Abstract
Background
Depression is the most common form of mental disorder in community and health care settings and current treatments are far from satisfactory. Vagus nerve stimulation (VNS) is an FDA-approved somatic treatment for treatment-resistant depression. However, the involvement of surgery has limited VNS only to patients who have failed to respond to multiple treatment options. Transcutaneous VNS (tVNS) is a relatively new, non-invasive VNS method based on the rationale that there is afferent / efferent vagus nerve distribution on the surface of the ear. The safe and low-cost characteristics of tVNS have the potential to significantly expand the clinical application of VNS.
Methods
In this study, we investigated how tVNS can modulate the default mode network (DMN) functional connectivity (FC) in mild or moderate major depressive disorder (MDD) patients. Forty-nine MDD patients were recruited, and received tVNS or sham tVNS (stVNS) treatments.
Result
34 patients completed the study and were included in data analysis. After one month of tVNS treatment, the 24-item Hamilton Depression Rating Scale (HAMD) score reduced significantly in the tVNS group as compared to the stVNS group. The FC between the DMN and anterior insula and parahippocampus decreased; the FC between the DMN and precuneus and orbital prefrontal cortex increased compared to stVNS. All these FC increases are also associated with HAMD reduction.
Conclusions
tVNS can significantly modulate the DMN FC of MDD patients; our results provide insights to elucidate the brain mechanism of tVNS treatment for MDD patients.
Keywords: Major depressive disorder, vagus nerve stimulation, transcutaneous vagus nerve stimulation, fMRI, default mode network, functional connectivity
Introduction
Major depressive disorder (MDD) is the fourth leading cause of disability worldwide (1), and is projected to become the second leading cause of disability worldwide by the year 2020 (2-3). Despite the critical need, current treatments for these disorders are far from satisfactory due to high non-response rate to treatments, high relapse rates, and frequent intolerable side effects (1, 3).
Vagus nerve stimulation (VNS) is a relatively new FDA-approved somatic treatment for treatment-resistant depression (TRD) that can produce significant and clinically meaningful antidepressant effects (1, 4-6). However, the involvement of surgery, perioperative risks, and potentially significant side effects have limited this treatment to patients who have been treated for depression in the past but have failed to respond to at least 4 prescribed medications and/or established somatic treatment options such as electroconvulsive therapy for MDD (7).
To overcome the potential barriers of applying VNS, a non-invasive VNS method, transcutaneous vagus nerve stimulation (tVNS) has been developed. The rationale for using tVNS on the ear is based on anatomical studies that suggest the ear is the only place on the surface of the human body where there is afferent vagus nerve distribution (8-9). Thus, direct stimulation of the afferent nerve fibers on the ear should produce an effect similar to classic VNS in reducing depressive symptoms, but without the burden of surgical intervention (10-11). In past years, tVNS has been applied to treat MDD (10) and other disorders such as epilepsy (12-13), and pre-diabetes (14). Yet, the underlying mechanism of tVNS remains unclear.
In past decades, with the aid of brain imaging tools, accumulating evidence has demonstrated that MDD is associated with structural and functional abnormalities in brain circuits involved in emotional processing, self-representation, reward, and external stimulus (stress, distress) interactions (15-22); these brain regions include the hippocampus, amygdala, anterior cingulated cortex, and medial prefrontal cortex. Interestingly, these brain regions also fall within the default mode network (DMN), a brain network believed to be involved in self-referential processing, affective cognition, and emotion regulation (23-24).
Thus, the DMN functional connectivity has drawn investigators’ attention in MDD research (25-29). Studies (30-36) found DMN functional connectivity changes in MDD patients, and these changes are associated with psychiatric measurements, such as rumination score, in MDD patients. For instance, studies (26, 30) have found increased functional connectivity of DMN with the subgenual anterior cingulate cortex (sACC) in MDD patients. After Transcranial Magnetic Stimulation treatment, the abnormal increase in functional connectivity of DMN and sACC was reduced (34). These studies demonstrated that the FC of DMN can be a useful tool in understanding the underlying mechanism of MDD treatment.
In this study, we investigated the resting state functional connectivity (rsFC) changes after the longitudinal tVNS as compared with sham tVNS (stVNS) in patients with mild or moderate depressive symptoms. We hypothesize that the longitudinal tVNS may significantly modulate the rsFC of DMN and reduce symptoms in MDD patients.
Methods and design
Study population
Forty-nine patients with mild or moderate MDD were recruited for the trial. ICD-10 Classification of Mental and Behavioral Disorders was used for diagnosis of MDD. Patients who voluntarily provided informed consent and met inclusion/exclusion criteria were enrolled in this study.
