddington AR, et al. A clinic-based interdisciplinary intervention for mothers of children newly diagnosed with cancer: a pilot study. Journal of Pediatric Pscyhology. 2012;37(10):1104–15. doi: 10.1093/jpepsy/jss093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar-King 2014 {published data only}.Naar-King S, Ellis D, King PS, Lam P, Cunningham P, Secord E, et al. Multisystemic therapy for high-risk African American adolescents with asthma: a randomized clinical trial. Journal of Consulting and Clinical Psychology. 2014;82(3):536–45. doi: 10.1037/a0036092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel 2009 {published data only}.Nansel TR, Anderson BJ, Laffel LM, Simons-Morton BG, Weissberg-Benchell J, Wysocki T, et al. A multi-site trial of a clinic-integrated intervention for promoting family management of pediatric type 1 diabetes: feasibility and design. Pediatric Diabetes. 2009;10(2):105–15. doi: 10.1111/j.1399-5448.2008.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel 2012 {published data only}.Nansel TR, Iannotti RJ, Liu A. Clinic-integrated behavioural intervention for families of youth with type 1 diabetes: randomized clinical trial. Pediatrics. 2012;129(4):1–8. doi: 10.1542/peds.2011-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng 2008 {published data only}.Ng SM, Li AM, Lou VW, Tso IF, Wan PY, Chan DF. Incorporating family therapy into asthma group intervention: a randomized waitlist-controlled trial. Family Process. 2008;47(1):115–30. doi: 10.1111/j.1545-5300.2008.00242.x. [DOI] [PubMed] [Google Scholar]
- Niebel 2000 {published data only}.Niebel G, Kallweit C, Lange I, Fölster-Holst R. Direct versus video-aided parent education in atopic eczema in childhood as a supplement to specialty physician treatment. A controlled pilot study [Direkte versus videover–mittelte elternschulung bei atopishchem ekzem in kinderslater als erganzung facharztlicher behandlung: eine kontrollierte pilotstudie] Hautarzt. 2000;51(6):401–11. doi: 10.1007/s001050051141. [DOI] [PubMed] [Google Scholar]
- Olivares 1997 {published data only}.Olivares J, Mendez FX, Ros M, Bermejo RM. Effects of a training program for parents with diabetic children with obstacles to comply with therapy [El cuidado de la diabetes mellitus insulino–dependiente: Efectos de un programa de modificacion de conducta en padres] Psicologia Conductual. 1997;5(2):219–35. [Google Scholar]
- Palermo 2009 {published data only}.Palermo TM, Wilson AC, Peters M, Lweandowski A, Somhegyi H. Randomized controlled trial of an Internet delivered family cognitive behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146(1–2):205–13. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins 2005 {published data only}.Robins PM, Smith SM, Glutting JJ, Bishop CT. A randomized controlled trial of a cognitive-behavioral family intervention for pediatric recurrent abdominal pain. Journal of Pediatric Psychology. 2005;30(5):397–408. doi: 10.1093/jpepsy/jsi063. [DOI] [PubMed] [Google Scholar]
- Sahler 2002 {published data only}.Sahler OJ, Varni JW, Fairclough DL, Butler RW, Noll RB, Dolgin MJ, et al. Problem-solving skills training for mothers of children with newly diagnosed cancer: a randomized trial. Developmental and Behavioural Pediatrics. 2002;23(2):77–86. doi: 10.1097/00004703-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Sahler 2005 {published data only}.Sahler OJ, Fairclough DL, Phipps S, Mulhern RK, Dolgin MJ, Noll RB, et al. Using problem-solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: Report of a multisite randomized trial. Journal of Consulting and Clinical Psychology. 2005;73(2):272–83. doi: 10.1037/0022-006X.73.2.272. [DOI] [PubMed] [Google Scholar]
- Sahler 2013 {published data only}.Sahler OJ, Dolgin MJ, Phipps S, Fairclough DL, Askins MA, Katz ER, et al. Specificity of problem solving skills training in mothers of children newly diagnosed with cancer: results of a multisite randomized clinical trial. Journal of Clinical Oncology. 2013;31(10):1329–35. doi: 10.1200/JCO.2011.39.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders 1994 {published data only}.Sandes MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. Journal of Consulting and Clinical Psychology. 1994;62(2):306–14. doi: 10.1037//0022-006x.62.2.306. [DOI] [PubMed] [Google Scholar]
- Saßman 2012 {published data only}.Saßmann H, Hair M, Danne T, Lange K. Reducing stress and supporting positive relations in families of young children with type 1 diabetes: A randomized controlled study for evaluating the effects of the DELFIN parenting program. BMC Pediatrics. 2012;12(152):2–11. doi: 10.1186/1471-2431-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seid 2010 {published data only}.Seid M, Varni JW, Gidwani P, Gelhard LR, Slymen DJ. Problem-solving skills training for vulnerable families of children with persistent asthma: report of a randomized trial on health-related quality of life outcomes. Journal of Pediatric Psychology. 2010;35(10):1133–43. doi: 10.1093/jpepsy/jsp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekarabi-Ahari 2012 {published data only}.Shekarabi-Ahari G, Younesi J, Borjali A, Ansari-Damavandi S. The effectiveness of group hope therapy on hope and depression of mothers with children suffering from cancer in Tehran. Iranian Journal of Cancer Prevention. 2012;5(4):183–8. [PMC free article] [PubMed] [Google Scholar]
- Stark 2005 {published data only}.Stark LJ, Davis AM, Janicke DM, Mackner LM, Hommel KA, Bean JA, et al. A randomized clinical trial of dietary calcium to improve bone accretion in children with juvenile rheumatoid arthritis. Pediatrics. 2006;148(4):501–7. doi: 10.1016/jpeds.2005.11.043. [DOI] [PubMed] [Google Scholar]
