ABSTRACT
Pancreatic ductal adenocarcinoma (PDAC) is highly infiltrated by CD4+T cells that express RORγt and IL-17 (TH17). Compelling evidence from the tumor microenvironment suggest that regulatory T cells (Treg) contribute to TH17 mediated inflammation. Concurrently, PDAC patients have elevated levels of pro-inflammatory cytokines that may lead to TH17 associated functional plasticity in Treg. In this study, we investigated the phenotype and functional properties of Treg in patients with PDAC. We report that PDAC patients have elevated frequency of FOXP3+Treg, which exclusively occurred within the FOXP3+RORγt+Treg compartment. The FOXP3+RORγt+Treg retained FOXP3+Treg markers and represented an activated subset. The expression of RORγt in Treg may indicate a phenotypic switch toward TH17 cells. However, the FOXP3+RORγt+Treg produced both TH17 and TH2 associated pro-inflammatory cytokines, which corresponded with elevated TH17 and TH2 immune responses in PDAC patients. Both the FOXP3+Treg and FOXP3+RORγt+Treg from PDAC patients strongly suppressed T cell immune responses, but they had impaired anti-inflammatory properties. We conclude that FOXP3+RORγt+Treg have a dual phenotype with combined pro-inflammatory and immunosuppressive activity, which may be involved in the pathogenesis of PDAC.
KEYWORDS: FOXP3, plasticity, RORγt, regulatory T Cells, suppression, surface Markers, Th2 cell, Th17 cell
Abbreviations
- CFSE
Carboxyfluorescein succinimidyl ester
- FOXP3
Forkhead box P3
- HD
Healthy donor
- IL
Interleukin
- INF
Interferon
- MFI
Median fluorescence intensity
- PBMC
Peripheral blood mononuclear cells
- PDAC
Pancreatic ductal adenocarcinoma
- Treg
Regulatory T cell
- RORγt
RAR related orphan receptor gammat
- TH cells
T helper cells
- Tresp cells
T responder cells.
Introduction
CD4+FOXP3+Treg cells (Treg) constitute a separate thymus-derived CD4+T cell lineage that is pivotal in maintaining immune tolerance.1 The dominant role of Treg in maintaining immune tolerance and homeostasis in humans is demonstrated in the fatal autoimmune disorder IPEX (immune dysregulation, polyendochrinopathy, enteropathy, X-linked syndrome), which is caused by mutations in the FOXP3 gene.1 Although Treg ensure a protective and balanced immunity to the host, they may also contribute to the suppression of antitumor immunity initiated by tumor-infiltrating and tumor-associated T cells (TILs and TALs).2 Therefore, analysis of Treg may be an interesting prognostication tool in many cancer types.3,4 However, high levels of Treg have also been reported to correlate with both poor and favorable prognosis in various cancer types, which suggests that Treg may have multiple effects on antitumor immunity.5
Pancreatic cancer is the fourth leading cause of cancer-related deaths,6 and is characterized by aggressive growth and poor prognosis even in early stage disease. Adenocarcinoma in the region of the pancreatic head can have different histological types, where the pancreaticobiliary type is most common and most aggressive.7 The putative origin of these tumors, either the pancreatic tissue or distal bile duct is difficult to establish with certainty and does not have prognostic significance given stage parity.8,9
PDAC is associated with chronic inflammation,10 and inflammation combined with expansion of Treg in peripheral blood and in the tumor tissue correlates with poor prognosis.11-13 In addition, infiltration of IL-17 producing TH17 and γδT cells into pancreatic stroma facilitates the initiation and progression of pancreatic intraepithelial neoplasia (PanIN) into PDAC.14 In colon cancer, the infiltration of TH17 cells and the expansion and conversion of Treg into pro-inflammatory IL17+Treg with reduced IL-10 secretion is associated with disease progression.15-18 This suggests that Treg not only suppress antitumor immunity, but they may also contribute to the inflammation.
Here, we show that the frequency of Treg is increased in the peripheral blood of PDAC patients compared to healthy blood donors. However, the expansion occurs exclusively within a subset of Treg that co-express FOXP3 and RORγt. Detailed phenotypic analyses revealed that the FOXP3+RORγt+Treg retained the FOXP3+Treg related markers and were a highly activated Treg subset. Treg from PDAC patients suppressed T cells, but they did not suppress inflammatory immune responses, and our results demonstrate that the expression of RORγt in FOXP3+Treg is associated with pro-inflammatory properties. Due to their suppressive activity of adaptive immune responses combined with pro-inflammatory activity, these cells may represent an attractive therapeutic target in PDAC patients. However, due to the small cohort presented in this study, this must be further investigated in a larger cohort of PDAC patients.
Results
FOXP3+RORγt+Treg expand in peripheral blood of PDAC patients
The frequency of CD4+CD25+Treg is elevated in both peripheral blood and in pancreatic tumors ranging from low-grade pancreatic intraepithelial neoplasia (PanIN) to highly invasive adenocarcinoma.11-13 To assess whether this expansion occurs within the Treg compartment and not in the FOXP3+ non-Treg population, we used the mutually exclusive marker CD127,19 to distinguish CD4+FOXP3+CD127−Treg (total Treg) from CD4+FOXP3+/− non-Treg cells (total TH cells) (Fig. S1). Total Treg frequency was significantly increased in peripheral blood mononuclear cells (PBMCs) of PDAC patients compared to that of HDs (Fig. 1A). A small fraction of IL17+FOXP3+Treg that co-express the FOXP3 and RORγt transcription factors has been shown to be present in peripheral blood from healthy donors (Fig. 1B).20-22 Recent reports suggest that inflammation associated with TH17 immune response in gastro-intestinal cancers can lead to accumulation of IL17+FOXP3+Treg and FOXP3+RORγt+Treg.15-17 TH17 associated inflammation has also been reported to fuel the progression of PDAC.14,23 However, appearance of FOXP3+RORγt+Treg has not been reported in PDAC patients. Here, we analyzed the expression of the TH17 lineage specific master transcription factor RORγt in the total Treg population and found that the frequency of Treg that co-express the FOXP3 and RORγt transcription factors was substantially elevated, whereas Treg that expressed FOXP3 alone were significantly decreased in PBMCs of PDAC patients compared to HDs (Fig. 1B and Fig. S2). More than 80% of the total Treg from PDAC patients and HDs were of the CD45RA− memory phenotype (data not shown). However, the presence of FOXP3+RORγt+Treg was elevated in both the CD45RA+ naïve and the CD45RA− memory compartments of the total Treg pool in PDAC patients compared to HDs (Fig. 1C). FOXP3 has been shown to inhibit the transcriptional activity of RORγt,24 and the increase in RORγt expression in FOXP3+RORγt+Treg from PDAC (Fig. 1D) could potentially indicate Treg lineage instability. However, the expression of FOXP3 was similar in the FOXP3+Treg and FOXP3+RORγt+Treg from PDAC patients and HDs (Fig. 1E). Taken together, these results suggest that FOXP3+RORγt+Treg constitute the expanding fraction of total FOXP3+Treg in PDAC patient peripheral blood.
Figure 1.