Inclusion criteria included: 1) Patient meets ICD-10 diagnosis standard: mild (2 typical + 2 other core symptoms), moderate (2 typical + 3 other core symptoms); 2) Patient is 16-70 years of age; 3) Patient stopped taking anti-depressive medication or other psychiatric medications 2 weeks before the intervention started; 4) Patient has a junior high school education and can understand the scales; 5) Patient has exhibited symptoms for at least 2 months, but no longer than 2 years.
Exclusion criteria included: 1) Patients with current addiction to drugs; 2) Patients with severe depression or suicidal thoughts; 3) Patients with other severe organic diseases, such as severe heart disease, kidney failure etc; 4) Patients who did not agree to sign the consent form.
Recruitment procedures
All patients were recruited using advertisements and by sending flyers to the four hospitals involved in the study. In this study, tVNS was the only treatment the patients received. Due to safety concerns and to increase the homogeneity of the study, we decided to include only patients with mild or moderate depressive symptoms. After passing a pre-screening, potentially eligible patients provided informed consent in the presence of a study physician.
We used a single blinded clinical trial to investigate the antidepressant effect of solo tVNS treatment. The first cohort all received tVNS. After demonstrating the effect of tVNS, we recruited the second cohort of patients who received four weeks of sham tVNS as a control in this study.
Intervention
After group randomization, all patients were trained to use the tVNS or stVNS device to apply stimulation. All subsequent treatments were self-administered by the patients at home. Patients were also instructed to complete a patient diary booklet each day to describe any side effects corresponding with or temporally related to treatment. The investigators checked all booklets at the 4-week assessments. All procedures performed in the stVNS treatment group were identical to the procedures for the tVNS group.
tVNS treatment
Location
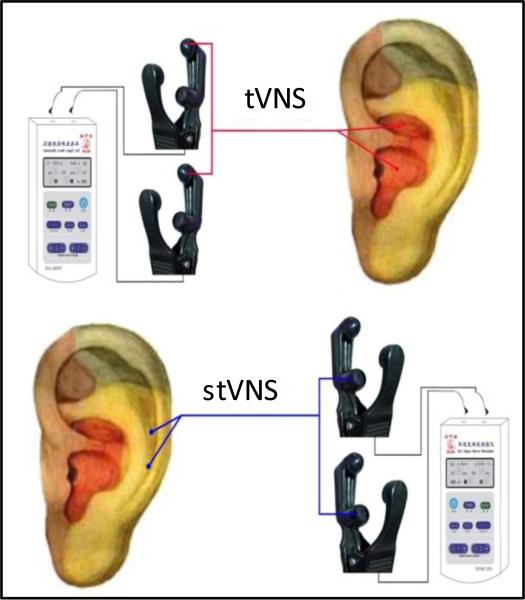
The points for tVNS are located in the auricular concha area where there are rich vagus nerve branch distributions (Figure 1).
Figure 1.
Locations of the stimulation electrodes on the auricular surface for tVNS and stVNS.
Intervention procedure
Patients took a seated position or lay on their side. After the stimulation points were disinfected according to standard practice, ear clips were attached to the ear area (auricular concha) at the stimulation site. Stimulation parameters included: 1) density wave adjusted to 20Hz, with a wave width less than 1ms, and 2) intensity adjusted based on the tolerance of the patient (~ 4-6 mA). Each treatment lasted 30 minutes and was carried out twice a day, at least 5 days per week for the duration of the treatment period (4 weeks).
StVNS treatment
Location
The stimulation points for stVNS are located at the superior scapha (outer ear margin midpoint) where there is no vagus nerve distribution (Figure 1).
Since all treatments were self-administered by the MDD patients, all patients were required to complete daily entries in a diary that was checked during assessments (at the end of the week 4) to enhance compliance.
Clinical outcomes and statistical analysis
All endpoints were measured at week 0 and week 4. The primary endpoint is the 24-item Hamilton Depression Rating Scale (HAMD). The secondary endpoints included the Hamilton Anxiety Rating Scale (HAMA), Self-Rating Anxiety Scale (SAS), and Self-Rating Depression Scale (SDS).