- *.Stark LJ, Janicke D, McGrath AM, Mackner L, Hommel KA, Lovell D. Prevention of osteoporosis: a randomized clinical trial to increase calcium intake in children with juvenile rheumatoid arthritis. Journal of Pediatric Psychology. 2005;30(5):377–86. doi: 10.1093/jpepsy/jsi061. [DOI] [PubMed] [Google Scholar]
- Stehl 2009 {published data only}.Stehl ML, Kazak AE, Alderfer MA, Rodriguez A, Hwang WT, Pai AL, et al. Conducting a randomized clinical trial of a psychological intervention for parents/caregivers of children with cancer shortly after diagnosis. Journal of Pediatric Psychology. 2009;34(8):803–16. doi: 10.1093/jpepsy/jsn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiouli 2014 {published data only}.Tsiouli E, Pavlopoulos V, Alexopoulos EC, Chrousos G, Darviri C. Short-term impact of a stress management and health promotion program on perceived stress, parental stress, health locus of control and cortisol levels in parents of children and adolescents with diabetes type 1: a pilot randomized controlled trial. Explore. 2014;10(2):88–98. doi: 10.1016/j.explore.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Wade 2006a {published data only}.Wade SL, Michaud L, Brown TM. Putting the pieces together: preliminary efficacy of a family problem-solving intervention for children with traumatic brain injury. Journal of Head Trauma Rehabilitation. 2006;21(1):59–67. doi: 10.1097/00001199-200601000-00006. [DOI] [PubMed] [Google Scholar]
- Wade 2006b {published data only}.Wade SL, Carey J, Wolfe CR. An online family intervention to reduce parental distress following pediatric brain injury. Journal of Consulting and Clinical Psychology. 2006;74(3):445–54. doi: 10.1037/0022-006X.74.3.445. [DOI] [PubMed] [Google Scholar]
- *.Wade SL, Carey J, Wolfe CR. The efficacy of an online cognitive-behavioral family intervention in improving child behavior and social competence following pediatric brain injury. Rehabilitation Psychology. 2006;51(3):179–89. [Google Scholar]
- Wade 2011 {published data only} *.Wade SL, Walz NC, Carey J, McMullen KM, Cass J, Mark E, et al. Effect on behavior problems of teen online problem-solving for adolescent traumatic brain injury. Pediatrics. 2011;128(4):e947–53. doi: 10.1542/peds.2010-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SL, Walz NC, Carey J, McMullen KM, Cass J, Mark E, et al. A randomized trial of teen online problem solving: efficacy in improving caregiver outcomes after brain injury. Health psychology. 2012;31(6):767–76. doi: 10.1037/a0028440. [DOI] [PubMed] [Google Scholar]
- Wysocki 1999 {published data only}.Wysocki T, Greco P, Harris MA, Bubb J, White NH. Behavior therapy for families of adolescents with diabetes: maintenance of treatment effects. Diabetes Care. 2001;24(3):441–6. doi: 10.2337/diacare.24.3.441. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, et al. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. Journal of Pediatric Psychology. 2000;25(1):23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]
- *.Wysocki T, Miller KM, Greco P, Harris MA, Harvey LM, Taylor A, et al. Behavior therapy for families of adolescents with diabetes: effects on directly observed family interactions. Behavior Therapy. 1999;30:507–25. [Google Scholar]
- Wysocki 2006 {published data only}.Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Mauras N, et al. Randomized trial of behavioral family systems therapy for diabetes: maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007;30(3):555–60. doi: 10.2337/dc06-1613. [DOI] [PubMed] [Google Scholar]
- *.Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. Journal of Pediatric Psychology. 2006;31(9):928–38. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Randomized, controlled trial of behavioral family systems therapy for diabetes: maintenance and generalization of effects on parent-adolescent communication. Behavior Therapy. 2008;39:33–46. doi: 10.1016/j.beth.2007.04.001. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Aleman 1992 {published data only}.Aleman Mendez S, Palacios AS. An integrated approach to the psychological features of the asthmatic child [Un adordaje integral de los aspectos psicologicos del niño asmatico] Allergologia et Immunopathologia. 1992;20(6):240–5. [PubMed] [Google Scholar]
- Anderson 1999 {published data only}.Anderson BJ, Ho J, Brackett J, Laffel LMB. An office-based intervention to maintain parent-adolescent teamwork in diabetes management: impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22(7):713–21. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- Bellin 2013 {published data only}.Bellin MH, Kub J, Frick KD, Bollinger ME, Tsoukleris M, Walker J, et al. Stress and quality of life in caregivers of inner-city minority children with poorly controlled asthma. Journal of Pediatric Health Care. 2013;27(2):127–34. doi: 10.1016/j.pedhc.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt 2004 {published data only}.Betancourt GP, Gutierrez de Pineres Scarpetta C. Psychological intervention pre-postsurgical program for cardiovascular pediatric patients. Saludarte. 2004;3(11):19–34. [Google Scholar]
- Borhani 2011 {published data only}.Borhani F, Najafi MK, Rabori ED, Sabzevari S. The effect of family-centred empowerment model on quality of life of school-aged children with thalassemia major. Iranian Journal of Nursing Midwifery Research. 2011;16(4):292–8. [PMC free article] [PubMed] [Google Scholar]
- Braga 2005 {published data only}.Braga L, Da Paz A, Jr, Ylvisaker M. Direct clinician-delivered versus indirect family-supported rehabilitation of children with traumatic brain injury: a randomized controlled trial. Brain Injury. 2005;19(10):819–31. doi: 10.1080/02699050500110165. [DOI] [PubMed] [Google Scholar]