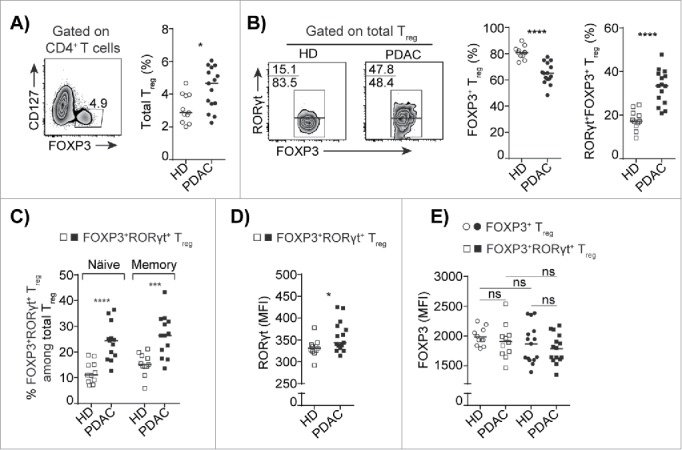
Increased frequency of FOXP3+RORγt+Treg in peripheral blood of PDAC patients. (A) A representative flow cytometry dot plot and compiled frequencies of CD4+FOXP3+CD127−Treg (total Treg) from PBMCs of HD and PDAC. (B) A representative flow cytometry dot plots and compiled frequencies of FOXP3+Treg and FOXP3+RORγt+Treg from PBMCs of HD and PDAC. (C) Percentages of FOXP3+RORγt+Treg in naïve (CD45RA+) and memory (CD45RA−) fraction of total Treg population from HD and PDAC PBMCs. (D) Expression level of RORγt in FOXP3+RORγt+Treg from PBMCs of HD and PDAC. (E) Expression level of FOXP3 in FOXP3+Treg and FOXP3+RORγt+Treg from PBMCs of HD and PDAC. HD (n=11) and PDAC (n=15). Horizontal bar represents median, each dot represents one patient. ns=non-significant, *p ≤ 0.05, ***p ≤ 0.001, ****p ≤ 0.0001.
FOXP3+RORγt+Treg represent a highly activated Treg subset with transition capabilities toward TH17 cells
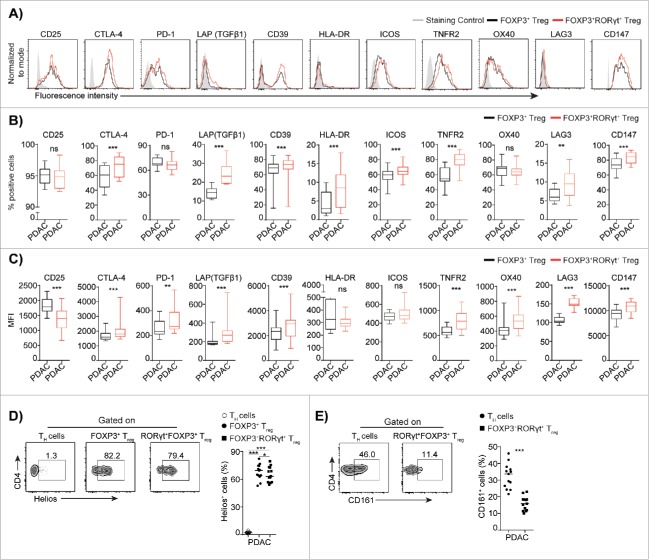
TH17 and Treg cells share a similar ontogeny, and the expression of RORγt could mark a transitional intermediate stage between Treg and TH17 cells.25 Therefore, we analyzed the expression of a panel of established Treg associated markers on circulating FOXP3+RORγt+Treg and compared their phenotype with that of FOXP3+Treg from PDAC patients (Fig. 2A to C). The phenotypic characterization demonstrated that FOXP3+RORγt+Treg is a unique Treg subset that has retained and upregulated a majority of the markers associated with FOXP3+Treg (Fig. 2B and C), which includes the inhibitory related markers CTLA-4, PD-1, LAP (TGFβ1), and CD39 and markers associated with activation that include HLA-DR, ICOS, TNFR2, OX40, LAG3, and CD147.26 Next, we used the thymus derived Treg marker Helios to study the origin of FOXP3+RORγt+Treg. Although the majority of FOXP3+RORγt+Treg were positive for Helios, we observed a significant decrease in the Helios positive subset compared to the FOXP3+Treg in PDAC patients (Fig. 2D), which indicates that the expansion takes place in the periphery. In contrast, TH cells as expected did not express Helios. Human TH17 cells have previously been shown to originate from CD161+CD4+T cell precursors,27 and we found that FOXP3+RORγt+Treg did not express CD161, which strongly indicates that FOXP3+RORγt+Treg from PDAC patients originate from FOXP3+Treg rather than from TH17 cells (Fig. 2E). However, we noticed that FOXP3+RORγt+Treg were positive for CD25, but the level of expression was downregulated (Fig. 2B and C). Expression of CD25 is required for the maintenance of the FOXP3+Treg pool in periphery,28 whereas IL-2 signaling through CD25 interferes with TH17 cells differentiation,29 and downregulation of CD25 indicates that FOXP3+RORγt+Treg may convert into TH17 cells. Together these results suggest that FOXP3+RORγt+Treg represent a distinct subset that is in a transition stage from Treg to TH17 cells.
Figure 2.
Phenotypic characteristics of FOXP3+RORγt+Treg from PDAC patients. Phenotypic analysis of PBMCs from PDAC shows the expression level of indicated markers on FOXP3+RORγt+ Treg compared to FOXP3+Treg. (A) A representative flow cytometry histogram from one donor is shown. The Box plots shows, (B) compiled frequencies and (C) expression level of indicated markers. (D) A representative flow cytometry dot plots and compiled frequencies of Helios+TH, FOXP3+Treg and FOXP3+RORγt+Treg from PDAC PBMCs. (E) A representative flow cytometry dot plots and compiled frequencies of CD161+TH, FOXP3+Treg, and FOXP3+RORγt+Treg from PDAC PBMCs. HD (n = 11) and PDAC (n = 13). Horizontal bar represents median, each dot represents one patient. ns = non-significant, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Expression of RORγt in FOXP3+Treg imprints pro-inflammatory features of TH2 and TH17 lineages
The RORγt transcription factor is essential for the generation of classical IL-17A producing CD4+TH cells.24 To investigate whether FOXP3+RORγt+Treg also display features of TH17 cells, we analyzed the production of cytokines by intracellular cytokine staining after 4 h of stimulation of PBMC. We found that the total Treg population from PDAC patients produced significantly increased levels of IL-17A when compared to that of HDs (Fig. 3A), whereas the production of the inhibitory cytokine IL-10, which is required for Treg to maintain immune homeostasis in the gastrointestinal tract (Fig. 3A),30-32 was significantly reduced from total Treg population from PDAC when compared to HDs. However, the production of other pro-inflammatory cytokines associated with TH2 and TH1 cells was not significantly altered comparing the total Treg from PDAC to that of HDs. Further analysis of intracellular cytokine profiles in FOXP3+Treg compared to FOXP3+RORγt+Treg from PDAC patients showed that only FOXP3+RORγt+Treg produced the TH17 associated cytokine IL-17A (Fig. 3B and C). However, the secretion of TNF-α, which is another TH17 associated cytokine, was significantly upregulated in FOXP3+RORγt+Treg, but was also produced by FOXP3+Treg. Unexpectedly, we found that FOXP3+RORγt+Treg, but not FOXP3+Treg were able to produce the TH2 associated cytokines IL-6 and IL-13 (Fig. 3B and C). The production of TH1 associated cytokine INFγ and the inhibitory cytokine IL-10 were produced at low levels by the FOXP3+Treg and FOXP3+RORγt+Treg (Fig. 3B and C). We also found that a significant fraction of FOXP3+RORγt+Treg were able to produce IL-17A, IL-6, IL-13, and TNF-α concurrently, whereas FOXP3+Treg mostly produced TNF-α, INFγ but little IL-10 and IL-6 (Fig. S3A). We found that in correspondence with their cytokine secretion pattern, the TH17 and TH2 associated chemokine receptors CCR4, CCR6, and intra-epithelial homing marker CD103 were significantly upregulated in FOXP3+RORγt+Treg compared to the FOXP3+Treg and the TH cells (Fig. 3D). Whereas, the expression of the TH1 associated chemokine receptor CXCR3 was not altered. Although the majority of the FOXP3+RORγt+Treg retained the lymphoid homing chemokine receptor CCR7, the expression was significantly reduced (Fig. 3D). These results demonstrate that FOXP3+RORγt+Treg are capable of producing both TH2 and TH17 associated cytokines and expresses chemokine receptors for homing to both non-lymphoid and lymphoid tissues.