Our analyses were based on the intention-to-treat principle. Statistical analysis was performed using SPSS 19.0 Software (SPSS Inc., Chicago, IL, USA). Repeated measurements were applied to compare primary and secondary outcomes. Age and gender were also included in the model as covariates.
fMRI Data acquisition and analysis
Each subject participated in identical fMRI scanning sessions before and after one month of treatment. The fMRI brain imaging acquisition was conducted on a 1.5 Tesla GE Signa MRI system equipped with the standard two channels’ Birdcage head coil. T1-weighted high-resolution structural images were acquired with the 3DFSPGR sequence (matrix 192 × 256, FOV 200 mm, flip angle 15 degree, slice thickness 1.4 mm). T2-weighted functional images encompassing the whole brain were acquired with the gradient echo EPI sequence (TE 30 ms, TR 2500 ms, matrix 64 × 64, FOV 240 mm, flip angle 90 degree, slice thickness 3.0 mm, gap 0.5 mm, 41 slices, paralleled by AC-PC line). Image collection was preceded by 4 dummy scans to allow for equilibration of the MRI signal. Two six-minute resting state fMRI scans were applied while the subjects were required to keep their eyes open.
We analyzed pre- and post-treatment resting state data using Independent Component Analysis in the FMRIB Software Library (FSL) (37-40). We first applied a band pass filter between 0.01 and 0.1 Hz to the functional time series, and then corrected for motion using MCFLIRT. We corrected slice timing and stripped the skull using the Brain Extraction Tool (BET), and smoothed it (full width at half maximum = 5 mm). Next, we registered the functional data to their respective skull stripped anatomical volume and further registered it to the MNI152 template using linear affine transformations with 12 degrees of freedom. We then concatenated the functional data into 4D data and performed a probabilistic independent component analysis using MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) (41) on the data set to identify 20 resting state networks. Using an algorithm, we searched for similarities between the default mode network (DMN) in our group-level networks and the template networks derived from 1414 healthy subjects to identify the corresponding network for our results (38).
Then, dual-regression was applied (38). Using the previously defined DMN as spatial regressors in a general linear model (GLM), we were able to extract the temporal dynamics associated with each spatial map. The resulting time courses served as temporal regressors in a GLM to generate subject-specific-maps of the whole brain for each subject. Finally, group analyses were performed using the whole-brain subject-specific network maps from the second GLM. The results represent the strength of FC for each voxel within the DMN.
To investigate the modulation effect of tVNS treatment, we compared the changes after treatment (post-pre) in the tVNS group to that of the stVNS group. To explore the association between the clinical outcomes and FC changes, we performed regression analyses using the network connectivity “change maps” between post- and pre-treatment and corresponding changes in clinical outcomes. For both analyses, age, gender were included in the model as non-interest covariates. To further control the potential residual effect of head motion, we also include average head motion difference (post-pre) for each individual as covariate during analysis (42). A threshold of voxel-wise Z > 2.6 and a corrected cluster significance threshold of P < 0.05 with more than 20 contiguous voxels was applied in data analysis.
Results
Out of 49 subjects (27 in the VNS group, 22 in the control group) recruited into the study, 35 patients (19 in VNS group, 16 in control group) completed the two fMRI scans (0 and 4 weeks). Out of 8 patients who dropped out in the VNS group, 4 dropped due to loss of contact, 3 due to disinterest, and 1 due to a scheduling conflict. Of the 7 patients who dropped out in the stVNS group, 3 dropped due to scheduling conflicts, 3 due to disinterest, and 1 due to poor data quality. There was no statistical difference in dropout rate between the two groups.
Of 35 subjects who completed the study, we excluded one additional subject in the tVNS group due to large head motion during data analysis. Of the 34 patients left for data analysis, there was no significant difference on head motion pre- and post-treatment between two groups (p = 0.89 and 0.95 between the tVNS and stVNS groups using two sample t-test).
Baseline characteristics of the two groups were shown in Supplementary Table 1. There was no significant difference between the two groups in age, gender, HAMD, HAMA, SDS and SAS at the beginning of the treatment.
The clinical outcome measurements of pre- and post-treatment are shown in Table 1. Repeated measurements showed that there was significant interaction between the treatment group (tVNS and stVNS) and treatment time (pre- and post-treatment) on all measured clinical outcomes (HAMD, p = 0.002; SAS: P = 0.013; SDS P =0.012) except HAMA, which showed a marginal significant difference (P =0.053). In addition, we also found a significant main effect of treatment time point (pre > post-treatment) in SDS (p = 0.019).
Table 1.