- Bruzzese 2008 {published data only}.Bruzzese J, Unikel L, Gallagher R, Evans D, Colland V. Feasilbility and impact of a school-based intervention for families of urban adolescents with asthma: results from a randomized pilot trial. Family Process. 2008;47(1):95–113. doi: 10.1111/j.1545-5300.2008.00241.x. [DOI] [PubMed] [Google Scholar]
- Burke 1997 {published data only}.Burke SO, Handley-Derry MH, Costello EA, Kauffmann E, Dillon MC. Stress-point intervention for parents of repeatedly hospitalized children with chronic conditions. Research in Nursing & Health. 1997;20(6):475–85. doi: 10.1002/(sici)1098-240x(199712)20:6<475::aid-nur2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Burke 2001 {published data only}.Burke SO, Harrison MB, Kauffmann E, Wong C. Effects of stress-point intervention with families of repeatedly hospitalized children. Journal of Family Nursing. 2001;7(2):128–58. [Google Scholar]
- Cakan 2007 {published data only}.Cakan N, Ellis DA, Templin T, Frey M, Naar-King S. The effects of weight status on treatment outcomes in a randomized clinical trial of multisystemic therapy for adolescents with type 1 diabetes and chronically poor metabolic control. Pediatric Diabetes. 2007;8(4):206–13. doi: 10.1111/j.1399-5448.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- Canino 2008 {published data only}.Canino G, Vila D, Normand ST, Acosta-Perez E, Ramirez R, Garcia P, et al. Reducing asthma health disparities in poor Puerto Rican children: the effectiveness of a culturally tailored family intervention. Journal of Allergy & Clinical Immunology. 2008;121(3):665–70. doi: 10.1016/j.jaci.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey 2008 {published data only}.Carey JC, Wade SL, Wolfe CR. Lessons learned: the effect of prior technology use on web-based interventions. Cyber Psychology & Behavior. 2008;11(2):188–95. doi: 10.1089/cpb.2007.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen 2013 {published data only}.Chen SH, Huang JL, Yeh KW, Tsai YF. Interactive support interventions for caregivers of asthmatic children. Journal of Asthma. 2013;50(6):649–57. doi: 10.3109/02770903.2013.794236. [DOI] [PubMed] [Google Scholar]
- Chernoff 2002 {published data only}.Chernoff RG, Ireys HT, DeVet KA, Kim YJ. A randomized, controlled trial of a community-based support program for families of children with chronic illness: pediatric outcomes. Archives of Pediatrics and Adolescent Medicine. 2002;156(6):533–9. doi: 10.1001/archpedi.156.6.533. [DOI] [PubMed] [Google Scholar]
- Chiang 2009 {published data only}.Chiang L, Ma W, Huang J, Tseng L, Hsueh K. Effect of relaxation-breathing training on anxiety and asthma signs/ symptoms of children with moderate-to-severe asthma: a randomized controlled trial. International Journal of Nursing Studies. 2009;46(8):1061–70. doi: 10.1016/j.ijnurstu.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Ellis 2007 {published data only}.Ellis DA, Naar-King S, Templin T, Frey MA, Cunningham PB. Improving health outcomes among youth with poorly controlled type 1 diabetes: the role of treatment fidelity in a randomized clinical trial of multisystemic therapy. Journal of Family Psychology. 2007;21(3):363–71. doi: 10.1037/0893-3200.21.3.363. [DOI] [PubMed] [Google Scholar]
- Ellis 2008 {published data only}.Ellis D, Naar-King S, Templin T, Frey M, Cunningham P, Sheidow A, et al. Multisystemic therapy for adolescents with poorly controlled type 1 diabetes: reduced diabetic ketoacidosis admissions and related costs over 24 months. Diabetes Care. 2008;31(9):1746–7. doi: 10.2337/dc07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans 1999 {published data only}.Evans R, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the national cooperative inner-city asthma study. Journal of Pediatrics. 1999;135(3):332–8. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- Fedele 2013 {published data only}.Fedele DA, Hullmann SE, Chaffin M, Kenner C, Fisher MJ, Kirk K, et al. Impact of a parent-based interdiscplinary intervention for mothers on adjustment in children newly diagnosed with cancer. Journal of Pediatric Psychology. 2013;38(5):531–40. doi: 10.1093/jpepsy/jst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field 1998 {published data only}.Field T, Henteleff T, Hernandez-Reif M, Martinez E, Mavunda K, Kuhn C, et al. Children with asthma have improved pulmonary functions after massage therapy. Journal of Pediatrics. 1998;132(5):854–8. doi: 10.1016/s0022-3476(98)70317-8. [DOI] [PubMed] [Google Scholar]
- Forsander 1995 {published data only} *.Forsander G. Family attitudes to different management regimens in diabetes mellitus. Practical Diabetes International. 1995;12(2):80–5. [Google Scholar]
- Forsander G, Persson B, Sundelin J, Berglund E, Snellman K, Hellström R. Metabolic control in children with insulin-dependent diabetes mellitus 5y after diagnosis. Early detection of patients at risk for poor metabolic control. Acta Paediatrica. 1998;87(8):857–64. doi: 10.1080/080352598750013635. [DOI] [PubMed] [Google Scholar]
- Forsander GA, Sundelin J. Comparison of two therapeutic regimes for diabetes-stricken children. Social and mental resources of the family are often crucial for the prognosis [Två behandlingsregimer vid diabetesdebut hos barn jämförda: Familjens sociala och mentalaresurser avgör ofta prognosen] Lakartidningen. 2001;98(48):5484–9. [PubMed] [Google Scholar]
- Forsander GA, Sundelin J, Persson B. Influence of the initial management regimen and family social situation on glycemic control and medical care in children with type I diabetes mellitus. Acta Paediatrica. 2000;89(12):1462–8. doi: 10.1080/080352500456651. [DOI] [PubMed] [Google Scholar]
- Sundelin JG, Forsander G, Mattson SE. Family-oriented support at the onset of diabetes mellitus: a comparison of two group conditions during 2 years following diagnosis. Acta Paediatrica. 1996;85(1):49–55. doi: 10.1111/j.1651-2227.1996.tb13889.x. [DOI] [PubMed] [Google Scholar]
