Abstract
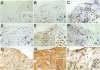
BACKGROUND--The formation of coronary artery neointima experimentally induced in piglets after cardiac transplantation is related to an immune-inflammatory reaction associated with increased expression of T cells and inflammatory mediators (tumour necrosis factor alpha and interleukin 1 beta) and upregulation of fibronectin. In vivo blockade of tumour necrosis factor alpha in rabbits after cardiac transplantation results in reduced neointimal formation. The objective of this study was to investigate the hypothesis that coronary restenosis after atherectomy or percutaneous balloon angioplasty is associated with a similar inflammatory cascade initiated by mechanical injury. METHODS--Specimens taken at coronary atherectomy were analysed from 16 patients. Nine had had the procedure performed twice, firstly, to remove a primary lesion, and secondly, to remove a restenotic lesion. Seven had percutaneous balloon angioplasty after removal of restenotic tissue. Coronary atherectomy specimens were analysed by immunohistochemistry for the presence of T cells, macrophages, major histocompatibility complex II, interleukin 1 beta, tumour necrosis factor alpha, fibronectin, and the receptor for hyaluronan mediated motility. RESULTS--The groups were clinically and angiographically similar with equivalent lumens before and after atherectomy. Restenotic lesions had increased expression of tumour necrosis factor alpha and fibronectin compared with the primary lesions (P < 0.05 for both). There was also a trend towards a greater number of T cells and increased expression of interleukin 1 beta. CONCLUSIONS--Restenosis is associated with increased expression of tumour necrosis factor alpha and fibronectin, suggesting that an immune-inflammatory reaction probably contributes to neointimal formation and may represent a form of wound healing and repair secondary to mechanical injury.
Full text
PDF


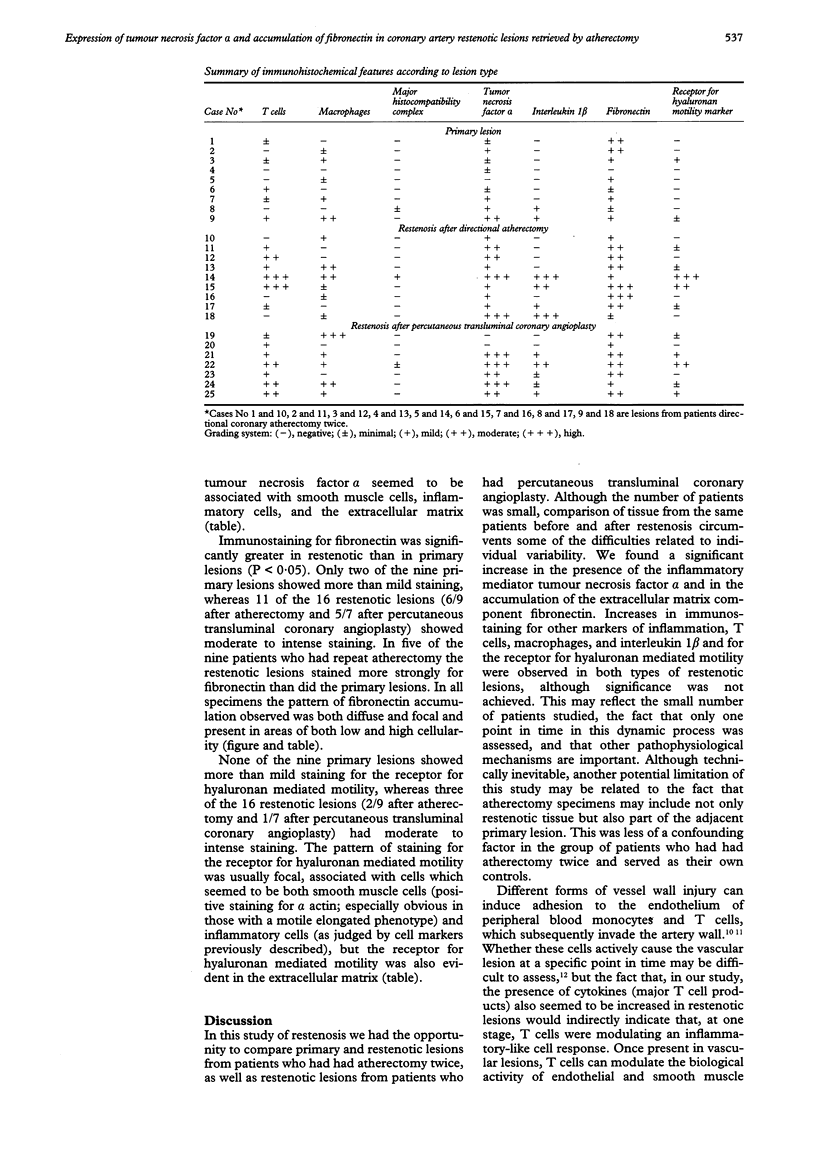


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman A. G., Cohen E. A., Kimball B. P., Bonan R., Ricci D. R., Webb J. G., Laramee L., Barbeau G., Traboulsi M., Corbett B. N. A comparison of directional atherectomy with balloon angioplasty for lesions of the left anterior descending coronary artery. N Engl J Med. 1993 Jul 22;329(4):228–233. doi: 10.1056/NEJM199307223290402. [DOI] [PubMed] [Google Scholar]
- Ager A., Humphries M. J. Use of synthetic peptides to probe lymphocyte--high endothelial cell interactions. Lymphocytes recognize a ligand on the endothelial surface which contains the CS1 adhesion motif. Int Immunol. 1990;2(10):921–928. doi: 10.1093/intimm/2.10.921. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Rabinovitch M. Developmentally regulated changes in extracellular matrix in endothelial and smooth muscle cells in the ductus arteriosus may be related to intimal proliferation. Lab Invest. 1991 Feb;64(2):187–199. [PubMed] [Google Scholar]
- Boudreau N., Turley E., Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991 Feb;143(2):235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Clausell N., Molossi S., Rabinovitch M. Increased interleukin-1 beta and fibronectin expression are early features of the development of the postcardiac transplant coronary arteriopathy in piglets. Am J Pathol. 1993 Jun;142(6):1772–1786. [PMC free article] [PubMed] [Google Scholar]