Clinical outcome measurements in each group (pre and post-treatment).
| Items | tVNS group | stVNS group | ||
|---|---|---|---|---|
| pre-treatment | Post-treatment | pre-treatment | Post-treatment | |
| HAMD* | 28.50±6.42 | 15.00±4.59 | 28.56±4.79 | 23.00±4.68 |
| HAMA | 19.61±5.88 | 11.67±7.06 | 16.88±4.19 | 13.44±3.71 |
| SAS score* | 56.56±8.69 | 42.83±10.90 | 58.50±10.71 | 54.44±5.72 |
| SDS score* | 66.33±11.11 | 50.56±12.05 | 66.94±9.28 | 61.19±10.02 |
indicates significant difference in pre- and post-treatment changes (P< 0.05) using repeated measurements after adjusting for age and gender.
fMRI result
The DMN obtained from ICA analysis is shown in Supplementary Figure 1. The network is consistent with previously published results (38). Brain regions observed in the network include the medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), precuneus, posterior cingulate cortex (PCC), and the bilateral parietal cortex.
The comparison on the post- and pre-treatment differences between the two groups showed that after treatment, patients in the tVNS group, as compared with patients in the stVNS group (tVNS > stVNS), showed significant FC differences between the DMN and the following regions: the bilateral fusiform gyrus and thalamus; the left orbital prefrontal cortex (OPFC), precuneus, temporoparietal junction; the right superior prefrontal cortex, dorsal lateral prefrontal cortex (DLPFC), middle temporal gyrus, and parahippocampus (Supplementary Table 2, Figure 2).
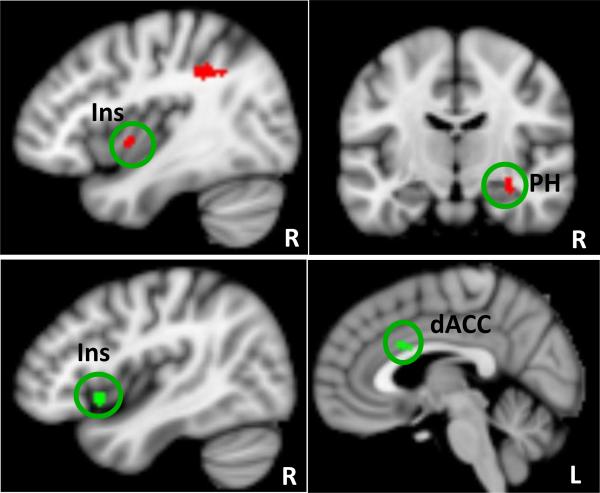
Figure 2.
Red (top row) indicates brain regions that showed significant FC decrease with the default mode network (DMN) in the tVNS group as compared to stVNS. Green color (bottom row) indicates brain regions whose DMN FC changes (post- minus pre-treatment) are positively associated with the corresponding HAMD score changes across all subjects. dACC: dorsal anterior cingulate cortex; Ins: insula; PH: parahippocampus; L: left; R: right.
The opposite comparison (stVNS > tVNS) showed significant FC differences between the DMN and the bilateral operculum and occipital cortex, left medial prefrontal cortex (MPFC), superior temporal gyrus, postcentral gyrus, occipitotemporal gyrus; right insula, parahippocampus / hippocampus, and inferior parietal lobule (Supplementary Table 2, Figure 3).
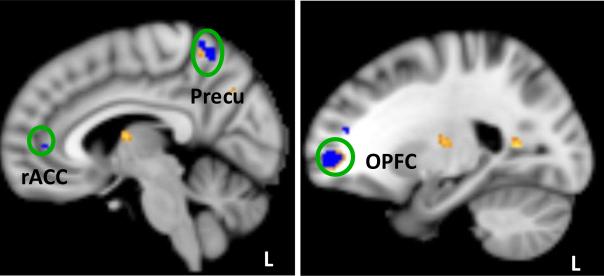
Figure 3.
Yellow indicates brain regions that showed significant FC increase with default mode network (DMN) in the tVNS group as compared to stVNS. Blue indicates brain regions whose DMN FC changes (post- minus pre-treatment) are negatively correlated with the corresponding HAMD score changes across all subjects. Precu: precuneus; OPFC: orbitoprefrontal cortex, L: left.