- Forsander 2003 {published data only}.Forsander G, Malmodin B, Eklund C, Persson B. Relationship between dietary intake in children with diabetes mellitus type I, their management at diagnosis, social factors, anthropometry and glycaemic control. Scandinavian Journal of Nutrition/Naringsforskning. 2003;47(2):75–84. [Google Scholar]
- Garbutt 2010 {published data only}.Garbutt JM, Banister C, Highstein G, Sterkel R, Epstein J, Bruns J, et al. Telephone coaching for parents of children with asthma: impact and lessons learned. Archives of Pediatrics and Adolescent Medicine. 2010;164(7):625–30. doi: 10.1001/archpediatrics.2010.91. [DOI] [PubMed] [Google Scholar]
- Gerber 2010 {published data only} *.Gerber W, Petermann F, Gerber-von Müller G, Dollwet M, Darabaneanu S, Niederberger U, et al. MIPAS-Family-evaluation of a new multi-modal behavioral training program for pediatric headaches: clinical effects and the impact on quality of life. Journal of Headache Pain. 2010;11(3):215–25. doi: 10.1007/s10194-010-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber WD, Muller GG, Petermann U, Niederberger U, Petermann F. Do behavioral medicine approaches have an effect on the quality of life and everyday competence of children suffering from chronic headaches? [Verbessern verhaltensmedizinische behandlungsstrategien die lebens–qualitat bei kindern mit chronischen kopfschmerzen? ] Zeitschrift fur Klinische Psychologie und Psychotherapie. 2009;38(4):231–9. [Google Scholar]
- Gerber WD, Petermann F, Müller G, Niederberger U, Rentmeister B, Siniatchkin M, et al. MIPAS-family: development and evaluation of a behavioural medicine programme for the treatment of chronic paediatric headaches. Verhaltenstherapie. 2008;18(4):247–55. [Google Scholar]
- Giallo 2008 {published data only}.Giallo R, Gavidia-Payne S. Evaluation of a family-based intervention for siblings of children with a disability or chronic illness. Australian e-Journal for the Advancement of Mental Health. 2008;7(2):1–13. [Google Scholar]
- Glang 2007 {published data only}.Glang A, McLaughlin K, Schroeder S. Using interactive multimedia to teach parent advocacy skills: an exploratory study. Journal of Head Trauma Rehabilitation. 2007;22(3):196–203. doi: 10.1097/01.HTR.0000271121.42523.3a. [DOI] [PubMed] [Google Scholar]
- Grey 2011 {published data only}.Grey M, Jaser SS, Whittemore R, Jeon S, Lindemann E. Coping skills training for parents of children with type 1 diabetes. Nursing Research. 2011;60(3):173–81. doi: 10.1097/NNR.0b013e3182159c8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groß 2013 {published data only}.Groß M, Warschburger P. Chronic abdominal pain: psychosocial strain and treatment-associated changes in coping. Verhaltenstherapie. 2013;23:80–89. [Google Scholar]
- *.Groß M, Warschburger P. Evaluation of a cognitive-behavioural pain management program for children with chronic abdominal pain: a randomzied controlled study. International journal of behavioural medicine. 2013;20:434–43. doi: 10.1007/s12529-012-9228-3. [DOI] [PubMed] [Google Scholar]
- Gulewitsch 2012 {published data only}.Gulewitsch MD, Schauer JS, Hautzinger M, Schlarb AA. Therapy of functional stomach ache in children. Concept, acceptance and first pilot results of a hypnotherapeutic-behavioral brief intervention [Therapie funktioneller Bauchschmerzenbei KindernKonzept, Akzeptanz und erste Pilotergebnisse einer hypnotherapeutisch–behavioralen Kurzintervention] Der Schmerz. 2012;26(2):160–7. doi: 10.1007/s00482-011-1139-8. [DOI] [PubMed] [Google Scholar]
- Gustafsson 1986 {published data only}.Gustafsson PA, Kjellman M, Cederblad M. Family therapy in the treatment of severe childhood asthma. Journal of Psychosomatic Research. 1986;30(3):369–74. doi: 10.1016/0022-3999(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Harris 2001 {published data only}.Harris MA, Greco P, Wysocki T, White TH. Family therapy with adolescents with diabetes: a litmus test for clinically meaningful change. Families, Systems & Health. 2001;19:159–68. [Google Scholar]
- Haus 1976 {published data only}.Haus BF, Thompson S. The effect of nursing intervention on a program of behavior modification by parents in the home. Journal of Psychiatric Nursing and Mental Health Services. 1976;14(8):9–16. [PubMed] [Google Scholar]
- Hernandez 1998 {published data only}.Hernandez NE, Kolb S. Effects of relaxation on anxiety in primary caregivers of chronically ill children. Pediatric Nursing. 1998;24(1):51–6. [PubMed] [Google Scholar]
- Hommel 2012 {published data only}.Hommel KA, Hente EA, Odell S, Herzer M, Ingerski LM, Guilfoyle SM, et al. Evaluation of a group-based behavioral intervention to promote adherence in adolescents with inflammatory bowel disease. European Journal of Gastroenterology and Hepatology. 2012;24(1):64–9. doi: 10.1097/MEG.0b013e32834d09f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell 1994 {published data only}.Hovell MF, Meltzer SB, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial. Chest. 1994;106(2):440–6. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- Humphreys 2000 {published data only}.Humphreys PA, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. Journal of Pediatric Gastroenterology and Nutrition. 2000;31(1):47–51. doi: 10.1097/00005176-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Ireys 1996 {published data only}.Ireys HT, Sills EM, Kolodner KB, Walsh BB. A social support intervention for parents of children with juvenile rheumatoid arthritis: results of a randomized trial. Journal of Pediatric Psychology. 1996;21(5):633–41. doi: 10.1093/jpepsy/21.5.633. [DOI] [PubMed] [Google Scholar]
