Abstract
Background & Aims
Bariatric surgery is associated with improved outcomes in subjects with severe obesity. We investigated the prognostic relevance of non-alcoholic steatohepatitis (NASH) and liver gene expression patterns in patients undergoing bariatric surgery.
Methods
We performed a retrospective analysis of 492 subjects who underwent gastric bypass bariatric surgery at a single center in Switzerland from January 1997 through December 2004; routine peri-operative liver biopsies were collected, analyzed histologically, and RNA was isolated. We collected data on overall survival and clinical and biochemical parameters and compared these with data from propensity score-matched subjects participating in the third National Health and Nutrition Examination Survey (NHANES III). We used liver biopsies to identify bariatric surgery patients with NASH; NHANES III participants with NASH were identified based on a hyperechogenic liver at ultrasound and increased alanine transaminase levels. We analyzed a 32-gene signature associated with NAFLD severity in the liver tissues collected from 47 bariatric surgery patients with NASH, and assessed its prognostic features using nearest template prediction and survival analysis.
Results
At baseline, the median body mass index of patients who underwent bariatric surgery was 43.6 kg/m2; based on histologic findings, 12% had NASH and 16% had fibrosis. During a median follow-up of 10.2 years after the surgery, 4.2% of the subjects died. In multivariable Cox regression, the presence of NASH (hazard ratio [HR], 2.9; P=.02) and arterial hypertension (HR, 3.9; P=.02) were associated with overall mortality. When bariatric surgery patients were matched with NHANES III participants, bariatric surgery reduced the risk of death during the follow-up period (HR, 0.54; P=.04). However, bariatric surgery patients with NASH did not have a reduced risk of death compared to NHANES III participants with NASH (HR, 0.90; P=.85). We identified an expression pattern of 32 genes in liver tissues from patients with NASH that was associated with increased risk of death in multivariable analysis (HR, 7.7; P=.045).
Conclusions
Histologically proven NASH is associated with increased risk of death within a median follow-up of 10.2 years after bariatric surgery, compared to patients who undergo bariatric surgery without NASH. The survival benefit of bariatric surgery in subjects with NASH may be reduced. A 32-gene expression pattern identified NASH patients who underwent bariatric surgery and had shorter survival times.
Keywords: post-operative complication, fatty liver disease, treatment, biomarker, NHANES III
Introduction
Obesity, defined as a body mass index (BMI) of over 30kg/m2, is a major public health issue affecting 34.9% of adults in the US and costing the US economy an estimated $148 billion in 2008, or almost 10% of total US healthcare spending1, 2. Globally, the prevalence of overweight (BMI greater than 25kg/m2) increased from 857 million to 2.1 billion, (29% to 37% of the world population) between 1980 and 2013 3. Obesity is strongly associated with non-alcoholic fatty liver disease (NAFLD), a clinicopathological spectrum ranging from hepatic steatosis, to non-alcoholic steatohepatitis (NASH) and cirrhosis. NAFLD is emerging as the most common liver disease in the Western world, with up to one third of adults affected in the US 4, 5. The prevalence of NAFLD in the setting of morbid obesity and bariatric surgery, ranges from 63–99% for steatosis >5%, 10–58% for NASH and 7–74% for fibrosis depending on definition, diagnostic criteria and the distribution of risk factors in the specific population studied 6
Bariatric surgery is now widely performed for severely obese subjects, and has been associated with weight reduction and an improvement in obesity-related conditions including type 2 diabetes, arterial hypertension and dyslipidaemia 7. Despite increasing data from case-control or cohort studies suggesting that bariatric surgery is associated with improved long-term survival, the mortality benefit depends on the risk-profile of the population with one study in Veterans Affairs medical centres reporting no benefit 8–10. Most prognostic studies in bariatric surgery-treated patients have focused on short-term postoperative outcomes 11, 12. Baseline factors associated with long-term mortality post bariatric surgery have not been fully established, although age, male sex, BMI over 50kg/m2, presence of comorbid conditions (including diabetes, cardiovascular disease) and active smoking have been proposed 9, 13, 14. In particular, despite the high prevalence of NAFLD/NASH in this population, its relevance in long-term outcome after bariatric surgery is unknown.
We aimed to study the prognostic relevance of histological and molecular parameters derived from liver biopsy specimens, on overall long-term survival in a well-characterized single-centre cohort of obese patients undergoing bariatric surgery.
Materials and Methods
Patient cohort (Geneva bariatric surgery cohort)
Five hundred severely obese patients consecutively underwent bariatric surgery (Roux-en-Y gastric bypass surgery) at Geneva University Hospital, a tertiary hospital serving the area around Geneva, Switzerland, between June 1997 and December 2004 (Geneva bariatric surgery cohort)15. During surgery, patients underwent systematic cholecystectomy and left-lobe (segment 3) wedge-resection or needle liver biopsy. Patients were excluded from the study if they had chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection at baseline, no available baseline liver biopsy tissue, or previous weight-losing surgery (Figure 1). Patients with significant substance abuse, including alcohol abuse (defined as more than 14 units of alcohol per week), were excluded from surgery (see Supplementary Methods for additional details). This study was approved by the Institutional Review Board of Geneva University Hospital (protocol number 14–125), and the requirement for informed consent from the patients was waived due to the retrospective nature of this study.