Regression analysis showed a positive association between the depression severity change as measured by HAMD (post- vs pre-treatment) score and the corresponding FC changes between the DMN and the left OPFC, middle occipital gyrus, right operculum, anterior insula, dorsal anterior cingulate cortex (dACC), inferior parietal lobule, inferior temporal gyrus, and cuneus (Supplementary Table 3 and Figure 2). A negative association between the depression severity change as measured by HAMD (post- vs pre-treatment) and the corresponding FC changes between the DMN and the bilateral DLPFC, fusiform, left MPFC, operculum, insula, temporoparietal junction, the right OPFC, rostral anterior cingulate cortex (rACC), precuneus, the middle temporal gyrus, and parahippocampus (Table 3 and Figure 3). The brain regions of the OPFC and precuneus overlap with the contrast comparing tVNS and stVNS (tVNS > stVNS) (Figure 3)
Discussions
In this study, we investigated the DMN rsFC changes before and after one month of tVNS treatment as compared with stVNS. The results showed that the HAMD and other clinical outcomes significantly decreased in the tVNS group as compared with stVNS. Further, we found tVNS can significantly modulate the rsFC of DMN in brain regions associated with emotion and affect. The rsFC changes in the DMN are also significantly associated with the MDD severity changes as indicated by HAMD score, implying that tVNS may achieve the treatment effect by modulating the FC of the DMN.
In a previous pilot study (10), Hein and colleagues investigated the short- term (2 weeks) therapeutic effect of taVNS patients suffering from major depression using an add-on design. They found that taVNS can significantly reduce the Beck Depression index (BDI) as compared with the sham condition. But there is no significant difference on the HAMD. In this study, tVNS could not only reduce HAMD and SDS, but also reduce the anxiety symptoms in these patients as indicated by SAS and HAMA (marginally significant) in comparison with stVNS at week 4. These results suggest that tVNS may also be extended to anxiety disorders in the future.
Interestingly, we found that the symptoms in the stVNS group also showed a significant decrease after treatment (HAMD p = 0.002, HAMA p = 0.001, SDS p = 0.03 using paired t-test to compare pre- and post-treatment outcomes). We speculate that this reflects the placebo effect produced by stVNS. The large placebo effect observed in our study is consistent with findings from previous clinical trials testing the efficacy of antidepressants (43).
MDD is a common and complex disease with unknown pathogenesis (21, 44). The monoamine hypothesis proposes that low levels of brain monoamines, such as serotonin, noradrenaline and dopamine, are responsible for the development of MDD (21, 44). Recently, accumulating evidence has suggested that cholinergic system is also involved in pathophysiology of depressive disorder (44-45). Specifically, it was believed that MDD derived from interaction of multiple genetic and environmental factors, which further caused high susceptibility to stress. Both chronic stress and depression are associated with cholinergic dysfunctions and structural and functional alterations in brain areas including the hippocampus, PFC and amygdala, which account for development of the cognitive symptoms of depression, such as attention deficit, memory impairment and negativity bias (44). Based on regional glucose metabolism in MDD patients, Merberg posed a limbic-cortical dysregulation model (46-47). Based on the model, MDD are associated with metabolism decreases in dorsal limbic (anterior and posterior cingulate) and neocortical regions (prefrontal, premotor, parietal cortex) and relative increases in ventral paralimbic areas (subgenual cingulate, anterior insula, hypothalamus, caudate). MDD remission requires the inhibition of overactive ventral areas and disinhibition of underactive dorsal regions.
Despite the clinical application of VNS / tVNS for MDD patients, its underlying mechanism is not fully understood (3, 48-49). Hypotheses are based on the impact of anatomy and function / neurotransmitter changes evoked by VNS / tVNS in mood control (50). The vagus nerve is a mixed nerve composed of about 80% afferent fibers. It is speculated that the antidepressant effects of VNS are partially attributed to the projection of afferent fibers to the nucleus tractus solitaries, which is further connected directly and indirectly with brain structures including the reticular formation in the amygdala, hypothalamus, insula, thalamus, orbitofrontal cortex, and other limbic regions responsible for mood and anxiety regulation (51-54). Interestingly, some of these brain regions are also believed to be involved in the pathogenesis and remission of MDD (44-47), which builds a basis for treatment with NVS. In addition, both clinical and animal studies indicate that VNS can produce changes of neurotransmitters implicated in the pathogenesis of MDD (55), including serotonin (56), norepinephrine (57), GABA, and glutamate (58).
The DMN is a network of brain regions that are active when the individual is not focused on the outside world and the brain is at wakeful rest. Previous studies have suggested that the DMN is associated with the self-referential system, affective cognition, and emotion regulation (25-26, 29). MDD patients are characterized by increase in self-focus and stress-sensitivity (59). Thus, accumulating evidence indicates that the DMN plays an important role in neuropathology of MDD (30-36, 60-62).