- Ireys 2001 {published data only}.Ireys HT, Chernoff R, DeVet KA, Kim Y. Maternal outcomes of a randomized controlled trial of a community-based support program for families of children with chronic illnesses. Archives of Pediatrics and Adolescent Medicine. 2001;155(7):771–7. doi: 10.1001/archpedi.155.7.771. [DOI] [PubMed] [Google Scholar]
- Jay 1990 {published data only}.Jay SM, Elliott CH. A stress inoculation program for parents whose children are undergoing painful medical procedures. Journal of Consulting and Clinical Psychology. 1990;58(6):799–804. doi: 10.1037//0022-006x.58.6.799. [DOI] [PubMed] [Google Scholar]
- Johnson 1987 {published data only}.Johnson MR, Whitt JK, Martin B. The effect of fantasy facilitation of anxiety in chronically ill and healthy children. Journal of Pediatric Psychology. 1987;12(2):273–84. doi: 10.1093/jpepsy/12.2.273. [DOI] [PubMed] [Google Scholar]
- Kamps 2008 {published data only}.Kamps JL, Rapoff MA, Roberts MC, Varela RE, Barnard M, Olson N. Improving adherence to inhaled corticosteroids in children with asthma: a pilot of a randomized clinical trial. Children’s Health Care. 2008;37(4):261–77. [Google Scholar]
- Kaslow 2000 {published data only}.Kaslow NJ, Collins MH, Rashid FL, Baskin ML, Griffith JR, Hollins L, et al. The efficacy of a pilot family psychoeducational intervention for pediatric sickle cell disease. Families, Systems, & Health. 2000;18(4):381–404. [Google Scholar]
- Katz 2014 {published data only}.Katz ML, Volkening LK, Butler DA, Anderson BJ, Laffel LM. Family-based psychoeducation and care ambassador intervention to improve glycemic control in youth with type 1 diabetes: a randomized trial. Pediatric Diabetes. 2014;15(2):142–50. doi: 10.1111/pedi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak 1996 {published data only}.Kazak AE, Penati B, Boyer BA, Himelstein B, Brophy P, Waibel K, et al. A randomized controlled prospective outcome study of a psychological and pharmacological intervention protocol for procedural distress in pediatric leukaemia. Journal of Pediatric Psychology. 1996;21(5):615–31. doi: 10.1093/jpepsy/21.5.615. [DOI] [PubMed] [Google Scholar]
- Kazak 2005 {published data only}.Kazak AE, Simms S, Alderfer MA, Rourke MT, Crump T, McClure K, et al. Feasibility and preliminary outcomes from a pilot study of a brief psychological intervention for families of children newly diagnose with cancer. Journal of Pediatric Psychology. 2005;30(8):644–55. doi: 10.1093/jpepsy/jsi051. [DOI] [PubMed] [Google Scholar]
- Ketchen 2006 {published data only}.Ketchen B, Hazzard A, Lassiter S, Barber N, Armistead L, Mentz R, et al. STARBRIGHT world: a pilot study of a home-based sickle cell psychoeducational intervention. Children’s Health Care. 2006;35(4):321–38. [Google Scholar]
- Klinnert 2005 {published data only}.Klinnert MD, Liu AH, Pearson MR, Ellison M, Budhiraja N, Robinson JL. Short-term impact of a randomized multifaceted intervention for wheezing infants in low-income families. Archives of Pediatric Adolescent Medicine. 2005;159(1):75–82. doi: 10.1001/archpedi.159.1.75. [DOI] [PubMed] [Google Scholar]
- Klinnert 2007 {published data only}.Klinnert MD, Liu AH, Pearson MR, Tong S, Strand M, Luckow A, et al. Outcome of a randomized multifaceted intervention with low-income families of wheezing infants. Archives of Pediatrics and Adolescent Medicine. 2007;161(8):783–90. doi: 10.1001/archpedi.161.8.783. [DOI] [PubMed] [Google Scholar]
- Kroner-Herwig 1998 {published data only}.Kroner-Herwig B, Mohn U, Pothmann R. Comparison of biofeedback and relaxation in the treatment of pediatric headache and the influence of parent involvement on outcome. Applied Psychophysiology and Biofeedback. 1998;23(3):143–57. doi: 10.1023/a:1022267104369. [DOI] [PubMed] [Google Scholar]
- Kupfer 2010 {published data only}.Kupfer J, Gieler U, Diepgen TL, Fartasch M, Lob-Corzilius T, Ring J, et al. Structured education program improves the coping with atopic dermatitis in children and their parents - a multicenter, randomized controlled trial. Journal of Psychosomatic Research. 2010;68(4):353–8. doi: 10.1016/j.jpsychores.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Kurowski 2013 {published data only} *.Kurowski BG, Wade SL, Kirkwood MW, Brown TM, Stancin T, Taylor G. Online problem-solving therapy for executive dysfunction after child traumatic brain injury. Pediatrics. 2013;132(1):158–65. doi: 10.1542/peds.2012-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowski BG, Wade SL, Kirkwood MW, Brown TM, Stancin T, Taylor HG. Long-term benefits of an early online problem solving intervention for executive dysfunction after traumatic brain injury in children. JAMA Pediatrics. 2014;168(6):523–31. doi: 10.1001/jamapediatrics.2013.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SL, Karver CL, Taylor GH, Cassedy A, Stancin T, Kirkwood MW, et al. Counselor-assisted problem solving improves caregiver efficacy following adolescent brain injury. Rehabilitation Psychology. 2014;59(1):1–9. doi: 10.1037/a0034911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasecki 2008 {published data only}.Lasecki K, Olympia D, Clark E, Jenson W, Heathfield LT. Using behavioral interventions to assist children with type 1 diabetes manage blood glucose levels. School Psychology Quarterly. 2008;23(3):389–406. [Google Scholar]
- Logan 1997 {published data only}.Logan S. Emotionally focused therapy improves marital adjustment in parents of children with chronically ill children. Child: Care, Health and Development. 1997;23(6):479–80. doi: 10.1111/j.1365-2214.1997.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Lyon 2013 {published data only}.Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. A longitudinal, randomized, controlled trial of advance care planning for teens with cancer: anxiety, depression, quality of life, advance directives, spirituality. Journal of Adolescent Health. 2014;54(6):710–7. doi: 10.1016/j.jadohealth.2013.10.206. [DOI] [PubMed] [Google Scholar]