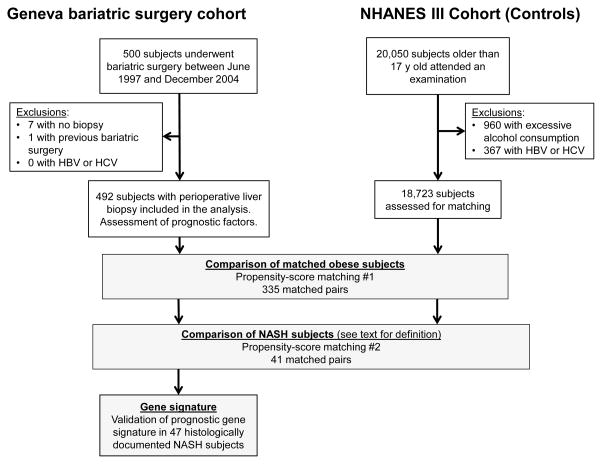
Figure 1.
Flowchart of patient inclusion in the Geneva cohort (left) and matching with the NHANES III cohort (right). A subset of 47 patients with histologically documented NASH was assessed for a liver-based gene signature.
Outcome assessment
The main outcome of interest was overall survival. Mortality data was obtained prospectively or, if the patient was lost to follow-up, it was assessed from the cantonal registry of Geneva. Cause of death was extracted from electronic medical records at our institution. Percentage excess BMI loss (EBMIL) was defined as ([preoperative BMI – postoperative BMI] × 100) / (preoperative BMI – 25). All endpoints, assessed variables and analyses were pre-planned (see Supplementary Methods for additional details).
Histological assessment of liver biopsy
All histological liver biopsy specimens were reviewed by an experienced liver histopathologist (LRB) blinded to clinical data. A threshold of 5% of hepatocytes with macrovesicular steatosis was required for the diagnosis of NAFLD. NASH was defined as an overall pattern of histological injury based on the presence of >5% macrovesicular steatosis, inflammation, and hepatocyte ballooning and not a NAFLD activity score (NAS) cut-off because previous reports underscore a suboptimal correlation between histological assessment of NASH and the semiquantitative NAS16, 17. NAS was determined based on steatosis grade, lobular inflammation, and hepatocellular ballooning 16 and the fibrosis score was assessed according to the NASH clinical research network (CRN) modified Brunt methodology 16.
Comparison with the NHANES III cohort
To assess whether overall survival in the Geneva cohort differed to a control obese population, we performed propensity-score matching of the Geneva cohort subjects to subjects from the third US National Health and Nutrition Examination Survey (NHANES III) 18 and compared survival of the subjects. A subset of the NHANES III cohort, who likely had NASH based on elevated ALT (>330 IU/L in men and >19 IU/L in women) and at least moderate hepatic steatosis by ultrasound (834 subjects: NHANES III NASH cohort), was propensity-score matched to the NASH subjects in the Geneva bariatric surgery cohort (see Supplementary Methods for additional details).
Tissue processing, RNA extraction and gene expression profiling
All NASH subjects with sufficient remaining formalin-fixed, paraffin-embedded (FFPE) hepatic tissue for RNA extraction (n=47) were included for gene expression profiling. Briefly, total RNA was extracted from five 5-μm-thick FFPE tissue sections using the High Pure RNA paraffin kit (Roche). Gene expression profiling was performed using 100–400ng total RNA by using nCounter Digital Analyzer system (NanoString) according to the manufacturer’s instructions. Raw transcript count data were log transformed and scaled by geometric mean of control probe data by using NanoString normalizer module implemented in GenePattern genomic analysis toolkit (www.broadinstitute.org/cancer/software/genepattern). Gene expression dataset is available at National Center for Biotechnology Information Gene Expression Omnibus database (accession number: GSE 69248).
Statistical and bioinformatics analysis
Continuous and categorical variables were tested using Wilcoxon rank-sum test and Fisher’s exact test, respectively. Association of the baseline variables with clinical outcomes were evaluated using uni- and multivariable Cox regression modelling. Propensity score-matching was performed non-parametrically using nearest-neighbour 1 to 1 matching, based on the following variables: BMI, sex, age, presence of diabetes, arterial hypertension, level of bilirubin, aspartate aminotransferase (AST), γ-glutamyltransferase (GGT) and total cholesterol (see Supplementary Methods for additional details).
A prognostic 32-gene signature reported in our previous study 19 was used to perform prognostic prediction by using nearest template prediction algorithm 20 implemented in GenePattern. Gene regulatory network was identified by using Multiscale Embedded Gene Co-expression Network Analysis (MEGENA) 21, 22. All statistical and bioinformatics analyses were performed using GenePattern and the R statistical package version 3.1 (www.r-project.org).