Figure 3.
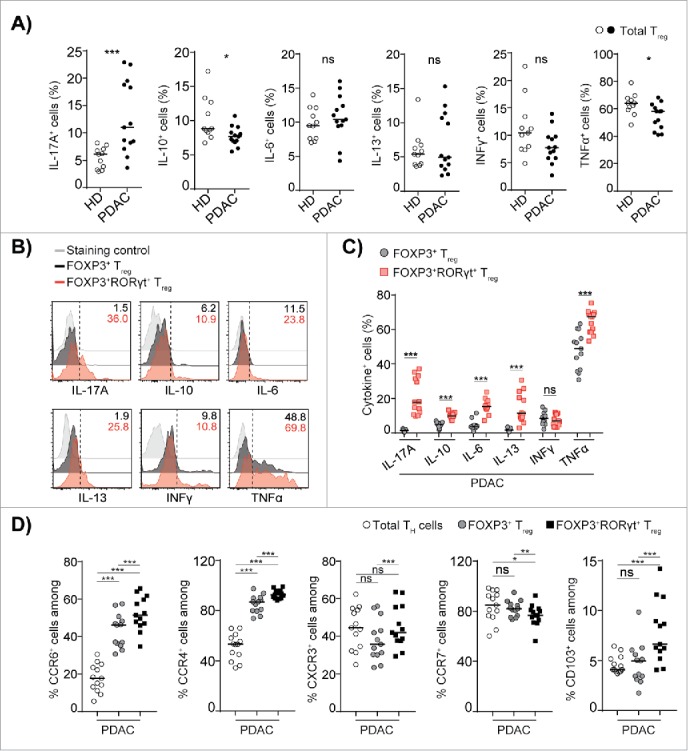
FOXP3+RORγt+Treg share pro-inflammatory cytokines and chemokine receptors of TH2 and TH17 lineages. (A) Compiled frequencies of IL-17A+, IL-10+, IL-6+, IL-13+, TNFα+, and INFγ+ total Treg from HD and PDAC PBMCs. (B) A representative overlaid flow histograms shows the expression levels of indicated cytokines in FOXP3+Treg and FOXP3+RORγt+Treg from PDAC PBMCs. (C) Compiled frequencies of indicated cytokines expressed by FOXP3+Treg and FOXP3+RORγt+Treg from PDAC PBMCs. (D) Compiled frequencies of expression of indicated non-lymphoid and lymphoid homing receptors on FOXP3+Treg and FOXP3+RORγt+Treg from PDAC PBMCs. HD (n = 11) and PDAC (n = 13). Horizontal bar represents median, each dot represents one patient. ns=non-significant, *p ≤ 0.05, ***p ≤ 0.001.
Higher frequency of RORγt+TH cells subset with features of both TH2 and TH17 lineages
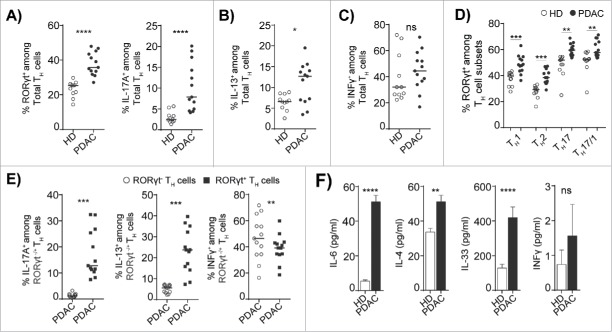
Next, we investigated the whether expansion of FOXP3+RORγt+Treg concurrently lead to an increase in TH2 and TH17 cells in PDAC patients. We found that the frequency of circulating RORγt+ and IL-17A producing TH17 cells in the total TH cell population was strongly increased in PDAC patients compared to HDs (Fig. 4A, right and left panels). Similarly, the circulating IL-13 producing TH2 cells were elevated in the total TH cell population in PDAC patients compared to HDs (Fig. 4B). However, there was no change in TH1 response as the INFγ producing total TH cells in PDAC patients were similar to the level in HDs (Fig. 4C). This indicates that there is a skewing from an antitumor TH1 immunity toward a pro-carcinogenic TH17 and TH2 responses in PDAC patients.33 We noticed that expression of RORγt was not only restricted to TH17 cells in PDAC and HDs, but was also expressed in TH1, TH2, and TH17/1 subsets (Fig. 4D). However, the expression level of RORγt was increased in all TH subsets identified in PDAC patients (Fig. 4D), which further supports the notion that there is a TH17 associated plasticity in PDAC patients. In humans, TH17 cells are highly plastic and contribute to inflammatory conditions,34 and owing to the elevated TH17 and TH2 responses in PDAC patients, we next analyzed the cytokine expression in RORγt− and RORγt+ TH cells. Notably, we found that the expression of the TH17 associated cytokine IL-17A and the TH2 associated cytokine IL-13 were strictly confined to the RORγt+TH cells (Fig. 4E). However, RORγt+TH cells and RORγt−TH cells expressed equal levels of INFγ (Fig. 4E). Furthermore, we confirmed that the PDAC patients had elevated levels of circulating TH17 and TH2 associated cytokines, which included IL-6, IL-4, and IL-33 with no change in the level of INFγ (Fig. 4F). Together, these results indicate that RORγt+TH cells appear to have a TH17/TH2 phenotype and are capable of producing the corresponding TH17 and TH2 cytokines. These findings mirrored the phenotypical features of FOXP3+RORγt+Treg and indicate that FOXP3+RORγt+Treg and RORγt+TH contribute to elevated TH17 and TH2 immune responses in PDAC patients.
Figure 4.