- *.Lyon ME, Jacobs S, Briggs L, Cheng YI, Wang J. Family-centered advance care planning for teens with cancer. JAMA Pediatrics. 2013;167(5):460–7. doi: 10.1001/jamapediatrics.2013.943. [DOI] [PubMed] [Google Scholar]
- Mendez 1997 {published data only}.Mendez FJ, Belendez M. Effects of a behavioral intervention on treatment adherence and stress management in adolescents with IDDM. Diabetes Care. 1997;20(9):1370–5. doi: 10.2337/diacare.20.9.1370. [DOI] [PubMed] [Google Scholar]
- Murphy 2012 {published data only}.Murphy HR, Wadham C, Hassler-Hurst J, Rayman G, Skinner TC. Randomized trial of a diabetes self-management education and family teamwork intervention in adolescents with type 1 diabetes. Diabetic Medicine. 2012;29(8):249–53. doi: 10.1111/j.1464-5491.2012.03683.x. [DOI] [PubMed] [Google Scholar]
- Nelson 2011 {published data only}.Nelson KA, Highstein GR, Garbutt J, Trinkaus K, Fisher E, Smith SR, et al. A randomized controlled trial of parental asthma coaching to improve outcomes among urban minority children. Archives of Pediatric Adolescent Medicine. 2011;165(6):520–6. doi: 10.1001/archpediatrics.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez 1999 {published data only}.Pérez MG, Feldman L, Caballero F. Effects of a self-management educational program for the control of childhood asthma. Patient Education and Counseling. 1999;36(1):47–55. doi: 10.1016/s0738-3991(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Rasoli 2008 {published data only}.Rasoli R, Etemadi A, Shafidabadi A, Delavar A. Comparing effectiveness of individual and marital emotionally focused intervention based on decreasing relationship distress of couples with chronically ill children. Journal of Family Research. 2008;3(3):683–96. [Google Scholar]
- Sanders 1989 {published data only}.Sanders MR, Rebgetz M, Morrison M, Bor W, Gordon A, Dadds M, et al. Cognitive-behavioral treatment of recurrent nonspecific abdominal pain in children: an analysis of generalization, maintenance, and side effects. Journal of Consulting and Clinical Psychology. 1989;57(2):294–300. doi: 10.1037//0022-006x.57.2.294. [DOI] [PubMed] [Google Scholar]
- Sanders 1996 {published data only}.Sanders MR, Cleghorn G, Shepherd RW, Patrick M. Predictors of clinical improvement in children with recurrent abdominal pain. Behavioural and Cognitive Psychotherapy. 1996;24:27–38. [Google Scholar]
- Satin 1989 {published data only}.Satin W, La Greca AM, Zigo MA, Skyler JS. Diabetes in adolescence: effects of multifamily group intervention and parent simulation of diabetes. Journal of Pediatric Psychology. 1989;14(2):259–75. doi: 10.1093/jpepsy/14.2.259. [DOI] [PubMed] [Google Scholar]
- Scholten 2011 {published data only}.Scholten L, Willemen AM, Grootenhuis MA, Maurice-Stam H, Schuengel C, Last BF. A cognitive behavioral based group intervention for children with a chronic illness and their parents: a multicentre randomized controlled trial. BMC Pediatrics. 2011;11(65):1–8. doi: 10.1186/1471-2431-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberg 2011 {published data only}.Sieberg CB, Flannery-Schroeder E, Plante W. Children with co-morbid recurrent abdominal pain and anxiety disorders: results from a multiple-baseline intervention study. Journal of Child Health Care. 2011;15(2):126–39. doi: 10.1177/1367493511401640. [DOI] [PubMed] [Google Scholar]
- Staab 2002 {published data only}.Staab D, Van Rueden U, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatric Allergy and Immunology. 2002;13(2):84–90. doi: 10.1034/j.1399-3038.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai 2010 {published data only}.Sullivan-Bolyai S, Bova C, Leung K, Trudeau A, Lee M, Gruppuso P. Social support to empower parents (STEP): an intervention for parents of young children newly diagnosed with type 1 diabetes. The Diabetes Educator. 2010;36(1):88–97. doi: 10.1177/0145721709352384. [DOI] [PubMed] [Google Scholar]
- Szczepanski 2010 {published data only}.Szczepanski R, Jaeschke R, Spindler T, Ihorst G, Forster J, ASEV Study Group Preschoolers’ and parents’ asthma education trial (P2AET) - a randomized controlled study. European Journal of Pediatrics. 2010;169(9):1051–60. doi: 10.1007/s00431-010-1173-z. [DOI] [PubMed] [Google Scholar]
- Van der Veek 2013 {published data only}.Van der Veek SMC, Derkx BHF, Benninga MA, Boer F, De Haan E. Cognitive behaviour therapy for pediatric functional abdominal pain: a randomized controlled trial. Pediatrics. 2013;132(5):1163–72. doi: 10.1542/peds.2013-0242. [DOI] [PubMed] [Google Scholar]
- Wade 2010 {published data only}.Wade SL, Walz NC, Carey J, Williams KM, Cass J, Herren L, et al. A randomized trial of teen online problem solving for improving executive function deficits following pediatric traumatic brain injury. Journal of Head Trauma Rehabilitation. 2010;25(6):409–15. doi: 10.1097/HTR.0b013e3181fb900d. [DOI] [PubMed] [Google Scholar]
- Walders 2006 {published data only}.Walders N, Kercsmar C, Schluchter M, Redline S, Lester Kirchner H, Drotar D. An interdisciplinary intervention for undertreated pediatric asthma. Chest. 2006;129(2):292–9. doi: 10.1378/chest.129.2.292. [DOI] [PubMed] [Google Scholar]
- Walker 1996 {published data only}.Walker JG, Johnson S, Manion I, Cloutier P. Emotionally focused marital intervention for couples with chronically ill children. Journal of Consulting and Clinical Psychology. 1996;64(5):1029–36. doi: 10.1037//0022-006x.64.5.1029. [DOI] [PubMed] [Google Scholar]