Results
Patient characteristics of the Geneva cohort
Eight of the 500 consecutively enrolled subjects were excluded due to absence of liver biopsy or prior bariatric surgery (Figure 1). Median baseline weight and BMI of the 492 patients included were 118kg and 43.6kg/m2 respectively, Table 1 summarizes other baseline characteristics. At baseline histology (84% wedge-resection biopsy), 89% had steatosis in more than 5% of hepatocytes, 12% had NASH and 4% had fibrosis stage 2 or greater (Table 2). Compared to non-NASH patients, patients with NASH were more likely to be male, diabetic, hypertensive, previous or current smokers and to be older, whereas subjects with fibrosis were more likely to be male and diabetic (Table 1).
Table 1.
Baseline characteristics of patients of the Geneva cohort and comparison of NASH versus non-NASH and fibrotic versus non-fibrotic subjects.
| Variable | All subjects
|
NASH comparison
|
Fibrosis comparison
|
Statistical comparison
|
|||
|---|---|---|---|---|---|---|---|
| NASH | non-NASH | Fibrosis | Non-fibrosis | NASH vs non-NASH, p-value | Fibrosis vs non-fibrosis, p-value | ||
| N | 492 | 59 | 433 | 77 | 415 | ||
| Clinical | |||||||
| Male sex, n (%) | 89 (18) | 22 (13) | 67 (16) | 26 (34) | 63 (15) | 0.004 | 0.007 |
| Age (y) | 41 (34–49) | 46 (40–53) | 40 (33–48) | 43.6 (36–50) | 40.3 (33–49) | 0.004 | 1.0 |
| Age (>50y), n (%) | 104 (21) | 20 (34) | 84 (19) | 19 (25) | 85 (21) | 0.4 | 1.0 |
| Weight (kg) | 118 (108–132) | 123 (161–174) | 117 (110–130) | 125 (112–140) | 117 (108–131) | 0.4 | 0.2 |
| BMI (kg/m2) | 43.6 (41–47) | 44 (42–49) | 43 (41–47) | 44.4 (42–48) | 43.2 (41–47) | 1.0 | 1.0 |
| BMI (>45kg/m2), n (%) | 181 (37) | 25 (42) | 156 (36) | 30 (39) | 151 (36) | 1.0 | 1.0 |
| Diabetes, n (%) | 82 (17) | 27 (46) | 55 (13) | 27 (35) | 55 (13) | 0.002 | 0.002 |
| Arterial Hypertension, n (%) | 155 (32) | 30 (51) | 125 (29) | 31 (40) | 124 (30) | 0.03 | 1.0 |
| OSA, n (%) | 67 (13) | 12 (20) | 55 (13) | 12 (16) | 55 (13) | 1.0 | 1.0 |
| Ever smoked (n=297), n (%) | 136 (46) | 35 (66) | 101 (41) | 43 (62) | 93 (41) | 0.03 | 0.051 |
| Biological | |||||||
| Platelets (×109/L) | 268 (226–310) | 255 (200–300) | 274 (240–310) | 256 (220–310) | 272 (230–310) | 1.0 | 1.0 |
| Albumin (g/L) | 36 (35–39) | 38 (35–39) | 36 (35–38) | 38 (35–39) | 36 (35–38) | 1.0 | 1.0 |
| Ferritin (ng/mL) | 74 (35–142) | 147 (90–310) | 65.5 (32–120) | 137 (55–280) | 70 (31–122) | 0.002 | 0.002 |
| Bilirubin (mg/dL) | 0.59 (0.47–0.76) | 0.65 (0.5–0.8) | 0.60 (0.5–0.8) | 0.64 (0.5–0.8) | 0.60 (0.5–0.8) | 1.0 | 1.0 |
| AST (IU/L) | 20 (16–27) | 30 (25–42) | 19 (15–24) | 28 (21–42) | 19 (15–24) | 0.002 | 0.002 |
| ALT (IU/L) | 26 (17–42) | 47 (34–70) | 24 (17–36) | 44 (23–78) | 24 (17–36) | 0.002 | 0.002 |
| AlkPh (IU/L) | 71 (60–84) | 72 (60–94) | 71 (60–84) | 74 (62–91) | 70 (60–84) | 1.0 | 1.0 |
| GGT (IU/L) | 30 (20–48) | 52 (36–92) | 27.5 (19–43) | 48 (34–85) | 27 (19–43) | 0.002 | 0.002 |
| Total cholesterol (mg/dL) | 200 (170–230) | 200 (180–240) | 200 (170–220) | 210 (170–250) | 200 (170–220) | 1.0 | 0.5 |
| HDL cholesterol (mg/dL) | 140 (110–170) | 150 (120–190) | 130 (110–160) | 150 (130–190) | 130 (100–170) | 1.0 | 0.6 |
| Triglycerides (mg/dL) | 120 (90–190) | 160 (115–210) | 115 (80–180) | 160 (115–230) | 115 (80–180) | 0.004 | 0.002 |
| HOMA-IR | 5.4 (3.6–8.5) | 8 (5.3–17) | 5.1 (3.4–8.2) | 8.7 (5.4–16) | 5.1 (3.4–7.9) | 0.002 | 0.002 |
AlkPh, alkaline phosphatase; GGT, γ-glutamyltransferase; HOMA-IR, homeostatic model assessment-insulin resistance; OSA, obstructive sleep apnoea.