A subset of RORγt+TH cells displays features of both TH2 and TH17 lineages. (A) Compiled frequencies of RORγt+ (left panel) IL-17A+ (right panel), (B) IL13+, and (C) INFγ+TH cells from HD and PDAC PBMCs. (D) Compiled frequency of RORγt expression in TH cell subsets from HD and PDAC PBMCs. (E) Compiled frequencies shows the IL-17A+, IL-13+, and INFγ+ RORγt− and RORγt+TH cells from PDAC PBMCs. HD (n = 11) and PDAC (n = 13). (F) Cytokines in HD and PDAC serum by ELISA HD (n = 10 to 11) and PDAC (n = 12 to 15). Error bars represents mean ± SEM, Horizontal bar represents median, each dot represents one patient. ns=non-significant, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, **** p ≤ 0.0001.
Treg from PDAC suppress T cell immune responses, but enhance inflammation
To examine the suppressive function of circulating Treg isolated from PDAC patients, we used CD4+FOXP3+CD127dim/− Treg (total Treg) and Treg depleted PBMCs from the same donor as Tresp cells in co-culture experiments. Treg from the PDAC patients strongly suppressed the proliferation of CD4+Tresp and CD8+Tresp cells at a similar potency as Treg from HDs (Fig. 5A). The suppression of Treg was dependent on the Treg: Tresp ratio and Treg from PDAC patients retained suppression at all the ratios tested (Fig. 5B). The Treg also suppressed significantly the secretion of IL-2, TNF-α, and IL-10, but increased the production of IL-17A (Fig. 5C). Treg in presence of TGFβ and IL-6 have previously been shown to promote IL-17A induction in TH cells.35-37 As already shown (Fig. 2B and C), TGFβ is expressed significantly higher in FOXP3+RORγt+Treg compared to FOXP3+Treg and are also able to secrete IL-6 (Fig. 3B and C), thus the FOXP3+RORγt+Treg may induce IL-17A secretion from TH cells by this mechanism. However, the expanded FOXP3+RORγt+Treg also expressed IL-17A at the same level as TH cells from PDAC patients, and both the FOXP3+RORγt+Treg and the TH cells may therefore contribute to the elevated level of IL-17A in the co-culture supernatants. Since FOXP3+RORγt+Treg, constitute more than 35% of total Treg population, the data suggest that PDAC Treg, which include both the FOXP3+Treg and the FOXP3+RORγt+Treg subsets are T cell immunosuppressive. Treg and mast cells share substantial co-localization in tissues and lymph nodes, and both cell types infiltrate the tumor microenvironment in PDAC.38 The interaction between Treg and mast cells enhances mast cell degranulation and promotes skewing of Treg from anti-inflammatory properties toward pro-inflammatory functions without losing its suppressive function.39 To test whether Treg enhance mast cell degranulation, we co-cultured Treg from patients and HDs with mast cell in a 1:1 ratio. Treg from PDAC patients significantly enhanced mast cell degranulation compared to Treg from HDs (Fig. 5D). Furthermore, we confirmed that PDAC patients have increased plasma tryptase activity compared to HDs (Fig. 5E), which indicates that there is enhanced mast cell degranulation in PDAC patients. Finally, we investigated the possibility to target the FOXP3+RORγt+Treg using the RORγt specific inhibitor digoxin, which inhibits the development of TH17 cells by antagonizing RORγt function and IL-17A production.40 We analyzed the production of cytokines by the FOXP3+RORγt+Treg compared to that of the FOXP3+Treg from digoxin pre-treated PBMCs from PDAC patients. We found that the production of IL-17A was strongly inhibited in the FOXP3+RORγt+Treg (Fig. 5F), whereas the production of IL-10 was largely unaltered in both the FOXP3+RORγt+Treg and the FOXP3+Treg (Fig. 5F). Furthermore, we found that the digoxin treated PBMCs demonstrated a significant decrease in the total Treg population and that this effect modestly influenced the composition of FOXP3+RORγt+Treg versus FOXP3+Treg (Fig. 5G). Similar results were observed for the RORγt+TH cells (data not shown). Thus, Treg from PDAC patients, which includes both FOXP3+Treg and FOXP3+RORγt+Treg, retain their T cell suppressive activity. However, the Treg population undergoes a shift from anti-inflammatory to pro-inflammatory activity, which can be inhibited using RORγt specific inhibitors.
Figure 5.
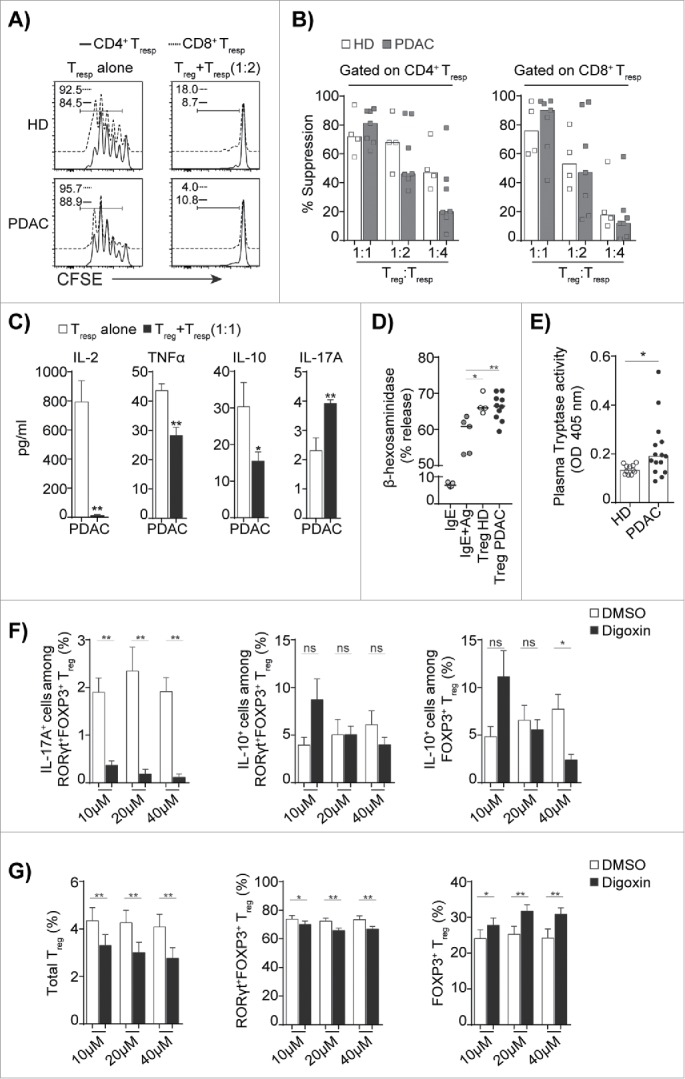
Treg that include FOXP3+Treg and FOXP3+RORγt+Treg from PDAC are immunosuppressive but enhance mast cell degranulation. (A) A representative overlaid flow histograms shows the suppressive capacity of Treg against CD4+Tresp (black), CD8+Tresp (blue) from HD and PDAC PBMCs. Left panel shows Tresp stimulated in the absence of Treg and right panel shows Tresp stimulated in presence of Treg (Treg:Tresp = 1:2 ratio). (B) Compiled percentage of suppression by Treg from HD and PDAC PBMCs with increasing Tresp to Treg ratio in the co-culture. (C) Cytokines from co-cultures of Treg and Tresp from PDAC PBMCs by ELISA. HD (n=4) and PDAC (n = 7). (D) Compiled percentage degranulation of LAD2 human mast cells sensitized with IgE alone, IgE + antigen cross-linked, or co-cultured with purified Treg (Treg:MC = 1:1 ratio) from HD and PDAC PBMCs. HD (n = 4) and PDAC (n = 10). (E) Tryptase activity of HD and PDAC serum was measured by spectrophotometric assay HD (n = 11) and PDAC (n = 15). (F) Compiled bar graph shows the IL-17A and IL-10 expressing FOXP3+RORγt+Treg and FOXP3+Treg, and (G) The frequency of total Treg, FOXP3+RORγt+Treg and FOXP3+Treg from PDAC PBMCs that were pretreated with indicated concentrations of the RORγt inhibitor digoxin or with dimethyl sulfoxide (DMSO) as control. PDAC (n = 10). Error bars represents mean ± SEM, horizontal bar represents median and each dot represents one patient. ns = non-significant, *p ≤ 0.05, **p ≤ 0.01.