Recently, brain imaging tools have been applied to investigate the fMRI signal change evoked by tVNS (63-65). In an early study, Kraus and colleagues (63) found that robust tVNS can induce fMRI signal decreases in limbic brain areas, including the amygdala, hippocampus, parahippocampal gyrus and middle and superior temporal gyrus, as well as an fMRI signal increase in the insula, precentral gyrus and thalamus. Psychometric assessment revealed a significant improvement in well-being after tVNS. The control intervention (earlobe stimulation) did not show similar fMRI or psychometric effects. In a follow-up study (65), the authors also studied the anterior wall and posterior wall auditory canal separately. The results showed that stimulation of the anterior wall evoked significant limbic deactivation at the parahippocampal gyrus and the posterior cingulate cortex, as well as other regions including the thalamus, locus coeruleus and solitary tract.
Consistent with the above brain imaging studies (63-65) that focus on the immediate brain response during tVNS, we found that after one month of tVNS treatment, the FC between the DMN and right anterior insula, and parahippocampus significantly decreased.
Anterior insula belong to a salience network, which is involved in detecting and orienting to both external and internal salient stimuli and events (66-67). Investigators believe that the anterior insula is critical in maintaining and updating representations of current and predictive salience (66, 68). Previous studies have suggested that the right anterior insula is involved in responses to salient stimuli via switching between the DMN-related self-referential network and executive network related to goal directed activities (66, 69).
The parahippocampus is a key region in the limbic system. Sheline and colleagues (70) showed that depressed subjects looking at negative pictures elicited a significantly greater increase in activity in the parahippocampus and hippocampus than control subjects. Dillon and colleagues (71) found that controls, not MDD patients, showed a stronger encoding response to reward tokens in the parahippocampus and the dopaminergic midbrain. They hypothesized that the weaker memory for positive material in MDD patients reflects blunted encoding responses in the dopaminergic midbrain and medial temporal lobes in MDD patients.
We also found that after tVNS treatment, the FC between the DMN and left precuneus, and the OPFC increased compared with stVNS. Furthermore, the DMN FC increase in these regions is also associated with the depression severity of the patients.
In a previous study, Li and colleagues (61) studied DMN resting state FC difference between MDD patients and healthy control subjects as well as the treatment effects of an antidepressant drug. They found that the FC between the posterior default mode subnetwork (similar to the DMN pattern derived from ICA in our study) and posterior cingulate cortex (PCC) were normalized (decreased) after antidepressant treatment. In our study, we found that the FC between the DMN and precuneus increased after tVNS stimulation, and the increase in FC is also associated with the depression severity reduction. This inconsistency may be due to different treatment modalities (61, 72).
In this study, we found that the increase of DMN FC with the rostral ACC and MPFC is associated with a reduction in depression severity. This is consistent with results from a recent study on the treatment effect of transcranial magnetic stimulation in depression (34). These results also provide direct support to the hypothesis (18, 47, 73), which suggests that the rACC / MPFC plays an important role in MDD treatment.
We found the DMN and OPFC FC increased after tVNS treatment. This increase is associated with depression severity reduction. The OPFC is connected to neuroanatomical structures directly involved in emotional and executive processing, such as the hippocampal formation, amygdala, ventral striatum, anterior cingulate, hypothalamus and medial temporal areas (74-76). Interestingly, we observed significant FC changes between the DMN and brain regions such as the parahippocampus, ACC, and medial temporal areas. These results suggest that the modulation of tVNS is not targeted to one particular region, but rather can influence brain region networks associated with emotion / affect regulation. This result is consistent with the brain imaging studies during VNS / tVNS that found activity changes in multiple brain regions (55, 63-65). Particularly, our study demonstrated that after longitudinal treatment, the FC between the DMN and some key regions in VNS pathways and mood regulation were significantly modified, and some of the FC changes are associated with depression symptom relief.
There are several limitations in this study. First, this is not a randomized clinical trial. We used this strategy mainly due to ethical concerns. As the first study to use tVNS alone on patients suffering from mild and moderate depression, we thought it would be wise to test the effectiveness of tVNS first. After demonstrating that tVNS could significantly reduce patients’ symptoms, we recruited a second cohort of patients to test if the treatment effect of tVNS was greater than that of the sham tVNS. Since the baseline characteristics were similar in two cohorts of patients, and the study is completed within one year, we do not expect the design will influence the validity of this study. Nevertheless, a randomized clinical trial is needed in the future.
Secondly, treatments in the study were self-administered by the MDD patients, and thus patient compliance may have influenced the observed results. To enhance compliance, all patients were required to complete daily entries in a diary that was checked during assessments. Nevertheless, this self-administration method provides direct evidence toward the feasibility of widespread application of the method used within the study, which could significantly reduce treatment expenses. Thirdly, the treatment was only four weeks in duration; hence, the results obtained only represent the short to mid-term effects. Further study is needed to evaluate the long-term effects of this treatment option. Also, objectively control of the tVNS application (e.g. recording of the quantity or load of applied impulses) was not included, which prevents us from calculate dose-response relationships. Finally, the sample size is relatively small, and further study with a larger sample size is needed to replicate our study findings.