Continuous variables: median (IQR). P-values adjusted for multiple testing (Bonferroni correction).
Table 2.
Baseline histological data of the Geneva cohort.
| Variable | All subjects | NASH | Fibrosis |
|---|---|---|---|
| N | 492 | 59 | 77 |
| NAFLD (steatosis >5%), n (%) | 439 (89) | 59 (100) | 73 (95) |
| NASH, n (%) | 59 (12) | 59 (100) | 47 (61) |
| Fibrosis, n (%) | 77 (16) | 47 (78) | 77 (100) |
| NAS scoring | |||
| Overall NAS score | 2 (1–3) | 5 (4–6) | 4 (2–5) |
| Steatosis | 2 (1–2) | 3 (2.5–3) | 3 (2–3) |
| Lobular Inflammation | 0 (0–0) | 1 (1–1) | 1 (0–2) |
| Ballooning | 0 (0–0) | 1 (1–2) | 1 (0–1) |
| Fibrosis, n (%) | |||
| stage 0 | 415 (84) | 12 (20) | 0 (0) |
| stage 1 | |||
| stage 1A | 30 (6.1) | 21 (36) | 30 (39) |
| stage 1B | 7 (1.4) | 6 (10) | 7 (9) |
| stage 1C | 22 (4.5) | 2 (3) | 22 (29) |
| stage 2 | 8 (1.6) | 8 (14) | 8 (10) |
| stage 3 | 9 (1.8) | 9 (15) | 9 (12) |
| stage 4 | 1 (0.2) | 1 (2) | 1 (1) |
NAS, non-alcoholic fatty liver disease activity score.
Continuous variables: median (IQR).
Prospectively assessed weight and BMI was available in 81%, 67%, 53% and 34% 1, 2, 3 and 5 years after bariatric surgery, respectively (Supplementary Table 1). Median weight loss and EBMIL at 1 year after bariatric surgery were 38kg and 74% respectively, similar to other reports and systematic reviews of bariatric surgery effectiveness7. No patient required bariatric surgery reversal. In univariable and multivariable analysis, EBMIL greater than 50% was not associated with features at liver histology but inversely correlated to male sex and baseline BMI greater than 45kg/m2 (Supplementary Table 2).
Overall Survival
Median follow-up of the Geneva cohort was 10.2 years (7.0–12 years). Overall, 21/492 (4.3%) individuals died during follow-up, causes of death were cerebrovascular disease in 2/21, non-hepatic neoplasia in 4/21 (all more than 5 years after bariatric surgery), sepsis in 7/21 (not at time of initial surgery), other causes in 7/21 patients (including pulmonary embolus, suicide, accident and one cirrhosis initially attributed to alcohol liver disease but reassessed as multifactorial aetiology due to the short history of alcohol consumption prior to death) and 3/21 unknown. When stratified by cause of death, NASH was associated with death from sepsis in univariable analysis along with other baseline factors (Supplementary Table 3) whereas fibrosis had borderline significance (p=0.067). Events were too rare in the other causes of death to test association with baseline factors.
Baseline factors associated with overall mortality in univariable survival were male sex, age greater than 50 years, presence of diabetes, arterial hypertension, presence of histological NASH and liver fibrosis (Table 3 and Figure 2A–B). In Cox regression multivariable stepwise AIC model selection only NASH (hazard ratio [HR] 2.9, p=0.02) and arterial hypertension (HR 3.9, p=0.02) were independently associated with overall mortality (Table 3). Neither liver fibrosis nor the interaction term between NASH and liver fibrosis were associated with overall mortality in the multivariable Cox regression.
Table 3.
Predictors of overall survival in the Geneva cohort.