Discussion
The FOXP3+Treg have diverse roles in the pathogenesis of human malignancies and have been associated with both favorable prognosis and progressive disease.2,5 The Treg population is heterogeneous and the composition could explain why Treg may have opposing effects on the course of the disease in cancer patients. Treg is one of the major T cell populations that infiltrate cancer tissues. Here, we demonstrate that FOXP3+Treg isolated from peripheral blood of patients with PDAC expand, but the expansion occurs only within the FOXP3+RORγt+Treg subset, which have combined inflammatory and immunosuppressive properties. This population may be particularly detrimental for the prognosis by suppressing adaptive antitumor immunity, whereas the pro-inflammatory nature may support malignant transformation and tumor growth by contributing to a microenvironment enriched in growth factors, pro-inflammatory cytokines, pro-angiogenic factors, and reactive oxygen species. Furthermore, recent evidence suggests that IL-17A signaling promotes the initiation of pancreatic intraepithelial neoplasia (PanIN) and disease progression.14 It is also interesting to note that immunosuppressive treatment in solid organ recipients and patients with chronic inflammatory diseases have been associated with an increased risk of developing pancreatic cancer.41, 42
Pro-carcinogenic processes that involve elevated TH17 immune response are involved in the pathogenesis of pancreatic, gastric, lung, and colon cancer.14,15,18,43,44 TH17 cells that express RORγt infiltrate tumor tissue in patients with PDAC, but Treg comprise a larger fraction of the total tumor infiltrating CD4+T cells compared to the TH17 cells. Even though FOXP3+Treg are a stable T cell lineage, phenotypic plasticity has been demonstrated in inflammatory conditions where the FOXP3+Treg may acquire pro-inflammatory properties. This suggests that the TH17 cells and the FOXP3+Treg may act in concert to propagate the disease. This is further supported by the identification of a small fraction of Treg that co-express FOXP3 and RORγt,20,21 which expands particularly in patients with chronic inflammation,42,43 and colon cancer.15-17
In our study, we found a significantly increased number of FOXP3+RORγt+Treg in peripheral blood of PDAC patients. The FOXP3+RORγt+Treg were present in both the naive and memory compartment, which indicates that these cells persist beyond the initial immune response and acquire the ability to persist and engage in a secondary immune response. At the molecular level, FOXP3 interacts with RORγt and antagonize its transcriptional function and subsequently hampers the induction of IL-17 production.24 This is thought to be the basis for the reciprocal balance between suppression of adaptive immunity versus inflammation. However, our results demonstrated that the level of expression of RORγt in FOXP3+RORγt+Treg was similar to the level in TH17 cells. This indicates that FOXP3 does not impair the RORγt function in the FOXP3+RORγt+Treg in these patients. Treg from healthy donors express equal level of two isoforms of FOXP3 by alternate splicing.45 The isoform lacking exon 2 does not interact with RORγt, and it is therefore likely that the expanding FOXP3+RORγt+Treg that we identified in PDAC patients may overexpress this FOXP3 isoform to facilitate FOXP3 and RORγt co-expression in Treg.
Phenotypic plasticity enables the Treg to downregulate FOXP3 expression and acquire TH cell properties.34 The enhanced expression of RORγt in Treg could also mark their potential plasticity toward TH17 cells. However, we found that the FOXP3+RORγt+Treg in PDAC patients retained the expression of FOXP3. The phenotypical features of FOXP3+RORγt+Treg have not been extensively reported, and our analyses herein demonstrate that both surface and intracellular markers associated with FOXP3+Treg activation and function were expressed either equally or at elevated levels in FOXP3+RORγt+Treg. Constitutive expression of LAG3, CTLA-4, and PD-1 has been shown to be essential for Treg suppressive function in the periphery.1 The enhanced expression of these receptors on Treg from PDAC patients could be associated with their suppressive function and blockade may lead to impaired Treg functions in both the FOXP3+RORγt+Treg and the FOXP3+Treg compartment and thereby improve T cell dependent antitumor immunity. Further, the expression of Helios, a marker for thymus derived FOXP3+Treg was expressed at a similar level in the FOXP3+RORγt+Treg as in the FOXP3+Treg, whereas the absence of the human TH17 marker CD161 excluded the possibility that these cells represent a subset that is derived from TH17 cells. Although FOXP3+RORγt+Treg retained FOXP3+Treg associated markers, the surface expression level of CD25 was significantly reduced in FOXP3+RORγt+Treg. The maintenance of FOXP3+Treg frequency in the periphery requires stable CD25 expression,28 and indicates that FOXP3+RORγt+Treg may undergo plasticity toward TH cells.
Treg inhibit inflammation in the gastrointestinal tract through secretion of IL-10.30-32 Our observations in PDAC patients suggest that FOXP3+Treg and FOXP3+RORγt+Treg produce less IL-10, whereas production of IL-17A was restricted to FOXP3+RORγt+Treg. Our observations also suggest that the FOXP3+RORγt+Treg were capable of expressing the poly-functional cytokines IL-6, IL-13, TNF-α which are associated with both TH17 and TH2 responses. It has previously been reported that IL-17A-secreting FOXP3+RORγt+ Treg and IL-10-secreting FOXP3+Treg are in equilibrium during infections and inflammation,46 however our findings in PDAC patients demonstrated that this balance is altered in the direction of pro-inflammatory function with production of TH17 and TH2 cytokines. The FOXP3+RORγt+Treg were also CD45RA- and upregulated the TH17 and TH2 associated non-lymphoid tissue homing receptors CCR6, CCR4, the intra-epithelial homing receptor CD103 and had significantly reduced expression of the lymphoid homing receptor CCR7 compared to the FOXP3+Treg and the TH cells. This suggests that the FOXP3+RORγt+Treg in PDAC are posed to migrate to inflamed tissue.47
Our results also show that the number of circulating RORγt and IL-17A expressing TH17 cells increase in parallel with expansion of FOXP3+RORγt+Treg in the PDAC patients. In addition, PDAC patients also had increased levels of circulating IL-13 expressing TH2 cells. Furthermore, we confirmed that not only the intracellular expression, but also the level of cytokines that are associated with TH17 and TH2 cells were elevated in peripheral blood of the PDAC patients. Unexpectedly, the expression of IL-13 was restricted to RORγt+TH cells. This further adds complexity to CD4+T cell mediated inflammatory responses in PDAC patients. TH17/TH2 hybrid TH cells were demonstrated to exacerbate inflammation in chronic asthma.48-50 Here, we show for the first time that the increased numbers of TH17 cells and TH2 cells in PDAC patients are confined to RORγt+TH cells that produce both IL-13 and IL-17A and represent TH17/TH2 hybrid cells. Furthermore, RORγt+TH cells also share features with FOXP3+RORγt+Treg both in terms of cytokine production and tissue trafficking receptors, which indicates that these cells co-localize at the same sites.