In summary, we found that tVNS can significantly reduce the severity of depression in patients. After tVNS, DMN FC showed significant changes in brain regions involved in emotional modulation. Some FC changes are also associated with depression severity changes in MDD patients. Our findings provide a framework of the brain network to further understand the mechanism of tVNS treatment in MDD patients.
Supplementary Material
Acknowledgements
This scientific work was supported by the Natural Science Foundation of Beijing China (No. 7111007) to Peijing Rong, Chinese National Natural Science Foundation (No. 30870668, 81273674) to Jiliang Fang, Chinese National Natural Science Foundation Research (No. 30973798) to Peijing Rong, grant of technology development research from the Ministry of Science and Technology (2011EG152313), and the National Twelfth Five-Year Plan of the National Science and Technology Support Program of China (2012BAF14B10). Jian Kong is supported by R01AT006364 (NIH/ NCCIH) and P01 AT006663 (NIH/NCCIH). We also thank Yunyao Ma, Chunhua Xu, Yingge Ma, Shaoyuan Li, Jingjun Zhao for their help in data collection and organization.
Footnotes
Trials registration:
Clinical Trials ChiCTR-TRC-11001201 http://www.chictr.org/cn/
Competing interests
All authors claim no conflict of interest.
References
- 1.Sackeim HA, Lisanby SH. physical treatments in psychiatry. In: Weissman MM, editor. Treatment of depression: bridging the 21 st century. Washington, DC: 2001. American psychiatric press. pp. 151–172. [Google Scholar]
- 2.Michaud CM, Murray CJ, Bloom BR. Burden of disease--implications for future research. Jama. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ. Vagus nerve stimulation: clinical results in depression. In: Schachter SC, Schmidt D, editors. Vagus nerve stimulaiton. Martin Dunitz; London: 2003. pp. 85–112. [Google Scholar]
- 4.George MS, Nahas Z, Bohning DE, Kozel FA, Anderson B, Chae JH, et al. Potential mechanisms of action of vagus nerve stimulaiton for depression. In: Schachter SC, Schmidt D, editors. Vagus nerve stimulaiton. Martin Dunitz; London: 2003. pp. 68–83. [Google Scholar]
- 5.Nemeroff CB, Mayberg HS, Krahl S E, McNamara J, Frazer A, Henry TR, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 6.Daban C, Martinez-Aran A, Cruz N, Vieta E. Safety and efficacy of Vagus Nerve Stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110:1–15. doi: 10.1016/j.jad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst. 2000;16:101–102. doi: 10.1007/s003810050021. [DOI] [PubMed] [Google Scholar]
- 8.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59:S3–14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 9.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–37. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 10.Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm. 2013;120:821–827. doi: 10.1007/s00702-012-0908-6. [DOI] [PubMed] [Google Scholar]
- 11.Rong PJ, Fang JL, Wang LP, Meng H, Liu J, Ma YG, et al. Transcutaneous vagus nerve stimulation for the treatment of depression: a study protocol for a double blinded randomized clinical trial. BMC Complement Altern Med. 2012;12:255. doi: 10.1186/1472-6882-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rong P, Liu A, Zhang J, Wang Y, He W, Yang A, et al. Transcutaneous vagus nerve stimulation for refractory epilepsy: a randomized controlled trial. Clin Sci (Lond) 2014 doi: 10.1042/CS20130518. doi:10.1042/CS20130518. [DOI] [PubMed] [Google Scholar]
- 13.Stefan H, Kreiselmeyer G, Kerling F, Kurzbuch K, Rauch C, Heers M, et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: A proof of concept trial. Epilepsia. 2012;53:e115–118. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang F, Dong J, Kong J, Wang H, Meng H, S paeth RB, et al. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement Altern Med. 2014;14:203. doi: 10.1186/1472-6882-14-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 16.Greicius M. Resting-state functional connectivity in neurops ychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 17.Northoff G, Wiebking C, Feinberg T, Panksepp J. The 'resting-state hypothesis' of major depressive disorder-a translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev. 2011;35:1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mwangi B, Ebmeier KP, Matthews K, Steele JD. Multi-centre diagnostic classification of individual structural neuroimaging scans from patients with major depressive disorder. Brain. 2012;135:1508–1521. doi: 10.1093/brain/aws084. [DOI] [PubMed] [Google Scholar]