| Variable | Number in each group
|
Univariable analysis
|
Mutivariable analysis
|
|||||
|---|---|---|---|---|---|---|---|---|
| Died, n (%) | Alive, n (%) | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Total n | 21 (4) | 471 (96) | ||||||
| Clinical | ||||||||
| Male sex | 9 (43) | 80 (17) | 3.8 | 1.6–9.0 | 0.001 | 2.0 | 0.8–4.8 | 0.14 |
| Age (> 50y) | 12 (57) | 92 (19) | 5.3 | 2.2–13 | <0.001 | 2.5 | 0.9–6.4 | 0.07 |
| BMI (> 45kg/m2) | 11 (52) | 170 (36) | 2.1 | 0.90–5.0 | 0.08 | |||
| Diabetes | 8 (38) | 74 (16) | 3.3 | 1.4–8.0 | 0.005 | - | - | - |
| Arterial hypertension | 16 (76) | 139 (30) | 7.9 | 2.9–22 | <0.001 | 3.9 | 1.2–12 | 0.02 |
| OSA | 4 (19) | 63 (13) | 1.6 | 0.53–4.7 | 0.41 | |||
| Ever smoked | 9 (45) | 127 (46) | 0.96 | 0.40–2.3 | 0.92 | |||
| Histology | ||||||||
| NAFLD (steatosis >5%) | 20 (95) | 419 (89) | 2.2 | 0.29–16 | 0.43 | |||
| NASH | 8 (38) | 51 (11) | 5.1 | 2.1–12 | <0.001 | 2.9 | 1.2–7.4 | 0.02 |
| Fibrosis | 8 (38) | 69 (15) | 3.5 | 1.5–8.6 | 0.003 | - | - | - |
| Biological | ||||||||
| Platelets (> ULN) | 2 (10) | 18 (14) | 0.79 | 0.18–3.4 | 0.75 | |||
| Albumin (< 35 g/L) | 7 (35) | 48 (38) | 0.93 | 0.42–2.7 | 0.89 | |||
| Ferritin (>1.5 × ULN)* | 1 (5) | 13 (3) | 1.51 | 0.20–11 | 0.69 | |||
| Bilirubin (> ULN) | 1 (5) | 7 (2) | 2.8 | 0.37–21 | 0.3 | |||
| AST (> ULN) | 2 (10) | 21 (5) | 2.3 | 0.53–9.8 | 0.25 | |||
| ALT (> ULN)+ | 16 (76) | 296 (64) | 1.9 | 0.68–5.1 | 0.22 | |||
| AlkPh (>ULN) | 1 (5) | 11 (2) | 2.4 | 0.32–18 | 0.37 | |||
| GGT (> ULN) | 14 (70) | 212 (47) | 2.6 | 0.98–6.7 | 0.054 | |||
| Total cholesterol (> 240mg/dL) | 4 (19) | 75 (17) | 1.2 | 0.41–3.6 | 0.73 | |||
| Triglycerides ((> 200mg/dL) | 4 (19) | 74 (17) | 1.1 | 0.36–3.1 | 0.91 | |||
| HOMA-IR (>3) | 15 (88) | 321 (81) | 2.1 | 0.46–9.2 | 0.33 | |||
| Outcome | ||||||||
| EBMIL > 50% | 14 (78) | 286 (72) | 1.9 | 0.62–5.8 | 0.27 | |||
AIC, Akaike information criteria; AlkPh, alkaline phosphatase; CI, confidence interval; EBMIL, excess BMI loss; GGT, γ-glutamyltransferase; HOMA-IR, homeostatic model assessment-insulin resistance HR, hazard ratio; OSA, obstructive sleep apnoea; ULN, upper limit of normal
Ferritin: >300ng/ml in women and > 450 ng/ml in men,
ALT: Men: ULN3<330 IU/L, Women: ULN3<319 IU/L
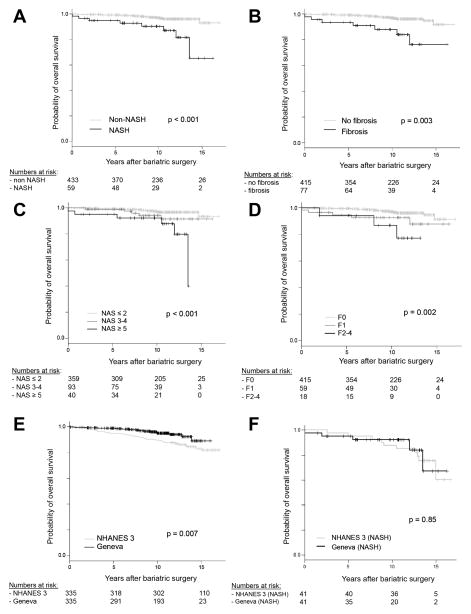
Figure 2.
A–D, Survival of the Geneva bariatric surgery cohort stratified by NASH, fibrosis, NAS score and fibrosis score. E-F, Survival comparison of propensity-score matched Geneva and NHANES III cohorts (n=335 in each cohort). F, Survival comparison of NASH subjects in the propensity-score matched Geneva and NHANES III cohorts (n=41 in each cohort).
To further clarify whether metabolic cofactors were driving the association between increased mortality and NASH, we performed a matched case-control analysis within the Geneva cohort. We matched the 59 NASH subjects with 59 non-NASH controls for age, presence of diabetes and arterial hypertension. This yielded 59 pairs of NASH cases and matched non-NASH controls with identical proportions of diabetes (46%), arterial hypertension (51%) and age greater than 50 years (34%) although, as expected, AST, ALT and GGT were significantly elevated in the NASH group (Supplementary Table 4). Despite matching for features of the metabolic syndrome, NASH at baseline biopsy was still associated with increased long-term mortality (HR=8.8, 95%CI 1.1–70, p=0.04).
When comparing baseline liver histology between subjects alive and dead at end of follow-up, deceased subjects had a higher proportion of NASH (38% versus 11%, p=0.01), fibrosis (38% versus 18%, p=0.045) a higher median NAS score (3 versus 2, p<0.001) and more advanced fibrosis (p=0.03) (Supplementary Table 5). In survival analysis, individuals with a NAS score ≥ 5 had significantly higher mortality than patients with a NAS score of ≤ 2 (p<0.001), whereas patients with fibrosis stage 1 and stage ≥ 2 had a significantly increased mortality than patients without fibrosis (p=0.048 and p=0.005 respectively) (Figure 2C–D and Supplementary Table 6).