We further assessed the suppressive activity of the Treg from the PDAC patients on CD4+ and CD8+T cells. In malignancies, this implies suppression of anticancer immunity. However, recent studies suggest that Treg promote TH17 differentiation and enhance their function.51 We found that Treg from PDAC patients were able to suppress the proliferation and IL-2 production of the TH cells completely, but the inhibitory effect on IL-17A, IL-10, and TNF-α secretion was compromised. This clearly indicates that Treg from PDAC patients enhance TH17 and TH2 responses, but suppress TH cells. Mast cells also infiltrate PDAC tissue,10 and the level of tryptase in peripheral blood was elevated. Treg interact with mast cells through the OX40 receptor,52 and mast cells have been shown to reduce the suppressive function of Treg and contribute to an additive effect to TH17 responses in cancer microenvironments.39,53 FOXP3+Treg and FOXP3+RORγt+Treg from PDAC patients expressed OX40 receptor and significantly enhanced degranulation from mast cells. Together, this shows that Treg retain their suppressive function toward TH cells while enhancing inflammation by inducing TH17 and TH2 responses in addition to stimulate mast cell degranulation. Finally, we show that specific inhibition of RORγt using digoxin,40 inhibited IL-17A production by FOXP3+RORγt+Treg. These findings indicate a potential future therapeutic strategy to target the inflammatory activity of the FOXP3+RORγt+Treg in the PDAC patients.
In conclusion, our identification, phenotypic, and functional characterization of FOXP3+RORγt+Treg provides an insight into their pro-inflammatory and suppressive functions in PDAC patients. We were able to confirm that FOXP3+RORγt+Treg and RORγt+TH cells exhibit overlapping functions and lead to TH17/TH2 associated immune responses and inflammation that likely contribute to the progression of the disease. Together, these results identify a cellular target that may prove to be therapeutically important in order to resolve inflammation and reverse the suppression of antitumor immunity in PDAC patients.
Materials and methods
Patient samples
Patient studies were approved by the Regional Ethics Committee of the South-Eastern Norway Regional Health Authority. Informed consent was obtained from 53 patients and blood samples were collected preoperatively. Fifteen of the patients treated with Whipple operation with curative intent had localized adenocarcinoma with pancreaticobiliary histology. No patients had received pre-surgical chemo- or radiotherapy. Patients with other tumor origin, histology, and advanced tumors were excluded from this analysis. Blood from age-matched healthy controls (HDs) were obtained from Oslo University Hospital Blood Center (Oslo, Norway). PBMCs were isolated by Ficoll-paque (Axis-Shield Poc AS) buoyant density medium centrifugation. PBMCs were cryopreserved in FBS with 10% DMSO.
Cell culture and purification
PBMCs were thawed and rested overnight at 37°C with 5% CO2 in RPMI 1640 with GlutaMax supplemented with 10% FCS, 1% penicillin-streptomycin (GIBCO), 1% sodium pyruvate, and 1% minimum non-essential amino acids before using it for immunophenotyping and functional assays. The Treg from rested PBMCs were purified using human CD4+CD25+CD127dim/− Treg isolation kit II (Miltenyi Biotech) according to the manufacturer's instructions. The Treg depleted CD3+T cells fraction from the same donor was used as Tresp cells. The LAD2 mast cell line was cultured in serum free StemPro→-34 SFM media (Life Technologies) supplemented with 2 mM L-Glutamine, 100 IU/mL penicillin, 50 μg/mL streptomycin, 100 ng/mL rhSCF (Peprotech) at 37°C with 5% CO2.
Treg suppression assays
Treg were pre-activated for 48 h using α-CD2/CD3/CD28 coated beads (T cell activation/Expansion Kit, Miltenyi Biotech) in a 1:2 ratio (beads to cells). Treg and CFSE (5µM/mL) labeled Tresp cells were mixed in a 1:1 to 1:4 (Treg:Tresp) ratio and stimulated with α-CD2/CD3/CD28 coated beads in a 1:5 ratio (beads:cells). CFSE dilution in Tresp was analyzed after 4 d of co-culture by flow cytometry. For mast cell degranulation inhibition by Treg, LAD2 cells were pre-sensitized over night with 100 ng/mL of biotin-conjugated human immunoglobulin E (IgE). Treg and LAD2 cells were mixed in 1:1 ratio in Tyrode's buffer and incubated for 10 min at 37°C, stimulated with Streptavidin (100 ng/mL, 30 min) and centrifuged. The supernatants and cell lysates (0.1% Triton X-100) were incubated with p-nitrophenyl n-acetyl-β-D-glucosaminide in 0.1 M citrate buffer (pH 4.6) for 60 min at 37°C and neutralized with 400 mM glycine (pH 10.7). Subsequently, β-hexosaminidase release was measured as described previously.54
Cytokine analysis
For intracellular cytokine staining, PBMCs were stimulated with phorbol 12-myristate 13-acetate (50ng/mL) and ionomycin (1 μg/mL) in presence of Brefeldin A (10μg/mL) for 4 h, in some experiments PBMCs were pretreated with 10–40 μM digoxin or equivalent dimethyl sulfoxide (DMSO) for 24 h prior to stimulation and analyzed by flow cytometry. The cytokines from plasma were quantified with ELISA kits (eBiosciences) or Bio-Plex Pro™ Human cytokine kit (Bio-Rad Laboratories) according to manufacturer's instructions.
Flow cytometry reagents
Antibodies used for human Treg phenotyping, α-CD3 PerCP 5.5 (UCHT1), α-CD4+ APCH7 (RPA-T4), α-CD25 V421 (M-A251), α-CD45RA V510 (HI100), α-CD127 BV786 (HIL-7R-M21), α-Foxp3 Ax488 (259D/C7), α-CD3 V421 (UCHT1), α-ICOS PE (DX29), α-HLA-DR PE (TU36), α-CD147 PE (HIM6), α-CXCR3 PE-Cy (1C6/CXCR3), α-CCR6 APC (11A9), α-CD38 APC (HIT2), α-IL-17A BV421 (N49–653), α-IL-13 BV421 (JES10–5A2), α-INFγ PE-Cy7 (B27), α-TNFα APC (6401.1111), and Human FOXP3 buffer were from BD Biosciences. α-CD161 PerCP 5.5 (HP-3G10), α-RORγt PE (AFKJS‐9), and α-IL-6 APC (MQ2–13A5) were from eBiosciences. α-RORγt PerCP (600380), and α-LAG3 APC (FAB2319A) was from R&D Systems. α-LAP PE (TW4–6H10), α-TNFRII PE (3G7A02), α-PD-1 PE-Cy7 (EH12.2H7), α-CD39 PE-Cy7 (A1), α-CD103 PE-Cy7 (Ber-ACT8), α-OX40 PE-Cy7 (Ber-ACT35), α-CCR7 PE-Cy7 (G043H7), α-Helios APC (22F6), α-CTLA-4 APC (L3D10), α-CCR4 APC (L291H4), and α-IL10 PE-Cy7 (JES3–9D7) were from BioLegend. All flow cytometry data was acquired with BD LSR Fortessa™ and analyzed using FlowJo™ version 10 (TreeStar Inc.).