- Clausell N., Molossi S., Sett S., Rabinovitch M. In vivo blockade of tumor necrosis factor-alpha in cholesterol-fed rabbits after cardiac transplant inhibits acute coronary artery neointimal formation. Circulation. 1994 Jun;89(6):2768–2779. doi: 10.1161/01.cir.89.6.2768. [DOI] [PubMed] [Google Scholar]
- Clausell N., Rabinovitch M. Upregulation of fibronectin synthesis by interleukin-1 beta in coronary artery smooth muscle cells is associated with the development of the post-cardiac transplant arteriopathy in piglets. J Clin Invest. 1993 Oct;92(4):1850–1858. doi: 10.1172/JCI116776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick C., Hoare K., Owens R., Hohn H. P., Hook M., Moore D., Cripps V., Austen L., Nance D. M., Turley E. A. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol. 1992 Jun;117(6):1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jättelä M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991 Jun;64(6):724–742. [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask K. K., Lash J. W. Precardiac cell migration: fibronectin localization at mesoderm-endoderm interface during directional movement. Dev Biol. 1986 Mar;114(1):87–101. doi: 10.1016/0012-1606(86)90385-4. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Libby P. Functional significance of human vascular smooth muscle cell-derived interleukin 1 in paracrine and autocrine regulation pathways. Exp Cell Res. 1992 Feb;198(2):283–290. doi: 10.1016/0014-4827(92)90381-h. [DOI] [PubMed] [Google Scholar]
- Molossi S., Clausell N., Rabinovitch M. Reciprocal induction of tumor necrosis factor-alpha and interleukin-1 beta activity mediates fibronectin synthesis in coronary artery smooth muscle cells. J Cell Physiol. 1995 Apr;163(1):19–29. doi: 10.1002/jcp.1041630104. [DOI] [PubMed] [Google Scholar]
- O'Brien E. R., Alpers C. E., Stewart D. K., Ferguson M., Tran N., Gordon D., Benditt E. P., Hinohara T., Simpson J. B., Schwartz S. M. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993 Aug;73(2):223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Samuel S. K., Hurta R. A., Spearman M. A., Wright J. A., Turley E. A., Greenberg A. H. TGF-beta 1 stimulation of cell locomotion utilizes the hyaluronan receptor RHAMM and hyaluronan. J Cell Biol. 1993 Nov;123(3):749–758. doi: 10.1083/jcb.123.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhonin B. V., Domogatsky S. P., Idelson G. L., Koteliansky V. E., Rukosuev V. S. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987 Sep;67(1):9–16. doi: 10.1016/0021-9150(87)90259-0. [DOI] [PubMed] [Google Scholar]
- Simons M., Leclerc G., Safian R. D., Isner J. M., Weir L., Baim D. S. Relation between activated smooth-muscle cells in coronary-artery lesions and restenosis after atherectomy. N Engl J Med. 1993 Mar 4;328(9):608–613. doi: 10.1056/NEJM199303043280903. [DOI] [PubMed] [Google Scholar]
- Speir E., Modali R., Huang E. S., Leon M. B., Shawl F., Finkel T., Epstein S. E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994 Jul 15;265(5170):391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- Walpola P. L., Gotlieb A. I., Langille B. L. Monocyte adhesion and changes in endothelial cell number, morphology, and F-actin distribution elicited by low shear stress in vivo. Am J Pathol. 1993 May;142(5):1392–1400. [PMC free article] [PubMed] [Google Scholar]