Comparison with matched obese subjects in NHANES III
In order to assess the effect of bariatric surgery on the survival of obese patients, the Geneva cohort was matched, using propensity-score matching, to 18723 subjects from the NHANES III cohort (Figure 1: propensity-score matching #1). This yielded 335 matched pairs of patients with similar baseline characteristics (Supplementary Table 7, Supplementary Figure 1A–B). Mortality was significantly reduced in the patients undergoing bariatric surgery in univariable and multivariable analysis (HR 0.54, p=0.035, Figure 2E, Supplementary Table 8).
To assess the potential impact of bariatric surgery on overall survival in patients with NASH, we matched the subset of patients in the Geneva cohort with histological NASH (n=59) with subjects from NHANES III with at least moderate steatosis on liver ultrasound and raised ALT (Figure 1: propensity-score matching #2). In the Geneva cohort, when compared to the histological gold standard, this surrogate marker of NASH had a sensitivity of 74%, specificity of 96%, positive predictive value of 83% and negative predictive value of 93% for the diagnosis of NASH. This index was confirmed to be prognostic in the Geneva cohort as the 145 subjects with steatosis and raised ALT had increased mortality (HR 3.7, p=0.003). This matching yielded 41 pairs of subjects with similar baseline characteristics despite higher ferritin in the NHANES III cohort (Supplementary Table 9, Supplementary Figure 2A–B). In this analysis, bariatric surgery was not significantly associated with reduced survival (Figure 2F), whereas older age was associated with worse survival (Supplementary Table 10).
Gene signature-based prognostication in NASH patients
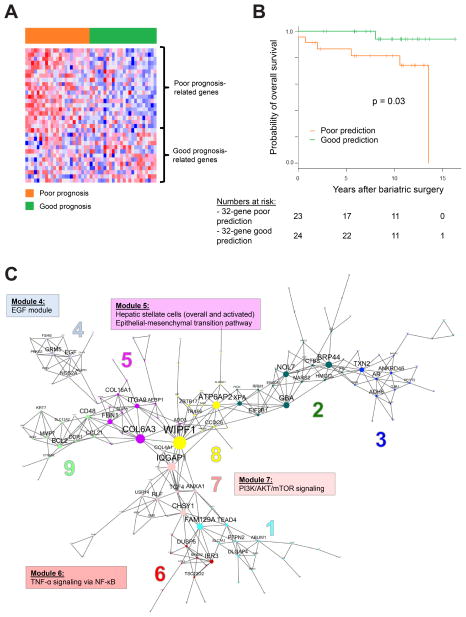
We next assessed whether molecular-based risk assessment in hepatic tissue of subjects undergoing bariatric surgery could improve risk stratification, especially in NASH patients. Forty-seven NASH subjects had available FFPE tissue blocks for transcriptome analysis. Median BMI and weight were 44.6kg/m2 and 125kg respectively, median NAS was 5 and seven subjects (15%) died during follow-up (Supplementary Table 11). A previously published 32-gene signature 19 (Supplementary Table 12) was found to be significantly associated with NAFLD severity in a previous cohort of 72 NAFLD subjects 23 (Supplementary Figure 3). The 32-gene poor prognosis prediction was associated with increased overall mortality in Geneva cohort NASH subjects (log-rank p=0.03). In multivariable Cox regression modelling, age greater than 50 years (HR 14, p=0.02) and 32-gene signature poor prognosis prediction (HR 9.0, p=0.045) were independently associated with increased overall mortality (Figure 3A–B, Supplementary Table 13).
Figure 3.
Gene expression signature prediction of overall survival in 47 NASH patients in the Geneva bariatric surgery cohort. A, 32-gene signature expression pattern, red and blue colors in the heatmap indicate high and low expression respectively. Each patient is represented as a column, individual genes are represented as rows. B, Survival of subjects based on their 32-gene prediction group. C, Gene regulatory network in NASH tissues. Each gene sub-module in the network is indicated by a different color and number. Enriched molecular pathways associated to 4 specific modules are highlighted.
EGF, epidermal growth factor; mTOR, mechanistic target of rapamycin
In the NASH gene expression profiles, MEGENA identified 9 distinct gene regulatory modules (Figure 3C, Supplementary Table 14). A tightly clustered gene module (no.4) was centred on a hub gene, epidermal growth factor (EGF), a fibrogenic and carcinogenic driver implicated as an HCC chemoprevention target in our previous study 24, which has led to initiation of a clinical trial of EGF receptor inhibitor, erlotinib (clinicaltrials.gov, trial number NCT02273362). Module no.6 was enriched with TNF-α signalling via NF-κB pathway (p<0.001), indicating presence of inflammatory pathway activation in NASH-affected liver (Supplementary Table 15). Module no.5 was enriched with collagen-encoding genes, TGF-β target genes such as TGFB1I1, epithelial-mesenchymal transition pathway (p=0.002), as well as 2 previously published hepatic stellate cell signatures 25, 26, evidencing activated fibrogenesis (p<0.05).