Statistics
p values were calculated using a two-tailed, non-parametric, Wilcoxon signed rank test or Mann–Whitney test (Wilcoxon rank-sum test) for respective paired and unpaired groups. Error bars represents mean ± SEM, horizontal line represent median, and each dot represents one donor. p ≤ 0.05 was considered statistically significant. All statistical values were generated using GraphPad Prism version 6.
Table 1.
The clinicopathological characteristics and immunological parameters in pancreatic cancer patients and healthy donors
| Parameters | PDAC | HD |
|---|---|---|
| Number (n) | 15 | 11 |
| Age (years)a | 69 (51–78) | 68 (65–69) |
| Male-female ratio | 8:7 | 7:4 |
| Tumor Stage (n)b | ||
| la | 1 | |
| lb | 1 | |
| lla | 4 | |
| llb | 8 | |
| lll | 1 | |
| Tumor Grade (n) | ||
| ll | 11 | |
| lll | 4 | |
| Residual Tumor (n) | ||
| 0 | 8 | |
| l | 7 | |
| Histology (n) Pancreaticobiliary | 15 | |
| Neoadjuvant/adjuvant therapy | 0/8 | |
| One-year overall surival, % | 80 | |
| CA19–9, U/mL | 649 (13–36831) | |
| PBMC, × 106 cells/mL | 2.12 (0.92–3.6) | 2.4 (1.84–4.24) |
| CD4+ cells, % | 51.3 (27.1–67.8) | 48.9 (33.1–56.9) |
| CD8+ cells, % | 34.7 (18.2–49.7) | 22.7 (13.1–45.3) |
| Among CD4+CD127+/−FOXP3+Treg | ||
| FOXP3+Treg , % | 65.1 (56–77.6) | 80.8 (73.4–90) |
| RORyt+FoxP3+Treg, % | 33.4 (21.7–47.8) | 17.2 (9.54–24.7) |
Abbreviations: PDAC, pancreatic ductal adenocarcinoma; HD, Healthy donor. The data are represented as median and range.
Age at diagnosis.
According to The American Joint Committee on Cancer.
Contributors
S.C. and E.M.A. designed the experiments; S.C. conducted and analyzed the experiments. S.C., K.T., E.M.A. interpreted the data. S.C. wrote the manuscript together with E.M.A. K.T. and E.M.A. obtained funding, supervised the research and edited the manuscript; H.H., K.J.L., and G.W. provided the patient materials and together with P.D.L contributed to data analysis and interpretation. M.H. did some of the cytokine quantification experiments. All authors reviewed the manuscript and approved the final version.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary Material
Acknowledgments
We thank Dr Arnold Kirshenbaum (Laboratory of Allergic Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA) for providing the LAD2 human mast cell line. We also thank the Oslo University Hospital Blood Center for providing healthy donor samples and Saranya Subramani (NCMM, University of Oslo) for spectrophotometry suggestions. This work was supported by the grants from the Research Council of Norway (221938, 204784 and 187615), the Norwegian Cancer Society (741746 and 419544), the KG Jebsen Foundation (2012/21 and 2012/23), and the South Eastern Norway Regional Health Authority (2010038).
References
- 1.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10:490-500; PMID:20559327; http://dx.doi.org/ 10.1038/nri2785 [DOI] [PubMed] [Google Scholar]
- 2.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol 2013; 34:33-40; PMID:22999714; http://dx.doi.org/ 10.1016/j.it.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin F, Ladoire S, Mignot G, Apetoh L, Ghiringhelli F. Human FOXP3 and cancer. Oncogene 2010; 29:4121-9; PMID:20498631; http://dx.doi.org/ 10.1038/onc.2010.174 [DOI] [PubMed] [Google Scholar]
- 4.Yaqub S, Aandahl EM. Inflammation Versus Adaptive Immunity in Cancer Pathogenesis. Crit Rev Oncog 2009; 15:43-63; PMID:20136627; http://dx.doi.org/ 10.1615/CritRevOncog.v15.i1-2.20 [DOI] [PubMed] [Google Scholar]
- 5.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res 2012; 18:3022-9; PMID:22510350; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-3216 [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11-30; PMID:23335087; http://dx.doi.org/ 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 7.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006; 244:10-5; PMID:16794383; http://dx.doi.org/ 10.1097/01.sla.0000217673.04165.ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, Clausen OP, Gladhaug IP. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer 2008; 8:170; PMID:18547417; http://dx.doi.org/ 10.1186/1471-2407-8-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandhu V, Bowitz Lothe IM, Labori KJ, Lingjaerde OC, Buanes T, Dalsgaard AM, Skrede ML, Hamfjord J, Haaland T, Eide TJ et al.. Molecular signatures of mRNAs and miRNAs as prognostic biomarkers in pancreatobiliary and intestinal types of periampullary adenocarcinomas. Mol Oncol 2015; 9:758-71; PMID:25579086; http://dx.doi.org/ 10.1016/j.molonc.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans A, Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front Physiol 2012; 3:270; PMID:22969725; http://dx.doi.org/ 10.3389/fphys.2012.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, Wang H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One 2014; 9:e91551; PMID:24637664; http://dx.doi.org/ 10.1371/journal.pone.0091551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T, Yanagimoto H, Satoi S, Toyokawa H, Hirooka S, Yamaki S, Yui R, Yamao J, Kim S, Kwon AH. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 2012; 41:409-15; PMID:22158072; http://dx.doi.org/ 10.1097/MPA.0b013e3182373a66 [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12:5423-34; PMID:17000676; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0369 [DOI] [PubMed] [Google Scholar]
- 14.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H et al.. Oncogenic Kras Activates a Hematopoietic-to-Epithelial IL-17 Signaling Axis in Preinvasive Pancreatic Neoplasia. Cancer Cell 2014; 25:621-37; PMID:24823639; http://dx.doi.org/ 10.1016/j.ccr.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM et al.. Expression of RORγt Marks a Pathogenic Regulatory T Cell Subset in Human Colon Cancer. Sci Transl Med 2012; 4:164ra59; http://dx.doi.org/ 10.1126/scitranslmed.3004566; PMID:2324174320952660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, Zhang B, Liu T, Yang P. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol 2011; 89:85-91; PMID:20952660; http://dx.doi.org/ 10.1189/jlb.0910506 [DOI] [PubMed] [Google Scholar]
- 17.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J et al.. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 2011; 186:4388-95; PMID:21357259; http://dx.doi.org/ 10.4049/jimmunol.1003251 [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Kim Min K, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu L-W et al.. Interleukin-17 Receptor A Signaling in Transformed Enterocytes Promotes Early Colorectal Tumorigenesis. Immunity; 41:1052-63; PMID:25526314; http://dx.doi.org/ 10.1016/j.immuni.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B et al.. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701-11; PMID:16818678; http://dx.doi.org/ 10.1084/jem.20060772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E et al.. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A 2009; 106:4793-8; PMID:19273860; http://dx.doi.org/ 10.1073/pnas.0900408106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A 2009; 106:8635-40; PMID:19439651; http://dx.doi.org/ 10.1073/pnas.0900621106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 2009; 113:4240-9; PMID:19171879; http://dx.doi.org/ 10.1182/blood-2008-10-183251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vizio B, Novarino A, Giacobino A, Cristiano C, Prati A, Ciuffreda L, Montrucchio G, Bellone G. Potential plasticity of T regulatory cells in pancreatic carcinoma in relation to disease progression and outcome. Exp Ther Med 2012; 4:70-8; PMID:23060925; http://dx.doi.org/ 10.3892/etm.2012.