- 20.Silbersweig D. Default mode subnetworks, connectivity, depression and its treatment: toward brain-based biomarker development. Biol Psychiatry. 2013;74:5–6. doi: 10.1016/j.biopsych.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Liu J, Zhong N, Qin Y, Zhou H, Li K. Changes in the brain intrinsic organization in both on-task state and post-task resting state. Neuroimage. 2012;62:394–407. doi: 10.1016/j.neuroimage.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 24.Andrews -Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckner RL, Sepulcre J, Taluk dar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Hermens DF, Hickies IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default Mode Network Mechanisms of Transcranial Magnetic Stimulation in Depression. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 36.Wu D, Yuan Y, Bai F, You J, Li L, Zhang Z. Abnormal functional connectivity of the default mode network in remitted late-onset depression. J Affect Disord. 2013;147:277–287. doi: 10.1016/j.jad.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Smith S M, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 38.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, S mith S M, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong J, Jensen K, Loiotile R, Cheetham A, Wey HY, Tan T, et al. Functional connectivity of frontoparietal network predicts cognitive modulation of pain. Pain. 2013;154:459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu D, Chang J, Freeman S, Tan Z, Xiao J, Gao Y, et al. Changes of functional connectivity in the left frontoparietal network following aphasic stroke. Front Behav Neurosci. 2014;8:167. doi: 10.3389/fnbeh.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beckmann CF, Smith S M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 42.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dagyte G, Den Boer JA, Trentani A. The cholinergic system and depression. Behav Brain Res. 2011;221:574–582. doi: 10.1016/j.bbr.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Graef S, Schonknecht P, Sabri O, Hegerl U. Cholinergic receptor subtypes and their role in cognition, emotion, and vigilance control: an overview of preclinical and clinical findings. Psychopharmacology (Berl) 2011;215:205–229. doi: 10.1007/s00213-010-2153-8. [DOI] [PubMed] [Google Scholar]
- 46.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 47.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 48.Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan S K, Davis S M, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 49.Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan S K, Davis SM, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 50.Mohr P, Rodriguez M, Slavickova A, Hanka J. The application of vagus nerve stimulation and deep brain stimulation in depression. Neuropsychobiology. 2011;64:170–181. doi: 10.1159/000325225. [DOI] [PubMed] [Google Scholar]
- 51.Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, Bohning DE. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36:219–227. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 52.Mu Q, Bohning DE, Nahas Z, Walker J, Anderson B, Johnson KA, et al. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol Psychiatry. 2004;55:816–825. doi: 10.1016/j.biopsych.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res. 2006;146:179–184. doi: 10.1016/j.pscychresns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Conway CR, Sheline YI, Chibnall JT, Bucholz RD, Price JL, Gangwani S, et al. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul. 2012;5:163–171. doi: 10.1016/j.brs.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 2000;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- 56.Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20:221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 57.Krahl S E, Clark KB, S mith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 58.Walker BR, Easton A, Gale K. Regulation of limbic motor seizures by GABA and glutamate transmission in nucleus tractus solitarius. Epilepsia. 1999;40:1051–1057. doi: 10.1111/j.1528-1157.1999.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012;12:181. doi: 10.1186/1471-244X-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng LL, et al. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, et al. Emotion-Dependent Functional Connectivity of the Default Mode Network in Adolescent Depression. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kraus T, Hosl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 64.Dietrich S, S mith J, Scherzinger C, Hofmann-Preiss K, Freitag T, Eisenkolb A, et al. [A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI]. Biomed Tech (Berl) 2008;53:104–111. doi: 10.1515/BMT.2008.022. [DOI] [PubMed] [Google Scholar]
- 65.Kraus T, Kiess O, Hosl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal - a pilot study. Brain Stimul. 2013;6:798–804. doi: 10.1016/j.brs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in s witching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dillon DG, Dobbins IG, Pizzagalli DA. Weak reward source memory in depression reflects blunted activation of VTA/SN and parahippocampus. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, et al. Modulation of cortical-limbic pathways in major depression: treatment-s pecific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 73.Mayberg HS, Brannan S K, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical res ponse. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 74.Jackowski AP, Araujo Filho GM, Almeida AG, Araujo CM, Reis M, Nery F, et al. The involvement of the orbitofrontal cortex in psychiatric disorders: an update of neuroimaging findings. Rev Bras Psiquiatr. 2012;34:207–212. doi: 10.1590/s1516-44462012000200014. [DOI] [PubMed] [Google Scholar]
- 75.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rolls ET. The orbitofrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1433–1443. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.