Discussion
In this retrospective, single-centre cohort, we have demonstrated that presence of NASH at baseline liver histology of severely obese subjects undergoing bariatric surgery is associated with increased long-term mortality compared to those without NASH. Of greater concern, despite reduced overall mortality for obese subjects undergoing bariatric surgery compared to matched, non-surgical, subjects, patients with NASH at baseline did not have improved survival after bariatric surgery. Importantly, we identified that a poor prognosis 32-gene signature prediction 19 was associated with NAFLD severity in a previously published cohort of 72 subjects with NAFLD 23 and could identify poor prognosis NASH subjects in the Geneva cohort with increased long-term mortality compared to control obese NASH subjects.
To our knowledge, our study is the first analysis of the relationship between perioperative findings at liver biopsy and long term outcomes after bariatric surgery. Previous reports have demonstrated that the beneficial effects of bariatric surgery are not only linked to a reduction of body weight but also to an improvement in metabolic parameters such as type 2 diabetes, hypertension and dyslipidaemia 7. Paired liver biopsy studies have also demonstrated a reduction of NASH prevalence over 5 years after surgery and improvement of fibrosis 27, 28. Nevertheless, despite these encouraging results, our data suggests that subjects with baseline NASH have an increased mortality after bariatric surgery and their overall survival may not improve after bariatric surgery despite no significant difference in weight loss. Further studies will have to examine whether persistence of diabetes was associated with NASH and long-term mortality. Interestingly, when performing a subgroup analysis on cause of death in our cohort, NASH was associated with mortality from sepsis. This could suggest that the association between NASH and mortality in our study captures a risk factor for non-hepatic mortality as underlined by the finding that only one person died from liver-related cause in our cohort. This could also explain why the association with overall mortality and NASH seemed stronger than the association with fibrosis, as opposed to longitudinal studies in non-bariatric surgery NAFLD subjects, indicating again that NASH could be a better predictor of overall, non-hepatic mortality whilst fibrosis may better predict liver-related mortality 29, 30. In addition, the finding that TNF-α signalling was enriched in one of the gene regulatory module, may suggest a link with altered intestinal permeability 31 and possibly a lack of reversal to a more favourable metabolic profile in a subgroup of individuals captured by the gene signature.
Potential limitations of our study include the selection of different populations for our bariatric surgery cohort and the US-based control NHANES III cohort although we performed careful propensity-score matching to minimize bias with well-matched major predictors of prognosis such as age, or features of the metabolic syndrome. Although the diagnosis of NASH could not be histologically documented in the control NHANES III cohort due to the absence of liver biopsy, we believe that our two-tier selection of patients, first by selecting subjects with steatosis and raised ALT then by propensity-matching these subjects to the histologically documented Geneva cohort NASH subjects, limited potential biases. In addition, we tested the diagnostic performance of this previously reported 32, 33 non-invasive definition of NASH in the Geneva cohort and confirmed that it was associated with prognosis. Finally, although potential bariatric surgery in the NHANES III control group could represent a bias, only 2.9% of adults eligible for bariatric surgery in the US underwent the procedure between 1998 and 2006, suggesting that the impact of potential bariatric surgery in the control NHANES III group would be relatively low 34.
In conclusion, we have shown that features found at baseline liver histology predict long-term overall survival in severely obese subjects undergoing bariatric surgery. Our results also suggest that a subgroup of subjects with NASH and a poor prognosis 32-gene signature is at particularly high risk of long-term mortality after bariatric surgery and may require specific management and follow-up. We believe these findings also reinforce the role of systematic perioperative liver biopsy in subjects undergoing bariatric surgery as a prognostic and diagnostic tool. Further experimental and clinical studies will be required to characterize and define these novel findings.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by grants from the FLAGS foundation, the Nuovo-Soldati Cancer Research Foundation and an advanced training grant from Geneva University Hospital to NG and by NIH/NIDDK R01 DK099558, Irma T. Hirschl Trust, and Dr. Harold and Golden Lamport Research Award to YH
Abbreviations
- AIC
Akaike information criteria
- ALT
alanine transaminase
- BMI
body mass index
- EBMIL
excess BMI loss
- EGF
epidermal growth factor
- FDR
false discovery rate
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HR
hazard ratio
- MEGENA
Multiscale Embedded Gene Co-expression Network Analysis
- mTOR
mechanistic target of rapamycin
- NAFLD
non-alcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
non-alcoholic steatohepatitis
- NHANES III
National Health and Nutrition Examination Survey
- OR
odds ratio
Footnotes
Disclosures
The authors disclose no conflicts
Transcript Profiling
Microarray data are available at National Center for Biotechnology Information Gene Expression Omnibus (GSE69248).