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY et al.. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008; 453:236-40; PMID:18368049; http://dx.doi.org/ 10.1038/nature06878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du R, Zhao H, Yan F, Li H. IL-17+Foxp3+ T cells: an intermediate differentiation stage between Th17 cells and regulatory T cells. J Leukoc Biol 2014; 96:39-48; PMID:24744433; http://dx.doi.org/ 10.1189/jlb.1RU0114-010RR [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Oppenheim JJ. Resolving the identity myth: key markers of functional CD4+FoxP3+ regulatory T cells. Int Immunopharmacol 2011; 11:1489-96; PMID:21635972; http://dx.doi.org/ 10.1016/j.intimp.2011.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R et al.. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med 2008; 205:1903-16; PMID:18663128; http://dx.doi.org/ 10.1084/jem.20080397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol 2007; 178:4062-71; PMID:17371960; http://dx.doi.org/ 10.4049/jimmunol.178.7.4062 [DOI] [PubMed] [Google Scholar]
- 29.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L et al.. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007; 26:371-81; PMID:17363300; http://dx.doi.org/ 10.1016/j.immuni.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 30.Huber S, Gagliani N, Esplugues E, O'Connor W Jr., Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY et al.. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011; 34:554-65; PMID:21511184; http://dx.doi.org/ 10.1016/j.immuni.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W et al.. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011; 34:566-78; PMID:21511185; http://dx.doi.org/ 10.1016/j.immuni.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999; 190:995-1004; PMID:10510089; http://dx.doi.org/ 10.1084/jem.190.7.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wormann SM, Diakopoulos KN, Lesina M, Algul H. The immune network in pancreatic cancer development and progression. Oncogene 2014; 33:2956-67; PMID:23851493; http://dx.doi.org/24211039 10.1038/onc.2013.257 [DOI] [PubMed] [Google Scholar]
- 34.Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol 2013; 25:305-12; PMID:24211039; http://dx.doi.org/ 10.1016/j.smim.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006; 24:179-89; PMID:16473830; http://dx.doi.org/ 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: Regulatory T cells induce CD4+CD25 -Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J Immunol 2007; 178:6725-9; PMID:17513718; http://dx.doi.org/ 10.4049/jimmunol.178.11.6725 [DOI] [PubMed] [Google Scholar]
- 37.Pandiyan P, Conti Heather R, Zheng L, Peterson Alanna C, Mathern Douglas R, Hernández-Santos N, Edgerton M, Gaffen Sarah L, Lenardo Michael J. CD4+CD25+Foxp3+ Regulatory T Cells Promote Th17 Cells In Vitro and Enhance Host Resistance in Mouse Candida albicans Th17 Cell Infection Model. Immunity 2011; 34:422-34; PMID:21435589; http://dx.doi.org/ 10.1016/j.immuni.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, Pedotti R, Pucillo CE, Colombo MP. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 2009; 114:2639-48; PMID:19643985; http://dx.doi.org/ 10.1182/blood-2009-05-220004 [DOI] [PubMed] [Google Scholar]
- 39.Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ et al.. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci U S A 2010; 107:6430-5; PMID:20308560; http://dx.doi.org/ 10.1073/pnas.0913683107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV et al.. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature 2011; 472:486-90; PMID:21441909; http://dx.doi.org/ 10.1038/nature09978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA et al.. SPectrum of cancer risk among us solid organ transplant recipients. JAMA 2011; 306:1891-901; PMID:22045767; http://dx.doi.org/ 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009; 6:699-708; PMID:19806144; http://dx.doi.org/ 10.1038/nrgastro.2009.177 [DOI] [PubMed] [Google Scholar]
- 43.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A 2014; 111:5664-9; PMID:24706787; http://dx.doi.org/ 10.1073/pnas.1319051111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iida T, Iwahashi M, Katsuda M, Ishida K, Nakamori M, Nakamura M, Naka T, Ojima T, Ueda K, Hayata K et al.. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol Rep 2011; 25:1271-7; PMID:21369705; http://dx.doi.org/ 10.3892/or.2010.1118 [DOI] [PubMed] [Google Scholar]
- 45.Du J, Huang C, Zhou B, Ziegler SF. Isoform-specific inhibition of ROR α-mediated transcriptional activation by human FOXP3. J Immunol 2008; 180:4785-92; PMID:18354202; http://dx.doi.org/ 10.4049/jimmunol.180.7.4785 [DOI] [PubMed] [Google Scholar]
- 46.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med 2008; 205:1381-93; PMID:18504307; http://dx.doi.org/ 10.1084/jem.20080034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, Krenn V, Schon MP, Scheffold A, Lowe JB et al.. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood 2005; 106:3097-104; PMID:16014565; http://dx.doi.org/ 10.1182/blood-2005-05-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallo E, Katzman S, Villarino AV. IL-13-producing Th1 and Th17 cells characterize adaptive responses to both self and foreign antigens. Eur J Immunol 2012; 42:2322-8; PMID:22684943; http://dx.doi.org/ 10.1002/eji.201142227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, Querci V, Angeli R, Matucci A, Fambrini M et al.. Identification of a novel subset of human circulating memory CD4+ T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol 2010; 125:222-30.e4; PMID:20109749; http://dx.doi.org/ 10.1016/j.jaci.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 50.Wang Y-H, Voo KS, Liu B, Chen C-Y, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu Y-J. A novel subset of CD4+ TH2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med 2010; 207:2479-91; PMID:20921287; http://dx.doi.org/ 10.1084/jem.20101376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Oppenheim JJ. Th17 cells and Tregs: unlikely allies. J Leukoc Biol 2014; 95:723-31; PMID:24563509; http://dx.doi.org/18993084 10.1189/jlb.1213633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP et al.. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 2008; 29:771-81; PMID:18993084; http://dx.doi.org/ 10.1016/j.immuni.2008.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganeshan K, Bryce PJ. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-β. J Immunol 2012; 188:594-603; PMID:22156492; http://dx.doi.org/ 10.4049/jimmunol.1102389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuehn HS, Radinger M, Gilfillan AM. Measuring Mast Cell Mediator Release. Curr Protoc Immunol. 2010; Chapter 7:Unit7.38; PMID:21053305; http://dx.doi.org/ 10.1002/0471142735.im0738s91 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.