- Warner 2011 {published data only}.Warner CM, Ludwig K, Sweeney C, Spillane C, Hogan L, Ryan J, et al. Treating persistent distress and anxiety in parents of children with cancer: an initial feasibility trial. Journal of Pediatric Oncology Nursing. 2011;28(4):224–30. doi: 10.1177/1043454211408105. [DOI] [PubMed] [Google Scholar]
- Wysocki 1997 {published data only}.Wysocki T, McDonell K, Harris MA, Elder Danda CL, Greco P, Bubb J, et al. Social validity of support group and behavior therapy interventions for families of adolescents with insulin-dependent diabetes mellitus. Journal of Pediatric Psychology. 1997;22(5):635–49. doi: 10.1093/jpepsy/22.5.635. [DOI] [PubMed] [Google Scholar]
Additional references
- American Psychological Association 2011.American Psychological Association. Publication Manual of the American Psychological Association. Washington: American Psychological Association; 2011. [Google Scholar]
- Anie 2012.Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database of Systematic Reviews. 2012(2) doi: 10.1002/14651858.CD001916.pub2. [DOI] [PubMed] [Google Scholar]
- Armour 2005.Armour TA, Norris SL, Jack L, Jr, Zhang X, Fisher L. The effectiveness of family interventions in people with diabetes mellitus: a systematic review. Diabetic Medicine. 2005;22(10):1295–305. doi: 10.1111/j.1464-5491.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Ashby 1999.Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106(3):529–50. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Bandura 1977.Bandura A. Social Learning Theory. New Jersey: Prentice-Hall; 1977. [Google Scholar]
- Bandura 1989.Bandura A. Human agency in social cognitive theory. American Psychologist. 1989;44(9):1175–84. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- Bargh 2008.Bargh JA, Morsella E. The unconscious mind. Perspectives on Psychological Science. 2008;3(1):73–9. doi: 10.1111/j.1745-6916.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck 1979.Beck AR, Rush AJ, Shaw B, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck 2011.Beck JS. Cognitive Behavior Therapy. New York: The Guilford Press; 2011. Introduction to cognitive behavior therapy; pp. 1–14. [Google Scholar]
- Bergin1975.Bergin AE, Suinn RM. Individual psychotherapy and behavior therapy. Annual Review of Psychology. 1975;26:509–56. doi: 10.1146/annurev.ps.26.020175.002453. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner 1979.Bronfenbreener U. The Ecology of Human Development: Experiments and Design and Nature. Massachusetts: Harvard University; 1979. [Google Scholar]
- Cancer Research UK 2014.Cancer Research UK. Childhood cancer key stats. www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/Childhoodcancers/ (Acessed 9th January 2015)
- Cohen 1992.Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Committee on Publication Ethics 2011.Committee on Publication Ethics. Code of conduct and best practice guidelines for journal editors. publicationethics.org/ (accessed 22 March 2012)
- D’Zurilla 1971.D’Zurilla TJ, Goldfried MR. Problem solving and behavior modification. Journal of Abnormal Psychology. 1971;78(1):107–26. doi: 10.1037/h0031360. [DOI] [PubMed] [Google Scholar]
- D’Zurilla 1982.D’Zurilla TJ, Nezu AM. Social problem solving in adults. In: Kendall PC, editor. Advances in Cognitive-Behavioral Research and Therapy. New York: Academic Press; 1982. pp. 202–74. [Google Scholar]
- D’Zurilla 1999.D’Zurilla TJ, Nezu AM. Problem-Solving Therapy: A Social Competence Approach to Clinical Intervention. New York: Springer-Verlag; 1999. [Google Scholar]
- D’Zurilla 2007.D’Zurilla TJ, Nezu AM. Problem-Solving Therapy A Positive Approach to Clinical Intervention. New York: Springer; 2007. [Google Scholar]
- Data Resource Center 2010.Data Resource Center. National survey of children with special health care needs: current health conditions and functional difficulties. childhealthdata.org/docs/cshcn/conditions-difficulties-09cshcnfeb-2-2012.pdf (accessed 5th 2015)
- Drotar 2010.Drotar D. Editorial: Guidance for submission and review of multiple publications derived from the same study. Journal of Pediatric Psychology. 2010;35(3):225–30. [Google Scholar]
- Eccleston 2014.Eccleston C, Palermo TM, Williams ACDC, Lewandowski Holley A, Morley S, Fisher E, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews. 2014;(5) doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersser 2014.Ersser SJ, Cowdell F, Latter S, Gardiner E, Flohr C, Thompson AR, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database of Systematic Reviews. 2014;(1) doi: 10.1002/14651858.CD004054.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher 2014.Fisher E, Heathcote L, Palermo TM, Williams ACDC, Lau J, Eccleston C. Systematic review and meta-analysis: psychological therapies for children with chronic pain. Journal of Pediatric Psychology. 2014;39(8):1–20. doi: 10.1093/jpepsy/jsu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliom 2002.Gilliom M, Shaw DS, Beck JE, Schonberg MA, Lukon JL. Anger regulation in disadvantaged preschool boys: strategies, antecedents, and the development of self-control. Developmental Psychology. 2002;38(2):222–35. doi: 10.1037//0012-1649.38.2.222. [DOI] [PubMed] [Google Scholar]
- Haley 1976.Haley J. Problem Solving Therapy. San Francisco: Jossey-Bass; 1976. [Google Scholar]
- Halfon 2010.Halfon N, Newackeck PW. Evolving notions of childhood chronic illness. JAMA. 2010;303(7):665–6. doi: 10.1001/jama.2010.130. [DOI] [PubMed] [Google Scholar]
- Henggeler 2003.Henggeler SW, Lee T. Multisystemic treatment of serious clinical problems. In: Kazdin E, Weisz JR, editors. Evidence-Based Psychotherapies for Children and Adolescents. New York: Guilford Press; 2003. pp. 301–24. [Google Scholar]
- Higgins 2011.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011 Available from www.cochrane-handbook.org.