Reviewer access link to the transcriptome profiling dataset: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=clsncgimvbajneb&acc=GSE69248
Author Contributions
study concept and design: NG, YH, JLF, LS, FN, LRB, EG
acquisition of data: NG, LS, MJ, WS, LRB, EG
analysis and interpretation of data: NG, YH, WS, EG
drafting of the manuscript: NG
critical revision of the manuscript for important intellectual content: all authors
obtained funding: NG, YH, JLF, LRB, EG
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual Medical Spending Attributable To Obesity: Payer-And Service-Specific Estimates. Health Affairs. 2009;28(5):w822–w31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–90. doi: 10.1038/nrgastro.2013.171. Epub 2013/09/18. [DOI] [PubMed] [Google Scholar]
- 6.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600–6. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Puzziferri N, Roshek TB, 3rd, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams TD, Gress RE, Smith SC, et al. Long-Term Mortality after Gastric Bypass Surgery. New England Journal of Medicine. 2007;357(8):753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 9.Sjöström L, Narbro K, Sjöström CD, et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. New England Journal of Medicine. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 10.Maciejewski ML, Livingston EH, Smith VA, et al. Survival among high-risk patients after bariatric surgery. JAMA. 2011;305(23):2419–26. doi: 10.1001/jama.2011.817. [DOI] [PubMed] [Google Scholar]
- 11.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–54. doi: 10.1056/NEJMoa0901836. Epub 2009/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas H, Agrawal S. Systematic Review of Obesity Surgery Mortality Risk Score—Preoperative Risk Stratification in Bariatric Surgery. Obesity Surgery. 2012;22(7):1135–40. doi: 10.1007/s11695-012-0663-7. [DOI] [PubMed] [Google Scholar]
- 13.Livingston EH, Huerta S, Arthur D, et al. Male Gender is a Predictor of Morbidity and Age a Predictor of Mortality for Patients Undergoing Gastric Bypass Surgery. Annals of Surgery. 2002;236(5):576–82. doi: 10.1097/00000658-200211000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arterburn D, Livingston EH, Schifftner T, et al. Predictors of long-term mortality after bariatric surgery performed in veterans affairs medical centers. Archives of Surgery. 2009;144(10):914–20. doi: 10.1001/archsurg.2009.134. [DOI] [PubMed] [Google Scholar]
- 15.Chassot G, Huber O, Koutny-Fong P, et al. Surgical treatment of obesity in 2006. Rev Med Suisse. 2006;2(70):1568–71. Epub 2006/07/15. Chirurgie de l’obesite en 2006. [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. doi: 10.1002/hep.20701. Epub 2005/05/26. [DOI] [PubMed] [Google Scholar]
- 17.Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–20. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control Prevention (CDC), National Center for Health Statistics (NCHS) U.S. Department of Health and Human Services, CDC, editor. National Health and Nutrition Examination Survey III, 1988–1994. Hyattsville, MD: 1998. [Google Scholar]
- 19.King LY, Canasto-Chibuque C, Johnson KB, et al. A genomic and clinical prognostic index for hepatitis C-related early-stage cirrhosis that predicts clinical deterioration. Gut. 2014 doi: 10.1136/gutjnl-2014-307862. Epub 2014/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshida Y. Nearest template prediction: a single-sample-based flexible class prediction with confidence assessment. PloS one. 2010;5(11):e15543. doi: 10.1371/journal.pone.0015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song W-M, Di Matteo T, Aste T. Building complex networks with Platonic solids. Physical Review E. 2012;85(4):046115. doi: 10.1103/PhysRevE.85.046115. [DOI] [PubMed] [Google Scholar]
- 22.Song W-M, Di Matteo T, Aste T. Nested hierarchies in planar graphs. Discrete Applied Mathematics. 2011;159(17):2135–46. [Google Scholar]
- 23.Moylan CA, Pang H, Dellinger A, et al. Hepatic gene expression profiles differentiate pre-symptomatic patients with mild versus severe nonalcoholic fatty liver disease (Severe NAFLD Gene Signature) Hepatology. 2013 doi: 10.1002/hep.26661. Epub 2013/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs BC, Hoshida Y, Fujii T, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59(4):1577–90. doi: 10.1002/hep.26898. Epub 2014/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drews F, Knobel S, Moser M, et al. Disruption of the latent transforming growth factor-beta binding protein-1 gene causes alteration in facial structure and influences TGF-beta bioavailability. Biochim Biophys Acta. 2008;1783(1):34–48. doi: 10.1016/j.bbamcr.2007.08.004. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Goossens N, Guo J, et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut. 2015 doi: 10.1136/gutjnl-2015-309655. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathurin P, Hollebecque A, Arnalsteen L, et al. Prospective Study of the Long-Term Effects of Bariatric Surgery on Liver Injury in Patients Without Advanced Disease. Gastroenterology. 2009;137(2):532–40. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 28.Lassailly G, Caiazzo R, Buob D, et al. Bariatric Surgery Reduces Features of Non-alcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–97. e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2014 doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 31.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–85. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011:343. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–12. doi: 10.1016/j.jhep.2008.06.018. Epub 2008/08/07. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen NT, Masoomi H, Magno CP, et al. Trends in Use of Bariatric Surgery, 2003–2008. Journal of the American College of Surgeons. 2011;213(2):261–6. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.