- Huertas-Ceballos 2008.Huertas-Ceballos AA, Logan S, Bennett C, Macarthur C. Psychosocial interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database of Systematic Reviews. 2008;(1) doi: 10.1002/14651858.CD003014.pub2. [DOI] [PubMed] [Google Scholar]
- James 1980.James W. The Principles of Psychology. New York: Henry Holt and Company; 1980. [Google Scholar]
- Jordan 2007.Jordan AL, Eccleston C, Osborn M. Being a parent of the adolescent with complex chronic pain: an interpretative phenomenological analysis. European Journal of Pain. 2007;11(1):49–56. doi: 10.1016/j.ejpain.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Karabulut 2013.Karabulut GS, Beer OF, Erginöz E, Kutlu T, Çoku ğ ra FC, Erka T. The incidence of irritable bowel syndrome in children using the Rome III criteria and the effect of trimebutine treatment. Journal of Neurogastroenterology and Motility. 2013;19(1):90–93. doi: 10.5056/jnm.2013.19.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law 2014.Law EF, Fisher E, Fales J, Noel M, Eccleston C. Systematic review and meta-analysis of parent and family-based interventions for children and adolescents with chronic medical conditions. Journal of Pediatric Psychology. 2014;39(8):866–886. doi: 10.1093/jpepsy/jsu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin 2010.Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, Van Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database of Systematic Reviews. 2010;(3) doi: 10.1002/14651858.CD004015.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan 2005.Logan DE, Scharff L. Relationships between family and parent characteristics and functional abilities in children with recurrent pain syndromes: an investigation of moderating effects on the pathway from pain to disability. Journal of Pediatric Psychology. 2005;30(8):698–707. doi: 10.1093/jpepsy/jsj060. [DOI] [PubMed] [Google Scholar]
- McBroom 2009.McBroom LA, Enriquez M. Review of family-centered interventions to enhance the health outcomes of children with type 1 diabetes. The Diabetes Educator. 2009;35(3):428–38. doi: 10.1177/0145721709332814. [DOI] [PubMed] [Google Scholar]
- McDougall 2004.McDougall J, King G, De Wit D, Miller LT, Honh S, Offord DR, et al. Chronic physical health conditions and disability among Canadian school-aged children: a national profile. Disability and Rehabilitation. 2004;26(1):35–45. doi: 10.1080/09638280410001645076. [DOI] [PubMed] [Google Scholar]
- Minuchin 1974.Minuchin S. Families & Family Therapy. Massachusetts: Harvard University; 1974. [Google Scholar]
- National Cancer Institute 2014.National Cancer Institute at the National Institutes of Health. Cancer in children and adolescents. www.cancer.gov/cancertopics/factsheet/Sites-Types/childhood (Accessed 9th January 2015)
- Nature 2009.Nature Editorial. Data’s shameful neglect. Nature. 2009;461(7261):145. doi: 10.1038/461145a. [DOI] [PubMed] [Google Scholar]
- Nock 2001.Nock MK, Kazdin AE. Parent expectancies for child therapy: assessment and relation to participation in treatment. Journal of Child and Family Studies. 2001;10(2):155–80. [Google Scholar]
- Pai 2006.Pai AL, Drotar D, Zebracki K, Moore M, Youngstorm E. A meta-analysis of the effects of psychological interventions in pediatric oncology on outcomes of psychological distress and adjustment. Journal of Pediatric Psychology. 2006;31(9):978–88. doi: 10.1093/jpepsy/jsj109. [DOI] [PubMed] [Google Scholar]
- Palermo 2000.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. Developmental and Behavioral Pediatrics. 2000;21(1):58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Palermo 2007.Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: an integrative approach. Pain. 2005;119(1–3):1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Palermo 2009a.Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an internet delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146(1–2):205–13. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo 2009b.Palermo TM, Eccleston C. Parents of children and adolescents with chronic pain. Pain. 2009;146(1–2):15–7. doi: 10.1016/j.pain.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RevMan 2014.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- Robin 1989.Robin AL, Foster SL. Negotiating Parent Adolescent Conflict: A Behavioral-Family Systems Approach. New York: Guilford; 1989. [Google Scholar]
- Sansom-Daly 2012.Sansom-Daly UM, Peate M, Wakefield CE, Bryant RA, Cohn RJ. A systematic review of psychological interventions for adolescents and young adults living with chronic illness. Health Psychology. 2012;31(3):380–93. doi: 10.1037/a0025977. [DOI] [PubMed] [Google Scholar]
- Savage 2014.Savage E, Beirne PV, Ni Chroinin M, Duff A, Fitzgerald T, Farrell D. Self-management education for cystic fibrosis. Cochrane Database of Systematic Reviews. 2014;(9) doi: 10.1002/14651858.CD007641.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz 2010.Schulz KF, Altman DG, Moher D, for the CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:698–702. [Google Scholar]
- Skinner 1953.Skinner BF. Science and Human Behaviour. Toronto: The Macmillan Company; 1953. Chapter 5: Operant behavior. [Google Scholar]
- Soo 2007.Soo C, Tate R. Psychological treatment for anxiety in people with traumatic brain injury. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD005239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleave 2010.Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303(7):623–30. doi: 10.1001/jama.2010.104. [DOI] [PubMed] [Google Scholar]
- Van der Lee 2007.Van der Lee JH, Mokkink LB, Grootenhuis MA, Heymans HS, Offringa M. Definitions and measurement of chronic health conditions in childhood. JAMA. 2007;297(24):2741–51. doi: 10.1001/jama.297.24.2741. [DOI] [PubMed] [Google Scholar]
- Wegner 1994.Wegner DM. Ironic processes of mental control. Psychological Review. 1994;101(1):34–52. doi: 10.1037/0033-295x.101.1.34. [DOI] [PubMed] [Google Scholar]
- WHO 2011.World Health Organization. World Health Statistics. WHO Library Cataloguing-in-Publication Data. 2011 [Google Scholar]
- Wicherts 2006.Wicherts JM, Borsboom D, Kats J, Molenaar D. The poor availability of psychological research data for reanalysis. American Psychologist. 2006;61(7):726–8. doi: 10.1037/0003-066X.61.7.726. [DOI] [PubMed] [Google Scholar]
- Wicherts 2011.Wicherts JM, Bakker M, Molenaar D. Willingness to share research data is related to the strength of the evidence and quality of reporting of statistical results. PLoS ONE. 2011;6(11):e26828. doi: 10.1371/journal.pone.0026828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams 2012.Williams ACDC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews. 2012;(11) doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Association of Medical Editors 2012.World Association of Medical Editors. Publication ethics policies for medical journals. www.wame.org (accessed 22 March 2012)
- Wysocki 2000.Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, et al. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. Journal of Pediatric Psychology. 2000;25(1):23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]
- Yates 2005.Yates SL, Morley S, Eccleston C, Williams A. A scale for rating the quality of psychological trials for pain. Pain. 2005;117(3):314–25. doi: 10.1016/j.pain.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Yorke 2005.Yorke J, Shuldham C. Family therapy for asthma in children. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD003272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Eccleston 2012a.Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database of Systematic Reviews. 2012;(2) doi: 10.1002/14651858.CD009660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston 2012b.Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database of Systematic Reviews. 2012;(8) doi: 10.1002/14651858.CD009660.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


